Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS) are shown to have neurotoxic effects on animals, but epidemiological evidence for associations between childhood PFAS exposure and neurodevelopment is inconclusive. We examined if childhood PFAS concentrations are associated with a diagnosis of autism spectrum disorder (ASD), developmental delay (DD), and other early concerns (OEC) in development.
Methods:
We included 551 children 2 to 5 years old from the CHildhood Autism Risks from Genetics and Environment (CHARGE) case-control study. Children were clinically diagnosed and classified as having ASD, DD, OEC, and typical development (TD). Fourteen PFAS were quantified in child serum samples collected when diagnostic assessments were performed. We used multinomial logistic regression models to investigate the cross-sectional associations of individual PFAS concentrations with neurodevelopmental outcomes and weighted quantile sum (WQS) regression models with repeated holdout validation to investigate the associations with PFAS mixtures.
Results:
Childhood perfluorooctanoic acid (PFOA) was associated with increased odds of ASD (odds ratio [OR] per ln ng/mL increase: 1.99, 95% confidence interval [CI]: 1.20, 3.29) and DD (OR: 2.16, 95% CI: 1.21, 3.84) versus TD. Perfluoroheptanoic acid (PFHpA) was associated with increased odds of ASD (OR: 1.61, 95% CI: 1.21, 2.13). However, perfluroundecanoic acid (PFUnDA) was associated with decreased odds of ASD (OR: 0.43, 95% CI: 0.26, 0.69). From mixture analyses, the WQS index was associated with increased odds of ASD (average OR: 1.57, 5th and 95th percentile: 1.16, 2.13). Child’s sex and homeownership modified associations of perfluorodecanoic acid (PFDA) with DD and ASD, respectively.
Conclusions:
In this case-control study, childhood PFOA, PFHpA, and a PFAS mixture was associated with increased odds of ASD, while PFUnDA was associated with decreased odds of ASD. Because we used concurrent measurements of PFAS, our results do not imply causal relationships and thus need to be interpreted with caution.
1. Background
Per- and polyfluoroalkyl substances (PFAS) are a group of synthetic, fluorinated chemicals that have been used in a variety of consumer products, including non-stick cookware, food packaging materials, fabric and carpet treatments, and firefighting foams, since the 1950s (ATSDR 2021). Infants and young children are exposed to PFAS through ingestion of breast milk, contaminated food and water, and house dust and soil (Fromme et al. 2010; Fromme et al. 2009; Kärrman et al. 2007; Trudel et al. 2008; Winkens et al. 2017; Wu and Kannan 2019). Due to their widespread exposure and persistence, several long-chain PFAS, including perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), perfluorohexane-1-sulfonic acid (PFHxS), and perfluorononanoic acid (PFNA), are frequently detected in the blood of children aged from 0 to 17 years (Duffek et al. 2020; Gump et al. 2011; Koponen et al. 2018; Schecter et al. 2012; Ye et al. 2018; Zhang et al. 2018b).
Early-life exposure to PFAS is shown to cause neurotoxic effects and behavioral changes in laboratory animals (Mariussen 2012). From repeated studies, male mice that were neonatally exposed to PFOA and PFOS showed persistent disturbances in spontaneous behaviors, such as reduced habituation and increased hyperactivity, in their adulthood (Hallgren et al. 2015; Johansson et al. 2008). They also failed to respond normally to nicotine injections, suggesting adverse effects of PFOA and PFOS on the developing cholinergic system (Johansson et al. 2008). PFOS exposure disturbed the cholinergic system in the brain of male neonatal mice by inducing reduction of transcriptional levels of several genes involved in acetylcholine signaling and metabolism after 24 hours (Hallgren et al. 2015). Neonatal exposure to PFOA and PFOS altered protein levels that are critical for normal brain development through their involvement in neuronal growth and survival and synaptogenesis in the neonatal brain (Johansson et al. 2009). Similar to PFOA and PFOS , PFHxS exposure to both male and female mice in the neonatal period was associated with alterations in spontaneous behaviors and habituation as well as functions of the cholinergic system in adulthood (Viberg et al. 2013).
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social interaction and communication skills and restricted, repetitive, and stereotypic behaviors and interests (American Psychiatric Association 2013). The prevalence of ASD in the United States (U.S.) has increased over the last decades, from 1 in 68 in 2010 to 1 in 44 children in 2018 (Baio 2014; Maenner et al. 2021). Exposure to a single compound or mixtures of environmental chemicals, especially during prenatal and early postnatal periods, can contribute to the development of ASD by disrupting thyroid functions which are critical for child brain development (Braun 2017; Hertz-Picciotto et al. 2018; Williams 2008). There is evidence that prenatal and early childhood exposures to PFAS interfere with thyroid hormone levels of newborns and children at age 1-5, respectively (Ballesteros et al. 2017; Lopez-Espinosa et al. 2012). Epidemiological studies showed null (Liew et al. 2014), adverse (Braun et al. 2014; Long et al. 2019; Lyall et al. 2018), or favorable (Oh et al. 2021a; Shin et al. 2020) associations between ASD risk and prenatal PFAS exposure, yet no previous study examined an association of ASD with PFAS exposure during postnatal periods. Early- to mid-childhood exposure to PFAS has been well studied for neurodevelopmental outcomes such as cognition, behaviors, and neuropsychological functioning, but the results are inconclusive, showing adverse (Harris et al. 2021; Harris et al. 2018; Oulhote et al. 2016; Vuong et al. 2021) or favorable (Stein et al. 2013; Vuong et al. 2019; Zhang et al. 2018a) associations.
Given the long half-lives of PFAS on the order of years (Bartell et al. 2010; Olsen et al. 2007; Wong et al. 2014), PFAS measured in 2- to 5-year-old children represent their exposures during the first few years of life, when the brain is still plastic, and its development is ongoing. The objective of this study was to elucidate if individual PFAS serum concentrations representing a cross-sectional look at ages 2-5 years, for at a set of compounds with half-lives of years, were associated with child neurodevelopmental outcomes. These outcomes are: ASD, developmental delay (DD), and other early concerns (OEC), as well as cognitive and adaptive functions. Child blood samples were collected on the same day that the child neurodevelopmental assessment was performed. We also examined the associations between exposure to a PFAS mixture and child neurodevelopment.
2. Methods
2.1. Study population
Children included in this study were participants in the Childhood Autism Risks from Genetics and Environment (CHARGE) study, a population-based case-control study (Hertz-Picciotto et al. 2006). Children who received services for ASD and DD were identified and recruited through Regional Centers that contract with the California Department of Developmental Services to provide services for individuals with developmental conditions. The general population controls were recruited through California birth files, frequency-matched to the ASD cases on child’s sex and age and Regional Center catchment area. Because ASD is approximately four times more prevalent in males, the study sought to recruit a similar ratio among controls: 80% males and 20% females. All children meeting the following eligibility criteria were included: a) aged between 2 and 5 years at study enrollment, b) born in California, c) living with at least one biological parent who speaks English or Spanish, and d) residing in one of the CHARGE study catchment areas. Study protocols were approved by the institutional review boards of the State of California and the University of California (UC), and written informed consent was obtained from the parents prior to collection of any data. Detailed information on study design, study population, recruitment, eligibility criteria, and data and sample collection is described elsewhere (Hertz-Picciotto et al. 2006). Though launched in 2003, the CHARGE study started collecting serum in 2009. Among the 889 CHARGE children who were enrolled between 2009 and 2017, we included 551 children who provided serum samples with sufficient volume for PFAS quantification.
2.2. Child neurodevelopmental assessment
Children 2 to 5 years old visited the UC Davis Medical Investigations of Neurodevelopmental Disorders (MIND) Institute for neurodevelopmental assessment.
