Abstract
The numbers of sulfate reducers in two Arctic sediments with in situ temperatures of 2.6 and −1.7°C were determined. Most-probable-number counts were higher at 10°C than at 20°C, indicating the predominance of a psychrophilic community. Mean specific sulfate reduction rates of 19 isolated psychrophiles were compared to corresponding rates of 9 marine, mesophilic sulfate-reducing bacteria. The results indicate that, as a physiological adaptation to the permanently cold Arctic environment, psychrophilic sulfate reducers have considerably higher specific metabolic rates than their mesophilic counterparts at similarly low temperatures.
Dissimilatory sulfate reduction is the most important bacterial process in anoxic marine sediments, accounting for up to half of the total organic carbon remineralization (4, 12, 21). Since more than 90% of the global sea floor is cold (<4°C [19]), sulfate reducers must be able to metabolize and grow at low ambient temperatures. Sulfate reduction rates (SRRs) in polar sediments may be similar to those of temperate environments (14, 21, 24, 28), but sulfate reducers active in polar sediments have not been isolated and studied.
Similar SRRs in cold and temperate sediments could be explained either by (i) the presence of more sulfate reducers in cold environments, thus compensating for lower per-cell SRRs (i.e., cell-specific SRRs) at low temperatures, or by (ii) comparable community sizes in both environments but higher specific respiration rates of psychrophiles relative to those of mesophiles at low temperatures. In the present study, both possibilities were investigated by quantifying sulfate reducers in two polar sediments as well as by comparing specific SRRs of new psychrophilic isolates to those of known mesophilic sulfate-reducing bacteria (SRB). Because the phylogeny and physiology of sulfate reducers living in polar sediments were previously unknown, we used the most-probable-number (MPN) method to count and subsequently isolate the most abundant cultivable sulfate reducers for further pure-culture studies.
Two permanently cold sediments, located off the coast of Svalbard, Hornsund (76°58′2"N, 15°34′5"E; in situ temperature, 2.6°C) and Storfjord (77°33′0"N, 19°05′0"E; in situ temperature, −1.7°C), were sampled during a cruise in September and October of 1995. For further information about sampling sites, see Kostka et al. (18). Sediment was collected with a multicorer, and one individual core (referred to as core A) was subsampled for enumeration of sulfate reducers by triplicate MPN series (2), SRR measurements by the whole-core method (11), and nucleic acid analysis (25). The subcores were sliced on the ship, and samples from five sediment layers between the surface and 30-cm depth (Fig. 1) were transferred to liquid medium (17) containing either lactate (20 mM) or acetate (15 mM). Additionally, single-dilution series with propionate (20 mM) or propanol (20 mM) were inoculated. The cultures were incubated at 4, 10, and 20°C in our laboratory, and growth of sulfate reducers was monitored by measuring sulfide production during the following 30 months.
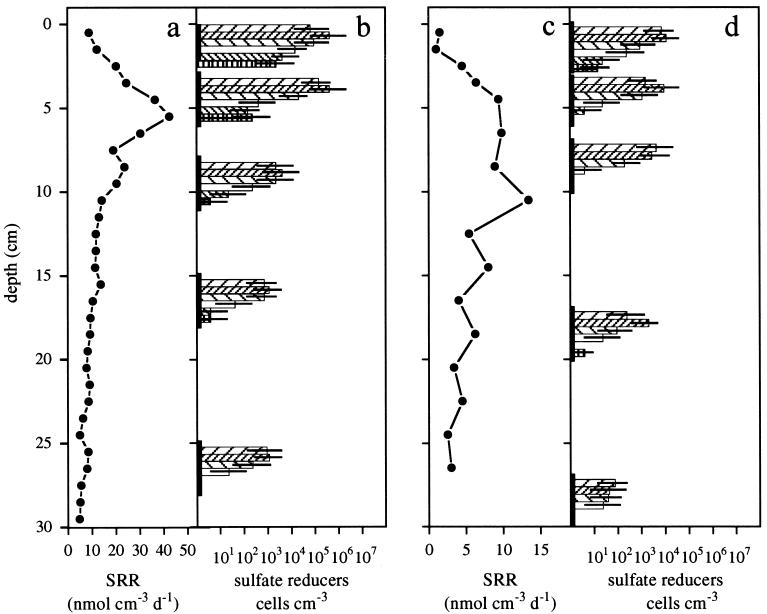
FIG. 1.
