Abstract
Objective:
Glucocorticoids (“steroids”) are frequently used in systemic lupus erythematosus (SLE). Prolonged use may contribute to racial/ethnic disparities in avoidable adverse outcomes. We examined racial/ethnic differences in longitudinal patterns of steroid use and dose.
Methods:
We identified Medicaid beneficiaries 18–65 years with incident SLE who received steroids for 12 months following the index date. Group-based trajectory modeling was used to identify patterns of daily prednisone-equivalent steroid doses. We examined demographic, clinical and healthcare utilization factors during the baseline period and used multinomial logistic regression to estimate the odds of belonging to the higher vs. lowest steroid dose trajectories over time.
Results:
We identified 6,314 individuals with SLE with ≥1 dispensed steroid prescription. The mean (SD) prednisone-equivalent dose was 7 (23) mg/day for Black, 7 (26) Hispanic, 7 (13) Asian, and 4 (10) for White individuals. Adjusted multinomial models demonstrated higher odds of belonging to the highest vs. lowest steroid trajectory for Black (OR 2.07, 95% CI 1.65–2.61), Hispanic (OR 1.81, 95% CI 1.38–2.39), and Asian (OR 2.42, 95% CI 1.53–3.83) vs. White individuals. Having >5 outpatient visits during the baseline period was associated with lower odds of being in the persistently high-dose steroid trajectory (OR 0.77; 95% CI 0.60–0.98).
Conclusion:
Black, Hispanic, and Asian (vs. White individuals) had higher odds of persistently high-dose steroid use. Sustained access to outpatient care and the development of standardized steroid-tapering regimens from clinical trials with diverse populations may be targets for intervention to mitigate disparities in steroid-related adverse outcomes.
Keywords: Systemic Lupus Erythematosus, Glucocorticoids, Disparities, Health Equity, Health Services Research
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can lead to irreversible multiorgan system damage. Glucocorticoids (“steroids”) are a mainstay of SLE treatment and are often required for disease control.1 Persistent steroid use among individuals with SLE is associated with potentially avoidable acute care use for adverse outcomes, including serious infections, osteoporotic fracture, avascular necrosis, and cardiovascular disease.2–6 Prior studies have demonstrated that reducing the cumulative steroid dose and in turn, minimizing steroid-related adverse outcomes can be achieved with the prompt initiation of steroid-sparing immunosuppressive agents, gradual steroid withdrawal, and access to routine evaluations at SLE-specialty centers.1,7,8 While steroids are often necessary for the immediate treatment of organ and life-threatening SLE manifestations, treatment guidelines suggest that efforts should be made to minimize steroid dose and duration, and to transition patients to steroid-sparing agents when possible.1,9
Several studies have formulated strategies to measure the adverse effects of long-term steroid use. A Glucocorticoid Toxicity Index (GTI) includes thirty-one weighted toxicity items such as hypertensive emergency, osteonecrosis, myopathy, or gastrointestinal perforations to allow for the measurement of steroid-induced complications.10 Additionally, a modified Delphi process identified steroid-related complications, including avascular necrosis, osteoporotic fracture, gastrointestinal bleeds, and uncontrolled diabetes mellitus that may be avoided if high-quality ambulatory care with preventive measures and appropriate steroid dose reduction were provided to individuals with SLE.11 Despite the availability of consensus guidelines and related tools, disparities have been identified in the use of steroid-sparing immunosuppressive agents, in the frequency of access to high-quality care, and in the burden of avoidable steroid-related adverse outcomes among Medicaid beneficiaries and other populations.1,12–14
In this study, we aimed to understand patterns of persistent steroid use that place individuals with SLE at high risk for potentially avoidable adverse outcomes and acute care use. We focused on individuals with SLE enrolled in Medicaid, the largest public insurance for low-income individuals in the U.S. Prior work has demonstrated that Medicaid beneficiaries with SLE receive lower quality care, have high rates of acute care use, and suffer from a disproportionate burden of potentially avoidable adverse outcomes.15–19 In addition, significant disparities exist in care quality and outcomes by race/ethnicity, particularly among Black compared to White individuals.15,16 We hypothesized, based on the known disparities in adverse outcomes, that Black individuals with SLE would have greater odds of persistent higher dose steroid use compared to White individuals and that modifiable factors such as infrequent outpatient visits would be associated with higher use.
2. Patients and Methods
Study Population and Patient Identification
Our data source was the Medicaid Analytic eXtract (MAX), which includes demographic information, billing codes, claims, and medication dispensing data for Medicaid beneficiaries between 2000–2010 from the 29 most populous U.S. states.20 We excluded individuals from Ohio and Michigan because drug dispensing data were not available in our dataset. We identified adults ages 18–65 years with incident SLE, defined as ≥3 ICD-9 codes (710.0) separated by ≥30 days with no SLE ICD-9 codes in the prior 24 months using a previously defined algorithm.21 We excluded individuals >65 years due to dual Medicare eligibility and the possibility of missing claims. The index date was defined as the date of the third ICD-9 code (Figure 1). We required 12 months of continuous Medicaid enrollment following the index date, defined as the date of the third SLE ICD-9 code. We restricted our cohort to include individuals with ≥1 claim for an oral steroid medication at any point during the 12-month follow-up period.
Figure 1:
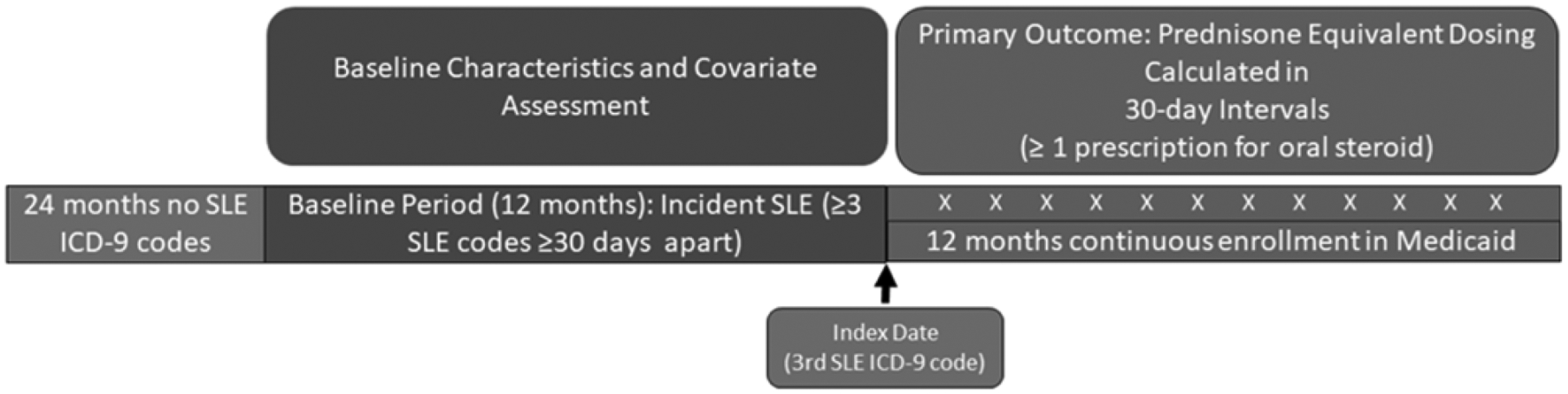
Study Design and Timeline
2.1. Primary Outcome
Our primary outcome was steroid use occurring on or 12 months after the index date. We defined this as ≥1 dispensing of an oral steroid medication (betamethasone, cortisone, dexamethasone, methylprednisolone, prednisolone, prednisone, and triamcinolone), which was then converted to a prednisone-equivalent dose. We calculated the mean daily prednisone-equivalent dose for each 30-day period based on prescription claims and converted the mean daily dose to the natural log as the distribution of doses was skewed toward higher values.
