Abstract
In the United States and globally, contaminant exposure in unregulated private-well point-of-use tapwater (TW) is a recognized public-health data gap and an obstacle to both risk-management and homeowner decision making. To help address the lack of data on broad contaminant exposures in private-well TW from hydrologically-vulnerable (alluvial, karst) aquifers in agriculturally-intensive landscapes, samples were collected in 2018–2019 from 47 northeast Iowa farms and analyzed for 35 inorganics, 437 unique organics, 5 in vitro bioassays, and 11 microbial assays. Twenty-six inorganics and 51 organics, dominated by pesticides and related transformation products (35 herbicide-, 5 insecticide-, and 2 fungicide-related), were observed in TW. Heterotrophic bacteria detections were near ubiquitous (94% of the samples), with detection of total coliform bacteria in 28% of the samples and growth on at least one putative-pathogen selective media across all TW samples. Health-based hazard index screening levels were exceeded frequently in private-well TW and attributed primarily to inorganics (nitrate, uranium). Results support incorporation of residential treatment systems to protect against contaminant exposure and the need for increased monitoring of rural private-well homes. Continued assessment of unmonitored and unregulated private-supply TW is needed to model contaminant exposures and human-health risks.
Keywords: tapwater contaminants, private wells, agricultural health, human health, organics, inorganics, microbial
Graphical Abstract
1. Introduction
Given the magnitude of anthropogenic (human-driven/-synthesized) chemicals in commerce and by extension in the environment (Wang et al., 2020) and the persistent challenges of geogenic contaminants (Lombard et al., 2021) and water-borne disease outbreaks (Collier et al., 2021; Craun et al., 2010), the paucity of realistically broad assessments of potentially co-occurring organic, inorganic, and microbial contaminants in point-of-use (POU) drinking water (tapwater, TW) is a growing concern in the United States (US) and globally (Bondy and Campbell, 2018; Bradley et al., 2018; Bradley et al., 2021a). In the US, many anthropogenic and naturally-occurring drinking-water contaminants and water-borne pathogens are regulated in public supplies (U.S. Environmental Protection Agency, 2018; U.S. Environmental Protection Agency, 2023) but not in private supplies (U.S. Environmental Protection Agency, 2021c), even though 40 million people in the US rely on private wells for drinking water (Dieter et al., 2018). The responsibility for protection, monitoring, and treatment of these private wells falls on property owners, who frequently lack the requisite knowledge and financial resources for effective risk management (Nigra, 2020; Rogan and Brady, 2009; Zheng and Flanagan, 2017).
Previous research has documented a range of contaminant concerns in unregulated private-well drinking water (Charrois, 2010; Focazio et al., 2006; Rogan and Brady, 2009). Due to high analytical costs, common-place confusion of organoleptic quality with safety, and a range of other socioeconomic factors, private-well water-quality data remain scarce and, where available, are typically limited to a few targeted (e.g., coliform bacteria) contaminants (Focazio et al., 2006; Seltenrich, 2017; Zheng and Flanagan, 2017). Consequently, the potential for unrecognized contaminant exposures and adverse health effects is notably elevated for private wells (American Academy of Pediatrics, 2009; Charrois, 2010; Rogan and Brady, 2009), as illustrated by a recent comparison of private- and public-supply TW exposures from a sole-source aquifer in a suburban/urban landscape (Bradley et al., 2021a). Comparable characterization of contaminant exposures in private wells in rural, agricultural landscapes is currently lacking.
The U.S. Geological Survey (USGS) collaborates with the U.S. Environmental Protection Agency (EPA), Food and Drug Administration (FDA), National Cancer Institute (NCI), National Institute of Environmental Health Science (NIEHS), tribal nations, universities, utilities, and communities to inform drinking-water exposure and water-supply data gaps by assessing TW inorganic/organic/microbial contaminant mixtures and associated drivers in a range of socioeconomic and source-water vulnerability settings across the US (Bradley et al., 2020; Bradley et al., 2018; Bradley et al., 2021a; Bradley et al., 2021b; Bradley et al., 2022). Herein, simultaneous exposures to an extensive suite of potential inorganic, organic, and microbial contaminants were assessed in TW from 47 rural farm homes in northeastern Iowa in 2018–19 to 1) provide initial insight into cumulative contaminant risk (Moretto et al., 2017; National Research Council, 1983) to human health of private-well TW in an agriculturally-intensive landscape overlying hydrologically-vulnerable aquifer sources and 2) further expand the national perspective on inorganic/organic/microbial contaminant exposures in POU TW by maintaining the same sampling protocol and an analytical toolbox similar to that employed in previous studies by this group across the US (Bradley et al., 2020; Bradley et al., 2018; Bradley et al., 2021a; Bradley et al., 2021b; Bradley et al., 2022; Bradley et al., 2023).
For this study, exposure was operationally represented as detections (and concentrations) of 35 inorganic and 448 organic (437 unique) analytes, 11 microbial groups, and 5 in vitro bioactivities in residential TW samples. Potential human-health risks of individual and aggregate TW exposures were explored based on 1) cumulative detections and concentrations of designed-bioactive (e.g., pesticides, pharmaceuticals) chemicals (Bradley et al., 2020; Bradley et al., 2018) and 2) cumulative Exposure-Activity Ratio(s) (∑EAR) (Blackwell et al., 2017) and hazard indices (HI) (Goumenou and Tsatsakis, 2019; U.S. Environmental Protection Agency, 2011) of cumulative benchmark-based Toxicity Quotients (∑TQ) (Corsi et al., 2019). In line with previous POU drinking-water studies by this research group (Bradley et al., 2018; Bradley et al., 2021a; Bradley et al., 2021b; Bradley et al., 2022) and others (Focazio et al., 2006; Rogan and Brady, 2009), simultaneous exposures to multiple inorganic, organic, and microbial constituents of potential human-health concern were expected to occur in these private-well TW samples (Hypothesis I). Consistent with hydrologically-vulnerable (alluvial, karst) aquifer sources in an agriculturally-intensive setting, agricultural pesticides and nutrients were expected to dominate TW exposures, with cumulative detections and concentrations generally higher than those observed in previous TW studies by this group in non-agricultural settings (Hypothesis II).
2. Materials and methods
2.1. Site selection and sample collection
A subset of 47 rural farm, private-well dependent, residences within an area of intensive crop (primarily corn, soybean) and animal (poultry, swine, cattle) agriculture in northeast Iowa (Table S1; Figure 1) were selected from enrollees in the Agricultural Health Study (AHS) (Alavanja et al., 1996) Biomarkers of Exposure and Effects in Agriculture (BEEA) subcohort (Hofmann et al., 2015). Additional participant eligibility criteria included active farming at the time of sample collection, permanent residence since 1995 (AHS inception), a private well screened (depth range: 8.5 – 88 m) in a hydrologically-vulnerable (karst or alluvial) aquifer setting, and proximity to an overnight shipping center. Untreated kitchen cold-water taps were sampled once between December 2018 and February 2019. Samples were collected at the residents’ convenience throughout the day without pre-cleaning, screen removal, or Lead and Copper Rule (U.S. Environmental Protection Agency, 2008; U.S. Environmental Protection Agency, 2020b) stagnant-sample protocols, as described (Romanok et al., 2018).
Figure 1.
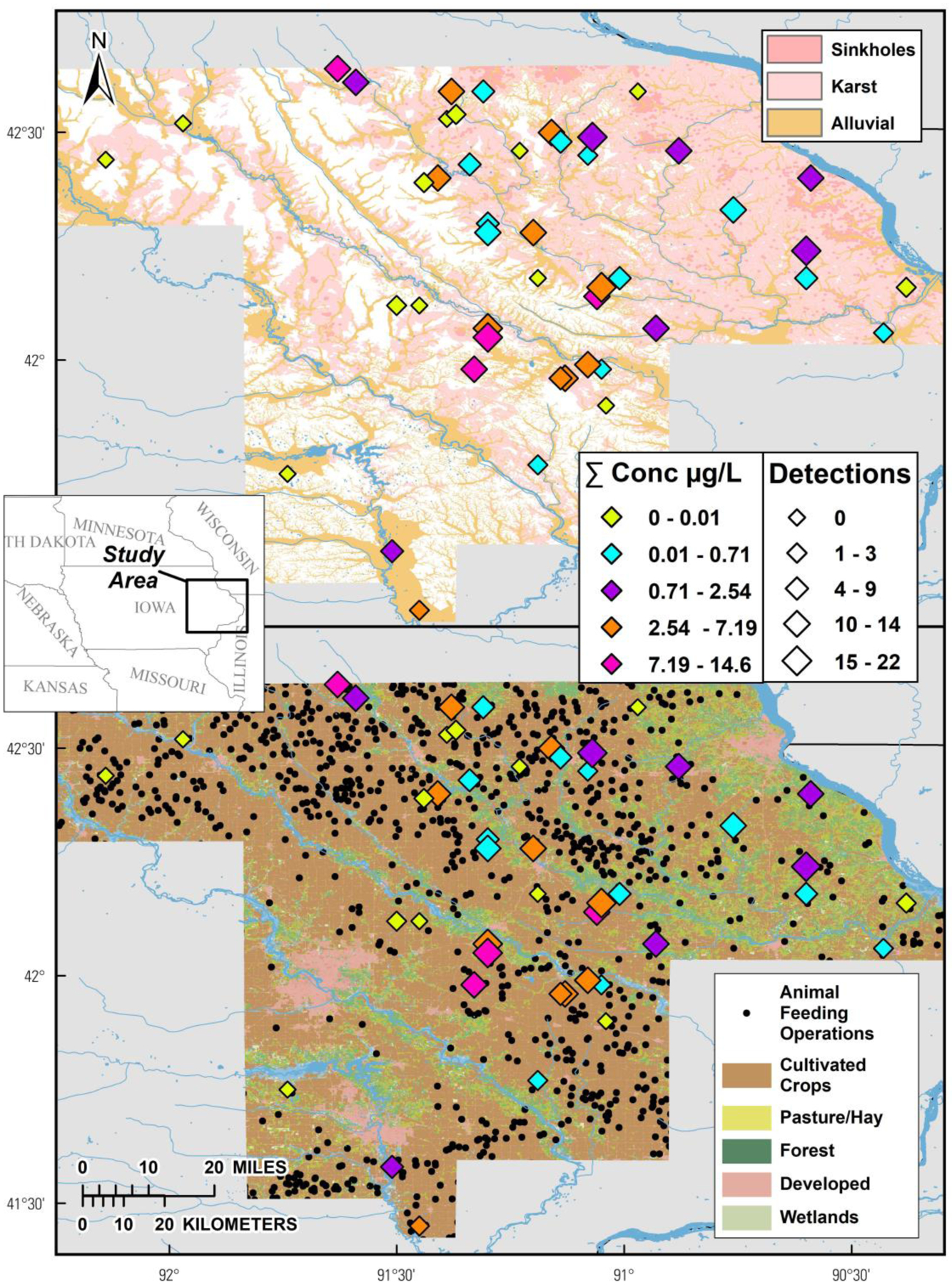
Cumulative (sum of all detected) concentrations (μg L−1) and numbers of organic compounds (diamonds, u) in samples of private-well tapwater collected during 2018–19 in northeast Iowa. Top: Color shadings indicate karst (Interstate Technology Regulatory Council, 2022) and alluvial (U.S. Geological Survey, 2002) aquifer areas. Bottom: Color shadings and black circles indicate land cover (U.S. Geological Survey, 2021) and animal feeding operations (Iowa Department of Natural Resources, 2021), respectively. Sample locations are anonymized.
