Abstract
Introduction
Occult or untreated gestational diabetes (GDM) is a well‐known risk factor for adverse perinatal outcomes and may contribute to antepartum stillbirth. We assessed the impact of screening for GDM on the rate of antepartum stillbirths in non‐anomalous pregnancies by conducting a population‐based study in 974 889 women in Austria.
Material and Methods
Our database was derived from the Austrian Birth Registry. Inclusion criteria were singleton live births and antepartum stillbirths ≥24+0 gestational weeks, excluding fetal congenital malformations, terminations of pregnancy and women with pre‐existing type 1 or 2 diabetes. Main outcome measures were (a) overall stillbirth rates and (b) stillbirth rates in women at high risk of GDM (i.e., women with a body mass index ≥30 kg/m2, history of previous intrauterine fetal death, GDM, previous macrosomic offspring) before (2008–2010, “phase I”) and after (2011–2019, “phase II”) the national implementation of universal GDM screening with a 75 g oral glucose tolerance test in Austrian pregnant women by 2011.
Results
In total, 940 373 pregnancies were included between 2008 and 2019, of which 2579 resulted in intrauterine fetal deaths at 33.51 ± 5.10 gestational weeks. After implementation of the GDM screening, a statistically significant reduction in antepartum stillbirth rates among non‐anomalous singletons was observed only in women at high risk for GDM (4.10‰ [95% confidence interval (CI) 3.09–5.43] in phase I vs. 2.96‰ [95% CI 2.57–3.41] in phase II; p = 0.043) but not in the general population (2.76‰ [95% CI 2.55–2.99] in phase I vs. 2.74‰ [95% CI 2.62–2.86] in phase II; p = 0.845). The number needed to screen with the oral glucose tolerance test to subsequently prevent one case of (non‐anomalous) intrauterine fetal death was 880 in the high‐risk and 40 000 in the general population.
Conclusions
The implementation of a universal GDM screening program in Austria in 2011 has not led to any significant reduction in antenatal stillbirths among non‐anomalous singletons in the general population. More international data are needed to strengthen our findings.
Keywords: epidemiology, gestational diabetes, high‐risk pregnancy, intrauterine fetal death, public health, screening, stillbirth
Abbreviations
- BMI
body mass index
- CI
confidence interval
- GDM
gestational diabetes
- IUFD
intrauterine fetal death
- OGTT
oral glucose tolerance test
- RR
relative risk
- GDM
screening program for gestational diabetes
Key message
The universal gestational diabetes screening program implemented in Austria in 2011 has not led to any significant reduction of antenatal stillbirths in non‐anomalous pregnancies ≥24/40 weeks among the general population, but reductions were seen in women considered high risk for developing gestational diabetes, with a number needed to screen of 880.
1. INTRODUCTION
Gestational diabetes (GDM) affects approximately one in six pregnancies worldwide and is considered a reflection of urbanization and hypernutrition. 1 , 2 It is characterized by maternal glucose intolerance, occurring as early as gestational week 16, leading to maternal hyperglycemia and eventually fetal hyperinsulinemia. 3 Traditionally, GDM has been perceived as an acute condition with short‐term consequences for both mother and fetus; however, recent studies have suggested long‐term consequences involving both metabolic and cardiac systems. 4 , 5 , 6 , 7 , 8 Common ways of detecting GDM are either a one‐step approach with a 2‐h oral glucose tolerance test (OGTT) or the two‐step Carpenter–Coustan screening, which includes an initial non‐fasting 1‐h glucose challenge followed by a 3‐h fasting OGTT if results are abnormal. 9 , 10
Occult or untreated GDM is a well‐known risk factor for pre‐eclampsia, fetal macrosomia, neonatal hypoglycemia, and antepartum stillbirth, especially in later gestational weeks. 2 Antepartum stillbirth is a devastating event that occurs with an incidence of about three to four per 1000 births in high‐income countries. 11 , 12 Since the recurrence risk of fetal death is as high as 22‐fold, 13 , 14 , 15 any clinically unexplained stillbirth should be thoroughly investigated for underlying maternal risk factors. 16 , 17 , 18 Maternal investigations include laboratory tests to detect direct causes of fetal demise, such as sepsis and occult bleeding, or to identify biomarkers that may refer to an associated pathology, such as GDM. One of the most common causes for antepartum stillbirth is placental dysfunction; however, GDM may also cause vascular damage, increasing the overall risk for fetal growth restriction and fetal asphyxia. 19 , 20 , 21 , 22 As maternal hyperglycemia is adequately reflected in the fetal circulation and causes high levels of insulin to be released from the fetal pancreas, the underlying pathophysiology for fetal death is thought to be linked to fetal metabolic acidosis, reducing placental oxygen supply and subsequently causing fetal death. 23
We aimed to evaluate the clinical impact of the introduction of a national screening program for GDM (GDM screening) on the stillbirth rate in Austria among the general female population and those at high risk of GDM. Our hypothesis was that universal GDM screening may have led to a reduction in stillbirth rates in both the general population and in high‐risk populations because of the early recognition and treatment of GDM and thus prevention of adverse pregnancy outcomes.
