Abstract
Animal brains evolved to optimize behavior in dynamic environments, flexibly selecting actions that maximize future rewards in different contexts. A large body of experimental work indicates that such optimization changes the wiring of neural circuits, appropriately mapping environmental input onto behavioral outputs. A major unsolved scientific question is how optimal wiring adjustments, which must target the connections responsible for rewards, can be accomplished when the relation between sensory inputs, action taken, and environmental context with rewards is ambiguous. The credit assignment problem can be categorized into context-independent structural credit assignment and context-dependent continual learning. In this perspective, we survey prior approaches to these two problems and advance the notion that the brain’s specialized neural architectures provide efficient solutions. Within this framework, the thalamus with its cortical and basal ganglia interactions serves as a systems-level solution to credit assignment. Specifically, we propose that thalamocortical interaction is the locus of meta-learning where the thalamus provides cortical control functions that parametrize the cortical activity association space. By selecting among these control functions, the basal ganglia hierarchically guide thalamocortical plasticity across two timescales to enable meta-learning. The faster timescale establishes contextual associations to enable behavioral flexibility, while the slower one enables generalization to new contexts.
Keywords: Meta-learning, Credit assignment, Continual learning, Thalamocortical interactions, Basal ganglia, Thalamus
Author Summary
Deep learning has shown great promise over the last decades, allowing artificial neural networks to solve difficult tasks. The key to success is the optimization process by which task errors are translated to connectivity patterns. A major unsolved question is how the brain optimally adjusts the wiring of neural circuits to minimize task error analogously. In our perspective, we advance the notion that the brain’s specialized architecture is part of the solution and spell out a path towards its theoretical, computational, and experimental testing. Specifically, we propose that the interaction between the cortex, thalamus, and basal ganglia induces plasticity in two timescales to enable flexible behaviors. The faster timescale establishes contextual associations to enable behavioral flexibility, while the slower one enables generalization to new contexts.
INTRODUCTION
Learning to flexibly choose appropriate actions in uncertain environments is a hallmark of intelligence (Miller & Cohen, 2001; Niv, 2009; Thorndike, 2017). When animals explore unfamiliar environments, they tend to reinforce actions that lead to unexpected rewards. A common notion in contemporary neuroscience is that such behavioral reinforcement emerges from changes in synaptic connectivity, where synapses that contribute to the unexpected reward are strengthened (Abbott & Nelson, 2000; Bliss & Lomo, 1973; Dayan & Abbott, 2005; Hebb, 2002; Whittington & Bogacz, 2019). A prominent model for connecting synaptic to behavioral reinforcement is dopaminergic innervation of basal ganglia (BG), where dopamine (DA) carries the reward prediction error (RPE) signals to guide synaptic learning (Bamford, Wightman, & Sulzer, 2018; Bayer & Glimcher, 2005; Montague, Dayan, & Sejnowski, 1996; Schultz, Dayan, & Montague, 1997). This circuit motif is thought to implement a basic form of the reinforcement learning algorithm (Houk, Davis, & Beiser, 1994; Morris, Nevet, Arkadir, Vaadia, & Bergman, 2006; Roesch, Calu, & Schoenbaum, 2007; Suri & Schultz, 1999; R. Sutton & Barto, 2018; R. S. Sutton & Barto, 1990; Wickens & Kotter, 1994), which has had much success in explaining simple Pavlovian and instrumental conditioning (Ikemoto & Panksepp, 1999; Niv, 2009; R. Sutton & Barto, 2018; R. S. Sutton & Barto, 1990). However, it is unclear how this circuit can reinforce the appropriate connections in complex natural environments where animals need to dynamically map sensory inputs to different action in a context-dependent way. If one naively credits all synapses with the RPE signals, the learning will be highly inefficient since different cues, contexts, and actions contribute to the RPE signals differently. To properly credit the cues, context, and actions that lead to unexpected reward is a challenging problem, known as the credit assignment problem (Lillicrap, Santoro, Marris, Akerman, & Hinton, 2020; Minsky, 1961; Rumelhart, Hinton, & Williams, 1986; Whittington & Bogacz, 2019).
One can roughly categorize the credit assignment into context-independent structural credit assignment and context-dependent continual learning. In structural credit assignment, animals may make decisions in a multi-cue environment and should be able to credit those cues that contribute to the rewarding outcome. Similarly, if actions are being chosen based on internal decision variables, then the underlying activity states must also be reinforced. In such cases, neurons that are selective to external cues or internal latent variables need to adjust their downstream connectivity based on its contribution of their downstream targets to the RPE. This is a challenging computation to implement because, for upstream neurons, the RPE will be dependent on downstream neurons that are several connections away. For example, a sensory neuron needs to know the action chosen in the motor cortex to selectively credit the sensory synapses that contribute to the action. In continual learning, animals not only need to appropriately credit the sensory cues and actions that lead to the reward but also need to credit the sensorimotor combination in the right context to retain the behaviors learned from different contexts and even to generalize to novel contexts. Therefore, animals can continually learn and generalize across different contexts while retaining behaviors in familiar contexts. For example, when one is in the United States, one learns to first look left before crossing the street, whereas in the United Kingdom, one learns to look right instead. However, after spending time in the United Kingdom, someone from the United States should not unlearn the behavior of looking left first when they return home because their brain ought to properly assign the credit to a different context. Furthermore, once one learns how to cross the street in the United States, it is much easier to learn how to cross the street in the United Kingdom because the brain flexibly generalize behaviors across contexts.
In this perspective, we will first go over common approaches from machine learning to tackle these two credit assignment problems. In doing so, we highlight the challenge in their efficient implementation within biological neural circuits. We also highlight some recent proposals that advance the notion of specialized neural hardware that approximate more general solutions for credit assignment (Fiete & Seung, 2006; Ketz, Morkonda, & O’Reilly, 2013; Kornfeld et al., 2020; Kusmierz, Isomura, & Toyoizumi, 2017; Lillicrap, Cownden, Tweed, & Akerman, 2016; Liu, Smith, Mihalas, Shea-Brown, & Sümbül, 2020; O’Reilly, 1996; O’Reilly, Russin, Zolfaghar, & Rohrlich, 2021; Richards & Lillicrap, 2019; Roelfsema & Holtmaat, 2018; Roelfsema & van Ooyen, 2005; Sacramento, Ponte Costa, Bengio, & Senn, 2018; Schiess, Urbanczik, & Senn, 2016; Zenke & Ganguli, 2018). Along these lines, we propose an efficient systems-level solution involving the thalamus and its interaction with the cortex and BG for these two credit assignment problems.
COMMON MACHINE LEARNING APPROACHES TO CREDIT ASSIGNMENT
One solution to structural credit assignment in machine learning is backpropagation (Rumelhart et al., 1986). Backpropagation recursively computes the vector-valued error signal for synapses based on their contribution to the error signal. There is much empirical success of backpropagation in surpassing human performance in supervised learning such as image recognition (He, Zhang, Ren, & Sun, 2016; Krizhevsky, Sutskever, & Hinton, 2012) and reinforcement learning such as playing the game of Go and Atari (Mnih et al., 2015; Schrittwieser et al., 2020; Silver et al., 2016; Silver et al., 2017). Additionally, comparing artificial networks trained with backpropagation with neural responses from the ventral visual stream of nonhuman primates shows comparable internal representations (Cadieu et al., 2014; Yamins et al., 2014). Despite its empirical success in superhuman-level performance and matching the internal representation of actual brains, backpropagation may not be straightforward to implement in biological neural circuits, as we explain below.
In its most basic form, backpropagation requires symmetric connections between neurons (forward and backward connections). Mathematically, we can write down the backpropagation in Equation 1:
| (1) |
where
E is the total error, ei is the vector error at layer i, Wi is the synaptic weight connecting layer i − 1 to layer i, and f is the nonlinearity. Intuitively, this is saying that the change of synaptic weight Wi is computed by a Hebbian learning rule between backpropagation error ei and activity from last layer f(ai−1), while the backpropagation error is computed by backpropagating the error in the next layer through symmetric feedback weights . Importantly, in this algorithm, error signals do not alter the activity of neurons in the preceding layers and instead operate independently from the feedforward activity. However, such arrangement is not observed in the brain; symmetric connections across neurons are not a universal feature of circuit organization, and biological neurons may encode both feedforward inputs and errors through changes in spike output (changes in activity; Crick, 1989; Richards & Lillicrap, 2019). Therefore, it is hard to imagine how the basic form of backpropagation (symmetry and error/activity separation) is physically implemented in the brain.
Furthermore, while an animal can continually learn to behave across different contexts, artificial neural networks trained by backpropagation struggle to learn and remember different tasks in different contexts: a problem known as catastrophic forgetting (French, 1999; Kemker, McClure, Abitino, Hayes, & Kanan, 2018; Kumaran, Hassabis, & McClelland, 2016; McCloskey & Cohen, 1989; Parisi, Kemker, Part, Kanan, & Wermter, 2019). Specifically, this problem occurs when the tasks are trained sequentially because the weights optimized for former tasks will be modified to fit the later tasks. One of the common solutions is to interleave the tasks from different contexts to jointly optimize performance across contexts by using an episodic memory system and replay mechanism (Kumaran et al., 2016; McClelland, McNaughton, & O’Reilly, 1995). This approach has received empirical success in artificial neural networks, including learning to play many Atari games (Mnih et al., 2015; Schrittwieser et al., 2020). However, since one needs to store past training data in memory to replay during learning, this approach demands a high computational overhead and can be is inefficient as the number of the contexts increases. On the other hand, humans and animals acquire diverse sensorimotor skills in different contexts throughout their life span: a feat that cannot be solely explained by memory replay (M. M. Murray, Lewkowicz, Amedi, & Wallace, 2016; Parisi et al., 2019; Power & Schlaggar, 2017; Zenke, Gerstner, & Ganguli, 2017). Therefore, biological neural circuits are likely to employ other solutions to continual learning in addition to memory replay.