Parents of children with ASD were administered the Autism Diagnostic Interview-Revised (ADI-R) (Le Couteur et al. 2003; Lord et al. 1997; Lord et al. 1994), and children were assessed using the Autism Diagnostic Observation Schedules-Generic (ADOS-G) (Lord et al. 2000) by clinical personnel who had achieved reliability on their respective instruments. Children with DD or recruited from the general population controls were screened for ASD symptoms using the Social Communication Questionnaire (SCQ) (Rutter et al. 2003). Those children whose SCQ score was above 14 were further administered the ADI-R and ADOS-G to assess for ASD diagnosis. For all children included in this study, cognitive functions were assessed using the Mullen Scales of Early Learning (MSEL) (Mullen 1997), and adaptive functions were assessed using the Vineland Adaptive Behavior Scales (VABS) (Sparrow et al. 1984). The MSEL scores on four subscales (Fine Motor, Visual Reception, Receptive Language, Expressive Language), which are combined to generate an Early Learning Composite (Composite) score. The VABS scores on four subscales (Socialization, Daily Living Skills, Motor Skills, Communication), are also combined to yield an Adaptive Behavior Composite (Composite) score. Composite and subscale scores for both MSEL and VABS are standardized with a normative mean of 100 and a standard deviation (SD) of 15. Higher MSEL and VABS scores indicate better cognitive and adaptive functions, respectively.
A final diagnosis of ASD was assigned when the child (1) met criteria on either the social or communication domains of the ADI-R and were within two points of meeting criteria in the other domain and (2) scored above the social and communication total cutoff for ASD on the ADOS module 1 or 2 (Risi et al. 2006). A diagnosis of DD was defined as having either one of the MSEL or VABS scores, but not both, less than 1.5 SD below the mean, having scores on the other instrument less than 2 SD below the mean, and scoring above 14 on the SCQ (Hertz-Picciotto et al. 2006). Among the children who did not meet the criteria for either ASD or DD in this study, children who previously received services for ASD or DD were classified as OEC, while children recruited from the general population controls who did not meet criteria for either ASD or DD were classified as typical development (TD). As a result, 551 children were categorized as ASD (n = 190), DD (n = 103), OEC (n = 78), and TD (n = 180).
2.3. Serum sample collection and PFAS quantification
Child blood samples were collected at the end of the neurodevelopmental assessment study visit. The mean child age at the sample collection was 3.4 years (range = 2 to 5 years). Whole blood samples were centrifuged, and the separated serum was transferred to a vial and frozen at −80 °C until analysis. For PFAS quantification, aliquots of the serum samples were shipped to the New York State Department of Health’s Wadsworth Center’s Human Health Exposure Analysis Resource Targeted Analysis Laboratory. The analytical method for 14 PFAS in child serum was previously described (Honda et al. 2018). Briefly, 0.25 mL of serum samples were aliquoted into 15 mL polypropylene tubes and spiked with 5 ng of 13C-labeled internal standard mixture and 0.7 mL of 1% ammonium formate (w/v) in methanol. The mixture was centrifuged for 5 min at 5000 rpm, and the supernatant was collected and passed through hybrid-solid-phase extraction cartridge (Phospholipid, 30 mg, 1 cc, Sigma-Aldrich, St. Louis, MO, USA). The cartridges were conditioned with 1 mL of methanol containing 1% ammonium formate (w/v). The samples were eluted through the cartridge and analyzed using an AB SCIEX™ 5500 electrospray triple quadrupole mass spectrometer (SCIEX, Framingham, MA, USA), interfaced with a Nexera X2 LC-30AD series high-performance liquid chromatography (Shimadzu, Kyoto, Japan). Details of the LC parameters and mass spectrometric conditions are described elsewhere (Honda et al. 2018).
Fourteen PFAS quantified included: PFOA, PFOS, PFHxS, PFNA, perfluorodecanoic acid (PFDA), perfluoro-n-pentanoic acid (PFPeA), perfluoroheptanoic acid (PFHpA), perfluoroundecanoic acid (PFUnDA), perfluorobutanesulfonic acid (PFBS), perfluorohexanoic acid (PFHxA), perfluorododecanoic acid (PFDoDA), N-methyl perfluorooctane sulfonamido acetic acid (MeFOSAA), ethyl perfluorooctane sulfonamido acetic acid (EtFOSAA), and perfluorooctanesulfonamide (PFOSA). For quality control, five replicates of procedural blanks and quality control samples (i.e., water spiked with native standards at 5 ng for all analytes and internal standards) were processed for each batch of 100 samples. In addition, two replicates of Standard Reference Material (SRM1957 and SRM1958, NIST, Gaithersburg, MD, USA), spiked with internal standards, containing certified values for PFOA, PFOS, PFHxS, PFNA, PFDA, PFHpA, and PFUnDA for SRM1957 and PFOA, PFOS, PFHxS, and PFNA for SRM1958 were processed. The limit of detection (LOD) was 0.02 ng/mL for PFOS, PFHxS, PFNA, PFDA, PFHpA, PFUnDA, PFBS, MeFOSAA, EtFOSAA, and PFOSA, and 0.05 ng/mL for PFOA, PFPeA, PFHxA, and PFDoDA. We used instrument-observed values for PFAS concentrations below the LOD, rather than imputing them with a single value, to avoid biased estimates (Richardson and Ciampi 2003; Schisterman et al. 2006).
2.4. Statistical analysis
Participant characteristics were compared by neurodevelopmental outcomes (ASD, DD, OEC, TD) using the chi-squared test for categorical variables or the Kruskal-Wallis test for continuous variables. MSEL and VABS Composite scores were compared by participant characteristics using the Wilcoxon rank-sum test or the Kruskal-Wallis test. Child serum PFAS concentrations were compared by neurodevelopmental outcomes using the Kruskal-Wallis test and by participant characteristics using the Wilcoxon rank-sum test or the Kruskal-Wallis test. Spearman’s correlation coefficients (rsp) were computed among PFAS concentrations. Among 14 PFAS quantified, 9 PFAS that were detected in greater than 74% of the serum samples were used in the regression analyses. Because instrument-observed values were used, some of the PFAS concentrations below the LOD contained negative values. Therefore, the minimum value of each PFAS concentration and a value of 0.01 were added to all PFAS concentrations to avoid negative and zero values (Bennett et al. 2022), which were then natural log (ln)-transformed to normalize the right-skewed distributions.
Multinomial logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between child serum PFAS concentrations and ASD, DD, and OEC, compared to TD. A false discovery rate (FDR) p-value was corrected per neurodevelopmental outcome to account for multiple comparisons. For the associations with continuous MSEL and VABS scores, multiple linear regression models were used to estimate regression coefficients (βs) and 95% CIs for Composite and each subscale. Among potential confounders a priori identified from a directed acyclic graph (Figure S1) (Hernán et al. 2004), those covariates that were associated with both exposures and outcomes (p < 0.20) were adjusted in the regression models. The covariates included in the final models were CHARGE frequency matching factors (child’s sex [female, male] and age at sampling [in years], recruitment regional center); sampling year (2009-2010, 2011-2013, 2014-2017); gestational age at delivery (< 37, ≥ 37 weeks), maternal factors (birthplace [U.S., non-U.S.], parity [1, 2, > 2], breastfeeding duration [never, ≤ 3, > 3 months], race/ethnicity [non-Hispanic white, Hispanic, Black/Asian/ multiracial], and homeownership [non-owner, owner] as an indicator of socioeconomic status [SES]). To achieve the most parsimonious model, maximum education in household, maternal age at delivery, and health insurance type at delivery, all of which represent or reflect SES, were excluded from the final models because homeownership was most strongly associated with outcomes. As four covariates had approximately 2% to 4% of the missing observations, we imputed them in a multiple imputation model by chained equations, which included all exposures, outcomes, and the selected covariates (White et al. 2011). We generated 20 imputed datasets and pooled the estimates across datasets (Graham et al. 2007; Rubin 2004). For sensitivity analysis, we ran the multinomial logistic regression and multiple linear regression models without imputing missing covariates.