2.2. Covariates
We examined demographic characteristics at the index date including age, sex (male or female), race/ethnicity as defined by the Centers for Medicare and Medicaid Services (CMS) during the years of the dataset (Black or African American, White, American Indian or Alaska Native (AI/AN), Asian, Native Hawaiian, and Pacific Islander, “some other race”, and Hispanic and Latino of any race), geographic region defined by the U.S. Census (Northeast, West, South, Midwest), and ZIP code median income using U.S. Census data.20 During the 12-month period prior to the index date, which we refer to as the “baseline period”, we identified comorbidities (Supplemental Table 1), medication use (immunosuppressives: mycophenolate mofetil/mycophenolic acid, azathioprine, methotrexate, rituximab, cyclosporine, cyclophosphamide, tacrolimus, sulfasalazine, abatacept; hydroxychloroquine); renal disease including chronic kidney disease22 and healthcare utilization (emergency department “ED” visits, outpatient visits, and hospitalizations; each categorized as 0 visits, 1–5 visits, and >5 visits). We also examined preventive care use during the baseline period using Current Procedural Terminology (CPT) codes for ever/never receipt of any vaccinations (influenza, pneumococcal, herpes zoster, meningitis, human papilloma virus, and hepatitis B), cancer screenings (colonoscopy, mammograms, pap tests), and bone density scans, as well as receipt of medications for Pneumocystis jirovecii (PJP) prophylaxis (trimethoprim-sulfamethoxazole, atovaquone, dapsone, pentamidine) (Supplemental Table 1). We then developed a composite measure of preventive care use that included receipt of 0, 1 or >1 vaccinations, cancer screenings, PJP prophylaxis, and bone density scans. To approximate SLE severity, we calculated the SLE risk adjustment index using baseline period covariates.14 This index has been shown to be a better predictor of inpatient mortality than the Charlson comorbidity index among individuals with SLE.23 We also examined immunosuppressive medication and hydroxychloroquine use, as well as healthcare utilization during the 12-month follow-up period, separately at 0–6 months and at 6–12 months, beginning the day following the index date to explore whether medication and healthcare use trends could explain simultaneous steroid use patterns. However, only baseline covariates were included in the multinomial models. Laboratory values were not included as they are not available in administrative claims data.
2.3. Statistical Analyses
2.3.1. Group-based Trajectory Models (GBTM)
Group-based trajectory modeling (GBTM) is a method of identifying distinct and relatively homogenous groups based upon the characteristics of the individuals in the cohort.23 We have used GBTM in prior studies to cluster individuals with SLE by distinctive longitudinal patterns of medication adherence and acute care use.24,25 In this study, we used GBTM to classify Medicaid beneficiaries with SLE by longitudinal patterns of steroid doses in the first 12-months following the SLE index date. We estimated GBTMs with 3–6 trajectories using monthly prednisone-equivalent oral steroid doses (natural log-transformed). We applied multinomial regression models to determine the probability that individuals would belong to a specific trajectory within a GBTM, based on their mean steroid dose at each 30-day interval. We assigned individuals to the trajectory of their highest probability. We compared the 3–6 trajectory GBTMs and chose the model that best fit the data.23,24,26 To determine the best fitting model, we considered each model’s Bayesian Information Criteria (BIC), the distribution of individuals per trajectory, the posterior probabilities (the mean probabilities of individuals belonging to the trajectory to which they were assigned, with goal of ≥80%), and the optimal clinical explanatory potential of the model. We first estimated GBTMs for our full cohort of steroid users and then stratified by racial/ethnic group (Black, White, Hispanic, and Asian) to examine potential differences in patterns and associated covariates.
2.3.2. Multivariable Multinomial Models
We used multivariable multinomial models to examine the odds of an individual belonging to any higher dose steroid use trajectory vs. the lowest steroid dose trajectory for the overall cohort by race/ethnicity adjusting for other demographic factors (age, sex, and region). We then additionally adjusted for income and SLE risk adjustment index and subsequently, for comorbidities, medication use, healthcare utilization, and preventive care to determine whether potential associations between race/ethnicity and trajectory would be attenuated. We then stratified our multivariable multinomial logistic regression models by race/ethnicity (White, Black, Hispanic, Asian). We were not able to examine models among AI/AN individuals due to small sample size. For these race/ethnicity-stratified models, covariate use was limited by sample size, particularly among the Asian subcohort. For the White, Black, and Hispanic subcohorts, we examined demographics, SLE risk adjustment index, comorbidities, healthcare utilization, and preventive care; for the Asian subcohort, we examined demographics and the SLE risk adjustment index.
2.4. Sensitivity Analyses
We examined steroid dose and use patterns among new steroid users, restricted to individuals with no steroid use in the 12-month baseline period prior to the index date. We constructed GBTMs for this subcohort and estimated multinomial models with the same covariates as our primary models. We also conducted a sensitivity analysis using the Garris algorithm, a measure of SLE disease severity. The Garris algorithm approximates disease severity in administrative claims data by combining elements of disease activity and cumulative damage based on the SLEDAI, SLAM, and BILAG measures of SLE disease activity. It also incorporates expert clinical opinion and use of SLE medications.27 We conducted analyses first using the Garris algorithm in its full form and then modified it by excluding steroids only, then excluding immunosuppressives only, and finally by excluding both steroids and immunosuppressives.
This study was approved by the Mass General Brigham (MGB) Institutional Review Board. Deidentified data were obtained from the CMS through a Data Use Agreement and cell sizes <11 are suppressed in accordance with their policies. SAS 9.4 (SAS Institute, Cary NC)28, and R 4.1.2 were used for these analyses.29
3. Results:
Within the cohort of 11,900 Medicaid beneficiaries with incident SLE with 12 months of continuous Medicaid enrollment between years 2000–2010, 6,314 had ≥1 dispensing for oral steroids on or within 12 months after the index date. The mean (SD) age in years of this steroid use cohort was 37 (12); 313 (5%) individuals were male, 2840 (45%) Black,1877 (30%) White,1147 (18%) Hispanic, 245 (4%) Asian, and 205 (3%) “other” including AI/AN, more than one race, or not recorded. Age distribution differed by race/ethnicity (Table 1). The largest percentages of Black and Hispanic patients were in the 18–34 years age range (50% and 46%, respectively), while the largest percentage of White and Asian patients were in the 35–50 years age range (43% and 41%, respectively). Immunosuppressive use during the baseline period was present in 9% of Black patients, 11% of White patients, 12% of Hispanic patients, and 19% of Asian patients. Baseline hydroxychloroquine use was present in 17% of the overall cohort, with similar distribution by race/ethnicity.
Table 1.
