Abstract
PURPOSE:
Transcutaneous auricular vagus nerve stimulation (taVNS) is a non-invasive neuromodulation technique that may improve oromotor skills when paired with feeding in at-risk infants, but effects on other motor function and how motor function relates to white matter (WM) microstructure are unknown.
METHODS:
In this prospective study, infants failing oral feeds and slated for gastrostomy tube (G-tube) placement received taVNS paired with bottle feeding daily for 2–3 weeks. The effects of taVNS-paired feeding on general and specific head movements were investigated using the Specific Test of Early infant motor Performance (STEP) and diffusion MRI obtained before and after taVNS treatment. Scores between and within groups (taVNS responders, attained full oral feeds; non-responders, received G-tubes) were compared.
RESULTS:
Performance on head movement items improved significantly in responders but not in non-responders (p < 0.05). Total STEP scores were significantly higher in responders after taVNS treatment than non-responders (p = 0.04). One STEP item, rolling by arm, was associated with significantly greater change in WM tract microstructure (p < 0.05) in the responders.
CONCLUSION:
These results suggest that pairing feeding with taVNS may affect specific head and neck movements to a greater extent in infants who are able to attain full oral feeds.
Keywords: Early motor movement, Oral-feed, STEP, Diffusion MRI, taVNS, infant development
1. Background
Non-invasive transcutaneous auricular vagal nerve stimulation (taVNS) paired with infant feeding is a novel therapeutic intervention for infants born preterm who exhibit motor or feeding delays or both [1, 2]. The vagus nerve comprises an extensive synaptic network of afferent and efferent projections integrating the central nervous system (CNS) and autonomic control circuitry of the brain stem to vital internal organs [3]. Vagal afferent projections, including those in the cervical and auricular branches, terminate in numerous nuclei, specifically, the locus coeruleus, raphe nuclei, thalami, and the basal forebrain. Electrical impulses delivered to these nuclei via the vagus nerve in the neck or the auricular branch at the ear result in neurotransmitter release that influences cortical plasticity [4–6].
Multiple preclinical and clinical studies in adults indicate that vagus nerve stimulation (VNS) drives cortical plasticity when combined with a simultaneous sensory or motor activity. Animal studies demonstrate that VNS reorganizes the motor cortex via acetylcholine release in the nucleus basalis, resulting in long-lasting functional performance [4–6]. Data from several recent small and large randomized controlled trials suggest that surgically implanted cervical VNS in conjunction with intensive task practice improves upper-motor function in adult stroke rehabilitation [7–11]. However, implanted cervical VNS is relatively expensive, requires surgery, and may result in adverse events such as wound infection, vocal cord palsy, or hoarseness [8–11]. Non-invasive taVNS targets the largely afferent auricular branch of the vagus nerve, is safe, and improves hand motor function in adult stroke survivors [8, 10, 12]. Further, randomized control trials of VNS and taVNS show greater motor improvements that lasted for at least three months in subacute stroke patients after rehabilitation paired with VNS compared with conventional rehabilitation alone [11, 13, 14].
In a first-of-its-kind infant study, it was demonstrated that taVNS is both feasible and associated with improved oromotor-feeding skills with attainment of full oral feeds in over half of infants failing oral feeds who were gastrostomy tube (G-tube) candidates [1, 2]. Brain stimulation via taVNS was delivered when the infant was actively sucking from a bottle once or twice daily for 2–3 weeks [1, 2]. Most recently, the US Food and Drug Administration designated the taVNS feeding system a Breakthrough Medical Device.
Aside from improved oromotor skills, the authors’ interests lie in evaluating the effect that taVNS treatment might have on other early motor skills. The Specific Test of Early infant motor Performance (STEP) is a novel developmental screening test constructed of ten itemized foundational movements crucial for development of refined motor skills, such as head control against gravity. The ten items on the STEP assess anti-gravity flexion and extension of the head and neck, movement in the arms and legs, and tone in the shoulder girdle and pelvis. Specific items on the STEP reflect the maturation of head movements in the first four months of life and encapsulate the infant’s ability to stabilize the head at midline in supine, prone, side-lying, and supported sitting [15].
To demonstrate the possible effects of this novel taVNS brain stimulation technique on CNS plasticity in a cohort of infants who failed to learn the fundamental motor sequence of feeding, diffusion MRI (dMRI) data and STEP assessments were obtained before and after the treatment course of taVNS-paired feeding. dMRI measures the random thermal movement of water molecules within the underlying tissue microstructure that make it acutely sensitive during the rapid progression, organization and myelination of developing white matter (WM). Of the myriad diffusion metrics, the fractional anisotropy (FA), a dimensionless rotational invariant expressing the water movement’s directional dependence, is the most commonly employed. The FA represents a surrogate biomarker of WM structural integrity and maturation. While studies have shown a correlation between dMRI measures and motor recovery in adult patients with chronic stroke, few studies have directly explored the relationship between FA values and motor learning changes after participation in a short-term, directed intervention [16–19].
It was postulated that circuit activity driven by taVNS-paired feeding would elicit cortical plasticity in and around WM tracts related to feeding (i.e., increased FA values) that correlate to infant motor skills as a whole. This substudy investigated whether this novel therapeutic intervention influenced 1) overall motor skills, 2) specific motor skills of neck and neck control, and 3) WM tracts involved in motor and sensorimotor functions. Moreover, it was of interest whether these skills were different in taVNS responders who attained full oral feeds versus taVNS non-responders who eventually required G-tube placement for oral feeds.
2. Methods
2.1. Design
This study was approved by the Institutional Review Board at the Medical University of South Carolina. Parental consent was obtained prior to enrollment into the taVNS paired-feeding study (Clinicaltrials.gov registration NCT# 04643808). Infants who had failed oral feeding trials and were referred for G-tube placement were recruited sequentially. Infants were either premature (born at <33 weeks gestational age [GA]) or suffered CNS injury such as hypoxic ischemic encephalopathy and were stable without significant respiratory support or unrepaired congenital anomalies that limited feeding volumes. Infants were deemed safe to attempt to take every feed by mouth by occupational or speech therapists.