2.2. Methods
Briefly, TW samples were analyzed by the 1) USGS using 7 target-organic (437 unique analytes), 3 inorganic (35 analytes), 3 field parameter, and 11 microbial methods (Table S2), as described (Bradley et al., 2021a; Romanok et al., 2018), 2) Center for Health Effects of Environmental Contamination and Iowa State Hygienic Laboratory at the University of Iowa for 6 neonicotinoid insecticides (acetamiprid, clothianidin, dinotefuran, imidacloprid, thiacloprid, thiamethoxam), as described (Evelsizer and Skopec, 2018; Thompson et al., 2021), 3) EPA using 3 in vitro bioassays targeting 3 (androgen [AR], estrogen [ER], and glucocorticoid [GR]) receptor classes (Medlock Kakaley et al., 2020; Medlock Kakaley et al., 2021), and 4) NCI using in vitro bioassays targeting 5 (AR, ER, GR, aryl hydrocarbon [AhR], thyroid hormone [TR]) receptor classes (Jones et al., 2020; Stavreva et al., 2012; Stavreva et al., 2016) (see SI for details/citations). A subset of 10 replicate TW samples was sent to the USGS Organic Chemical Research Laboratory for comparative analysis of the 6 neonicotinoid analytes, as described (Hladik and Calhoun, 2012). All results are in Tables S3–S6 and in Meppelink et al (2023).
2.3. Quality assurance
Quantitative (≥limit of quantitation, ≥LOQ) and semi-quantitative (between LOQ and long-term method detection limit, MDL (Childress et al., 1999; U.S. Environmental Protection Agency, 2020a)) results were treated as detections (Childress et al., 1999; Foreman et al., 2021; Mueller et al., 2015). Chemical quality-assurance/quality-control included 5 field blanks (inorganics, organics) as well as laboratory blanks (inorganics, organics), spikes (organics), and stable-isotope surrogates (organics). The median organic surrogate recovery (Table S4c) was 99.8% (interquartile range: 84–106%). Only sulfate (0.5 mg L−1), calcium (0.01 mg L−1), and hexamethylinetetramine (0.14 μg L−1) were detected in blanks at concentrations in the range observed in TW samples; corresponding results were censored at twice the maximum blank concentrations, as footnoted (Tables S3 and S4a). Microbial quality-assurance/quality-control included 4 field blanks as well as 8 laboratory blanks; microbial results were censored based on blanks, as footnoted (Tables S5a–b).
2.4. Statistics
Differences (centroids and dispersions) between TW-sample groups were assessed by nonparametric one-way PERMANOVA (n = 9999 permutations) on Euclidean distance (Paleontological Statistics, PAST, v. 4.03) (Hammer et al., 2001). Relations between detections/concentrations of selected TW contaminants were assessed by Spearman rho (ρ) correlation (SigmaPlot, v. 13, Systat Inc., Palo Alto, California). Organic-contaminant concentration patterns and potential contributing factors were assessed across all sites using principal component analysis (PCA) to reduce multivariate dimensionality (SigmaPlot, v. 13).
2.5. Risk assessments
Screening-level assessments (Goumenou and Tsatsakis, 2019; U.S. Environmental Protection Agency, 2011) of cumulative effects potentials were based on cumulative Exposure-Activity Ratio(s) (∑EAR) (Blackwell et al., 2017) and HI (Goumenou and Tsatsakis, 2019; U.S. Environmental Protection Agency, 2011; U.S. Environmental Protection Agency, 2012) of cumulative benchmark-based Toxicity Quotients (∑TQ) (Corsi et al., 2019), as described (Bradley et al., 2020; Bradley et al., 2018; Bradley et al., 2021a; Bradley et al., 2021b). ToxEval v. 1.2.0 (De Cicco et al., 2018) of the open source statistical software R (R Development Core Team, 2019) was used to sum (non-interactive concentration addition model (Altenburger et al., 2018; Cedergreen et al., 2008; Stalter et al., 2020)) individual ToxCast™ based (U.S. Environmental Protection Agency National Center for Computational Toxicology, 2019; U.S. Environmental Protection Agency National Center for Computational Toxicology, 2020) exposure-activity ratio(s) (EAR) or benchmark-based toxicity quotient(s) (TQ), respectively. For the latter, the most protective human-health benchmark (i.e., lowest benchmark concentration) among maximum contaminant level(s) (MCL) goal(s) (MCLG) (U.S. Environmental Protection Agency, 2018; U.S. Environmental Protection Agency, 2023), World Health Organization (WHO) Guideline Value(s) (GV) and provisional GV (pGV) (World Health Organization (WHO), 2011), EPA Human Health Benchmark(s) for Pesticides (HHBP) (U.S. Environmental Protection Agency, 2021b), USGS Health-Based Screening Level(s) (HBSL) (Norman et al., 2018), and state drinking-water MCL or health advisory(ies) (DWHA) was used, with MCLG of zero (no safe-exposure level for sensitive sub-populations, including infants, children, elderly, and immunocompromised) (U.S. Environmental Protection Agency, 2021a; U.S. Environmental Protection Agency, 2023) set to the method reporting limit or 1 μg L−1 for Pb (Lanphear et al., 2016). Cumulative EAR (∑EAR) and TQ (∑TQ) results, ToxCast exclusions, and benchmarks are summarized in Tables S7–S8 (additional details in SI).
3. Results and discussion
Regulated and unregulated chemical (inorganic, organic) and microbial contaminants were frequently detected in TW samples in this agriculturally-intensive, hydrologically-vulnerable northeast Iowa study area (Tables S3–S5; Figures 1, 2, 3, 4), with two or more detections commonly observed per sample (Hypothesis I). Fifty-one (12%) of the 437 unique organic-indicator analytes assessed in this study were detected at least once, with detections per sample ranging 0 – 22 (median: 8). Pesticide-related compounds comprised 82% (42/51) of TW contaminant detections. Twenty-seven (77%) of the 35 inorganic analytes were detected. Heterotrophic bacteria detections were near ubiquitous (94% of the samples), with detection of total coliform bacteria in 28% of the samples and frequent detection of growth on at least one putative-pathogen selective media across all TW samples.
Figure 2.
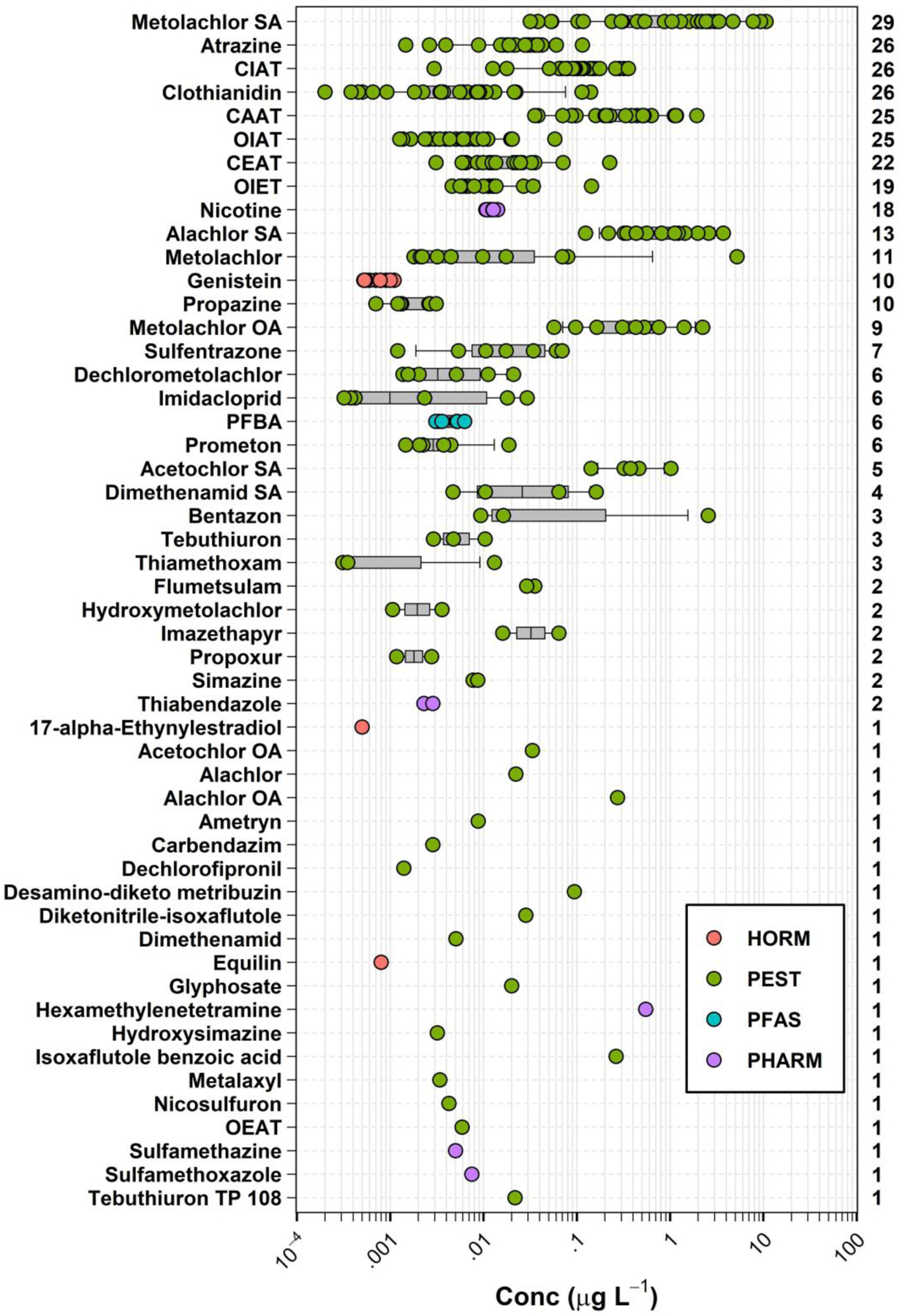
Detected concentrations (circles, μg L−1) and number of sites (right axis) for 52 organic analytes (left axis, in order of decreasing total detections) detected in samples of private-supply tapwater collected during 2018–19 in northeast Iowa. Circles (●) are data for individual samples. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively. Symbol colors identify hormone (HORM), pesticide (PEST), per/polyfluoroalkyl substances (PFAS), and pharmaceutical (PHARM) classes.
