Abstract
The striatum receives abundant glutamatergic afferents from the cortex and thalamus. These inputs play a major role in the functions of the striatal neurons in normal conditions, and are significantly altered in pathological states, such as Parkinson’s disease. This review summarizes the current knowledge of the connectivity of the corticostriatal and thalamostriatal pathways, with emphasis on the most recent advances in the field. We also discuss novel findings regarding structural changes in cortico- and thalamostriatal connections that occur in these connections as a consequence of striatal loss of dopamine in parkinsonism.
Introduction
The main input nucleus of the basal ganglia, the striatum, is composed of spiny projection neurons (SPNs), and several types of interneurons (Kreitzer 2009; Tepper et al. 2018). The striatal SPNs project to the output nuclei of the basal ganglia (internal globus pallidus (GPi) and substantia nigra reticulata (SNr)) or to the external segment of the globus pallidus (GPe) establishing the direct (dSPNs) and indirect (iSPNs) pathways, respectively (Albin et al. 1989). These pathways are further identified by the selective expression of dopamine (DA) receptors, with D1 receptors (D1Rs) expressed on dSPNs and D2 receptors (D2Rs) on iSPNs, respectively (Gerfen and Surmeier 2011). The striatum receives substantial dopaminergic inputs from the substantia nigra compacta (SNc), GABAergic inputs from the GPe and massive glutamatergic afferents from most cortical regions and several thalamic nuclei. The latter are the focus of this review.
The striatal interneurons include cholinergic interneurons (ChINs) and a diversity of GABAergic interneurons (Assous and Tepper 2019a; Kreitzer 2009; Tepper and Bolam 2004; Tepper et al. 2010). Three main groups of GABAergic interneurons have been traditionally defined: parvalbumin (PV)-expressing (also known as fast spiking interneurons or FSIs), neuropeptide Y and somatostatin (NPY/SOM)-expressing (also termed low-threshold spike or LTS interneurons), and calretinin (CR)-expressing (Kawaguchi et al. 1995) interneurons. The use of transgenic mice has allowed the characterization of new subtypes of striatal GABAergic interneurons according to their molecular, physiological and pharmacological properties (Burke et al. 2017), such as the tyrosine hydroxylase (TH) expressing and NPY-neurogliaform interneurons (Assous and Tepper 2019b). In the last years, genomic-based approaches have served to identify novel striatal cell types which are species unique. For example, in primates a new type of striatal interneuron has been described, which is not found in rodents (Krienen et al. 2020), and SPNs have been subdivided into more than the two traditional ‘direct’ and ‘indirect’ pathway categories, based on their molecular definition and anatomical localization (He et al. 2021).
Besides the anatomical subdivisions (described in the next section), the striatum has been segmented into histochemically defined compartments known as striosomes (‘patches’) and matrix, which are characterized by expression of various neurochemical markers, connectivity patterns and functional roles (Brimblecombe and Cragg 2017; Crittenden and Graybiel 2011).
This review aims to provide an update on the most recent advances on our understanding of the anatomy and connectivity of the corticostriatal and thalamostriatal connections. We also discuss how the loss of dopaminergic innervation to the striatum, which is the main pathological hallmark of parkinsonism, is associated with changes in cortico- and thalamostriatal connections. The current knowledge about the anatomical and functional organization of cortical and thalamic inputs to the striatum is extensive, and in the last few years, the use of viral based tracing (including trans-synaptic tracing with deletion mutant-rabies virus, e.g. Lavin et al. 2020) in combination with transgenic mice lines has provided a more nuanced understanding of the specific cell types that participate in these pathways (Smith et al. 2022). In Table 1 we present a summary of recent studies that have used viral vectors as tracers to map connections to the striatum in rodents and non-human primates. For a more extensive covering of earlier studies in which traditional tracer methods have been used, the reader is directed to previous reviews that have been published on this topic (e.g., Mathai and Smith 2011; Shepherd 2013; Smith et al. 2014a; Bradfield et al. 2013; Galvan et al. 2016; Stayte et al. 2021).
Table 1:
Use of retrograde viral vectors to map connectivity to the striatum
| Study and species | Viral vector* | Animals | Injection site | Type of neuron targeted | Main sources of cortical inputs identified | Thalamic afferents identified |
|---|---|---|---|---|---|---|
| Studies using rabies virus to achieve trans-synaptic followed by retrograde tracing | ||||||
| (Wall et al. 2013) | AAV-EF1a-FLEX-GTB or AAV-FLEX-hGTB; (EnvA)-deltaG- Rabies | D1R-Cre mice | Dorsal striatum (tendency to lateral half) | dSPNs | S1, S2, M1, M2, insular, orbitofrontal; cingulate | PF main source, non-PF contribute |
| D2R-Cre mice | iSPNs | S1, S2, M1, M2, insular, orbitofrontal | PF main source, non-PF | |||
| (Guo et al. 2015) | AAV-DIO -TVA and AAV-DIO-RG; (EnvA)-deltaG-Rabies | D1R-Cre mice | Dorsomedial striatum | dSPNs | Cingulate, M1, M2, S1 | PF, non-PF |
| D2R-Cre mice | iSPNs | Cingulate, M1, M2, S1 | PF, non-PF | |||
| Chat-Cre mice | ChINs | Cingulate, M2 | PF, non-PF | |||
| (Smith et al. 2016) | AAV5-EF1a-Flex-TVA and AAV8-CA-Flex-RG; (EnvA)-deltaG-Rabies | Plxnd1-OG1 (matrix-Cre) mice | Dorsal striatum (no distinction medial vs lateral) | Matrix (preferentially SPNs) | More limbic and sensory cortical inputs to matrix than patch, but both compartments receive inputs from all areas | PF, non-PF |
| Sepw1-NP67 (patch-Cre) mice | Patch (preferentially dSPNs) | |||||
| (Klug et al. 2018) | AAV5-TVA: AAV8-CA-RG; (EnvA)-deltaG-Rabies | ChAT-IRES-Cre mice | Dorsal striatum (no distinction medial vs lateral) | ChINs | Cingulate, M2, S1 | PF |
| PV-Cre mice | PV | Cingulate, M1, M2, S1 | Non-PF | |||
| (Choi et al. 2019) | AAV5-CAG-DIO-TVA; AAV8-CAG-DIO-G; (EnvA)-deltaG-rabies | Drd1a-Cre mice | Dorsomedial striatum | dSPNs | Cingulate, Orbitofrontal, M2, M1, S1 | PF main source, non-PF contribute |
| PV-2a-Cre mice | PV | Cingulate, Orbitofrontal, M2, M1, S1, retrosplenial | PF main source, non-PF contribute | |||
| SST-IRES-Cre mice | SST+ LTSIs. | Cingulate, Orbitofrontal, M2, M1, S1 | PF main source, non-PF contribute | |||
| (Melendez-Zaidi et al. 2019) | AAV9-EF1a-DIO-HTB; (EnvA)-deltaG-rabies | SST-IRES-Cre | Dorsal striatum | LTSIs | Cingulate, M2 | PF |
| Studies using AAVretro (AAVrg) to achieve retrograde tracing | ||||||
| (Tervo et al. 2016) | AAVrg-hSyn-Cre | Rosa26-LSL-H2B-EGFP mice | Dorsomedial striatum | Unspecific | Perirhinal, ectorhinal temporal, auditory | PF, non-PF |
| (Weiss et al. 2020) | AAVrg-CMV | Rhesus macaques | Caudate nucleus (head) and putamen | Unspecific | Prefrontal, orbitofrontal, premotor, cingulate, supplemental motor, M1, insular | Non-PF** |
| (Albaugh et al. 2020) | AAVrg-hSyn | Rats | Dorsal striatum | Unspecific | Mulitple cortical regions | PF, non-PF |
| Rhesus macaques | Putamen | Multiple cortical regions, most prominent in frontal regions. | Negligible** | |||
For simplicity the reporter proteins used are not mentioned in this table
Note that the lack of thalamostriatal inputs identified with AAVrg in rhesus macaques contrasts with previous literature findings, and likely indicates a pathway selectivity of the vector. See main text for further details.
Corticostriatal Inputs
Anatomical organization of corticostriatal inputs
The corticostriatal inputs have been well described for decades and present an anatomo-functional organization based on the cortical origin of those inputs (Alexander et al. 1986; Pan et al. 2010). Thus, the striatum of the rodent and primate is classically subdivided into three functional territories. In the primate, we distinguish the posterior putamen, connected to the primary motor cortex (M1), the premotor areas and the somatosensory cortex, which forms the motor striatum; the anterior putamen, along with the body and the head of the caudate nucleus, connected to the premotor, prefrontal, parietal and temporal areas, which correspond to the associative striatum; the ventral part of the anterior regions of the caudate nucleus and putamen, including the nucleus accumbens (NAcc), connected to the orbitofrontal cortex, anterior cingulate cortex and amygdala, which form the limbic striatum (Alexander et al. 1986; Selemon and Goldman-Rakic 1985; Kunzle 1977, 1975; Goldman and Nauta 1977). In the rodent, these territories correspond to the dorsolateral striatum (receiving inputs primarily from motor and somatosensory cortices), the dorsomedial striatum (which receives a majority of inputs from frontal and associative cortices) and the NAcc (which receives inputs from limbic cortices) (McGeorge and Faull 1989; Voorn et al. 2004). Although traditional findings indicate that striosomes and matrix compartments receive preferentially inputs from limbic and sensorimotor cortex, respectively (Crittenden and Graybiel 2011), recent work using retrograde virus as tracers and transgenic mice has shown that both matrix and striosomes receive cortical information from sensorimotor, limbic and associative regions (Table 1, Smith et al. 2016).
Most of the cortical neurons projecting to the striatum are small to medium sized pyramidal neurons in layers 3 and 5. In rodents, two main categories of corticostrial neurons have been distinguished: the intratelencephalic (IT) neurons mainly located in layer 3 and upper layer 5 and the pyramidal tract (PT) neurons located in the lower layer 5 (Levesque et al. 1996a; Levesque et al. 1996b; Reiner et al. 2003; Landry et al. 1984; Wilson 1987; Levesque and Parent 1998)
The axonal projection of IT neurons innervates the ipsilateral and contralateral cortex and striatum, while the PT neurons innervate the brainstem and spinal cord and give rise to axon collaterals that innervate the striatum. Anatomical tracing studies have also identified this distinction in population of corticostriatal projection neurons in monkeys (Parent and Parent 2006). For example, in the macaque brain, with the use of retrograde tracer injections in the caudate, contribution of layer 3 in the temporal cortex and the prefrontal cortex was found to be correlated with the density of the corticostriatal cells (Arikuni and Kubota 1986; Saint-Cyr et al. 1990). Note, however, that only few PT neurons sparsely branch to the striatum, and these neurons do not innervate other forebrain, midbrain or spinal cord regions (Smith et al. 2014b). This is in stark contrast to rats, in which many PT neurons show a prominent collateralization to the striatum (Sinopoulou et al. 2022).
In the last few years, progress has been made towards a more nuanced description of the corticostriatal projection. By using retrograde tracers, it was shown that the laminar pattern of corticostriatal projections differs according to the striatal area targeted in macaque monkeys. Thus, projections from the superior temporal cortex to the head of the caudate arise from layers 3 and 5, whereas those targeting the tail of the caudate originate in layers 3 and 6 (Griggs et al. 2017). Similarly, other study that examined corticostriatal projections from the frontal motor areas and the frontal operculum, showed that, after injections of retrograde tracer in the motor putamen, the proportion of corticostriatal labeled neurons in layers 3 and/or 6 was comparable or even stronger than in layer 5. Furthermore, within these regions, the laminar distribution pattern of corticostriatal labeled neurons largely varied independently of their density and projecting area, but likely according to the target striatal zone (Borra et al. 2021). The authors emphasized that layer 6 could be a source of corticostriatal inputs. Given that layer 6 is traditionally host to corticothalamic or corticocortical pyramidal cells (Chen et al. 2009; Llano and Sherman 2008; Bourassa and Deschenes 1995; Bourassa et al. 1995; Deschenes et al. 1998; Thomson 2010), these results suggest that these corticostriatal pyramidal cells represent a newly described class of layer 6 neuron. Also, a novel connection was recently described in the mouse auditory cortex, originating from layer 4 (which had been considered to be involved exclusively in intracortical circuits) to the striatum (Bertero et al. 2022).
The laminar pattern of the corticostriatal projection, which appears to be more complex than previously described, suggests an elaborated information processing, in which different aspects of motor and nonmotor functions provide converging input to the striatum. Indeed, the different striatal functional territories seem to combine information from various cortical layers and areas, possibly differing in terms of information coding, timing, and direction of information flow. Such view challenges the classic notion of segregated functional loops (Alexander et al. 1986).
Recently, the study of neural circuits has been revolutionized using transcriptomics to examine genetic expression at the regional or single cell level. These studies complement anatomical tracer studies by examining the gene expression patterns across brain structures that are functionally related, such as the cortex and the striatum. Using human and primate brain transcriptional atlases, Anderson et al (2018) demonstrated that spatial patterns of gene expression show strong correspondence with limbic (including NAcc and orbital frontal cortex) and motor (including the dorsal putamen and motor, auditory and sensory cortices) corticostriatal functional networks (Anderson et al. 2018). These observations, which are consistent across humans and evolutionarily conserved in non-human primates, suggest that functionally coupled cortex and striatum regions shared profiles of gene expression. Along the same line, a recent neuroimaging study comparing data from human and non-human primate, provided evidence for a correspondence between corticostriatal functional networks (Liu et al. 2021). Although the study reports significant similarity in the corticostriatal resting state functional connectivity between human and macaque rostral caudate and putamen, it also revealed cross-species differences in the connectivity of the dorsal caudate with prefrontal regions, somatosensory cortex, and visual areas of cortex.
Neuronal targets of corticostriatal inputs
As described above, the striatum internal circuitry is composed by an heterogenous neuronal population. The cellular and ultrastructural targets of the corticostriatal inputs provide another level of complexity to these projections.
The cortical inputs target preferentially the SPNs by forming asymmetric synapses at the level of the dendritic spines (Somogyi et al. 1981; Frotscher et al. 1981; Kemp and Powell 1971; Smith and Bolam 1990). Studies using retrograde tracers have found that in the dorsolateral striatum of rats, striatonigral (dSPNs) preferentially receive input from IT cortical neurons, whereas striatopallidal neurons (iSPNs) receive greater input from PT cortical neurons (Lei et al. 2004; Deng et al. 2015). Electron microscopy studies and physiological recordings have shown that neurons in many of the cortical input areas connect with both types of SPNs, as well as with ChINs and GABAergic interneurons (Lei et al. 2004; Lapper and Bolam 1992; Assous and Tepper 2019a). In rodents in particular, the development of genetics tools with numerous Cre-expressing mouse lines has provided the opportunity to study how the corticostriatal connections are established within the striatum, revealing a non-uniform and highly specific organization of diverse cortical inputs onto the striatal interneurons and SPNs. Using rabies virus-based trans-synaptic tracing methods injected in the dorsal striatum of mice, it has been shown that both types of SPNs and ChINs receive monosynaptic inputs from discrete brain areas in the cortex (Table 1, Guo et al. 2015; Wall et al. 2013). In the dorsal striatum, these inputs arise from areas involved in the sensory and motor processing, but also from limbic areas such as the cingulate cortex and the amygdala.