Therefore, to solve these two credit assignment problems in the brain, one needs to seek different solutions. One of the pitfalls of backpropagation is that it is a general algorithm that works on any architecture. However, actual brains are collections of specialized hardware put together in a specialized way. It can be conceived that through clever coordination between different cell types and different circuits, the brains can solve the credit assignment problem by leveraging its specialized architectures. Along this line of ideas, many investigators have proposed cellular (Fiete & Seung, 2006; Kornfeld et al., 2020; Kusmierz et al., 2017; Liu et al., 2020; Richards & Lillicrap, 2019; Sacramento et al., 2018; Schiess et al., 2016) and circuit-level mechanisms (Lillicrap et al., 2016; O’Reilly, 1996; Roelfsema & Holtmaat, 2018; Roelfsema & van Ooyen, 2005) to assign credit appropriately. In this perspective, we would like to advance the notion that the specialized hardware arrangement also happens at the system level and propose that the thalamus and its interaction with basal ganglia and the cortex serve as a system-level solution for these three types of credit assignment.
A PROPOSAL: THALAMOCORTICAL–BASAL GANGLIA INTERACTIONS ENABLE META-LEARNING TO SOLVE CREDIT ASSIGNMENT
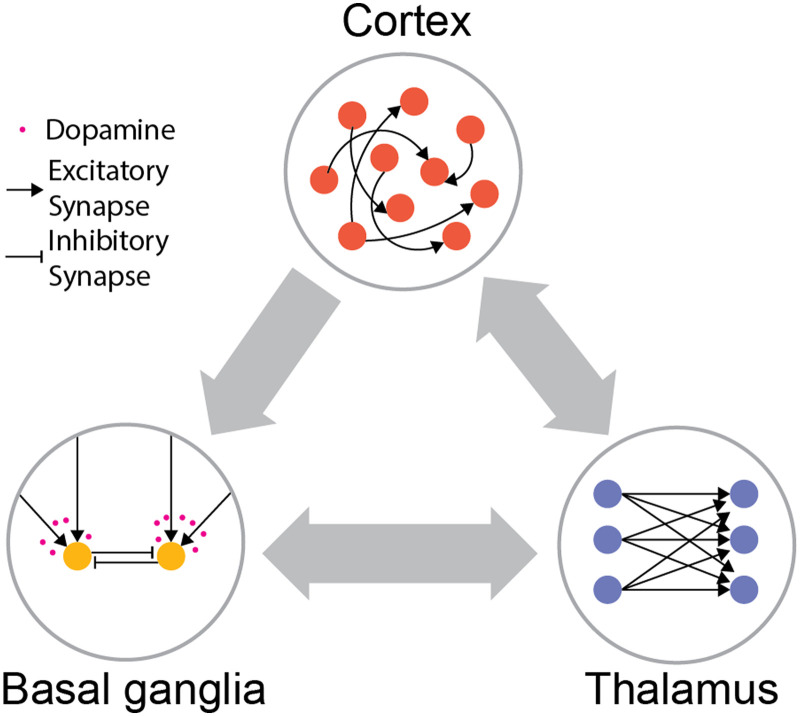
To motivate the notion of thalamocortical–basal ganglia interactions being a potential solution for credit assignment, we will start with a brief introduction. The cortex, thalamus, and basal ganglia are the three major components of the mammalian forebrain—the part of the brain to which high-level cognitive capacities are attributed to (Alexander, DeLong, & Strick, 1986; Badre, Kayser, & D’Esposito, 2010; Cox & Witten, 2019; Makino, Hwang, Hedrick, & Komiyama, 2016; Miller, 2000; Miller & Cohen, 2001; Niv, 2009; Seo, Lee, & Averbeck, 2012; Wolff & Vann, 2019). Each of these components has its specialized internal architectures; the cortex is dominated by excitatory neurons with extensive lateral connectivity profiles (Fuster, 1997; Rakic, 2009; Singer, Sejnowski, & Rakic, 2019), the thalamus is grossly divided into different nuclei harboring mostly excitatory neurons devoid of lateral connections (Harris et al., 2019; Jones, 1985; Sherman & Guillery, 2005), and the basal ganglia are a series of inhibitory structures driven by excitatory inputs from the cortex and thalamus (Gerfen & Bolam, 2010; Lanciego, Luquin, & Obeso, 2012; Nambu, 2011) (Figure 1). A popular view within system neuroscience stipulates that BG and the cortex underwent different learning paradigms, where BG is involved in reinforcement learning while the cortex is involved in unsupervised learning (Doya, 1999, 2000). Specifically, the input structure of the basal ganglia known as the striatum is thought to be where reward gated plasticity takes place to implement reinforcement learning (Bamford et al., 2018; Cox & Witten, 2019; Hikosaka, Kim, Yasuda, & Yamamoto, 2014; Kornfeld et al., 2020; Niv, 2009; Perrin & Venance, 2019). One such evidence is the high temporal precision of DA activity in the striatum. To accurately attribute the action that leads to positive RPE, DA is released into the relevant corticostriatal synapses. However, DA needs to disappear quickly to prevent the next stimulus-response combination from being reinforced. In the striatum, this elimination process is carried out by dopamine active transporter (DAT) to maintain a high temporal resolution of DA activity on a timescale of around 100 ms–1 s to support reinforcement learning (Cass & Gerhardt, 1995; Ciliax et al., 1995; Garris & Wightman, 1994). In contrast, although the cortex also has dopaminergic innervation, cortical DAT expression is low and therefore DA levels may change at a timescale that is too slow to support reinforcement learning (Cass & Gerhardt, 1995; Garris & Wightman, 1994; Lapish, Kroener, Durstewitz, Lavin, & Seamans, 2007; Seamans & Robbins, 2010) but instead supports other processes related to learning (Badre et al., 2010; Miller & Cohen, 2001). In fact, ample evidence indicates that cortical structures undergo Hebbian-like long-term potentiation (LTP) and long-term depression (LTD; Cooke & Bear, 2010; Feldman, 2009; Kirkwood, Rioult, & Bear, 1996). However, despite the unsupervised nature of these processes, cortical representations are task-relevant and include appropriate sensorimotor mappings that lead to rewards (Allen et al., 2017; Donahue & Lee, 2015; Enel, Wallis, & Rich, 2020; Jacobs & Moghaddam, 2020; Petersen, 2019; Tsutsui, Hosokawa, Yamada, & Iijima, 2016). How could this arise from an unsupervised process? One possible explanation is that basal ganglia activate the appropriate cortical neurons during behaviors and the cortical network collectively consolidates high-reward sensorimotor mappings via Hebbian-like learning (Andalman & Fee, 2009; Ashby, Ennis, & Spiering, 2007; Hélie, Ell, & Ashby, 2015; Tesileanu, Olveczky, & Balasubramanian, 2017; Warren, Tumer, Charlesworth, & Brainard, 2011). Previous computational accounts of this process have emphasized a consolidation function for the cortex in this process, which naively would beg the question of why duplicate a process that seems to function well in the basal ganglia and perhaps include a lot of details of the associated experience?
Figure 1. .
Distinct architectures of cortex, thalamus, and basal ganglia. Cortex is largely composed of excitatory neurons with extensive recurrent connectivity. Thalamus consists of mostly excitatory neurons without lateral connections. Basal ganglia consist of mostly inhibitory neurons driven by cortical and thalamic inputs, and the corticostriatal plasticity is modulated by dopamine.
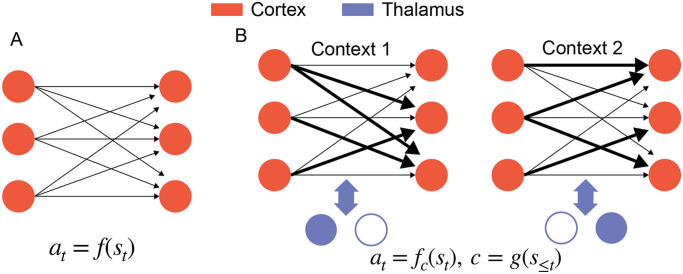
The answer to this question is the core of our proposal. We propose that the learning process is not a duplication, but instead that the reinforcement process in the basal ganglia selects thalamic control functions that subsequently activate cortical associations to allow flexible mappings across different contexts (Figure 2).
Figure 2. .
Two views of learning in the cortex. (A) One possible view is that the Hebbian cortical plasticity consolidates the sensorimotor mapping from BG to learn a stimulus-action mapping at = f(st). (B) We propose that thalamocortical systems perform meta-learning by consolidating the teaching signals from BG to learn a context-dependent mapping at = fc(st), where the context c is computed by past stimulus history and represented by different thalamic activities.
To understand this proposition, we need to take a closer look at the involvement of these distinct network elements in task learning. Learning in basal ganglia happens in corticostriatal synapses where the basic form of reinforcement learning is implemented. Specifically, the coactivation of sensory and motor cortical inputs generates eligibility traces in corticostriatal synapses that get captured by the presence or absence of DA (Fee & Goldberg, 2011; Fiete, Fee, & Seung, 2007; Kornfeld et al., 2020). This reinforcement learning algorithm is fast at acquiring simple associations but slow at generalization to other behaviors. On the other hand, the cortical plasticity operates in a much slower timescale but seems to allow flexible behaviors and fast generalization (Kim, Johnson, Cilles, & Gold, 2011; Mante, Sussillo, Shenoy, & Newsome, 2013; Miller, 2000; Miller & Cohen, 2001). How does the cortex exhibit slow synaptic plasticity and flexible behaviors at the same time? An explanatory framework is meta-learning (Botvinick et al., 2019; Wang et al., 2018), where the flexibility arises from network dynamics and the generalization emerges from slow synaptic plasticity across different contexts. In other words, synaptic plasticity stores a higher order association between contexts and sensorimotor associations while the network dynamics switches between different sensorimotor associations based on this higher order association. However, properly arbitrating between synaptic plasticity and network dynamics to store such higher order association is a nontrivial task (Sohn, Meirhaeghe, Rajalingham, & Jazayeri, 2021). We propose that the thalamocortical system learns these dynamics, where the thalamus provides control nodes that parametrize the cortical activity association space. Basal ganglia inputs to the thalamus learn to select between these different control nodes, directly implementing the interface between weight adjustment and dynamical controls. Our proposal rests on the following three specific points.