Depth profile of SRRs in Hornsund (a) and Storfjord (c) at in situ temperatures and MPN counts of SRB in Hornsund (b) and Storfjord (d) sediments. MPN series were incubated at different temperatures with either lactate ( , 20°C; ▨, 10°C; ▧, 4°C) or acetate (□, 20°C; ▧, 10°C; ⊞, 4°C). Horizontal bars represent 95% confidence intervals, and vertical bars indicate the depths of sediments used for MPN enrichments.
At both sampling sites, the maximum MPN counts of SRB occurred in the top 6 cm of the sediment. In particular in Storfjord, the highest SRRs occurred at a deeper layer than the maximum cell counts (Fig. 1). Below that depth, cell numbers decreased sharply. Maximum cell numbers were generally detected in MPN series incubated at 10°C with lactate as the substrate (Fig. 1b and d). Higher cell numbers at 10°C than at 20°C indicate that the majority of cultivable sulfate reducers in the sediment are unable to grow at 20°C, thus providing the first microbiological evidence for a predominantly psychrophilic sulfate reducer community in a marine sediment. Maximum MPNs with acetate as the substrate were 10- to 100-fold lower than those with lactate as the substrate for cultures and were always highest at 20°C. These results are probably due to extremely slow growth of acetate oxidizers at 4 and 10°C and not to a mesophilic acetate-oxidizing SRB community. This conclusion is supported by the facts that the first positive enrichments of samples collected at Storfjord, incubated at 4 and 10°C on acetate, were detected after more than 6 months and that counts increased slowly during the following 2 years.
In contrast to this microbiological evidence for a community with a psychrophilic growth potential (optimum temperature, below 20°C), Sagemann et al. (24) measured the highest SRRs for Hornsund and Storfjord sediments at 27°C. These process rate measurements seem to contradict our results from MPN counts. However, Isaksen and Jørgensen (9) demonstrated that a moderately psychrophilic SRB had an optimum temperature for sulfate reduction (28°C) 10°C higher than that for growth (18°C). This result indicates that the observed maximum SRRs at 27°C in the Svalbard sediments might still be assigned to a psychrophilic community.
MPN counts yielded no evidence for a larger community size of cultivable sulfate reducers in Arctic sediments relative to temperate sediments since maximum cell counts, e.g. 4.3 × 105 cells cm−3 for Hornsund sediments (Fig. 1b), are in the range of those reported previously for temperate marine sediments (2 × 105 to 2 × 106 cells cm−3 (13, 20, 27). Furthermore, parallel slot blot hybridizations indicate that numbers of SRB in Hornsund and Storfjord are comparable to those in temperate sediments (25, 26). If the community size and the SRRs in Arctic and temperate habitats are similar, then SRRs per cell must be comparable too, irrespective of the temperature difference.
To test this possibility, pure cultures of Arctic SRB were isolated from the highest dilution steps of the MPN enrichments by the modified deep-agar dilution technique (10). At 20°C, only three pure cultures could be isolated because most enrichments did not continue to grow after a transfer to fresh medium. None of these isolates is able to grow at the in situ temperature of the sampling sites, providing further evidence that the community active in the sediments is psychrophilic. At 4 and 10°C, 30 different strains were isolated from the MPN enrichments. Based on a preliminary physiological and phylogenetic characterization, 19 psychrophilic strains were selected for further studies. All strains except LSv22 had optimum temperatures below 20°C, and only three isolates grew at 26°C (Table 1). More relevant, however, is that they are the first isolates that grow at a typical temperature for polar sediments, i.e., the freezing point of seawater, −1.8°C (Table 1). Doubling times at −1.8°C were 4 to 6 days for the lactate-grown strains LSv54, LSv514 and LSv21 but more than 5 weeks for the acetate- and propionate-grown strains ASv26 and PSv29 (16).
TABLE 1.