2. MATERIAL AND METHODS
2.1. Diabetes screening in Austria
Austria has about 8.84 million inhabitants, with a female proportion of 50.8%, of which about 2.5 million are in their reproductive years. 11 The Austrian public health system covers nearly the entire population and provides access to health care services across the country. 24 The Mother–Child Booklet program, introduced in 1974 by the Austrian Ministry of Health, is an obligatory license that every pregnant woman receives upon confirmation of a viable pregnancy by her gynecologist. 25 Its aim is to ensure thorough and concise antenatal surveillance via five gynecological examinations, three ultrasound scans and relevant maternal blood tests. The book further documents the infant’s health checks after delivery until the age of 5 years. All examinations are compulsory if parents are to receive governmental social benefits and financial support from the state.
In 2011, the insights gained from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study led an expert commission of the Mother–Child Booklet to expand the maternal laboratory investigations to include a universal obligatory 75 g OGTT at 24–28 gestational weeks. This screening enables the detection of GDM in pregnant populations at both low and high risk for metabolic disorders and associated complications. The test results are usually checked by the gynecologist, who initiates monitoring and treatment upon abnormal results. 26 Prior to the universal GDM screening as introduced within the Mother–Child Booklet, women were screened only randomly or upon signs of GDM (eg maternal obesity or rapid weight gain, fetal macrosomia, polyhydramnios, obesity, family history of diabetes).
2.2. Study design and population
The Austrian Birth Registry (ABR) is a prospective nationwide registry of all live‐ and stillborn deliveries in Austria that was founded in 2008. It retrieves maternal and fetal characteristics from the electronic database ViewPoint (General Electric Company) of all obstetrical departments in Austria, including registered home deliveries. For this population analysis, data were retrospectively derived from the ABR. Data were checked for integrity and consistency, and all patient data were de‐identified prior to analyses.
For this study, we included all singleton live‐ and stillborn deliveries ≥24+0 gestational weeks between January 1, 2008, and December 31, 2019, in Austria. We excluded women with pre‐existing type 1 or 2 diabetes and stillborn fetuses with congenital anomalies, following termination of pregnancy or intrapartum or perinatal death (see the flowchart in Figure 1).
FIGURE 1.
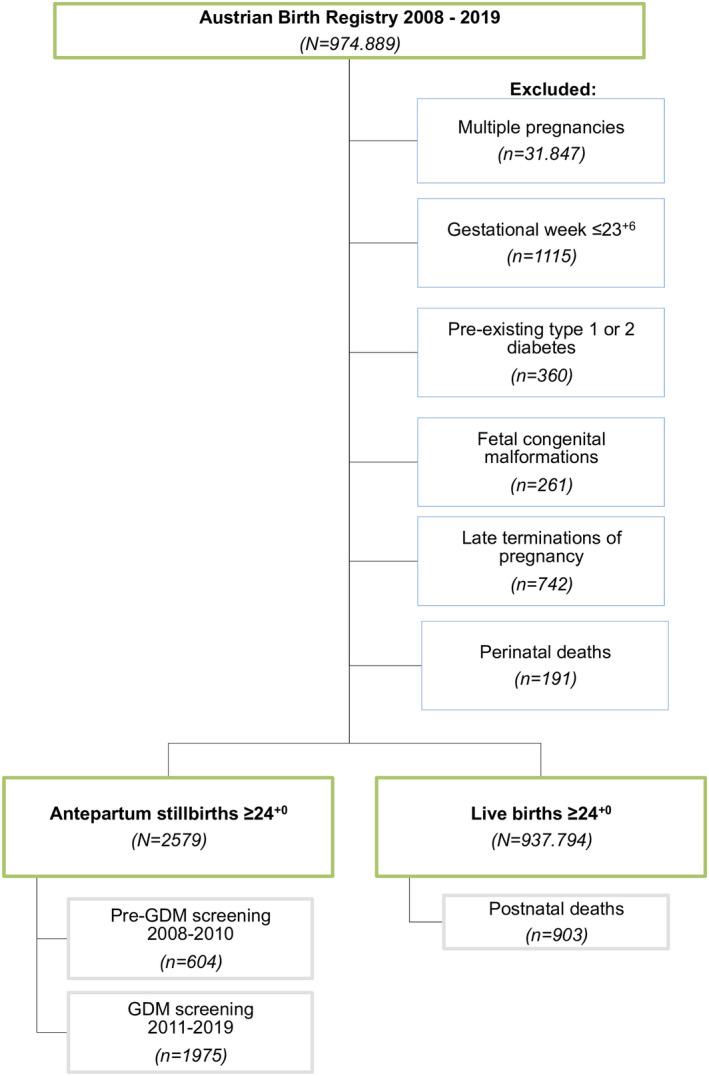
Flowchart showing the eligibility and selection of the study population in Austria between 2008 and 2019
We included all cases available since the implementation of the ABR in 2008, resulting in a 12‐year study period, which we divided into two phases as per the introduction of the GDM screening program in 2011 (phase I: January 1, 2008, to December 31, 2010; phase II: January 1, 2011, to December 31, 2019).