To examine the associations of mixtures of nine PFAS with child neurodevelopment, we performed weighted quantile sum (WQS) regression analyses with repeated holdout validation, accounting for multicollinearity and nonlinear relationships (Carrico et al. 2015; Lazarevic et al. 2019). After randomly splitting the data into training (40%) and validation (60%) sets, an average of empirical weights of each PFAS across the 100 bootstrap samples was estimated and applied to quartiles of PFAS concentrations to yield a WQS index, represented by the total body burden. We then examined associations of the WQS index with neurodevelopmental outcomes using the multinomial logistic regression models and with MSEL and VABS Composite scores using multiple linear regression models, respectively. We adjusted for the same covariate set as before, but without multiple imputation. The process of splitting participants into training and validation sets was repeated 100 times, resulting in 100 effect estimates. We constrained the inference in a positive direction for neurodevelopmental outcomes and in a negative direction for cognitive and adaptive functions to test the hypothesis that a PFAS mixture was associated with increased odds of ASD, DD, and OEC and poorer performance on the MSEL and VABS, i.e., lower Composite scores. When at least 95% of the holdouts resulted in increased odds, per diagnosis group, chemical weight distributions were interpreted. Chemicals with 50% of repetitions above a threshold of 0.11 (1/number of PFAS detected greater than 74% of samples = 1/9) were defined as being possible contributors (Bennett et al. 2022; Carrico et al. 2015).
For associations between individual PFAS concentrations and each of the outcomes, we investigated effect modification by child’s sex, homeownership (as a proxy of SES), and breastfeeding duration, based on suggestive evidence from previous studies (Forns et al. 2020; Oh et al. 2021b; Oulhote et al. 2016; Skogheim et al. 2021; Stein et al. 2014; Vuong et al. 2019; 2021). We compared the regression coefficients in the stratified analyses and evaluated a p-value for the interaction term between each PFAS concentration and each potential effect modifier. We also stratified the multiple linear regression analyses for MSEL and VABS scores by neurodevelopmental outcomes: ASD (n = 190) and non-ASD (n = 361). To assess the confounding effect of breastfeeding duration, we ran the final multinomial logistic regression and multiple linear regression models without adjusting for breastfeeding duration. Statistical analyses were conducted using R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria), including R packages “mice”, “nnet”, and “gWQS” (Renzetti et al. 2021; Ripley et al. 2016; Van Buuren and Groothuis-Oudshoorn 2011). Associations with p < 0.05 were considered as statistically significant, and those with p < 0.10 after FDR correction as borderline significant.
3. Results
3.1. Participant characteristics by neurodevelopmental outcomes
This study population consisted of 190 ASD, 103 DD, 78 OEC, and 180 TD children (Table 1). Seventeen pairs of twins and 9 families with 2 siblings enrolled in the study were included. Among 551 children, approximately 74% were males and 82% were born full-term. Because children with ASD and TD were frequency-matched on child’s sex, they were more likely to be male than those with DD and OEC. The goal was to age-match within 6 months, but children with ASD were older than children with TD. Children with ASD tended to be the firstborn child and recruited in later years compared to those with TD, DD, or OEC. Mothers of children with TD were more likely to be non-Hispanic white, own a home, be born in the U.S., and have insurance at delivery and also more likely to have breastfed longer than 3 months compared to those of children with other diagnoses. There was no difference in demographic characteristics between the CHARGE mother-child pairs included and excluded from this study (Table S1).
Table 1.
Participant characteristics of 551 mother-child pairs by neurodevelopmental outcomes and MSEL and VABS Composite scores by characteristics in the CHARGE study.
| Characteristic | All (n = 551) a |
TD (n = 180) |
ASD (n = 190) |
DD (n = 103) |
OEC (n = 78) |
p b | MSEL Composite | p c | VABS Composite | p c |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Freq (%) | Freq (%) | Freq (%) | Freq (%) | Freq (%) | Mean (SD) | Mean (SD) | ||||
| Child sex | <0.001 | 0.147 | 0.052 | |||||||
| Female | 145 (26.3) | 32 (17.8) | 42 (22.1) | 41 (39.8) | 30 (38.5) | 77 (31) | 73 (21) | |||
| Male | 406 (73.7) | 148 (82.2) | 148 (77.9) | 62 (60.2) | 48 (61.5) | 82 (28) | 77 (20) | |||
| Sampling year | <0.001 | 0.015 | <0.001 | |||||||
| 2009 – 2010 | 180 (32.7) | 65 (36.1) | 48 (25.3) | 33 (32.0) | 34 (43.6) | 84 (28) | 81 (22) | |||
| 2011 – 2013 | 214 (38.8) | 82 (45.6) | 46 (24.2) | 57 (55.3) | 29 (37.2) | 81 (29) | 77 (20) | |||
| 2014 – 2017 | 157 (28.5) | 33 (18.3) | 96 (50.5) | 13 (12.6) | 15 (19.2) | 75 (28) | 69 (18) | |||
| Gestational age at delivery | <0.001 | 0.074 | 0.045 | |||||||
| < 37 weeks | 86 (15.6) | 14 (7.8) | 22 (11.6) | 27 (26.2) | 23 (29.5) | 76 (27) | 71 (17) | |||
| ≥ 37 weeks | 452 (82.0) | 162 (90.0) | 163 (85.8) | 72 (69.9) | 55 (70.5) | 81 (29) | 77 (21) | |||
| Mother’s age at delivery | 0.179 | 0.103 | 0.072 | |||||||
| < 30 years | 247 (44.8) | 77 (42.8) | 94 (49.5) | 43 (41.7) | 33 (42.3) | 78 (29) | 75 (21) | |||
| 30 – 35 years | 156 (28.3) | 60 (33.3) | 50 (26.3) | 23 (22.3) | 23 (29.5) | 84 (28) | 79 (21) | |||
| ≥ 35 years | 148 (26.9) | 43 (23.9) | 46 (24.2) | 37 (35.9) | 22 (28.2) | 80 (29) | 74 (19) | |||
| Parity | 0.006 | 0.452 | 0.875 | |||||||
| 1 | 204 (37.0) | 55 (30.6) | 91 (47.9) | 29 (28.2) | 29 (37.2) | 80 (28) | 75 (19) | |||
| 2 | 209 (37.9) | 73 (40.6) | 67 (35.3) | 40 (38.8) | 29 (37.2) | 79 (29) | 76 (21) | |||