Growth characteristics and specific SRRs of psychrophilic SRB measured at the in situ temperatures of their habitats
| Strain | Substratea | Incubation temp (°C) | Specific SRR (fmol cell−1 day−1) | Growth at each temp (°C)
|
||||
|---|---|---|---|---|---|---|---|---|
| −1.8 | 4 | 15 | 20 | 26 | ||||
| Hornsund | ||||||||
| LSv20 | Lactate | 2.6 | 14.0 ± 0.6 | + | + | + | + | − |
| LSv21 | Lactate | 2.6 | 2.7 ± 0.7 | + | + | + | + | − |
| LSv22 | Lactate | 2.6 | 13.0 ± 2.0 | + | + | + | + | + |
| LSv23 | Lactate | 2.6 | 2.3 ± 0.6 | + | + | + | + | − |
| LSv24 | Lactate | 2.6 | 11.0 ± 0.8 | + | + | + | + | − |
| LSv25 | Lactate | 2.6 | 2.8 ± 1.1 | + | + | + | + | − |
| LSv26 | Lactate | 2.6 | 6.9 ± 0.5 | + | + | + | + | + |
| LSv27 | Lactate | 2.6 | 2.6 ± 0.3 | + | + | N.D.b | − | − |
| LSv28 | Lactate | 2.6 | 2.6 ± 0.2 | + | + | + | − | − |
| PlSv28 | Propanol | 2.6 | 2.5 ± 1.4 | + | + | + | − | − |
| PSv29 | Propionate | 2.6 | 41.9 ± 23.4 | + | + | − | − | − |
| ASv25 | Acetate | 2.6 | 25.3 ± 0.3 | + | + | + | + | − |
| ASv26 | Acetate | 2.6 | 3.8 ± 1.0 | + | + | − | − | − |
| ASv28 | Acetate | 2.6 | 11.3 ± 0.9 | + | + | + | + | + |
| Storfjord | ||||||||
| LSv514 | Lactate | −1.7 | 3.6 ± 0.4 | + | + | + | + | − |
| LSv52 | Lactate | −1.7 | 7.6 ± 3.7 | + | + | + | + | − |
| LSv53 | Lactate | −1.7 | 0.9 ± 0.4 | + | + | + | + | − |
| LSv54 | Lactate | −1.7 | 1.9 ± 0.2 | + | + | + | − | − |
| LSv55 | Lactate | −1.7 | 6.2 ± 0.8 | + | + | + | − | − |
Carbon substrate used for isolation and for measurements of specific SRRs.
N.D., not determined.
Values are means ± standard deviations for three cultures. The mean specific SRRs were 10.2 and 4.0 fmol cell−1 day−1 for the Hornsund strains and Storfjord strains, respectively.
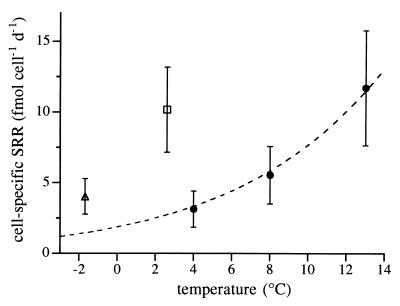
To compare SRRs of psychrophiles and mesophiles at the temperatures of their respective habitats, the specific SRRs of psychrophilic SRB were measured at the in situ temperatures of the Arctic sediments (2.6 and −1.7°C) and SRRs for 9 mesophiles were measured at 4, 8, and 13°C, temperatures in the range normally encountered in temperate sediments. All cultures were grown to the exponential growth phase, and rates were measured with the radiotracer method as described elsewhere (16). Specific SRRs of psychrophiles at 2.6 and −1.7°C varied between 1 and 42 fmol cell−1 day−1 (Table 1). All mesophiles reduced sulfate at 4°C, although only Desulfobacter hydrogenophilus was able to grow at that temperature. Specific SRRs of all mesophiles except D. hydrogenophilus (Table 2) increased exponentially with increasing temperatures but were still comparable to those found for the psychrophiles at temperatures 6 to 10°C lower. Since it is difficult to directly compare rates for mesophiles and psychrophiles at low temperatures because their growth temperature ranges do not overlap, we fitted mean rates for mesophiles by the Arrhenius equation: rate = A · exp(−Ea · [R · T]−1), where A is a constant, Ea is apparent activation energy, R is the gas constant, and T is absolute temperature expressed in Kelvins. The fit was extrapolated to <0°C and compared to rates for psychrophiles (Fig. 2). Calculated rates for mesophiles at 2.6 and −1.7°C were three- to fourfold lower than the measured rates for psychrophiles at the same temperatures (Fig. 2). The comparison of biomass-specific SRRs yielded similar differences (data not shown). These differences indicate that psychrophilic SRB are adapted to low temperatures not only because their minimum growth temperatures are at or below in situ temperatures but also because their metabolic rates are comparable to those of mesophiles at temperatures 6 to 10°C higher. Many studies have demonstrated that organisms active at low temperature differ physiologically from their counterparts in warmer environments (reference 22 and references therein). Cell membranes of psychrophiles tend to contain more unsaturated fatty acids (3, 5) and short-chain fatty acids (3) than membranes of mesophiles. Changes in the membrane composition might lead to a more efficient solute uptake at low temperatures (23). Furthermore, psychrophiles synthesize enzymes with high catalytic activities at low temperatures (8) and produce more enzymes when the temperature decreases (7). Different enzymes or enzyme levels could be one explanation for the comparable SRRs for psychrophiles and mesophiles at different temperatures.