2.3. Definitions
GDM was defined upon abnormal OGTT test results: basal plasma glucose ≥92 mg/dl; 1‐h‐glucose ≥180 mg/dl; 2‐h‐glucose ≥153 mg/dl. 26 Women at high risk of GDM were defined upon meeting one or more of the following criteria 27 : maternal body mass index (BMI) ≥30 kg/m2; history of one or more previous intrauterine fetal death (IUFD); history of previous GDM, and previous delivery of macrosomic infant (birthweight ≥4500 g). BMI was defined as underweight (≤18.5 kg/m2), normal weight (18.6–24.9 kg/m2), pre‐obesity (25.0–29.9 kg/m2), and obesity (≥30.0 kg/m2).
2.4. Statistical analyses
Categorical data are given as absolute (n) and relative frequencies (%). The stillbirth rate is presented as per 1000 births (‰). Continuous data were compared with the unpaired t‐test and the Mann–Whitney U test, respectively. Categorical data were compared with the Chi‐squared test. Log‐binomial regression analyses were carried out to calculate risk ratios (RRs) with 95% confidence interval (CIs) to estimate the effect of the introduction of the GDM screening (binary variable: “0” for the pre‐GDM screening period, “1” for the GDM screening period). A two‐sided p‐value <0.05 was considered statistically significant. Tests were done with STATA (version 16.0, StataCorp LLC). Figures were designed with GraphPad Prism (version 9.3 for Mac, GraphPad Software).
2.5. Ethical approval
The study was approved by the Ethics Committee of the Medical University of Vienna (registration number 1154/2019) on March 12, 2019, and complied with the Declaration of Helsinki. Participants' written consent was not required according to the Austrian Federal Act on Protection of Personal Data (DSG 2000).
3. RESULTS
3.1. Sample characteristics
After consideration of the inclusion and exclusion criteria (Figure 1), the live birth population sample consisted of 937 794 singletons at a mean gestational age of 39.39 ± 1.78 weeks, with 482 400 (51.44%) male and 454 852 (48.50%) female newborns (542 [0.06%] unreported sex). Maternal age was 30.28 ± 5.40 years, and mean BMI was 23.77 ± 4.74 kg/m2.
The sample of antepartum stillbirths between 2008 and 2019 included 2579 singletons (1292 [50.10%] males; 1278 [49.55%] females; nine [0.35%] unreported sex), with a mean gestational age of 33.51 ± 5.10 weeks. Mean maternal age at time of stillbirth was 30.58 ± 6.03 years, and BMI was 24.43 ± 4.94 kg/m2.
3.2. GDM screening and stillbirth rate
Table 1 shows the total sample characteristics before and after the introduction of the GDM screening. In comparison with the pre‐screening period (phase I), women in the total Austrian population were older, more frequently nulliparous, had higher BMI, and had babies with greater infant birthweight and height during phase II (Table 1).
TABLE 1.
Sample characteristics of the included total Austrian pregnant population (N = 940 373) before (“pre‐GDM screening,” i.e., January 2008 to December 2010) and after (“GDM screening,” i.e., January 2011 to December 2019) implementation of the universal gestational diabetes screening program
| Pre‐GDM screening | GDM screening | ||||
|---|---|---|---|---|---|
| 2008–2010 | 2011–2019 | ||||
| N = 218 702 | N = 721 671 | ||||
| Baseline characteristics | Estimates | 95% CI | Estimates | 95% CI | p‐value a |
| Maternal | |||||
| Maternal age (years) | 29.82 ± 5.59 | 29.80–29.84 | 30.42 ± 5.34 | 30.41–30.43 | <0.001 |
| BMI (kg/m2) | 22.50 (20.40–25.50) | 22.50–22.50 | 22.70 (20.50–25.90) | 22.70–22.70 | <0.001 |
| BMI category b | |||||
| Underweight | 7824 (6.25%) | 6.11–6.38 | 36 683 (6.17%) | 6.11–6.23 | <0.001 |
| Normal | 81 963 (65.43%) | 65.17–65.70 | 372 508 (62.98%) | 62.86–63.11 | |
| Pre‐obesity | 24 225 (19.34%) | 19.12–19.56 | 119 993 (20.29%) | 20.18–20.39 | |
| Obesity | 11 248 (8.98%) | 8.82–9.14 | 62 498 (10.57%) | 10.49–10.65 | |
| Parity | |||||
| Nullip | 106 667 (48.77%) | 48.56–48.98 | 361 765 (50.13%) | 50.01–50.24 | <0.001 |
| 1–3 | 108 251 (49.50%) | 49.29–49.77 | 340 053 (47.12%) | 47.01–47.24 | |
| ≥4 | 3784 (1.73%) | 1.67–1.79 | 19 853 (2.75%) | 2.71–2.79 | |
| Gestational diabetes | 6336 (2.90%) | 2.83–2.97 | 31 619 (4.38%) | 4.33–4.43 | <0.001 |
| Smoker | 22 346 (10.22%) | 10.09–10.35 | 59 573 (8.25%) | 8.19–8.32 | <0.001 |
| Fetal | |||||
| Fetal sex c | |||||
| Male | 112 086 (51.3%) | 51.08–51.50 | 371 606 (51.5%) | 51.40–51.63 | 0.064 |
| Female | 106 437 (48.7%) | 48.50–48.92 | 349 693 (48.5%) | 48.37–48.60 | |
| Birthweight (g) | 3350 (3340–3665) | 3350–3355 | 3362 (3050–3670) | 3360–3365 | <0.001 |
| Birth height (cm) | 50.47 ± 2.74 | 50.46–50.48 | 50.64 ± 2.75 | 50.64–50.65 | <0.001 |
Abbreviations: BMI, body mass index; CI, confidence interval; GDM, gestational diabetes; IQR, interquartile range, SD, standard deviation.