Baseline Characteristics By Race Among Individuals With Incident SLE Enrolled in Medicaid, 2000–2010 Who Received Steroids In the First 12 Months Following Diagnosis
| Overall | Black | Hispanic | Asian | White | Other | |
|---|---|---|---|---|---|---|
| N (% of total) | 6314 | 2840 (45) | 1147 (18) | 245 (4) | 1877 (30) | 205 (3) |
| Mean (SD) age | 37 (12) | 35 (11) | 37 (12) | 38 (13) | 40 (12) | 42 (12) |
| Age Group – N (%) | ||||||
| 18–34 years | 2757 (44) | 1433 (50) | 523 (46) | 94 (38) | 652 (35) | 55 (27) |
| 35–50 years | 2548 (40) | 1087 (38) | 459 (40) | 101 (41) | 812 (43) | 89 (43) |
| 51–65 years | 1009 (16) | 320 (11) | 165 (15) | 50 (20) | 413 (22) | 61 (30) |
| Male – N (%) | 313 (5) | 106 (4) | 60 (5) | * | 117 (6) | * |
| Female – N (%) | 6001 (95) | 2734 (96) | 1087 (95) | * | 1760 (94) | * |
| Region – N (%) | ||||||
| Midwest | 832 (13) | 434 (15) | 61 (5) | * | 319 (17) | * |
| Northeast | 1500 (24) | 599 (21) | 341 (30) | * | 450 (24) | * |
| South | 2501 (40) | 1462 (51) | 263 (23) | * | 695 (37) | * |
| West | 1481 (23) | 345 (12) | 482 (42) | * | 413 (22) | * |
| SLE Risk Adjustment Index – mean (SD) | 1.2 (2.3) | 1.3 (2.4) | 1.3 (2.4) | 1.3 (2.2) | 1.1 (2.2) | 1.4 (2.6) |
| Medication Use – N (%) | ||||||
| Hydroxychloroquine | 1093 (17) | 484 (17) | 217 (19) | 39 (16) | 311 (17) | 42 (20) |
| Glucocorticoid | ||||||
| Baseline period | ||||||
| Exactly 1 dispensed | 1374 (22) | 620 (22) | 245 (21) | 63 (26) | 405 (22) | 41 (20) |
| >1 dispensed | 2001 (32) | 841 (30) | 377 (33) | 75 (31) | 624 (33) | 84 (41) |
| None dispensed | 2939 (47) | 1379 (49) | 525 (46) | 107 (44) | 848 (45) | 80 (39) |
| Immunosuppressivesⱡ | 681 (11) | 266 (9) | 139 (12) | 46 (19) | 199 (11) | 31 (15) |
| Comorbidities and Pregnancy – N (%) | ||||||
| Diabetes | 627 (10) | 250 (9) | 119 (10) | 26 (11) | 207 (11) | 25 (12) |
| Cardiovascular Disease | 2151 (34) | 1015 (36) | 344 (30) | 76 (31) | 633 (34) | 83 (40) |
| Pregnancy | 603 (10) | 290 (10) | 128 (11) | * | 152 (8) | * |
| Chronic Kidney Disease | 40 (1) | 17 (1) | * | * | * | * |
| Health Care Utilization mean (SD) | ||||||
| ED visits | ||||||
| 0 | 2960 (47) | 1287 (45) | 561 (49) | * | 867 (46) | * |
| 1–5 | 2686 (43) | 1249 (44) | 507 (44) | * | 764 (41) | * |
| >5 | 668 (11) | 304 (11) | 79 (7) | * | 246 (13) | * |
| Outpatient Visits | ||||||
| 0 | 1488 (24) | 760 (27) | 279 (24) | 45 (18) | 369 (20) | 35 (17) |
| 1–5 | 2051 (32) | 1002 (35) | 401 (35) | 76 (31) | 517 (28) | 55 (27) |
| >5 | 2775 (44) | 1078 (38) | 467 (41) | 124 (51) | 991 (53) | 115 (56) |
| Hospitalizations | ||||||
| 0 | 4810 (76) | 2143 (75) | 886 (77) | 189 (77) | 1438 (77) | 154 (75) |
| 1–5 | 760 (12) | 331 (12) | 157 (14) | 39 (16) | 213 (11) | 20 (10) |
| >5 | 744 (12) | 366 (13) | 104 (9) | 17 (7) | 226 (12) | 31 (15) |
| Preventive Care | ||||||
| Vaccinations ≥1 | 443 (7) | 125 (4) | 72 (6) | 23 (9) | 191 (10) | 32 (16) |
| Pneumocystis jirovecii ppx | 769 (12) | 339 (12) | 147 (13) | 21 (9) | 230 (12) | 32 (16) |
| Bone Density Scan | 60 (1) | 19 (1) | 12 (1) | * | 22 (1) | * |
| Zip Code Median Household Annual Income – N (%) | ||||||
| Above $41,749.50 | 2894 (46) | 1021 (36) | 573 (50) | 170 (69) | 1038 (55) | 92 (45) |
| Below $41,749.50 | 3420 (54) | 1819 (64) | 574 (50) | 75 (31) | 839 (45) | 113 (55) |
Value is too low to report per data use agreement
Mycophenolate, mofetil/mycophenolic acid, azathioprine, methotrexate, rituximab, cyclosporine, cyclophosphamide, tacrolimus, sulfasalazine, abatacept; hydroxychloroquine; and pneumocystis prophylaxis: trimethoprim-sulfamethoxazole, atovaquone, dapsone, pentamidine
ppx=prophylaxis
3.1. Steroid Use Patterns and Group-Based Trajectory Models – Overall Cohort
Beginning at the index date, the mean (SD) prednisone equivalent dose for the full cohort was 6 (20) mg/day averaged over 12 months. The mean (SD) prednisone equivalent dose excluding months when individuals were not prescribed steroids was 17 (29) mg/day. The mean (SD) prednisone equivalent steroid doses beginning at the index date (excluding the months that individuals were not prescribed steroids) among Black individuals was 18 (33) mg/month, Hispanic individuals 17 (39) mg/month, Asian individuals 17 (17) mg/month, and White individuals 14 (14) mg/month.
We compared characteristics for 3–6 group trajectory models and found that both the 3- and 4-group models demonstrated an acceptable fit for our data with similar BICs, posterior probabilities >80% and reasonable distributions between the different trajectories. We chose the 4-group model (Figure 2) because it approximated theoretical clinical scenarios where Group A represents persistently high-dose steroid use among patients with the highest disease activity, Group B represents those with initial high-dose steroid use to treat active disease followed by a taper, Group C represents low steroid dose use that increases with more activity, and finally Group D represents persistently low-dose or occasional use (hereafter referred to as “persistently-low steroid dose trajectory”) of steroids for mild or quiescent disease.
Figure 2:
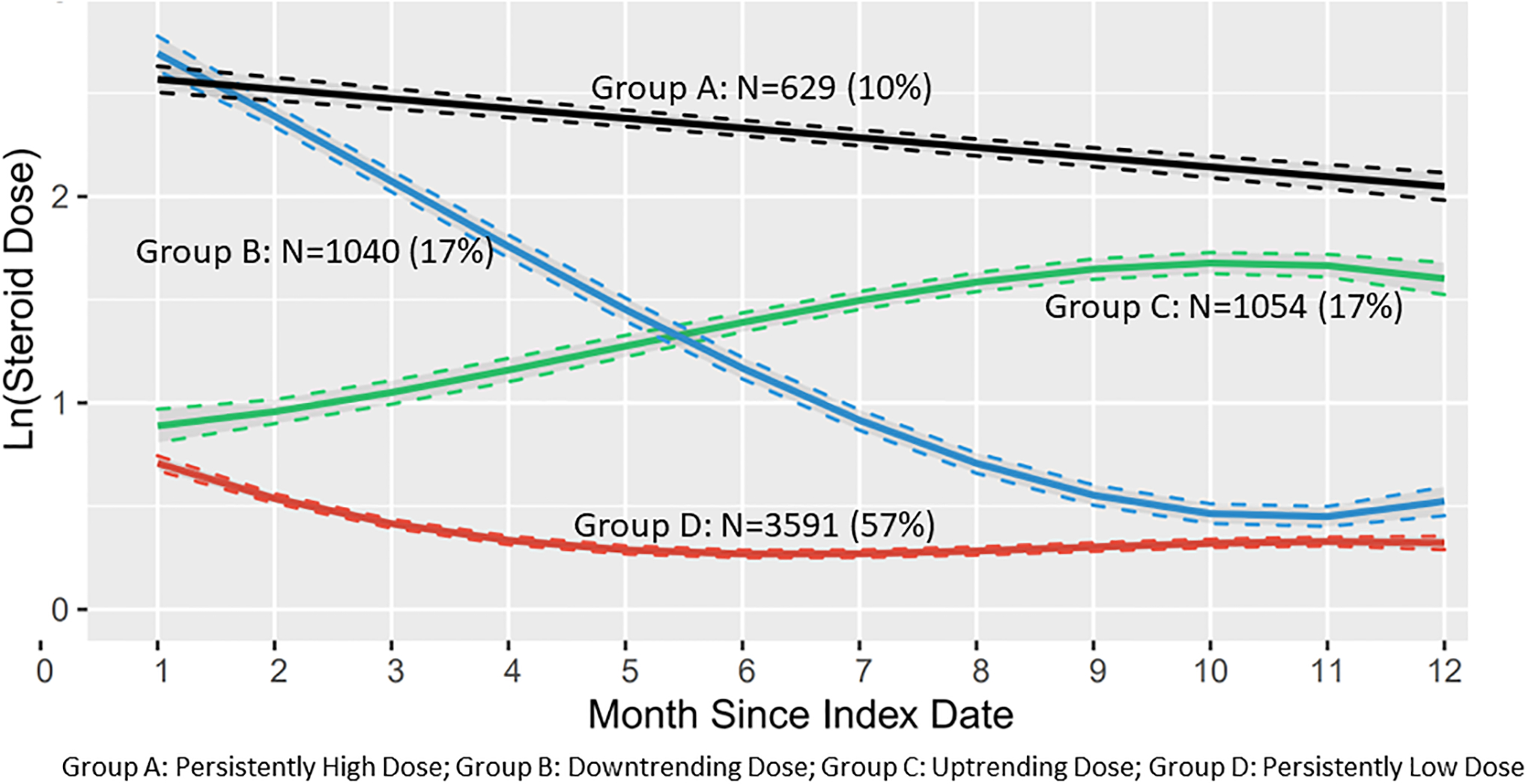
Group-Based Steroid Dose Trajectories of Overall Cohort (N=6314)
In the four-group trajectory model (Figure 2) there were 629 individuals (10%) in Group A with a mean steroid dose of 19 (43) mg/day. In Group B there were 1040 individuals (17%) with a mean steroid dose of 10 (16) mg/day. Group C had 1054 individuals (17%) with a mean steroid dose of 8 (26) mg/day). There were 3591 individuals (57%) in Group D with a mean steroid dose of 2 (6) mg/day. Baseline period characteristics stratified by the four trajectories are presented in Supplemental Table 2.