TABLE 2.
Specific SRRs of mesophilic SRB at different temperatures
| Strain | DSMZa strain no. | Substrateb | Specific SRR
(fmol cell−1 day−1)c
at:
|
||
|---|---|---|---|---|---|
| 4°C | 8°C | 13°C | |||
| Desulfobacter postgatei | 2043 | Acetate | 11.0 ± 1.6 | 19.4 ± 1.4 | 37.9 ± 5.9 |
| D. hydrogenophilus | 3380 | Hydrogen | 8.0 ± 0.3 | 7.8 ± 2.8 | 20.0 ± 3.3 |
| Desulfobulbus sp. 3pr10 | 2058 | Propionate | 4.2 ± 0.1 | 6.2 ± 0.36 | 12.2 ± 0.6 |
| Desulfovibrio salexigens | 2636 | Lactate | 0.7 ± 0.06 | 1.4 ± 0.07 | 3.9 ± 0.4 |
| Desulfovibrio vulgaris | 1744 | Lactate | 0.4 ± 0.05 | 0.8 ± 0.06 | 2.1 ± 0.1 |
| Desulfobacterium autotrophicum | 3382 | Lactate | 1.6 ± 0.07 | 2.9 ± 0.2 | 4.4 ± 0.4 |
| Desulfofustis glycolicus | 9705 | Glycolate | 0.3 ± 0.01 | 0.5 ± 0.06 | 1.1 ± 0.1 |
| Desulfococcus niacini | 2650 | Nicotinate | 1.2 ± 0.05 | 2.0 ± 0.24 | 4.0 ± 0.7 |
| Desulfosarcina variabilis | 2060 | Benzoate | 0.7 ± 0.4 | 9.0 ± 2.3 | 20.0 ± 0.6 |
All strains were obtained from the Deutsche Sammlung für Mikroorganismen und Zellkulturen (DSMZ), Braunschweig, Germany.
Carbon substrates used for isolation and for measurements of specific SRRs.
Values are means ± standard deviations for three cultures. The mean specific SRRs were 3.1, 5.6, and 11.7 fmol cell−1 day−1 at 4, 8, and 13°C, respectively. Measurements of specific SRR were made in 15-ml Hungate tubes except for D. hydrogenophilus, which was incubated in flat 50-ml culture flasks to enhance hydrogen diffusion into the aqueous phase.
FIG. 2.
Mean values of specific SRRs of 10 mesophilic sulfate reducers (closed circles) determined at 4, 8, and 13°C, 14 psychrophiles from Hornsund sediments (open square), and 5 psychrophiles from Storfjord sediments (open triangle). Dashed line represents the Arrhenius fit of specific SRRs for mesophiles. Bars represent standard deviations of the means for all strains.
The calculated activation energy (Ea) of mesophilic SRB was 90.6 kJ/mol, which is within the range (23 to 132 kJ/mol) determined previously for sulfate reduction in temperate sediments (1, 6, 29) and close to the values (74 and 85 kJ/mol) calculated from specific SRR between 0 and 30°C for a Desulfovibrio desulfuricans strain (15). Thus, we suppose that the specific SRRs measured in pure cultures are representative for mesophilic sulfate reducers of temperate sediments. However, the possibility that measured rates for mesophiles were biased by the inability of most strains to grow at the low experimental temperatures cannot be ruled out. This problem could not be avoided in our use of culture collection strains because mesophilic marine sulfate reducers that are able to grow at temperature as low as 0°C are almost unknown.