Estimates are presented as mean ± SD, n (%), or median (interquartile range) unless otherwise indicated.
p‐values were calculated using the Mann–Whitney U test, the unpaired t‐test and the Chi‐squared test, respectively.
Missing values N = 223 631. BMI categories: underweight (≤18.5 kg/m2), normal (18.6–24.9 kg/m2), pre‐obesity (25.0–29.9 kg/m2), obesity (≥30.0 kg/m2).
Missing values N = 551.
In the pre‐screening phase I (2008–2010), the antepartum stillbirth rate among non‐anomalous singletons was 2.76‰ (95% CI 2.55–2.99). Following the introduction of the GDM screening in 2011, in phase II (2011–2019), the antepartum stillbirth rate was 2.74‰ (95% CI 2.62–2.86; p = 0.845). No differences in maternal and fetal characteristics in the stillbirth cohort were seen between the pre‐screening and screening periods (Table 2).
TABLE 2.
Sample characteristics of the stillbirth cohort (N = 2579) in non‐anomalous pregnancies ≥24+0 gestational weeks before (“pre‐GDM screening,” i.e., January 2008 to December 2010) and after (“GDM screening,” i.e., January 2011 to December 2019) implementation of the universal gestational diabetes screening program in Austria
| Pre‐GDM screening | GDM screening | ||||
|---|---|---|---|---|---|
| 2008–2010 | 2011–2019 | ||||
| N = 604 | N = 1975 | ||||
| Baseline characteristics | Estimates | 95% CI | Estimates | 95% CI | p‐value a |
| Maternal | |||||
| Antepartum stillbirth rate | 2.76‰ | 2.55–2.99 | 2.74‰ | 2.62–2.86 | 0.845 |
| Maternal age (years) | 30.20 ± 6.33 | 29.69–30.70 | 30.70 ± 5.93 | 30.43–30.96 | 0.129 |
| BMI (kg/m2) | 22.9 (20.2–27.0) | 22.16–23.74 | 23.5 (20.8–27.0) | 23.20–23.90 | 0.171 |
| BMI‐category b | |||||
| Underweight | 20 (8.16%) | 5.32–12.33 | 71 (5.16%) | 4.11–6.47 | 0.072 |
| Normal | 142 (57.96%) | 51.67–64.00 | 782 (56.87%) | 54.24–59.47 | |
| Pre‐obesity | 47 (19.18%) | 14.71–24.62 | 346 (25.16%) | 22.94–27.53 | |
| Obesity | 36 (14.69%) | 10.78–19.72 | 176 (12.80%) | 11.13–14.67 | |
| Parity | |||||
| Nullip | 329 (54.47%) | 50.47–58.41 | 1034 (52.35%) | 50.15–54.55 | 0.660 |
| 1–3 | 250 (41.39%) | 37.52–45.37 | 856 (43.34%) | 41.17–45.54 | |
| ≥4 | 25 (4.14%) | 02.81–06.06 | 85 (4.31%) | 3.50–5.29 | |
| Gestational diabetes | 14 (2.31%) | 01.38–03.88 | 63 (3.19%) | 2.50–4.06 | 0.270 |
| Smoker | 83 (13.74%) | 11.22–16.73 | 192 (9.72%) | 8.49–11.11 | 0.005 |
| Fetal | |||||
| Fetal sex c | |||||
| Male | 294 (49.08%) | 45.09–53.08 | 998 (50.63%) | 48.43–52.84 | 0.506 |
| Female | 305 (50.92%) | 46.91–54.91 | 973 (49.37%) | 47.16–51.57 | |
| Stillbirth age (weeks) | 34.0 (29.0–38.0) | 33.00–35.00 | 34.0 (29.0–38.0) | 33.00–34.00 | 0.382 |
| Stillbirth weight (g) | 1895 (1030–2770) | 1770–2050 | 1927.5 (1056–2810) | 1820–2000 | 0.777 |
| Stillbirth height (cm) | 43.39 ± 7.57 | 42.78–44.00 | 43.46 ± 7.73 | 43.11–43.80 | 0.757 |
Abbreviations: BMI, body mass index; CI, confidence interval; GDM, gestational diabetes; IQR, interquartile range; SD, standard deviation.
Estimates are presented as mean ± SD, n (%), or median (interquartile range) unless otherwise indicated.
p‐values were calculated using the Mann–Whitney U test, the unpaired t‐test and the Chi‐squared test, respectively.
Missing values N = 959. BMI categories: underweight (≤18.5 kg/m2), normal (18.6–24.9 kg/m2), pre‐obesity (25.0–29.9 kg/m2), obesity (≥30.0 kg/m2).
Missing values N = 9.
Assessing antepartum stillbirth risk in women at high risk for GDM, the antepartum stillbirth rate was 4.10‰ (95% CI 3.09–5.43) during phase I and 2.96‰ (95% CI 2.57–3.41; p = 0.043) during phase II; the overlapping CIs indicate a minimal difference. Despite a total increase in the prevalence of women considered high risk for GDM over the years (5.40 vs. 9.10%; p < 0.001), the demographic characteristics of high‐risk women experiencing stillbirth were similar regarding age, BMI, and gestational week at stillbirth (Table 3).