Modified rabies virus tracing was also used to study the cortical input patterns onto striatal ChINs and PV-positive GABAergic interneurons. The rabies virus injections were performed in the dorsal striatum of transgenic mice in which Cre recombinase was expressed in either ChAT or PV-positive neurons (Table 1, Klug et al. 2018). The tracing results revealed that both types of striatal interneurons received a vast majority of inputs from the cortex. The ChINs receive inputs from the cingulate cortex and the secondary motor cortex (M2) while the PV-expressing interneurons receive inputs from similar cortical regions with the addition of a substantial primary motor and primary somatosensory cortex inputs. This suggests a role for PV-positive interneurons in sensorimotor integration, while ChINs are contacted by associative regions of cortex. Moreover, in this study, the authors identified that the cortical neurons projecting to the ChINs and PV-expressing interneurons originate from cortical layers 2/3, 5 and 6.
Johansson and Silberberg (2020) examined whether the synaptic inputs from the primary sensory (S1) and primary motor (M1) cortices targeted specific cell types in the mouse dorsolateral striatum. (Johansson and Silberberg). The organization of corticostriatal inputs was mapped onto five types of striatal neurons (SPNs and interneurons) to determine their synaptic strength, short term plasticity and receptor composition. By optogenetically activating the ipsilateral or contralateral inputs while simultaneously whole-cell recording from the SPNs and neighboring interneurons, it was found that ipsi- and contralateral S1 and M1 inputs target dSPNs and iSPNs, but the types of co-activated interneurons are unique for each cortical input. PV interneurons receive the strongest inputs from S1 and M1, NPY/SOM interneurons are primarily excited by contralateral M1, and ChINs receive no input from contralateral M1 and S1. Moreover, S1 afferents densely target the dorsolateral striatum, but rarely contact NPY/SOM and ChINs, while M1 ipsilateral afferents innervate the dorsolateral and dorsomedial striatum and frequently form functional synapses onto NPY/SOM and ChINs (Figure 1). These anatomical findings support behavioral studies identifying the dorsolateral striatum as the neural substrate for sensorimotor integration, whereas the dorsomedial striatum acts as an associative territory central to instrumental conditioning and behavioral flexibility. Transgenic mouse lines and optogenetics slice electrophysiology has also been used to assess the cellular origin of corticostriatal inputs. With these tools, a recent study described an excitatory circuit by which IT and PT corticostriatal inputs differentially impact the SPNs activity in the dorsolateral striatum of mice (Morgenstern et al. 2022). This circuit is predominantly constituted by a polysynaptic connection from cortical neurons to ChINs to glutamate-releasing axons, running in parallel to the monosynaptic connection from the cortex to the SPNs. In this circuit, responses are preferentially evoked by PT neurons, rather than IT neurons, because of a stronger PT-cholinergic interneurons strength (Figure 2) (Morgenstern et al. 2022). This study, combined with the data from Johansson and Silberberg (2020), showing that ChINs in the dorsolateral striatum are exclusively contacted by ipsilateral cortical axons, indicate that ChINs receive predominant PT inputs. In the same way, the PV-positive interneurons receive a strong connectivity from IT neurons.
Figure 1: Synaptic strength and innervation of four inputs to five striatal cell types.
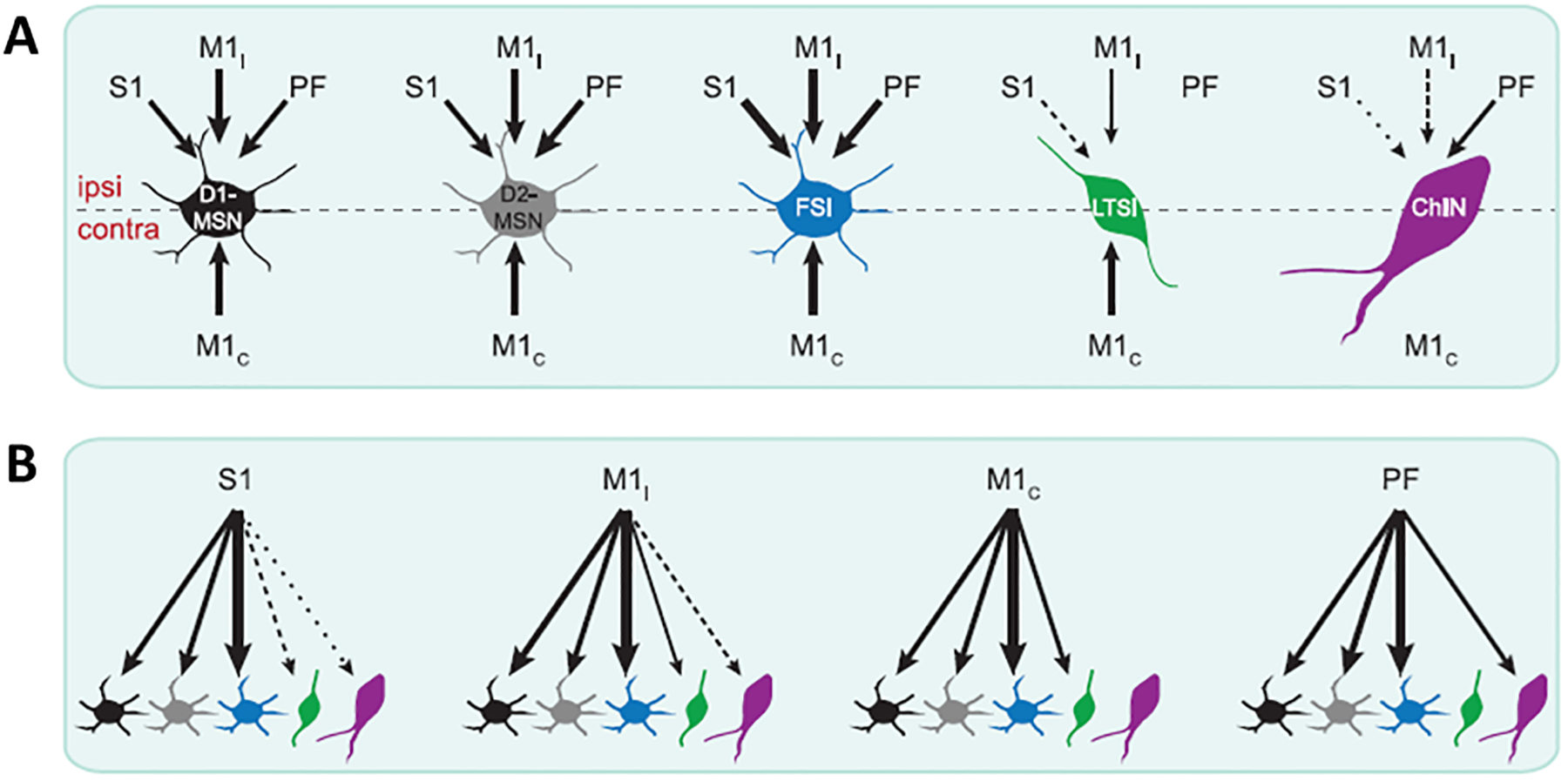
A: Schematic summarizing which input is most robustly exciting each cell type. Primary sensory (S1), primary motor (M1) (ipsi (M1i) or contralateral (M1c)) and prefrontal (PF) cortex projection to the different striatal projecting neurons (D1-MSN; D2-MSN), Fast-spiking interneurons (FSI), low threshold spiking interneurons (LTSI) and cholinergic interneurons (ChIN). B: schematic illustrating which cell types are most robustly excited by each input. The thickness of the arrow reflect how strong responding neurons are excited by each input (from Johansson and Silberberg 2020).
Figure 2: PT neurons amplify excitatory inputs to striatum through ChIs.
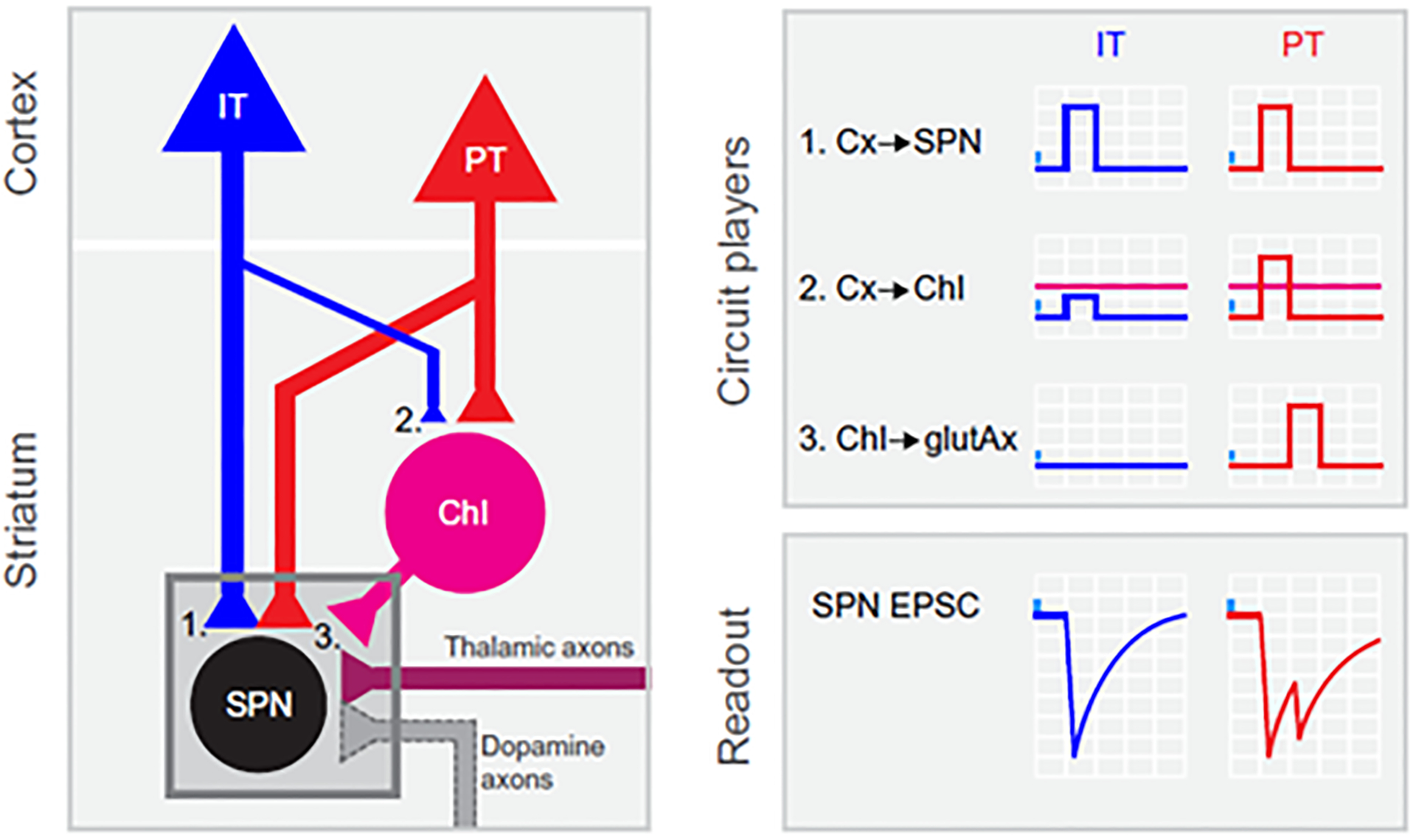
Summary of the results: left, circuit diagram proposed for corticostriatal connectivity. IT and PT cortical neurons project to both projection neurons (SPNs) (1) and ChINs (2). While PT➔SPN (1, red) and PT➔ChI (2, red) input strength is similar, IT➔ChI (2, blue) connection is weaker than IT➔SPN (1, blue). Within the striatum, ChIs convey excitation to SPNs by recruiting long-range glutamate-releasing terminals reaching DLS through α4-containing nicotinic receptors (3). Right: Schematic of the activation of the different circuit players upon IT or PT photostimulation and its impact on the recorded SPNs. Magenta horizontal lines represent ChI spiking threshold (from Morgenstern et al. 2022).
The improved understanding and mapping of the corticostriatal projections in relation with the neuronal diversity of the striatum allowed us to clarify the role those local microcircuits play in the processing of information within the striatum. The evidence currently available shows that this processing is not limited to a simple summation of cortical inputs onto SPNs. Intra-striatal connectivity, provided by all striatal interneurons and by local axon collaterals of SPNs, directly modulates SPNs’ synaptic inputs and excitability. This complex organization of the corticostriatal inputs is likely to be the basis of an elaborate processing of signals and may have differential implications on behavior and plasticity. Future studies in which the identified corticostriatal pathways are differentially manipulated in vivo in rodent or non-human primate models, could help assess the functional significance of the distinct cortical origins and targets of the cortical inputs to the striatum.
Thalamostriatal Inputs
Anatomical organization of thalamic inputs
Neurons in the caudal intralaminar nuclei (centromedian/parafascicular (CM/PF) complex) are the main source of thalamic inputs to the striatum. In primates, the CM and PF are two separate nuclei, while in rodents, the caudal intralaminar nuclei are a single structure known as PF, the lateral part of which has been considered as the homologue of the primate CM (Jones 2007).
The CM/PF projections to the striatum are topographically organized and display a clear functional segregation. In primates, the CM projects to the post-commissural (sensorimotor) putamen, while the rostral and caudal portions of the PF project to the NAcc and to the caudate (limbic and associative striatum respectively) (Galvan et al. 2016; Smith et al. 2004). An equivalent segregation can be found in rodents, in which the lateral part of the PF projects to the dorsolateral (sensorimotor) striatum and the medial PF projects to the dorsomedial (associative) striatum (Berendse and Groenewegen 1990; Mandelbaum et al. 2019). Recent studies in the mouse have revealed further details of the PF-striatal projection, showing that this pathway is composed of various neuronal subclasses, each with distinct transcriptional and physiological characteristics, and each reciprocally connected with specific functional cortical regions (Mandelbaum et al. 2019). Thus, ‘medial’ PF neurons project to the matrix compartment of the medial striatum, and to limbic cortical regions; neurons in the central portion of PF project to dorsomedial striatum and to limbic and associate regions of cortex; while lateral PF neurons project to dorsolateral striatum and to somatosensory regions of cortex (Mandelbaum et al. 2019). The functional segregation of the PF-striatal pathways was confirmed using monosynaptic retrograde rabies virus and chemogenetic manipulation (Zhang et al. 2022). These studies showed that connection from the PF to the dorsal striatum is involved in motor behaviors, while PF projections to the ventral (limbic) striatum (NAcc) have a role in mood (Zhang et al. 2022).