2.2. Intervention
Infants received taVNS paired with bottle feeds once or twice a day for 2–3 weeks. The taVNS treatment was delivered via a left ear electrode with electrical pulses at 25 Hertz, a 500 microseconds pulse width, and current intensity at 0.1 miliAmpere below the infant’s perceptual threshold [1, 2, 20]. The microcurrent stimulation was delivered while the infant was actively sucking and was off during periods of rest or burping; bottle-feeding sessions lasted up to 30 minutes. Infants were categorized as treatment ‘responders’ to taVNS treatment if they achieved full oral feeds (>120ml/kg/d) with sufficient weight gain for nursery discharge. The clinical results and a detailed description of the stimulation intervention have been reported in prior studies [1, 2, 20].
2.3. Outcome measures
2.3.1. STEP
Early motor abilities were measured via the STEP, which was administered by trained pediatric occupational therapists before the start of taVNS intervention (pre-STEP) and upon completion of the intervention (post-STEP) in the neonatal intensive care unit. The STEP’s ten itemized foundational movements can determine if an infant is at risk for developmental delays as early as term and 3 months of age [2]. The STEP takes both qualitative and quantitative aspects of the infant’s movements into consideration and clearly discriminates among preterm infants of different motor abilities [15, 21, 22] (Fig. 1). Excellent sensitivity and specificity of the STEP cut-off scores at term (≤16) and 3 months (≤22) were previously demonstrated to predict the Bayley-III gross motor performance at 12 months [15]. When compared with the Test of Infant Motor Performance, the STEP showed better prediction of delays at 12 months [15].
Fig. 1.
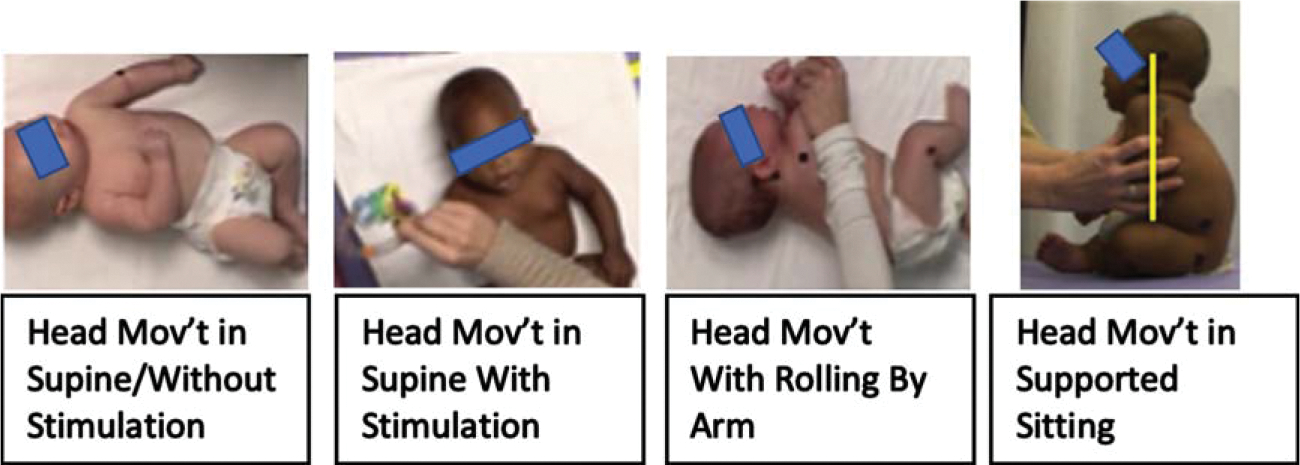
STEP items related to head and neck movements.
2.3.2. Neuroimaging
dMRI data were acquired pre- and post-taVNS treatment on a Skyra 3T MRI (Siemens Healthineers, Erlangen, Germany) immediately following feeding with swaddling to induce natural sleep. A diffusional kurtosis imaging (DKI) [23] protocol utilized b-values of b = 0, 1000 and 2000 s/mm2, (3 mm)3 isotropic voxels, echo time = 122 ms, repetition time = 6700 ms with 30 diffusion-encoding directions per non-zero b-value and 40 contiguous axial slices per image volume. Additionally, 9 non-diffusion weighted (b = 0 s/mm2) images were also acquired with matching imaging parameters.
Image volumes and their corresponding diffusion-encoding directions that were corrupted by motion or signal dropout were manually removed prior to image processing. The DESIGNER pipeline [24], reformatted into Python 3 [25], was employed for the full DKI analysis. The pipeline includes Marchenko-Pastur-PCA denoising [26], Gibbs artifact correction [27], eddy correction [28, 29], Gaussian smoothing with a kernel 1.25 times the voxel size [30] and Rician noise bias correction [31]. The output of DESIGNER is tensor-derived diffusion and kurtosis metrics; the FA was specifically focused in on for this investigation.
2.4. Data analysis
2.4.1. Change in total STEP scores and STEP item scores between responders and non-responders
Total STEP scores were compared from pre-taVNS to post-taVNS in the responders and non-responders groups by paired t-test. The change in total STEP scores between responder and non-responder groups was compared by independent t-test and normalized for the weeks of development between assessments (ΔSTEP/weeks). To determine specific movements that might be responsive to taVNS-paired feeding intervention, the change in specific STEP item scores within each group was compared by paired t-test and between the two groups by independent t-test, normalized for weeks of development (ΔSTEP/weeks). Statistical analyses were conducted using IBM SPSS (version 21).