| > 2 | 120 (21.8) | 47 (26.1) | 28 (14.7) | 28 (27.2) | 17 (21.8) | 83 (28) | 76 (21) | |||
| Mother’s pre-pregnancy BMI | 0.985 | 0.562 | 0.574 | |||||||
| Normal/underweight | 286 (51.9) | 97 (53.9) | 100 (52.6) | 51 (49.5) | 38 (48.7) | 81 (29) | 76 (20) | |||
| Overweight | 120 (21.8) | 40 (22.2) | 41 (21.6) | 23 (22.3) | 16 (20.5) | 80 (29) | 76 (20) | |||
| Obese | 134 (24.3) | 41 (22.8) | 45 (23.7) | 28 (27.2) | 20 (25.6) | 78 (28) | 75 (21) | |||
| Vitamin intake during the first month of pregnancy | 0.150 | 0.026 | 0.007 | |||||||
| No | 159 (28.9) | 50 (27.8) | 65 (34.2) | 29 (28.2) | 15 (19.2) | 77 (30) | 73 (21) | |||
| Yes | 329 (59.7) | 117 (65.0) | 106 (55.8) | 57 (55.3) | 49 (62.8) | 83 (28) | 78 (21) | |||
| Mother’s race/ethnicity | <0.001 | <0.001 | <0.001 | |||||||
| Non-Hispanic White | 317 (57.5) | 126 (70.0) | 96 (50.5) | 47 (45.6) | 48 (61.5) | 87 (28) | 80 (21) | |||
| Hispanic | 133 (24.1) | 28 (15.6) | 45 (23.7) | 39 (37.9) | 21 (26.9) | 70 (25) | 70 (19) | |||
| Black, Asian, multiracial | 101 (18.3) | 26 (14.4) | 49 (25.8) | 17 (16.5) | 9 (11.5) | 74 (29) | 71 (18) | |||
| Maximum education in household | 0.041 | <0.001 | <0.001 | |||||||
| Less than college credit | 271 (49.2) | 78 (43.3) | 91 (47.9) | 64 (62.1) | 38 (48.7) | 76 (27) | 73 (19) | |||
| Bachelor’s degree | 179 (32.5) | 69 (38.3) | 66 (35.7) | 23 (22.3) | 21 (26.9) | 84 (29) | 78 (21) | |||
| Graduate or professional degree | 101 (18.3) | 33 (18.3) | 33 (17.4) | 16 (15.5) | 19 (24.4) | 86 (28) | 80 (20) | |||
| Homeownership | 0.005 | <0.001 | <0.001 | |||||||
| Non-owner | 223 (40.5) | 55 (30.6) | 82 (43.2) | 49 (47.6) | 37 (47.4) | 75 (27) | 72 (19) | |||
| Owner | 304 (55.2) | 118 (65.6) | 102 (53.7) | 47 (45.6) | 37 (47.4) | 85 (29) | 79 (21) | |||
| Mother’s birthplace | 0.049 | <0.001 | <0.001 | |||||||
| U.S. | 431 (78.2) | 152 (84.4) | 140 (73.7) | 76 (73.8) | 63 (80.8) | 83 (28) | 77 (21) | |||
| Non-U.S. | 120 (21.8) | 28 (15.6) | 50 (26.3) | 27 (26.2) | 15 (19.2) | 72 (28) | 70 (18) | |||
| Health insurance type at delivery | 0.007 | <0.001 | <0.001 | |||||||
| No insurance | 139 (25.2) | 32 (17.8) | 55 (28.9) | 36 (35.0) | 16 (20.5) | 69 (26) | 69 (19) | |||
| Insurance | 394 (71.5) | 140 (77.8) | 129 (67.9) | 65 (63.1) | 60 (76.9) | 84 (28) | 78 (20) | |||
| Breastfeeding duration | <0.001 | <0.001 | <0.001 | |||||||
| Never | 42 (8.0) | 9 (5.2) | 16 (8.7) | 12 (12.8) | 5 (6.6) | 73 (29) | 69 (18) | |||
| ≤ 3 months | 168 (31.9) | 32 (18.4) | 76 (41.5) | 35 (37.2) | 25 (32.9) | 72 (28) | 70 (19) | |||
| > 3 months | 317 (60.2) | 133 (76.4) | 91 (49.7) | 47 (50.0) | 46 (60.5) | 87 (27) | 80 (20) | |||
| Recruitment regional center | 0.264 | 0.251 | 0.217 | |||||||
| Alta, Far Northern, Redwood Coast | 305 (55.4) | 101 (56.1) | 108 (56.8) | 58 (56.3) | 38 (48.7) | 81 (29) | 76 (20) | |||
| North Bay, East Bay, San Andreas, Golden Gate | 104 (18.9) | 41 (22.8) | 27 (14.2) | 18 (17.5) | 18 (23.1) | 83 (30) | 78 (22) | |||
| Valley Mountain, Central Valley, Kern | 142 (25.8) | 38 (21.1) | 55 (28.9) | 27 (26.2) | 22 (28.2) | 78 (27) | 73 (19) | |||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||||
| Child’s age at sampling (years) | 3.4 (0.8) | 3.2 (0.8) | 3.5 (0.7) | 3.4 (0.7) | 3.3 (0.8) | 0.004 | ||||
Missing (frequency, %): gestational age at delivery (13, 2%), parity (18, 3%), mother’s pre-pregnancy BMI (11, 2%), vitamin intake during the first month of pregnancy (63, 11%), homeownership (24, 4%), health insurance type at delivery (18, 3%), breastfeeding duration (24, 4%)
P-values from the chi-squared test for categorical variables or the Kruskal-Wallis test for continuous variables
P-values from the Wilcoxon rank-sum test for binary variables or the Kruskal-Wallis test for other categorical variables
Children with TD had higher mean MSEL and VABS Composite scores than those with ASD (107 vs. 63 for MSEL and 98 vs. 62 for VABS) (Table S2). By definition, the TD group had higher scores than the DD group for MSEL (107 vs. 55) and VABS (98 vs. 59). Composite scores differed by several participant characteristics (Table 1). MSEL and VABS Composite scores were higher in children who were recruited in earlier years and born at or after 37 weeks of pregnancy and whose mother took prenatal vitamins during the first month of pregnancy, was non-Hispanic white, held a bachelor’s or higher degree, owned a home, was born in the U.S., had insurance at delivery, and breastfed their child longer than 3 months.
3.2. Child serum PFAS concentrations
PFOA, PFOS, PFHxS, and PFNA were detected in 100% of the child serum samples, and PFDA, PFPeA, PFHpA, PFUnDA, and MeFOSAA were detected in 99.6, 99.3, 96.0, 74.6, and 81.1% of the samples, respectively (Table 2). The detection frequencies of PFBS, PFHxA, PFDoDA, EtFOSAA, and PFOSA were less than 30%. Median concentrations of several PFAS were different by the neurodevelopmental outcomes. Children with TD had higher medians of PFOA, PFOS, PFHxS, PFNA, and PFUnDA compared to those with ASD, higher medians of PFDA and PFUnDA than those with ASD and OEC, and higher median PFHpA than those with DD and OEC. On the contrary, children with DD and OEC had higher median PFPeA compared to those with TD. Among 9 PFAS detected in greater than 74% of the samples, PFAS compounds were weakly to moderately correlated with each other (rsp = 0.15 to 0.69), except for PFPeA (rsp = −0.02 to 0.14) (Figure S2). There were differences in median PFAS concentrations by participant characteristics (Table S3). Most PFAS concentrations tended to be higher in children recruited in earlier years and born at or after 37 weeks of pregnancy and whose mother was normal or underweight at pre-pregnancy, non-Hispanic white, and had longer breastfeeding duration.
Table 2.
Distributions of PFAS concentrations in 551 child serum samples.