To begin to understand the steroid dose patterns observed, we examined healthcare and medication utilization stratified by steroid dose trajectory during the follow-up period for all individuals and then grouped by race/ethnicity. For all trajectories, we observed a trend toward greater proportions of individuals with >5 outpatient visits over the 12-month follow-up period. During the baseline period, the persistently low steroid dose trajectory (Group D) had the highest proportion of individuals with >5 outpatient visits (Supplemental Table 3). The pattern observed for hydroxychloroquine dispensing in all four steroid dose trajectories revealed a peak dispensing of hydroxychloroquine during the 0–6 month interval followed by a decline in dispensing during the final 6–12 month interval (Supplemental Table 4). Immunosuppressive dispensing increased from the baseline period to its maximum dispensing over the subsequent 6 months, however dispensing of immunosuppressives declined during the 6–12-month interval for all trajectories, except for the uptrending steroid dose trajectory, Group C (Supplemental Table 5). Among individuals in the highest steroid dose trajectory, Group A, immunosuppressive use increased over the 12-month follow-up period. However, the increased percentage of use was least pronounced among Black individuals compared to White, Asian and Hispanic individuals. While percentages continued to increase among White, Asian and Hispanic individuals, they decreased among Black individuals from 0–6 months to 6–12 months (Supplemental Table 6).
3.2. Multivariable Multinomial Models – Overall Cohort
We estimated multivariable multinomial logistic regression models for the overall cohort (Table 2) to determine the odds of an individual belonging to each of the higher steroid dose trajectories (Groups A-C) compared with the persistently low steroid dose trajectory (Group D). We first examined models by demographic factors only (Supplemental Table 7). Males had higher odds of being Groups A-C vs Group D compared to females. Black (OR 2.18, 95% CI 1.75–2.73), Hispanic (OR 1.93, 95% CI 1.47–2.53), and Asian (OR 2.55, 95% CI 1.62–4.01) individuals had higher odds of being in the persistently high steroid dose trajectory Group A vs Group D compared to White individuals.
Table 2.
Multinomial Logistic Regression Model Examining the Odds of Belonging to Group A, Group B, or Group C compared to Group D Steroid Dose Trajectories for Individuals with Incident SLE Receiving Steroids (N=6314)
| Covariates | Group D Low-Dose (Ref) |
Group C Uptrending Dose Odds ratio (95% CI) |
Group B Downtrending Dose Odds ratio (95% CI) |
Group A High Dose Odds ratio (95% CI) |
|---|---|---|---|---|
| Age (ref=18–34 years) | ||||
| 35–50 | 0.84 (0.71–0.98) | 0.56 (0.47–0.66) | 0.62(0.50–0.75) | |
| 51–65 | 0.98 (0.78–1.22) | 0.45 (0.35–0.57) | 0.64 (0.48–0.85) | |
| Sex (ref=female) | - | |||
| Male | 1.40 (1.02–1.92) | 1.45 (1.05–2.00) | 1.83 (1.28–2.60) | |
| Race/Ethnicity(ref=White) | ||||
| Black | - | 1.57 (1.32–1.87) | 1.72 (1.44–2.06) | 2.07 (1.65–2.61) |
| Asian | - | 1.61 (1.10–2.36) | 2.25 (1.55–3.26) | 2.42 (1.53–3.83) |
| Hispanic | - | 1.43 (1.15–1.77) | 1.55 (1.24–1.94) | 1.81 (1.38–2.39) |
| Other | - | 1.42 (0.96–2.10) | 1.00 (0.61–1.61) | 1.76 (1.07–2.92) |
| SLE Risk-Adjustment Index | - | 1.03 (0.99–1.07) | 1.07 (1.04–1.11) | 1.11 (1.06–1.15) |
| Pregnancy | - | 0.77 (0.58–1.01) | 1.15 (0.91–1.44) | 0.92 (0.68–1.25) |
| Cardiovascular Disease | - | 0.97 (0.82–1.16) | 1.23 (1.03–1.46) | 1.22 (0.99–1.51) |
| Diabetes | - | 0.85 (0.66–1.11) | 0.69 (0.52–0.91) | 0.70 (0.51–0.97) |
| Chronic Kidney Disease * | - | 2.33 (1.04–5.22) | 1.13 (0.46–2.80) | 1.21 (0.44–3.29) |
| Hydroxychloroquine | - | 1.16 (0.97–1.39) | 0.93 (0.77–1.14) | 0.84 (0.66–1.07) |
| Immunosuppressives | - | 1.92 (1.56–2.37) | 1.44 (1.14–1.83) | 1.91 (1.46–2.49) |
| Outpatient Visits (ref=0) | ||||
| 1–5 | - | 0.90 (0.74–1.09) | 1.07 (0.88–1.30) | 0.90 (0.71–1.14) |
| >5 | 0.74 (0.60–0.90) | 0.98 (0.80–1.21) | 0.77 (0.60–98) | |
| ED Visits (ref = 0) | ||||
| 1–5 | - | 1.10 (0.94–1.30) | 1.12 (0.98–1.36) | 1.22 (1.00–1.49) |
| >5 | - | 0.95 (0.72–1.25) | 0.95 (0.73–1.24) | 0.85 (0.60–1.19) |
| Hospitalizations (Ref = 0) | ||||
| 1–5 | - | 1.02 (0.81–1.29) | 1.06 (0.84–1.33) | 1.07 (0.81–1.41) |
| >5 | - | 1.07 (0.82–1.39) | 1.46 (1.15–1.86) | 1.50 (1.13–2.01) |
| Preventive Care (ref=no vaccines) | - | |||
| At least 1 Vaccine | - | 0.79 (0.59–1.06) | 0.73 (0.53–0.99) | 0.67 (0.46–0.99) |
| Bone Density Scan | 1.96 (1.06–3.62) | 0.67 (0.25–1.75) | 0.95 (0.36–2.53) | |
| Pneumocystis jirovecii ppx | 1.09 (0.87–1.35) | 0.99 (0.80–1.24) | 1.07 (0.82–1.40) |
Includes CKD III-V and ESRD, does not include lupus nephritis
Also adjusted for zip code median income and region
ppx=prophylaxis
We additionally adjusted the model by the baseline zip code median income, SLE risk adjustment index, comorbidities, medication use, healthcare utilization, and preventive care variables (Table 2). Statistically significant associations between race/ethnicity and steroid-dose were only modestly attenuated after adding these variables (<13% for all race/ethnic groups). Higher SLE risk adjustment indices were associated with higher odds of being in Groups A-C vs. Group D. Individuals on immunosuppressives at baseline also had greater odds of being in Groups A-C vs. Group D compared to those not on immunosuppressives at baseline (Table 2). Individuals who had >5 hospitalizations during the baseline period (vs. no hospitalizations) had higher odds of being in Group A vs. D (OR 1.50, 95% CI 1.13–2.01). Having >5 outpatient visits during the baseline period was associated with lower odds of being in the persistently high dose trajectory, Group A (0.77, 95% CI 0.60–0.98) vs. Group D Patients who received ≥1 vaccine in the baseline period (vs. no vaccinations) had lower odds (OR 0.67 95% CI 0.46–0.99) of being in the Group A vs. Group D. We observed higher odds of being in the uptrending steroid dose trajectory vs. the persistently low steroid dose trajectory for individuals who received (vs. did not receive) a bone density scan during the baseline period (OR 1.96, 95% 1.06–3.62). In a model with the preventive care composite measure, we observed significantly lower odds of being in the persistently high vs. low steroid dose trajectory among individuals who received >1 preventive care measure vs. no measure (OR 0.66, 95% CI 0.46–0.95). Being in the Midwestern (OR 1.41, 95% CI 1.07–1.86) or Northeastern region (OR 1.88, 95% CI 1.50–2.35) of the U.S. vs the Southern region was associated with higher odds of being in the persistently high steroid dose trajectory (Group A) vs. the lowest dose trajectory (Group D).