2.4.2. FA study template
A neonatal FA study template was generated based on a user-chosen subject FA image (i.e., target) using the optimized method described by Ball et al. [32]. Briefly, all FA images were brain-extracted [33] and registered to the target FA image using a 6 and then 12 degree-of-freedom registration [34]. The resulting affine transformation matrices were concatenated and served as the initialization for non-linear warping of each subject’s FA image to the target FA image [35, 36].
The study-specific FA template resulted from taking the average across all target FA aligned images. This mean FA template was then registered to the Johns Hopkins University neonatal FA template [37] to create the final standard space FA template using similar steps outlined above. The core WM voxels common to all subjects were identified and, using FA <0.15, a binary WM skeleton mask was generated.
The change in FA (ΔFA) after completing taVNS treatment was obtained by taking the difference of the post- and pre-treatment FA maps. Subjects were then dichotomized by total and individual STEP item scores and labeled high (total score >16; item score of 2 or 3) or low performing (total score ≤16; item score of 0 or 1) on the STEP.
A tract-based spatial statistics (TBSS) analysis [38] was then performed for each dichotomized ΔFA set separately while adjusting for the GA difference from pre- to post-taVNS dMRI scans. Individual STEP items tested were head supported sitting, prone, pull to sit, rolling by arm and rolling by leg. A two-sided t-test was employed using the randomize function of FSL, which is a method to perform voxelwise non-parametric permutation inference testing along with threshold-free cluster enhancement to correct for multiple comparisons [39].
3. Results
3.1. Participants
A total of 27 of the 35 infants in the taVNS feeding study had at least one completed STEP performed before or after taVNS treatment (15 non-responders, 12 responders) (Fig.2). There were no significant differences between the two groups in GA, birth weight, age at enrollment, or length of time attempting full intended volume for oral feeds prior to receiving taVNS intervention (Table 1). However, there were statistically significant differences in the number of taVNS treatments and days to achieve full oral feeds. Infants in the responder group required fewer taVNS treatments (p = 0.04) and were able to achieve full oral feeds within an average of 12.7 days. The STEP subgroup included more non-responders than responders, unlike the parent study of 35 infants in which 54% responded to taVNS treatment.
Fig. 2.

CONSORT flow diagram of the study participants.
Table 1.
Patient demographics and characteristics
| All subjects (n = 27) | Responders (full PO Feed) (n =12) | Non- responders (G-tube, n =15) | p-valuea | |
|---|---|---|---|---|
|
| ||||
| Sex | 16 F/11 M | 7F/5M | 9F/6M | |
| GA at birth (weeks) | 31.27 ± 4.7 | 31.1 ± 5.3 | 31.42 ± 4.3 | ns |
| Birth weight (g) | 1737 ± 1091 | 1753 ± 1218 | 1725 ± 1021 | ns |
| GA at enrollment (wks) | 42.4 ± 3.7 | 42.9 ± 3.9 | 41.9 ± 3.6 | ns |
| Days trying po, prior to taVNS | 38.9 ± 17.9 | 39.5 ± 25.0 | 38.5 ± 10.1b | ns |
| Days until full oral feeds from taVNS start | NA c | 10.2 ± 5.6 | NA c | |
| Total number of taVNS sessions | 23.5 ± 11.2 | 18.6 ± 8.0 | 27.5 ± 12.0 | 0.04* |
| Total number of taVNS days | 15.4 ± 5.2 | 12.7 ± 5.0 | 17.7 ± 4.2 | 0.01* |
| taVNS-paired feed 1X day | 11 | 5 | 6 | ns |
| taVNS-paired feed 2X day | 16 | 7 | 9 | ns |
| GA at first MRI scan | 42.6 ± 3.7 | 43.1 ± 4.0 | 42.2 ± 3.5 | ns |
| STEP score pre-taVNS | 13.7 ± 2.9 | 12.5 ± 2.0^ | 14.5 ± 3.0 | 0.07 |
| STEP score post-taVNS | 15.5 ± 3.1 | 16.8 ± 4.7^ | 15.4 ± 3.0 | ns |
| Clinical Sepsis | 12 | 5 | 7 | ns |
| Hypoxic Ischemic Encephalopathy (HIE) | 6 | 4 | 2 | ns |
| Periventricular Leukomalacia (PVL) | 5 | 2 | 3 | ns |
| Intraventricular hemorrhage (IVH) Grade | I (9) | I (5) | I (4) | ns |
| II (1) | III (1) | II (1) | ||
| III (1) | IV (1) | |||
| IV (1) | ||||
| Other Central Nervous System (CNS) problems | 7 | 3 | 4 | ns |
Data presented as mean ± standard deviation.
Significant at p < 0.05 level
p = 0.005 between pre- and post-STEP in Responders.
p-values from independent t-test or chi-square test between Responders and Non-responders.
Data are not applicable.
Patient 11: was transferred patient from another hospital and had >30 days of attempting PO.
3.2. STEP assessment
Of the total 27 infants, 19 had the STEP performed before and after taVNS treatment (12 non-responders, 7 responders). The average number of days between the pre- and post-STEP tests were similar between the responders (15.3) and non-responders (23.1) (p = 0.22). The STEP scores before taVNS were not significantly different by responder group pre-taVNS treatment (Table 2). Infants who were responders had a significantly greater increase in total STEP score (mean 1.7±2.1) from pre- to post-taVNS treatment than infants who were non-responders and required a G-tube (0.0±0.85, p = 0.04, paired t-test, Fig. 3A, Table 2). Expected change in total STEP score for high performing infants was 1 point over 2 weeks [15]. In addition, responders had significantly greater increase in four specific STEP items directly related to movements in the head and neck compared with non-responders. These included head in supine with visual stimulation (p = 0.01), head in supine with no visual stimulation (p = 0.03), rolling elicited by arm (p = 0.04), and head movements in supported sitting (p = 0.01) (Fig. 3 B–D, Table 3). No changes were observed in the non-responder group from before to after treatment in total STEP scores or in any specific STEP item scores (Table 3).
