Abstract
Adherence of Clostridium difficile to Vero cells under anaerobic conditions was increased by a high sodium concentration, calcium-rich medium, an acidic pH, and iron starvation. The level of adhesion of nontoxigenic strains was comparable to that of toxigenic strains. Depending on the bacterial culture conditions, Vero cells could bind to one, two, or three bacterial surface proteins with molecular masses of 70, 50, and 40 kDa.
Clostridium difficile is recognized as the major causative agent of pseudomembranous and antibiotic-associated colitis (14). Its pathogenicity is mediated by two exotoxins, toxins A (308 kDa) and B (207 kDa), which both damage human colonic mucosa in vitro and are potent cytotoxic enzymes (24). Other potential virulence factors include the capsule (9), proteolytic enzymes (17, 18, 28, 29), flagella (32), fimbriae (4), and an adhesin(s) potentially involved in mucus and cell association (2, 5, 12, 16).
Proliferation of C. difficile in the colon results from the suppression of members of the normal microbiota (i.e., suppression of colonization resistance) during or after antibiotherapy. A colonic ecosystem in equilibrium is characterized by a pH at the luminal surface of around 6.2 to 6.8 (11); an osmolarity of 310 mosM (1); low oxygen tension (1); a temperature of 37°C; calcium and magnesium concentrations of 5 and 2 mM, respectively; (30), and a low level of free iron (7). Oral intake of antibiotics and the subsequent disturbance of this equilibrium can profoundly alter any of these parameters. In this study, we investigated whether environmental conditions deviating from physiological ones constitute a stimulus for C. difficile that could drive it to increased adherence and subsequent colonization.
The effect of environmental stresses on C. difficile adherence was investigated in vitro in the Vero cell adherence model (16) by using either different culture or adherence assay conditions. Toxigenic C. difficile isolate 79-685 was used as the reference strain in all of the adherence assays. Other isolates used are listed in Table 1. Before adherence assays, bacteria were subcultured twice for 24 h each time in an anaerobic chamber at 37°C in tryptone-glucose-yeast extract (TGY) broth (Difco) that had been prereduced prior to use.
TABLE 1.
Serogroups, toxin production, and origins of C. difficile isolates studied
| Strain | Sero-groupa | Production of toxins A and Bb | Origin | Source |
|---|---|---|---|---|
| 79-685 | S3 | + | Adult with PMCc | VPIb |
| VPI-10463 | G | + | Adult with wound abscess | Institut de Bactéri-ologiee |
| 90-111 | D | 0 | Child carrier | Jean Verdier Hospitalf |
| 93-136 | D | 0 | Child carrier | |
| 93-226 | D | 0 | Child carrier | |
| 93-296 | D | 0 | Child carrier | |
| 93-379 | D | 0 | Adult carrier |
All cell adherence assays were performed as previously described by us (16), in accordance with two protocols: (i) in an aerobic atmosphere enriched with 10% CO2 (partially aerobic conditions) with bacteria exposed to a heat shock (20 min at 60°C) prior to contact with Vero cells (12) and (ii) under strict anaerobic conditions with no heat shock. Bacteria and cells were incubated together for 1 h or more at 37°C under aerobic (with CO2) or strict anaerobic conditions. Nonadherent bacteria were eliminated by five washings in PBS (10 mM phosphate buffer, 150 mM NaCl), pH 7.0, and the cells were fixed and stained with May-Grünwald-Giemsa stain (Sigma). The adhesion index is given as the average number of adhering bacteria (counted at a magnification of ×1,000) per cell ± the standard deviation from at least three different assays. The significance of differences between various treatments was assessed by Student’s t test.