3.4. Group-Based Trajectory Models – Race/Ethnicity Stratified
We examined 3- and 4-group trajectory models stratified by race. We chose the 4-group model for each race/ethnicity group (Figure 3). In the GBTM for the cohort of Black individuals (N= 2840), 10% were in the persistently high steroid dose trajectory with a mean (SD) dose of 21 (59) mg/day. In the cohort of Hispanic individuals (N=1147) 11% were in persistently high dose group, at a mean steroid dose of 19 (19) mg/day; among Asian individuals (N=245) 20% were in the persistently high dose group, at mean of dose 17 (18) mg/day; and in the cohort of White individuals (N= 1877) 7% at a mean (SD) dose 16 mg/day (15). The 4-group GBTM model for Asian individuals was unlike the other 4-group GBTMs stratified by race/ethnicity, in that it did not have a persistently high steroid dose trajectory, rather it had two downtrending trajectories; one with an early decrease in steroid dose over time and the other with a later decrease in steroid dose over time (Figure 3).
Figure 3:
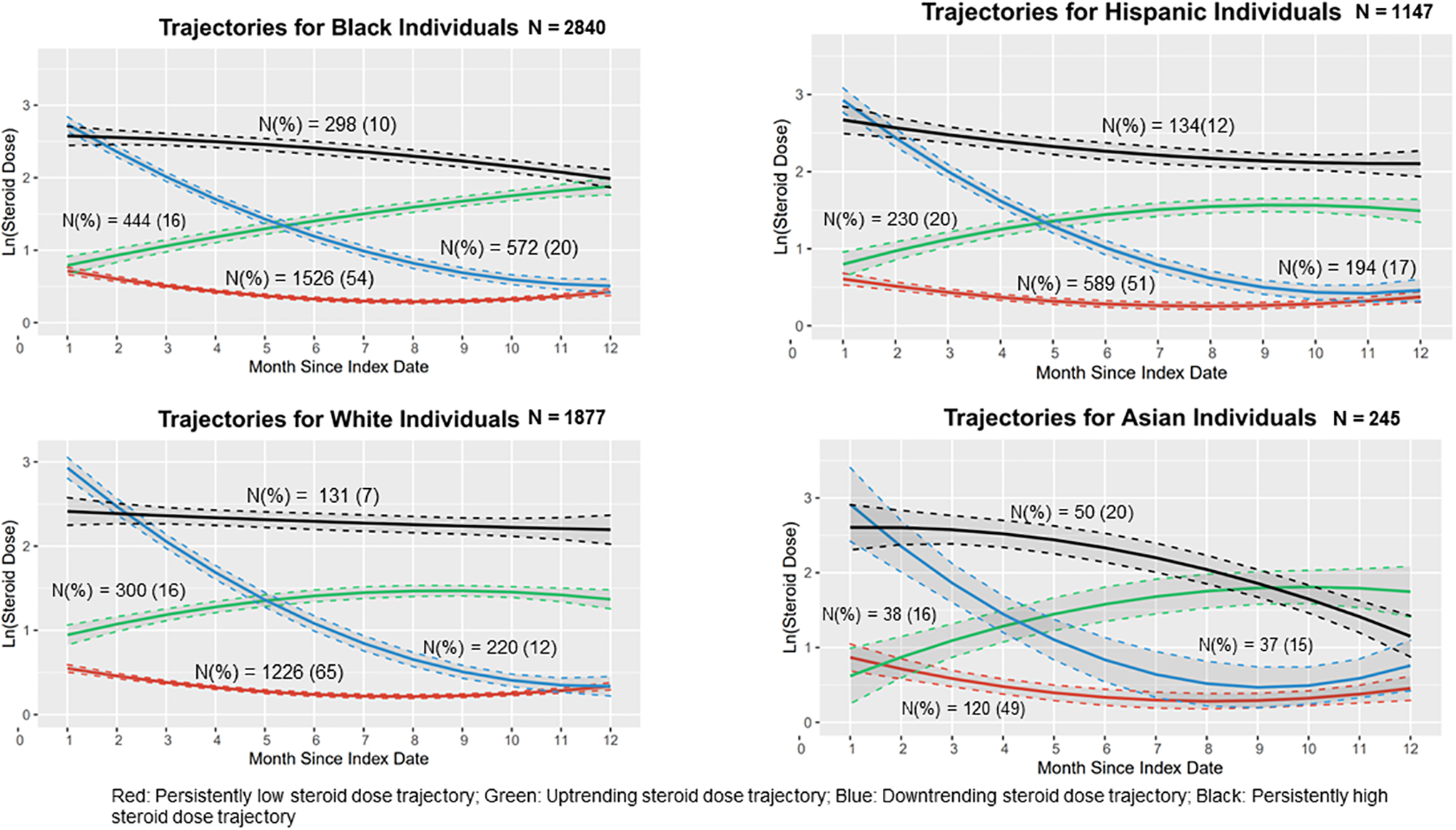
Group-Based Steroid Dose Trajectories Stratified by Race/Ethnicity
We examined healthcare utilization at baseline and during follow-up among individuals grouped by race/ethnicity. Within the persistently high steroid dose trajectory group, White individuals had the highest proportion of individuals accessing >5 outpatient visits at baseline (54%) compared to Asian (39%), Black (38%), and Hispanic (35%) individuals. With respect to healthcare utilization measures, Black and Hispanic individuals had fewer outpatient visits compared to White individuals in every steroid dose trajectory, at baseline and throughout the follow-up periods.
3.5. Multinomial Models – Race/Ethnicity Stratified
In separate regression models stratified by race/ethnicity (Table 3), Black (OR 1.09, 95% CI 1.02–1.15) and White (OR 1.10, 95% CI 1.04–1.20) individuals had higher odds of being in the persistently high steroid dose trajectory with higher SLE risk adjustment indices. Black patients with >5 hospitalizations at baseline had higher odds of being in the persistently high steroid trajectory (OR 1.90, 95% CI 1.25–2.88) vs. the persistently low steroid dose trajectory. Odds ratios for steroid use trajectories for hospitalizations at baseline were not statistically significant for any other racial group.
Table 3.
Multinomial Logistic Regression Model Examining the Odds of Belonging to Different Steroid Dose Trajectories for Individuals with Incident SLE Receiving Steroids Grouped by Race* (N=6323)
| Covariatesⱡ | Low-Dose (Ref) |
Uptrending Dose Odds ratio (95% CI) |
Downtrending Dose Odds ratio (95% CI) |
High Dose Odds ratio (95% CI) |
|---|---|---|---|---|
| Black Individuals | ||||
| Geographic Region (ref=South) | ||||
| Midwest | - | 1.18 (0.86–1.61) | 1.19 (0.89–1.58) | 1.53 (1.06–2.21) |
| Northeast | - | 1.34 (1.02–1.76) | 1.30 (1.01–1.68) | 2.05 (1.50–2.81) |
| West | - | 1.04 (0.72–1.48) | 1.34 (0.97–1.84) | 1.27 (0.82–1.95) |
| SLE Risk-Adjustment Index | - | 1.04 (0.98–1.10) | 1.09 (1.04–1.15) | 1.09 (1.02–1.15) |
| Hydroxychloroquine | - | 1.09 (0.82–1.44) | 0.78 (0.59–1.04) | 0.68 (0.47–1.01) |
| Immunosuppressives | - | 1.54 (1.08–2.20) | 1.55 (1.10–2.20) | 1.93 (1.28–2.90) |
| Outpatient Visits (ref=0) | ||||
| 1–5 | - | 0.98 (0.74–1.30) | 0.99 (0.76–1.29) | 0.93 (0.66–1.30) |
| >5 | 0.85 (0.63–1.15) | 0.98 (0.74–1.30) | 0.83 (0.58–1.18) | |
| ED Visits (ref = 0) | ||||
| 1–5 | - | 0.98 (0.77–1.26) | 1.23 (0.98–1.55) | 1.03 (0.76–1.39) |
| >5 | - | 0.95 (0.63–1.44) | 1.08 (0.74–1.56) | 0.75 (0.46–1.24) |
| Hospitalizations (Ref = 0) | ||||
| 1–5 | - | 1.16 (0.81–1.66) | 0.97 (0.70–1.34) | 1.29 (0.86–1.95) |
| >5 | - | 1.04 (0.69–1.56) | 1.43 (1.02–2.01) | 1.90 (1.25–2.88) |
| Preventive Care (ref=none) | ||||
| At least 1 Vaccine | - | 0.53 (0.29–0.97) | 0.69 (0.41–1.13) | 0.56 (0.28–1.10) |
| Bone Density Scan | 1.84 (0.53–6.38) | 1.25 (0.31–4.96) | 2.50 (0.68–9.04) | |
| Pneumocystis jirovecii ppx | 1.24 (0.90–1.73) | 1.03 (0.76–1.41) | 1.42 (0.97–2.06) | |
| Hispanic Individuals | ||||
| Geographic Region (ref=South) | ||||
| Midwest | - | 1.24 (0.57–2.70) | 1.10 (0.49–2.46) | 1.73 (0.72–4.18) |
| Northeast | - | 1.26 (0.81–1.97) | 0.98 (0.61–1.58) | 1.69 (0.97–2.96) |
| West | - | 1.19 (0.77–1.83) | 1.05 (0.67–1.64) | 1.17 (0.67–2.05) |
| SLE Risk-Adjustment Index | - | 1.01 (0.93–1.10) | 1.08 (0.99–1.17) | 1.11 (1.01–1.22) |
| Hydroxychloroquine | - | 1.26 (0.84–1.87) | 0.86 (0.54–1.36) | 0.87 (0.50–1.49) |
| Immunosuppressives | - | 1.85 (1.15–2.97) | 1.45 (0.85–2.49) | 1.77 (0.96–3.27) |
| Outpatient Visits (ref=0) | ||||
| 1–5 | - | 0.75 (0.50–1.11) | 1.08 (0.69–1.71) | 0.99 (0.60–1.64) |
| >5 | 0.45 (0.29–0.70) | 0.86 (0.53–1.38) | 0.65 (0.38–1.13) | |
| ED Visits (ref = 0) | ||||
| 1–5 | - | 1.53 (1.08–2.16) | 1.17 (0.80–1.69) | 1.51 (0.98–2.33) |
| >5 | - | 1.31 (0.64–2.68) | 0.69 (0.32–1.47) | 0.99 (0.42–2.37) |
| Hospitalizations (Ref = 0) | ||||
| 1–5 | - | 0.70 (0.41–1.19) | 0.88 (0.53–1.47) | 0.85 (0.46–1.57) |
| >5 | - | 1.08 (0.56–2.07) | 1.26 (0.67–2.38) | 1.54 (0.76–3.15) |
| Preventive Care (ref=none) | ||||
| At least 1 Vaccine | - | 1.22 (0.63–2.35) | 1.02 (0.50–2.09) | 1.07 (0.46–2.48) |
| Bone Density Scans | 1.84 (0.53–6.38) | 1.25 (0.31–4.96) | 2.48 (0.68–9.04) | |
| Pneumocystis jirovecii ppx | 1.24 (0.90–1.73) | 1.03 (0.76–1.41) | 1.42 (0.97–2.06) | |
| White Individuals | ||||
| Geographic Region (ref=South) | ||||
| Midwest | - | 1.04 (0.69–1.58) | 1.22 (0.80–1.86) | 1.29 (0.72–2.28) |