First, building on a line of the literature that shows diverse thalamocortical interaction in sensory, cognitive, and motor cortex, we propose that thalamic output may be described as control functions over cortical computations. These control functions can be purely in the sensory domain like attentional filtering, in the cognitive domain like manipulating working memory, or in the motor domain like preparation for movement (Bolkan et al., 2017; W. Guo, Clause, Barth-Maron, & Polley, 2017; Z. V. Guo et al., 2017; Mukherjee et al., 2020; Rikhye, Gilra, & Halassa, 2018; Saalmann & Kastner, 2015; Schmitt et al., 2017; Tanaka, 2007; Wimmer et al., 2015; Zhou, Schafer, & Desimone, 2016). These functions directly relate thalamic activity patterns to different cortical dynamical regimes and thus offer a way to establish higher order association between context and sensorimotor mapping within the thalamocortical pathways. Second, based on previous studies on direct and indirect BG pathways that influence most cortical regions (Hunnicutt et al., 2016; Jiang & Kim, 2018; Nakajima, Schmitt, & Halassa, 2019; Peters, Fabre, Steinmetz, Harris, & Carandini, 2021), we propose that BG hierarchically selects these thalamic control functions to influence activities of the cortex toward rewarding behavioral outcomes. Lastly, we propose that thalamocortical structure consolidates the selection of BG through a two-timescale Hebbian learning process to enable meta-learning. Specifically, the faster corticothalamic plasticity learns the higher order association that enables flexible contextual switching with different thalamic patterns (Marton, Seifikar, Luongo, Lee, & Sohal, 2018; Rikhye et al., 2018), while the slower cortical plasticity learns the shared representations that allow generalization to new behaviors. Below, we will go over the supporting literature that leads us to this proposal.
MORE GENERAL ROLES OF THALAMOCORTICAL INTERACTION AND BASAL GANGLIA
Classical literature has emphasized the role of the thalamus in transmitting sensory inputs to the cortex. This is because some of the better studied thalamic pathways are those connected to sensors on one end and primary cortical areas on another (Hubel & Wiesel, 1961; Lien & Scanziani, 2018; Reinagel, Godwin, Sherman, & Koch, 1999; Sherman & Spear, 1982; Usrey, Alonso, & Reid, 2000). From that perspective, thalamic neurons being devoid of lateral connection transmit their inputs (e.g., from the retina in the case of the lateral geniculate nucleus, LGN) to the primary sensory cortex (V1 in this same example case), and the input transformation (center-surround to oriented edges) occurs within the cortex (Hoffmann, Stone, & Sherman, 1972; Hubel & Wiesel, 1962; Lien & Scanziani, 2018; Usrey et al., 2000). In many cases, these formulations of thalamic “relay” have generalized to how motor and cognitive thalamocortical interactions may be operating. However, in contrast to the classical relay view of the thalamus, more recent studies have shown diverse thalamic functions in sensory, cognitive, and motor processing (Bolkan et al., 2017; W. Guo et al., 2017; Z. V. Guo et al., 2017; Rikhye et al., 2018; Saalmann & Kastner, 2015; Schmitt et al., 2017; Tanaka, 2007; Wimmer et al., 2015; Zhou et al., 2016). For example in mice, sensory thalamocortical transmission can be adjusted based on prefrontal cortex (PFC)-dependent, top-down biasing signals transmitted through nonclassical basal ganglia pathways involving the thalamic reticular nucleus (TRN; Nakajima et al., 2019; Phillips, Kambi, & Saalmann, 2016; Wimmer et al., 2015). Interestingly, these task-relevant PFC signals themselves require long-range interactions with the associative mediodorsal (MD) thalamus to be initiated, maintained, and flexibly switched (Rikhye et al., 2018; Schmitt et al., 2017; Wimmer et al., 2015). One can also observe nontrivial control functions in the motor thalamus. Motor preparatory activities in the anterior motor cortex (ALM) show persistent activities that predicted future actions. Interestingly, the motor thalamus also shows similar preparatory activities that predict future actions and by optogenetically manipulating the motor thalamus activities, the persistent activities in ALM quickly diminished (Z. V. Guo et al., 2017). Recently, Mukherjee, Lam, Wimmer, and Halassa (2021) discovered two cell types within MD thalamus differentially modulate the cortical evidence accumulation dynamics depending on whether the evidence is conflicting or sparse to boost the signal-to-noise ratio in decision-making. Based on the above studies, we propose that the thalamus provides a set of control functions to the cortex. Specifically, cortical computations may be flexibly switched to different dynamical modes by activating a particular thalamic output that corresponds to that mode.
On the other hand, the selective role of BG in motor and cognitive control also has dominated the literature because thalamocortical–basal ganglia interaction is the most well studied in frontal systems (Cox & Witten, 2019; Makino et al., 2016; McNab & Klingberg, 2008; Monchi, Petrides, Strafella, Worsley, & Doyon, 2006; Seo et al., 2012). However, classical and contemporary studies have recognized that all cortical areas, including primary sensory areas, project to the striatum (Hunnicutt et al., 2016; Jiang & Kim, 2018; Peters et al., 2021). Similarly, the basal ganglia can project to the more sensory parts of the thalamus through lesser studied pathways to influence the sensory cortex (Hunnicutt et al., 2016; Nakajima et al., 2019; Peters et al., 2021). Specifically, a nonclassical BG pathway projects to TRN, which in turn modulates the activities of LGN to influence sensory thalamocortical transmission (Nakajima et al., 2019). On the other hand, it has also been argued that BG is involved in gating working memory (McNab & Klingberg, 2008; Voytek & Knight, 2010). This shows that BG has a much more general role than classical action and action strategy selection. Therefore, combining with our proposals on thalamic control functions, we propose that BG hierarchically selects different thalamic control functions to influence all cortical areas in different contexts through reinforcement learning.
Furthermore, there are series of the work that indicates the role of BG to guide plasticity in thalamocortical structures (Andalman & Fee, 2009; Fiete et al., 2007; Hélie et al., 2015; Mehaffey & Doupe, 2015; Tesileanu et al., 2017). In particular, there is evidence that BG is critical for the initial learning and less involved in the automatic behaviors once the behaviors are learned across different species. In zebra finches, the lesion of BG in adult zebra finch has little effect on song production, but the lesion of BG in juvenile zebra finch prevents the bird from learning the song (Fee & Goldberg, 2011; Scharff & Nottebohm, 1991; Sohrabji, Nordeen, & Nordeen, 1990). Similar patterns can be observed in people with Parkinson’s disease. Parkinson’s patients who have a reduction of DA and striatal defects have troubles in solving procedural learning tasks but can produce automatic behaviors normally (Asmus, Huber, Gasser, & Schöls, 2008; Soliveri, Brown, Jahanshahi, Caraceni, & Marsden, 1997; Thomas-Ollivier et al., 1999). This behavioral evidence suggests that thalamocortical structures consolidate the learning from BG as the behaviors become more automatic. Furthermore, on the synaptic level, a songbird learning circuit also demonstrates this cortical consolidation motif (Mehaffey & Doupe, 2015; Tesileanu et al., 2017). In a zebra finch, the premotor nucleus HVC (a proper name) projects to the motor nucleus robust nucleus of the arcopallium (RA) to produce the song. On the other hand, RA also receives BG nucleus Area X mediated inputs from the lateral nucleus of the medial nidopallium (LMAN). The latter pathway is believed to be a locus of reinforcement learning in the songbird circuit. By burst stimulating both input pathways in different time lags, one can discover that HVC-RA and LMAN-RA underwent opposite plasticity (Mehaffey & Doupe, 2015). This suggests that the learning is gradually transferred from LMAN-RA to HVC-RA pathway (Fee & Goldberg, 2011; Mehaffey & Doupe, 2015; Tesileanu et al., 2017). This indicates a general role of BG as the trainer for cortical plasticity.
THE THALAMOCORTICAL STRUCTURE CONSOLIDATES THE BG SELECTIONS ON THALAMIC CONTROL FUNCTIONS IN DIFFERENT TIMESCALES TO ENABLE META-LEARNING
In this section, in addition to BG’s role as the trainer for cortical plasticity, we further propose that BG is the trainer in two different timescales for thalamocortical structures to enable meta-learning. The faster timescale trainer trains the corticothalamic connections to select the appropriate thalamic control functions in different contexts, while the slower timescale trainer trains the cortical connections to form a task-relevant and generalizable representation.
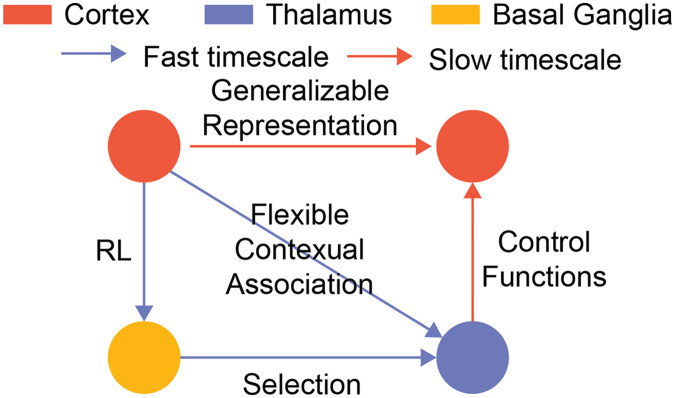
From the songbird example, we see how thalamocortical structures can consolidate simple associations learned through the basal ganglia. To enable meta-learning, we propose that this general network consolidation motif operates over two different timescales within thalamocortical–basal ganglia interactions (Figure 3). First, combining the idea of thalamic outputs as control functions over cortical network activity patterns and the basal ganglia selecting such functions, we frame learning in basal ganglia as a process that connects contextual associations (higher order) with the appropriate dynamical control that maximizes reward at the sensorimotor level (lower order). Under this framing, corticothalamic plasticity consolidates the higher order association within a fast timescale. This allows flexible switching between different thalamic control functions in different contexts. On the other hand, the cortical plasticity consolidates the sensorimotor association over a slow timescale to allow shared representation that can generalize across different contexts. As the thalamocortical structures learn the higher order association, the behaviors become less BG-dependent and the network is able to switch between different thalamic control functions to induce different sensorimotor mappings in different contexts. By having two learning timescales, animals can conceivably both adapt quickly in changing environments with fast learning of corticothalamic connections and maintain the important information across the environment in the cortical connections. One should note that this separation of timescales is independent from different timescales across cortex (Gao, van den Brink, Pfeffer, & Voytek, 2020; J. D. Murray et al., 2014). While different timescales across cortex allows animals to process information differentially, the separation of corticothalmic and cortical plasticity allows the thalamocortical system to learn the higher contextual association to modulate cortical dynamics flexibly.