Table 2.
Total STEP scores and specific STEP items between responder and non-responder infants who had both pre- and post-STEP scores; Mean (SD)
| Responders (full PO Feed) (n = 7) | Non-responders (G-tube) (n = 12) | P-value | |
|---|---|---|---|
|
| |||
| GA at pre-STEP | 41.5 ± 1.8 | 41.8 ± 3.2 | 0.83 |
| GA at post-STEP | 44.3 ± 2.5 | 44.9 ± 2.9 | 0.66 |
| Total pre-STEP scores | 12.6 ± 1.6 | 14.6 ± 3.0 | 0.12 |
| Total change in STEP scores a | 1.7 ± 2.1 | 0.0 ± 0.85 | 0.04* |
| Head in supine with visual stimulation a | 0.35 ± 0.31 | 0.03 ± 0.30 | 0.03* |
| Head in supine with no visual stimulation a | 0.22 ± 0.26 | 0.00 ± 0.14 | 0.03* |
| Head movement rolling by arm a | 0.22 ± 0.21 | 0.07 ± 0.19 | 0.14 |
| Head movement supported sitting a | 0.39 ± 0.32 | 0.03 ± 0.21 | 0.01* |
Data presented as difference in means (post item score - pre item score) ± standard deviation.
Significant at P < 0.05 level.
Change in STEP scores divided by weeks between STEP, normalized by weeks of development. Independent t-test was used in all analysis.
Fig. 3.
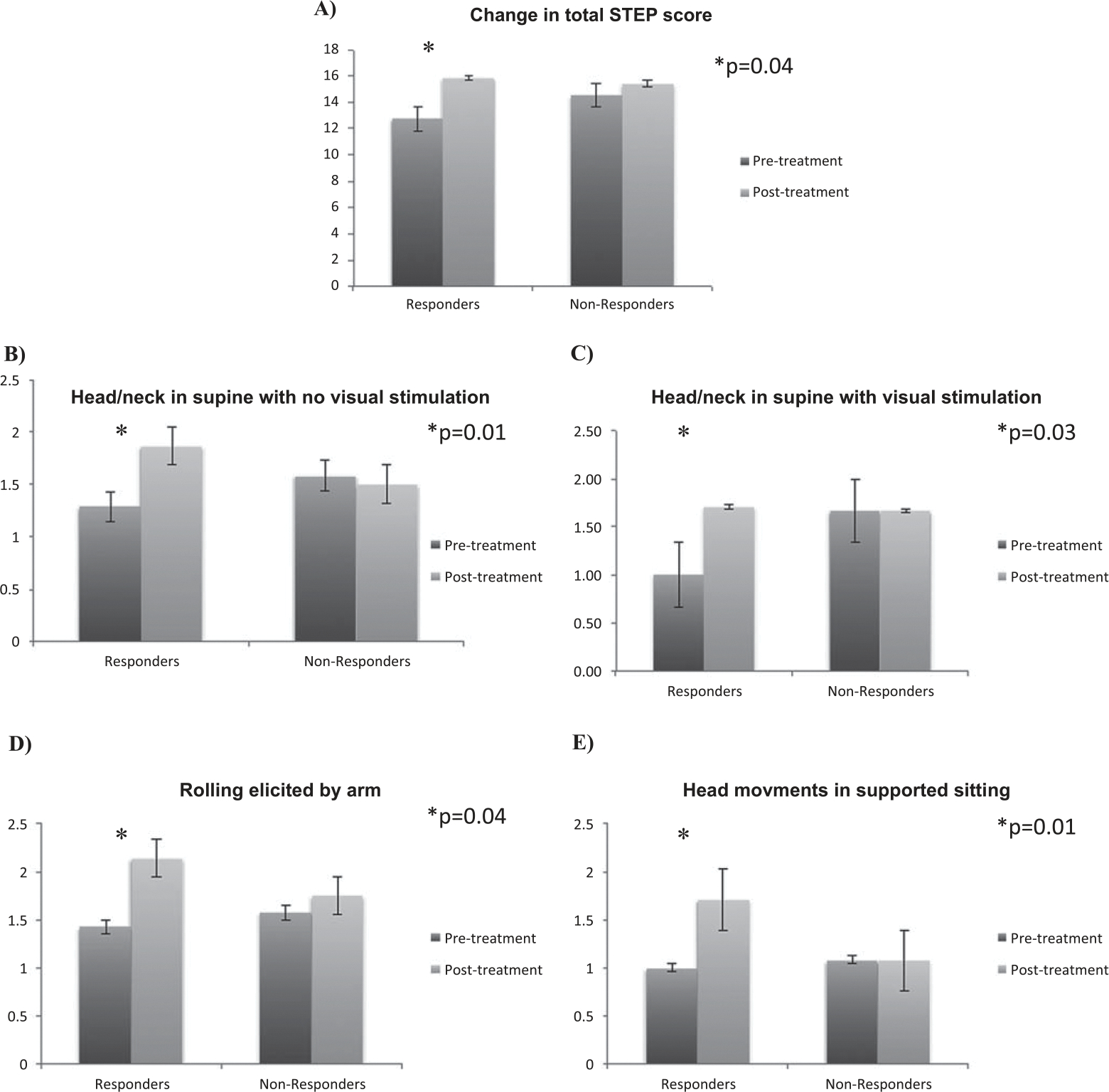
Difference in the change in total STEP score (A) and specific STEP items (B, C, D, E) between the taVNS Reponders versus Non-Responders.
Table 3.