Figure 3.
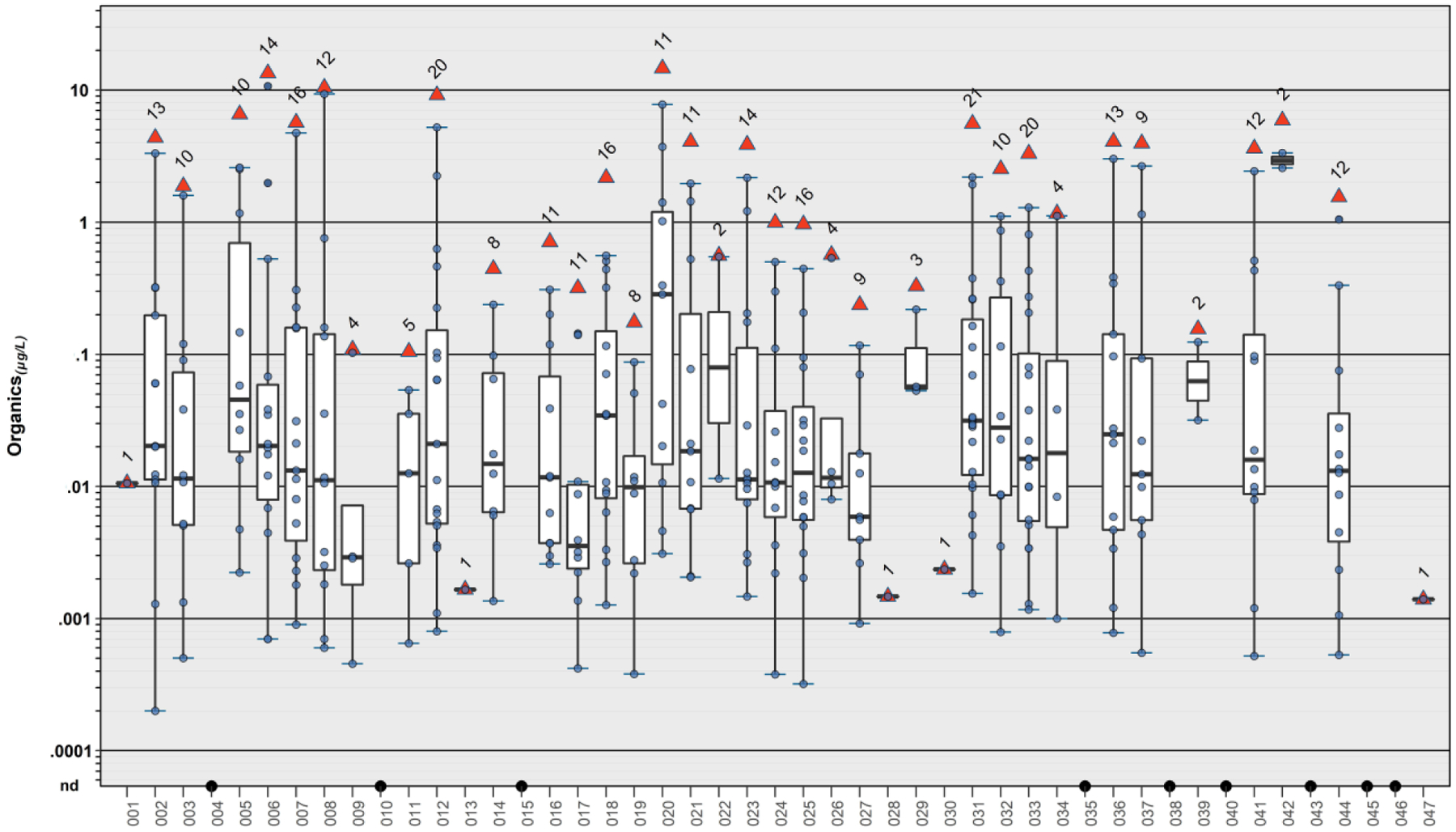
Individual (circles, ●) and cumulative (sum of all detected; red triangles, ▲) concentrations (μg L−1) of 51 organic analytes detected in samples of private-supply tapwater collected during 2018–19 in northeast Iowa. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively. Numbers above each boxplot indicate total detected organic analytes. “nd” indicates not detected.
Figure 4.
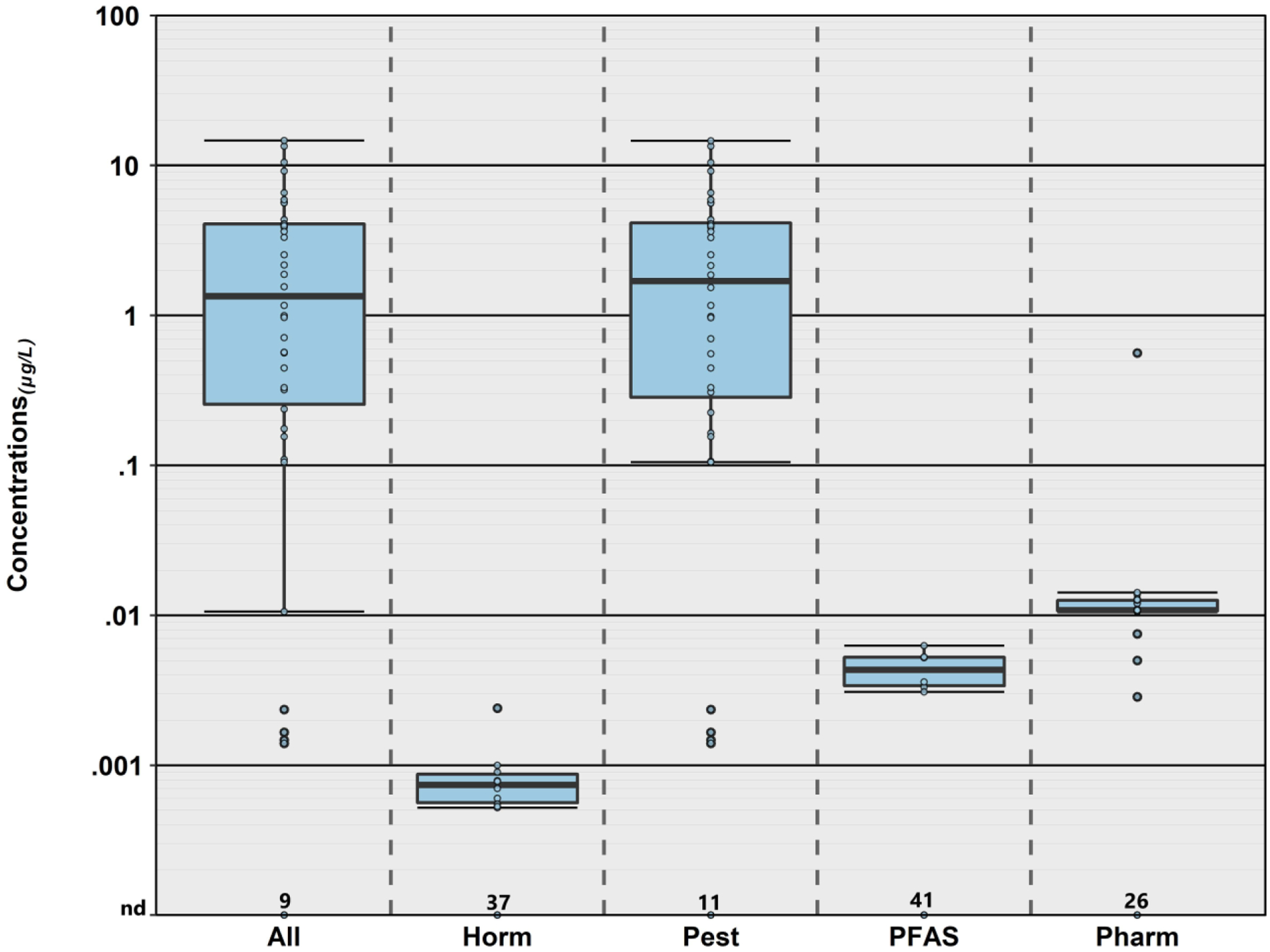
Cumulative concentration (μg L−1) of all organic analytes and classes of organic analytes detected in samples of private-supply tapwater collected during 2018–19 in northeast Iowa. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively. “nd” indicates not detected. HORM, PEST, PFAS, and PHARM indicate hormone, pesticide, per/polyfluoroalkyl substances, and pharmaceutical classes, respectively.
Herein we provide Safe Drinking Water Act (SDWA) MCL for regulatory context, but the organ/organism-level human-health effects of individual contaminant exposures are screened based on MCLG and other human-health advisories, which generally provide a margin-of-exposure concentration below which there is no known risk to the health of presumptive “most vulnerable” (e.g., infants, children, pregnant women, elderly, immune-compromised) sub-populations (U.S. Environmental Protection Agency, 2021a). MCL are set as close to the MCLG as feasible, considering technical and financial drinking-water treatment limitations (U.S. Environmental Protection Agency, 2021a), and are enforceable only in public supplies (U.S. Environmental Protection Agency, 2018; U.S. Environmental Protection Agency, 2023).
3.1. TW exposure-benchmark comparisons – inorganics
Few exceedances of human-health advisories for inorganics were observed in TW samples in this study, with the notable exception of nitrate-nitrogen (NO3-N) concentrations (Table S3a, Figure S1). NO3-N was near (2 samples >9.5 mg L−1) or above (15 samples) the MCLG (10 mg L−1) established to protect against bottle-fed infant (<6 months) methemoglobinemia (U.S. Environmental Protection Agency, 2018; U.S. Environmental Protection Agency, 2023), in 36% (17/47) of collected TW samples. Elevated groundwater NO3-N concentrations are well-documented in the US Midwest, including Iowa (Kolpin et al., 1994). Importantly, TW exposures to <MCLG NO3-N concentrations recently have been associated with several adverse outcomes (Ward et al., 2005; Ward et al., 2018) including specific cancers (Jones et al., 2016; Jones et al., 2017; Quist et al., 2018), thyroid disease (Aschebrook-Kilfoy et al., 2012), and neural tube defects (Brender et al., 2013). NO3-N concentrations >2 mg L−1 were observed in 49% (23/47) of TW samples. While microorganisms, including fecal indicator bacteria and potential human bacterial pathogens, were detected in this study, the general lack of human-use pharmaceutical co-contaminants other than nicotine (Table S4a) and corresponding lack of relation between NO3-N concentrations and pharmaceutical detections/concentrations (Figure S2) indicate that human-waste infrastructures (septic systems) were not primary drivers of elevated TW NO3-N concentrations. Strong correlations between NO3-N concentrations and pesticide-related contaminant detections (Spearman rho: ρ ≥ 0.823; p-value < 0.0001) or concentrations (Spearman rho: ρ ≥ 0.744; p-value < 0.0001) indicate that agricultural treatments (e.g., inorganic/organic fertilizers or animal waste) were probable sources of elevated TW NO3-N concentrations.