Figure 3. .
Two-timescale learning in thalamocortical structures. We propose that one can learn the thalamocortical structure to enable meta-learning by applying the general network motif in two different timescales. First, one can learn the corticothalamic connections by applying the motif on the blue loop with a faster timescale. This allows the network to consolidate flexible switching behaviors. Second, one can learn the cortical connections by applying the motif on the orange loop in a slower timescale. This allows cortical neurons to develop a task-relevant shared representation that can generalize across contexts.
Some anatomical observations support this idea. The thalamostriatal neurons have a more modulatory role to the cortical dynamics in a diffusive projection, while thalamocortical neurons have a more driver role to the cortical dynamic in a topographically restricted dense projection (Sherman & Guillery, 2005). This indicates that thalamostriatal neurons might serve as the role of control functions in the faster consolidation loop with the feedback to striatum to conduct credit assignment. On the other hand, thalamocortical neurons might be more involved in the slower consolidation loop with the feedback to striatum coming from the cortex to train the common cortical representation across contexts.
In summary, this two-timescale network consolidation scheme provides a general way for BG to guide plasticity in the thalamocortical architecture to enable meta-learning and thus solves structural credit assignment as a special case. Along these lines, experimental evidence supports the notion that when faced with multisensory inputs, the BG can selectively disinhibit a modality-specific subnetwork of the thalamic reticular nucleus (TRN) to filter out the sensory inputs that are not relevant to the behavior outcomes and thus solve the structural credit assignment problem.
In the discussion above, we discuss our proposal under a general formulation of thalamic control functions. In the next section, we will specify other thalamic control functions suggested by recent studies and observe how they can solve continual learning under this framework as well.
THE THALAMUS SELECTIVELY AMPLIFIES FUNCTIONAL CORTICAL CONNECTIVITY AS A SOLUTION TO CONTINUAL LEARNING AND CATASTROPHIC FORGETTING
One of the pitfalls of the artificial neural network is catastrophic forgetting. If one trains an artificial neural network on a sequence of tasks, the performance on the older task will quickly deteriorate as the network learns the new task (French, 1999; Kemker et al., 2018; Kumaran et al., 2016; McCloskey & Cohen, 1989; Parisi et al., 2019). On the other hand, the brain can achieve continual learning, the ability to learn different tasks in different contexts without catastrophic forgetting and even generalize the performance to novel context (Lewkowicz, 2014; M. M. Murray et al., 2016; Power & Schlaggar, 2017; Zenke, Gerstner, & Ganguli, 2017). There are three main approaches in machine learning to deal with catastrophic forgetting. First, one can use the regularization method to mostly update the weights that are less important to the prior tasks (Fernando et al., 2017; Jung, Ju, Jung, & Kim, 2018; Kirkpatrick et al., 2017; Li & Hoiem, 2018; Maltoni & Lomonaco, 2019; Zenke, Poole, & Ganguli, 2017). This idea is inspired by experimental and theoretical studies on how synaptic information is selectively protected in the brain (Benna & Fusi, 2016; Cichon & Gan, 2015; Fusi, Drew, & Abbott, 2005; Hayashi-Takagi et al., 2015; Yang, Pan, & Gan, 2009). However, it is unclear how to biologically compute the importance of each synapse to prior tasks nor how to do global regularization locally. Second, one can also use a dynamic architecture in which the network expands the architecture by allocating a subnetwork to train with the new information while preserving old information (Cortes, Gonzalvo, Kuznetsov, Mohri, & Yang, 2017; Draelos et al., 2017; Rusu et al., 2016; Xiao, Zhang, Yang, Peng, & Zhang, 2014). However, this type of method is not scalable since the number of neurons needs to scale linearly with the number of tasks. Lastly, one can use a memory buffer to replay past tasks to avoid catastrophic forgetting by interleaving the experience of the past tasks with the experience of the present task (Kemker & Kanan, 2018; Kumaran et al., 2016; McClelland et al., 1995; Shin, Lee, Kim, & Kim, 2017). However, this type of method cannot be the sole solution, as the memory buffer needs to scale linearly with the number of tasks and potentially the number of trials.
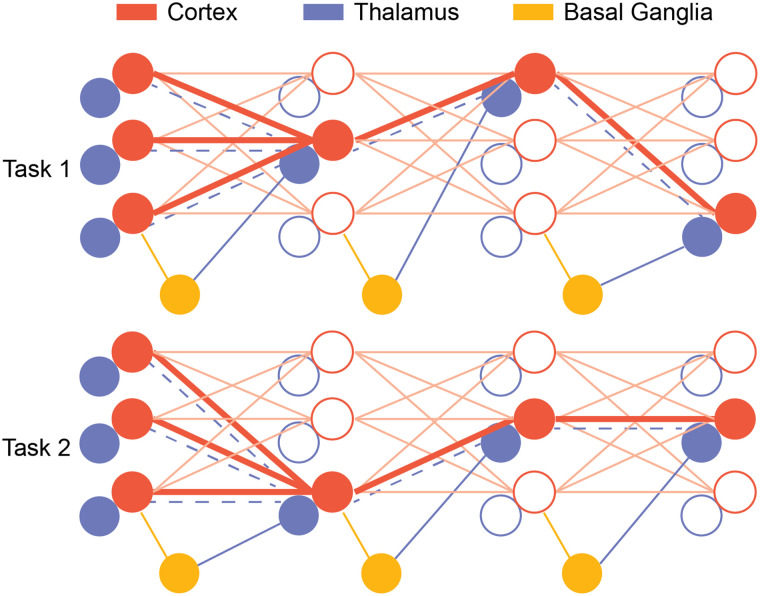
We propose that the thalamus provides another way to solve continual learning and catastrophic forgetting via selectively amplifying parts of the cortical connections in different contexts (Figure 4). Specifically, we propose that a population of thalamic neurons topographically amplify the connectivity of cortical subnetworks as their control functions. During a behavioral task, BG selects subsets of the thalamus that selectively amplify the connectivity of cortical subnetworks. Because of the reinforcement learning in BG, the subnetwork that is the most relevant to the current task will be more preferentially activated and updated. By selecting only the relevant subnetwork to activate in one context, the thalamus protects other subnetworks that can have useful information in another context from being overwritten. The corticothalamic structures can then consolidate these BG-guided flexible switching behaviors via our proposed network motif, and the switching becomes less BG-dependent. Furthermore, our proposed solution has implications on generalization as well. Different tasks can have principles in common that can be transferred. For example, although the rules of chess and Go are very different, players in both games all need to predict what the other players are going to do and counterattack based on the prediction. Since BG selects the subnetwork at each hierarchy that is most relevant to the current tasks, in addition to selecting different subnetworks to prevent catastrophic forgetting, BG can also select subnetworks that are beneficial to both tasks as well to achieve generalization. Therefore, the cortex can develop a modular hierarchical representation of the world that can be easily generalized.
Figure 4. .
A thalamocortical architecture with interaction with BG for continual learning. During task execution, BG selects thalamic neurons that amplify the relevant cortical subnetwork. This protects other parts of the network that are important for another context from being overwritten. When the other task comes, BG selects other thalamic neurons and since the synapses are protected from the last task, animals can freely switch from different tasks without forgetting the previous tasks. Furthermore, as the corticothalamic synapses learn how to select the right thalamic neurons in a different context (blue dashed line), task execution can become less BG dependent.
The idea of protecting relevant information from the past tasks to be overwritten has been applied before computationally and has decent success in combating catastrophic forgetting in deep learning (Kirkpatrick et al., 2017). Experimentally, we also have found that thalamic neurons selectively amplify the cortical connectivity to solve the continual learning problem. In a task where the mice need to switch between different sets of task cues that guided the attention to the visual or auditory target, the performance of the mice does not deteriorate much after switching to the original context, which is an indication of continual learning (Rikhye et al., 2018). Through electrophysiological recording of PFC and mediodorsal thalamic nucleus (MD) neurons, we discovered that PFC neurons preferentially code for the rule of the attention, while MD neurons preferentially code for the contexts of different sets of the cues. Thalamic neurons that encode the task-relevant context translate this neural representation into the amplification of cortical activity patterns associated with that context (despite the fact that cortical neurons themselves only encode the context implicitly). These experimental observations are consistent with our proposed solution: By incorporating the thalamic population that can selectively amplify connectivity of cortical subnetworks, the thalamus and its interaction with cortex and BG solve the continual learning problem and prevent catastrophic forgetting.
CONCLUSION
In summary, in contrast to the traditional relay view of the thalamus, we propose that thalamocortical interaction is the locus of meta-learning where the thalamus provides cortical control functions, such as sensory filtering, working memory gating, or motor preparation, that parametrize the cortical activity association space. Furthermore, we propose a two-timescale learning consolidation framework in which BG hierarchically selects these thalamic control functions to enable meta-learning, solving the credit assignment problem. The faster plasticity learns contextual associations to enable rapid behavioral flexibility, while the slower plasticity establishes cortical representation that generalizes. By considering the recent observation of the thalamus selectively amplifying functional cortical connectivity, the thalamocortical–basal ganglia network is able to flexibly learn context-dependent associations without catastrophic forgetting while generalizing to the new contexts. This modular account of the thalamocortical interaction may seem to be in contrast with the recent proposed dynamical perspectives (Barack & Krakauer, 2021) on thalamocortical interaction in which the thalamus shapes and constrains the cortical attractor landscapes (Shine, 2021). We would like to argue that both the modular and the dynamical perspectives are compatible with our proposal. The crux of the perspectives is that the thalamus provides control functions that parametrize cortical dynamics, and these control functions can be of modular nature or of dynamical nature depending on their specific input-output connectivity. Flexible behaviors can be induced by selecting either the control functions that amplify the appropriate cortical subnetworks or those that adjust the cortical dynamics to the appropriate regimes.