TABLE 3.
Sample characteristics of women considered at high risk of developing gestational diabetes (N = 77 210) and their associated stillbirth rates in non‐anomalous pregnancies ≥24+0 gestational weeks (“pre‐GDM screening,” i.e., January 2008 to December 2010) and after (“GDM screening” i.e., January 2011 to December 2019) implementation of the universal gestational diabetes screening program in Austria
| Pre‐GDM screening | GDM screening | ||||
|---|---|---|---|---|---|
| 2008–2010 | 2011–2019 | ||||
| Baseline characteristics | Estimates | 95% CI | Estimates | 95% CI | p‐value a |
| Women at high risk for GDM | 11 712 (5.4) | 5.26–5.45 | 65 498 (9.1) | 9.01–9.14 | <0.001 |
| Antepartum stillbirths, n (‰) | 48 (4.10) | 3.09–5.43 | 194 (2.96) | 2.57–3.41 | 0.043 |
| Maternal age (years) | 32 (26.5–36.0) | 28.69–35.00 | 31 (27.0–36.0) | 31.00–32.15 | 0.883 |
| Maternal BMI (kg/m2) | 32.8 (31.2–35.85) | 31.43–34.29 | 32.9 (31.1–35.8) | 32.00–33.65 | 0.695 |
| Stillbirth age (weeks) | 34.0 (30.0–38.0) | 31.69–37.00 | 35.5 (30.0–39.0) | 35.00–36.00 | 0.579 |
| Fetal sex | |||||
| Male | 19 (39.6) | 26.69–54.11 | 112 (57.7) | 50.62–64.54 | 0.024 |
| Female | 29 (60.4) | 45.89–73.31 | 82 (42.3) | 35.46–49.38 | |
Abbreviations: BMI, body mass index; CI, confidence interval; GDM, gestational diabetes. Estimates are presented as n (%) or median (interquartile range) unless otherwise indicated.
p‐values were calculated using the unpaired t‐test, the Mann–Whitney U test and the Chi‐squared test, respectively.
Figure 2 illustrates the antepartum stillbirth rates in the total population and in women considered at high risk of GDM (“high‐risk population”). In Austria, the most relevant risk factors for stillbirth were obesity (RR 1.40 [95% CI 1.20–1.63]; p < 0.001), pre‐obesity (RR 1.34 [95% CI 1.19–1.50]; p < 0.001), nicotine consumption (RR 1.31 [95% CI 1.13–1.52]; p < 0.001), history of previous stillbirth (RR 1.27 [95% CI 1.04–1.57]; p = 0.021), and nulliparity (RR 1.13 [95% CI 1.02–1.25]; p = 0.017). In women considered at high risk of GDM, GDM screening reduced the risk of stillbirth (RR 0.68 [95% CI 0.50–0.94]; p = 0.019), whereas a history of previous stillbirth increased the risk (RR 1.94 [95% CI 1.36–2.75]; p < 0.001).
FIGURE 2.
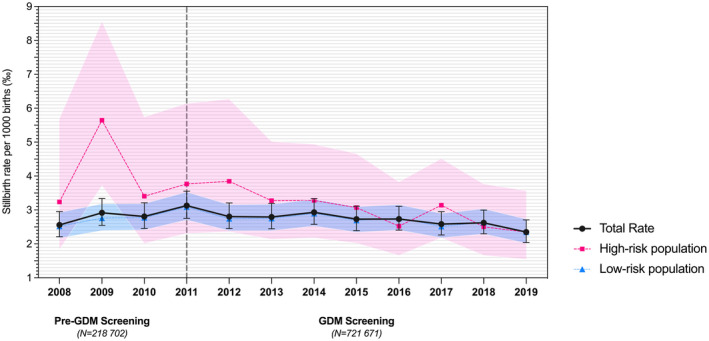
Antepartum stillbirth rates per 1000 births (‰) in non‐anomalous singleton pregnancies ≥24+0 gestational weeks before (“pre‐GDM screening,” i.e., January 1, 2008, to December 31, 2010) and after (“GDM screening,” i.e., January 1, 2011, to December 31, 2019) the implementation of the universal gestational diabetes (GDM) screening program in Austria (vertical gray dotted line). Total population (N = 940 373): Mean values are represented by dots, and black error bars indicate 95% confidence interval (CIs). High‐risk population (N = 77 210), i.e., women at high risk for developing GDM: Mean values are represented by squares, and red‐colored error bands indicate 95% CIs. Low‐risk population (N = 863 163), i.e., women at low risk for developing GDM: Mean values are represented by triangles, and blue‐colored error bands indicate 95% CIs
3.3. Numbers needed to screen by OGTT to prevent one case of IUFD
In the general population, the GDM screening yielded an absolute stillbirth risk reduction of 0.003% and a relative stillbirth risk reduction of 0.91%. In women at high risk of GDM, the screening yielded an absolute stillbirth risk reduction of 0.11% and a relative stillbirth risk reduction of 27.73%. Therefore, in the general population, 40 000 Austrian women would need to be screened using OGTT to prevent one case of (non‐anomalous) stillbirth, whereas the number needed to screen in the high‐risk population is 880.
