Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive non-Hodgkin’s lymphoma (NHL). 30 ~ 40% of DLBCL patients were resistant to the standard R-CHOP regimen or recurrence after remission. It is currently believed that drug resistance is the main cause of the recurrence and refractory of DLBCL (R/R DLBCL). With the increased understanding of DLBCL biology, tumor microenvironment and epigenetics, some new therapies and drugs like molecular and signal pathway target therapy, chimeric antigen receptor (CAR) T-cell therapy, immune checkpoint inhibitors, antibody drug-conjugate and tafasitamab have been used for R/R DLBCL. This article will review the drug resistance mechanism and novel targeted drugs and therapies of DLBCL.
Keywords: relapse and refractory DLBCL, drug resistance, targeted therapy, CD47, NF-κB, CAR-T therapy, antibody drug-conjugate
Background
Lymphoma is a group of heterogeneous hematological malignancies, which are classified into Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL). Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive NHL. DLBCL can be divided into germinal center B-cell-like (GCB) subgroup and non-GCB subgroup according to the origin of cells. The latter includes activated B-cell-like (ABC) subgroup and type 3 DLBCL.1 The immunochemotherapy of rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has greatly improved the remission rate of DLBCL, which can reach 70 ~ 80%. However, 30~40% of patients still show poor response to treatment or disease recurrence. Such patients usually have a poor prognosis, and the 2-year overall survival (OS) is only 20% ~ 40%.2 MYC and BCL-2 and/or BCL-6 rearrangements can occur in 5% ~ 10% of DLBCL (also known as double-hit or triple-hit lymphoma (DHL/THL)),3 with a dismal event-free survival (EFS) and OS using standard immunochemotherapy regimens.4 It is currently believed that drug resistance is the main cause of the recurrence and refractory of DLBCL.2 The tumor microenvironment plays an important role in the progress of the DLBCL. The interaction between DLBCL cells and different components and vessels in the environment is closely related to drug resistance.5 Individual tumor cells can release in the blood and lymphatic system, and are transferred to the peripheral tissue and adapt to the new microenvironment, leading to residual disease and relapse.6 The apoptotic tumor cells can release circulating tumor DNA (ctDNA), which has become a characteristic tumor biomarker.
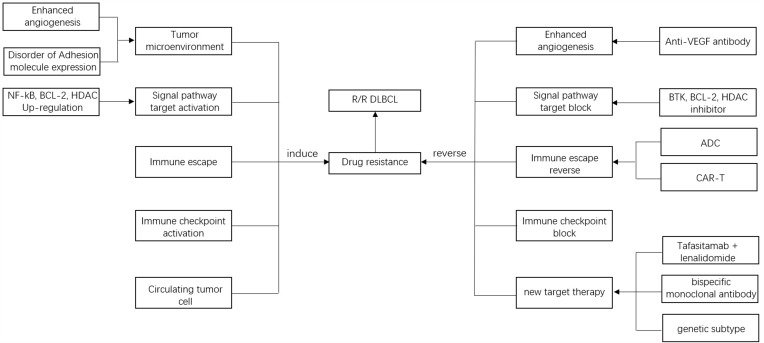
In recent years, some new targeted agents for relapse and refractory DLBCL (R/R DLBCL) have been used in clinical practice, and more novel predictive markers and therapies are being actively explored. This article will review the drug resistance mechanism and novel targeted drugs and treatments of DLBCL (Figure 1).
Figure 1.
Drug resistance mechanisms and novel targeted drugs and treatments of DLBCL.
Tumor Microenvironment
The tumor microenvironment mainly includes immune cells, extracellular matrix, fibroblasts, lymphocytes, inflammatory factors, and signal molecules, which are closely related to the differentiation of B cell and the occurrence and progression of DLBCL.7 The prognosis of DLBCL is influenced by different components in the microenvironment. Disorder of adhesion molecule expression and enhanced angiogenesis causes immune escape of tumor cells,8 which induces tumor cell proliferation.9 This may be an important mechanism for tumor resistance. Anti-angiogenic and microenvironment targeted therapies have become an important strategy for the treatment of lymphoma.6
Studies have compared the microenvironment components of DLBCL patients who were sensitive to the R-CHOP regimen and those with drug resistance, and found that there were certain differences in genes and proteins in the microenvironment of the two groups.10 Xu-Monette et al analyzed the expression of immune markers (PD-1, PD-L1, PD-L2, CD20, etc.) in the tumor and microenvironment of 405 patients with DLBCL, the results showed that the lack of T cells and/or NK cells in the microenvironment was associated with poor prognosis, and high expression of PD-L1/PD-L2 in DLBCL cells was associated with good prognosis.11 In addition, the high expression of PD-1 in other cells in the microenvironment (such as T cells, NK cells, phagocyte) was associated with poor prognosis.11 These factors may be involved in the resistance to standard immunochemotherapy. A study with 939 DLBCL patients treated with R-CHOP was conducted by Boustred R et al, exploring the relationship between CD47 expression and the prognosis of DLBCL. The results showed that high CD47 expression was associated with poor OS (P=0.001).12 Subgroup analysis showed that this correlation was more obvious in non-GCB DLBCL patients (P = 0.004), while there was no significant difference in OS between the high and low CD47 expression groups in GCB DLBCL (P = 0.058), which indicates that non-GCB DLBCL patients may benefit from CD47 targeted therapy.12