Other TW inorganic results of note included infrequent detections of uranium (U) and generally low fluoride (F) concentrations. The redox-reactive geogenic radionuclide U was detected in four TW samples in this study. No level of exposure is considered safe to vulnerable sub-populations (i.e., MCLG zero) (U.S. Environmental Protection Agency, 2018; U.S. Environmental Protection Agency, 2023). Drinking-water U is associated with nephrotoxicity (Magdo et al., 2007; Seldén et al., 2009) and osteotoxicity (Kurttio et al., 2005) in humans, inhibition of DNA-repair mechanisms in human embryonic kidney 293 (HEK293) cells (Cooper et al., 2016), and estrogen-receptor effects in mice (Raymond-Whish et al., 2007). Consistent with national surveys (DeSimone et al., 2015; McMahon et al., 2020), F concentrations observed in these TW samples were well below the EPA MCL (U.S. Environmental Protection Agency, 2018; U.S. Environmental Protection Agency, 2023) for toxic effects. However, almost all samples also were below the US Public Health Service optimum of 0.7 mg L−1 to prevent dental caries in children (2015), consistent with previous concerns for the dental health of children on private wells in the US (American Academy of Pediatrics, 2009). Concentrations of F were <0.6 mg L−1 in all but two TW samples and <0.3 mg L−1 in 85% (40/47) of TW samples; supplementation is recommended from 3–16 years of age for children with TW F <0.6 mg L−1, beginning at 6 months if TW F is <0.3 mg L−1 F (American Academy of Pediatrics: Committee on Nutrition, 1995; Kohn et al., 2001).
3.2. TW exposure-benchmark comparisons - organics
Among the 51 organic analytes detected in this study (Figure 3), 27 (53%) were detected in ≤ 2 samples, with 21 (41%) detected only once. At least one organic analyte was detected in 81% (38/47) of the TW samples, with more than one detected in 70% (33/47) of samples (Figure 4, Table S4a). Consistent with the hydrologically-vulnerable (alluvial, karst) aquifer sources in an agriculturally-intensive setting and with Hypothesis II, on average (median) 91% of cumulative detections (IQR: 83–100%) and 100% of concentrations (IQR: 100–100%) in TW samples with detectable organics were attributable to pesticide-related (parent or transformation product) contaminants, and maximum cumulative pesticide detections and concentrations were more than an order of magnitude higher than observed in public- or private-supply TW samples in previous studies by this group, using the same pesticide analytical method (Bradley et al., 2020; Bradley et al., 2018; Bradley et al., 2021a; Bradley et al., 2021b).
TW samples contained on average 8 pesticides (IQR: 1–10; range: 0–21), with median cumulative concentrations of 0.56 μg L−1 (IQR: not detected (nd)–3.9 μg L−1; range: nd–14.6 μg L−1). In general, the most frequently detected organic analytes were herbicide-related (e.g., acetochlor, atrazine, metolachlor) and primarily transformation products, consistent with previous findings for groundwater samples in Iowa (Kolpin et al., 2000). Three neonicotinoid insecticides (clothianidin, imidacloprid, thiamethoxam) also were detected in more than 5% of the TW samples, with clothianidin observed in more than half. Frequent TW exposures to neonicotinoid insecticides are emerging human-health concerns (Cimino et al., 2017; Thompson et al., 2020), driven by widespread adoption of neonicotinoid seed treatment for crop-pest management (Douglas and Tooker, 2015; Tooker et al., 2017). Other frequently detected organics included the pharmaceutical nicotine, the phytoestrogen genistein, and the PFAS compound perfluorobutanoate (PFBA), detected in 38%, 21%, and 13% of samples, respectively.
Among the 51 detected organics, only 4 have EPA promulgated MCLG and none were exceeded. No differences (p ≥ 0.264) in cumulative concentrations of organics or organics sub-classes were observed between alluvial and karst TW sources (Figures 1 and S3). Consistent with predominant herbicide-intensive corn/soybean crop agriculture across the study area, PCA (Figure S4) revealed central clustering and no apparent differences (α = 0.05) for multivariate concentration profiles, except for two sites distinguished by co-occurring estrogenic-organic detections and almost two orders of magnitude higher detected metolachlor concentration (AHS 012) and by the highest concentrations and simultaneous detections of three neonicotinoid analytes (AHS 031). Frequent and simultaneous detections of multiple pesticides in TW samples raise concerns for potential adverse human-health effects and demonstrate the need for improved understanding of the implications of long-term TW exposures to mixtures of pesticides and other commonly co-occurring organic contaminants.
3.3. TW exposure-benchmark comparisons - microbial
Elevated (>100 CFU 100 mL−1) heterotrophic bacteria plate counts (HPC) were common in this study, exceeding the quantitation limit (“too numerous to count” >2400 CFU 100 mL−1) in 23% of samples (Table S5a). HPC bacteria occur naturally in the environment, are commonly detected in private-well TW, and are not an intrinsic health concern, but do provide a useful indication of system maintenance (U.S. Environmental Protection Agency, 2018; U.S. Environmental Protection Agency, 2023), which for private wells would include regular disinfection (U.S. Environmental Protection Agency, 2021c). Common detection of total coliform bacteria (28%) and frequent detection of growth on at least one putative-pathogen selective media across all TW samples raise concerns for human health. The MCLG for total coliforms in TW is zero (U.S. Environmental Protection Agency, 2018; U.S. Environmental Protection Agency, 2023). Two TW samples were positive for total coliform bacteria and Escherichia coli (E. coli; fecal indicator bacteria), a result which, if confirmed, would represent a MCL violation in a public-supply setting. Detections of Salmonella (12 samples) and Campylobacter (1 sample) spp., common causes of foodborne bacterial diarrheal diseases (Eng et al., 2015; Silva et al., 2011), support concerns for adverse TW microbial exposures in this agriculturally-intensive area. Widespread (24% of samples) growth on oxacillin-resistant staphylococci selective media, including “too numerous to count” (10% of samples), indicates the presence of antibiotic-resistant microorganisms, a growing public-health (Laxminarayan et al., 2013) and drinking-water quality concern (Ashbolt et al., 2013). Total coliform bacteria, E. coli, Salmonella spp., Campylobacter spp., staphylococci, and antibiotic-resistant staphylococci are well-documented in livestock and poultry wastes (U.S. Environmental Protection Agency, 2013) and are acknowledged human-exposure concerns in nearby groundwater drinking-water supplies, especially unmonitored private wells (Borchardt et al., 2021; Burch et al., 2021), due to infiltration from waste storage lagoons (Chee-Sanford et al., 2001) and agricultural land applications (Givens et al., 2016). These results reiterate the inherent human-health challenge of unmonitored TW (DeSimone et al., 2015; Focazio et al., 2006; MacDonald Gibson and Pieper, 2017; Rogan and Brady, 2009) and support previous calls for systematic private-well monitoring (Zheng and Flanagan, 2017), including for microbial contamination.
3.4. TW in vitro bioactivities
Net biological activities were observed by NCI and EPA in some TW samples using distinct in vitro methodologies. For NCI bioassays (Table S6a), 5 of the 47 (11%) TW samples exhibited significant (p < 0.05) receptor bioactivity, with AhR and AR bioactivities in 3 samples each (co-occurring in 2 samples) and ER bioactivity in another. Results for 2 other samples indicated borderline (0.05 ≤ p ≤ 0.1) AR and GR bioactivity. For EPA bioassays (Table S6b), ER activity was detected in 6 (13%) samples above the T47D-KBluc minimum detectable concentration (MDC; 0.0164 ng 17b-Estradiol Equivalents (E2Eq) L−1); detected ER activity did not exceed a previously developed drinking water effects-based trigger value (1 ng E2Eq L−1) for adverse effects (Brand et al., 2013). No AR or GR bioactivities were detected above corresponding bioassay MDC. The results indicated the potential for net biological effects of some TW exposures in this agriculturally-intensive area and the need for further investigation of potential contaminant drivers of the observed activities.
3.5. TW aggregated screening assessments
Cumulative-exposure effects of potential human-health interest were screened using two bioactivity-weighted approaches (∑EAR, ∑TQ) based on detected TW analytes. Both approaches 1) are constrained by the analytical scope (437 organics, 32 inorganics), which, while extensive, is an orders-of-magnitude underestimate of the organic chemicals in commercial use and by extension in the environment (Wang et al., 2020), 2) are limited to available weighting-factors (ToxCast ACC and human-health benchmarks, respectively), and 3) assume cumulative effects are reasonably approximated by concentration addition (Backhaus, 2016; Cedergreen et al., 2008; Ermler et al., 2011; Medlock-Kakaley et al., 2018; Sigurnjak Bureš et al., 2021; Stalter et al., 2020). The ∑EAR approach (Blackwell et al., 2017; Bradley et al., 2021a) leverages ToxCast high-throughput exposure-effects data (Filer et al., 2017; Richard et al., 2016) to estimate potential cumulative activity of organics at sensitive molecular endpoints, but not all predicted pathway responses are necessarily adverse at organ/organism scales and ToxCast has no coverage of inorganic contaminants (Schroeder et al., 2016). We aggregated contaminant bioactivity ratios across all endpoints without restriction to recognized modes of action as a precautionary screening for further investigation of potential effects (Bradley et al., 2021a; Bradley et al., 2021b) (i.e., as a lower bound estimate of in vivo adverse effect levels (Paul Friedman et al., 2020)), but this approach may not accurately reflect apical effects (Blackwell et al., 2017; Schroeder et al., 2016). The ∑TQ approach assesses effects of simultaneous inorganic and organic exposures, is targeted at apical human-health effects, but is limited to existing health benchmarks.