4. DISCUSSION
In this population‐based study, we aimed to assess the impact of screening for GDM on the risk reduction of antepartum stillbirths >24 weeks of gestation in non‐anomalous singleton pregnancies, excluding women with known type 1 or 2 diabetes mellitus. A longitudinal change of demographic patterns was observed in the general Austrian population, consistent with international trends, moving towards advanced maternal age, increasing body weight, lower parity, and increased neonatal weight and height. At the same time, in the Austrian cohort of women experiencing antepartum stillbirth, these demographic characteristics remained unchanged over time. The prevalence of women identified as at risk of GDM has increased by nearly 4% over this time, and the identification by OGTT of women with gestational diabetes has also increased, which is consistent with previous literature. 28 Although the overall antepartum stillbirth rate remained unchanged in the general population, our study indicates that GDM screening reduced the number of stillbirths in women considered at high risk of GDM. However, critical review of our data indicates that the CIs in our Austrian high‐risk population reflect the paucity of data, so more data are needed to strengthen our findings.
One example of such data is the Midlands and North of England Stillbirth Study (MiNESS), a case–control study that explored the effect of being at risk of GDM and screening for GDM on late stillbirth rates ≥28 weeks of gestation. 29 The authors found that women at risk of GDM overall experienced a modestly increased risk of late stillbirth (adjusted odds ratio [aOR] 1.17 [95% CI 0.87–1.57]). Among these, women at risk of GDM who were not screened for GDM experienced a 44% higher risk of late IUFD than did those not at risk (aOR 1.44 [95% CI 1.01–2.06]), whereas those at risk who did undergo GDM screening had a risk of stillbirth similar to that of women without any risk factors (aOR 0.98 [95% CI 0.70–1.36]). These results are in accordance with a previous analysis that reviewed the risks of maternal conditions on stillbirth: The risk of stillbirth was highest among women with pre‐existing diabetes (aOR 3.21 [95% CI 3.06–3.38]) and among women with diabetes and chronic hypertension (aOR 3.79 [95% CI 3.4–4.2]) but lower in women with GDM (aOR 0.73 [95% CI 0.69–0.76]). 30 Maternal risk factors for IUFD also rose with increasing BMI and advanced maternal age. 31
In 2015, Koivunen et al. showed that the implementation of universal GDM screening in Finland led to a significant increase in primarily mild forms of GDM. 32 Furthermore, the authors found that GDM screening led to decreased birthweight and macrosomia rates, whereas the prevalence of neonatal hypoglycemia increased. 28
Despite the intensity of resources required for the implementation and conduct of a universal GDM screening in a population, research findings are supportive because of the reductions in maternal and fetal harm in the long term. 33 Our study has limitations inherent to its retrospective study design, with the earliest date possible for data acquisition being 2008. The multicenter setting meant it was difficult to ascertain whether data were completely and accurately entered into the databases across delivery units, with subsequent missing data (eg BMI). In addition, we could not control for whether women with underlying GDM having experienced an IUFD had actually received adequate antihyperglycemic treatment or whether all pregnant women in Austria had undergone screening. In addition, we acknowledge the lack of certain variables, such as cause of fetal death, OGTT test results, and family history for diabetes, which is a well‐known factor associated with the recurrent risk of GDM. 27 Another limitation may be failure to control for other maternal co‐morbidities known to be associated with stillbirth, such as sickle‐cell disease or anti‐phospholipid syndrome, in the general population. 34 Although we observed a temporal reduction of nicotine consumption in the general population, which is known to be a very important measure in reducing stillbirth, 35 we acknowledge that smoking history was self‐reported by the woman and may therefore be subject to recall bias or unwillingness to honestly disclose harmful lifestyle habits to healthcare professionals. Furthermore, we acknowledge the potential for unmeasured temporal confounding: A before‐and‐after design is prone to such bias because decreasing stillbirth prevalence could be due to factors external to the screening, eg improvements in care or in the health of the background population at risk. Furthermore, the lack of a transition period following implementation of the OGTT may be a potential methodological limitation. Finally, the pre‐screening period was shorter than the GDM screening period (i.e., 3 vs. 9 years): This also limits the ability to estimate the trend in the pre‐screening period, which would be useful to remove the trend effects from the screening effects. This is analogous to having an “exposure” whose outcomes are more precisely estimated for the exposed group than for the unexposed group.
Despite these limitations, our study is the first nationwide analysis to examine the impact of universal GDM screening on stillbirth risk in a central European population. The clear inclusion and exclusion criteria for singleton antepartum stillbirths ≥24+0 weeks limit the heterogeneity in the underlying pathomechanisms of fetal death. We also consider the number of undiagnosed GDM neglectable because of the obligatory setting of the GDM screening within the Mother–Child Booklet and the nationwide access to health care facilities, including in remote areas. Finally, although the time frame of the first study period was short, no major public health changes occurred in Austria between 2008 and 2010, excluding potential further confounders.