Vascular endothelial growth factor (VEGF) is an angiogenic factor, which is involved in tumorigenesis and immune escape,13 and the treatment targeting VEGF and VEGF receptors has achieved significant effect in solid tumors (such as non-small cell lung cancer and ovarian cancer).14 Shahini et al found that VEGF-A was highly expressed in DLBCL patients with high score of International Prognostic Index (IPI).15 Sang et al analyzed the specimens of 65 DLBCL patients and found that patients with elevated VEGF had a higher probability of extranodal involvement, high IPI, Myc/Bcl-2 double expression and high Ki-67.16 In addition, they created a V–IPI model based on VEGF and IPI. Patients were divided into low-risk, low-intermediate-risk, high-intermediate-risk and high-risk groups according to this model, the cumulative progress-free survival (PFS) of four groups were 94.4%, 74.1%, 40.6% and 14.8% (P < 0.05), the cumulative OS were 100%, 100%, 42.4% and 0% (P < 0.05), respectively.16 Drugs with anti-VEGF activity may be promising in the treatment of DLBCL after further exploration. Bevacizumab, an anti-VEGF monoclonal antibody, shows definite effect in several solid tumors, such as non-squamous non-small cell lung cancer17 and metastatic colorectal cancer.18 The efficacy and tolerance of bevacizumab combination with R-CHOP (RA-CHOP) have also been explored in DLBCL. A Phase III trial conducted by Seymour et al compared the efficacy and safety of RA-CHOP and R-CHOP in 787 patients with DLBCL. The median follow-up time of the R-CHOP and the RA-CHOP was 23.7 and 23.6 months, respectively. The results showed that median PFS for RA-CHOP and R-CHOP was 40.2 and 42.9 months, respectively.19 There was no significant difference in proportion of deaths of the two groups. Moreover, the incidence of cardiac events in the RA-CHOP was higher.19
Potential Molecular and Signal Pathway Target Therapy
BCR/NF-κB Pathway Inhibitors
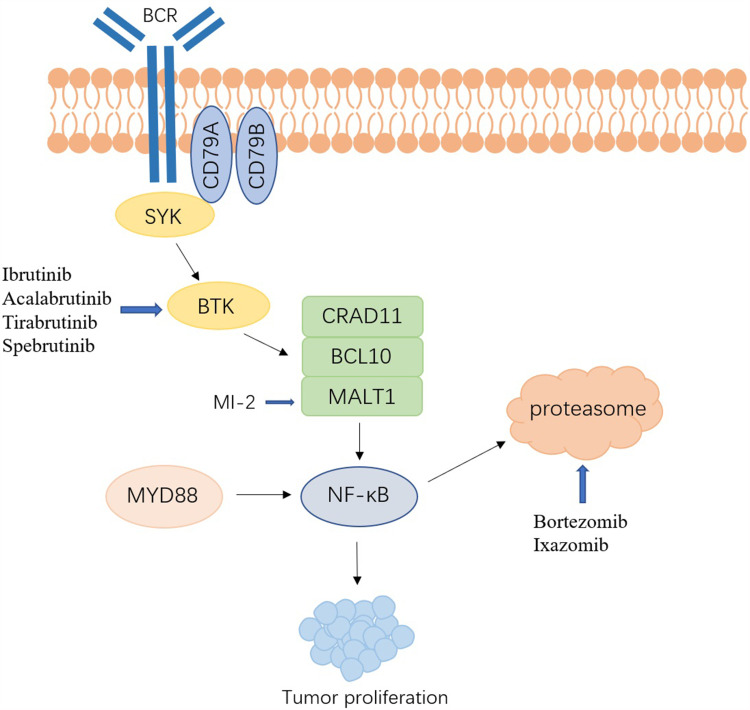
B-cell receptor (BCR) is a membrane-bound antibody expressing on the surface of B cells, which transduces signals through non-covalent binding with CD79A-CD79B heterodimer.20,21 Bruton’s tyrosine kinase (BTK) is a regulator of the BCR signaling pathway, and nuclear factor-κB (NF-κB) is a transcription factor downstream of BTK.22 The activation of NF-κB and BCR signals caused by various driving factors plays a key role in the growth, proliferation, and apoptosis of DLBCL, and may be involved in mediating tumor resistance (Figure 2).22,23 Studies have found that ABC-DLBCL was usually accompanied by continuous activation of the NF-κB signaling pathway, which can make DLBCL cells insensitive to chemotherapeutic drugs and block cell apoptosis.23
Figure 2.
Schematic depiction of BCR signaling pathway and targeted drugs in DLBCL.
Caspase recruitment domain family member 11 (CARD11) is a cytoplasmic scaffold protein, which forms CBM multiprotein complex together with MALT1 and BCL10, and participates in the regulation of the activation of the BCR and NF-κB pathway.24,25 Studies have found that CARD11 has a higher expression in ABC-DLBCL compared to other subgroups, and CARD11 mutations can occur in about 10% of ABC-DLBCL.24,26 MI-2 is the first MALT1 inhibitor and has shown antitumor activity against ABC-DLBCL both in vivo and in vitro.27 Ducharme et al performed whole exome sequencing (WES) on pathological biopsy specimens of 32 patients with primary cutaneous diffuse large B cell lymphoma, leg type (PCLBCL-LT).28 The results suggested that mutations involving BCR pathway had close connections with the invasiveness of disease. Patients with CD79A/B, MYD 88 and/or CARD11 mutations had short EFS and OS.28
BTK is the key link between BCR and NF-κB, so it is the main drug target for B cell malignancies.29 Ibrutinib is the first-generation selective BTK inhibitor that has a cytotoxic effect on ABC-DLBCL by reducing the activity of the NF-κB pathway.30 A clinical trial conducted by Wilson et al compared the efficacy of ibrutinib between ABC-DLBCL and GCB-DLBCL. The results showed that 37% (14/38) of ABC-DLBCL patients achieved complete or partial responses, while only 5% (1/20) of GCB-DLBCL patients achieved complete or partial responses.31 In addition, this study found that ABC-DLBCL patients with BCR mutations and/or MYD 88 mutations had a higher response rate to ibrutinib.31 This result provides promising prospects for ibrutinib-based personalized treatment in patients with ABC-DLBCL. In a double-blind phase III trial involving 838 patients with non-GCB DLBCL, patients were divided into ibrutinib + R-CHOP group (n = 419) and placebo + R-CHOP group (n = 419). The study found that the efficacy of ibrutinib is related to the patient’s age. Among patients younger than 60 years old, the ibrutinib + R-CHOP group had a better EFS (HR, 0.579), PFS (HR, 0.556), and OS (HR, 0.330). However, patients over 60 years old have reduced tolerance to ibrutinib + R-CHOP regimen, and the incidence of adverse effects is higher, which leads to worse EFS and OS.32 Cases of ibrutinib resistance have been reported, and more selective second- and third-generation BTK inhibitors (acalabrutinib, tirabrutinib, spebrutinib, etc.) are being actively explored and clinically validated.33,34
The proteasome inhibitor bortezomib can inhibit the activation of the NF-κB pathway; therefore, some researchers have tried bortezomib combined with R-CHOP to treat non-GCB DLBCL, but the effect is not satisfactory.35 A meta-analysis studied the efficacy and adverse effects of bortezomib-containing regimens and standard R-CHOP regimens in ABC-DLBCL. The results showed that compared with standard R-CHOP regimens, bortezomib-containing regimens could not improve OS, and would increase the risk of peripheral neuropathy.36 Davies et al conducted a prospective clinical trial to compare the efficacy of R-CHOP and bortezomib combined with R-CHOP on DLBCL. A total of 1128 cases were included and the follow-up time was 30 months. The results showed that bortezomib combined with R-CHOP did not improve the PFS (P=0.28).37
Ixazomib is an oral second-generation proteasome inhibitor. Compared with bortezomib, it has better pharmacokinetics and anti-tumor activity. Liu et al confirmed the efficacy of ixazomib in DLBCL in vitro and in vivo, and found that ixazomib can induce tumor cell apoptosis by activating checkpoint kinase 2 (CHK2).38 The anti-tumor activity of ixazomib can be enhanced by inhibiting CHK2, and after further verification in the future, ixazomib combined with CHK2 inhibitors may provide options for the treatment of R/R DLBCL.38