Change in total and specific STEP items from pre- to post-taVNS between the Responder and Non-Responder groups, Mean (SD)
| Responders (full PO Feed) (n = 7) |
Non-responders (g-tube) (n = 12) |
|||||
|---|---|---|---|---|---|---|
| Pre-STEP | Post-STEP | P-value | Pre-STEP | Post-STEP | P-value | |
|
| ||||||
| Total change in STEP | 12.6 ± 1.6 | 15.9 ± 3.9 | 0.04* | 14.6 ± 3.0 | 15.4 ± 3.0 | ns |
| Head in supine with visual stimulation | 1.0 ± 0.0 | 1.7 ± 0.5 | 0.01* | 1.6 ± 0.6 | 1.7 ± 0.5 | ns |
| Head in supine with no visual stimulation | 1.3 ± 0.5 | 1.7 ± 0.7 | 0.03* | 1.6 ± 0.5 | 1.5 ± 0.7 | ns |
| Head movement with rolling by arm | 1.4 ± 0.5 | 2.1 ± 0.4 | 0.04* | 1.6 ± 0.5 | 1.7 ± 0.6 | ns |
| Head movement supported sitting | 1.0 ± 0.6 | 1.7 ± 0.8 | 0.01* | 1.2 ± 0.6 | 1.3 ± 0.6 | ns |
Significant at P <0.05 level. Paired t-test was used in all analysis.
While taVNS treatment was performed over 2–3 weeks, gains in motor skills or tone might be observed even in this relatively short time period. Analyzing the change in STEP scores normalized by weeks between the pre- and post-STEP assessments (Table 2), there was still a significant difference in the change in total STEP score (p = 0.04) and in three items related to head and neck movement: head in supine with visual stimulation (p = 0.03), head in supine with no visual stimulation (p = 0.03), and head movements in supported sitting (p = 0.01).
3.3. Neuroimaging
Of the total 19 infants with STEP scores, 18 had an MRI performed before and after taVNS treatment (9 non-responders, 9 responders). To determine if the improvements in STEP score were related to WM tract plasticity changes, TBSS analyses of ΔFA between pre- and post-taVNS treatment were performed and dichotomized by high and low performance on total and individual STEP item scores, adjusting for GA between the two MRI scans. There was a significantly greater ΔFA within multiple WM regions (p < 0.05) after taVNS treatment in infants who performed well in rolling by arm compared to infants who performed poorly (Fig. 4). The regions highlighted as different in the rolling by arm analysis correspond to primarily left sided portions of major WM tracts: the anterior limb of internal capsule (ALIC), external capsule (EC), inferior fronto-occipital fasciculus (IFOF), and superior longitudinal fasciculus (SLF) (Fig. 4). The TBSS analysis dichotomized by total STEP performance had no significant findings.
Fig. 4.

WM tract regions are different following TBSS comparing high and low performing infants in Rolling by Arm (panel A) and Rolling by Leg (panel B) with WM tract atlas regions for refence (panel C). (A) Red-yellow voxels denote the WM tracts with significantly greater positive ΔFA in infants who performed well on STEP item Rolling by Arm item after taVNS treatment vs infants who performed poorly (p < 0.05). The left hemispheric tracts were: anterior and posterior limbs of internal capsule (ALIC and PLIC), external capsule (EC), inferior frontooccipital fasciculus (IFOF), and corona radiata (CR). (B) Regions in red denote WM tracts with significantly greater ΔFA in infants who performed well on STEP item Rolling by Leg item after taVNS treatment vs infants who performed poorly (p < 0.08). The right hemispheric WM clusters correspond to parts of the EC, as well as the ALIC and PLIC, frontal corona radiata, cortico-spinal tract and lower optic radiations. (C) Colored regions from the John’s Hopkins neonatal WM atlas for reference and orientation to the relative location of the IFOF (green), EC (yellow), ALIC (blue), PLIC (light-blue), CR (pink) and optic radiations (red).
A trend to greater ΔFA in infants who performed well on the item Rolling by Leg pre- and post- taVNS treatment (p = 0.1, threshold for TBSS) was observed (Fig. 4). In contrast to rolling by arm, the highlighted WM clusters were heavily right hemispheric with a few clusters of significant voxels along the left optic radiation. The right-sided WM clusters correspond to regions of the EC and ALIC as well as the posterior limb of internal capsule. The SLF, frontal corona radiata and cortico-spinal tract were also included in the trending WM regions.
4. Discussion
In this open label study, the possible effects of taVNS paired with oral feeding on the general and specific movements of the head and neck was investigated in a cohort of infants who failed to learn the fundamental motor sequence of feeding. Splitting the group into responders and non-responders allowed comparison of STEP assessments before and after the treatment course of taVNS-paired feeding and investigation of performance on specific STEP items to dMRI data. These results reveal that, although pre-taVNS STEP scores were not different between groups, responders had greater changes in motor function over the short 2–3 week paired taVNS intervention when compared to non-responders.
These intriguing data show that this improvement in overall motor function is largely driven by increases in head and neck control. While bottle-feeding involves coordination of 22 oral-facial muscles for successful intake, the muscles of the head and neck are also activated during feeding and may be affected by the activity of bottle feeding. These results suggest that when feeding is paired with taVNS, these movements may be affected to a greater extent in infants who are able to attain full oral feeds. As STEP total and rolling by arm item scores are not significantly different between responders and non-responders pre-taVNS paired bottle feeding, it is conceivable that taVNS paired with bottle feeding facilitated integration of these other motor circuits. Additionally, from dMRI, FA revealed greater improvements in motor tract WM integrity after taVNS treatment in infants who were high performing on specific STEP items related to head and neck control, as demonstrated during an axial rolling movement. This suggests that tissue anisotropy is increasing more rapidly in high performing infants than in their low performing counterparts, suggesting that increased WM maturation in specific WM tracts is likely reflecting induced neuroplasticity after taVNS-paired bottle feeding.