Causes for antepartum stillbirth in the second and third trimester of pregnancy are diverse and vary in their prevalence according to environmental, geographical, and socio‐economic influences. 19 , 36 The correct identification of causative vs contributory factors is of paramount importance and should be regarded as the core of a patient‐centered and evidence‐based practice following fetal bereavement. 37 Gold standards to elucidate the cause of fetal death include fetal autopsy, placental histology, and genetic analyses. 38 , 39 To investigate underlying risk factors, current guidelines recommend maternal laboratory tests to rule out inherited or acquired thrombophilia, anti‐phospholipid syndrome, thyroid disorders, and occult diabetes. In 2017, Page et al. investigated the usefulness of tests within the frame of the post‐mortem workup and found that the total point estimate of finding the cause of fetal death through glucose screening was 1.6% (95% CI 0.7–3.1). 40 Although our study could not confirm the clinical impact of universal GDM screening at 24–28 gestational weeks on stillbirth rates ≥24 gestational weeks in the general population, it did show an effect in the high‐risk population. It is speculated that early hyperglycemia, as may be present in women at high risk of GDM, may elicit epigenetic changes and influence fetal and placental programming, which might eventually lead to placental dysfunction. 21 , 41
In view of the wide spectrum of medical sequalae associated with GDM, universal GDM screening has been widely accepted as an important public health measure. However, more data are needed to verify the robust effects of GDM screening and total stillbirth reduction in early and late gestational weeks.
5. CONCLUSION
In Austria, the implementation of a universal GDM screening program in 2011 has not led to any significant reduction in antenatal stillbirths among non‐anomalous singletons >24 weeks of gestation.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception, planning and carrying out of the research. DAM conceived the study and wrote the first draft of this paper. DAM, BP, WO, HL and HK conceived the study design. SN, HH, WO and HL helped with acquisition of data. BP, SN, WO and HL conducted statistical analyses. All authors contributed to critically revising the manuscript and approved the final version.
Muin DA, Pfeifer B, Helmer H, et al. Universal gestational diabetes screening and antepartum stillbirth rates in Austria—A population‐based study. Acta Obstet Gynecol Scand. 2022;101:396–404. doi: 10.1111/aogs.14334
REFERENCES
- 1. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saravanan P. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020;8:793‐800. [DOI] [PubMed] [Google Scholar]
- 3. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991‐2002. [DOI] [PubMed] [Google Scholar]
- 4. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta‐analysis. Diabetologia. 2019;62:905‐914. [DOI] [PubMed] [Google Scholar]
- 5. Daly B, Toulis KA, Thomas N, et al. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: a population‐based cohort study. PLoS Med. 2018;15:e1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lowe WL Jr, Lowe LP, Kuang A, et al. Maternal glucose levels during pregnancy and childhood adiposity in the hyperglycemia and adverse pregnancy outcome follow‐up study. Diabetologia. 2019;62:598‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang J, Zhang C, Chavarro JE, et al. Lifestyle changes and long‐term weight gain in women with and without a history of gestational diabetes mellitus: a prospective study of 54,062 women in the Nurses' health study II. Diabetes Care. 2022;45:348‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun J, Kim GR, Lee SJ, Kim HC. Gestational diabetes mellitus and the role of intercurrent type 2 diabetes on long‐term risk of cardiovascular events. Sci Rep. 2021;11:21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. 2. Classification and Diagnosis of Diabetes: Standards of medical Care in Diabetes‐2020. Diabetes Care. 2020;43:S14‐S31. [DOI] [PubMed] [Google Scholar]
- 10. ACOG Practice Bulletin No. 190: Gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49‐e64. [DOI] [PubMed] [Google Scholar]
- 11.Statistik Austria. 2020. http://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/bevoelkerung/geborene/index.html.
- 12. Flenady V, Wojcieszek AM, Middleton P, et al. Stillbirths: recall to action in high‐income countries. Lancet. 2016;387:691‐702. [DOI] [PubMed] [Google Scholar]
- 13. Lamont K, Scott NW, Jones GT, Bhattacharya S. Risk of recurrent stillbirth: systematic review and meta‐analysis. BMJ. 2015;350:h3080. [DOI] [PubMed] [Google Scholar]
- 14. Herring AH, Reddy U. Recurrence risk of stillbirth in the second pregnancy. BJOG. 2010;117:1173‐1174. [DOI] [PubMed] [Google Scholar]
- 15. Bhattacharya S, Prescott GJ, Black M, Shetty A. Recurrence risk of stillbirth in a second pregnancy. BJOG. 2010;117:1243‐1247. [DOI] [PubMed] [Google Scholar]
- 16. Royal College of Obstetricians and Gynaecologists . Late Intrauterine Fetal Death and Stillbirth Green–top Guideline No. 55. 2010.
- 17. American College of Obstetricians and Gynecologists . ACOG Practice Bulletin Clinical Management Guidelines No. 102. 2009.
- 18. Perinatal Society of Australia and New Zealand . Clinical Practice Guideline for Care Around Stillbirth and Neonatal Death, Version 3.1, March 2018.