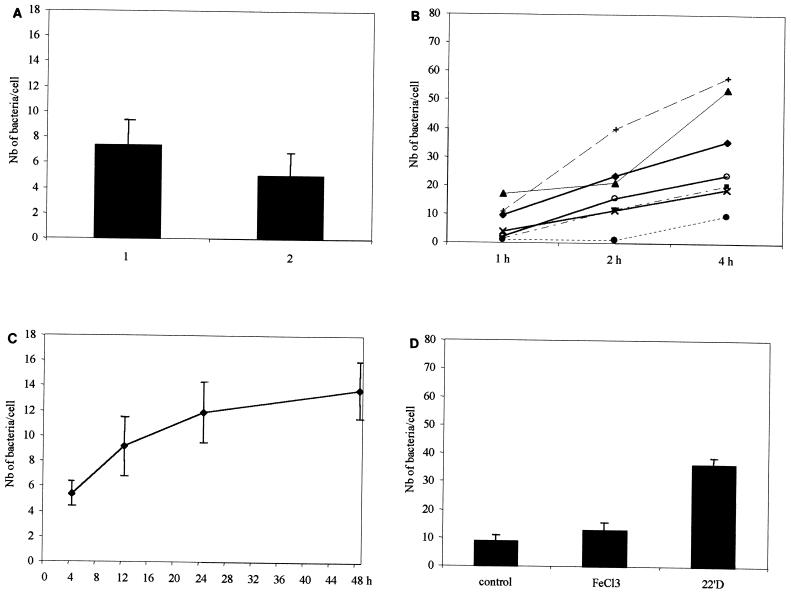
When our assays were performed under partially aerobic conditions after a heat shock, the adherence level was slightly lower than that observed under strict anaerobic conditions with no heat shock, due to the death of some bacteria (Fig. 1A), but similar adherence profiles were always observed for all of the environmental factors tested. Only results obtained under strict anaerobic conditions with no heat shock are shown in the figures.
FIG. 1.
(A) Adherence of C. difficile to Vero cells under anaerobic conditions (part 1) or after a heat shock under aerobic (with CO2) conditions (part 2). (B) Adherence to Vero cells of seven C. difficile strains as a function of time. Symbols for toxigenic strains: ⧫, 79-685; ■, VPI-10409. Symbols for nontoxigenic strains: ▴, 90-111; ×, 93-136; ○, 93-226; ●, 93-296; +, 93-379. (C) Effect of growth phase on the adherence of C. difficile to Vero cells. (D) Effect of iron in the culture medium on the adherence of C. difficile to Vero cells. The control was TGY, the concentration of FeCl3 was 50 μM, and that of 2,2′-dipyridyl (22′D) was 200 μM. Nb, number.
Toxins.
To compare the kinetics of adherence of five nontoxigenic and two toxigenic C. difficile strains (Table 1), the bacteria were incubated with Vero cells for 1 to 4 h. All of the C. difficile strains tested were adherent, and there was no significant interstrain variability in adhesion levels (P < 0.05) (Fig. 1B). Thus, cell adherence, in contrast to mucus association, as shown by us (15) and Borriello et al. (3, 5), does not appear to be promoted by toxins.
Growth phase.
To study the effect of the growth phase on C. difficile adherence, bacteria were cultivated under anaerobic conditions for 4 (early exponential phase), 12 (early stationary phase), 24, or 48 (stationary phase) h prior to the cell adherence assay. As shown in Fig. 1C, expression of adherence was growth phase dependent, reaching a maximum late in stationary phase. Stationary phase can be considered a stress that can trigger expression of pathogenic determinants (8).
Iron.
To investigate the effect of iron starvation on the adherence capacity of C. difficile, bacteria were cultured (i) under iron-rich conditions created by adding 50 μM FeCl3 (Sigma) to TGY broth or (ii) in iron-limited medium prepared by adding 200 μM 2,2′-dipyridyl (Sigma) to TGY broth. Bacteria were subsequently washed with PBS, and adherence assays were performed as described above. As shown in Fig. 1D, bacteria collected from iron-limited cultures displayed significantly higher Vero cell adherence than did those collected from iron-rich or control cultures (P < 0.05). An iron-poor environment could drive C. difficile toward colonization of target tissues, which should facilitate acquisition of iron from tissues later damaged by the pathogenic process.