This is the first study in infants to demonstrate that early taVNS treatment for feeding delays may be associated with improvements in critical early motor movements, especially those related to maturity of antigravity head and neck control. The findings are consistent with the improvement in motor function when VNS was paired with a functional task in adult stroke survivors, demonstrating a generalized, long-lasting effect on motor performance [8, 11, 40]. In addition, studies have shown that VNS paired with intensive motor practice in rats poststroke promotes reorganization of the primary cortex corresponding to the task footprint and results in greater functional improvement compared to motor practice alone [7]. When VNS was paired with wheel spin activity, cortical representation of the distal forelimb increased, whereas with the lever press activity, cortical representation of more proximal forelimb activity increased compared to rats who practiced the activity alone [41].
While cortical reorganization cannot be directly measured, the maturation of WM tracts involved in these motor activities can be measured via FA. The FA imparts information on the directional dependence of water movement, such that highly aligned, tightly packed fiber bundles will have a large FA (closer to 1) whereas crossing fiber regions or loosely aligned bundles will be representative of lower FA values (closer to 0). The interpretation of FA is complicated by the crossing fiber problem, in which two high FA fibers may cross at right angles resulting in FA = 0, which is not a correct measure of the water movement. However, axonal alignment and progressive rapid myelination during early development has a role in the clear and dramatic increase in FA seen within the WM of the genu and splenium of the corpus callosum. This FA increase stems directly from decreased water mobility across the lipid membrane now sheathing the axons. In healthy development, this FA increase begins to plateau at approximately two to five years of age. In the current study, STEP movements that involve head and neck control were increased in responders versus non-responders after only a brief taVNS paired oral feeding intervention. In addition, greater maturity in the axial rolling items was associated with greater ΔFA values in several major WM tracts involved in coordination of sensorimotor responses (ALIC, EC, IFOF and SLF).
Studies have also shown the importance of post-term axial rolling, in which the infant’s whole body is turned from supine to/from prone and the infant aligns head and neck with the trunk. A new assessment termed the Motor Optimality Score for infants, which is based on the work of Prechtl, includes axial rolling as one of the items [42, 43]. In infants with neurodevelopmental impairment and cerebral palsy (CP), rolling to the side and antigravity movements were decreased compared with typically developing infants [44]. In addition, this study found that movements of the arms and legs to midline, which require antigravity flexion, emerge earlier in neurotypical infants. The item rolling to the side can also help discriminate between grades of CP severity using the Gross Motor Function Classification System (GMFCS), specifically between children classified with GMFCS I-II vs. GMFCS III-V unilateral vs. bilateral CP [44].
Two prior studies have also determined a link between an infant’s feeding abilities and early motor performance using the General Movements Assessment (GMA) [45, 46]. The GMA assesses the quality of spontaneous movements, namely fidgety movements (FM), in the first five months and has been used to predict CP [47–49]. When combined with MRI and neurological examination, the GMA has shown excellent sensitivity and specificity for detecting CP in high-risk infants [50]. Nieuwenhuis et al. [45] showed that abnormal FMs are associated with uncoordinated sucking patterns near term equivalent age (TEA) [45]. Moreover, infants with abnormal GMA scores at term were observed to have a higher risk of developing oromotor-feeding impairment at 12 months [46]. Their data indicated only a weak association of brain abnormalities on structural MRI at TEA and future oromotor-feeding impairment. Nevertheless, taken together, these studies suggest a link between an infant’s early motor performance and early feeding abilities [45, 46].
The current study is limited by the open label design and the relatively small sample size. However, in this first-in-infants study on the impact of taVNS on at-risk infants, multi-modal data was collected on each infant enrolled in the study. This allowed for in-depth analysis of specific early infant motor movements, which have been shown to be significant in other published studies [37, 42]. In addition, the important relationship of dMRI metrics to motor learning changes in infant head control after a brief but focused motor-paired taVNS intervention was explored.
5. Conclusion
This study provides proof-of-concept that early neuromodulation paired with the motor task of feeding in at-risk infants may have positive and potentially long-lasting effects on motor movements required for head and neck control. When taVNS is delivered while the infant is actively engaging muscles of the head and neck during bottle-feeding, significant changes were observed not just in feeding behaviors but also in other early head and neck movements. The long-term impact of taVNS treatment in these and hopefully future study infants and how improvements in early head and neck movements may impact overall motor skill development later in life will continue to be documented. In addition, future studies should determine the potential long-term impact of taVNS paired with oromotor activity on speech and language domains.
Acknowledgments
Funding for this study was provided by the National Institutes of Health National Center of Neuromodulation for Rehabilitation NIH/NICHD Grant #P2CHD086844; National Institutes of Health Center of Biomedical Research Excellence (COBRE) in Stroke Recovery, Grant #P20GM109040; and NIH NINDS F31NS108623 (H. Moss). We would like to acknowledge the following people who contributed to this study: Sarah Huffman, Morgan Dancy, Will Devries, Sean Thompson and Georgia O’Leary.
Abbreviations
- ALIC
Anterior limb of internal capsule
- CNS
Central nervous system
- CP
Cerebral palsy
- DKI
Diffusional kurtosis imaging
- dMRI
Diffusion MRI
- EC
External capsule
- FA
Fractional anisotropy
- FM
Fidgety movements
- GA
Gestational age
- G-tube
Gastrostomy tube
- GMA
General Movements Assessment
- GMFCS
Gross Motor Function Classification System
- IFOF
Inferior frontooccipital fasciculus
- SLF
Superior longitudinal fasciculus
- STEP
Specific Test of Early infant motor Performance
- TEA
Term equivalent age
- taVNS
transcutaneous auricular vagus nerve stimulation
- TBSS
Tract-based spatial statistics
- VNS
Vagus nerve stimulation
- WM
White matter
Footnotes
Conflict of interest
The authors have no conflicts of interest to report.