Approximately half (30) of the 51 detected organic compounds had exact Chemical Abstract Services (CAS) number matches in the ToxCast invitroDBv3.2 database (Table S7b). However, the highest EAR (0.55) and the only ∑EAR exceeding the level expected to modulate molecular targets in vitro (i.e., solid red ∑EAR = 1 line, Figure 5) in this study was for a TW sample (AHS 012) containing 5.23 03B3μg L−1 metolachlor, and co-occurring detections of EE2 and equilin. Exceedance of ∑EAR = 0.001 (precautionary screening-level threshold of interest) in more than half (26/47) of the samples indicated that further investigation of the cumulative biological activity of TW exposures in this agriculturally-intensive area is warranted.
Figure 5.
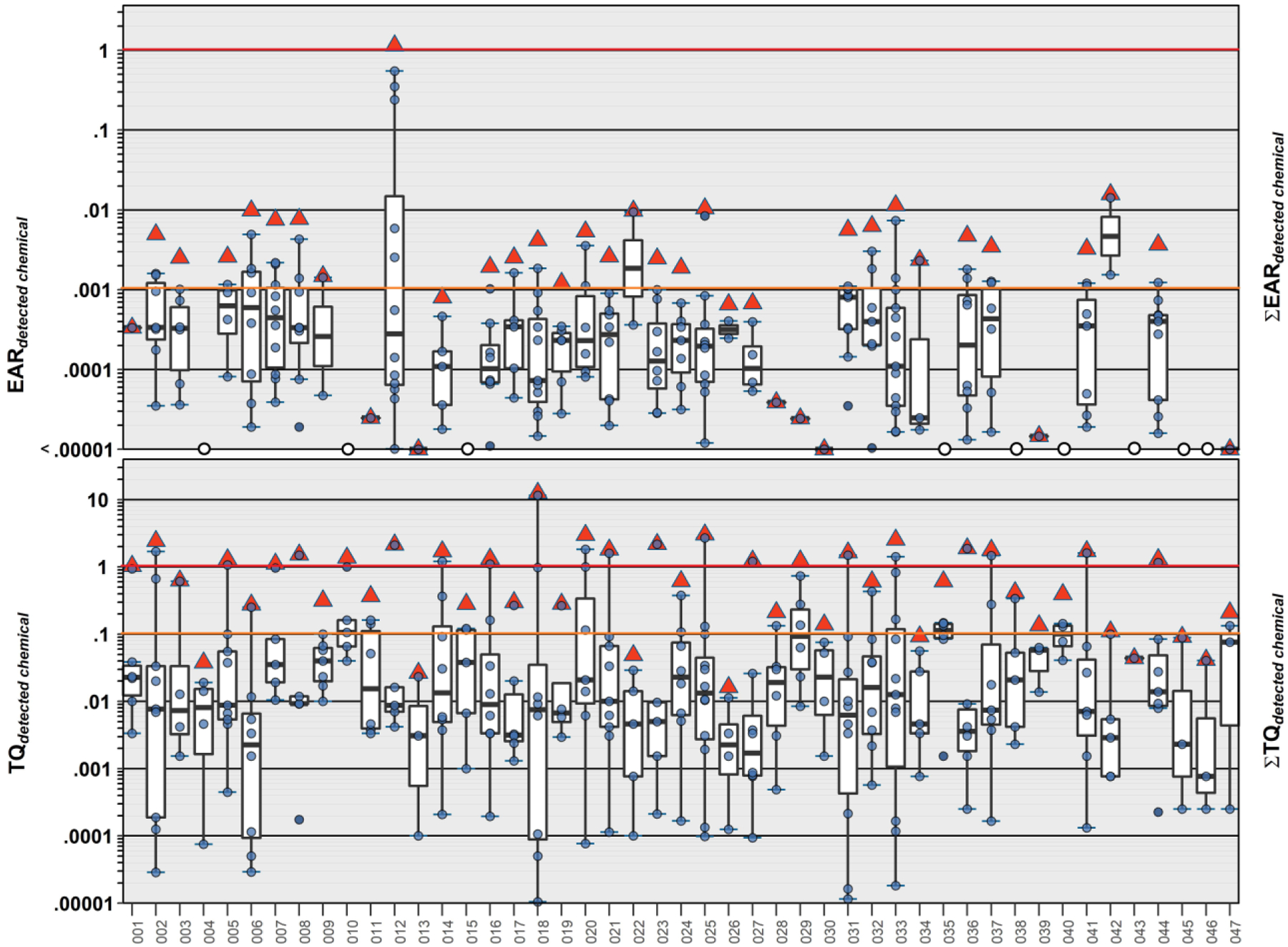
Top. Individual EAR values (circles, ●) and cumulative EAR (∑EAR, sum of all detected; red triangles, ▲) across all assays for 43 organic analytes detected in samples of private-supply tapwater collected during 2018–19 in northeast Iowa. Red and orange lines indicate concentrations shown to modulate effects in vitro (EAR = 1) and effects-screening-level thresholds (EAR = 0.001), respectively. Bottom. Human health benchmark-based individual TQ values (circles) and cumulative TQ (∑TQ, sum of all detected; red triangles, ▲) for inorganic and organic analytes listed in Table S11 and detected in samples of private-supply tapwater. Red and orange lines indicate benchmark equivalent concentrations (TQ = 1) and effects-screening-level threshold of concern (TQ = 0.1), respectively. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively, for both plots.
Approximately 83% (39/47) of the TW samples in this study exceeded the ∑TQ = 0.1 HI screening threshold of potential concern and 47% (22/47) exceeded ∑TQ = 1 (Figure 5; Table S8b). These ∑TQ results indicate high probabilities of aggregated risks in private-well TW samples in this agriculturally-intensive area, when considering exposures to both organic and inorganic contaminants. Consistent with the above discussion of individual contaminant benchmark comparisons and the lack of human-health benchmarks for most of the organic contaminants detected in TW in this study, ∑TQ was driven primarily by inorganics, notably by NO3-N, which exceeded the MCLG (MCL) in 32% (15/47) of collected TW samples, and less frequently (4/47) by U, for which there is no known safe level of exposure to vulnerable subpopulations (MCLG zero (U.S. Environmental Protection Agency, 2018)). Other notable ∑TQ results included the elevated alachlor exposure in a single sample and common-place simultaneous exposures to multiple pesticides. The results of this and previous (Bradley et al., 2020; Bradley et al., 2018; Bradley et al., 2021a; Bradley et al., 2021b) studies by this group reiterate the inherent human-health challenge of unmonitored TW (DeSimone et al., 2015; Focazio et al., 2006; MacDonald Gibson and Pieper, 2017; Rogan and Brady, 2009; Zheng and Flanagan, 2017) and the potential importance of systematic private-supply monitoring (Zheng and Flanagan, 2017), with an analytical scope that reflects the breadth of inorganic and organic environmental contamination (Bradley et al., 2017; Glassmeyer et al., 2017; Moschet et al., 2014; Schaider et al., 2016; Schaider et al., 2014).
4. Conclusions
Improved understandings of TW contaminant exposures based on more environmentally realistic and directly comparable POU exposure characterizations like this and others by this group (Bradley et al., 2020; Bradley et al., 2018; Bradley et al., 2021a; Bradley et al., 2021b; Bradley et al., 2022), in a range of source-water vulnerability settings, are essential to public health, because drinking-water is a biological necessity and, consequently, a high-vulnerability vector for human contaminant exposures (Dai et al., 2017). The paucity of information on private-well contaminant exposures is a recognized public-health data gap and a fundamental obstacle to private-supply risk-management and decision-making (Bradley et al., 2021a; Bradley et al., 2021b; Zheng and Flanagan, 2017). The current results address a critical lack of directly comparable data on broad contaminant exposures in TW in agriculturally-developed settings and document commonplace simultaneous exposures to inorganic, organic, and microbial contaminants of human-health concern. Based on these one-time spatial synoptic results, further spatial coverage and, importantly, assessment of temporal variability are warranted to more fully characterize POU exposures. The results indicate that incorporation of well-maintained, residential treatment systems could substantially protect against unrecognized contaminant exposures in private-well homes, including in agriculturally developed areas. Several POU (and point-of-entry) treatment technologies are effective in reducing TW exposures to many of the contaminants identified in this study (Wu et al., 2021). However, given the common-place simultaneous exposures to multiple inorganic, organic, and microbial contaminants in the study area, broadly effective single-stage treatment technologies, such as RO, or multi-stage/multi-filtration systems (sediment filter, redox media, activated carbon, ion exchange, RO, UV disinfection, etc) warrant consideration. More broadly, the results corroborate the importance of continued systematic, quantitative assessments of contaminant exposures and associated bioactivities in TW, especially in unregulated and unmonitored locations (Baken et al., 2018; Braun and Gray, 2017; Gross and Birnbaum, 2017; Lanphear, 2017; Schriks et al., 2010), to support models of drinking-water contaminant exposures and related risks at the point of use.
Supplementary Material
Highlights.
Private-well tapwater contaminant exposures are a global public-health data gap
47 home tapwaters assessed in hydrologically-vulnerable ag-intensive northeast Iowa
437 organics/35 inorganics/11 microbial indicators/5 bioactivities analyzed
51 organics (primarily pesticides)/26 inorganics/microbial indicators detected
Common exceedances of human risk screening level indicate increased monitoring need
Acknowledgments
We thank the Agricultural Health Study participants for their support of this drinking-water research. Researchers would also like to acknowledge Amy Miller, Kate Torres, Sarah Woodruff, Himanshi Singh, and Marsha Dunn (Westat, Rockville, Maryland) for study coordination and data management, Abigail Flory (Westat, Rockville, Maryland) and Bernard T. Nolan (USGS) for land use and hydrogeological metrics, and the State Hygienic Laboratory at the University of Iowa for neonicotinoid analysis. This research was conducted and funded by the Environmental Health Program of the USGS Ecosystems Mission Area, University of Iowa Center for Health Effects of Environmental Contamination, Iowa Institute of Public Health Research and Policy, NIOSH-funded Heartland Center for Occupational Health and Safety (Training Grant No. T42OH008491), and NIEHS-funded Environmental Health Sciences Research Center (Grant No. P30 ES005605). Support for the Biomarkers of Exposure and Effects in Agriculture (BEEA) study, including the selection and outreach to potentially eligible participants, was provided by the Intramural Research Program of the National Institutes of Health, National Cancer Institute (Z01 CP 010119). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The findings and conclusions in this article do not necessarily represent the views or policies of the US Environmental Protection Agency, National Institute of Environmental Health Sciences, or National Cancer Institute. This report contains CAS Registry Numbers, which is a registered trademark of the American Chemical Society. CAS recommends the verification of the CASRNs through CAS Client ServicesSM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRedIT
Paul Bradley: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing - Original Draft, Writing - Review & Editing. Dana Kolpin: Conceptualization, Methodology, Project administration, Writing - Review & Editing. Darrin Thompson: Conceptualization, Methodology, Project administration, Writing - Review & Editing. Kristin Romanok: Data curation, Formal analysis, Investigation, Visualization, Writing - Review & Editing. Kelly Smalling: Formal analysis, Visualization, Writing – Review & Editing. Sara Breitmeyer: Visualization. Mary Cardon: Investigation. David Cwiertny: Writing – Review & Editing. Nicola Evans: Investigation, Writing – Review & Editing. William Field: Writing – Review & Editing. Michael Focazio: Writing – Review & Editing. Laura Beane Freeman: Writing – Review & Editing. Carrie Givens: Investigation, Writing – Review & Editing. James Gray: Investigation, Writing – Review & Editing. Gordon Hager: Writing – Review & Editing. Michelle Hladik: Investigation, Writing – Review & Editing. Jonathan Hofmann: Writing – Review & Editing. Rena Jones: Writing – Review & Editing. Leslie Kanagy: Investigation. Rachael Lane: Investigation, Writing – Review & Editing. Blaine McCleskey: Investigation, Writing – Review & Editing. Danielle Medgyesi: Writing – Review & Editing. Elizabeth Medlock-Kakaley: Formal analysis, Investigation, Writing – Review & Editing. Shannon Meppelink: Data curation, Investigation, Writing - Review & Editing. Michael Meyer: Investigation. Diana Stavreva: Investigation, Writing – Review & Editing. Mary Ward: Writing – Review & Editing.