Besides the CM/PF, the striatum receives significant inputs from other thalamic nuclei, mainly from the rostral intralaminar (including the central lateral (CL), paracentral, central medial), midline, the mediodorsal, the motor nuclei (ventral anterior-ventral lateral (VA/VL) complex) and the pulvinar (Berendse and Groenewegen 1990; Beckstead 1984; Smith and Parent 1986; Groenewegen and Berendse 1994; Alloway et al. 2014), but it is now clear that most of the thalamus projects to some degree to the striatum (Hunnicutt et al. 2016). As is the case for the projections from the CM/PF, other thalamic nuclei (from here on termed ‘non-CM/PF’ or ‘non-PF’ in the case of rodents) display a topographical organization and preserve functional segregation. For instance, the dorsal midline thalamic nuclei project to the NAcc while the rostral intralaminar group targets preferentially the dorsal striatum (Berendse and Groenewegen 1990; Van der Werf et al. 2002; Elena Erro et al. 2002; Gimenez-Amaya et al. 2000; Nakano et al. 1990; Parent et al. 1983; de las Heras et al. 1999).
Studies that integrated large-scale viral tracing databases showed that thalamic projections to the striatum overlap with functionally related cortical projections, and help delineate distinct functional regions in the striatum in mice (Hunnicutt et al. 2016). Similarly, in primates the sensorimotor putamen receives overlapping inputs from motor areas of thalamus and cortex that are interconnected (McFarland and Haber 2000), and convergent connectivity patterns have been proposed for projections from the VA and frontal cortices with associative regions of the striatum (Haber and Calzavara 2009). Such arrangements support interactions of functionally related corticostriatal and thalamostriatal projections.
At the compartmental level, the thalamic input to the striatum targets both the matrix and the striosome compartments, but the inputs to each of these compartments originate from different thalamic nuclei. Studies in primates and in rodents using traditional anatomical tracers found that CM/PF projections target mostly the matrix compartment, while inputs from non-CM/PF nuclei terminate in the matrix and striosomes (Herkenham and Pert 1981; Sadikot et al. 1992b; Berendse and Groenewegen 1990; Fujiyama et al. 2019; Ragsdale and Graybiel 1991; Raju et al. 2006). This pattern has been confirmed in more recent rodent studies that used viral vectors tracers combined with single-cell reconstruction (Unzai et al. 2017) or with transgenic mice in which Cre is selectively expressed in the matrix or striosome (Smith et al. 2016). The latter study found that both compartments receive comparable inputs rom PF, but associative thalamic nuclei project preferentially to striosomes (Smith et al. 2016).
Neuronal targets of thalamostriatal inputs
Thalamostriatal inputs contact SPNs and multiple types of striatal interneurons. Although earlier studies were not able to discriminate SPNs subtypes, the use of bacterial artificial chromosome (BAC) transgenic mice has allowed identification of SPNs from the direct (D1R-expressing) or indirect (D2R-expressing) pathways (Doig et al. 2010; Lei et al. 2013). These studies found that neurons of both pathways receive similar proportions of thalamic terminals (Doig et al. 2010; Lei et al. 2013). While some evidence suggests a slight preference for direct pathway neurons (Lei et al. 2013; Sidibe and Smith 1996), recent studies in rodents using rabies virus to trans-synaptically trace projections to specific striatal neuronal subtypes, have confirmed that SPNs in direct and indirect pathways receive thalamic inputs (from PF as well as from other nuclei) in comparable proportions (Guo et al. 2015; Wall et al. 2013).
Among striatal interneurons, ChINs receive prominent projections from the CM, as demonstrated by tract tracing (i.e., Sidibe and Smith 1999; Lapper and Bolam 1992) and rabies viral vectors studies (Guo et al. 2015; Wall et al. 2013). Thalamic contacts are found from proximal to distal dendrites of ChINs, indicating that thalamic inputs can regulate the activity of these neurons at many locations along their dendritic tree (Doig et al. 2014). As we discuss below, the thalamic modulation of ChINs has a tight interaction with dopaminergic inputs from the nigrostriatal pathway. According to Tanimura et al. (2019), the inputs to ChINs arise only from PF, but not other thalamic nuclei. Furthermore, the PF neurons that contact these interneurons represent a particular neuronal population, that, in contrast to PF neurons that innervate SPNs, lack expression of the retinol binding protein 4 (Rbp4, a retinol transporter) (Tanimura et al. 2019).
Several other types of striatal interneuron receive thalamic contacts, albeit to a lesser extent than ChINs. In primates, CM terminals make synaptic contact with parvalbumin- and somatostatin-positive dendrites (but not with calretinin-positive elements) (Sidibe and Smith 1999). In rodents PV-expressing interneurons receive thalamic inputs on proximal dendrites (Rudkin and Sadikot 1999; Nakano et al. 2018; Zheng et al. 2021), although most of these contacts arise from non-PF nuclei, with a large contribution from motor thalamic nuclei (Nakano et al. 2018). Other types of interneurons that receive contacts from the thalamus are described below, along with comparisons of the cortical inputs to these neurons.
Electron microscopy (EM) studies have analyzed at the ultrastructural level the connectivity patterns of thalamic input to the striatum. Projections from the CM/PF nuclei contact preferentially the dendritic shafts of SPNs, in contrast to inputs from other (non-CM/PF) nuclei, which terminate on the spines of these neurons (Raju et al. 2006; Lacey et al. 2007) Concordant with these observations, most of the thalamic terminals localized in striosomes (known to originate mostly from non-CM/PF nuclei, see above) synapse onto dendritic spines, while thalamic terminals in the matrix compartment, which arise preferentially from CM/PF neurons, tend to terminate onto dendritic shafts (Raju et al. 2006; Fujiyama et al. 2019).
As discussed in previous reviews, the thalamostriatal inputs have been found to play major roles in conveying sensory-related information to the striatum, modulating attention-related cognitive processes, such as behavioral switching and goal-directed action selections (Stayte et al. 2021; Galvan et al. 2016; Alloway et al. 2017; Yamanaka et al. 2018). One of the main roles of the thalamostriatal CM/PF pathway, may be to gate corticostriatal transmission by modulating the activity of ChINs, and through this interaction regulate learning processes related to flexibility of behavior and attentional set-shifting (Kato et al. 2021).
Differences and similarities between corticostriatal and thalamostriatal connections
Direct comparisons of the characteristics of cortico- and thalamostriatal terminals has been facilitated by the discovery that these two inputs preferentially express a different vesicular glutamate transporter, that allows identification of these terminals at the molecular level. Cortical terminals express vGluT1, while thalamic terminals, along with most of other subcortical glutamate structures, express vGluT2 (Fremeau et al. 2004).
Immunohistochemistry against vGluT1 and vGluT2 has frequently been used to identify cortical and thalamic inputs, respectively and increase our understanding of corticostriatal and thalamostriatal connectivity. However, it is important to bear in mind that some neurons in principal relay and association thalamic nuclei (but not in midline and intralaminar nuclei) that project to the striatum co-express mRNAs for vGluT1 and vGluT2 (Barroso-Chinea et al. 2007; Barroso-Chinea et al. 2008), and thus it cannot be ruled out that vGluT1 may be expressed, at least to a low level, in these thalamic terminals (see also Graziano et al. 2008).
Striatal compartments
The traditional description of the connectivity of matrix and striosome compartments state that the matrix receives, preferentially, sensorimotor and associative circuits, while the striosomes receive inputs from limbic regions of cortex (Kincaid and Wilson 1996; Eblen and Graybiel 1995; Gerfen 1989; Jimenez-Castellanos and Graybiel 1987; Crittenden and Graybiel 2011). This view has been updated with the use of novel viral vector tracing methods and genetic-based approaches.
To identify brain-wide differences in the afferents received by the matrix and striosome, viral tracing studies were conducted in transgenic mice (Smith et al. 2016). At a compartmental level, the matrix was found to receive significantly more inputs from cortex, while the striosomes received more inputs from subcortical regions (including thalamus). Other recent study used immunohistochemistry to directly compare vGluT1 and vGluT2 in matrix and striosome compartments (Fujiyama et al. 2019), and reported that, in contrast to the findings with the viral tracing methods by Smith et al (2016), vGluT1-positive (cortical) terminals were homogenously expressed in striosome and matrix compartment. These discrepancies could be due to different sensitivity of the viral tracing and the immunohistochemistry methods. On the other hand, vGluT2 (thalamic) terminals were found to be enriched in the matrix (Fujiyama et al. 2019). It is likely, however, that most of these inputs arise from CM/PF because, as noted above, the thalamic inputs from non-CM/PF nuclei present a homogenous distribution in matrix and striosomes.
Predominance of cortical over thalamic inputs
Based on the size of the cortical mantle, it would be expected that the inputs from the cortex to the striatum outnumber those from the thalamus. In general, studies that have quantified vGluT1- and vGluT2-expressing terminals in the striatum report that the proportion of cortical (vGluT1-positive) terminals is higher than the thalamic (vGluT2-positive) terminals, but the specific proportions vary among studies and species (Smith et al. 2014a). More recent studies using trans-synaptic viral vectors similarly showed that the dorsal striatum receives a more abundant innervation from the cortex than from the thalamus (Wall et al. 2013; Guo et al. 2015). Interestingly, two recent studies in non-human primates using a retrograde viral vector (AAVrg) found that injections of this virus in the caudate and putamen of macaques resulted in abundant transduction of neurons in the cortex (as well as in many subcortical regions known to project to the striatum), but there was an striking lack of expression of the transgene in thalamic neurons (Table 1, Albaugh et al. 2020; Weiss et al. 2020), although the same AAV vector leads to prominent labeling in intralaminar and other thalamic nuclei in rodents (Tervo et al. 2016; Albaugh et al. 2020), indicating a possible pathway and species specificity of the AAVrg vector and highlighting the importance of careful characterization of viral vectors for inter-species studies.
As we described next, the prevalence of the two types of excitatory inputs also differs among striatal cell types. Most striatal neurons receive synaptic contacts from cortex and thalamus, but the strength of these connections is cell specific, as explained by Assous et al. (2019).
Connections with SPNs
SPNs of both direct and indirect pathway receive higher proportion of excitatory inputs from cortex than from thalamus (Doig et al. 2010; Huerta-Ocampo et al. 2014). However, individual SPNs, from both the direct and indirect pathway, receive convergent inputs from cortex and thalamus (Huerta-Ocampo et al. 2014; Doig et al. 2010), in agreement with functional studies showing that single SPNs from either pathways respond to both thalamic and cortical stimulations (e.g., Arias-Garcia et al. 2018).
Cortical and thalamic terminals display a clear differential pattern of synaptic contacts onto SPNs. In rodent and primates, cortical inputs terminate on dendritic spines, and similarly thalamic inputs from non-CM/PF nuclei are also established on spines (Fig. 3) (Raju et al. 2008). However, as noted above, CM/PF inputs make synapses on dendritic shafts (Raju et al. 2006). Thus, the CM/PF complex provides the only source of axodendritic glutamatergic terminals to SPNs, underscoring a significant role in regulating the activities of these neurons.
Figure 3: Summary of thalamic and cortical inputs to striatum.
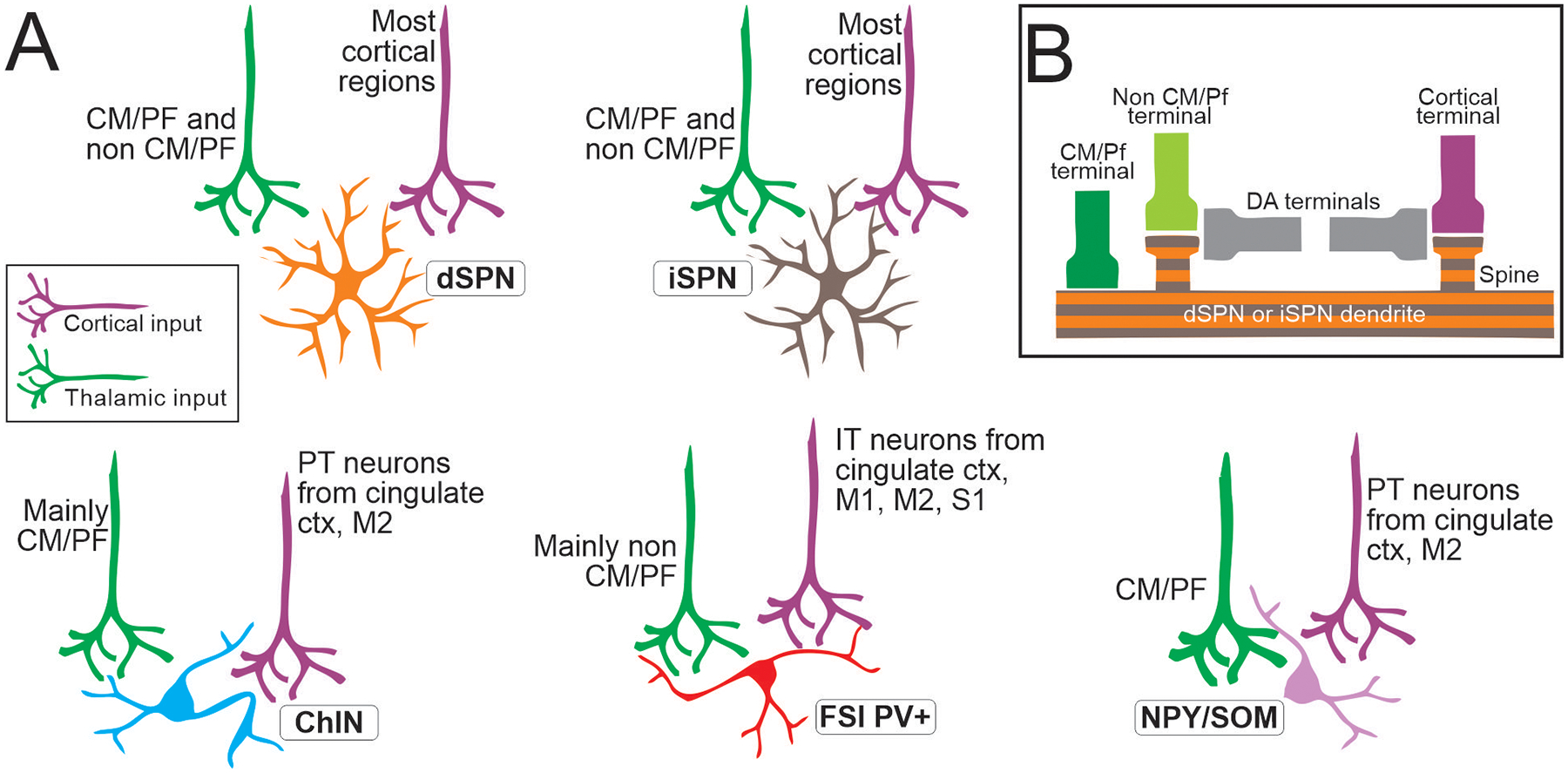
(A) Inputs to each type of SPN and to 3 main classes of interneurons. The origins of cortical inputs are indicated for interneurons when known, according to viral tracing studies in rodents. (B) Synaptic pattern of connectivity of thalamic and cortical inputs onto SPNs. See main text for abbreviations (figured made in part with BioRender)
Connections with cholinergic interneurons
In contrast to SPNs, ChINs, receive a much more prominent glutamatergic innervation from thalamic than cortical sources (Doig et al. 2014). Electron microscopic observations showed that an individual ChI could receive synapses from cortical and thalamic afferents (Doig et al. 2014). Although the prominence of thalamic inputs to ChINs has been well documented with tracing and functional experiments (Assous and Tepper 2019a), trans-synaptic retrograde tracing in transgenic mice expressing Cre in ChINs found a higher number of cortical than thalamic neurons projecting to these cells (Guo et al. 2015), still cortical inputs tended to make fewer inputs to ChINs compared to SPNs (Guo et al. 2015). It is important to keep in mind that monosynaptic tracing using rabies virus reveals anatomical connectivity but does not inform about the efficacy of these connections. The higher number of cortical than thalamic neurons projecting to ChINs reported in rabies tracing studies, coupled to the reported strength of thalamic connections onto striatal cholinergic interneurons, could suggest that each thalamic projection establishes numerous synaptic contacts with a single ChIN, while cortical neurons may only make a few contacts on each of these cells. This view is supported by single-cell tracing and electron microscope studies (Doig et al. 2014; Sadikot et al. 1992a). Selective optogenetic stimulation of thalamic or cortical inputs onto ChINs show, however, that both inputs exert similar responses on single ChINs (Arias-Garcia et al. 2018; Mamaligas et al. 2019) indicating that either of these glutamatergic sources could influence cholinergic activity in the striatum.