Acknowledgments
We thank the cruise leader, Donald E. Canfield, and the crew of the RV Jan Mayen for a successful Svalbard cruise. We are grateful to Kerstin Sahm and Friedrich Widdel for help during the isolation of the studied strains and for critical discussions and to Bo Thamdrup for help with the computer software.
This work was supported by the Max Planck Society, Germany.
REFERENCES
- 1.Aller R C, Yingst J Y. Relationships between microbial distributions and the anaerobic decomposition of organic matter in surface sediments of Long Island Sound, USA. Mar Biol. 1980;56:29–42. [Google Scholar]
- 2.American Public Health Association. Standard methods for the examination of water and wastewater, including bottom sediment and sludge. Washington, D.C: American Public Health Association; 1969. [Google Scholar]
- 3.Bhakoo M, Herbert R A. The effects of temperature on the fatty acid and phospholipid composition of four obligately psychrophilic Vibriospp. Arch Microbiol. 1979;121:121–127. [Google Scholar]
- 4.Canfield D E, Jørgensen B B, Fossing H, Glud R, Gundersen J, Ramsing N B, Thamdrup B, Hansen J W, Nielsen L P, Hall P O J. Pathways of organic carbon oxidation in three continental margin sediments. Mar Geol. 1993;113:27–40. doi: 10.1016/0025-3227(93)90147-n. [DOI] [PubMed] [Google Scholar]
- 5.Chan M, Himes R H, Akagi J M. Fatty acid composition of thermophilic, mesophilic, and psychrophilic clostridia. J Bacteriol. 1971;106:876–881. doi: 10.1128/jb.106.3.876-881.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crill P M, Martens C S. Biogeochemical cycling in an organic-rich coastal marine basin. 6. Temporal and spatial variations in sulfate reduction rates. Geochim Cosmochim Acta. 1987;51:1175–1186. [Google Scholar]
- 7.Feller G, Narinx E, Arpigny J L, Zekhnini Z, Swings J, Gerday C. Temperature dependence of growth, enzyme secretion and activity of psychrophilic Antarctic bacteria. Appl Microbiol Biotechnol. 1994;41:477–479. [Google Scholar]
- 8.Feller G, Payan F, Theys F, Qian M, Haser R, Gerday C. Stability and structural analysis of α-amylase from the Antarctic psychrophile Alteromonas haloplanctisA23. Eur J Biochem. 1994;222:441–447. doi: 10.1111/j.1432-1033.1994.tb18883.x. [DOI] [PubMed] [Google Scholar]
- 9.Isaksen M F, Jørgensen B B. Adaptation of psychrophilic and psychrotrophic sulfate-reducing bacteria to permanently cold marine environments. Appl Environ Microbiol. 1996;62:408–414. doi: 10.1128/aem.62.2.408-414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isaksen M F, Teske A. Desulforhopalus vacuolatusgen. nov., sp. nov., a new moderately psychrophilic sulfate-reducing bacterium with gas vacuoles isolated from a temperate estuary. Arch Microbiol. 1996;166:160–168. [Google Scholar]
- 11.Jørgensen B B. A comparison of methods for the quantification of bacterial sulfate reduction in coastal marine sediments I. Measurement with radiotracer techniques. Geomicrobiol J. 1978;1:11–27. [Google Scholar]
- 12.Jørgensen B B. Mineralization of organic matter in the sea bed—the role of sulphate reduction. Nature. 1982;296:643–645. [Google Scholar]
- 13.Jørgensen B B, Bak F. Pathways and microbiology of thiosulfate transformations and sulfate reduction in a marine sediment (Kattegat, Denmark) Appl Environ Microbiol. 1991;57:847–856. doi: 10.1128/aem.57.3.847-856.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jørgensen B B, Bang M, Blackburn T H. Anaerobic mineralization in marine sediments from the Baltic Sea-North Sea transition. Mar Ecol Prog Ser. 1990;59:39–54. [Google Scholar]
- 15.Kaplan I R, Rittenberg S C. Microbiological fractionation of sulphur isotopes. J Gen Microbiol. 1964;34:195–212. doi: 10.1099/00221287-34-2-195. [DOI] [PubMed] [Google Scholar]