BCL2 Inhibitors
Studies have found that BCL2 overexpression is not only involved in the pathogenesis of NHL, but also related to immunochemotherapy resistance.2 Venetoclax (ABT-199) is the first BCL2 inhibitor approved for R/R chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML), and the efficacy and safety in DLBCL patients are under active clinical trial exploration.39 The Phase II CAVALLI trial studied the efficacy and safety of venetoclax combined with R-CHOP in DLBCL, the CR rate at the end of treatment was 69%.40 This trial showed that compared with R-CHOP, venetoclax combined with R-CHOP could improve PFS. Although the regimen increased grade 3/4 hematologic adverse events (86%), it did not increase related mortality.40
TP53 gene is an important tumor suppressor gene, which is related to apoptosis or cell cycle regulation.4,41 About 22% of DLBCL patients may have TP53 mutations, and the prognosis of these patients is generally poor.42 In addition, studies have found that TP53 mutation is related to venetoclax resistance in DLBCL patients.43
HDAC Inhibitors
CD20 is expressed on the surface of B cells and plays an important role in the proliferation and differentiation of B cells.44 It is currently the main target of DLBCL immunotherapy. Studies have found that the expression of CD20 is highly heterogeneous among patients, and as the course of treatment with rituximab-containing regimens increases, the expression of CD20 gradually decreases, resulting in a decrease in the sensitivity to treatment.45 Drug combination has become the main strategy to overcome drug resistance and improve the efficacy of immunochemotherapy. Histone deacetylases (HDACs) are a class of proteases, which play an important role in the regulation of gene expression and are related to the occurrence and drug resistance of lymphoma.46 The abnormal expression of HDAC can make histone acetylation unregulated, which leads to the inhibition of gene transcription and the decrease of CD20 expression, thereby mediating immune evasion.45
B cell is also a type of antigen-presenting cell (APC), which expresses MHC class I and class II molecules and presents antigens to CD4 and CD8 T cells.47 B-cell lymphoma cells usually down-regulate the expression of MHC class I and class II to reduce the recognition of T cells, thereby causing immune evasion.45,48 Some patients with DLBCL may have varying degrees of decreased expression of MHC class I and/or class II.49 Studies have found that HDACs inhibitors can induce the expression of MHC class I, MHC class II and CD20 in DLBCL cell lines.50–52 Entinostat is an oral selective HDAC1 inhibitor. In vitro study conducted by Sarah Frys et al showed that entinostat can increase the expression of CD20 and adhesion molecules in B-cell lymphoma, and has a synergistic effect with rituximab.53 HDAC6 overexpression often occurs in DLBCL. Studies have shown that HDAC6 inhibitors can promote DLBCL cell apoptosis by inhibiting the MET/PI3K/AKT signaling pathway.54,55 In addition, it can significantly up-regulate the expression of CD20 in B-cell lymphoma and enhance the efficacy of anti-CD20 monoclonal antibodies by inhibiting HDAC6.56 Chidamide is a new oral HDAC inhibitor that can selectively inhibit the activity of HDAC1, 2, 3 and 10.57 Guan et al proved that chidamide can significantly increase the expression of CD20 in DLBCL cells and has a synergistic anti-tumor effect with rituximab in vitro and in vivo.58 CKD-581 is a new HDACs inhibitor with promising application prospect. Kim et al found that CKD-581 can reduce the expression of MYC and other anti-apoptotic proteins (such as BCL-2, BCL-6, and BCL-XL) in DHL and THL cell lines.59 Currently, some clinical trials of HDACs inhibitors are being actively carried out, and initial achievement has been made.60,61
Chimeric Antigen Receptor (CAR) T-Cell Therapy
CAR-T therapy is a novel and effective immunotherapy, which has shown significant efficacy in patients with relapsed and refractory hematological tumors,62 and provides a promising treatment strategy for patients with R/R DLBCL. The patient’s T cells are genetically modified to express CAR that targets their own lymphoma, and then bind to the antigen on the surface of the lymphoma cell to exert anti-tumor activity.63 The adverse effects of CAR-T therapy mainly include cytokine release syndrome (CRS), neurotoxicity, and panhematopenia.64
CD19 is a cell surface molecule found in B-cell leukemia and lymphoma. Anti-CD19 CAR-T therapy has shown good efficacy in patients with refractory and relapsed acute lymphoblastic leukemia (ALL) and B-NHL. Tisagenlecleucel, axicabtagene ciloleucel (axi-cel) and lisocabtagene maraleucel (liso-cel) are anti-CD19 CAR T-cells that have been used for R/R DLBCL, which have been evaluated in clinical trials (Table 1).65–67 In a phase IIa clinical trial, 93 patients with R/R DLBCL received tisagenlecleucel treatment. The median follow-up time was 14 months, and the overall response rate was 52%. Among them, 40% of patients achieved complete response and 12% of patients achieved partial response, the one-year relapse-free survival (RFS) is estimated to be 65%.68 The most common grade 3 or 4 adverse events include CRS (22%) and neurotoxicity (12%), the remaining adverse reactions are mainly anemia and neutropenia, infection, etc.68 A multicenter, single-arm, phase II trial conducted by Stephen J Schuster et al evaluated the efficacy and safety of tisagenlecleucel.69 The study enrolled 115 patients with R/R DLBCL who received tisagenlecleucel infusion, and the median follow-up time was 40.3 months. The overall response rate was 53% (61), 39% (45) of patients had complete response, and the incidence of CRS was 27% (31), there were no reports of treatment-related deaths.69
Table 1.