| PFAS a | LOD (ng/mL) | % detect | 50th (5th, 95th) percentiles (ng/mL) | p-value (vs. TD) b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| All (n = 551) | TD (n = 180) | ASD (n = 190) | DD (n = 103) | OEC (n = 78) | ASD | DD | OEC | |||
| PFOA | 0.05 | 100 | 2.36 (0.93, 6.77) | 2.58 (1.14, 6.50) | 2.20 (0.91, 6.30) | 2.22 (0.90, 9.45) | 2.13 (0.90, 6.31) | 0.01 | 0.10 | 0.13 |
| PFOS | 0.02 | 100 | 2.45 (0.93, 11.0) | 2.58 (1.13, 10.7) | 2.01 (0.81, 8.01) | 2.62 (0.86, 11.0) | 2.90 (0.99, 13.6) | <0.01 | 0.35 | 0.99 |
| PFHxS | 0.02 | 100 | 0.64 (0.24, 3.41) | 0.72 (0.28, 3.07) | 0.59 (0.20, 3.05) | 0.59 (0.23, 3.48) | 0.71 (0.24, 4.24) | 0.01 | 0.14 | 0.98 |
| PFNA | 0.02 | 100 | 0.85 (0.29, 2.60) | 0.92 (0.41, 3.24) | 0.71 (0.26, 2.49) | 0.92 (0.33, 2.49) | 0.93 (0.29, 2.42) | <0.01 | 0.99 | 0.33 |
| PFDA | 0.02 | 99.6 | 0.16 (0.07, 0.48) | 0.17 (0.08, 0.50) | 0.14 (0.06, 0.49) | 0.15 (0.07, 0.44) | 0.15 (0.07, 0.34) | <0.01 | 0.06 | 0.02 |
| PFPeA | 0.05 | 99.3 | 0.57 (0.22, 1.25) | 0.56 (0.22, 1.14) | 0.51 (0.20, 1.33) | 0.62 (0.24, 1.44) | 0.65 (0.22, 1.17) | 0.67 | 0.02 | 0.04 |
| PFHpA | 0.02 | 96.0 | 0.20 (0.03, 0.87) | 0.22 (0.04, 0.72) | 0.23 (0.03, 1.00) | 0.16 (0.02, 0.86) | 0.18 (<LOD, 1.04) | 0.65 | 0.04 | 0.02 |
| PFUnDA | 0.02 | 74.6 | 0.04 (<LOD, 0.15) | 0.05 (<LOD, 0.16) | 0.03 (<LOD, 0.13) | 0.04 (<LOD, 0.13) | 0.04 (<LOD, 0.18) | <0.01 | 0.06 | 0.03 |
| PFBS | 0.02 | 29.6 | <LOD (<LOD, 0.07) | <LOD (<LOD, 0.06) | <LOD (<LOD, 0.10) | <LOD (<LOD, 0.06) | <LOD (<LOD, 0.06) | - | - | - |
| PFHxA | 0.05 | 16.2 | <LOD (<LOD, 0.28) | <LOD (<LOD, 0.24) | <LOD (<LOD, 0.43) | <LOD (<LOD, <LOD) | <LOD (<LOD, 0.15) | - | - | - |
| MeFOSAA | 0.02 | 81.1 | 0.12 (<LOD, 1.64) | 0.12 (<LOD, 1.45) | 0.10 (<LOD, 1.56) | 0.14 (<LOD, 2.26) | 0.13 (<LOD, 2.14) | 0.06 | 0.24 | 0.66 |
| EtFOSAA | 0.02 | 17.1 | <LOD (<LOD, 0.07) | <LOD (<LOD, 0.07) | <LOD (<LOD, 0.06) | <LOD (<LOD, 0.07) | <LOD (<LOD, 0.10) | - | - | - |
PFDoDA and PFOSA, which were detected in 4.2% and 0.7% of the samples, respectively, were excluded from the table
P-values from the Wilcoxon rank-sum test were not calculated for PFAS that were detected in less than 30% of the samples.
3.3. Associations of child serum PFAS concentrations with child neurodevelopmental outcomes and MSEL and VABS scores
After adjusting for selected covariates, child serum PFOA concentrations were associated with increased odds of ASD (OR per ln ng/mL increase: 1.99, 95% CI: 1.20, 3.29) and DD (OR: 2.16, 95% CI: 1.21, 3.84) compared to TD (Table 3). PFHpA was associated with increased odds of ASD (OR: 1.61, 95% CI: 1.21, 2.13). On the other hand, PFUnDA was associated with decreased odds of ASD (OR: 0.43, 95% CI: 0.26, 0.69) compared to TD. After FDR correction, the associations of ASD with PFOA, PFHpA, and PFUnDA remained significant, while those of DD with PFOA became borderline significant.
Table 3.
Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of ASD (n = 190), DD (n = 103), and OEC (n = 78) versus TD (n = 180) in association with child serum PFAS concentrations and p-values with and without FDR correction for multiple tests.
| PFAS (ng/mL) a | ASD vs. TD | DD vs. TD | OEC vs. TD | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| OR b (95% CI) | Unadj. p-value | FDR corrected p-value | OR b (95% CI) | Unadj. p-value | FDR corrected p-value | OR b (95% CI) | Unadj. p-value | FDR corrected p-value | |
| PFOA | 1.99 (1.20, 3.29) | 0.007 | 0.022 | 2.16 (1.21, 3.84) | 0.009 | 0.083 | 1.15 (0.61, 2.18) | 0.658 | 0.658 |
| PFOS | 0.89 (0.59, 1.35) | 0.586 | 0.586 | 1.37 (0.89, 2.12) | 0.152 | 0.228 | 1.39 (0.87, 2.22) | 0.165 | 0.371 |
| PFHxS | 1.22 (0.84, 1.78) | 0.297 | 0.383 | 1.52 (1.00, 2.32) | 0.053 | 0.158 | 1.41 (0.92, 2.18) | 0.115 | 0.345 |
| PFNA | 0.75 (0.48, 1.17) | 0.203 | 0.365 | 0.88 (0.53, 1.46) | 0.621 | 0.798 | 0.74 (0.42, 1.30) | 0.299 | 0.449 |
| PFDA | 0.78 (0.49, 1.24) | 0.298 | 0.383 | 0.95 (0.56, 1.62) | 0.861 | 0.969 | 0.61 (0.33, 1.10) | 0.100 | 0.345 |
| PFPeA | 1.48 (0.96, 2.28) | 0.074 | 0.167 | 1.59 (0.93, 2.74) | 0.092 | 0.208 | 1.15 (0.67, 1.97) | 0.608 | 0.658 |
| PFHpA | 1.61 (1.21, 2.13) | 0.001 | 0.005 | 1.00 (0.72, 1.39) | 0.993 | 0.993 | 0.81 (0.58, 1.14) | 0.234 | 0.420 |
| PFUnDA | 0.43 (0.26, 0.69) | 0.001 | 0.005 | 0.59 (0.34, 1.00) | 0.052 | 0.158 | 0.62 (0.35, 1.10) | 0.102 | 0.345 |
| MeFOSAA | 1.08 (0.89, 1.31) | 0.451 | 0.508 | 1.19 (0.95, 1.50) | 0.135 | 0.228 | 1.11 (0.87, 1.42) | 0.413 | 0.531 |
Nine PFAS that were detected in > 74% of the samples were individually included in the multinomial regression models.
Multinomial logistic regression models were adjusted for CHARGE frequency matching factors (child’s sex and age at sampling, recruitment regional center), sampling year, gestational age at delivery, mother’s birthplace, parity, mother’s race/ethnicity, homeownership, and breastfeeding duration.
When MSEL and VABS scores were used as outcomes, PFOA was consistently associated with decreased Composite and subscale scores (β per ln ng/mL increase: −6.82 to −4.91 for MSEL; β: −4.51 to −2.45 for VABS) (Figure 1). PFPeA similarly was associated with decreased MSEL Composite (β: −4.29, 95% CI: −8.42, −0.17) and Fine Motor scores (β: −4.73, 95% CI: −8.58, −0.88) and lower VABS Composite and subscale scores (β: −3.39 to −2.77), except for Motor Skills. A third PFAS, PFHpA, was consistently associated with lower MSEL and VABS Composite and subscale scores (β: −5.13 to −3.28 for MSEL; β: −2.86 to −1.92 for VABS). In contrast, PFUnDA was associated with increased MSEL and VABS Composite and subscale scores (β: 4.61 to 7.34 for MSEL; β: 3.51 to 4.95 for VABS). In sensitivity analyses performing the regression analyses without imputing missing covariates, the results did not change (Table S4 and Figure S3).
Figure 1.