AUTHOR CONTRIBUTIONS
Mien Wang: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing. Michael M. Halassa: Conceptualization; Funding acquisition; Methodology; Supervision; Writing – review & editing.
FUNDING INFORMATION
Michael M. Halassa, National Institute of Mental Health (https://dx.doi.org/10.13039/100000025), Award ID: 5R01MH120118-02.
TECHNICAL TERMS
- Reward prediction error:
A quantity represented by the difference between the expected reward and actual reward.
- Credit assignment:
A computational problem to determine which stimulus, action, internal states, and context lead to outcome.
- Continual learning:
A computational problem to learn tasks sequentially to both learn new tasks faster and not forget old tasks.
- Backpropagation:
An algorithm to compute the error gradient of an artificial neural network through chain rules.
- Catastrophic forgetting:
A phenomenon in which the network forgets about the previous tasks upon learning new tasks.
- Meta-learning:
A learning paradigm in which a network learns how to learn more efficiently.
REFERENCES
- Abbott, L. F., & Nelson, S. B. (2000). Synaptic plasticity: Taming the beast. Nature Neuroscience, 3, 1178–1183. 10.1038/81453, [DOI] [PubMed] [Google Scholar]
- Alexander, G. E., DeLong, M. R., & Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9, 357–381. 10.1146/annurev.ne.09.030186.002041, [DOI] [PubMed] [Google Scholar]
- Allen, W. E., Kauvar, I. V., Chen, M. Z., Richman, E. B., Yang, S. J., Chan, K., … Deisseroth, K. (2017). Global representations of goal-directed behavior in distinct cell types of mouse neocortex. Neuron, 94(4), 891–907. 10.1016/j.neuron.2017.04.017, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andalman, A. S., & Fee, M. S. (2009). A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proceedings of the National Academy of Sciences, 106(30), 12518–12523. 10.1073/pnas.0903214106, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby, F. G., Ennis, J. M., & Spiering, B. J. (2007). A neurobiological theory of automaticity in perceptual categorization. Psychological Review, 114(3), 632–656. 10.1037/0033-295X.114.3.632, [DOI] [PubMed] [Google Scholar]
- Asmus, F., Huber, H., Gasser, T., & Schöls, L. (2008). Kick and rush: Paradoxical kinesia in Parkinson disease. Neurology, 71(9), 695. 10.1212/01.wnl.0000324618.88710.30, [DOI] [PubMed] [Google Scholar]
- Badre, D., Kayser, A. S., & D’Esposito, M. (2010). Frontal cortex and the discovery of abstract action rules. Neuron, 66(2), 315–326. 10.1016/j.neuron.2010.03.025, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford, N. S., Wightman, R. M., & Sulzer, D. (2018). Dopamine’s effects on corticostriatal synapses during reward-based behaviors. Neuron, 97(3), 494–510. 10.1016/j.neuron.2018.01.006, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barack, D. L., & Krakauer, J. W. (2021). Two views on the cognitive brain. Nature Reviews Neuroscience, 22(6), 359–371. 10.1038/s41583-021-00448-6, [DOI] [PubMed] [Google Scholar]
- Bayer, H. M., & Glimcher, P. W. (2005). Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron, 47(1), 129–141. 10.1016/j.neuron.2005.05.020, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benna, M. K., & Fusi, S. (2016). Computational principles of synaptic memory consolidation. Nature Neuroscience, 19(12), 1697–1706. 10.1038/nn.4401, [DOI] [PubMed] [Google Scholar]
- Bliss, T. V., & Lomo, T. (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. Journal of Physiology, 232(2), 331–356. 10.1113/jphysiol.1973.sp010273, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkan, S. S., Stujenske, J. M., Parnaudeau, S., Spellman, T. J., Rauffenbart, C., Abbas, A. I., … Kellendonk, C. (2017). Thalamic projections sustain prefrontal activity during working memory maintenance. Nature Neuroscience, 20(7), 987–996. 10.1038/nn.4568, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick, M., Ritter, S., Wang, J. X., Kurth-Nelson, Z., Blundell, C., & Hassabis, D. (2019). Reinforcement learning, fast and slow. Trends in Cognitive Sciences, 23(5), 408–422. 10.1016/j.tics.2019.02.006, [DOI] [PubMed] [Google Scholar]
- Cadieu, C. F., Hong, H., Yamins, D. L. K., Pinto, N., Ardila, D., Solomon, E. A., … DiCarlo, J. J. (2014). Deep neural networks rival the representation of primate IT cortex for core visual object recognition. PLoS Computational Biology, 10(12), 1–18. 10.1371/journal.pcbi.1003963, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass, W. A., & Gerhardt, G. A. (1995). In vivo assessment of dopamine uptake in rat medial prefrontal cortex: Comparison with dorsal striatum and nucleus accumbens. Journal of Neurochemistry, 65(1), 201–207. 10.1046/j.1471-4159.1995.65010201.x, [DOI] [PubMed] [Google Scholar]
- Cichon, J., & Gan, W. B. (2015). Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature, 520(7546), 180–185. 10.1038/nature14251, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax, B. J., Heilman, C., Demchyshyn, L. L., Pristupa, Z. B., Ince, E., Hersch, S. M., … Levey, A. I. (1995). The dopamine transporter: Immunochemical characterization and localization in brain. Journal of Neuroscience, 15(3 Pt. 1), 1714–1723. 10.1523/JNEUROSCI.15-03-01714.1995, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke, S. F., & Bear, M. F. (2010). Visual experience induces long-term potentiation in the primary visual cortex. Journal of Neuroscience, 30(48), 16304–16313. 10.1523/JNEUROSCI.4333-10.2010, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes, C., Gonzalvo, X., Kuznetsov, V., Mohri, M., & Yang, S. (2017). AdaNet: Adaptive structural learning of artificial neural networks. In Proceedings of the 34th international conference on machine learning (Vol. 70, pp. 874–883). Retrieved from https://proceedings.mlr.press/v70/cortes17a.html [Google Scholar]
- Cox, J., & Witten, I. B. (2019). Striatal circuits for reward learning and decision-making. Nature Reviews Neuroscience, 20(8), 482–494. 10.1038/s41583-019-0189-2, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick, F. (1989). The recent excitement about neural networks. Nature, 337(6203), 129–132. 10.1038/337129a0, [DOI] [PubMed] [Google Scholar]
- Dayan, P., & Abbott, L. F. (2005). Theoretical neuroscience: Computational and mathematical modeling of neural systems. MIT Press. [Google Scholar]
- Donahue, C. H., & Lee, D. (2015). Dynamic routing of task-relevant signals for decision making in dorsolateral prefrontal cortex. Nature Neuroscience, 18(2), 295–301. 10.1038/nn.3918, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya, K. (1999). What are the computations of the cerebellum, the basal ganglia and the cerebral cortex? Neural Networks, 12(7–8), 961–974. 10.1016/S0893-6080(99)00046-5, [DOI] [PubMed] [Google Scholar]
- Doya, K. (2000). Complementary roles of basal ganglia and cerebellum in learning and motor control. Current Opinion in Neurobiology, 10(6), 732–739. 10.1016/S0959-4388(00)00153-7, [DOI] [PubMed] [Google Scholar]
- Draelos, T. J., Miner, N. E., Lamb, C. C., Cox, J. A., Vineyard, C. M., Carlson, K. D., … Aimone, J. B. (2017). Neurogenesis deep learning: Extending deep networks to accommodate new classes. In 2017 international joint conference on neural networks (IJCNN) (pp. 526–533). 10.1109/IJCNN.2017.7965898 [DOI] [Google Scholar]
- Enel, P., Wallis, J. D., & Rich, E. L. (2020). Stable and dynamic representations of value in the prefrontal cortex. eLife, 9, e54313. 10.7554/eLife.54313, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee, M. S., & Goldberg, J. H. (2011). A hypothesis for basal ganglia–dependent reinforcement learning in the songbird. Neuroscience, 198, 152–170. 10.1016/j.neuroscience.2011.09.069, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, D. E. (2009). Synaptic mechanisms for plasticity in neocortex. Annual Review of Neuroscience, 32, 33–55. 10.1146/annurev.neuro.051508.135516, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando, C., Banarse, D., Blundell, C., Zwols, Y., Ha, D., Rusu, A. A., … Wierstra, D. (2017). Pathnet: Evolution channels gradient descent in super neural networks. CoRR, abs/1701.08734. Retrieved from https://arxiv.org/abs/1701.08734. 10.48550/arXiv.1701.08734 [DOI] [Google Scholar]
- Fiete, I. R., Fee, M. S., & Seung, H. S. (2007). Model of birdsong learning based on gradient estimation by dynamic perturbation of neural conductances. Journal of Neurophysiology, 98(4), 2038–2057. 10.1152/jn.01311.2006, [DOI] [PubMed] [Google Scholar]
- Fiete, I. R., & Seung, H. S. (2006). Gradient learning in spiking neural networks by dynamic perturbation of conductances. Physical Review Letters, 97, 048104. 10.1103/PhysRevLett.97.048104, [DOI] [PubMed] [Google Scholar]
- French, R. M. (1999). Catastrophic forgetting in connectionist networks. Trends in Cognitive Sciences, 3(4), 128–135. 10.1016/S1364-6613(99)01294-2, [DOI] [PubMed] [Google Scholar]
- Fusi, S., Drew, P. J., & Abbott, L. F. (2005). Cascade models of synaptically stored memories. Neuron, 45(4), 599–611. 10.1016/j.neuron.2005.02.001, [DOI] [PubMed] [Google Scholar]
- Fuster, J. (1997). The prefrontal cortex: Anatomy, physiology, and neuropsychology of the frontal lobe. Lippincott-Raven. Retrieved from https://books.google.com/books?id=YupqAAAAMAAJ [Google Scholar]