Clinical Outcomes of Patients with DLBCL Treated with Anti-CD19 CAR-T Therapy
| Stage | Number | Median Follow-Up Time (Months) | ORR (%) | CRR (%) | Grade≥3 CRS (%) | Grade≥3 Neurotoxicity (%) |
|---|---|---|---|---|---|---|
| Tisagenlecleucel | ||||||
| Phase IIa68 | 93 | 14 | 53 | 40 | 22 | 12 |
| Phase II69 | 115 | 40.3 | 53 | 39 | 26 | - |
| Axicabtagene ciloleucel | ||||||
| Phase II70 | 111 | 15.4 | 82 | 54 | 13 | 28 |
| Phase II73 | 42 | 15.9 | 89 | 78 | 8 | 23 |
| Phase II71 | 101 | 27.1 | 83 | 59 | 11 | 32 |
| Phase III72 | 180 | 24.9 | 83 | 65 | 6 | 21 |
| Lisocabtagene maraleucel | ||||||
| Phase III67 | 92 | 6.2 | - | - | 1 | 4 |
Abbreviations: ORR, overall response rate; CRR, complete response rate; CRS, cytokine release syndrome.
In a multicenter phase II trial, 111 patients with R/R DLBCL and primary mediastinal B-cell lymphoma were enrolled. All patients were treated with axi-cel. The median follow-up was 15.4 months, and the overall response rate was 82%, 54% of patients had complete response, and the 18-month OS was 52%.70 The incidence of CRS and neurotoxicity accounted for 13% and 28%, respectively.70 The ZUMA-1 study conducted by Frederick L Locke et al evaluated the long-term safety and activity of axi-cel in patients with R/R DLBCL. The median follow-up time was 27.1 months. Among 101 evaluable patients, 59 (58%) patients achieved complete response and 25 (25%) patients had partial response, the objective response rate was 83%6 999er, the 24-month EFS is estimated to be 72.0%.71 The ZUMA-7 study showed that the 24-month EFS of axi-cel group had improved significantly than standard second-line therapy (salvage immunochemotherapy followed by autologous hematopoietic stem cell transplantation) group (41% vs 16%, P<0.001). The complete response rate of the axi-cel group and the standard second-line therapy group was 65% and 32%, respectively.72 The ZUMA-12 study evaluated the efficacy of axi-cel in high-risk DLBCL as first-line therapy, and the complete response rate (78%) and objective response rate (89%) were encouraging, and the 12-month estimated PFS, EFS and OS rate were 75%, 73% and 91%, respectively.73
TRANSFORM trial conducted by Manali Kamdar et al compared the efficacy of liso-cel and standard second-line therapy in R/R DLBCL, 184 patients with R/R DLBCL were enrolled.67 The results showed that the median EFS of the liso-cel group (10.1 months) was significantly improved compared to the control group (2.3 months), and the adverse effects were manageable.67
A phase III trial compared the efficacy of tisagenlecleucel and standard second-line therapy in aggressive B-cell lymphoma, the results showed that tisagenlecleucel have limited efficacy and was not satisfactory.74 Some patients cannot benefit from CAR-T therapy, which may be related to mechanisms of drug resistance.
The problems in product manufacturing may be the potential barriers of the remission of CAR-T therapy. The inability to collect enough T cells can affect the quality of CAR T cell products, which may be related to the reduction of the number of T cells caused by previous chemotherapy.75 Early collection of T cells in high-risk patients may become a way to solve this problem. Treatment-related antigen loss or modulation plays an important role in DLBCL immune escape.76 Down-regulation or loss of CD19 expression is the key mechanism of resistance to initially effective CAR-T therapy.77 In addition, abnormal differentiation and dysfunction of T cells are related to poor response to CAR-T therapy.78
Immune Checkpoint Inhibitors (ICIs)
Programmed death receptor 1 (PD-1)/programmed death ligand-1 (PD-L1) immune checkpoints play an important role in immune evasion and drug resistance of lymphoma.79,80 Anti-PD-1 monoclonal antibody nivolumab was evaluated in several studies; however, the efficacy was not satisfactory.81,82 Therefore, the combination of nivolumab and other targeted drugs such as ibrutinib is also being explored.