| Northeast | - | 1.67 (1.18–2.37) | 1.62 (1.10–2.37) | 2.36 (1.46–3.81) |
| West | - | 1.87 (1.31–2.66) | 1.11 (0.72–1.73) | 1.59 (0.92–2.74) |
| SLE Risk-Adjustment Index | - | 1.04 (0.96–1.12) | 1.08 (1.00–1.17) | 1.10 (1.00–1.20) |
| Hydroxychloroquine | - | 1.32 (0.95–1.85) | 1.35 (0.92–1.98) | 1.29 (0.80–2.09) |
| Immunosuppressives | - | 2.86 (1.98–4.12) | 1.10 (0.65–1.87) | 2.20 (1.29–3.74) |
| Outpatient Visits (ref=0) | ||||
| 1–5 | - | 1.09 (0.74–1.61) | 1.12 (0.70–1.77) | 1.14 (0.64–2.04) |
| >5 | 1.00 (0.68–1.48) | 1.37 (0.88–2.14) | 1.01 (0.57–1.79) | |
| ED Visits (ref = 0) | ||||
| 1–5 | - | 0.81 (0.60–1.10) | 0.70 (0.49–0.99) | 1.10 (0.72–1.68) |
| >5 | - | 0.74 (0.46–1.19) | 0.54 (0.32–0.91) | 0.66 (0.33–1.33) |
| Hospitalizations (Ref = 0) | ||||
| 1–5 | - | 1.31 (0.86–2.00) | 1.69 (1.07–2.68) | 1.01 (0.54–1.91) |
| >5 | - | 0.96 (0.59–1.57) | 1.82 (1.14–2.92) | 1.60 (0.89–2.88) |
| Preventive Care (ref=none) | ||||
| At least 1 Vaccine | - | 0.66 (0.41–1.05) | 0.51 (0.28–0.92) | 0.66 (0.34–1.26) |
| Bone Density Scan | 3.14 (1.17–8.43) | 1.56 (0.39–6.18) | <0.001 (<0.001–>999) | |
| Pneumocystis carinii ppx | 0.72 (0.46–1.12) | 1.27 (0.83–1.95) | 0.74 (0.40–1.38) | |
Also adjusted for age, sex, income, and comorbidities (cardiovascular disease, diabetes, pregnancy, renal disease)
Sample size for the subcohort of Asian Individuals was too small for the fully adjusted multinomial models
ppx=prophylaxis
Among White individuals, receipt of a bone density scan during the baseline period was associated with higher odds of being in the uptrending steroid dose trajectory (OR 3.14, 95% CI 1.17–8.43) vs. the persistently low steroid dose trajectory. Odds ratios for bone density scans at baseline were not statistically significant for any other racial/ethnic group.
Immunosuppressive use during the baseline period among Black individuals was associated with having higher odds of being in each of the higher steroid dose trajectory groups vs. the persistently low steroid dose trajectory (OR 1.93, 95% CI 1.28–2.90, persistently high dose vs. persistently low dose). Immunosuppressive use among White individuals at baseline was associated with higher odds of being in the uptrending (OR 2.86, 95% CI 1.98–4.12) and persistently high dose steroid trajectories (OR 2.20, 95% CI 1.29–3.74) vs. persistently low steroid dose trajectory. Among Hispanic individuals, immunosuppressive use at baseline was associated with higher odds of being in the uptrending steroid dose trajectory vs. the persistently low steroid dose trajectory (OR 1.85, 95% CI 1.15–2.96). We did not observe associations between baseline hydroxychloroquine use and steroid dose trajectory among the racial/ethnic subpopulations. Black individuals residing in the Midwestern (OR 1.53, 95% CI 1.06–2.21) and Northeastern (OR 2.05, 95% CI 1.50–2.81) regions demonstrated higher odds of being in the persistently high dose steroid trajectory vs. the persistently low steroid dose trajectory compared to Black individuals residing in the Southern region of the U.S.
3.6. Sensitivity Analyses
We excluded 3375 individuals who had steroid use during the baseline period and restricted our analysis to new steroid users (N=2939). We constructed GBTMs of new steroid users with 4-group trajectories to parallel our primary analyses (Supplemental Figure 1). The persistently low steroid dose trajectory group included 64% of the new users, the uptrending steroid dose trajectory included 13% of new users, the downtrending steroid dose trajectory included 15% of new steroid users, and the persistently high dose trajectory included 8%. There were slightly more individuals in the persistently low steroid dose group among new steroid users than in the original cohort (64 vs. 57%), and slightly fewer individuals in persistently high steroid dose group (8 vs. 10%). The demographic findings in the multinomial models of new steroid users were consistent with the overall cohort; Black and Hispanic individuals had higher odds of being in any trajectory vs. the persistently low steroid dose trajectory compared to White individuals after adjusting for sociodemographic and disease severity variables (Supplemental Table 9).
We constructed multinomial models adjusting for the SLE disease severity using the Garris algorithm, which was initially applied in full form, then modified to exclude steroids and immunosuppressive.27 Each model had nearly identical findings by race/ethnicity and healthcare utilization. As expected, moderate and severe vs. mild SLE using the Garris algorithm was associated with higher odds of belonging to the persistently high steroid dose trajectory vs. lower steroid dose trajectories.
4. Discussion
Among Medicaid beneficiaries with incident SLE who had oral steroids dispensed on or during the 12 months following the index date, race/ethnicity was strongly associated with distinctive longitudinal steroid dose patterns. We observed that Black and Hispanic individuals with SLE had significantly higher odds of persistent higher steroid dose dispensings compared with White individuals after accounting for SLE severity, comorbidities, other medication use, and health care utilization. Prolonged persistently high steroid dose use is associated with higher odds of acute care use for potentially avoidable adverse outcomes including serious infections and gastrointestinal bleeding. Avoidable adverse outcomes are also higher among Black compared to White individuals. Steroid dosing patterns may highlight a modifiable target to help mitigate racial/ethnic disparities in avoidable adverse outcomes.