Connections with GABAergic interneurons
Less is known about the relative contributions of thalamic and cortical inputs for non-cholinergic striatal interneurons since few studies have compared side by side glutamatergic inputs to GABAergic interneurons. In part, this lack of knowledge is due to the difficulty in identifying the several types of striatal interneurons (Assous and Tepper 2019b).
Among GABAergic interneurons, PV-positive cells have received most experimental attention. It has been known for many years that these cells receive inputs from cortical and thalamic regions (reviewed in Assous and Tepper 2019b), but the specific proportions of each type of input may vary among species, since the thalamic afferents to these interneurons appear to be more prominent in non-human primates than in rodents (Sidibe and Smith 1999; Rudkin and Sadikot 1999). Functional studies have demonstrated that both cortical and thalamic inputs modulate the activity of these interneurons (Assous and Tepper 2019a). Furthermore, the use of trans-synaptic tracing with rabies virus in transgenic mice expressing Cre in PV-positive cells confirmed that cortical regions contribute more afferents to these cells than thalamic ones (Table 1, Choi et al. 2019). Similarly, immunofluorescence and electron microscope studies found that both vGluT1- and vGluT2-positive terminals make close appositions with the soma and dendrites of PV-positive cells in the striatum, however, in contrast to previous views, vGluT2-expressing terminals showed a tendency to prefer proximal than distal dendrites, perhaps hinting at a stronger thalamic control of PV-positive interneurons than previously thought (Zheng et al. 2021). In agreement with the anatomical studies, functional optogenetic stimulation studies have shown that both cortical and thalamic inputs modulate the activity of FSIs (Arias-Garcia et al. 2018), although the effects of each pathway are distinct and mediated by the interneurons intrinsic and synaptic properties (Sciamanna et al. 2015).
NPY/SOM-interneurons receive cortical inputs, but until recently the extent of the thalamic innervation they receive had been debated (Choi et al. 2019; Assous and Tepper 2019a). Using rabies virus to map monosynaptic connections in SOM-Cre and NPY-Cre transgenic mice, Melendez-Zaidi and colleagues (2019, Table 1) showed that these neurons receive very similar connections as those received by ChINs. That is, cortical projections to NPY/SOM cells arise from cingulate cortex and M2, while thalamic projections originate from PF (Melendez-Zaidi et al. 2019). Complementary functional optogenetic studies showed that activation of cingulate cortex terminals evoked spiking in LTS interneurons, but, regardless of the PF connections mapped onto these cells, activation of PF axons had either no effect or induced inhibition of firing. Such paradoxical effect was shown to be mediated by connections of PF terminals onto other interneurons, prominently ChINs (Melendez-Zaidi et al. 2019), in agreement with previous studies suggesting that the functional thalamic connection to NPY/SOM interneurons is indirect and mediated by other interneurons (Assous et al. 2017). The study by Melendez-Zaidi et al. underscores the intricacy of the intrastriatal circuitry and, from a technical perspective, provides an illustrative example of how retrograde tracing methods not always translate into functional connectivity.
A recently identified type of striatal interneurons, the neuropeptide Y-expressing neurogliaform (NGF) interneurons (Ibanez-Sandoval et al. 2011) display a larger response to optogenetic stimulation of thalamostriatal than cortical inputs, and these responses are mediated, at least in part, by ChINs (Assous et al. 2017). The anatomical connections from the cortex or thalamus to these cells remain to be examined.
The functional consequences of the convergence of cortico- and thalamostriatal inputs onto individual (or ensembles) of striatal neurons are still unclear. As noted by Xiao and Roberts (2021), while the role of the corticostriatal pathway in performing motor sequences is well-established, the involvement of the thalamostriatal inputs in motor learning and motor performance remains to be more fully explored (Xiao and Roberts 2021). However, recent studies have provided evidence that cortical and thalamic inputs jointly contribute to learning and execution of motor skills (Wolff et al. 2022; Lee et al. 2019). A schematic summary of the anatomical differences between cortico- and thalamostriatal connections is shown in figure 3.
Interactions of cortico- and thalamostriatal terminals with dopamine inputs
The dopaminergic inputs from the substantia nigra compacta play a major role in the modulation of striatal activity, not only by directly acting on striatal SPNs and interneurons, but also by closely regulating the functions of cortico- and thalamostriatal terminals (Zhai et al. 2019). According to the classic model (Albin et al. 1989), activation of D1Rs receptors activates dSPNs, whereas activation of D2Rs inhibits iSPNs. However, it is important to keep in mind that this functional separation into the two types of SPNs remains partial, given interactions among striatal neurons, non-complete segregation of DA receptor expression in dSPNs and iSPNs, and expression of DA receptors in interneurons (Bertran-Gonzalez et al. 2008; Matamales et al. 2009; Gangarossa et al. 2013; Gagnon et al. 2017; Maurice et al. 2004; Salgado et al. 2005; Rivera et al. 2002; Holly et al. 2021).
The position of the dopaminergic afferent at the base of the dendritic spines of SPNs facilitates its modulatory role with respect to the glutamatergic excitatory inputs which form synapses at the spine head (Smith and Bolam 1990). Indeed, the modulatory effect of dopamine on corticostriatal synapses has been extensively studied, and recent publications have reviewed this subject focusing on its functional implications (e.g., Bamford et al. 2018; Westbrook et al. 2021).
The organization of the corticostriatal inputs on the matrix and striosome compartments has been studied, but many questions remain unanswered. For instance, our knowledge of the mechanism that regulate striosome and matrix output is limited (Brimblecombe and Cragg 2017; Prager and Plotkin 2019). However, progress has been made concerning the dopaminergic modulation of the corticostriatal input between the two different compartments. An unexplored possibility is that there are intrinsic differences in how striosome and matrix SPNs transform converging excitatory inputs into somatic state-transitions and how this process is modulated by dopamine. Using transgenic mice in ex vivo slice recordings, Prager and colleagues (2020) showed that responses to convergent glutamatergic inputs are differentially modulated by DA in the matrix and striosome compartments. Activation of D1Rs in dSPNs prolonged the duration of dendritically evoked depolarization in the matrix but shortened the duration in striosomes. This result suggests that elevations in DA levels in the striatum will reduce the time window during which dSPNs in striosomes can fire action potentials, while simultaneously prolonging this time window for matrix dSPNs, biasing ongoing striatal output away from striosomes and toward the matrix (Prager et al. 2020). In the macaque monkey, a recent study using single nucleus RNA sequencing revealed at least nine distinct genetically identified subtypes of SPNs distributed within the striosome or matrix, each displaying distinct combination of DA receptors (He et al. 2021). This new finding contributes to the heterogeneity and complexity of the striatal population and dopaminergic receptors that participate in the dopaminergic modulation of cortical afferents on the striatal projection neurons.
Electron microscope analysis have shown that most corticostriatal and non-CM/PF thalamic terminals are directly contacted by a dopaminergic terminal while simultaneously converge on, or are in close proximity to, individual spines of SPNs (Moss and Bolam 2008), indicating that both types of terminals are positioned to be affected by synaptically released dopamine. In contrast, inputs from the CM/PF (which, as noted above, occur on the shafts of dendrites) are not directly apposed by dopaminergic terminals (Raju et al. 2006; Moss and Bolam 2008). However, given the lattice-like arrangement of dopaminergic axons in the striatum, which allows for most striatal structures to be in close proximity (within 1 microm) to a dopaminergic synapse (Moss and Bolam 2008), and considering that dopaminergic transmission can occur not only by synaptic released dopamine but also by spill-over of synaptically released dopamine (Moss and Bolam 2008; Cragg and Rice 2004; Arbuthnott and Wickens 2007), it is likely that most glutamatergic synapses from cortical, CM/PF and other thalamic nuclei are regulated by dopaminergic innervation (Arbuthnott 2014). The corollary is that the loss of dopaminergic inputs that characterize Parkinson’s disease would impact the majority of glutamatergic inputs in the striatum.
In turn, both cortical and thalamic inputs to the striatum can modulate dopaminergic transmission, through an indirect mechanism that involves ChINs. Electrical or optogenetic stimulation of thalamo- or corticostriatal axons elicits firing of ChINs, ensuing activation of nicotinic receptors on dopaminergic terminals (Threlfell et al. 2012) and release of dopamine. (Ding et al. 2010; Kosillo et al. 2016). Dopamine release can, subsequently, affect the response of ChINs to thalamic stimulation, which is characterized by a burst of spikes, followed by a pause. The pause component is dopamine dependent, as demonstrated by pharmacological blockade with D2R antagonists, which attenuates the pause, while the dopamine transporter antagonist cocaine enhances it (Ding et al. 2010). Such effect illustrates the tight interactions between glutamatergic and dopaminergic terminals in the striatum.
Morphological and connectivity changes in the corticostriatal and thalamostriatal pathways in parkinsonism
The degeneration of the dopaminergic neurons in the ventrolateral part of the substantia nigra pars compacta (SNc) in Parkinson’s disease (PD) leads to an imbalanced excitability between direct and indirect pathway SPNs, and it is generally considered that the motor deficits that characterize PD originate from this imbalance (Albin et al. 1989; DeLong 1990). The loss of striatal dopamine, however, also triggers a series of adaptive (and maladaptive) events throughout the basal ganglia and thalamocortical circuits that exacerbate the uneven activation of these pathways (Galvan et al. 2015). Many of these alterations involve the cortical and thalamic inputs to the striatum.
Loss of dendritic spines in SPNs and alterations in striatal glutamatergic synapses
As has been repeatedly documented in animal models of parkinsonism and in humans with PD, the progressive loss of the nigrostriatal pathway leads to a major loss of dendritic spines (reviewed in Villalba and Smith 2013, 2018). The dendritic spine loss on SPNs appears to be an early plastic change correlated with the degree of nigrostriatal DA innervation.
Numerous studies in animal models suggest that the synaptic architecture of SPNs undergoes a complex re-organization in the parkinsonian state, including recent reports confirming that the loss of striatal innervation of DA causes major synaptic dysfunction with marked dendritic tree atrophy and severe decrease in spine density in both types of SPNs (Gagnon et al. 2017; Lieberman et al. 2018; Graves and Surmeier 2019). However, the literature is still ambivalent as to whether dSPNs and iSPNs are equally affected. Graves et al. (2019) postulate that the time course of the dopaminergic denervation could explain the discrepancies, since in 6-OHDA treated rodents dSPNs display a delayed spine loss later after the lesion compared to iSPNs (Graves and Surmeier 2019). Thus, the study of the morphology and functional activity in the partially dopaminergic-denervated striatum is essential to understand the progression of parkinsonian symptoms. Pitx3-deficient aphakia mice, a genetic model of partial dopaminergic innervation that occurs in a dorso-ventral gradient in the striatum, provide an ideal tool to study structural and functional plasticity under different degrees of dopaminergic innervation (Hwang et al. 2003). Using this model, recent data established that DA modulates striatal morphology in a concentration dependent manner. Indeed, the spine loss in dSPN and iSPN decrease from dorsal to ventral striatum of these mice, following the pattern of decrease in DA levels (Alberquilla et al. 2020). This modulatory effect seems to be mediated through D1Rs, which inactivation induces a spine density reduction and increased firing in dSPN and, remarkably, also affect iSPNs by reducing their dendritic tree complexity and spine density, without changing their firing rate (Suarez et al. 2020).
Plasticity of the corticostriatal pathway
Given that most glutamatergic afferents synapse on the spines of SPNs (where they interact with dopaminergic axons, Moss and Bolam 2008), it is not surprising that glutamatergic synapses and glutamatergic transmission are substantially affected as a consequence of DA denervation. Loss of glutamatergic synapses onto SPNs were first reported in rodent models of parkinsonism (Ingham et al. 1998), and similar observations were later obtained in non-human primate models (Villalba and Smith 2018).
What is the origin of the glutamatergic terminals that are lost? As we described above, both cortical and nonCM/PF thalamic glutamatergic terminals establish synapses on SPN spines. The identity of the glutamatergic neurons affected in parkinsonism has been studied with vGluT1 and vGluT2 markers. No significant change in the prevalence of vGluT2-immunoreactive terminals was found in the putamen of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated parkinsonian monkeys (Villalba et al. 2019), or 6-OHDA treated rodents (Zhang et al. 2013), but the number of vGluT1-positive synapses was reduced in the rodent study (Zhang et al. 2013), suggesting that the loss of glutamatergic synapses was driven by deafferentation of cortical inputs (Arbuthnott 2014). This may, however, be an oversimplification because the lack of significant changes in vGluT2-positive terminals could point to compensatory mechanisms in the thalamostriatal pathway in this pathological condition. For instance, both vGluT1 and vGluT2-positive terminals in the striatum show profound ultrastructural changes, such as increases in volume or alterations in synaptic areas (Villalba and Smith 2011). The likelihood of compensatory or plastic mechanisms is also supported by the fact that in PD patients (Halliday 2009), as well as in MPTP-treated monkeys (Villalba et al. 2014), there is a loss of neurons in the CM/PF. Loss of PF neurons has also been reported in some rodent models of parkinsonism (Aymerich et al. 2006; Ghorayeb et al. 2002; Freyaldenhoven et al. 1997; but see Henderson et al. 2005).