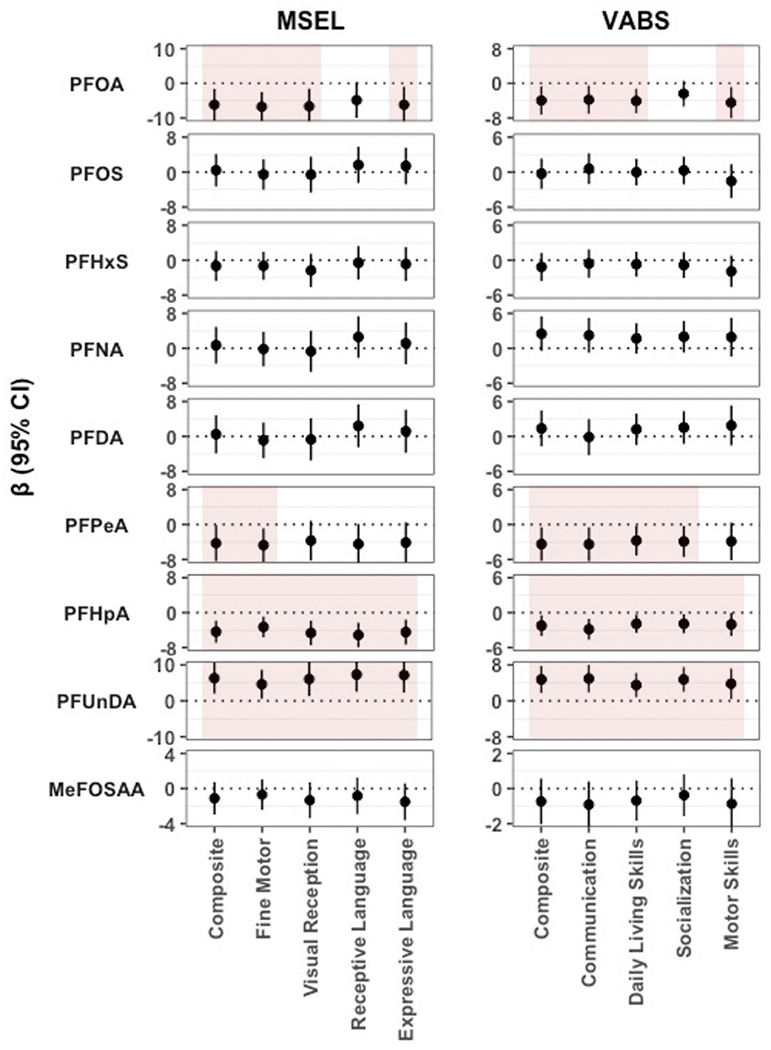
Estimated mean differences (β) in MSEL and VABS Composite and subscale scores per ln ng/mL increase in child serum PFAS concentrations. Multiple linear regression models were adjusted for CHARGE frequency matching factors (child’s sex and age at sampling, recruitment regional center), sampling year, gestational age at delivery, mother’s birthplace, parity, mother’s race/ethnicity, homeownership, and breastfeeding duration. Shaded areas indicate estimates with a p-value < 0.05.
From repeated holdout WQS regression analyses, the PFAS index constrained in the positive direction was associated with increased odds of ASD (average OR: 1.57, 5th and 95th percentile: 1.16, 2.13) (Table 4). The PFAS index in the negative direction was associated with decreased MSEL (average β: −3.45, 5th and 95th percentile: −6.44, −0.47) and VABS Composite scores (average β: −3.34, 5th and 95th percentile: −6.37, −0.31). For the associations of PFAS mixtures with ASD as well as MSEL and VABS Composite scores, PFHpA, PFPeA, and PFOA were common possible contributors (Figure S4).
Table 4.
Associations between combined child PFAS exposure and neurodevelopmental outcomes (ASD, DD, OEC versus TD) and MSEL and VABS Composite scores from repeated holdout weighted quantile sum (WQS) regression.
| Outcome a | Direction | OR or β b | Mean | Median | 5th PCT | 95th PCT |
|---|---|---|---|---|---|---|
| ASD vs. TD | Positive | OR | 1.57 | 1.59 | 1.16 | 2.13 |
| DD vs. TD | Positive | OR | 1.22 | 1.23 | 0.81 | 1.85 |
| OEC vs. TD | Positive | OR | 1.02 | 1.03 | 0.68 | 1.53 |
|
| ||||||
| MSEL Composite | Negative | β | −3.45 | −3.55 | −6.44 | −0.47 |
| VABS Composite | Negative | β | −3.34 | −3.21 | −6.37 | −0.31 |
Outcome (n) for repeated holdout WQS regression: ASD (174), TD (166), DD (87), OEC (73), MSEL Composite (499), VABS Composite (500)
Regression models were adjusted for CHARGE frequency matching factors (child’s sex and age at sampling, recruitment regional center), sampling year, gestational age at delivery, mother’s birthplace, parity, mother’s race/ethnicity, homeownership, and breastfeeding duration
Note: Weight distributions of individual PFAS from each 100 repetitions of WQS are presented in Figure S4.
Effect modification by child’s sex was observed for the association between PFDA and DD, with increased odds among females (OR: 2.44, 95% CI: 0.80, 7.50) and decreased odds among males (OR: 0.56, 95% CI: 0.28, 1.15; p-value for interaction = 0.02) (Table S5). The associations of PFDA with MSEL and VABS Composite scores were also modified, showing adverse associations among females (β: −6.7 for MSEL, −2.3 for VABS) and favorable associations among males (β: 3.9 for MSEL, 3.3 for VABS; p-values for interaction < 0.04) (Table S6). We also observed that the associations of PFDA with ASD as well as MSEL and VABS Composite scores were modified by homeownership (p-values for interaction < 0.04) (Table S7 and Table S8). Among homeowners, PFDA was associated with decreased odds of ASD (OR: 0.50, 95% CI: 0.26, 0.96) and higher MSEL and VABS scores (β: 4.3 for MSEL, 4.1 for VABS), whereas among those not owning a home, odds of ASD were increased (OR: 1.64, 95% CI: 0.71, 3.78), and those of cognitive and adaptive skills were reduced (β: −3.8 for MSEL, −2.3 for VABS). The associations of PFOA, PFOS, and PFUnDA with MSEL Composite scores were also modified by homeownership, with favorable associations among homeowners (p-values for interaction < 0.07). There was no consistent effect modification by breastfeeding duration (never or ≤ 3 months versus > 3 months) (Table S9 and Table S10).
When stratifying the associations between PFAS and MSEL and VABS scores by children with and without ASD, most of the associations were not statistically significant in both sub-groups (Figure S5). However, among children with ASD, MSEL and VABS scores tended to be poorer with increasing exposures to PFHpA and better with increasing exposures to PFUnDA. Among children without ASD (i.e., DD, OEC, and TD), MSEL and VABS scores indicated poorer skills with increasing exposures to PFOA and PFPeA and better skills with increasing exposures to PFUnDA. When performing the analyses without adjusting for the breastfeeding duration, the associations between PFOA and increased odds of ASD (OR: 1.15, 95% CI: 0.74, 1.79) and DD (OR: 1.26, 95% CI: 0.75, 2.12) as well as decreased MSEL (β: −2.05 to 0.91) and VABS (β: −1.27 to 0.47) scores moved toward the null and were no longer statistically significant (Table S11 and Figure S6). The associations for PFPeA, PFHpA, and PFUnDA for neurodevelopmental outcomes and MSEL and VABS scores remained similar.
4. Discussion
In this case-control study, we examined if PFAS concentrations in child serum samples collected at 2 to 5 years of age were associated with increased odds of ASD, DD, and OEC as well as with reduced cognitive and adaptive abilities, assessed using the MSEL and VABS, respectively. Childhood PFOA was associated with increased odds of ASD and DD, and PFHpA was associated with increased odds of ASD. PFOA and PFHpA were also associated with decreased scores on cognitive and adaptive functions, and additionally, PFPeA was associated with lower adaptive function scores. However, PFUnDA was associated with decreased odds of ASD and DD and higher scores on cognitive and adaptive abilities. A PFAS mixture, highly weighted by PFPeA and PFHpA, was associated with increased odds of ASD. A PFAS mixture, which was weighted with PFOA, PFPeA, PFHpA, and MeFOSAA, was associated with poorer cognitive and adaptive functions. Child’s sex modified the associations of PFDA with odds of DD and MSEL and VABS scores, showing that among females, increasing exposure tended to be towards an association with higher odds of the DD diagnosis and poorer scores whereas males with decreased odds and better scores. Furthermore, the associations of PFDA with odds of ASD and MSEL and VABS scores were modified by homeownership, showing that homeowners tended to have decreased odds of neurodevelopmental diagnoses and higher skills, whereas non-owners tended to have increased odds of diagnoses and poorer skills.