Anti-CD47 antibodies can produce antitumor effects by inducing cell phagocytosis.83 Hu5F9-G4 is a CD47-blocking monoclonal antibody. A phase Ib clinical trial conducted by Advani et al studied the efficacy of Hu5F9-G4 antibody in NHL, and verified the synergistic effect of Hu5F9-G4 and rituximab.84 Twenty-two patients (15 with DLBCL and 7 with follicular lymphoma) were enrolled in this study, the objective response rate was 50%, complete response rate was 36%, and the objective response rate and complete response rate of 15 patients with DLBCL patients were 40% and 33%, respectively.84
Antibody Drug-Conjugate (ADC)
ADC is a novel compound with proved efficacy on DLBCL or promising activity, which formed by the combination of cytotoxic drugs with antibodies that targeted surface antigens of tumor cell.85 ADC selectively deliver effective cytotoxic drugs through antibodies to malignant cells expressing specific antigen, thereby improving the specificity and efficacy of chemotherapy drugs. Polatuzumab vedotin is a novel ADC targeting CD79b and delivers monomethyl auristatin E (MMAE).86 Polatuzumab vedotin was evaluated in a phase III trial in 879 patients with intermediate-risk or high-risk DLBCL. The patients were randomly divided into two groups, receiving pola-R-CHP and R-CHOP treatment, respectively. The results showed that 2-year EFS was significantly higher in pola-R-CHP group than that in R-CHOP group (76.7% vs 70.2%, P = 0.02).87 Loncastuximab tesirine is a CD19-targeted ADC and delivers cytotoxic alkylating agent, SG3199.88 A phase II trial studied the efficacy and safety of loncastuximab tesirine in R/R DLBCL. A total of 145 patients received at least one dose of loncastuximab tesirine, with an overall response rate of 48.3%.89 There were no loncastuximab tesirine-associated deaths.
Tafasitamab Combined with Lenalidomide
Tafasitamab is a humanized monoclonal antibody with an optimized FC domain, targeting CD19. Because the response rates of tafasitamab as a single agent are low, the combined drug therapy is being developed. To date, the tafasitamab combined with lenalidomide regimen shows promising anti-tumor activity.90 A phase II trial (L-MIND) observed the efficacy and safety of tafasitamab combined with lenalidomide in R/ R DLBCL. Eighty-one patients were enrolled and the median follow-up was 13.2 months, objective response rate was 60%, complete response rate was 43%.91 The major adverse effects of grades 3 and 4 were neutropenia (39[48%]) and thrombocytopenia (14[17%]).
In addition, a novel targeted drug bispecific monoclonal antibody (bsAb) shows promising efficacy in invasive lymphoma. BsAb is a new type of monoclonal antibody that can recognize two different antigens or epitopes simultaneously, the two unique fragments on anti-CD20xCD3 bsAb can be combined with CD3 on T-cells and tumor-associated antigens, thereby enhancing cytotoxicity92,93 Mosunetuzumab, glofitamab, epcoritamab, plamotamab and odronextamab are anti-CD20xCD3 bsAb currently evaluated in clinical trials.
At present, the next-generation sequencing (NGS) has been widely used in the precise diagnosis and treatment of hematological malignancy, which gives people a deep understanding of DLBCL at genome level.94 Recently, several studies have proposed new genetic subtypes based on the distinct genotypic of DLBCL. Schmitz et al have proposed four genetic subgroups, namely MCD (based on the co-occurrence of MYD88L265P and CD79B mutations), BN2 (based on BCL6 fusions and NOTCH2 mutations), N1 (based on NOTCH1 mutations), and EZB (based on EZH2 mutations and BCL2 translocations); moreover, they found that MCD and N1 have a worse prognosis compared with the other two subtypes.95 Based on the previous four subtypes, they added A53 (characterized by TP53 mutations and lack) and ST2 (SGK1 and TET2 mutations) subtypes recently.96 Based on genetic subtypes, gene-oriented treatment provides personalized therapeutic strategies for DLBCL. Studies have shown that the MCD subtype can benefit from BTK-inhibitor ibrutinib combined with R-CHOP.97,98
Conclusion
With the widespread application of targeted therapy, some patients are becoming resistant to novel drugs. People start to pay more attention to the mechanisms of resistance and their reversal in order to find more effective treatments. The early identification of high-risk patients and the choice of personalized targeted therapy is still a major clinical challenge.
The increased understanding of DLBCL biology, tumor microenvironment, epigenetics, and genetic subtypes will bring more new therapeutic strategies and gene-oriented treatment. In the future, these novel and effective targeted drugs and immunotherapy will make the long-term survival of patients with R/R DLBCL more promising. More precision and personalized treatments need to be evaluated in larger-scale clinical trials and the selection of targeted drugs according to the genetic subgroup is worth further studies.