Sodium chloride.
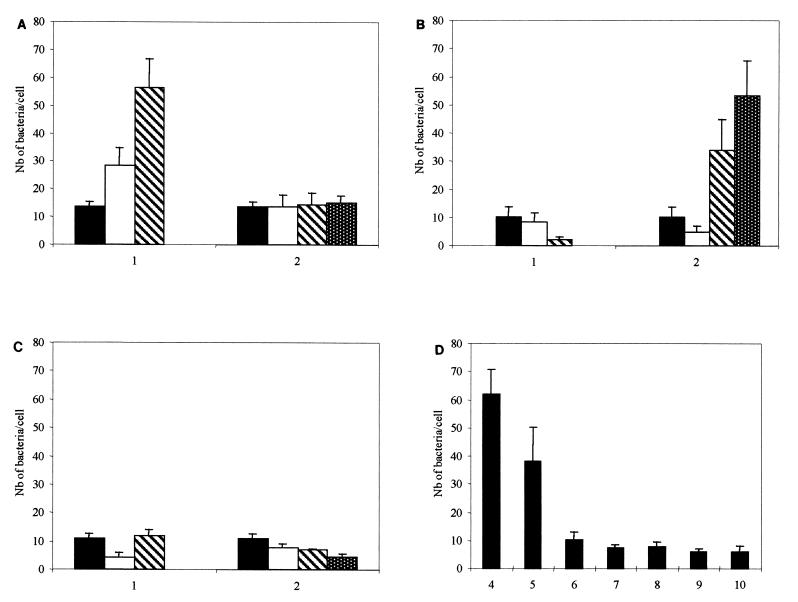
Osmolarity is one environmental factor which regulates the interaction of enteric microorganisms with eucaryotic cells (6, 20, 23, 25–27, 31). To investigate whether C. difficile adherence to cells is osmoregulated, bacteria were cultivated in TGY broth containing 50 (low osmolarity), 150 (medium osmolarity), or 500 (hyperosmolarity) mM NaCl. As shown in Fig. 2A, part 1, adherence of bacteria grown in the presence of 500 mM NaCl was increased three- to fourfold, as opposed to 50 and 150 mM NaCl (P < 0.05). In contrast, adherence of C. difficile grown in the presence of 50 mM NaCl was not influenced by modifications of the sodium concentration of the adherence assay medium (Fig. 2A, part 2).
FIG. 2.
Effects of cations and pH on C. difficile adherence to Vero cells. (A) Part 1, NaCl concentrations in growth medium: ■ (control [TGY broth]), 50 mM; □, 150 mM; ▧, 500 mM. Part 2, NaCl concentrations in adhesion medium: ■, control; □, 50 mM; ▧, 150 mM; ▩, 500 mM. (B) Part 1, CaCl2 concentrations in growth medium: ■ (control [TGY broth]), 1 mM; □, 12.5 mM; ▧, 25 mM. Part 2, CaCl2 concentration in adhesion medium: ■, control; □, 1 mM; ▧, 12.5 mM; ▩, 25 mM. (C) Part 1 MgCl2 concentrations in growth medium: ■ (control [TGY broth]), 1 mM; □, 22.5 mM; ▧, 45 mM. Part 2, MgCl2 concentrations in adhesion medium: ■, control; □, 1 mM; ▧, 22.5 mM; ▩, 45 mM. (D) Treatment of C. difficile at pHs 4 to 10 before adhesion assay. Nb, number.
Interestingly, when bacteria were grown at high NaCl concentrations, morphological modifications were evident; i.e., they were short and surrounded by viscous material (data not shown). High osmolarity may induce physicochemical changes in the bacterial surface, especially hydrophobicity (19, 34), thus changing adherence properties. Alternatively, high osmolarity, along with other environmental stimuli (O2, temperature, and starvation) can change DNA superhelicity, thus increasing transcription of adherence-related genes (13).
Calcium and magnesium.