How the loss of CM/PF neurons contributes to changes in striatal morphology in parkinsonism remains to be defined (Villalba et al. 2019). From a functional point of view, the loss of DA alters the functional plasticity of glutamatergic synapses, strengthening those synapses on iSPNs, and weakening those on dSPNs (Shen et al. 2022).
The plastic changes in glutamatergic terminals and transmission in the striatum have been extensively investigated, and recently, studies have taken advantage of genetic models of parkinsonism. For example, rodents with mutations or deletions of the PINK1 gene (which encodes for the protein PTEN induced kinase 1) present motor symptoms and alpha-synuclein aggregates in several brain regions as cerebral cortex, dorsal striatum and substantia nigra (Dave et al. 2014). In Pink1 knock-out (KO) rats, there is an increase of excitatory glutamatergic transmission onto striatal SPNs (Ren et al. 2019), which is likely due to exacerbated firing of glutamatergic presynaptic neurons, an effect observed at early stages of the pathology, at the same time as the first appearance of the motor deficits (Creed et al. 2021). These results indicate that the increased glutamate could be a consequence of heightened glutamatergic synaptic transmission into the SPNs, a notion is supported by the findings that the expression of AMPA or NMDA glutamate receptors was unchanged in in the mutant animals compared to wildtype controls (Creed et al. 2021), and that electron microscopy analysis revealed an increase in excitatory synapses at the early stages (4 months). Interestingly, at a later stage (6 months) the presynaptic glutamatergic transmission returns to normal levels, even though motor symptoms persist. It is plausible that the normalization in glutamate levels is mediated by an increase in D2R density, which has been reported to occur at this stage in Pink1 KO rats (Sun et al. 2013), perhaps providing a compensatory response to the functional impairment of dopamine-mediated neuromodulation, and subsequent increase in glutamate release, observed at 4 months. Thus, this animal model suggests a plastic balance between the dopaminergic and glutamatergic modulatory deficit in the striatum during the progression of parkinsonism
Despite the changes in glutamatergic transmission, the functions of cortical neurons that project to the striatum (at least those in the primary motor cortex) do not show changes in excitability or firing rates, in contrast to cortical neurons that project to the pyramidal tract (Chen et al. 2021; Pasquereau and Turner 2011). However, other aspects of corticostriatal activity could be altered. For instance, simultaneous local field potential (LFP) recordings in motor cortex and striatum of control and DA depleted hemi-parkinsonian rats (Jiang et al. 2019) provided evidence that DA depletion induces abnormal enhanced high beta oscillations in the corticostriatal circuit and, consistent with previous data, showed that excessive synchronization in this band was sufficient to generate marked akinesia in these animals (Beck et al. 2016). In agreement with involvement of the corticostriatal pathology in the motor deficits, modulation of this pathway could have antiparkinsonian effects. Using transcranial magnetic stimulation (rTMS) on a rat model of early parkinsonism, a recent study found that this treatment rescued functional and structural alterations specific to the early symptomatic stage of experimental parkinsonism, including reversion of dendritic spine loss (Natale et al. 2021). The rTMS-induced plastic changes were accompanied by amelioration of motor symptoms (Natale et al. 2021).
Foffani and Obeso (2018) have proposed that the corticostriatal pathway is not only involved in PD pathophysiology but could, in fact, be a major contributor in the degeneration of vulnerable dopaminergic neurons. They pose that corticostriatal activity over a lifespan, specifically in motor areas projecting to the posterior putamen, represents a critical somatotopic source contributing to striatal dysfunction (Foffani and Obeso 2018). Activity in the corticostriatal pathway could, thus, promote secretion of striatal extracellular alpha-synuclein, favoring its aggregation at dopaminergic synapses and leading ultimately to retrograde neurodegeneration of nigrostriatal neurons, and to the focal motor onset of PD. In support of this hypothesis, it has been shown that in both control and alpha-synuclein overexpressing transgenic mice, the majority of physiological alpha-synuclein in the striatum is localized at corticostriatal glutamatergic terminals (Totterdell and Meredith 2005; Taguchi et al. 2016; Emmanouilidou et al. 2016). In this scenario, the plasticity of corticostriatal projections would play a key role in the course of PD. Functional studies in vivo are needed to clearly establish the relation between the progression of parkinsonian motor symptoms and the functional changes between the motor cortex, the striatum and the SNc.
Plasticity of the thalamostriatal pathway
Thalamic inputs are also involved in the complex remodeling that takes place on glutamatergic excitatory synapses as a consequence of the dopaminergic depletion. In dopamine-depleted mice, functional connectivity (assessed by synaptic strength to stimulation) is reduced at thalamic inputs that contact dSPNs, but not at terminals in contact with iSPNs, an alteration that probably contributes to the imbalance between direct and indirect pathways (Parker et al. 2016). Although this study is consistent with the classic ‘imbalance’ hypothesis of parkinsonism (DeLong 1990), no discrimination was made between the projections from the PF and the neighboring CL nucleus. It has, in fact, been traditionally challenging to assess the specific roles of thalamic nuclei to the striatum is because the anatomical proximity of PF and surrounding areas makes it difficult to stereotaxically target one nucleus without affecting the ones surrounding it. To achieve selective targeting of PF, Tanimura and colleagues (2019) used an intelligent approach. An AAV carrying a Cre-off channelrhodopsin-2 (ChR2) was injected into the PF of transgenic mice in which the Cre-recombinase is expressed in the adjacent CL nucleus, but not in PF, resulting in ChR2 expression only in PF-striatal projections (Tanimura et al. 2019). This strategy revealed that, in parkinsonian mice, the functional connectivity of PF-striatal terminals was enhanced. This effect was mediated indirectly by ChINs, which responses to PF stimulation are also enhanced in parkinsonian conditions (Tanimura et al. 2019; but see Aceves Buendia et al. 2019). In a follow-up study, Tanimura et al. (2022) explored contributions of non-PF thalamic terminals by conducting selective optogenetic stimulation of CL-striatal terminals. They found reduced evoked synaptic currents in SPNs, but a greater reduction was observed in dSPNs, suggesting that the projections from CL to the striatum experience a pathway-specific remodeling (Tanimura et al. 2022). These studies helped assemble a more complete picture of the alterations that occur in cortical and thalamic inputs in rodent models of parkinsonism (Figure 4), which should be confirmed and complemented in the future with studies in other models, including primates.
Figure 4: Model of remodeling of cortico and thalamic terminals in parkinsonism.
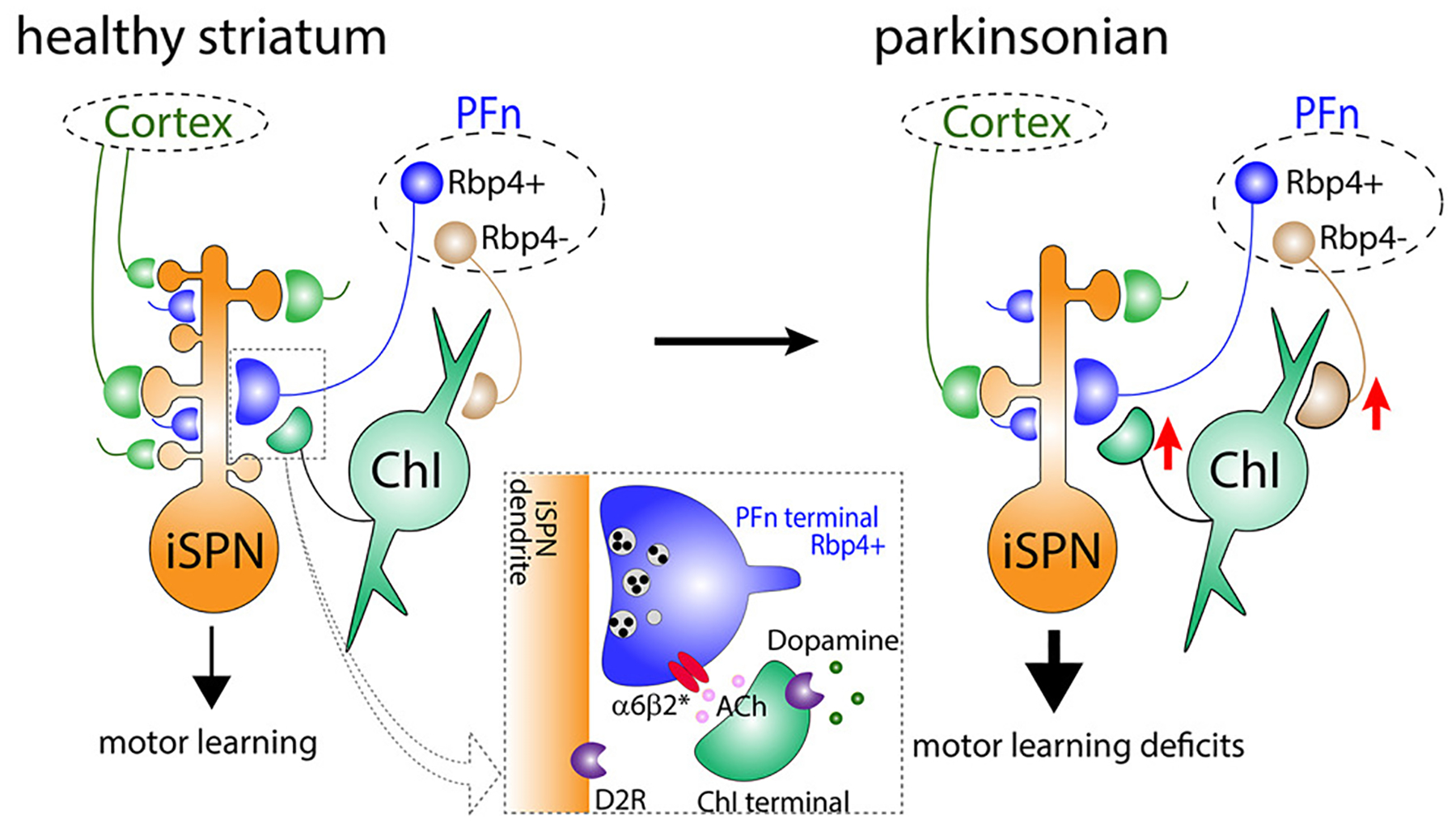
According to studies in unilaterally treated 6-OHDA treated mice, in the parkinsonian state thalamic neurons exert a more potent activation of iSPNs than in normal animals. This increase is mediated by PF connections onto ChINs, which activate, through acetylcholine release, presynaptic nicotinic receptors on PF terminals (from Tanimura et al. 2019)
As could be expected given the aberrant thalamostriatal connectivity in parkinsonism, chemogenetic inhibition of the intralaminar thalamic nuclei, or optogenetic silencing of thalamic terminals in the striatum, ameliorates the motor deficits in parkinsonian mice (Parker et al. 2016). In agreement with the report by Parker et al (2016) Tanimura and colleagues found that inhibition of thalamic terminals (in this case, selective to PF inputs) ameliorated the motor deficits in parkinsonian mice (Tanimura et al. 2019). In parkinsonian rodents, selective inhibition of PF neurons that project to the dorsal striatum (using chemogenetic modulation and targeting of these neurons with rabies virus and Cre-dependent expression of inhibitory chemogenetic receptors), improves locomotion, while inhibition of PF neurons projecting to NAcc restored decreased preference for sucrose (interpreted as a depressive-like behavior in mice) (Zhang et al. 2022). This data suggests that each of the segregated functional pathways from the PF to the striatum could have different roles in motor and non-motor parkinsonian deficits.
The restoration of parkinsonian deficits by chemogenetic- or optogenetic-mediated inhibition of PF-striatal pathway are consistent with earlier studies showing that the use of high-frequency deep brain stimulation (DBS) of the PF or PF lesions improves movement in rodent models of parkinsonism (Kerkerian-Le Goff et al. 2009; Jouve et al. 2010). Although these results are promising and warrant more studies of neuromodulation of the thalamostriatal pathway to alleviate parkinsonism, initial studies in non-human primates showed only a limited effect of lesions of CM/PF on the motor deficits induced by MPTP (Lanciego et al. 2008).
Closing remarks
Our knowledge of the cortico- and thalamostriatal pathways has been expanded in the last few years thanks to novel techniques including viral vector tracing, genomics and the availability of transgenic mice. We have started to understand that the connectivity patterns to the striatum are strongly determined by the specific cell types, establishing complex microcircuits that can be selectively modulated with optogenetics and chemogenetics. These methods are being used to examine the unique functions of these circuits, and how they contribute to pathological conditions such as parkinsonism. Although these advances are exciting, studies should be expanded to other species, in particular non-human primates in which the anatomy and circuitry of the basal ganglia-thalamocortical circuits is closer to humans. Such studies would be essential to translate to humans the clinical potential encompassed in the selective modulation of these circuits.
Acknowledgments
The authors’ research is funded in part by NIH grant P50NS123103, and infrastructure grant to the Emory National Primate Research Center (P51OD011132).