- Gao, R., van den Brink, R. L., Pfeffer, T., & Voytek, B. (2020). Neuronal timescales are functionally dynamic and shaped by cortical microarchitecture. eLife, 9, e61277. 10.7554/eLife.61277, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris, P. A., & Wightman, R. M. (1994). Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: An in vivo voltammetric study. Journal of Neuroscience, 14(1), 442–450. 10.1523/JNEUROSCI.14-01-00442.1994, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen, C., & Bolam, J. (2010). The neuroanatomical organization of the basal ganglia. Handbook of Behavioral Neuroscience, 20, 3–28. 10.1016/B978-0-12-374767-9.00001-9 [DOI] [Google Scholar]
- Guo, W., Clause, A. R., Barth-Maron, A., & Polley, D. B. (2017). A corticothalamic circuit for dynamic switching between feature detection and discrimination. Neuron, 95(1), 180–194. 10.1016/j.neuron.2017.05.019, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Z. V., Inagaki, H. K., Daie, K., Druckmann, S., Gerfen, C. R., & Svoboda, K. (2017). Maintenance of persistent activity in a frontal thalamocortical loop. Nature, 545(7653), 181–186. 10.1038/nature22324, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, J. A., Mihalas, S., Hirokawa, K. E., Whitesell, J. D., Choi, H., Bernard, A., … Zeng, H. (2019). Hierarchical organization of cortical and thalamic connectivity. Nature, 575(7781), 195–202. 10.1038/s41586-019-1716-z, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takagi, A., Yagishita, S., Nakamura, M., Shirai, F., Wu, Y. I., Loshbaugh, A. L., … Kasai, H. (2015). Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature, 525(7569), 333–338. 10.1038/nature15257, [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, K., Zhang, X., Ren, S., & Sun, J. (2016). Deep residual learning for image recognition. In 2016 IEEE conference on computer vision and pattern recognition (CVPR) (pp. 770–778). 10.1109/CVPR.2016.90 [DOI] [Google Scholar]
- Hebb, D. (2002). The organization of behavior: A neuropsychological theory. Taylor & Francis. Retrieved from https://books.google.com/books?id=gUtwMochAI8C [Google Scholar]
- Hikosaka, O., Kim, H. F., Yasuda, M., & Yamamoto, S. (2014). Basal ganglia circuits for reward value-guided behavior. Annual Review of Neuroscience, 37, 289–306. 10.1146/annurev-neuro-071013-013924, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, K. P., Stone, J., & Sherman, S. M. (1972). Relay of receptive-field properties in dorsal lateral geniculate nucleus of the cat. Journal of Neurophysiology, 35(4), 518–531. 10.1152/jn.1972.35.4.518, [DOI] [PubMed] [Google Scholar]
- Houk, J. C., Davis, J. L., & Beiser, D. G. (1994). Adaptive critics and the basal ganglia. In Models of information processing in the basal ganglia (pp. 215–232). MIT Press. 10.7551/mitpress/4708.003.0018 [DOI] [Google Scholar]
- Hubel, D. H., & Wiesel, T. N. (1961). Integrative action in the cat’s lateral geniculate body. Journal of Physiology, 155, 385–398. 10.1113/jphysiol.1961.sp006635, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel, D. H., & Wiesel, T. N. (1962). Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. Journal of Physiology, 160, 106–154. 10.1113/jphysiol.1962.sp006837, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnicutt, B. J., Jongbloets, B. C., Birdsong, W. T., Gertz, K. J., Zhong, H., & Mao, T. (2016). A comprehensive excitatory input map of the striatum reveals novel functional organization. eLife, 5, e19103. 10.7554/eLife.19103, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélie, S., Ell, S. W., & Ashby, F. G. (2015). Learning robust cortico-cortical associations with the basal ganglia: An integrative review. Cortex, 64, 123–135. 10.1016/j.cortex.2014.10.011, [DOI] [PubMed] [Google Scholar]
- Ikemoto, S., & Panksepp, J. (1999). The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Research Reviews, 31(1), 6–41. 10.1016/S0165-0173(99)00023-5, [DOI] [PubMed] [Google Scholar]
- Jacobs, D. S., & Moghaddam, B. (2020). Prefrontal cortex representation of learning of punishment probability during reward-motivated actions. Journal of Neuroscience, 40(26), 5063–5077. 10.1523/JNEUROSCI.0310-20.2020, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H., & Kim, H. F. (2018). Anatomical inputs from the sensory and value structures to the tail of the rat striatum. Frontiers in Neuroanatomy, 12, 30. 10.3389/fnana.2018.00030, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, E. G. (Ed.). (1985). The thalamus. Springer US. 10.1007/978-1-4615-1749-8 [DOI] [Google Scholar]
- Jung, H., Ju, J., Jung, M., & Kim, J. (2018). Less-forgetful learning for domain expansion in deep neural networks. In AAAI conference on artificial intelligence. Retrieved from https://www.aaai.org/ocs/index.php/AAAI/AAAI18/paper/view/17073 [Google Scholar]
- Kemker, R., & Kanan, C. (2018). FearNet: Brain-inspired model for incremental learning. In International conference on learning representations. Retrieved from https://openreview.net/forum?id=SJ1Xmf-Rb [Google Scholar]
- Kemker, R., McClure, M., Abitino, A., Hayes, T., & Kanan, C. (2018). Measuring catastrophic forgetting in neural networks. In AAAI conference on artificial intelligence. Retrieved from https://aaai.org/ocs/index.php/AAAI/AAAI18/paper/view/16410 [Google Scholar]
- Ketz, N., Morkonda, S. G., & O’Reilly, R. C. (2013). Theta coordinated error-driven learning in the hippocampus. PLoS Computational Biology, 9(6), 1–9. 10.1371/journal.pcbi.1003067, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C., Johnson, N. F., Cilles, S. E., & Gold, B. T. (2011). Common and distinct mechanisms of cognitive flexibility in prefrontal cortex. Journal of Neuroscience, 31(13), 4771–4779. 10.1523/JNEUROSCI.5923-10.2011, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick, J., Pascanu, R., Rabinowitz, N., Veness, J., Desjardins, G., Rusu, A. A., … Hadsell, R. (2017). Overcoming catastrophic forgetting in neural networks. Proceedings of the National Academy of Sciences, 114(13), 3521–3526. 10.1073/pnas.1611835114, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood, A., Rioult, M. C., & Bear, M. F. (1996). Experience-dependent modification of synaptic plasticity in visual cortex. Nature, 381(6582), 526–528. 10.1038/381526a0, [DOI] [PubMed] [Google Scholar]
- Kornfeld, J., Januszewski, M., Schubert, P., Jain, V., Denk, W., & Fee, M. (2020). An anatomical substrate of credit assignment in reinforcement learning. bioRxiv. 10.1101/2020.02.18.954354 [DOI] [Google Scholar]
- Krizhevsky, A., Sutskever, I., & Hinton, G. E. (2012). ImageNet classification with deep convolutional neural networks. In Advances in neural information processing systems (Vol. 25). Curran Associates, Inc. Retrieved from https://proceedings.neurips.cc/paper/2012/file/c399862d3b9d6b76c8436e924a68c45b-Paper.pdf [Google Scholar]
- Kumaran, D., Hassabis, D., & McClelland, J. L. (2016). What learning systems do intelligent agents need? Complementary learning systems theory updated. Trends in Cognitive Sciences, 20(7), 512–534. 10.1016/j.tics.2016.05.004, [DOI] [PubMed] [Google Scholar]
- Kusmierz, L., Isomura, T., & Toyoizumi, T. (2017). Learning with three factors: modulating Hebbian plasticity with errors. Current Opinion in Neurobiology, 46, 170–177. 10.1016/j.conb.2017.08.020, [DOI] [PubMed] [Google Scholar]
- Lanciego, J. L., Luquin, N., & Obeso, J. A. (2012). Functional neuroanatomy of the basal ganglia. Cold Spring Harbor Perspectives in Medicine, 2(12), a009621. 10.1101/cshperspect.a009621, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapish, C. C., Kroener, S., Durstewitz, D., Lavin, A., & Seamans, J. K. (2007). The ability of the mesocortical dopamine system to operate in distinct temporal modes. Psychopharmacology, 191(3), 609–625. 10.1007/s00213-006-0527-8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowicz, D. J. (2014). Early experience and multisensory perceptual narrowing. Developmental Psychobiology, 56(2), 292–315. 10.1002/dev.21197, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z., & Hoiem, D. (2018). Learning without forgetting. IEEE Transactions on Pattern Analysis and Machine Intelligence, 40(12), 2935–2947. 10.1109/TPAMI.2017.2773081, [DOI] [PubMed] [Google Scholar]
- Lien, A. D., & Scanziani, M. (2018). Cortical direction selectivity emerges at convergence of thalamic synapses. Nature, 558(7708), 80–86. 10.1038/s41586-018-0148-5, [DOI] [PubMed] [Google Scholar]
- Lillicrap, T. P., Cownden, D., Tweed, D. B., & Akerman, C. J. (2016). Random synaptic feedback weights support error backpropagation for deep learning. Nature Communications, 7, 13276. 10.1038/ncomms13276, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillicrap, T. P., Santoro, A., Marris, L., Akerman, C. J., & Hinton, G. (2020). Backpropagation and the brain. Nature Reviews Neuroscience, 21(6), 335–346. 10.1038/s41583-020-0277-3, [DOI] [PubMed] [Google Scholar]
- Liu, Y. H., Smith, S., Mihalas, S., Shea-Brown, E., & Sümbül, U. (2020). A solution to temporal credit assignment using cell-type-specific modulatory signals. bioRxiv. 10.1101/2020.11.22.393504 [DOI] [Google Scholar]