We examined whether concentrations of calcium and magnesium deviating from physiological values could influence C. difficile adherence to cells. Variations in the calcium concentration in the growth medium did not influence adherence (Fig. 2B, part 1). On the other hand, adherence was increased 13-fold when the adherence assay was performed in the presence of 12.5 mM CaCl2 and 17-fold when it was performed in the presence of 25 mM CaCl2, compared with the standard condition (P < 0.05) (Fig. 2B, part 2). This augmentation could be linked to modification of the net positive charge of the C. difficile surface (19); thus, calcium could simply play a nonspecific role in adherence.
Magnesium chloride did not modify adherence at any of the concentrations tested (1, 22.5, and 45 mM), whether present in the growth or adherence medium (Fig. 2C). The absence of an effect due to MgCl2 suggests that the phenomenon observed with other chlorides is solely due to the cation part of the salt.
Acid shock.
pH variations in the colon could occur due to alimentary variations, drugs (11), as a result of inflammation (21), or other, as yet unknown, factors. C. difficile spores or vegetative forms could therefore encounter acidic or basic pH in the intestine (1). To determine the effect of pH on C. difficile adherence, bacteria were washed and resuspended in PBS with pHs ranging from 4 to 10 and incubated for 45 min at 37°C. The low pH of the bacterial suspension was rapidly neutralized during the incubation with Vero cells, excluding the hypothesis that variations of C. difficile adherence could be due to modification of Vero cell receptors. Viability of Vero cells after incubation at pH extremes was confirmed with trypan blue dye (Sigma). As shown in Fig. 2D and 3B, maximal adhesion occurred at pH 4 with an 8.3-fold increase (P < 0.05) compared with standard conditions (pH 6.8; Fig. 2D). The increase in adherence caused by an acidic pH could result from (i) unmasking of a bacterial cell surface component with adhesin activity (22) or (ii) induction of expression of the adhesin itself.
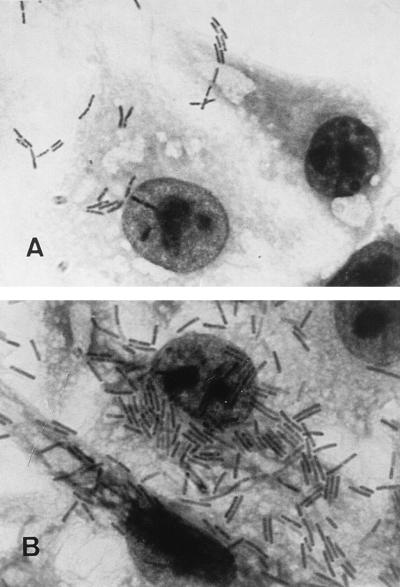
FIG. 3.
Patterns of C. difficile adherence to Vero cells examined by light microscopy (magnification, ×1,000). Panels: A, control (C. difficile treated at pH 7.0 before adhesion assay); B, C. difficile treated at pH 4 before adhesion assay.
Adhesins involved in cell attachment.
We investigated whether expression of C. difficile adhesive proteins is modified under conditions that were found, as described above, to alter cell adherence. Extraction of surface proteins and separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis were performed as described by Wexler et al. (33). Subsequently, proteins were electrically transferred to a polyvinylidene fluoride membrane (Millipore) which was blocked with 5% bovine serum albumin at 37°C for 4 h, washed with PBS, and incubated for 90 min at 37°C under 5% CO2 with Vero cells metabolically labeled with l-[35S]methionine (Amersham) for 4 h and resuspended in minimum essential medium (Life Technologies) (105 cells/cm2 of membrane). After washing with PBS, protein-bound cells on the blotted membrane were detected by exposure of the membrane to Kodak Biomax photographic film.