Access to sustained high quality outpatient care may contribute to the disparities observed in steroid use patterns. Medicaid-based insurance status is a proxy for low socioeconomic status in the US. Individuals with SLE in lower socioeconomic status groups are less likely to identify a rheumatologist as their primary SLE provider and are more likely to travel significantly further for their care.30 For context, in a prior study of Medicaid beneficiaries with SLE, individuals had an average of 4 visits to a rheumatologist and 5 visits to a generalist during a 12-month follow up period.28 We observed that individuals with >5 outpatient visits during the baseline period had lower odds of being in the persistently high steroid dose trajectory. More than half of the White (53%) and Asian (51%) individuals in this cohort had >5 outpatient visits during the 12-month baseline period compared to 38% and 41% for Black and Hispanic patients, respectively. While our dataset did not allow us to distinguish between primary care and subspecialty visits, this suggests that Black and Hispanic individuals have below-average access to outpatient care compared to all Medicaid beneficiaries with SLE. Among White individuals who received a bone density scan during the baseline period, we observed higher odds of the belonging to the uptrending steroid dose trajectory vs. the persistently low steroid dose trajectory, likely reflecting an anticipation that the individual would require long-term steroid treatment. However, there were no statistically significant associations between bone density scan at baseline and dose trajectory for any other race/ethnicity. Our findings suggest that lack of sustained access to outpatient care and high quality preventive care is a modifiable factor that may contribute to racial differences in steroid use patterns. We did not have access to specific information regarding where individuals in this study were receiving their SLE care. Proximity to medical centers may contribute to steroid use patterns and warrants further study.
Individuals with SLE managed by non-rheumatologists are rarely prescribed hydroxychloroquine, an inexpensive, relatively well-tolerated medication that remains central to SLE disease control.2,31–33 We did not find a statistically significant association between baseline hydroxychloroquine use and steroid trajectory although hydroxychloroquine use has been shown to have a steroid-sparing effect.34 We found that hydroxychloroquine dispensing was suboptimal, peaking at 44%, which is consistent with prior studies among Medicaid beneficiaries with SLE.17,24 Dispensing increased in the first 6 months following the index date across all racial/ethnic groups, but decreased in the subsequent 6 months suggesting nonadherence and/or inadequate access to sustained care.27,31 While hydroxychloroquine in some cases may have been prescribed but not filled due to factors such as concerns about medication side effects, since it is usually well tolerated, it may be more plausible that hydroxychloroquine is consistently under prescribed over time in this population. We hypothesize that Medicaid beneficiaries not only have inadequate access to consistent primary care, but also to high-quality rheumatologic specialty care, which may disproportionately affect people of color.
As expected, immunosuppressive dispensing patterns were associated with steroid dose trajectory, likely reflecting higher SLE disease activity. However, there was significant variability in immunosuppressive use over time and by race/ethnicity. Black individuals had the lowest utilization of immunosuppressives at baseline compared to all other racial/ethnic groups. We observed variability in immunosuppressive use longitudinally between Black individuals and all other racial/ethnic groups in the persistently high dose steroid trajectory where immunosuppressive use increased among White, Asian, and Hispanic individuals, but decreased among Black individuals. Nonadherence may be a factor here, which is in line with prior studies that have demonstrated higher odds of nonadherence among Black compared to White individuals with SLE.24,25,35 Additional studies are needed to explore potential associations between immunosuppressive use and the quality of patient-provider communication, frequency of contact with specialty providers, the effect of physician-patient racial concordance, experiences of racial discrimination and patient satisfaction with care.
We found that residing in the South was associated with lower odds of being in the persistently high steroid dose trajectory (Group A) compared to the living in the Northeast and Midwest especially for Black individuals. Interestingly, a prior study in the Medicaid SLE population demonstrated that patients who receive care in the South compared to other US regions have lower odds of receiving high-quality care as measured by performance on three measures of health care quality: receipt of immunosuppressive, renal-protective antihypertensive, and antimalarial medications.13 Additional studies are needed to explore potentially modifiable factors associated with regional differences in steroid dosing patterns by race/ethnicity.
This study has several strengths. We included a large, diverse population of individuals with SLE. Comprehensive demographic information, claims and pharmacy dispensing information was available for this population. We examined patterns of steroid use and dose in an incident SLE population using a rigorous method to uncover latent trajectories. This has greater explanatory potential than pure measures of mean doses over time. This novel method of exploring patterns of steroid use allowed us to consider nuances in racial/ethnic differences in use and to examine longitudinal patterns of potentially modifiable factors including healthcare and medication use.34
There are limitations to this work. First, measures of disease activity or reasons for persistent steroid use are not available in administrative claims data. Second, we used the SLE risk adjustment index, and in sensitivity analyses, the Garris algorithm, as markers for disease severity, but they may not capture all markers of disease severity or damage accrual. When compared to the SLICC Damage Index (SDI)36, the SLE risk adjustment index and Garris algorithm do not include skin disease or pulmonary disease.12,37 The SLE risk adjustment index captures more of the domains included in the SDI (such as malignancy and diabetes) than the Garris algorithm does, which is why we used it in our primary models (Supplemental Table 10). Third, a small of number of individuals with the most severe SLE may have been excluded at baseline if their SLE ICD-9 codes were less than 30 days apart. Fourth, racial and ethnic categories are social constructs that have changed over time. Hispanic ethnicity was included as a distinct racial/ethnic group by the Centers for Medicare and Medicaid Services (CMS) during the years of this study (2000–2010). CMS has since changed how race/ethnicity is categorized however race (White, Non-White, Black) is not available as a separate category for individuals described as Hispanic in this analysis. We were also unable to examine patterns of use specifically among AI/AN individuals due to sample size.37 Fifth, Medicaid is the largest public insurer in the United States and although this study examines a large and diverse population, it may not be generalizable all individuals with SLE. Finally, data presented here are from 2000–2010, but racial/ethnic disparities persist, and we believe it to be unlikely that significant secular changes have occurred that would make our findings inapplicable to the present day.
Inconsistencies in immunosuppressive use and the number of outpatient visits between Black individuals compared to all other racial/ethnic groups in the persistently high steroid dose trajectory during the baseline and follow up periods suggest that further studies are needed to elucidate the root cause of the disparate patterns. Early and appropriate initiation of steroid-sparing therapies and adequate access to outpatient care may be critical to reducing steroid use in the long-term and may lead to a decrease in preventable adverse outcomes and acute care use. Currently, no evidence from a randomized clinical trial exists for glucocorticoid tapering regimens in SLE to form the basis for a gold standard in steroid tapering. Therefore, rigorous studies in racially, ethnically, and socioeconomically diverse patient populations are needed to define appropriate steroid tapering regimens and to define standard-of-care procedures for steroid-sparing agent use across all racial/ethnic groups.4,12 Furthermore, to close the racial/ethnic gap in steroid-related adverse events among individuals with SLE, intensive efforts should be made to ensure access to high-quality outpatient rheumatologic services. Greater equity may be achieved with the development of a gold standard for steroid-tapering regimens and with the establishment of health care policies that incentivize providers to adhere to the latest standards of quality measures for the care of all individuals SLE.
Supplementary Material
Funding:
This study was funded by NIH NIAMS K23 AR071500 (Feldman). Dr. Chandler receives support from NIH NIAMS T32 5T32AI007512-35 (Oettgen and Geha). Dr.Costenbader is supported by NIH K24 AR066109. The content of this article is solely the responsibility of the authors and does not necessarily represent the official view of the sources providing funding support to the authors.
Disclosures:
Dr. Feldman receives research support through Brigham and Women’s Hospital from Pfizer Pharmaceuticals and Bristol Myers Squibb Foundation for studies unrelated to this work. She also serves on the Medical and Scientific Advisory Board of the Lupus Foundation of America and during this project served on the Board of Directors of the American College of Rheumatology. She serves as a research consultant on grants to the American College of Rheumatology, the Lupus Foundation of America, and the University of Alabama for work unrelated to the content of this article (All <$10,000). Dr. Kim received research support to Brigham and Women’s Hospital from Pfizer, AbbVie, Roche, and Bristol Myers Squibb for unrelated studies but is now fully employed by Bristol Myers Squibb. Dr. Costenbader receives research support from Exagen, Gilead and Merck. She has consulted for Astra Zeneca, Glaxo Smith Kline, Amgen, Lilly, Janssen and Neutrolis. (All < $10,000.).