The case-control design of this study did not allow a causal interpretation for the associations observed between PFAS and the outcomes considered based on the fact that the neurodevelopmental assessment and the blood sample collection were performed on the same day. Most of the children had already been diagnosed prior to the assessment. In fact, the cross-sectional data collection opens the possibility of reverse causation if the different dietary and behavior patterns of the children with ASD or DD could have influenced the child’s PFAS levels (Shin et al. 2020). On the other hand, PFAS quantified at ages 2-5 represent exposures beginning in at least as far back as the early postnatal period and may extend even into the prenatal period, due to their long half-lives: estimated at 2.1-10.1 years for PFOA, 3.3-27 for PFOS, 4.7-35 years for PFHxS, and 2.5-4.3 years for PFNA (ATSDR 2021). PFAS in child’s serum or plasma showed moderate to strong correlations between 3 months and 2 years (rsp = 0.82 for PFOA, 0.76 for PFOS, 0.82 for PFHxS, 0.68 for PFNA, 0.46 for PFDA) (van Beijsterveldt et al. 2022) and between 1.5 and 5 years (rsp = 0.57 for PFOA, 0.69 for PFOS, 0.75 for PFHxS, 0.49 for PFNA, 0.41 for PFDA) (Blomberg et al. 2021), and weak to moderate correlations of birth (i.e., cord serum) with 1.5 years (rsp = 0.35 to 0.44), 3 years (Pearson’s correlation coefficients [rp] = −0.19 to 0.46), and 5 years (rsp = 0.21 to 0.42) (Blomberg et al. 2021; Kingsley et al. 2018). Therefore, our findings warrant further investigations regarding effects of PFAS exposures during the first few years of life on child neurodevelopment.
We observed that increased odds of an ASD diagnosis and cognitive and adaptive functions were adversely associated with PFOA and PFHpA but favorably associated with PFUnDA. The mixed associations depending on the PFAS compound may be partly explained by their different exposure sources. In this study, child concentrations of PFUnDA were relatively weakly correlated with those of PFOA (rsp = 0.36) and PFHpA (rsp = 0.21), compared to the correlations between PFOA and PFHpA (rsp = 0.62), implicating slightly different exposure sources of PFUnDA. PFUnDA has a longer carbon chain (C 11), compared to PFOA (C 8) and PFHpA (C 5) with a higher bioaccumulation factor (Chen et al. 2018; Liu et al. 2019). In studies conducted in Europe and Asia, the dietary intake of PFUnDA was predominated by the consumption of fish products due to the bioaccumulative property, while that of PFOA and PFHpA originated from various food categories, such as dairy, fruits, and vegetable products, (Eriksson et al. 2013; Fujii et al. 2015; Heo et al. 2014; Johansson et al. 2014; Klenow et al. 2013; Vestergren et al. 2012). Many long-chain perfluorocarboxylates can also originate from hepatic biotransformation of fluorotelomer alcohols, which are frequently used in paper products and textiles (Sinclair et al. 2007). In this study population, diet and fish consumption data were collected but not adjusted for because changes in the diet questionnaire in the CHARGE study midway through this study period (i.e., in 2011) presented obstacles to the use of these data. However, among children who had fish consumption data from either the old or new questionnaire (n = 416), the percentage of those who consumed fish before 2 years was higher in the TD group (54%), compared to ASD (31%) and DD (44%) groups. In an earlier report from the CHARGE Study, children with ASD were shown to be far less likely to consume fish, including tuna, other ocean fish, or freshwater fish, than the children with TD (Hertz-Picciotto et al. 2010). Therefore, the residual confounding by diet, including fish consumption, may have affected the mixed associations between PFOA or PFHpA and PFUnDA.
Breastfeeding is a primary exposure pathway to PFAS for infants (Zheng et al. 2021). The duration of breastfeeding was positively associated with PFAS concentrations in 2- to 5-year-old children (Gyllenhammar et al. 2018; Kingsley et al. 2018; Mogensen et al. 2015; Papadopoulou et al. 2016). In this study population, we also observed that most of the PFAS concentrations were higher in the children with longer breastfeeding duration, showing positive correlations (range = 0.14 to 0.43), except for PFPeA and MeFOSAA (Table S3 and Table S12). Due to the highest correlations between PFOA and breastfeeding duration, we observed that the associations between childhood PFOA and increased odds of ASD and DD and decreased MSEL and VABS scores became nonsignificant after excluding breastfeeding duration from the models. Breastfeeding, a major determinant of PFAS concentrations in early childhood, is also associated with better child neurodevelopment, including ASD and cognitive development (Bar et al. 2016), and hence, should be considered a likely confounder of the relationship between childhood PFAS and neurodevelopment.
Previous epidemiological studies focused on the associations of the risk of ASD or DD with prenatal PFAS exposure rather than childhood PFAS exposure as pregnancy is often thought to be the most sensitive window for brain development (Buck Louis et al. 2019). Still, developmental exposures to neurotoxicants during the first few years of life have been emphasized in association with child neurodevelopment (Hertz-Picciotto et al. 2018; Lyall et al. 2014; Selevan et al. 2000; Volk et al. 2013). However, epidemiologic evidence on childhood PFAS exposure and neurodevelopmental scores, assessing cognitive functioning and behavioral difficulties at various ages, is inconclusive. For example, in the mid-Ohio-Valley population who were highly exposed to PFOA, PFOA in child serum samples collected at age 2-8 was associated with higher IQ and better visual-spatial processing at 6-12 years of age (Stein et al. 2013). A Cincinnati study that collected child serum samples at ages 3 and 8 years observed that PFOA, PFOS, and PFNA were associated with better reading skills at 5 and 8 years of age (Zhang et al. 2018a). In the same population, PFNA at age 3 was associated with higher IQ and perceptual reasoning and less attention problems at 8 years of age, while concurrent PFNA was associated with poorer activity of daily living at age 8 (Vuong et al. 2019; 2021).
Numerous other studies observed associations similar to those of the current study: A Boston-area birth cohort study observed that PFOA, PFOS, and PFHxS at 6-10 were associated cross-sectionally with lower visual-motor abilities and greater parent-reported behavioral difficulties (Harris et al. 2021; Harris et al. 2018). In the Faroese population, PFOA, PFNA, and PFDA at 5 years were associated with parent-reported behavioral difficulties at 7 years of age (Oulhote et al. 2016). Only the Faroese birth cohort investigated the joint effects of a chemical mixture, combining PFAS, mercury, and polychlorinated biphenyls, at age 5 on cognitive and behavioral functions at 7 years of age (Oulhote et al. 2019). They observed that the mixture concentrations were associated with higher scores of parent-reported behavioral difficulties, which was consistent with our findings. Some of the variations across studies could be due to the sources of PFAS, for instance, fish consumption can be quite high in countries where most inhabitants live near coasts, and is associated with improved cognitive abilities.