- Makino, H., Hwang, E. J., Hedrick, N. G., & Komiyama, T. (2016). Circuit mechanisms of sensorimotor learning. Neuron, 92(4), 705–721. 10.1016/j.neuron.2016.10.029, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltoni, D., & Lomonaco, V. (2019). Continuous learning in single-incremental-task scenarios. Neural Networks, 116, 56–73. 10.1016/j.neunet.2019.03.010, [DOI] [PubMed] [Google Scholar]
- Mante, V., Sussillo, D., Shenoy, K. V., & Newsome, W. T. (2013). Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature, 503(7474), 78–84. 10.1038/nature12742, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton, T. F., Seifikar, H., Luongo, F. J., Lee, A. T., & Sohal, V. S. (2018). Roles of prefrontal cortex and mediodorsal thalamus in task engagement and behavioral flexibility. Journal of Neuroscience, 38(10), 2569–2578. 10.1523/JNEUROSCI.1728-17.2018, [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland, J. L., McNaughton, B. L., & O’Reilly, R. C. (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102(3), 419–457. 10.1037/0033-295X.102.3.419, [DOI] [PubMed] [Google Scholar]
- McCloskey, M., & Cohen, N. J. (1989). Catastrophic interference in connectionist networks: The sequential learning problem. In Bower G. H. (Ed.), Psychology of learning and motivation (Vol. 24, pp. 109–165). Academic Press. 10.1016/S0079-7421(08)60536-8 [DOI] [Google Scholar]
- McNab, F., & Klingberg, T. (2008). Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience, 11(1), 103–107. 10.1038/nn2024, [DOI] [PubMed] [Google Scholar]
- Mehaffey, W. H., & Doupe, A. J. (2015). Naturalistic stimulation drives opposing heterosynaptic plasticity at two inputs to songbird cortex. Nature Neuroscience, 18(9), 1272–1280. 10.1038/nn.4078, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, E. K. (2000). The prefontral cortex and cognitive control. Nature Reviews Neuroscience, 1(1), 59–65. 10.1038/35036228, [DOI] [PubMed] [Google Scholar]
- Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. 10.1146/annurev.neuro.24.1.167, [DOI] [PubMed] [Google Scholar]
- Minsky, M. (1961). Steps toward artificial intelligence. Proceedings of the IRE, 49(1), 8–30. 10.1109/JRPROC.1961.287775 [DOI] [Google Scholar]
- Mnih, V., Kavukcuoglu, K., Silver, D., Rusu, A. A., Veness, J., Bellemare, M. G., … Hassabis, D. (2015). Human-level control through deep reinforcement learning. Nature, 518(7540), 529–533. 10.1038/nature14236, [DOI] [PubMed] [Google Scholar]
- Monchi, O., Petrides, M., Strafella, A. P., Worsley, K. J., & Doyon, J. (2006). Functional role of the basal ganglia in the planning and execution of actions. Annals of Neurology, 59(2), 257–264. 10.1002/ana.20742, [DOI] [PubMed] [Google Scholar]
- Montague, P. R., Dayan, P., & Sejnowski, T. J. (1996). A framework for mesencephalic dopamine systems based on predictive Hebbian learning. Journal of Neuroscience, 16(5), 1936–1947. 10.1523/JNEUROSCI.16-05-01936.1996, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, G., Nevet, A., Arkadir, D., Vaadia, E., & Bergman, H. (2006). Midbrain dopamine neurons encode decisions for future action. Nature Neuroscience, 9(8), 1057–1063. 10.1038/nn1743, [DOI] [PubMed] [Google Scholar]
- Mukherjee, A., Bajwa, N., Lam, N. H., Porrero, C., Clasca, F., & Halassa, M. M. (2020). Variation of connectivity across exemplar sensory and associative thalamocortical loops in the mouse. eLife, 9, e62554. 10.7554/eLife.62554, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, A., Lam, N. H., Wimmer, R. D., & Halassa, M. M. (2021). Thalamic circuits for independent control of prefrontal signal and noise. Nature, 600(7887), 100–104. 10.1038/s41586-021-04056-3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, J. D., Bernacchia, A., Freedman, D. J., Romo, R., Wallis, J. D., Cai, X., … Wang, X. J. (2014). A hierarchy of intrinsic timescales across primate cortex. Nature Neuroscience, 17(12), 1661–1663. 10.1038/nn.3862, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, M. M., Lewkowicz, D. J., Amedi, A., & Wallace, M. T. (2016). Multisensory processes: A balancing act across the lifespan. Trends in Neurosciences, 39(8), 567–579. 10.1016/j.tins.2016.05.003, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, M., Schmitt, L. I., & Halassa, M. M. (2019). Prefrontal cortex regulates sensory filtering through a basal ganglia-to-thalamus pathway. Neuron, 103(3), 445–458. 10.1016/j.neuron.2019.05.026, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu, A. (2011). Somatotopic organization of the primate basal ganglia. Frontiers in Neuroanatomy, 5, 26. 10.3389/fnana.2011.00026, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv, Y. (2009). Reinforcement learning in the brain. Journal of Mathematical Psychology, 53(3), 139–154. 10.1016/j.jmp.2008.12.005 [DOI] [Google Scholar]
- O’Reilly, R. C. (1996). Biologically plausible error-driven learning using local activation differences: The generalized recirculation algorithm. Neural Computation, 8(5), 895–938. 10.1162/neco.1996.8.5.895 [DOI] [Google Scholar]
- O’Reilly, R. C., Russin, J. L., Zolfaghar, M., & Rohrlich, J. (2021). Deep predictive learning in neocortex and pulvinar. Journal of Cognitive Neuroscience, 33(6), 1158–1196. 10.1162/jocn_a_01708, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi, G. I., Kemker, R., Part, J. L., Kanan, C., & Wermter, S. (2019). Continual lifelong learning with neural networks: A review. Neural Networks, 113, 54–71. 10.1016/j.neunet.2019.01.012, [DOI] [PubMed] [Google Scholar]
- Perrin, E., & Venance, L. (2019). Bridging the gap between striatal plasticity and learning. Current Opinion in Neurobiology, 54, 104–112. 10.1016/j.conb.2018.09.007, [DOI] [PubMed] [Google Scholar]
- Peters, A. J., Fabre, J. M. J., Steinmetz, N. A., Harris, K. D., & Carandini, M. (2021). Striatal activity topographically reflects cortical activity. Nature, 591, 420–425. 10.1038/s41586-020-03166-8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, C. C. H. (2019). Sensorimotor processing in the rodent barrel cortex. Nature Reviews Neuroscience, 20(9), 533–546. 10.1038/s41583-019-0200-y, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, J. M., Kambi, N. A., & Saalmann, Y. B. (2016). A subcortical pathway for rapid, goal-driven, attentional filtering. Trends in Neurosciences, 39(2), 49–51. 10.1016/j.tins.2015.12.003, [DOI] [PubMed] [Google Scholar]
- Power, J. D., & Schlaggar, B. L. (2017). Neural plasticity across the lifespan. Wiley Interdisciplinary Reviews: Developmental Biology, 6(1), e216. 10.1002/wdev.216, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic, P. (2009). Evolution of the neocortex: A perspective from developmental biology. Nature Reviews Neuroscience, 10(10), 724–735. 10.1038/nrn2719, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinagel, P., Godwin, D., Sherman, S. M., & Koch, C. (1999). Encoding of visual information by LGN bursts. Journal of Neurophysiology, 81(5), 2558–2569. 10.1152/jn.1999.81.5.2558, [DOI] [PubMed] [Google Scholar]
- Richards, B. A., & Lillicrap, T. P. (2019). Dendritic solutions to the credit assignment problem. Current Opinion in Neurobiology, 54, 28–36. 10.1016/j.conb.2018.08.003, [DOI] [PubMed] [Google Scholar]
- Rikhye, R. V., Gilra, A., & Halassa, M. M. (2018). Thalamic regulation of switching between cortical representations enables cognitive flexibility. Nature Neuroscience, 21(12), 1753–1763. 10.1038/s41593-018-0269-z, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema, P. R., & Holtmaat, A. (2018). Control of synaptic plasticity in deep cortical networks. Nature Reviews Neuroscience, 19(3), 166–180. 10.1038/nrn.2018.6, [DOI] [PubMed] [Google Scholar]
- Roelfsema, P. R., & van Ooyen, A. (2005). Attention-gated reinforcement learning of internal representations for classification. Neural Computation, 17(10), 2176–2214. 10.1162/0899766054615699, [DOI] [PubMed] [Google Scholar]
- Roesch, M. R., Calu, D. J., & Schoenbaum, G. (2007). Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nature Neuroscience, 10(12), 1615–1624. 10.1038/nn2013, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumelhart, D. E., Hinton, G. E., & Williams, R. J. (1986). Learning representations by back-propagating errors. Nature, 323(6088), 533–536. 10.1038/323533a0 [DOI] [Google Scholar]
- Rusu, A. A., Rabinowitz, N. C., Desjardins, G., Soyer, H., Kirkpatrick, J., Kavukcuoglu, K., … Hadsell, R. (2016). Progressive neural networks. CoRR, abs/1606.04671. Retrieved from https://arxiv.org/abs/1606.04671. 10.48550/arXiv.1606.04671 [DOI] [Google Scholar]
- Saalmann, Y. B., & Kastner, S. (2015). The cognitive thalamus. Frontiers in Systems Neuroscience, 9, 39. 10.3389/fnsys.2015.00039, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacramento, J., Ponte Costa, R., Bengio, Y., & Senn, W. (2018). Dendritic cortical microcircuits approximate the backpropagation algorithm. In Advances in neural information processing systems (Vol. 31, pp. 8735–8746). Curran Associates, Inc. Retrieved from https://proceedings.neurips.cc/paper/2018/file/1dc3a89d0d440ba31729b0ba74b93a33-Paper.pdf [Google Scholar]