- 16.Knoblauch, C., and B. B. Jørgensen. Effect of temperature on sulfate reduction, growth rate, and growth yield in five psychrophilic sulfate-reducing bacteria from Arctic sediments. In Environmental Microbiology, vol. 1, in press. Blackwell Science, Ltd., Oxford, United Kingdom. [DOI] [PubMed]
- 17.Knoblauch, C., K. Sahm, and B. B. Jørgensen. Psychrophilic sulfate-reducing bacteria isolated from permanently cold Arctic marine sediments: description of Desulfofrigus oceanense gen. nov., sp. nov., Desulfofrigus fragile sp. nov., Desulfofaba gelida gen. nov., sp. nov., Desulfotalea psychrophila gen. nov., sp. nov., and Desulfotalea arctica, sp. nov. Int. J. Syst. Bacteriol., in press. [DOI] [PubMed]
- 18.Kostka J E, Thamdrup B, Glud R N, Canfield D E. Rates and pathways of carbon oxidation in permanently cold Arctic sediments. Mar Ecol Prog Ser. 1999;180:7–21. [Google Scholar]
- 19.Levitus S, Boyer T. World ocean atlas. Vol. 4. Washington, D.C: Temperature. U.S. Department of Commerce; 1994. [Google Scholar]
- 20.Lillebæk R. Application of antisera raised against sulfate-reducing bacteria for indirect immunofluorescent detection of immunoreactive bacteria in sediment from the German Baltic Sea. Appl Environ Microbiol. 1995;61:3436–3442. doi: 10.1128/aem.61.9.3436-3442.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nedwell D B, Walker T R, Ellis-Evans J C, Clarke A. Measurements of seasonal rates and annual budgets of organic carbon fluxes in an Antarctic coastal environment at Signy Island, South Orkney Islands, suggest a broad balance between production and decomposition. Appl Environ Microbiol. 1993;59:3989–3995. doi: 10.1128/aem.59.12.3989-3995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell N J, Hamamoto T. Psychrophiles. In: Horikoshi K, Grant W D, editors. Extremophiles: microbial life in extreme environments. New York, N.Y: John Wiley & Sons; 1998. pp. 25–45. [Google Scholar]
- 23.Russell N J. Cold adaptation of microorganisms. Philos Trans R Soc Lond B Biol Sci. 1990;326:595–611. doi: 10.1098/rstb.1990.0034. [DOI] [PubMed] [Google Scholar]
- 24.Sagemann J, Jørgensen B B, Greef O. Temperature dependence and rates of sulfate reduction in cold sediments of Svalbard, Arctic Ocean. Geomicrobiol J. 1998;15:85–100. [Google Scholar]
- 25.Sahm K, Knoblauch C, Amann R I. Phylogenetic affiliation and quantification of psychrophilic sulfate-reducing isolates in marine Arctic sediments. Appl Environ Microbiol. 1999;65:3976–3981. doi: 10.1128/aem.65.9.3976-3981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahm K, MacGregor B J, Jørgensen B B, Stahl D A. Sulphate reduction and vertical distribution of sulphate-reducing bacteria quantified by rRNA slot-blot hybridization in a coastal marine sediment. Environ Microbiol. 1999;1:65–74. doi: 10.1046/j.1462-2920.1999.00007.x. [DOI] [PubMed] [Google Scholar]
- 27.Teske A, Wawer C, Muyzer G, Ramsing N B. Distribution of sulfate-reducing bacteria in a stratified fjord (Mariager Fjord, Denmark) as evaluated by most-probable-number counts and denaturing gradient gel electrophoresis of PCR-amplified ribosomal DNA fragments. Appl Environ Microbiol. 1996;62:1405–1415. doi: 10.1128/aem.62.4.1405-1415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thamdrup B, Fossing H, Jørgensen B B. Manganese, iron, and sulfur cycling in a coastal marine sediment, Aarhus Bay, Denmark. Geochim Cosmochim Acta. 1994;58:5115–5129. [Google Scholar]
- 29.Westrich J T, Berner R A. The effect of temperature on rates of sulfate reduction in marine sediments. Geomicrobiol J. 1988;6:99–117. [Google Scholar]