References:
- 1.Fanouriakis A, Kostopoulou M, Cheema K, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 2020;79(6):713–723. DOI: 10.1136/annrheumdis-2020-216924. [DOI] [PubMed] [Google Scholar]
- 2.Alarcon GS, McGwin G, Bertoli AM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 2007;66(9):1168–72. DOI: 10.1136/ard.2006.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zonana-Nacach A, Barr SG, Magder LS, Petri M. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum 2000;43(8):1801–1808. (In English). DOI: Doi . [DOI] [PubMed] [Google Scholar]
- 4.Yee CS, Su L, Toescu V, et al. Birmingham SLE cohort: outcomes of a large inception cohort followed for up to 21 years. Rheumatology 2015;54(5):836–843. (In English). DOI: 10.1093/rheumatology/keu412. [DOI] [PubMed] [Google Scholar]
- 5.Bultink IEM. Bone Disease in Connective Tissue Disease/Systemic Lupus Erythematosus. Calcified Tissue Int 2018;102(5):575–591. (In English). DOI: 10.1007/s00223-017-0322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber MRW, Clarke AE. Systemic lupus erythematosus and risk of infection. Expert Rev Clin Immu 2020;16(5):527–538. (In English). DOI: 10.1080/1744666x.2020.1763793. [DOI] [PubMed] [Google Scholar]
- 7.van Vollenhoven RF, Mosca M, Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Annals of the Rheumatic Diseases 2014;73(6):958–967. (In English). DOI: 10.1136/annrheumdis-2013-205139. [DOI] [PubMed] [Google Scholar]
- 8.Tselios K, Gladman DD, Su JD, Urowitz MB. Gradual Glucocorticosteroid Withdrawal Is Safe in Clinically Quiescent Systemic Lupus Erythematosus. Acr Open Rheumatol 2021;3(8):550–557. (In English). DOI: 10.1002/acr2.11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology Guidelines for Screening, Treatment, and Management of Lupus Nephritis. Arthrit Care Res 2012;64(6):797–808. (In English). DOI: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miloslavsky EM, Naden RP, Bijlsma JWJ, et al. Development of a Glucocorticoid Toxicity Index (GTI) using multicriteria decision analysis. Annals of the Rheumatic Diseases 2017;76(3):543–546. (In English). DOI: 10.1136/annrheumdis-2016-210002. [DOI] [PubMed] [Google Scholar]
- 11.Feldman CH, Speyer C, Ashby R, et al. Development of a Set of Lupus-Specific, Ambulatory Care-Sensitive, Potentially Preventable Adverse Conditions: A Delphi Consensus Study. Arthrit Care Res 2021;73(1):146–157. (In English). DOI: 10.1002/acr.24095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmajuk G, Tonner C, Yazdany J. Factors associated with access to rheumatologists for Medicare patients. Semin Arthritis Rheu 2016;45(4):511–518. (In English). DOI: 10.1016/j.semarthrit.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yazdany J, Feldman CH, Liu J, Ward MM, Fischer MA, Costenbader KH. Quality of Care for Incident Lupus Nephritis Among Medicaid Beneficiaries in the United States. Arthrit Care Res 2014;66(4):617–624. (In English). DOI: 10.1002/acr.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward MM. Hospital experience and mortality in patients with systemic lupus erythematosus. Arthritis Rheum 1999;42(5):891–898. (In English). DOI: Doi . [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Puerta JA, Barbhaiya M, Guan HS, Feldman CH, Alarcon GS, Costenbader KH. Racial/Ethnic Variation in All-Cause Mortality Among United States Medicaid Recipients With Systemic Lupus Erythematosus. Arthritis Rheumatol 2015;67(3):752–760. (In English). DOI: 10.1002/art.38981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams JN, Xu C, Costenbader KH, Bermas BL, Pace LE, Feldman CH. Racial Differences in Contraception Encounters and Dispensing Among Female Medicaid Beneficiaries With Systemic Lupus Erythematosus. Arthrit Care Res 2021;73(10):1396–1404. (In English). DOI: 10.1002/acr.24346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pryor KP, Barbhaiya M, Costenbader KH, Feldman CH. Disparities in Lupus and Lupus Nephritis Care and Outcomes Among US Medicaid Beneficiaries. Rheum Dis Clin N Am 2021;47(1):41–53. (In English). DOI: 10.1016/j.rdc.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SK, Barbhaiya M, Fischer MA, et al. Lipid Testing and Statin Prescriptions Among Medicaid Recipients With Systemic Lupus Erythematosus or Diabetes Mellitus and the General Medicaid Population. Arthrit Care Res 2019;71(1):104–115. (In English). DOI: 10.1002/acr.23574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman CH, Xu C, Costenbader KH. Avoidable Acute Care Use for Vaccine-Preventable Illnesses Among Medicaid Beneficiaries With Lupus. Arthrit Care Res 2021;73(9):1236–1242. (In English). DOI: 10.1002/acr.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bureau UC. 2010 Census of Population and Housing, Population and Housing Unit Counts, CPH-2–1. 2012.
- 21.Feldman CH, Hiraki LT, Liu J, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 20002004. Arthritis Rheum 2013;65(3):753–763. (In English). DOI: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus 2010;19(6):741–743. (In English). DOI: 10.1177/0961203309356289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychol Methods 1999;4(2):139–157. (In English). DOI: Doi 10.1037/1082-989x.4.2.139. [DOI] [PubMed] [Google Scholar]
- 24.Feldman CH, Collins J, Zhang Z, et al. Dynamic patterns and predictors of hydroxychloroquine nonadherence among Medicaid beneficiaries with systemic lupus erythematosus. Semin Arthritis Rheu 2018;48(2):205–213. (In English). DOI: 10.1016/j.semarthrit.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldman CH, Collins J, Zhang Z, et al. Azathioprine and Mycophenolate Mofetil Adherence Patterns and Predictors Among Medicaid Beneficiaries With Systemic Lupus Erythematosus. Arthrit Care Res 2019;71(11):1419–1424. (In English). DOI: 10.1002/acr.23792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagin DS, Odgers CL. Group-Based Trajectory Modeling in Clinical Research. Annu Rev Clin Psycho 2010;6:109–138. (In English). DOI: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 27.Garris C, Jhingran P, Bass D, Engel-Nitz NM, Riedel A, Dennis G. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ 2013;16(5):667–77. DOI: 10.3111/13696998.2013.778270. [DOI] [PubMed] [Google Scholar]
- 28.SAS Institute Inc. In: Inc. SI, ed. SAS® 9.4 Statments Reference ed. Cary, NC: SAS Institute Inc; 2013. [Google Scholar]
- 29.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. [Google Scholar]
- 30.Yazdany J, Gillis JZ, Trupin L, et al. Association of socioeconomic and demographic factors with utilization of rheumatology subspecialty care in systemic lupus erythematosus. Arthritis Rheum 2007;57(4):593–600. DOI: 10.1002/art.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmajuk G, Yazdany J, Trupin L, Yelin E. Hydroxychloroquine Treatment in a Community-Based Cohort of Patients With Systemic Lupus Erythematosus. Arthrit Care Res 2010;62(3):386–392. (In English). DOI: 10.1002/acr.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill A, Wang J, Levi J, Heath K, Fortunak J. Minimum costs to manufacture new treatments for COVID-19. J Virus Erad 2020;6(2):61–69. (https://www.ncbi.nlm.nih.gov/pubmed/32405423). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakiya R, Kameda T, Nakashima S, et al. Efficacy and Safety of Hydroxychloroquine Therapy for Systemic Lupus Erythematosus Patients Depend on Administration Dose. Intern Med 2020;59(17):2105–2112. DOI: 10.2169/internalmedicine.4317-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arora SYJ. Use of Quality Measures to Identify Disparities in Health Care for Systemic Lupus Erythematosus. Rheum Dis Clin N Am 2020;46(4):623–638. DOI: doi: 10.1016/j.rdc.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun K, Eudy AM, Criscione-Schreiber LG, et al. Racial Disparities in Medication Adherence between African American and Caucasian Patients With Systemic Lupus Erythematosus and Their Associated Factors. Acr Open Rheumatol 2020;2(7):430–437. DOI: 10.1002/acr2.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the systemic lupus international collaborating clinics American College of Rheumatology Damage Index for Systemic Lupus Erythematosus. Arthritis Rheum 1996;39(3):363–369. (In English). DOI: DOI 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 37.Wickham H. Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