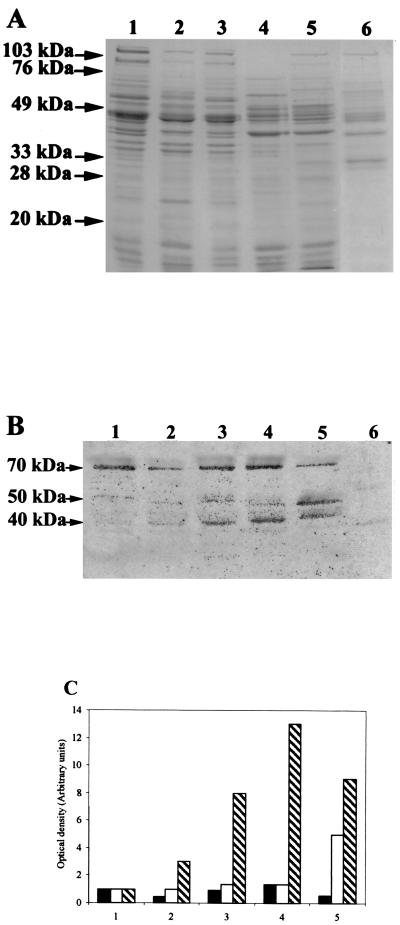
Three surface proteins with molecular masses of 70, 50, and 40 kDa able to bind to Vero cells were observed (Fig. 4A and B). The binding of the 70-kDa protein to Vero cells varied little as a function of the environmental conditions, in contrast to that of the 40-kDa protein, whose expression was enhanced by acidic or osmotic shock and iron limitation. Bacteria cultured in NaCl-rich medium also overexpressed the 50-kDa protein (Fig. 4B and C).
FIG. 4.
Adhesins involved in cell attachment. (A) Surface proteins from C. difficile grown under various conditions (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). (B) Proteins on membrane which bound Vero cells (autoradiography). (C) Optical density determined by densitometric scanning of autoradiograms for the 70-kDa (■), 50-kDa (□), and 40-kDa (▧) proteins (expressed in comparison with the control). Lanes: 1, non-heat-shocked C. difficile; 2, C. difficile heat shocked under partial anaerobic conditions; 3, C. difficile treated at pH 4; 4, C. difficile grown in the presence of 200 μM 2,2′-dipyridyl; 5, C. difficile grown in the presence of 500 mM NaCl; 6, negative control (Clostridium indolis proteins [nonadherent, normal-flora bacterial strain]).
We have identified several C. difficile genes encoding heat shock proteins (e.g., DnaK and GroEL), some of which may turn out to be identical to the three proteins described here.
Acknowledgments
This work was supported in part by the FAIR Program of the European Union (CT95-0433) and by the ACC-SV6 program of the Ministère de l’Education Nationale, de l’Enseignement Supérieur, et de la Recherche of France.
REFERENCES
- 1.Bermudez L E, Petrofsky M, Goodman J. Exposure to low oxygen tension and increased osmolarity enhance the ability of Mycobacterium aviumto enter intestinal epithelia (HT-29) cells. Infect Immun. 1997;65:3768–3773. doi: 10.1128/iai.65.9.3768-3773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borriello S P. Clostridium difficileand its toxins in the gastrointestinal tract in health and disease. Res Clin Forums. 1979;1:33–35. [Google Scholar]
- 3.Borriello S P. Pathogenesis of Clostridium difficileinfection of the gut. J Med Microbiol. 1990;33:207–215. doi: 10.1099/00222615-33-4-207. [DOI] [PubMed] [Google Scholar]
- 4.Borriello S P, Davies H A, Barclay F E. Detection of fimbriae amongst strains of Clostridium difficile. FEMS Microbiol Lett. 1988;49:65–67. [Google Scholar]
- 5.Borriello S P, Welch A R, Barclay F E, Davies M A. Mucosal association by Clostridium difficilein the hamster gastrointestinal tract. J Med Microbiol. 1988;25:191–196. doi: 10.1099/00222615-25-3-191. [DOI] [PubMed] [Google Scholar]
- 6.Cacalano G, Kays M, Saiman L, Prince A. Production of the Pseudomonas aeruginosaneuraminidase is increased under hyperosmolar conditions and is regulated by genes involved in alginate expression. J Clin Investig. 1992;89:1866–1874. doi: 10.1172/JCI115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conte M P, Longhi C, Buonfiglio V, Polidoro M, Seganti L. The effect of iron on the invasiveness of Escherichia coli carrying the inv gene of Yersinia pseudotuberculosis. J Med Microbiol. 1994;40:236–240. doi: 10.1099/00222615-40-4-236. [DOI] [PubMed] [Google Scholar]