Abbreviations
- ChAT
Choline Acetyltransferase
- ChIN
Cholinergic interneuron
- ChR2
Channelrhodopsin 2
- Cing
Cingulate cortex
- CL
Central lateral nucleus
- CM
Centromedian
- D1R
D1-type dopamine receptor
- D2R
D2-type dopamine receptor
- DA
Dopamine
- dSPN
Direct pathway spinal projection neuron
- FSI
Fast-spiking interneuron
- iSPN
Indirect pathway spinal projection neuron
- IT
Intratelencephalic cortical neurons
- KO
Knock-out
- LTSI
Low-thresholed spike interneuron
- M1
Primary motor cortex
- M2
Secondary motor cortex
- NAcc
Nucleus Accumbens
- NPY
Neuropeptid Y
- PF
Parafascicular
- PINK1
PTEN induced kinase 1
- PT
Pyramidal tract cortical neurons
- PV
Parvalbumin
- S1
Primary sensory cortex
- S2
Secondary sensory cortex
- SOM
Somatostatin
- SPN
Spinal Projection Neuron
- VA
Ventral anterior
References
- Aceves Buendia JJ, Tiroshi L, Chiu WH, Goldberg JA (2019) Selective remodeling of glutamatergic transmission to striatal cholinergic interneurons after dopamine depletion. The European journal of neuroscience 49 (6):824–833. doi: 10.1111/ejn.13715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albaugh DL, Smith Y, Galvan A (2020) Comparative analyses of transgene expression patterns after intra-striatal injections of rAAV2-retro in rats and rhesus monkeys: A light and electron microscopic study. The European journal of neuroscience 52 (12):4824–4839. doi: 10.1111/ejn.15027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberquilla S, Gonzalez-Granillo A, Martin ED, Moratalla R (2020) Dopamine regulates spine density in striatal projection neurons in a concentration-dependent manner. Neurobiol Dis 134:104666. doi: 10.1016/j.nbd.2019.104666 [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12 (10):366–375. doi: 10.1016/0166-2236(89)90074-x [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381 [DOI] [PubMed] [Google Scholar]
- Alloway KD, Smith JB, Mowery TM, Watson GDR (2017) Sensory Processing in the Dorsolateral Striatum: The Contribution of Thalamostriatal Pathways. Front Syst Neurosci 11:53. doi: 10.3389/fnsys.2017.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloway KD, Smith JB, Watson GDR (2014) Thalamostriatal projections from the medial posterior and parafascicular nuclei have distinct topographic and physiologic properties. Journal of Neurophysiology 111 (1):36–50. doi: 10.1152/jn.00399.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KM, Krienen FM, Choi EY, Reinen JM, Yeo BTT, Holmes AJ (2018) Gene expression links functional networks across cortex and striatum. Nat Commun 9 (1):1428. doi: 10.1038/s41467-018-03811-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott GW (2014) Thalamostriatal synapses-another substrate for dopamine action? Prog Brain Res 211:1–11. doi: 10.1016/B978-0-444-63425-2.00001-5 [DOI] [PubMed] [Google Scholar]
- Arbuthnott GW, Wickens J (2007) Space, time and dopamine. Trends in Neurosciences 30 (2):62–69 [DOI] [PubMed] [Google Scholar]
- Arias-Garcia MA, Tapia D, Laville JA, Calderon VM, Ramiro-Cortes Y, Bargas J, Galarraga E (2018) Functional comparison of corticostriatal and thalamostriatal postsynaptic responses in striatal neurons of the mouse. Brain Struct Funct 223 (3):1229–1253. doi: 10.1007/s00429-017-1536-6 [DOI] [PubMed] [Google Scholar]
- Arikuni T, Kubota K (1986) The organization of prefrontocaudate projections and their laminar origin in the macaque monkey: A retrograde study using HRP-gel. The Journal of comparative neurology 244 (4):492–510. doi: 10.1002/cne.902440407 [DOI] [PubMed] [Google Scholar]
- Assous M, Kaminer J, Shah F, Garg A, Koos T, Tepper JM (2017) Differential processing of thalamic information via distinct striatal interneuron circuits. Nat Commun 8:15860. doi: 10.1038/ncomms15860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assous M, Tepper JM (2019a) Cortical and thalamic inputs exert cell type-specific feedforward inhibition on striatal GABAergic interneurons. J Neurosci Res 97 (12):1491–1502. doi: 10.1002/jnr.24444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assous M, Tepper JM (2019b) Excitatory extrinsic afferents to striatal interneurons and interactions with striatal microcircuitry. The European journal of neuroscience 49 (5):593–603. doi: 10.1111/ejn.13881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymerich MS, Barroso-Chinea P, Perez-Manso M, Munoz-Patino AM, Moreno-Igoa M, Gonzalez-Hernandez T, Lanciego JL (2006) Consequences of unilateral nigrostriatal denervation on the thalamostriatal pathway in rats. The European journal of neuroscience 23 (8):2099–2108 [DOI] [PubMed] [Google Scholar]
- Bamford NS, Wightman RM, Sulzer D (2018) Dopamine’s Effects on Corticostriatal Synapses during Reward-Based Behaviors. Neuron 97 (3):494–510. doi: 10.1016/j.neuron.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso-Chinea P, Castle M, Aymerich MS, Lanciego JL (2008) Expression of vesicular glutamate transporters 1 and 2 in the cells of origin of the rat thalamostriatal pathway. Journal of chemical neuroanatomy 35 (1):101–107. doi: 10.1016/j.jchemneu.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Castle M, Aymerich MS, Perez-Manso M, Erro E, Tunon T, Lanciego JL (2007) Expression of the mRNAs encoding for the vesicular glutamate transporters 1 and 2 in the rat thalamus. The Journal of comparative neurology 501 (5):703–715. doi: 10.1002/cne.21265 [DOI] [PubMed] [Google Scholar]
- Beck MH, Haumesser JK, Kuhn J, Altschuler J, Kuhn AA, van Riesen C (2016) Short- and long-term dopamine depletion causes enhanced beta oscillations in the cortico-basal ganglia loop of parkinsonian rats. Exp Neurol 286:124–136. doi: 10.1016/j.expneurol.2016.10.005 [DOI] [PubMed] [Google Scholar]
- Beckstead RM (1984) The thalamostriatal projection in the cat. The Journal of comparative neurology 223 (3):313–346. doi: 10.1002/cne.902230302 [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ (1990) Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. The Journal of comparative neurology 299 (2):187–228. doi: 10.1002/cne.902990206 [DOI] [PubMed] [Google Scholar]
- Bertero A, Verrillo L, Apicella AJ (2022) A Novel Layer 4 Corticofugal Cell Type/Projection Involved in Thalamo-Cortico-Striatal Sensory Processing. J Neurosci 42 (8):1383–1405. doi: 10.1523/jneurosci.1738-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA (2008) Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 28 (22):5671–5685. doi:28/22/5671 10.1523/JNEUROSCI.1039-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra E, Rizzo M, Gerbella M, Rozzi S, Luppino G (2021) Laminar Origin of Corticostriatal Projections to the Motor Putamen in the Macaque Brain. J Neurosci 41 (7):1455–1469. doi: 10.1523/JNEUROSCI.1475-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa J, Deschenes M (1995) Corticothalamic projections from the primary visual cortex in rats: a single fiber study using biocytin as an anterograde tracer. Neuroscience 66 (2):253–263. doi: 10.1016/0306-4522(95)00009-8 [DOI] [PubMed] [Google Scholar]
- Bourassa J, Pinault D, Deschenes M (1995) Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. The European journal of neuroscience 7 (1):19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x [DOI] [PubMed] [Google Scholar]
- Bradfield LA, Bertran-Gonzalez J, Chieng B, Balleine BW (2013) The thalamostriatal pathway and cholinergic control of goal-directed action: interlacing new with existing learning in the striatum. Neuron 79 (1):153–166. doi: 10.1016/j.neuron.2013.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimblecombe KR, Cragg SJ (2017) The Striosome and Matrix Compartments of the Striatum: A Path through the Labyrinth from Neurochemistry toward Function. ACS Chem Neurosci 8 (2):235–242. doi: 10.1021/acschemneuro.6b00333 [DOI] [PubMed] [Google Scholar]
- Burke DA, Rotstein HG, Alvarez VA (2017) Striatal Local Circuitry: A New Framework for Lateral Inhibition. Neuron 96 (2):267–284. doi: 10.1016/j.neuron.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Abrams S, Pinhas A, Brumberg JC (2009) Morphological heterogeneity of layer VI neurons in mouse barrel cortex. The Journal of comparative neurology 512 (6):726–746. doi: 10.1002/cne.21926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Daniels S, Kim Y, Chu HY (2021) Cell Type-Specific Decrease of the Intrinsic Excitability of Motor Cortical Pyramidal Neurons in Parkinsonism. J Neurosci 41 (25):5553–5565. doi: 10.1523/JNEUROSCI.2694-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Holly EN, Davatolhagh MF, Beier KT, Fuccillo MV (2019) Integrated anatomical and physiological mapping of striatal afferent projections. The European journal of neuroscience 49 (5):623–636. doi: 10.1111/ejn.13829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Rice ME (2004) DAncing past the DAT at a DA synapse. Trends Neurosci 27 (5):270–277. doi: 10.1016/j.tins.2004.03.011 [DOI] [PubMed] [Google Scholar]
- Creed RB, Roberts RC, Farmer CB, McMahon LL, Goldberg MS (2021) Increased glutamate transmission onto dorsal striatum spiny projection neurons in Pink1 knockout rats. Neurobiol Dis 150:105246. doi: 10.1016/j.nbd.2020.105246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR, Graybiel AM (2011) Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat 5:59. doi: 10.3389/fnana.2011.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KD, De Silva S, Sheth NP, Ramboz S, Beck MJ, Quang C, Switzer RC 3rd, Ahmad SO, Sunkin SM, Walker D, Cui X, Fisher DA, McCoy AM, Gamber K, Ding X, Goldberg MS, Benkovic SA, Haupt M, Baptista MA, Fiske BK, Sherer TB, Frasier MA (2014) Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiol Dis 70:190–203. doi: 10.1016/j.nbd.2014.06.009 [DOI] [PubMed] [Google Scholar]
- de las Heras S, Mengual E, Gimenez-Amaya JM (1999) Double retrograde tracer study of the thalamostriatal projections to the cat caudate nucleus. Synapse 32 (2):80–92. doi: [pii] [DOI] [PubMed] [Google Scholar]
- DeLong MR (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13 (7):281–285. doi: 10.1016/0166-2236(90)90110-V [DOI] [PubMed] [Google Scholar]
- Deng Y, Lanciego J, Kerkerian-Le-Goff L, Coulon P, Salin P, Kachidian P, Lei W, Del Mar N, Reiner A (2015) Differential organization of cortical inputs to striatal projection neurons of the matrix compartment in rats. Front Syst Neurosci 9:51. doi: 10.3389/fnsys.2015.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Veinante P, Zhang ZW (1998) The organization of corticothalamic projections: reciprocity versus parity. Brain research Brain research reviews 28 (3):286–308. doi: 10.1016/s0165-0173(98)00017-4 [DOI] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ (2010) Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron 67 (2):294–307. doi:S0896–6273(10)00475–7 10.1016/j.neuron.2010.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig NM, Magill PJ, Apicella P, Bolam JP, Sharott A (2014) Cortical and thalamic excitation mediate the multiphasic responses of striatal cholinergic interneurons to motivationally salient stimuli. J Neurosci 34 (8):3101–3117. doi: 10.1523/JNEUROSCI.4627-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig NM, Moss J, Bolam JP (2010) Cortical and Thalamic Innervation of Direct and Indirect Pathway Medium-Sized Spiny Neurons in Mouse Striatum. J Neurosci 30 (44):14610–14618. doi: 10.1523/jneurosci.1623-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eblen F, Graybiel AM (1995) Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J Neurosci 15 (9):5999–6013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elena Erro M, Lanciego JL, Gimenez-Amaya JM (2002) Re-examination of the thalamostriatal projections in the rat with retrograde tracers. Neurosci Res 42 (1):45–55 [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Minakaki G, Keramioti MV, Xylaki M, Balafas E, Chrysanthou-Piterou M, Kloukina I, Vekrellis K (2016) GABA transmission via ATP-dependent K+ channels regulates alpha-synuclein secretion in mouse striatum. Brain 139 (Pt 3):871–890. doi: 10.1093/brain/awv403 [DOI] [PubMed] [Google Scholar]
- Foffani G, Obeso JA (2018) A Cortical Pathogenic Theory of Parkinson’s Disease. Neuron 99 (6):1116–1128. doi: 10.1016/j.neuron.2018.07.028 [DOI] [PubMed] [Google Scholar]
- Fremeau RT Jr., Voglmaier S, Seal RP, Edwards RH (2004) VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci 27 (2):98–103. doi: 10.1016/j.tins.2003.11.005 S0166223603003813 [DOI] [PubMed] [Google Scholar]
- Freyaldenhoven TE, Ali SF, Schmued LC (1997) Systemic administration of MPTP induces thalamic neuronal degeneration in mice. Brain Res 759 (1):9–17. doi:S0006–8993(97)00045–0 [DOI] [PubMed] [Google Scholar]
- Frotscher M, Rinne U, Hassler R, Wagner A (1981) Termination of cortical afferents on identified neurons in the caudate nucleus of the cat. A combined Golgi-EM degeneration study. Exp Brain Res 41 (3–4):329–337. doi: 10.1007/BF00238890 [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Unzai T, Karube F (2019) Thalamostriatal projections and striosome-matrix compartments. Neurochem Int 125:67–73. doi: 10.1016/j.neuint.2019.01.024 [DOI] [PubMed] [Google Scholar]
- Gagnon D, Petryszyn S, Sanchez MG, Bories C, Beaulieu JM, De Koninck Y, Parent A, Parent M (2017) Striatal Neurons Expressing D1 and D2 Receptors are Morphologically Distinct and Differently Affected by Dopamine Denervation in Mice. Sci Rep 7:41432. doi: 10.1038/srep41432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Devergnas A, Wichmann T (2015) Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front Neuroanat 9:5. doi: 10.3389/fnana.2015.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Villalba RM, Wichmann T, Smith Y (2016) Chapter 24 - The Thalamostriatal Systems in Normal and Disease States. In: Steiner H, Tseng KY (eds) Handbook of Behavioral Neuroscience, vol 24. Elsevier, pp 477–493. doi: 10.1016/B978-0-12-802206-1.00024-6 [DOI] [Google Scholar]
- Gangarossa G, Espallergues J, Mailly P, De Bundel D, de Kerchove d’Exaerde A, Herve D, Girault JA, Valjent E, Krieger P (2013) Spatial distribution of D1R- and D2R-expressing medium-sized spiny neurons differs along the rostro-caudal axis of the mouse dorsal striatum. Frontiers in neural circuits 7:124. doi: 10.3389/fncir.2013.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR (1989) The neostriatal mosaic: striatal patch-matrix organization is related to cortical lamination. Science 246 (4928):385–388. doi: 10.1126/science.2799392 [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34:441–466. doi: 10.1146/annurev-neuro-061010-113641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorayeb I, Fernagut PO, Hervier L, Labattu B, Bioulac B, Tison F (2002) A ‘single toxin-double lesion’ rat model of striatonigral degeneration by intrastriatal 1-methyl-4-phenylpyridinium ion injection: a motor behavioural analysis. Neuroscience 115 (2):533–546. doi:S0306452202004013 [DOI] [PubMed] [Google Scholar]
- Gimenez-Amaya JM, de las Heras S, Erro E, Mengual E, Lanciego JL (2000) Considerations on the thalamostriatal system with some functional implications. Histol Histopathol 15 (4):1285–1292 [DOI] [PubMed] [Google Scholar]
- Goldman PS, Nauta WJ (1977) An intricately patterned prefronto-caudate projection in the rhesus monkey. The Journal of comparative neurology 72 (3):369–386 [DOI] [PubMed] [Google Scholar]