Data availability
The associated data are published in a USGS data release (Meppelink et al., 2023) at https://doi.org/10.5066/P9IYT37H.
References
- Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, et al. The Agricultural Health Study. Environmental Health Perspectives 1996; 104: 362–369. 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenburger R, Scholze M, Busch W, Escher BI, Jakobs G, Krauss M, et al. Mixture effects in samples of multiple contaminants–An inter-laboratory study with manifold bioassays. Environment international 2018; 114: 95–106. 10.1016/j.envint.2018.02.013. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics. Policy statement: Drinking water from private wells and risks to children. Pediatrics 2009; 123: 1599–1605. 10.1542/peds.2009-0751. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics: Committee on Nutrition. Fluoride supplementation for children: Interim policy recommendations. Pediatrics 1995; 95: 777–777. 10.1542/peds.95.5.777. [DOI] [PubMed] [Google Scholar]
- Aschebrook-Kilfoy B, Heltshe SL, Nuckols JR, Sabra MM, Shuldiner AR, Mitchell BD, et al. Modeled nitrate levels in well water supplies and prevalence of abnormal thyroid conditions among the Old Order Amish in Pennsylvania. Environmental Health 2012; 11: 6. 10.1186/1476-069X-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbolt NJ, Amézquita A, Backhaus T, Borriello P, Brandt KK, Collignon P, et al. Human Health Risk Assessment (HHRA) for environmental development and transfer of antibiotic resistance. Environmental Health Perspectives 2013; 121: 993–1001. 10.1289/ehp.1206316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus T Environmental Risk Assessment of Pharmaceutical Mixtures: Demands, Gaps, and Possible Bridges. The AAPS Journal 2016; 18: 804–813. 10.1208/s12248-016-9907-0. [DOI] [PubMed] [Google Scholar]
- Baken KA, Sjerps RMA, Schriks M, van Wezel AP Toxicological risk assessment and prioritization of drinking water relevant contaminants of emerging concern. Environment International 2018; 118: 293–303. 10.1016/j.envint.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Blackwell BR, Ankley GT, Corsi SR, De Cicco LA, Houck KA, Judson RS, et al. An” EAR” on environmental surveillance and monitoring: A case study on the use of exposure-activity ratios (EARs) to prioritize sites, chemicals, and bioactivities of concern in Great Lakes waters. Environmental Science & Technology 2017; 51: 8713–8724. 10.1021/acs.est.7b01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy SC, Campbell A Water quality and brain function. International Journal of Environmental Research and Public Health 2018; 15. 10.3390/ijerph15010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt MA, Stokdyk JP, Kieke BA, Muldoon MA, Spencer SK, Firnstahl AD, et al. Sources and risk factors for nitrate and microbial contamination of private household wells in the fractured dolomite aquifer of northeastern Wisconsin. Environmental Health Perspectives 2021; 129: 067004. 10.1289/EHP7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Argos M, Kolpin DW, Meppelink SM, Romanok KM, Smalling KL, et al. Mixed organic and inorganic tapwater exposures and potential effects in greater Chicago area, USA. Science of The Total Environment 2020; 719: 137236. 10.1016/j.scitotenv.2020.137236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Journey C, Romanok K, Barber L, Buxton HT, Foreman WT, et al. Expanded target-chemical analysis reveals extensive mixed-organic-contaminant exposure in USA streams. Environmental Science & Technology 2017; 51: 4792–4802. 10.1021/acs.est.7b00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Kolpin DW, Romanok KM, Smalling KL, Focazio MJ, Brown JB, et al. Reconnaissance of mixed organic and inorganic chemicals in private and public supply tapwaters at selected residential and workplace sites in the United States. Environmental Science & Technology 2018; 52: 13972–13985. 10.1021/acs.est.8b04622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, LeBlanc DR, Romanok KM, Smalling KL, Focazio MJ, Cardon MC, et al. Public and private tapwater: Comparative analysis of contaminant exposure and potential risk, Cape Cod, Massachusetts, USA. Environment International 2021a; 152: 106487. 10.1016/j.envint.2021.106487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Padilla IY, Romanok KM, Smalling KL, Focazio MJ, Breitmeyer SE, et al. Pilot-scale expanded assessment of inorganic and organic tapwater exposures and predicted effects in Puerto Rico, USA. Science of The Total Environment 2021b; 788: 147721. 10.1016/j.scitotenv.2021.147721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Romanok KM, Smalling KL, Focazio MJ, Charboneau R, George CM, et al. Tapwater exposures, effects potential, and residential risk management in northern plains nations. Environmental Science and Technology Water 2022; 2: 1772–1788. 10.1021/acsestwater.2c00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Romanok KM, Smalling KL, Focazio MJ, Evans N, Fitzpatrick SC, et al. Bottled water contaminant exposures and potential human effects. Environment International 2023; 171: 107701. 10.1016/j.envint.2022.107701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand W, de Jongh CM, van der Linden SC, Mennes W, Puijker LM, van Leeuwen CJ, et al. Trigger values for investigation of hormonal activity in drinking water and its sources using CALUX bioassays. Environment International 2013; 55: 109–118. 10.1016/j.envint.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Braun JM, Gray K Challenges to studying the health effects of early life environmental chemical exposures on children’s health. PLOS Biology 2017; 15: e2002800. 10.1371/journal.pbio.2002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender JD, Weyer PJ, Romitti PA, Mohanty BP, Shinde MU, Vuong AM, et al. Prenatal nitrate intake from drinking water and selected birth defects in offspring of participants in the National Birth Defects Prevention Study. Environmental Health Perspectives 2013; 121: 1083–1089. 10.1289/ehp.1206249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch TR, Stokdyk JP, Spencer SK, Kieke BA, Firnstahl AD, Muldoon MA, et al. Quantitative microbial risk assessment for contaminated private wells in the fractured dolomite aquifer of Kewaunee County, Wisconsin. Environmental Health Perspectives 2021; 129: 067003. 10.1289/EHP7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedergreen N, Christensen AM, Kamper A, Kudsk P, Mathiassen SK, Streibig JC, et al. A review of independent action compared to concentration addition as reference models for mixtures of compounds with different molecular target sites. Environmental Toxicology and Chemistry 2008; 27: 1621–1632. 10.1897/07-474.1. [DOI] [PubMed] [Google Scholar]
- Charrois JWA Private drinking water supplies: Challenges for public health. Canadian Medical Association Journal 2010; 182: 1061–1064. 10.1503/cmaj.090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee-Sanford JC, Aminov RI, Krapac IJ, Garrigues-Jeanjean N, Mackie RI Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Applied and Environmental Microbiology 2001; 67: 1494–1502. 10.1128/AEM.67.4.1494-1502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress C, Foreman W, Conner B, Maloney T New reporting procedures based on long-term method detection levels and some considerations for interpretations of water-quality data provided by the U.S. Geological Survey National Water Quality Laboratory. U.S. Geological Survey Open-File Report 99–193 1999; 19 p. 10.3133/ofr99193. [DOI] [Google Scholar]
- Cimino AM, Boyles AL, Thayer KA, Perry MJ Effects of neonicotinoid pesticide exposure on human health: A systematic review. Environmental Health Perspectives 2017; 125: 155–162. 10.1289/EHP515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier SA, Deng L, Adam EA, Benedict KM, Beshearse EM, Blackstock AJ, et al. Estimate of burden and direct healthcare cost of infectious waterborne disease in the United States. Emerging Infectious Diseases, 2021; 27: 140–149. 10.3201/eid2701.190676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KL, Dashner EJ, Tsosie R, Cho YM, Lewis J, Hudson LG Inhibition of poly(ADP-ribose)polymerase-1 and DNA repair by uranium. Toxicology and Applied Pharmacology 2016; 291: 13–20. 10.1016/j.taap.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi SR, De Cicco LA, Villeneuve DL, Blackwell BR, Fay KA, Ankley GT, et al. Prioritizing chemicals of ecological concern in Great Lakes tributaries using high-throughput screening data and adverse outcome pathways. Science of the Total Environment 2019; 686: 995–1009. 10.1016/j.scitotenv.2019.05.457. [DOI] [PubMed] [Google Scholar]
- Craun GF, Brunkard JM, Yoder JS, Roberts VA, Carpenter J, Wade T, et al. Causes of outbreaks associated with drinking water in the United States from 1971 to 2006. Clinical Microbiology Reviews 2010; 23: 507–528. https://doilorg/10.1128/CMR.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D, Prussin AJ, Marr LC, Vikesland PJ, Edwards MA, Pruden A Factors shaping the human exposome in the built environment: opportunities for engineering control. Environmental Science & Technology 2017; 51: 7759–7774. 10.1021/acs.est.7b01097. [DOI] [PubMed] [Google Scholar]
- De Cicco L, Corsi SR, Villeneuve D, Blackwell BR, Ankley GT toxEval: Evaluation of measured concentration data using the ToxCast high-throughput screening database or a user-defined set of concentration benchmarks. R package version 1.0.0 2018. accessed May 1, 2018 https://owi.usgs.gov/R/gran.html [Google Scholar]
- DeSimone LA, McMahon PB, Rosen MR The quality of our Nation’s waters: water quality in Principal Aquifers of the United States, 1991–2010. U. S. Geological Survey Circular 1360 2015; 161 p. 10.3133/cir1360. [DOI] [Google Scholar]
- Dieter CA, Maupin MA, Caldwell RR, Harris MA, Ivahnenko TI, Lovelace JK, et al. Estimated use of water in the United States in 2015. U. S. Geological Survey Circular 1441 2018; 76 p. 10.3133/cir1441. [DOI] [Google Scholar]