- 8.Conte M P, Longhi C, Polidoro M, Petrone G, Buonfiglio V, di Santo S, Papi E, Seganti L, Visca P, Valenti P. Iron availability affects entry of Listeria monocytogenesinto the enterocytelike cell line Caco-2. Infect Immun. 1996;64:3925–3929. doi: 10.1128/iai.64.9.3925-3929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies H A, Borriello S P. Detection of capsule in strains of Clostridium difficile. Microb Pathog. 1990;9:141–146. doi: 10.1016/0882-4010(90)90088-8. [DOI] [PubMed] [Google Scholar]
- 10.Delmee M, Homel M, Wauters G. Serogrouping of Clostridium difficilestrains by slide agglutination. J Clin Microbiol. 1985;21:323–327. doi: 10.1128/jcm.21.3.323-327.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans D F, Pye G, Bramley R, Clark A G, Dyson T J, Hardcastle J D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eveillard M, Fourel V, Barc M-C, Keineis S, Coconier M-H, Karjalainen T, Bourlioux P, Servin A. Identification and characterization of adhesive factors of Clostridium difficile involved in adhesion to human colonic enterocyte-like Caco-2 and mucus-secreting HT29 cells in culture. Mol Microbiol. 1993;7:371–381. doi: 10.1111/j.1365-2958.1993.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 13.Galán J E, Curtiss R., III Expression of Salmonella typhimuriumgenes required for invasion is regulated by changes in DNA supercoiling. Infect Immun. 1990;58:1879–1885. doi: 10.1128/iai.58.6.1879-1885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George W L. Antimicrobial agent associated colitis and diarrhea: historical background and clinical aspects. Rev Infect Dis. 1984;6:208–213. doi: 10.1093/clinids/6.supplement_1.s208. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Trevino M, Boureau H, Karjalainen T, Bourlioux P. Clostridium difficile adherence to mucus: results of an in vivo and ex vivoassay. Microb Ecol Health Dis. 1996;9:329–334. [Google Scholar]
- 16.Karjalainen T, Barc M-C, Collignon A, Trollé S, Boureau H, Cotte-Laffitte J, Bourlioux P. Cloning of a genetic determinant from Clostridium difficileinvolved in adherence to tissue culture cells and mucus. Infect Immun. 1994;62:4347–4355. doi: 10.1128/iai.62.10.4347-4355.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karjalainen T, Collignon A, Barc M-C, Bourlioux P. Molecular cloning of the collagenase gene of Clostridium difficile. In: Duerden P I, et al., editors. Medical and environmental aspects of anaerobes. Northwood, United Kingdom: Science Reviews; 1995. pp. 407–412. [Google Scholar]
- 18.Karjalainen T, Poilane I, Collignon A, Barc M-C, Gomez-Trevino M, Boureau H, Bourlioux P. Clostridium difficilevirulence: correlation between toxigenicity, adherence, enzyme production and serogroup. Microecol Ther. 1995;25:157–163. [Google Scholar]
- 19.Krishna M M, Powell N B L, Borriello S P. Cell surface properties of Clostridium difficile: haemagglutination, relative hydrophobicity and charge. J Med Microbiol. 1996;44:115–123. doi: 10.1099/00222615-44-2-115. [DOI] [PubMed] [Google Scholar]