Acknowledgments
This work was supported by the Key Department of Jiangsu Province (ZDXKB201620), and the Guo Qinglong 2016 Nanjing Emerging Industry Guidance Special Fund (YY2016120701).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545 [DOI] [PubMed] [Google Scholar]
- 2.Klener P, Klanova M. Drug resistance in non-Hodgkin lymphomas. Int J Mol Sci. 2020;21(6):2081. doi: 10.3390/ijms21062081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giacomelli AO, Yang X, Lintner RE, et al. Mutational processes shape the landscape of TP53 mutations in human cancer. Nat Genet. 2018;50(10):1381–1387. doi: 10.1038/s41588-018-0204-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fornecker LM, Muller L, Bertrand F, et al. Multi-omics dataset to decipher the complexity of drug resistance in diffuse large B-cell lymphoma. Sci Rep. 2019;9(1):895. doi: 10.1038/s41598-018-37273-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solimando AG, Annese T, Tamma R, et al. New insights into diffuse large B-cell lymphoma pathobiology. Cancers. 2020;12(7):1869. doi: 10.3390/cancers12071869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del PA, Schioppa T, Tiberio L, et al. Leukocyte trafficking in tumor microenvironment. Curr Opin Pharmacol. 2017;35:40–47. doi: 10.1016/j.coph.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 8.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80. doi: 10.1126/science.aaa6204 [DOI] [PubMed] [Google Scholar]
- 9.Todorovic BM, Jelicic J, Mihaljevic B, et al. Gene mutation profiles in primary diffuse large B cell lymphoma of central nervous system: next generation sequencing analyses. Int J Mol Sci. 2016;17(5):683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Li LR. R-CHOP resistance in diffuse large B-cell lymphoma: biological and molecular mechanisms. Chin Med J. 2020;134(3):253–260. doi: 10.1097/CM9.0000000000001294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu-Monette ZY, Xiao M, Au Q, et al. Immune profiling and quantitative analysis decipher the clinical role of immune-checkpoint expression in the tumor immune microenvironment of DLBCL. Cancer Immunol Res. 2019;7(4):644–657. doi: 10.1158/2326-6066.CIR-18-0439 [DOI] [PubMed] [Google Scholar]
- 12.Bouwstra R, He Y, de Boer J, et al. CD47 expression defines efficacy of rituximab with CHOP in Non-Germinal Center B-cell (Non-GCB) diffuse large B-cell Lymphoma Patients (DLBCL), but not in GCB DLBCL. Cancer Immunol Res. 2019;7(10):1663–1671. doi: 10.1158/2326-6066.CIR-18-0781 [DOI] [PubMed] [Google Scholar]
- 13.Brito A, Delamain MT, Fanelli MF, et al. Angiogenesis’ related genetic variants alter clinical features and prognosis of diffuse large B-cell lymphoma patients. Tumour Biol. 2021;43(1):129–140. doi: 10.3233/TUB-211510 [DOI] [PubMed] [Google Scholar]
- 14.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264. doi: 10.1016/j.cell.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahini L, Gasparov S, Petrusevska G, et al. Clinical significance of VEGF-A and microvessel density in diffuse large B-Cell lymphoma and low-grade follicular lymphoma. Acta Clin Croat. 2017;56(4):588–593. doi: 10.20471/acc.2017.56.04.02 [DOI] [PubMed] [Google Scholar]
- 16.Sang W, Zhou H, Qin Y, et al. Risk stratification model based on VEGF and International Prognostic Index accurately identifies low-risk diffuse large B-cell lymphoma patients in the rituximab era. Int J Hematol. 2021;114(2):189–198. doi: 10.1007/s12185-021-03145-3 [DOI] [PubMed] [Google Scholar]
- 17.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: aVAil. J Clin Oncol. 2009;27(8):1227–1234. doi: 10.1200/JCO.2007.14.5466 [DOI] [PubMed] [Google Scholar]
- 18.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305 [DOI] [PubMed] [Google Scholar]
- 19.Seymour JF, Pfreundschuh M, Trneny M, et al. R-CHOP with or without bevacizumab in patients with previously untreated diffuse large B-cell lymphoma: final MAIN study outcomes. Haematologica. 2014;99(8):1343–1349. doi: 10.3324/haematol.2013.100818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young RM, Phelan JD, Wilson WH, et al. Pathogenic B-cell receptor signaling in lymphoid malignancies: new insights to improve treatment. Immunol Rev. 2019;291(1):190–213. doi: 10.1111/imr.12792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ondrisova L, Mraz M. Genetic and non-genetic mechanisms of resistance to BCR signaling inhibitors in B cell malignancies. Front Oncol. 2020;10:591577. doi: 10.3389/fonc.2020.591577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young RM, Shaffer AR, Phelan JD, et al. B-cell receptor signaling in diffuse large B-cell lymphoma. Semin Hematol. 2015;52(2):77–85. doi: 10.1053/j.seminhematol.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turturro F. Constitutive NF- κ B activation underlines major mechanism of drug resistance in relapsed refractory diffuse large B cell lymphoma. Biomed Res Int. 2015;2015:484537. doi: 10.1155/2015/484537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenz G, Davis RE, Ngo VN, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319(5870):1676–1679. doi: 10.1126/science.1153629 [DOI] [PubMed] [Google Scholar]
- 25.Juilland M, Thome M. Holding all the CARDs: how MALT1 controls CARMA/CARD-dependent signaling. Front Immunol. 2018;9:1927. doi: 10.3389/fimmu.2018.01927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunleavy K, Erdmann T, Lenz G. Targeting the B-cell receptor pathway in diffuse large B-cell lymphoma. Cancer Treat Rev. 2018;65:41–46. doi: 10.1016/j.ctrv.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 27.Fontan L, Goldstein R, Casalena G, et al. Identification of MALT1 feedback mechanisms enables rational design of potent antilymphoma regimens for ABC-DLBCL. Blood. 2021;137(6):788–800. doi: 10.1182/blood.2019004713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ducharme O, Beylot-Barry M, Pham-Ledard A, et al. Mutations of the B-cell receptor pathway confer chemoresistance in primary cutaneous diffuse large B-cell lymphoma leg type. J Invest Dermatol. 2019;139(11):2334–2342. doi: 10.1016/j.jid.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 29.Gabizon R, London N. Fast and clean BTK inhibitor. J Med Chem. 2020. 63. 10:5100–5101. doi: 10.1021/acs.jmedchem.0c00597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou K, Yu Z, Jia Y, et al. Efficacy and safety of ibrutinib in diffuse large B-cell lymphoma: a single-arm meta-analysis. Crit Rev Oncol Hematol. 2020;152:103010. doi: 10.1016/j.critrevonc.2020.103010 [DOI] [PubMed] [Google Scholar]