References
- [1].Badran BW, Jenkins DD, DeVries WH, Dancy M, Summers PM, Mappin GM, et al. Transcutaneous auricular vagus nerve stimulation (taVNS) for improving oromotor function in newborns. Brain Stimul. 2018;11(5):1198–200. doi: 10.1016/j.brs.2018.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Badran BW, Jenkins DD, Cook D, Thompson S, Dancy M, DeVries WH, et al. Transcutaneous auricular vagus nerve stimulation-paired rehabilitation for oromotor feeding problems in newborns: An open-label pilot study. Front Hum Neurosci. 2020;14:77. doi: 10.3389/fnhum.2020.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res. 2018;11:203–13. doi: 10.2147/JIR.S163248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther. 2006;318(2):890–8. doi: 10.1124/jpet.106.104166 [DOI] [PubMed] [Google Scholar]
- [5].Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci. 2004;118(1):79–88. doi: 10.1037/0735-7044.118.1.79 [DOI] [PubMed] [Google Scholar]
- [6].Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59(6 Suppl 4):S3–14. doi: 10.1212/wnl.59.6_suppl_4.s3 [DOI] [PubMed] [Google Scholar]
- [7].Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL, et al. Reorganization of motor cortex by vagus nerve stimulation requires cholinergic innervation. Brain Stimul. 2016;9(2):174–81. doi: 10.1016/j.brs.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, et al. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke. 2016;47(1):143–50. doi: 10.1161/STROKEAHA.115.010477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Engineer ND, Kimberley TJ, Prudente CN, Dawson J, Tarver WB, Hays SA. Targeted vagus nerve stimulation for rehabilitation after stroke. Front Neurosci. 2019;13:280. doi: 10.3389/fnins.2019.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kimberley TJ, Pierce D, Prudente CN, Francisco GE, Yozbatiran N, Smith P, et al. Vagus nerve stimulation paired with upper limb rehabilitation after chronic stroke. Stroke. 2018;49(11):2789–92. doi: 10.1161/STROKEAHA.118.022279 [DOI] [PubMed] [Google Scholar]
- [11].Dawson J, Liu CY, Francisco GE, Cramer SC, Wolf SL, Dixit A, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): A randomised, blinded, pivotal, devicetrial. Lancet. 2021;397(10284):1545–53.doi: 10.1016/S0140-6736(21)00475-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ben-Menachem E, Revesz D, Simon BJ, Silberstein S. Surgically implanted and non-invasive vagus nerve stimulation: A review of efficacy, safety and tolerability. Eur J Neurol. 2015;22(9):1260–8. doi: 10.1111/ene.12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Redgrave JN, Moore L, Oyekunle T, Ebrahim M, Falidas K, Snowdon N, et al. Transcutaneous auricular vagus nerve stimulation with concurrent upper limb repetitive task practice for poststroke motor recovery: A pilot study. J Stroke Cerebrovasc Dis. 2018;27(7):1998–2005. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.056 [DOI] [PubMed] [Google Scholar]
- [14].Wu D, Ma J, Zhang L, Wang S, Tan B, Jia G. Effect and safety of transcutaneous auricular vagus nerve stimulation on recovery of upper limb motor function in subacute ischemic stroke patients: A randomized pilot study. Neural Plast. 2020;2020:8841752. doi: 10.1155/2020/8841752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gower L, Jenkins D, Fraser JL, Ramakrishnan V, Coker-Bolt P. Early developmental assessment with a short screening test, the STEP, predicts one-year outcomes. J Perinatol. 2019;39(2):184–92. doi: 10.1038/s41372-018-0234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74(4):280–7. doi: 10.1212/WNL.0b013e3181ccc6d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Puig J, Blasco G, Schlaug G, Stinear CM, Daunis-I-Estadella P, Biarnes C, et al. Diffusion tensor imaging as a prognostic biomarker for motor recovery and rehabilitation after stroke. Neuroradiology. 2017;59(4):343–51. doi: 10.1007/s00234-017-1816-0 [DOI] [PubMed] [Google Scholar]
- [18].Boespflug EL, Storrs JM, Allendorfer JB, Lamy M, Eliassen JC, Page S. Mean diffusivity as a potential diffusion tensor biomarker of motor rehabilitation after electrical stimulation incorporating task specific exercise in stroke: A pilot study. Brain Imaging Behav. 2014;8(3):359–69. doi: 10.1007/s11682-011-9144-1 [DOI] [PubMed] [Google Scholar]
- [19].Song J, Nair VA, Young BM, Walton LM, Nigogosyan Z, Remsik A, et al. DTI measures track and predict motor function outcomes in stroke rehabilitation utilizing BCI technology. Front Hum Neurosci. 2015;9:195. doi: 10.3389/fnhum.2015.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Badran BW, Yu AB, Adair D, Mappin G, DeVries WH, Jenkins DD, et al. Laboratory administration of transcutaneous auricular vagus nerve stimulation (taVNS): Technique, targeting, and considerations. J Vis Exp. 2019;(143):10.3791/58984. doi: 10.3791/58984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bentzley JP, Coker-Bolt P, Moreau NG, Hope K, Ramakrishnan V, Brown T, et al. Kinematic measurement of 12-week head control correlates with 12-month neurodevelopment in preterm infants. Early Hum Dev. 2015;91(2):159–64. doi: 10.1016/j.earlhumdev.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shehee L, Coker-Bolt P, Barbour A, Moss H, Brown T, Jenkins D. Predicting motor outcomes with 3 month prone hip angles in premature infants. J Pediatr Rehabil Med. 2016;9(3):231–6. doi: 10.3233/PRM-160384 [DOI] [PubMed] [Google Scholar]