- 20.Kunin C M, Hua T H, Guerrant R L, Bakaletz L O. Effect of salicylate, bismuth, osmolytes, and tetracycline resistance on expression of fimbriae by Escherichia coli. Infect Immun. 1994;62:2178–2186. doi: 10.1128/iai.62.6.2178-2186.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J-Y, Caparon M. An oxygen-induced but protein F-independent fibronectin-binding pathway in Streptococcus pyogenes. Infect Immun. 1996;64:413–421. doi: 10.1128/iai.64.2.413-421.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S G, Kim C, Ha Y C. Successful cultivation of a potentially pathogenic coccoid organism with trophism for gastric mucin. Infect Immun. 1997;65:49–54. doi: 10.1128/iai.65.1.49-54.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mai G T, McCormack J G, Seow W K, Pier G B, Jackson L A, Thong Y H. Inhibition of adherence of mucoid Pseudomonas aeruginosaby alginase, specific monoclonal antibodies, and antibiotics. Infect Immun. 1993;61:4338–4343. doi: 10.1128/iai.61.10.4338-4343.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riegler M, Sedivy R, Pothoulakis C, Hamilton G, Zacherl J, Bischof G, Cosentini E, Feil W, Schiessel R, LaMont J T, et al. Clostridium difficiletoxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Investig. 1995;95:2004–2011. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saiman L, Cacalano G, Gruenert D, Prince A. Comparison of adherence of Pseudomonas aeruginosato respiratory epithelial cells from cystic fibrosis patients and healthy subjects. Infect Immun. 1992;60:2808–2814. doi: 10.1128/iai.60.7.2808-2814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saiman L, Ishimoto K, Lory S, Prince A. The effect of piliation and exoproduct expression on the adherence of Pseudomonas aeruginosato respiratory epithelial monolayers. J Infect Dis. 1990;161:541–548. doi: 10.1093/infdis/161.3.541. [DOI] [PubMed] [Google Scholar]
- 27.Sauvage M, Eloumi N, Capmau M L, Hulen C. Inhibition de la biosynthèse des alginates chez les souches mucoides de Pseudomonas aeruginosa. Pathol Biol. 1991;39:606–612. [PubMed] [Google Scholar]
- 28.Seddon S V, Borriello S P. Proteolytic activity of Clostridium difficile. J Med Microbiol. 1992;36:307–311. doi: 10.1099/00222615-36-5-307. [DOI] [PubMed] [Google Scholar]
- 29.Seddon S V, Hemingway I, Borriello S P. Hydrolytic enzyme production by Clostridium difficileand its relationship to toxin production and virulence in the hamster model. J Med Microbiol. 1990;31:169–174. doi: 10.1099/00222615-31-3-169. [DOI] [PubMed] [Google Scholar]
- 30.Tamura G S, Kuypers J M, Smith S, Raff H, Rubens C E. Adherence of group B streptococci to cultured epithelial cells: roles of environmental factors and bacterial surface components. Infect Immun. 1994;62:2450–2458. doi: 10.1128/iai.62.6.2450-2458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tartera C, Metcalf E S. Osmolarity and growth phase overlap in regulation of Salmonella typhiadherence to and invasion of human intestinal cells. Infect Immun. 1993;61:3084–3089. doi: 10.1128/iai.61.7.3084-3089.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tasteyre, A., M.-C. Barc, T. Karjalainen, P. Dodson, S. Hyde, N. Powell, S. Bulteau, P. Bourlioux, and P. Borriello. Identification, characterization, and expression of a gene encoding flagellin of Clostridium difficile. Submitted for publication.
- 33.Wexler H, Mulligan M E, Finegold S M. Polyacrylamide gel electrophoresis patterns produced by Clostridium difficile. Rev Infect Dis. 1984;6:S229–S234. doi: 10.1093/clinids/6.supplement_1.s229. [DOI] [PubMed] [Google Scholar]
- 34.Wood-Helie S J, Dalton H P, Shadomy S. Hydrophobic and adherence properties of Clostridium difficile. Eur J Clin Microbiol. 1986;5:441–445. doi: 10.1007/BF02075701. [DOI] [PubMed] [Google Scholar]