- 31.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–926. doi: 10.1038/nm.3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Younes A, Sehn LH, Johnson P, et al. Randomized Phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in Non-Germinal Center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285–1295. doi: 10.1200/JCO.18.02403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George B, Chowdhury SM, Hart A, et al. Ibrutinib resistance mechanisms and treatment strategies for B-cell lymphomas. Cancers. 2020;12(5):1328. doi: 10.3390/cancers12051328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu J, Liu C, Tsui ST, et al. Second-generation inhibitors of Bruton tyrosine kinase. J Hematol Oncol. 2016;9(1):80. doi: 10.1186/s13045-016-0313-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubio AJ, Bencomo-Alvarez AE, Young JE, et al. 26S proteasome Non-ATPase regulatory subunits 1 (PSMD1) and 3 (PSMD3) as putative targets for cancer prognosis and therapy. Cells. 2021;10(9):2390. doi: 10.3390/cells10092390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Z, Chen X, Li Z, et al. The role of bortezomib in newly diagnosed diffuse large B cell lymphoma: a meta-analysis. Ann Hematol. 2018;97(11):2137–2144. doi: 10.1007/s00277-018-3435-1 [DOI] [PubMed] [Google Scholar]
- 37.Davies A, Cummin TE, Barrans S, et al. Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, Phase 3 trial. Lancet Oncol. 2019;20(5):649–662. doi: 10.1016/S1470-2045(18)30935-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W, Chen J, Tamayo AT, et al. Preclinical efficacy and biological effects of the oral proteasome inhibitor ixazomib in diffuse large B-cell lymphoma. Oncotarget. 2018;9(1):346–360. doi: 10.18632/oncotarget.20378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35(8):826–833. doi: 10.1200/JCO.2016.70.4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morschhauser F, Feugier P, Flinn IW, et al. A Phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600–609. doi: 10.1182/blood.2020006578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bykov V, Eriksson SE, Bianchi J, et al. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18(2):89–102. doi: 10.1038/nrc.2017.109 [DOI] [PubMed] [Google Scholar]
- 42.Xu-Monette ZY, Wu L, Visco C, et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2012;120(19):3986–3996. doi: 10.1182/blood-2012-05-433334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esteve-Arenys A, Valero JG, Chamorro-Jorganes A, et al. The BET bromodomain inhibitor CPI203 overcomes resistance to ABT-199 (venetoclax) by downregulation of BFL-1/A1 in in vitro and in vivo models of MYC+/BCL2+ double hit lymphoma. Oncogene. 2018;37(14):1830–1844. doi: 10.1038/s41388-017-0111-1 [DOI] [PubMed] [Google Scholar]
- 44.Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. N Engl J Med. 2012;366(21):2008–2016. doi: 10.1056/NEJMct1114348 [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Waschke BC, Woolaver RA, et al. HDAC inhibitors overcome immunotherapy resistance in B-cell lymphoma. Protein Cell. 2020;11(7):472–482. doi: 10.1007/s13238-020-00694-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sermer D, Pasqualucci L, Wendel HG, et al. Emerging epigenetic-modulating therapies in lymphoma. Nat Rev Clin Oncol. 2019;16(8):494–507. doi: 10.1038/s41571-019-0190-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Charette M, Marabelle A, Houot R. Turning tumour cells into antigen presenting cells: the next step to improve cancer immunotherapy? Eur J Cancer. 2016;68:134–147. doi: 10.1016/j.ejca.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 48.de Charette M, Houot R. Hide or defend, the two strategies of lymphoma immune evasion: potential implications for immunotherapy. Haematologica. 2018;103(8):1256–1268. doi: 10.3324/haematol.2017.184192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nijland M, Veenstra RN, Visser L, et al. HLA dependent immune escape mechanisms in B-cell lymphomas: implications for immune checkpoint inhibitor therapy? Oncoimmunology. 2017;6(4):e1295202. doi: 10.1080/2162402X.2017.1295202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang Y, Ortega-Molina A, Geng H, et al. CREBBP inactivation promotes the development of HDAC3-dependent lymphomas. Cancer Discov. 2017;7(1):38–53. doi: 10.1158/2159-8290.CD-16-0975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng S, Hu Q, Zhang H, et al. HDAC3 inhibition upregulates PD-L1 expression in B-cell lymphomas and augments the efficacy of Anti-PD-L1 therapy. Mol Cancer Ther. 2019;18(5):900–908. doi: 10.1158/1535-7163.MCT-18-1068 [DOI] [PubMed] [Google Scholar]
- 52.Cycon KA, Mulvaney K, Rimsza LM, et al. Histone deacetylase inhibitors activate CIITA and MHC class II antigen expression in diffuse large B-cell lymphoma. Immunology. 2013;140(2):259–272. doi: 10.1111/imm.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frys S, Simons Z, Hu Q, et al. Entinostat, a novel histone deacetylase inhibitor is active in B-cell lymphoma and enhances the anti-tumour activity of rituximab and chemotherapy agents. Br J Haematol. 2015;169(4):506–519. doi: 10.1111/bjh.13318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Z, Cai Y, Yang Y, et al. Activation of MET signaling by HDAC6 offers a rationale for a novel ricolinostat and crizotinib combinatorial therapeutic strategy in diffuse large B-cell lymphoma. J Pathol. 2018;246(2):141–153. doi: 10.1002/path.5108 [DOI] [PubMed] [Google Scholar]
- 55.Yang J, Li D, Zhou J. Histone deacetylase 6 as a therapeutic target in B cell-associated hematological malignancies. Front Pharmacol. 2020;11:971. doi: 10.3389/fphar.2020.00971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bobrowicz M, Dwojak M, Pyrzynska B, et al. HDAC6 inhibition upregulates CD20 levels and increases the efficacy of anti-CD20 monoclonal antibodies. Blood. 2017;130(14):1628–1638. doi: 10.1182/blood-2016-08-736066 [DOI] [PubMed] [Google Scholar]
- 57.Gao S, Li X, Zang J, et al. Preclinical and clinical studies of chidamide (CS055/HBI-8000), an orally available subtype-selective HDAC inhibitor for cancer therapy. Anticancer Agents Med Chem. 2017;17(6):802–812. doi: 10.2174/1871520616666160901150427 [DOI] [PubMed] [Google Scholar]