- Douglas MR, Tooker JF Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in U.S. field crops. Environmental Science & Technology 2015; 49: 5088–5097. 10.1021/es506141g. [DOI] [PubMed] [Google Scholar]
- Eng S-K, Pusparajah P, Ab Mutalib N-S, Ser H-L, Chan K-G, Lee L-H Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Frontiers in Life Science 2015; 8: 284–293. 10.1080/21553769.2015.1051243. [DOI] [Google Scholar]
- Ermler S, Scholze M, Kortenkamp A The suitability of concentration addition for predicting the effects of multi-component mixtures of up to 17 anti-androgens with varied structural features in an in vitro AR antagonist assay. Toxicology and Applied Pharmacology 2011; 257: 189–197. 10.1016/j.taap.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Evelsizer V, Skopec M Pesticides, Including Neonicotinoids, in Drained Wetlands of Iowa’s Prairie Pothole Region. Wetlands 2018; 38: 221–232. 10.1007/s13157-016-0796-x. [DOI] [Google Scholar]
- Filer DL, Kothiya P, Setzer RW, Judson RS, Martin MT tcpl: the ToxCast pipeline for high-throughput screening data. Bioinformatics 2017; 33: 618–620. 10.1093/bioinformatics/btw680. [DOI] [PubMed] [Google Scholar]
- Focazio MJ, Tipton D, Dunkle SS, Geiger LH The chemical quality of self-supplied domestic well water in the United States. Ground Water Monitoring and Remediation 2006; 26: 92–104. 10.1111/j.1745-6592.2006.00089.x. [DOI] [Google Scholar]
- Foreman WT, Williams TL, Furlong ET, Hemmerle DM, Stetson SJ, Jha VK, et al. Comparison of detection limits estimated using single- and multi-concentration spike-based and blank-based procedures. Talanta 2021; 228: 122139. 10.1016/j.talanta.2021.122139. [DOI] [PubMed] [Google Scholar]
- Givens CE, Kolpin DW, Borchardt MA, Duris JW, Moorman TB, Spencer SK Detection of hepatitis E virus and other livestock-related pathogens in Iowa streams. Science of The Total Environment 2016; 566–567: 1042–1051. 10.1016/j.scitotenv.2016.05.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassmeyer ST, Furlong ET, Kolpin DW, Batt AL, Benson R, Boone JS, et al. Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States. Science of the Total Environment 2017; 581–582: 909–922. 10.1016/j.scitotenv.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumenou M, Tsatsakis A Proposing new approaches for the risk characterisation of single chemicals and chemical mixtures: The source related Hazard Quotient (HQS) and Hazard Index (HIS) and the adversity specific Hazard Index (HIA). Toxicology Reports 2019; 6: 632–636. 10.1016/j.toxrep.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross L, Birnbaum LS Regulating toxic chemicals for public and environmental health. PLOS Biology 2017; 15: e2004814. 10.1371/journal.pbio.2004814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer Ø, Harper DA, Ryan PD PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 2001; 4: 9. https://palaeo-electronica.org/2001_1/past/issue1_01.htm. [Google Scholar]
- Hladik ML, Calhoun DL Analysis of the herbicide diuron, three diuron degradates, and six neonicotinoid insecticides in water–method details and application to two Georgia streams. U.S. Geological Survey Scientific Investigations Report 2012–5206 2012; 9 p. 10.3133/sir20125206. [DOI] [Google Scholar]
- Hofmann JN, Beane Freeman LE, Lynch CF, Andreotti G, Thomas KW, Sandler DP, et al. The Biomarkers of Exposure and Effect in Agriculture (BEEA) Study: Rationale, design, methods, and participant characteristics. Journal of Toxicology and Environmental Health, Part A 2015; 78: 1338–1347. 10.1080/15287394.2015.1091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interstate Technology Regulatory Council. PFAS — Per- and Polyfluoroalkyl Substances. 2022. accessed March 30, 2022
- Iowa Department of Natural Resources. 2021 Animal Feeding Operations that are registered, permitted, or monitored by the Iowa Department of Natural Resources, 2021. https://services1.arcgis.com/ZIL9uO234SBBPGL7/arcgis/rest/services/Animal_Feeding_Operations/FeatureServer.
- Jones RR, Stavreva DA, Weyer PJ, Varticovski L, Inoue-Choi M, Medgyesi DN, et al. Pilot study of global endocrine disrupting activity in Iowa public drinking water utilities using cell-based assays. Science of The Total Environment 2020; 714: 136317. 10.1016/j.scitotenv.2019.136317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RR, Weyer PJ, DellaValle CT, Inoue-Choi M, Anderson KE, Cantor KP, et al. Nitrate from drinking water and diet and bladder cancer among postmenopausal women in Iowa. Environ Health Perspect 2016; 124: 1751–1758. 10.1289/EHP191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RR, Weyer PJ, DellaValle CT, Robien K, Cantor KP, Krasner S, et al. Ingested nitrate, disinfection by-products, and kidney cancer risk in older women. Epidemiology 2017; 28: 703–711. 10.1097/EDE.0000000000000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn WG, Maas WR, Malvitz DM, Presson SM, Shaddix KK Recommendations for using fluoride to prevent and control dental caries in the United States. 2001. https://stacks.cdc.gov/view/cdc/5160.
- Kolpin DW, Burkart MR, Thurman EM Herbicides and nitrate in near-surface aquifers in the midcontinental United States, 1991. U.S. Geological Survey Water-Supply Paper 2413 1994; p. 10.3133/wsp2413. http://pubs.er.usgs.gov/publication/wsp2413 [DOI] [Google Scholar]
- Kolpin DW, Thurman EM, Linhart SM Finding minimal herbicide concentrations in ground water? Try looking for their degradates. Science of The Total Environment 2000; 248: 115–122. 10.1016/S0048-9697(99)00535-5. [DOI] [PubMed] [Google Scholar]
- Kurttio P, Komulainen H, Leino A, Salonen L, Auvinen A, Saha H Bone as a possible target of chemical toxicity of natural uranium in drinking water. Environmental health perspectives 2005; 113: 68. 10.1289/ehp.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear B, Lowry J, Ahdoot S, Baum C, Bernstein A, Bole A, et al. Prevention of childhood lead toxicity: Policy statement of the American Academy of Pediatrics Council on Environmental Health. Pediatrics 2016; 138: e20161493. 10.1542/peds.2016-1493. [DOI] [PubMed] [Google Scholar]
- Lanphear BP Low-level toxicity of chemicals: No acceptable levels? PLoS Biology 2017; 15: e2003066. 10.1371/journal.pbio.2003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance—the need for global solutions. The Lancet infectious diseases 2013; 13: 1057–1098. 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- Lombard MA, Scannell Bryan M, Jones DK, Bulka C, Bradley P, Backer L, et al. Machine learning models of arsenic in private wells throughout the conterminous United States as a tool for exposure assessment in human health studies. Environmental Science & Technology 2021. 10.1021/acs.est.0c05239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald Gibson J, Pieper K Strategies to improve private-well water quality: A North Carolina perspective. Environmental health perspectives 2017; 125: 076001–076001. 10.1289/EHP890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdo HS, Forman J, Graber N, Newman B, Klein K, Satlin L, et al. Grand rounds: Nephrotoxicity in a young child exposed to uranium from contaminated well water. Environmental Health Perspectives 2007; 115: 1237. 10.1289/ehp.9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon PB, Brown CJ, Johnson TD, Belitz K, Lindsey BD Fluoride occurrence in United States groundwater. Science of The Total Environment 2020; 732: 139217. 10.1016/j.scitotenv.2020.139217. [DOI] [PubMed] [Google Scholar]
- Medlock Kakaley EK, Blackwell BR, Cardon MC, Conley JM, Evans N, Feifarek DJ, et al. De facto water reuse: Bioassay suite approach delivers depth and breadth in endocrine active compound detection. Science of the Total Environment 2020; 699: 134297. 10.1016/j.scitotenv.2019.134297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock Kakaley EK, Cardon MC, Evans N, Iwanowicz LR, Allen JM, Wagner E, et al. In vitro effects-based method and water quality screening model for use in pre- and post-distribution treated waters. Environmental Science and Technology 2021. 10.1016/j.scitotenv.2020.144750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock-Kakaley E, Cardon MC, Gray LE, Hartig PC, Wilson VS Generalized concentration addition model predicts glucocorticoid activity bioassay responses to environmentally detected receptor-ligand mixtures. Toxicological Sciences 2018; 168: 252–263. 10.1093/toxsci/kfy290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meppelink SM, Bradley PM, Romanok KM, Kolpin DW Target-chemical concentrations, exposure activity ratios, and bioassay results for assessment of mixed-organic/inorganic chemical exposures in northeast Iowa private-well tapwater, 2018–19. U.S. Geological Survey data release 10.5066/P9IYT37H. 2023; p., [DOI] [Google Scholar]
- Moretto A, Bachman A, Boobis A, Solomon KR, Pastoor TP, Wilks MF, et al. A framework for cumulative risk assessment in the 21st century. Critical Reviews in Toxicology 2017; 47: 85–97. 10.1080/10408444.2016.1211618. [DOI] [PubMed] [Google Scholar]
- Moschet C, Wittmer I, Simovic J, Junghans M, Piazzoli A, Singer H, et al. How a complete pesticide screening changes the assessment of surface water quality. Environmental Science & Technology 2014; 48: 5423–5432. 10.1021/es500371t. [DOI] [PubMed] [Google Scholar]
- Mueller DK, Schertz TL, Martin JD, Sandstrom MW Design, analysis, and interpretation of field quality-control data for water-sampling projects. U.S. Geological Survey Techniques and Methods Book 4 Chapter C4 2015; 54 p. 10.3133/tm4C4. [DOI] [Google Scholar]
- National Research Council. Risk Assessment in the Federal Government: Managing the Process. Washington, DC: The National Academies Press, 1983. 10.17226/366. [DOI] [PubMed] [Google Scholar]
- Nigra AE Environmental racism and the need for private well protections. Proceedings of the National Academy of Sciences 2020; 117: 17476–17478. 10.1073/pnas.2011547117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JE, Toccalino PL, Morman SA Health-Based Screening Levels for evaluating water-quality data (2nd ed.). 2018. accessed February 10, 2020 [Google Scholar]