- [23].Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23(7):698–710. doi: 10.1002/nbm.1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ades-Aron B, Veraart J, Kochunov P, McGuire S, Sherman P, Kellner E, et al. Evaluation of the accuracy and precision of the diffusion parameter EStImation with Gibbs and NoisE removal pipeline. Neuroimage 2018;183:532–43. doi: 10.1016/j.neuroimage.2018.07.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dhiman S, Teves JB, Thorn KE, McKinnon ET, Moss HG, Adisetiyo V, et al. PyDesigner: A pythonic implementation of the DESIGNER pipeline for diffusion tensor and diffusional kurtosis imaging. bioRxiv. 2021:2021.10.20.465189. [Google Scholar]
- [26].Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394–406. doi: 10.1016/j.neuroimage.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kellner E, Dhital B, Kiselev VG, Reisert M. Gibbs-ringing artefact removal based on local subvoxel-shifts. Magn Reson Med. 2016;76(5):1574–81. doi: 10.1002/mrm.26054 [DOI] [PubMed] [Google Scholar]
- [28].Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–78. doi: 10.1016/j.neuroimage.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage. 2016;141:556–72. doi: 10.1016/j.neuroimage.2016.06.058 [DOI] [PubMed] [Google Scholar]
- [30].Tabesh A, Jensen JH, Ardekani BA, Helpern JA. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med. 2011;65(3):823–36. doi: 10.1002/mrm.22655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gudbjartsson H, Patz S. The rician distribution of noisy MRI data. Magn Reson Med. 1995;34(6):910–4. doi: 10.1002/mrm.1910340618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ball G, Counsell SJ, Anjari M, Merchant N, Arichi T, Doria V, et al. An optimised tract-based spatial statistics protocol for neonates: Applications to prematurity and chronic lung disease. Neuroimage. 2010;53(1):94–102. doi: 10.1016/j.neuroimage.2010.05.055 [DOI] [PubMed] [Google Scholar]
- [33].Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–55. doi: 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- [35].Andersson JLR, Jenkinson M, Smith S. Non-Linear Registration Aka Spatial Normalisation. FMRIB Technial Report TR07JA2. Oxford: FMRIB Centre; 2007. Available from: https://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf [Google Scholar]
- [36].Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6 [DOI] [PubMed] [Google Scholar]
- [37].Oishi K, Mori S, Donohue PK, Ernst T, Anderson L, Buchthal S, et al. Multi-contrast human neonatal brain atlas: Application to normal neonate development analysis. Neuroimage. 2011;56(1):8–20. doi: 10.1016/j.neuroimage.2011.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–505. doi: 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- [39].Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92(100):381–97. doi: 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Meyers EC, Solorzano BR, James J, Ganzer PD, Lai ES, Rennaker RL, et al. Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery. Stroke. 2018;49(3):710–7. doi: 10.1161/STROKEAHA.117.019202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, et al. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb Cortex. 2012;22(10):2365–74. doi: 10.1093/cercor/bhr316 [DOI] [PubMed] [Google Scholar]
- [42].Einspieler C, Prechtl HF. Prechtl’s assessment of general movements: A diagnostic tool for the functional assessment of the young nervous system. Ment Retard Dev Disabil Res Rev. 2005;11(1):61–7. doi: 10.1002/mrdd.20051 [DOI] [PubMed] [Google Scholar]
- [43].Bruggink JLM, Einspieler C, Butcher PR, Stremmelaar EF, Prechtl HFR, Bos AF . Quantitative aspects of the early motor repertoire in preterm infants: Do they predict minor neurological dysfunction at school age? Early Hum Dev. 2009;85(1):25–36. doi: 10.1016/j.earlhumdev.2008.05.010 [DOI] [PubMed] [Google Scholar]
- [44].Einspieler C, Bos AF, Krieber-Tomantschger M, Alvarado E, Barbosa VM, Bertoncelli N, et al. Cerebral palsy: Early markers of clinical phenotype and functional outcome. J Clin Med. 2019;8(10):1616. doi: 10.3390/jcm8101616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nieuwenhuis T, da Costa SP, Bilderbeek E, Geven WB, van der Schans CP, Bos AF. Uncoordinated sucking patterns in preterm infants are associated with abnormal general movements. J Pediatr. 2012;161(5):792–8. doi: 10.1016/j.jpeds.2012.04.032 [DOI] [PubMed] [Google Scholar]
- [46].Sanchez K, Morgan AT, Slattery JM, Olsen JE, Lee KJ, Anderson PJ, et al. Neuropredictors of oromotor feeding impairment in 12month-old children. Early Hum Dev. 2017;111:49–55. doi: 10.1016/j.earlhumdev.2017.05.012 [DOI] [PubMed] [Google Scholar]
- [47].Morgan C, Romeo DM, Chorna O, Novak I, Galea C, Del Secco S, et al. The pooled diagnostic accuracy of neuroimaging, general movements, and neurological examination for diagnosing cerebral palsy early in high-risk infants: A case control study. J Clin Med. 2019;8(11):1879. doi: 10.3390/jcm8111879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Prechtl HF. Qualitative changes of spontaneous movements in fetus and preterm infant are a marker of neurological dysfunction. Early Hum Dev. 1990;23(3):151–8. doi: 10.1016/0378-3782(90)90011-7 [DOI] [PubMed] [Google Scholar]
- [49].Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, et al. Early, accurate diagnosis and early intervention in cerebral palsy: Advances in diagnosis and treatment. JAMA Pediatr. 2017;171(9):897–907. doi: 10.1001/jamapediatrics.2017.1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kwong AKL,Fitzgerald TL,Doyle LW,Cheong JLY,Spittle AJ . Predictive validity of spontaneous early infant movement for later cerebral palsy: A systematic review. Dev Med Child Neurol. 2018;60(5):480–9. doi: 10.1111/dmcn.13697 [DOI] [PubMed] [Google Scholar]