- Graves SM, Surmeier DJ (2019) Delayed Spine Pruning of Direct Pathway Spiny Projection Neurons in a Mouse Model of Parkinson’s Disease. Front Cell Neurosci 13:32. doi: 10.3389/fncel.2019.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano A, Liu XB, Murray KD, Jones EG (2008) Vesicular glutamate transporters define two sets of glutamatergic afferents to the somatosensory thalamus and two thalamocortical projections in the mouse. The Journal of comparative neurology 507 (2):1258–1276. doi: 10.1002/cne.21592 [DOI] [PubMed] [Google Scholar]
- Griggs WS, Kim HF, Ghazizadeh A, Costello MG, Wall KM, Hikosaka O (2017) Flexible and Stable Value Coding Areas in Caudate Head and Tail Receive Anatomically Distinct Cortical and Subcortical Inputs. Front Neuroanat 11:106. doi: 10.3389/fnana.2017.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW (1994) The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends Neurosci 17 (2):52–57 [DOI] [PubMed] [Google Scholar]
- Guo Q, Wang D, He X, Feng Q, Lin R, Xu F, Fu L, Luo M (2015) Whole-brain mapping of inputs to projection neurons and cholinergic interneurons in the dorsal striatum. PLoS One 10 (4):e0123381. doi: 10.1371/journal.pone.0123381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Calzavara R (2009) The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull 78 (2–3):69–74. doi:S0361–9230(08)00342–0 10.1016/j.brainresbull.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM (2009) Thalamic changes in Parkinson’s disease. Parkinsonism Relat Disord 15 Suppl 3:S152–155. doi:S1353–8020(09)70804–1 10.1016/S1353-8020(09)70804-1 [DOI] [PubMed] [Google Scholar]
- He J, Kleyman M, Chen J, Alikaya A, Rothenhoefer KM, Ozturk BE, Wirthlin M, Bostan AC, Fish K, Byrne LC, Pfenning AR, Stauffer WR (2021) Transcriptional and anatomical diversity of medium spiny neurons in the primate striatum. Curr Biol 31 (24):5473–5486 e5476. doi: 10.1016/j.cub.2021.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JM, Schleimer SB, Allbutt H, Dabholkar V, Abela D, Jovic J, Quinlivan M (2005) Behavioural effects of parafascicular thalamic lesions in an animal model of parkinsonism. Behav Brain Res 162 (2):222–232. doi:S0166–4328(05)00100–2 10.1016/j.bbr.2005.03.017 [DOI] [PubMed] [Google Scholar]
- Herkenham M, Pert CB (1981) Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature 291 (5814):415–418 [DOI] [PubMed] [Google Scholar]
- Holly EN, Davatolhagh MF, Espana RA, Fuccillo MV (2021) Striatal low-threshold spiking interneurons locally gate dopamine. Curr Biol 31 (18):4139–4147 e4136. doi: 10.1016/j.cub.2021.06.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Ocampo I, Mena-Segovia J, Bolam JP (2014) Convergence of cortical and thalamic input to direct and indirect pathway medium spiny neurons in the striatum. Brain Struct Funct 219 (5):1787–1800. doi: 10.1007/s00429-013-0601-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnicutt BJ, Jongbloets BC, Birdsong WT, Gertz KJ, Zhong H, Mao T (2016) A comprehensive excitatory input map of the striatum reveals novel functional organization. Elife 5. doi: 10.7554/eLife.19103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DY, Ardayfio P, Kang UJ, Semina EV, Kim KS (2003) Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res 114 (2):123–131. doi: 10.1016/s0169-328x(03)00162-1 [DOI] [PubMed] [Google Scholar]
- Ibanez-Sandoval O, Tecuapetla F, Unal B, Shah F, Koos T, Tepper JM (2011) A novel functionally distinct subtype of striatal neuropeptide Y interneuron. J Neurosci 31 (46):16757–16769. doi: 10.1523/JNEUROSCI.2628-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Taggart P, Arbuthnott GW (1998) Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci 18 (12):4732–4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Yan Y, Wang K, Wei J, Su W, Jia J (2019) Brain state-dependent alterations of corticostriatal synchronized oscillations in awake and anesthetized parkinsonian rats. Brain Res 1717:214–227. doi: 10.1016/j.brainres.2019.04.019 [DOI] [PubMed] [Google Scholar]
- Jimenez-Castellanos J, Graybiel AM (1987) Subdivisions of the dopamine-containing A8-A9-A10 complex identified by their differential mesostriatal innervation of striosomes and extrastriosomal matrix. Neuroscience 23 (1):223–242. doi: 10.1016/0306-4522(87)90285-5 [DOI] [PubMed] [Google Scholar]
- Johansson Y, Silberberg G (2020) The Functional Organization of Cortical and Thalamic Inputs onto Five Types of Striatal Neurons Is Determined by Source and Target Cell Identities. Cell Rep 30 (4):1178–1194 e1173. doi: 10.1016/j.celrep.2019.12.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG (2007) The Thalamus. Cambridge University Press, New York, NY [Google Scholar]
- Jouve L, Salin P, Melon C, Kerkerian-Le Goff L (2010) Deep brain stimulation of the center median-parafascicular complex of the thalamus has efficient anti-parkinsonian action associated with widespread cellular responses in the basal ganglia network in a rat model of Parkinson’s disease. J Neurosci 30 (29):9919–9928. doi:30/29/9919 10.1523/JNEUROSCI.1404-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Nishizawa K, Kobayashi K (2021) Thalamostriatal System Controls the Acquisition, Performance, and Flexibility of Learning Behavior. Frontiers in Systems Neuroscience 15. doi: 10.3389/fnsys.2021.729389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC (1995) Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci 18 (12):527–535. doi:0166223695983748 [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP (1971) The structure of the caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc Lond B Biol Sci 262 (845):383–401. doi: 10.1098/rstb.1971.0102 [DOI] [PubMed] [Google Scholar]
- Kerkerian-Le Goff L, Bacci JJ, Jouve L, Melon C, Salin P (2009) Impact of surgery targeting the caudal intralaminar thalamic nuclei on the pathophysiological functioning of basal ganglia in a rat model of Parkinson’s disease. Brain Res Bull 78 (2–3):80–84. doi:S0361-9230(08)00291-8 10.1016/j.brainresbull.2008.08.010 [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Wilson CJ (1996) Corticostriatal innervation of the patch and matrix in the rat neostriatum. The Journal of comparative neurology 374 (4):578–592. doi: [DOI] [PubMed] [Google Scholar]
- Klug JR, Engelhardt MD, Cadman CN, Li H, Smith JB, Ayala S, Williams EW, Hoffman H, Jin X (2018) Differential inputs to striatal cholinergic and parvalbumin interneurons imply functional distinctions. Elife 7. doi: 10.7554/eLife.35657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosillo P, Zhang YF, Threlfell S, Cragg SJ (2016) Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons. Cereb Cortex. doi: 10.1093/cercor/bhw252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC (2009) Physiology and pharmacology of striatal neurons. Annu Rev Neurosci 32:127–147. doi: 10.1146/annurev.neuro.051508.135422 [DOI] [PubMed] [Google Scholar]
- Krienen FM, Goldman M, Zhang Q, R CHDR, Florio M, Machold R, Saunders A, Levandowski K, Zaniewski H, Schuman B, Wu C, Lutservitz A, Mullally CD, Reed N, Bien E, Bortolin L, Fernandez-Otero M, Lin JD, Wysoker A, Nemesh J, Kulp D, Burns M, Tkachev V, Smith R, Walsh CA, Dimidschstein J, Rudy B, L SK, Berretta S, Fishell G, Feng G, McCarroll SA (2020) Innovations present in the primate interneuron repertoire. Nature 586 (7828):262–269. doi: 10.1038/s41586-020-2781-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzle H (1975) Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res 88 (2):195–209. doi:0006–8993(75)90384–4 [pii] [DOI] [PubMed] [Google Scholar]
- Kunzle H (1977) Projections from the primary somatosensory cortex to basal ganglia and thalamus in the monkey. Exp Brain Res 30 (4):481–492. doi: 10.1007/BF00237639 [DOI] [PubMed] [Google Scholar]
- Lacey CJ, Bolam JP, Magill PJ (2007) Novel and distinct operational principles of intralaminar thalamic neurons and their striatal projections. J Neurosci 27 (16):4374–4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Rodriguez-Oroz MC, Blesa FJ, Alvarez-Erviti L, Guridi J, Barroso-Chinea P, Smith Y, Obeso JA (2008) Lesion of the centromedian thalamic nucleus in MPTP-treated monkeys. Mov Disord 23 (5):708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry P, Wilson CJ, Kitai ST (1984) Morphological and electrophysiological characteristics of pyramidal tract neurons in the rat. Exp Brain Res 57 (1):177–190. doi: 10.1007/BF00231144 [DOI] [PubMed] [Google Scholar]
- Lapper SR, Bolam JP (1992) Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience 51 (3):533–545. doi:0306–4522(92)90293-B [DOI] [PubMed] [Google Scholar]
- Lavin TK, Jin L, Lea NE, Wickersham IR (2020) Monosynaptic Tracing Success Depends Critically on Helper Virus Concentrations. Front Synaptic Neurosci 12:6. doi: 10.3389/fnsyn.2020.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Bakhurin KI, Claar LD, Holley SM, Chong NC, Cepeda C, Levine MS, Masmanidis SC (2019) Gain Modulation by Corticostriatal and Thalamostriatal Input Signals during Reward-Conditioned Behavior. Cell Rep 29 (8):2438–2449 e2434. doi: 10.1016/j.celrep.2019.10.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Deng Y, Liu B, Mu S, Guley NM, Wong T, Reiner A (2013) Confocal laser scanning microscopy and ultrastructural study of VGLUT2 thalamic input to striatal projection neurons in rats. The Journal of comparative neurology 521 (6):1354–1377. doi: 10.1002/cne.23235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A (2004) Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci 24 (38):8289–8299. doi: 10.1523/JNEUROSCI.1990-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M, Charara A, Gagnon S, Parent A, Deschenes M (1996a) Corticostriatal projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain Res 709 (2):311–315 [DOI] [PubMed] [Google Scholar]
- Levesque M, Gagnon S, Parent A, Deschenes (1996b) Axonal arborizations of corticostriatal and corticothalamic fibers arising from the second somatosensory area in the rat. Cereb Cortex 6 (6):759–770 [DOI] [PubMed] [Google Scholar]
- Levesque M, Parent A (1998) Axonal arborization of corticostriatal and corticothalamic fibers arising from prelimbic cortex in the rat. Cereb Cortex 8 (7):602–613. doi: 10.1093/cercor/8.7.602 [DOI] [PubMed] [Google Scholar]
- Lieberman OJ, McGuirt AF, Mosharov EV, Pigulevskiy I, Hobson BD, Choi S, Frier MD, Santini E, Borgkvist A, Sulzer D (2018) Dopamine Triggers the Maturation of Striatal Spiny Projection Neuron Excitability during a Critical Period. Neuron 99 (3):540–554 e544. doi: 10.1016/j.neuron.2018.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Eickhoff SB, Caspers S, Wu J, Genon S, Hoffstaedter F, Mars RB, Sommer IE, Eickhoff CR, Chen J, Jardri R, Reetz K, Dogan I, Aleman A, Kogler L, Gruber O, Caspers J, Mathys C, Patil KR (2021) Functional parcellation of human and macaque striatum reveals human-specific connectivity in the dorsal caudate. Neuroimage 235:118006. doi: 10.1016/j.neuroimage.2021.118006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Sherman SM (2008) Evidence for nonreciprocal organization of the mouse auditory thalamocortical-corticothalamic projection systems. The Journal of comparative neurology 507 (2):1209–1227. doi: 10.1002/cne.21602 [DOI] [PubMed] [Google Scholar]
- Mamaligas AA, Barcomb K, Ford CP (2019) Cholinergic Transmission at Muscarinic Synapses in the Striatum Is Driven Equally by Cortical and Thalamic Inputs. Cell Rep 28 (4):1003–1014 e1003. doi: 10.1016/j.celrep.2019.06.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbaum G, Taranda J, Haynes TM, Hochbaum DR, Huang KW, Hyun M, Umadevi Venkataraju K, Straub C, Wang W, Robertson K, Osten P, Sabatini BL (2019) Distinct Cortical-Thalamic-Striatal Circuits through the Parafascicular Nucleus. Neuron 102 (3):636–652 e637. doi: 10.1016/j.neuron.2019.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau JM, Valjent E, Herve D, Girault JA (2009) Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS One 4 (3):e4770. doi: 10.1371/journal.pone.0004770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai A, Smith Y (2011) The corticostriatal and corticosubthalamic pathways: two entries, one target. So what? Front Syst Neurosci 5:64. doi: 10.3389/fnsys.2011.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, Surmeier DJ (2004) D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci 24 (46):10289–10301. doi: 10.1523/JNEUROSCI.2155-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Haber SN (2000) Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J Neurosci 20 (10):3798–3813. doi:20/10/3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL (1989) The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 29 (3):503–537 [DOI] [PubMed] [Google Scholar]
- Melendez-Zaidi AE, Lakshminarasimhah H, Surmeier DJ (2019) Cholinergic modulation of striatal nitric oxide-producing interneurons. The European journal of neuroscience 50 (11):3713–3731. doi: 10.1111/ejn.14528 [DOI] [PubMed] [Google Scholar]
- Morgenstern NA, Isidro AF, Israely I, Costa RM (2022) Pyramidal tract neurons drive amplification of excitatory inputs to striatum through cholinergic interneurons. Sci Adv 8 (6):eabh4315. doi: 10.1126/sciadv.abh4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J, Bolam JP (2008) A dopaminergic axon lattice in the striatum and its relationship with cortical and thalamic terminals. J Neurosci 28 (44):11221–11230. doi: 10.1523/JNEUROSCI.2780-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Hasegawa Y, Tokushige A, Nakagawa S, Kayahara T, Mizuno N (1990) Topographical projections from the thalamus, subthalamic nucleus and pedunculopontine tegmental nucleus to the striatum in the Japanese monkey, Macaca fuscata. Brain Res 537 (1–2):54–68. doi:0006–8993(90)90339-D [pii] [DOI] [PubMed] [Google Scholar]
- Nakano Y, Karube F, Hirai Y, Kobayashi K, Hioki H, Okamoto S, Kameda H, Fujiyama F (2018) Parvalbumin-producing striatal interneurons receive excitatory inputs onto proximal dendrites from the motor thalamus in male mice. J Neurosci Res 96 (7):1186–1207. doi: 10.1002/jnr.24214 [DOI] [PubMed] [Google Scholar]
- Natale G, Pignataro A, Marino G, Campanelli F, Calabrese V, Cardinale A, Pelucchi S, Marcello E, Gardoni F, Viscomi MT, Picconi B, Ammassari-Teule M, Calabresi P, Ghiglieri V (2021) Transcranial Magnetic Stimulation Exerts “Rejuvenation” Effects on Corticostriatal Synapses after Partial Dopamine Depletion. Mov Disord 36 (10):2254–2263. doi: 10.1002/mds.28671 [DOI] [PubMed] [Google Scholar]
- Pan WX, Mao T, Dudman JT (2010) Inputs to the dorsal striatum of the mouse reflect the parallel circuit architecture of the forebrain. Front Neuroanat 4:147. doi: 10.3389/fnana.2010.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Mackey A, De Bellefeuille L (1983) The subcortical afferents to caudate nucleus and putamen in primate: a fluorescence retrograde double labeling study. Neuroscience 10 (4):1137–1150 [DOI] [PubMed] [Google Scholar]
- Parent M, Parent A (2006) Single-axon tracing study of corticostriatal projections arising from primary motor cortex in primates. The Journal of comparative neurology 496 (2):202–213 [DOI] [PubMed] [Google Scholar]