- Paul Friedman K, Gagne M, Loo L-H, Karamertzanis P, Netzeva T, Sobanski T, et al. Utility of in vitro bioactivity as a lower bound estimate of in vivo adverse effect levels and in risk-based prioritization. Toxicological Sciences 2020; 173: 202–225. 10.1093/toxsci/kfz201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist AJL, Inoue-Choi M, Weyer PJ, Anderson KE, Cantor KP, Krasner S, et al. Ingested nitrate and nitrite, disinfection by-products, and pancreatic cancer risk in postmenopausal women. International Journal of Cancer 2018; 142: 251–261. 10.1002/ijc.31055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Version 3.5.2 2019; p., https://www.R-project.orghttps://www.R-project.org
- Raymond-Whish S, Mayer LP, O’Neal T, Martinez A, Sellers MA, Christian PJ, et al. Drinking water with uranium below the US EPA water standard causes estrogen receptor–dependent responses in female mice. Environmental health perspectives 2007; 115: 1711. 10.1289/ehp.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard AM, Judson RS, Houck KA, Grulke CM, Volarath P, Thillainadarajah I, et al. ToxCast chemical landscape: paving the road to 21st century toxicology. Chemical Research in Toxicology 2016; 29: 1225–1251. 10.1021/acs.chemrestox.6b00135. [DOI] [PubMed] [Google Scholar]
- Rogan WJ, Brady MT Drinking water from private wells and risks to children. Pediatrics 2009; 123: e1123–e1137. 10.1542/peds.2009-0752. [DOI] [PubMed] [Google Scholar]
- Romanok KM, Kolpin DW, Meppelink SM, Argos M, Brown J, DeVito M, et al. Methods used for the collection and analysis of chemical and biological data for the Tapwater Exposure Study, United States, 2016–17. U.S. Geological Survey Open-File Report 2018–1098 2018; 81 p. 10.3133/ofr20181098. [DOI] [Google Scholar]
- Schaider LA, Ackerman JM, Rudel RA Septic systems as sources of organic wastewater compounds in domestic drinking water wells in a shallow sand and gravel aquifer. Science of the Total Environment 2016; 547: 470–481. 10.1016/j.scitotenv.2015.12.081. [DOI] [PubMed] [Google Scholar]
- Schaider LA, Rudel RA, Ackerman JM, Dunagan SC, Brody JG Pharmaceuticals, perfluorosurfactants, and other organic wastewater compounds in public drinking water wells in a shallow sand and gravel aquifer. Science of the Total Environment 2014; 468: 384–393. 10.1016/j.scitotenv.2013.08.067. [DOI] [PubMed] [Google Scholar]
- Schriks M, Heringa MB, van der Kooi MME, de Voogt P, van Wezel AP Toxicological relevance of emerging contaminants for drinking water quality. Water Research 2010; 44: 461–476. 10.1016/j.watres.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Schroeder AL, Ankley GT, Houck KA, Villeneuve DL Environmental surveillance and monitoring—The next frontiers for high- throughput toxicology. Environmental Toxicology and Chemistry 2016; 35: 513–525. 10.1002/etc.3309. [DOI] [PubMed] [Google Scholar]
- Seldén AI, Lundholm C, Edlund B, Högdahl C, Ek B-M, Bergström BE, et al. Nephrotoxicity of uranium in drinking water from private drilled wells. Environmental Research 2009; 109: 486–494. 10.1016/j.envres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Seltenrich N Unwell: The public health implications of unregulated drinking water. Environmental health perspectives 2017; 125: 114001–114001. 10.1289/EHP2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurnjak Bureš M, Cvetnić M, Miloloža M, Kučić Grgić D, Markić M, Kušić H, et al. Modeling the toxicity of pollutants mixtures for risk assessment: a review. Environmental Chemistry Letters 2021. 10.1007/s10311-020-01107-5. [DOI] [Google Scholar]
- Silva J, Leite D, Fernandes M, Mena C, Gibbs P, Teixeira P Campylobacter spp. as a foodborne pathogen: A review. Frontiers in Microbiology 2011; 2. 10.3389/fmicb.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalter D, O’Malley E, von Gunten U, Escher BI Mixture effects of drinking water disinfection by-products: implications for risk assessment. Environmental Science: Water Research and Technology 2020; 6: 2341–2351. 10.1039/C9EW00988D. [DOI] [Google Scholar]
- Stavreva DA, George AA, Klausmeyer P, Varticovski L, Sack D, Voss TC, et al. Prevalent glucocorticoid and androgen activity in US water sources. Scientific Reports 2012; 2: 937. 10.1038/srep00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavreva DA, Varticovski L, Levkova L, George AA, Davis L, Pegoraro G, et al. Novel cell-based assay for detection of thyroid receptor beta-interacting environmental contaminants. Toxicology 2016; 368–369: 69–79. 10.1016/j.tox.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DA, Kolpin DW, Hladik ML, Barnes KK, Vargo JD, Field RW Prevalence of neonicotinoids and sulfoxaflor in alluvial aquifers in a high corn and soybean producing region of the Midwestern United States. Science of The Total Environment 2021; 782: 146762. 10.1016/j.scitotenv.2021.146762. [DOI] [Google Scholar]
- Thompson DA, Lehmler H-J, Kolpin DW, Hladik ML, Vargo JD, Schilling KE, et al. A critical review on the potential impacts of neonicotinoid insecticide use: current knowledge of environmental fate, toxicity, and implications for human health. Environmental Science Processes & Impacts 2020; 22: 1315–1346. 10.1039/c9em00586b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooker JF, Douglas MR, Krupke CH Neonicotinoid seed treatments: limitations and compatibility with integrated pest management. Agricultural & Environmental Letters 2017; 2: ael2017.08.0026. 10.2134/ael2017.08.0026. [DOI] [Google Scholar]
- U.S. Environmental Protection Agency. Lead and Copper Rule: A quick reference guide. 2008. accessed March 3, 2022 https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=60001N8P.txt
- U.S. Environmental Protection Agency. EPA’s National-scale Air Toxics Assessment, An Overview of Methods for EPA’s National-Scale Air Toxics Assessment. 2011; p. https://www.epa.gov/sites/production/files/2015-10/documents/2005-nata-tmd.pdf.
- U.S. Environmental Protection Agency. Sustainable Futures / Pollution Prevention (P2) Framework Manual. EPA-748-B12–001. 2012; 326 p. https://www.epa.gov/sustainable-futures/sustainable-futures-p2-framework-manual.
- U.S. Environmental Protection Agency. Literature review of contaminants in livestock and poultry manure and implications for water quality. EPA 820-R-13–002. 2013; 137 p. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100H2NI.txt.
- U.S. Environmental Protection Agency. 2018 Edition of the Drinking Water Standards and Health Advisories. EPA 822-F-18–001. 2018; 20 p. https://www.epa.gov/sites/production/files/2018-03/documents/dwtable2018.pdf. [Google Scholar]
- U.S. Environmental Protection Agency. 40 C.F.R. § 136: Guidelines establishing test procedures for the analysis of pollutants. 40 C.F.R. § 136. U.S Environmental Protection Agency, Washington, DC, 2020a, pp. 319–322. http://www.ecfr.gov/cgi-bin/text-idx?SID=3c78b6c8952e5e79268e429ed98bad84&mc=true&node=pt40.23.136&rgn=div5. [Google Scholar]
- U.S. Environmental Protection Agency. 40 C.F.R. § 141 and § 142: National Primary Drinking Water Regulations: Lead and Copper Rule Revisions - Pre-Publication Version. 40 C.F.R. § 141/142. U.S Environmmental Protection Agency,, Washington, DC, 2020b. https://www.regulations.gov/)https://www.regulations.gov/). [Google Scholar]
- U.S. Environmental Protection Agency. How EPA Regulates Drinking Water Contaminants. 2021a. accessed July 11, 2021
- U.S. Environmental Protection Agency. Human Health Benchmarks for Pesticides (HHBPs). 2021b. accessed December 17, 2021
- U.S. Environmental Protection Agency. Private drinking water wells. 2021c. accessed March 30, 2021
- U.S. Environmental Protection Agency. National Primary Drinking Water Regulations. 2023. accessed January 9, 2023
- U.S. Environmental Protection Agency National Center for Computational Toxicology. ToxCast Database InvitroDBv3.1 2019. accessed, at 10.23645/epacomptox.6062623.v3 [DOI]
- U.S. Environmental Protection Agency National Center for Computational Toxicology. ToxCast Database invitroDBv3.3 2020. accessed, at 10.23645/epacomptox.6062623.v5 [DOI]
- U.S. Geological Survey. Alluvial and Glacial Aquifers. 2002. accessed, at https://water.usgs.gov/lookup/getspatial?alluvial_and_glacial_aquifers
- U.S. Geological Survey. National Land Cover Database (NLCD) 2019 Edition, 2021. 10.5066/P9KZCM54. [DOI]
- U.S. Public Health Service. U.S. Public Health Service recommendation for fluoride concentration in drinking water for the prevention of dental caries. Public Health Reports 2015; 130: 318–331. 10.1177/003335491513000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Walker GW, Muir DCG, Nagatani-Yoshida K Toward a global understanding of chemical pollution: a first comprehensive analysis of national and regional chemical inventories. Environmental Science & Technology 2020; 54: 2575–2584. 10.1021/acs.est.9b06379. [DOI] [PubMed] [Google Scholar]
- Ward MH, deKok TM, Levallois P, Brender J, Gulis G, Nolan BT, et al. Workgroup report: Drinking-water nitrate and health - recent findings and research needs. Environmental Health Perspectives 2005; 113: 1607–1614. 10.1289/ehp.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MH, Jones RR, Brender JD, De Kok TM, Weyer PJ, Nolan BT, et al. Drinking water nitrate and human health: an updated review. International journal of environmental research and public health 2018; 15: 1557. 10.3390/ijerph15071557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO). Guidelines for drinking-water quality, Fourth edition incorporating the first addendum, 2011. https://apps.who.int/iris/rest/bitstreams/1080656/retrieve. [PubMed]
- Wu J, Cao M, Tong D, Finkelstein Z, Hoek EMV A critical review of point-of-use drinking water treatment in the United States. npj Clean Water 2021; 4: 40. 10.1038/s41545-021-00128-z. [DOI] [Google Scholar]
- Zheng Y, Flanagan SV The case for universal screening of private well water quality in the U.S. and testing requirements to achieve it: Evidence from arsenic. Environmental Health Perspectives 2017; 125: 085002. https://doi.org/0.1289/EHP629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The associated data are published in a USGS data release (Meppelink et al., 2023) at https://doi.org/10.5066/P9IYT37H.