- 58.Guan XW, Wang HQ, Ban WW, et al. Novel HDAC inhibitor Chidamide synergizes with Rituximab to inhibit diffuse large B-cell lymphoma tumour growth by upregulating CD20. Cell Death Dis. 2020;11(1):20. doi: 10.1038/s41419-019-2210-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SJ, Kim UJ, Yoo HY, et al. Anti-cancer effects of CKD-581, a potent histone deacetylase inhibitor against diffuse large B-cell lymphoma. Int J Mol Sci. 2020;21(12):4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Persky DO, Li H, Rimsza LM, et al. A phase I/II trial of vorinostat (SAHA) in combination with rituximab-CHOP in patients with newly diagnosed advanced stage diffuse large B-cell lymphoma (DLBCL): SWOG S0806. Am J Hematol. 2018;93(4):486–493. doi: 10.1002/ajh.25010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Assouline SE, Nielsen TH, Yu S, et al. Phase 2 study of panobinostat with or without rituximab in relapsed diffuse large B-cell lymphoma. Blood. 2016;128(2):185–194. doi: 10.1182/blood-2016-02-699520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sermer D, Brentjens R. CAR T-cell therapy: full speed ahead. Hematol Oncol. 2019;37(Suppl 1):95–100. doi: 10.1002/hon.2591 [DOI] [PubMed] [Google Scholar]
- 63.Abramson JS. Anti-CD19 CAR T-cell therapy for B-cell non-Hodgkin lymphoma. Transfus Med Rev. 2020;34(1):29–33. doi: 10.1016/j.tmrv.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 64.Hopfinger G, Jager U, Worel N. CAR-T cell therapy in diffuse large B cell lymphoma: hype and hope. Hemasphere. 2019;3(2):e185. doi: 10.1097/HS9.0000000000000185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. doi: 10.1016/S0140-6736(22)00662-6 [DOI] [PubMed] [Google Scholar]
- 68.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 69.Schuster SJ, Tam CS, Borchmann P, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(10):1403–1415. doi: 10.1016/S1470-2045(21)00375-2 [DOI] [PubMed] [Google Scholar]
- 70.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, Phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. doi: 10.1056/NEJMoa2116133 [DOI] [PubMed] [Google Scholar]
- 73.Neelapu SS, Dickinson M, Munoz J, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. 2022;28(4):735–742. doi: 10.1038/s41591-022-01731-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bishop MR, Dickinson M, Purtill D, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629–639. doi: 10.1056/NEJMoa2116596 [DOI] [PubMed] [Google Scholar]
- 75.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16(6):372–385. doi: 10.1038/s41571-019-0184-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shalabi H, Kraft IL, Wang HW, et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica. 2018;103(5):e215–e218. doi: 10.3324/haematol.2017.183459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–1295. doi: 10.1158/2159-8290.CD-15-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng J, Zhao L, Zhang Y, et al. Understanding the mechanisms of resistance to CAR T-cell therapy in malignancies. Front Oncol. 2019;9:1237. doi: 10.3389/fonc.2019.01237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol. 2017;14(4):203–220. doi: 10.1038/nrclinonc.2016.168 [DOI] [PubMed] [Google Scholar]
- 80.Godfrey J, Tumuluru S, Bao R, et al. PD-L1 gene alterations identify a subset of diffuse large B-cell lymphoma harboring a T-cell-inflamed phenotype. Blood. 2019;133(21):2279–2290. doi: 10.1182/blood-2018-10-879015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ansell SM, Minnema MC, Johnson P, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: a single-arm, Phase II study. J Clin Oncol. 2019;37(6):481–489. doi: 10.1200/JCO.18.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a Phase Ib study. J Clin Oncol. 2016;34(23):2698–2704. doi: 10.1200/JCO.2015.65.9789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang W, Fan Y, Li M, et al. Therapy strategy of CD47 in diffuse large B-cell lymphoma (DLBCL). Dis Markers. 2021;2021:4894022. doi: 10.1155/2021/4894022 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Advani R, Flinn I, Popplewell L, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med. 2018;379(18):1711–1721. doi: 10.1056/NEJMoa1807315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Teicher BA, Chari RV. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011;17(20):6389–6397. doi: 10.1158/1078-0432.CCR-11-1417 [DOI] [PubMed] [Google Scholar]
- 86.Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155–165. doi: 10.1200/JCO.19.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med. 2022;386(4):351–363. doi: 10.1056/NEJMoa2115304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Furqan F, Hamadani M. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma: a review of clinical data. Ther Adv Hematol. 2022;13:1554005271. doi: 10.1177/20406207221087511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790–800. doi: 10.1016/S1470-2045(21)00139-X [DOI] [PubMed] [Google Scholar]
- 90.Cheson BD, Nowakowski G, Salles G. Diffuse large B-cell lymphoma: new targets and novel therapies. Blood Cancer J. 2021;11(4):68. doi: 10.1038/s41408-021-00456-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salles G, Duell J, Gonzalez BE, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978–988. doi: 10.1016/S1470-2045(20)30225-4 [DOI] [PubMed] [Google Scholar]
- 92.Gonzalez BE. Role of bispecific antibodies in relapsed/refractory diffuse large B-cell lymphoma in the CART era. Front Immunol. 2022;13:909008. doi: 10.3389/fimmu.2022.909008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sawalha Y. Relapsed/refractory diffuse large B-cell lymphoma: a look at the approved and emerging therapies. J Pers Med. 2021;11(12):1345. doi: 10.3390/jpm11121345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pedrosa L, Fernandez-Miranda I, Perez-Callejo D, et al. Proposal and validation of a method to classify genetic subtypes of diffuse large B cell lymphoma. Sci Rep. 2021;11(1):1886. doi: 10.1038/s41598-020-80376-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378(15):1396–1407. doi: 10.1056/NEJMoa1801445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wright GW, Huang DW, Phelan JD, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell. 2020;37(4):551–568. doi: 10.1016/j.ccell.2020.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen R, Zhou D, Wang L, et al. MYD88(L265P) and CD79B double mutations type (MCD type) of diffuse large B-cell lymphoma: mechanism, clinical characteristics, and targeted therapy. Ther Adv Hematol. 2022;13:1543990599. doi: 10.1177/20406207211072839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilson WH, Wright GW, Huang DW, et al. Effect of ibrutinib with R-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer Cell. 2021;39(12):1643–1653. doi: 10.1016/j.ccell.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]