- Parker PR, Lalive AL, Kreitzer AC (2016) Pathway-Specific Remodeling of Thalamostriatal Synapses in Parkinsonian Mice. Neuron 89 (4):734–740. doi: 10.1016/j.neuron.2015.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquereau B, Turner RS (2011) Primary motor cortex of the parkinsonian monkey: differential effects on the spontaneous activity of pyramidal tract-type neurons. Cereb Cortex 21 (6):1362–1378. doi:bhq217 10.1093/cercor/bhq217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Dorman DB, Hobel ZB, Malgady JM, Blackwell KT, Plotkin JL (2020) Dopamine Oppositely Modulates State Transitions in Striosome and Matrix Direct Pathway Striatal Spiny Neurons. Neuron 108 (6):1091–1102 e1095. doi: 10.1016/j.neuron.2020.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Plotkin JL (2019) Compartmental function and modulation of the striatum. J Neurosci Res 97 (12):1503–1514. doi: 10.1002/jnr.24522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale CW Jr., Graybiel AM (1991) Compartmental organization of the thalamostriatal connection in the cat. The Journal of comparative neurology 311 (1):134–167 [DOI] [PubMed] [Google Scholar]
- Raju DV, Ahern TH, Shah DJ, Wright TM, Standaert DG, Hall RA, Smith Y (2008) Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of parkinsonism. The European journal of neuroscience 27 (7):1647–1658. doi:EJN6136 10.1111/j.1460-9568.2008.06136.x [DOI] [PubMed] [Google Scholar]
- Raju DV, Shah DJ, Wright TM, Hall RA, Smith Y (2006) Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. The Journal of comparative neurology 499 (2):231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Jiao Y, Del Mar N, Laverghetta AV, Lei WL (2003) Differential morphology of pyramidal tract-type and intratelencephalically projecting-type corticostriatal neurons and their intrastriatal terminals in rats. The Journal of comparative neurology 457 (4):420–440. doi: 10.1002/cne.10541 [DOI] [PubMed] [Google Scholar]
- Ren X, Hinchie A, Swomley A, Powell DK, Butterfield DA (2019) Profiles of brain oxidative damage, ventricular alterations, and neurochemical metabolites in the striatum of PINK1 knockout rats as functions of age and gender: Relevance to Parkinson disease. Free Radic Biol Med 143:146–152. doi: 10.1016/j.freeradbiomed.2019.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A, Alberti I, Martin AB, Narvaez JA, de la Calle A, Moratalla R (2002) Molecular phenotype of rat striatal neurons expressing the dopamine D5 receptor subtype. The European journal of neuroscience 16 (11):2049–2058 [DOI] [PubMed] [Google Scholar]
- Rudkin TM, Sadikot AF (1999) Thalamic input to parvalbumin-immunoreactive GABAergic interneurons: organization in normal striatum and effect of neonatal decortication. Neuroscience 88 (4):1165–1175. doi:S0306–4522(98)00265–6 [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, Francois C (1992a) Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. The Journal of comparative neurology 315 (2):137–159. doi: 10.1002/cne.903150203 [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, Smith Y, Bolam JP (1992b) Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a light and electron microscopic study of the thalamostriatal projection in relation to striatal heterogeneity. The Journal of comparative neurology 320 (2):228–242. doi: 10.1002/cne.903200207 [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Ungerleider LG, Desimone R (1990) Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey. The Journal of comparative neurology 298 (2):129–156. doi: 10.1002/cne.902980202 [DOI] [PubMed] [Google Scholar]
- Salgado H, Tecuapetla F, Perez-Rosello T, Perez-Burgos A, Perez-Garci E, Galarraga E, Bargas J (2005) A reconfiguration of CaV2 Ca2+ channel current and its dopaminergic D2 modulation in developing neostriatal neurons. J Neurophysiol 94 (6):3771–3787. doi:00455.2005 10.1152/jn.00455.2005 [DOI] [PubMed] [Google Scholar]
- Sciamanna G, Ponterio G, Mandolesi G, Bonsi P, Pisani A (2015) Optogenetic stimulation reveals distinct modulatory properties of thalamostriatal vs corticostriatal glutamatergic inputs to fast-spiking interneurons. Sci Rep 5:16742. doi: 10.1038/srep16742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS (1985) Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci 5 (3):776–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Zhai S, Surmeier DJ (2022) Striatal synaptic adaptations in Parkinson’s disease. Neurobiol Dis 167:105686. doi: 10.1016/j.nbd.2022.105686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM (2013) Corticostriatal connectivity and its role in disease. Nat Rev Neurosci 14 (4):278–291. doi: 10.1038/nrn3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidibe M, Smith Y (1996) Differential synaptic innervation of striatofugal neurones projecting to the internal or external segments of the globus pallidus by thalamic afferents in the squirrel monkey. The Journal of comparative neurology 365 (3):445–465 [DOI] [PubMed] [Google Scholar]
- Sidibe M, Smith Y (1999) Thalamic inputs to striatal interneurons in monkeys: synaptic organization and co-localization of calcium binding proteins. Neuroscience 89 (4):1189–1208 [DOI] [PubMed] [Google Scholar]
- Sinopoulou E, Rosenzweig ES, Conner JM, Gibbs D, Weinholtz CA, Weber JL, Brock JH, Nout-Lomas YS, Ovruchesky E, Takashima Y, Biane JS, Kumamaru H, Havton LA, Beattie MS, Bresnahan JC, Tuszynski MH (2022) Rhesus macaque versus rat divergence in the corticospinal projectome. Neuron. doi: 10.1016/j.neuron.2022.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Bolam JP (1990) The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci 13 (7):259–265 [DOI] [PubMed] [Google Scholar]
- Smith JB, Klug JR, Ross DL, Howard CD, Hollon NG, Ko VI, Hoffman H, Callaway EM, Gerfen CR, Jin X (2016) Genetic-Based Dissection Unveils the Inputs and Outputs of Striatal Patch and Matrix Compartments. Neuron 91 (5):1069–1084. doi: 10.1016/j.neuron.2016.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JB, Smith Y, Venance L, Watson GDR (2022) Editorial: Thalamic Interactions With the Basal Ganglia: Thalamostriatal System and Beyond. Frontiers in Systems Neuroscience 16. doi: 10.3389/fnsys.2022.883094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Galvan A, Ellender TJ, Doig N, Villalba RM, Huerta-Ocampo I, Wichmann T, Bolam JP (2014a) The thalamostriatal system in normal and diseased states. Front Syst Neurosci 8:5. doi: 10.3389/fnsys.2014.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Parent A (1986) Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus). Neuroscience 18 (2):347–371 [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M (2004) The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci 27 (9):520–527. doi: 10.1016/j.tins.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Smith Y, Wichmann T, DeLong MR (2014b) Corticostriatal and mesocortical dopamine systems: do species differences matter? Nat Rev Neurosci 15 (1):63–63. doi: 10.1038/nrn3469-c1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Bolam JP, Smith AD (1981) Monosynaptic cortical input and local axon collaterals of identified striatonigral neurons. A light and electron microscopic study using the Golgi-peroxidase transport-degeneration procedure. The Journal of comparative neurology 195 (4):567–584. doi: 10.1002/cne.901950403 [DOI] [PubMed] [Google Scholar]
- Stayte S, Dhungana A, Vissel B, Bradfield LA (2021) Parafascicular Thalamic and Orbitofrontal Cortical Inputs to Striatum Represent States for Goal-Directed Action Selection. Front Behav Neurosci 15:655029. doi: 10.3389/fnbeh.2021.655029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez LM, Solis O, Sanz-Magro A, Alberquilla S, Moratalla R (2020) Dopamine D1 Receptors Regulate Spines in Striatal Direct-Pathway and Indirect-Pathway Neurons. Mov Disord 35 (10):1810–1821. doi: 10.1002/mds.28174 [DOI] [PubMed] [Google Scholar]
- Sun J, Kouranova E, Cui X, Mach RH, Xu J (2013) Regulation of dopamine presynaptic markers and receptors in the striatum of DJ-1 and Pink1 knockout rats. Neurosci Lett 557 Pt B:123–128. doi: 10.1016/j.neulet.2013.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K, Watanabe Y, Tsujimura A, Tanaka M (2016) Brain region-dependent differential expression of alpha-synuclein. The Journal of comparative neurology 524 (6):1236–1258. doi: 10.1002/cne.23901 [DOI] [PubMed] [Google Scholar]
- Tanimura A, Du Y, Kondapalli J, Wokosin DL, Surmeier DJ (2019) Cholinergic Interneurons Amplify Thalamostriatal Excitation of Striatal Indirect Pathway Neurons in Parkinson’s Disease Models. Neuron 101 (3):444–458 e446. doi: 10.1016/j.neuron.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Tanimura A, Shen W, Wokosin D, Surmeier DJ (2022) Pathway-Specific Remodeling of Thalamostriatal Synapses in a Mouse Model of Parkinson’s Disease. Mov Disord. doi: 10.1002/mds.29030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP (2004) Functional diversity and specificity of neostriatal interneurons. Curr Op Neurobiol 14 (6):685–692 [DOI] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Ibanez-Sandoval O, Tecuapetla F, Faust TW, Assous M (2018) Heterogeneity and Diversity of Striatal GABAergic Interneurons: Update 2018. Front Neuroanat 12:91. doi: 10.3389/fnana.2018.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Tecuapetla F, Koos T, Ibanez-Sandoval O (2010) Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat 4:150. doi: 10.3389/fnana.2010.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo DG, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, Huang CC, Gerfen CR, Schiller J, Dudman JT, Hantman AW, Looger LL, Schaffer DV, Karpova AY (2016) A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 92 (2):372–382. doi: 10.1016/j.neuron.2016.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM (2010) Neocortical layer 6, a review. Front Neuroanat 4:13. doi: 10.3389/fnana.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ (2012) Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75 (1):58–64. doi: 10.1016/j.neuron.2012.04.038 [DOI] [PubMed] [Google Scholar]
- Totterdell S, Meredith GE (2005) Localization of alpha-synuclein to identified fibers and synapses in the normal mouse brain. Neuroscience 135 (3):907–913. doi: 10.1016/j.neuroscience.2005.06.047 [DOI] [PubMed] [Google Scholar]
- Unzai T, Kuramoto E, Kaneko T, Fujiyama F (2017) Quantitative Analyses of the Projection of Individual Neurons from the Midline Thalamic Nuclei to the Striosome and Matrix Compartments of the Rat Striatum. Cereb Cortex 27 (2):1164–1181. doi: 10.1093/cercor/bhv295 [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ (2002) The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain research Brain research reviews 39 (2–3):107–140 [DOI] [PubMed] [Google Scholar]
- Villalba RM, Pare JF, Lee S, Lee S, Smith Y (2019) Thalamic degeneration in MPTP-treated Parkinsonian monkeys: impact upon glutamatergic innervation of striatal cholinergic interneurons. Brain Struct Funct 224 (9):3321–3338. doi: 10.1007/s00429-019-01967-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Smith Y (2011) Differential structural plasticity of corticostriatal and thalamostriatal axo-spinous synapses in MPTP-treated Parkinsonian monkeys. The Journal of comparative neurology 519 (5):989–1005. doi: 10.1002/cne.22563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Smith Y (2013) Differential striatal spine pathology in Parkinson’s disease and cocaine addiction: a key role of dopamine? Neuroscience 251:2–20. doi: 10.1016/j.neuroscience.2013.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Smith Y (2018) Loss and remodeling of striatal dendritic spines in Parkinson’s disease: from homeostasis to maladaptive plasticity? J Neural Transm (Vienna) 125 (3):431–447. doi: 10.1007/s00702-017-1735-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Wichmann T, Smith Y (2014) Neuronal loss in the caudal intralaminar thalamic nuclei in a primate model of Parkinson’s disease. Brain Struct Funct 219 (1):381–394. doi: 10.1007/s00429-013-0507-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM (2004) Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27 (8):468–474. doi: 10.1016/j.tins.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Wall NR, De La Parra M, Callaway EM, Kreitzer AC (2013) Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron 79 (2):347–360. doi: 10.1016/j.neuron.2013.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AR, Liguore WA, Domire JS, Button D, McBride JL (2020) Intra-striatal AAV2.retro administration leads to extensive retrograde transport in the rhesus macaque brain: implications for disease modeling and therapeutic development. Sci Rep 10 (1):6970. doi: 10.1038/s41598-020-63559-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook A, Frank MJ, Cools R (2021) A mosaic of cost-benefit control over cortico-striatal circuitry. Trends Cogn Sci 25 (8):710–721. doi: 10.1016/j.tics.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ (1987) Morphology and synaptic connections of crossed corticostriatal neurons in the rat. The Journal of comparative neurology 263 (4):567–580. doi: 10.1002/cne.902630408 [DOI] [PubMed] [Google Scholar]
- Wolff SBE, Ko R, Olveczky BP (2022) Distinct roles for motor cortical and thalamic inputs to striatum during motor skill learning and execution. Sci Adv 8 (8):eabk0231. doi: 10.1126/sciadv.abk0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Roberts TF (2021) What Is the Role of Thalamostriatal Circuits in Learning Vocal Sequences? Frontiers in neural circuits 15:724858. doi: 10.3389/fncir.2021.724858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Hori Y, Minamimoto T, Yamada H, Matsumoto N, Enomoto K, Aosaki T, Graybiel AM, Kimura M (2018) Roles of centromedian parafascicular nuclei of thalamus and cholinergic interneurons in the dorsal striatum in associative learning of environmental events. J Neural Transm (Vienna) 125 (3):501–513. doi: 10.1007/s00702-017-1713-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S, Shen W, Graves SM, Surmeier DJ (2019) Dopaminergic modulation of striatal function and Parkinson’s disease. J Neural Transm (Vienna) 126 (4):411–422. doi: 10.1007/s00702-019-01997-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Meredith GE, Mendoza-Elias N, Rademacher DJ, Tseng KY, Steece-Collier K (2013) Aberrant Restoration of Spines and their Synapses in L-DOPA-Induced Dyskinesia: Involvement of Corticostriatal but Not Thalamostriatal Synapses. J Neurosci 33 (28):11655–11667. doi: 10.1523/JNEUROSCI.0288-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Roy DS, Zhu Y, Chen Y, Aida T, Hou Y, Shen C, Lea NE, Schroeder ME, Skaggs KM, Sullivan HA, Fischer KB, Callaway EM, Wickersham IR, Dai J, Li X-M, Lu Z, Feng G (2022) Targeting thalamic circuits rescues motor and mood deficits in PD mice. Nature. doi: 10.1038/s41586-022-04806-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Sun L, Liu B, Huang Z, Zhu Y, Chen T, Jia L, Li Y, Lei W (2021) Morphological Study of the Cortical and Thalamic Glutamatergic Synaptic Inputs of Striatal Parvalbumin Interneurons in Rats. Neurochem Res 46 (7):1659–1673. doi: 10.1007/s11064-021-03302-4 [DOI] [PubMed] [Google Scholar]