- Scharff, C., & Nottebohm, F. (1991). A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: Implications for vocal learning. Journal of Neuroscience, 11(9), 2896–2913. 10.1523/JNEUROSCI.11-09-02896.1991, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiess, M., Urbanczik, R., & Senn, W. (2016). Somato-dendritic synaptic plasticity and error-backpropagation in active dendrites. PLoS Computational Biology, 12(2), 1–18. 10.1371/journal.pcbi.1004638, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt, L. I., Wimmer, R. D., Nakajima, M., Happ, M., Mofakham, S., & Halassa, M. M. (2017). Thalamic amplification of cortical connectivity sustains attentional control. Nature, 545(7653), 219–223. 10.1038/nature22073, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrittwieser, J., Antonoglou, I., Hubert, T., Simonyan, K., Sifre, L., Schmitt, S., … Silver, D. (2020). Mastering Atari, Go, chess and shogi by planning with a learned model. Nature, 588(7839), 604–609. 10.1038/s41586-020-03051-4, [DOI] [PubMed] [Google Scholar]
- Schultz, W., Dayan, P., & Montague, P. R. (1997). A neural substrate of prediction and reward. Science, 275(5306), 1593–1599. 10.1126/science.275.5306.1593, [DOI] [PubMed] [Google Scholar]
- Seamans, J. K., & Robbins, T. W. (2010). Dopamine modulation of the prefrontal cortex and cognitive function. In The dopamine receptors (pp. 373–398). Totowa, NJ: Humana Press. 10.1007/978-1-60327-333-6_14 [DOI] [Google Scholar]
- Seo, M., Lee, E., & Averbeck, B. B. (2012). Action selection and action value in frontal-striatal circuits. Neuron, 74(5), 947–960. 10.1016/j.neuron.2012.03.037, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, S. M., & Guillery, R. W. (2005). Exploring the thalamus and its role in cortical function (2nd ed.). MIT Press. [Google Scholar]
- Sherman, S. M., & Spear, P. D. (1982). Organization of visual pathways in normal and visually deprived cats. Physiological Reviews, 62(2), 738–855. 10.1152/physrev.1982.62.2.738, [DOI] [PubMed] [Google Scholar]
- Shin, H., Lee, J. K., Kim, J., & Kim, J. (2017). Continual learning with deep generative replay. In Advances in neural information processing systems (Vol. 30). Curran Associates, Inc. Retrieved from https://proceedings.neurips.cc/paper/2017/file/0efbe98067c6c73dba1250d2beaa81f9-Paper.pdf [Google Scholar]
- Shine, J. M. (2021). The thalamus integrates the macrosystems of the brain to facilitate complex, adaptive brain network dynamics. Progress in Neurobiology, 199, 101951. 10.1016/j.pneurobio.2020.101951, [DOI] [PubMed] [Google Scholar]
- Silver, D., Huang, A., Maddison, C. J., Guez, A., Sifre, L., van den Driessche, G., … Hassabis, D. (2016). Mastering the game of Go with deep neural networks and tree search. Nature, 529(7587), 484–489. 10.1038/nature16961, [DOI] [PubMed] [Google Scholar]
- Silver, D., Schrittwieser, J., Simonyan, K., Antonoglou, I., Huang, A., Guez, A., … Hassabis, D. (2017). Mastering the game of Go without human knowledge. Nature, 550(7676), 354–359. 10.1038/nature24270, [DOI] [PubMed] [Google Scholar]
- Singer, W., Sejnowski, T., & Rakic, P. (2019). The neocortex. MIT Press. Retrieved from https://books.google.com/books?id=aL60DwAAQBAJ. 10.7551/mitpress/12593.001.0001 [DOI] [Google Scholar]
- Sohn, H., Meirhaeghe, N., Rajalingham, R., & Jazayeri, M. (2021). A network perspective on sensorimotor learning. Trends in Neurosciences, 44(3), 170–181. 10.1016/j.tins.2020.11.007, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji, F., Nordeen, E. J., & Nordeen, K. W. (1990). Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behavioral and Neural Biology, 53(1), 51–63. 10.1016/0163-1047(90)90797-A, [DOI] [PubMed] [Google Scholar]
- Soliveri, P., Brown, R. G., Jahanshahi, M., Caraceni, T., & Marsden, C. D. (1997). Learning manual pursuit tracking skills in patients with Parkinson’s disease. Brain, 120(Pt. 8), 1325–1337. 10.1093/brain/120.8.1325, [DOI] [PubMed] [Google Scholar]
- Suri, R. E., & Schultz, W. (1999). A neural network model with dopamine-like reinforcement signal that learns a spatial delayed response task. Neuroscience, 91(3), 871–890. 10.1016/S0306-4522(98)00697-6, [DOI] [PubMed] [Google Scholar]
- Sutton, R., & Barto, A. (2018). Reinforcement learning: An introduction. MIT Press. Retrieved from https://books.google.com/books?id=sWV0DwAAQBAJ [Google Scholar]
- Sutton, R. S., & Barto, A. G. (1990). Time-derivative models of Pavlovian reinforcement. In Learning and computational neuroscience: Foundations of adaptive networks (pp. 497–537). MIT Press. [Google Scholar]
- Tanaka, M. (2007). Cognitive signals in the primate motor thalamus predict saccade timing. Journal of Neuroscience, 27(44), 12109–12118. 10.1523/JNEUROSCI.1873-07.2007, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesileanu, T., Olveczky, B., & Balasubramanian, V. (2017). Rules and mechanisms for efficient two-stage learning in neural circuits. eLife, 6, e20944. 10.7554/eLife.20944, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Ollivier, V., Reymann, J. M., Le Moal, S., Schück, S., Lieury, A., & Allain, H. (1999). Procedural memory in recent-onset Parkinson’s disease. Dementia and Geriatric Cognitive Disorders, 10(2), 172–180. 10.1159/000017100, [DOI] [PubMed] [Google Scholar]
- Thorndike, E. (2017). Animal intelligence: Experimental studies. Taylor & Francis. Retrieved from https://books.google.com/books?id=1_hADwAAQBAJ. 10.4324/9781351321044 [DOI] [Google Scholar]
- Tsutsui, K., Hosokawa, T., Yamada, M., & Iijima, T. (2016). Representation of functional category in the monkey prefrontal cortex and its rule-dependent use for behavioral selection. Journal of Neuroscience, 36(10), 3038–3048. 10.1523/JNEUROSCI.2063-15.2016, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey, W. M., Alonso, J. M., & Reid, R. C. (2000). Synaptic interactions between thalamic inputs to simple cells in cat visual cortex. Journal of Neuroscience, 20(14), 5461–5467. 10.1523/JNEUROSCI.20-14-05461.2000, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek, B., & Knight, R. T. (2010). Prefrontal cortex and basal ganglia contributions to visual working memory. Proceedings of the National Academy of Sciences, 107(42), 18167–18172. 10.1073/pnas.1007277107, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. X., Kurth-Nelson, Z., Kumaran, D., Tirumala, D., Soyer, H., Leibo, J. Z., … Botvinick, M. (2018). Prefrontal cortex as a meta-reinforcement learning system. Nature Neuroscience, 21(6), 860–868. 10.1038/s41593-018-0147-8, [DOI] [PubMed] [Google Scholar]
- Warren, T. L., Tumer, E. C., Charlesworth, J. D., & Brainard, M. S. (2011). Mechanisms and time course of vocal learning and consolidation in the adult songbird. Journal of Neurophysiology, 106(4), 1806–1821. 10.1152/jn.00311.2011, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington, J. C. R., & Bogacz, R. (2019). Theories of error back-propagation in the brain. Trends in Cognitive Sciences, 23(3), 235–250. 10.1016/j.tics.2018.12.005, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens, J. R., & Kotter, R. (1994). Cellular models of reinforcement. In Models of information processing in the basal ganglia. MIT Press. 10.7551/mitpress/4708.003.0017 [DOI] [Google Scholar]
- Wimmer, R. D., Schmitt, L. I., Davidson, T. J., Nakajima, M., Deisseroth, K., & Halassa, M. M. (2015). Thalamic control of sensory selection in divided attention. Nature, 526(7575), 705–709. 10.1038/nature15398, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff, M., & Vann, S. D. (2019). The cognitive thalamus as a gateway to mental representations. Journal of Neuroscience, 39(1), 3–14. 10.1523/JNEUROSCI.0479-18.2018, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, T., Zhang, J., Yang, K., Peng, Y., & Zhang, Z. (2014). Error-driven incremental learning in deep convolutional neural network for large-scale image classification. In ACM multimedia. 10.1145/2647868.2654926 [DOI] [Google Scholar]
- Yamins, D. L., Hong, H., Cadieu, C. F., Solomon, E. A., Seibert, D., & DiCarlo, J. J. (2014). Performance-optimized hierarchical models predict neural responses in higher visual cortex. Proceedings of the National Academy of Sciences, 111(23), 8619–8624. 10.1073/pnas.1403112111, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, G., Pan, F., & Gan, W. B. (2009). Stably maintained dendritic spines are associated with lifelong memories. Nature, 462(7275), 920–924. 10.1038/nature08577, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke, F., & Ganguli, S. (2018). SuperSpike: Supervised learning in multilayer spiking neural networks. Neural Computation, 30(6), 1514–1541. 10.1162/neco_a_01086, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke, F., Gerstner, W., & Ganguli, S. (2017). The temporal paradox of Hebbian learning and homeostatic plasticity. Current Opinion in Neurobiology, 43, 166–176. 10.1016/j.conb.2017.03.015, [DOI] [PubMed] [Google Scholar]
- Zenke, F., Poole, B., & Ganguli, S. (2017). Continual learning through synaptic intelligence. In Proceedings of the 34th international conference on machine learning (Vol. 70, pp. 3987–3995). Retrieved from https://proceedings.mlr.press/v70/zenke17a.html [PMC free article] [PubMed] [Google Scholar]
- Zhou, H., Schafer, R. J., & Desimone, R. (2016). Pulvinar-cortex interactions in vision and attention. Neuron, 89(1), 209–220. 10.1016/j.neuron.2015.11.034, [DOI] [PMC free article] [PubMed] [Google Scholar]