- 19. Flenady V, Koopmans L, Middleton P, et al. Major risk factors for stillbirth in high‐income countries: a systematic review and meta‐analysis. Lancet. 2011;377:1331‐1340. [DOI] [PubMed] [Google Scholar]
- 20. Aldahmash WM, Alwasel SH, Aljerian K. Gestational diabetes mellitus induces placental vasculopathies. Environ Sci Pollut Res Int 2021. doi: 10.1007/s11356-021-17267-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21. Franzago M, Fraticelli F, Stuppia L, Vitacolonna E. Nutrigenetics, epigenetics and gestational diabetes: consequences in mother and child. Epigenetics. 2019;14:215‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carrasco‐Wong I, Moller A, Giachini FR, et al. Placental structure in gestational diabetes mellitus. Biochim Biophys Acta Mol Bas Dis. 2020;1866:165535. [DOI] [PubMed] [Google Scholar]
- 23. Silver RM, Varner MW, Reddy U, et al. Work‐up of stillbirth: a review of the evidence. Am J Obstet Gynecol. 2007;196:433‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bachner F, Bobek J, Habimana K. Austria—Health System review 2018. Vol. 20 No. 3 2018. http://www.euro.who.int/__data/assets/pdf_file/0009/382167/hit‐austria‐eng.pdf?ua=1 [PubMed]
- 25.Bundesministerium für Soziales G, Pflege und Konsumentenschutz,. Mutter‐Kind‐Pass 2020. http://www.sozialministerium.at/Themen/Gesundheit/Eltern‐und‐Kind/Mutter‐Kind‐Pass.html
- 26. Deutsche Gesellschaft für Gynäkologie und Geburtshilfe e.V. Gestationsdiabetes mellitus (GDM), Diagnostik, Therapie und Nachsorge 2018 [Graviditetsdiabetes mellitus (GDM), diagnos, terapi och eftervård 2018 ] In German. updated February 28, 2018; cited 2020 5.10.2020]. http://www.awmf.org/leitlinien/detail/ll/057‐008.html
- 27. National Institute for Health and Care Excellence (NICE) . Diabetes in pregnancy: management from preconception to the postnatal period. London: © Nice 2019.; 2015 Aug. [PubMed]
- 28. Koivunen S, Torkki A, Bloigu A, et al. Towards national comprehensive gestational diabetes screening—consequences for neonatal outcome and care. Acta Obstet Gynecol Scand. 2017;96:106‐113. [DOI] [PubMed] [Google Scholar]
- 29. Stacey T, Tennant P, McCowan L, et al. Gestational diabetes and the risk of late stillbirth: a case‐control study from England, UK. BJOG. 2019;126:973‐982. [DOI] [PubMed] [Google Scholar]
- 30. Patel EM, Goodnight WH, James AH, Grotegut CA. Temporal trends in maternal medical conditions and stillbirth. Am J Obstet Gynecol. 2015;212(673):e1‐e11. [DOI] [PubMed] [Google Scholar]
- 31. Man J, Hutchinson JC, Ashworth M, Heazell AE, Jeffrey I, Sebire NJ. Stillbirth and intrauterine fetal death: contemporary demographic features of >1000 cases from an urban population. Ultrasound Obstet Gynecol. 2016;48:591‐595. [DOI] [PubMed] [Google Scholar]
- 32. Koivunen S, Kajantie E, Torkki A, et al. The changing face of gestational diabetes: the effect of the shift from risk factor‐based to comprehensive screening. Eur J Endocrinol. 2015;173:623‐632. [DOI] [PubMed] [Google Scholar]
- 33. O’Sullivan EP, Avalos G, O’Reilly M, Dennedy MC, Gaffney G, Dunne F. Atlantic diabetes in pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia. 2011;54:1670‐1675. [DOI] [PubMed] [Google Scholar]
- 34. Townsend R, Sileo FG, Allotey J, et al. Prediction of stillbirth: an umbrella review of evaluation of prognostic variables. BJOG. 2021;128:238‐250. [DOI] [PubMed] [Google Scholar]
- 35. Lau YZ, Widdows K, Roberts SA, et al. Assessment of the quality, content and perceived utility of local maternity guidelines in hospitals in England implementing the saving babies' lives care bundle to reduce stillbirth. BMJ Open Qual. 2020;9:e000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lawn JE, Blencowe H, Waiswa P, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387:587‐603. [DOI] [PubMed] [Google Scholar]
- 37. Nijkamp JW, Sebire NJ, Bouman K, Korteweg FJ, Erwich J, Gordijn SJ. Perinatal death investigations: what is current practice? Semin Fetal Neonatal Med. 2017;22:167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Korteweg FJ, Erwich JJ, Timmer A, et al. Evaluation of 1025 fetal deaths: proposed diagnostic workup. Am J Obstet Gynecol. 2012;206:53.e1‐53.e12. [DOI] [PubMed] [Google Scholar]
- 39. Korteweg FJ, Gordijn SJ, Timmer A, et al. The tulip classification of perinatal mortality: introduction and multidisciplinary inter‐rater agreement. BJOG. 2006;113:393‐401. [DOI] [PubMed] [Google Scholar]
- 40. Page JM, Christiansen‐Lindquist L, Thorsten V, et al. Diagnostic tests for evaluation of stillbirth: results from the stillbirth collaborative research network. Obstet Gynecol. 2017;129:699‐706. [DOI] [PubMed] [Google Scholar]
- 41. Liu ZN, Jiang Y, Liu XQ, et al. MiRNAs in gestational diabetes mellitus: potential mechanisms and clinical applications. J Diabetes Res. 2021;2021:4632745. [DOI] [PMC free article] [PubMed] [Google Scholar]