Our study provided additional evidence on sexually dimorphic associations of childhood PFDA with neurodevelopment. We observed increased odds of DD and decreased MSEL and VABS scores among females, while decreased odds of DD and increased MSEL and VABS scores among males. As this study enrolled fewer female children (n = 145) than male children (n = 406), stratified analyses of neurodevelopmental outcomes by child’s sex should be interpreted cautiously. Still, it is noteworthy that we observed consistent sex-specific associations not only for the odds of DD but also for MSEL and VABS scores. The underlying mechanism remains unclear, but it was suggested that PFAS may disrupt functions of sex hormones, having sexually dimorphic effects on neurodevelopment (Kjeldsen and Bonefeld-Jørgensen 2013). Similar to our findings, a previous study reported that concurrent PFOS, PFHxS, and PFNA were adversely associated with autism screening and externalizing problems among females at 7 years of age, but not or favorably associated among males (Oulhote et al. 2016). Another study also observed adverse associations between PFOA at age 2-8 and mother-reported executive functions and behavioral assessment among females and favorable associations among males at 6-12 years of age (Stein et al. 2014). However, in the Cincinnati birth cohort, concurrent PFOS was associated with better IQ and cognitive functions only among 8-year-old males (Vuong et al. 2019), and PFOA and PFNA at age 3 and 8 were associated with greater externalizing and internalizing problems, aggression, and depression among males, compared to females (Vuong et al. 2021). Other studies did not find evidence of heterogeneity of PFAS associations with neurodevelopment by child’s sex (Harris et al. 2021; Harris et al. 2018; Stein et al. 2013).
Homeownership, as a proxy of SES, modified associations of PFDA with ASD and cognitive and adaptive scores and those of PFOA and PFUnDA with cognitive scores, showing favorable associations among homeowners and adverse associations among those who did not own a home. In this study population, child PFAS concentrations and neurodevelopmental outcomes as well as cognitive and adaptive scores differed by SES. For example, serum concentrations of most PFAS were higher among children whose parents owned a home, had higher educational attainment (i.e., Bachelor’s degree or higher), and had an insurance at delivery (Table S3). These children also accounted for a lower percentage of ASD, DD, and OEC groups, compared to the TD group, and had higher scores for cognitive and adaptive functions (Table 1). In line with our findings, several studies also observed the effect modification of associations between early-life PFAS and neurodevelopmental outcomes by maternal education, another measure of SES. In a Norwegian cohort, a diagnosis of ASD was associated with higher prenatal maternal PFOA and PFOS and lower PFHxS and PFUnDA among children whose mother had higher education (Skogheim et al. 2021). In a meta-analysis of European population-based studies, higher odds of attention deficit hyperactivity disorder were associated with early-life exposure to PFOA and PFOS among children born to low-educated mothers (Forns et al. 2020). However, because the evidence on effect modification by SES is limited and mixed, further studies are needed to confirm our findings.
This study has several limitations. As mentioned above, PFAS were quantified in child serum samples that were collected at the end of the visit for neurodevelopmental assessment. Therefore, our findings based on concurrent exposure measurements should not be interpreted as a causal relationship. Moreover, the estimates from the sub-group analyses were unstable due to the small subsample size, thus the results should be interpreted cautiously. On the other hand, a major strength of this study is the clinical confirmation of ASD, DD, and OEC diagnoses and the assessment of cognitive and adaptive functions using established reliable assessment tools. We accounted for an extensive list of potential confounders, including demographic, maternal, and perinatal characteristics and breastfeeding duration, contributing to the scientific rigor of this analysis. Furthermore, because 9 PFAS compounds were widely detected (> 74%) in the study samples, we could evaluate associations of PFAS mixtures with neurodevelopment. We also found of associations with adverse neurodevelopmental outcomes in relation to two short-chain PFAS (i.e., PFHpA and PFPeA) for which neurotoxic effects have not been well- studied. Because epidemiological evidence was also limited partly due to their low detection rates, our findings warrant further studies of these two compounds in association with child neurodevelopment. This large study provided robust results identifying several PFAS compounds measured in early life that were associated with neurodevelopmental conditions as well as of cognitive and adaptive skills.
5. Conclusions
In this case-control study that quantified PFAS in child serum samples and assessed neurodevelopment at 2 to 5 years of age, PFOA was associated with increased odds of ASD and DD, whereas PFUnDA with decreased odds. PFHpA was associated with increased odds of ASD. Similarly, PFOA and PFHpA showed associations with poorer cognitive and adaptive skills, while PFUnDA showed associations with stronger skills. Higher concentrations of the PFAS mixture were associated with increased odds of ASD and decreased scores of cognitive and adaptive functions, with PFHpA, PFPeA, and PFOA as major contributors. There was evidence of effect modification of the associations between PFDA and neurodevelopment by child’s sex and homeownership, which should be replicated in larger samples. Although a prenatal period may be the most susceptible window of PFAS exposure for neurodevelopment, this study suggests that early postnatal periods need further scrutiny.
Supplementary Material
Acknowledgements
Authors would like to acknowledge the CHARGE investigators, staff, and most of all, the participants for helping make this research possible.
Competing interests
RJS has received lodging for the Baby Siblings Research Consortium Meeting; travel and lodging for invited talks at the University of Sherbrooke, Sherbrooke, Québec, Canada; the University of California Santa Cruz. Santa Cruz, California (Lodging); Epigenomics 2016, Puerto Rico (Lodging); Neurotoxicity Society & International Neurotoxicology Association, Florianópolis, Brazil; RISE 2017 Second International Meeting on Environmental Health in Strasbourg. Strasbourg, France. RJS also received funding from Autism Speaks grant to develop an online autism environmental questionnaire and from Simons Foundation. Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health (NIH), under Award Number UG3OD023365, UH3OD023365 (Bennett, Hertz-Picciotto, Schwizer). Lab analysis of serum PFAS was supported by funding from the National Institute of Environmental Health Sciences (NIEHS) to the Wadsworth Center-Children’s Health Exposure Analysis Resource (U2CES026542-01) (Kannan). This research was also supported through other NIH grants (R01ES020392, R24ES028533, P30ES023513, U2CES026555, U2CES026560, U54HD079125, P50HD103526), U.S Environmental Protection Agency (83543201), and the UC Davis MIND Institute.
List of abbreviations
- ADI-R
Autism Diagnostic Interview-Revised
- ADOS-G
Autism Diagnostic Observation Schedules-Generic
- ASD
autism spectrum disorder
- CHARGE
CHildhood Autism Risks from Genetics and Environment
- CI
confidence interval
- Composite
Early Learning Composite/Adaptive Behavior Composite
- DD
developmental delay
- EtFOSAA
ethyl perfluorooctane sulfonamido acetic acid
- ln
natural log
- LOD
limit of detection
- MeFOSAA
N-methyl perfluorooctane sulfonamido acetic acid
- MIND
Medical Investigations of Neurodevelopmental Disorders
- MSEL
Mullen Scales of Early Learning
- OEC
other early concerns
- OR
odds ratio
- PFAS
per- and polyfluoroalkyl substances
- PFBS
perfluorobutanesulfonic acid
- PFDA
perfluorodecanoic acid
- PFDoDA
perfluorododecanoic acid
- PFHpA
perfluoroheptanoic acid
- PFHxA
perfluorohexanoic acid
- PFHxS
perfluorohexane-1-sulfonic acid
- PFNA
perfluorononanoic acid
- PFOA
perfluorooctanoic acid
- PFOS
perfluorooctanesulfonic acid
- PFOSA
perfluorooctanesulfonamide
- PFPeA
perfluoro-n-pentanoic acid
- PFUnDA
perfluoroundecanoic acid
- rsp
Spearman’s correlation coefficients
- SCQ
Social Communication Questionnaire
- SD
standard deviation
- SES
socioeconomic status
- TD
typical development
- UC
University of California
- U.S.
United States
- VABS
Vineland Adaptive Behavior Scales
- WQS
weighted quantile sum
Footnotes
Ethics approval and consent to participate
The CHARGE study protocol and this study were approved by the institutional review boards (IRB) for the State of California and the University of California-Davis (UC-Davis). Participants provided written informed consent before collection of any data.
Consent for publication
Not applicable.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://
Availability of data and material
Lab and epidemiological data are hosted at the Human Health Exposure Analysis Resources (HHEAR) Data Center Repository (https://hheardatacenter.mssm.edu/).
References
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). American Psychiatric Pub. [Google Scholar]
- ATSDR, 2021. Toxicological Profile for Perfluoroalkyls. Agency for Toxic Substances and Disease Registry. Atlanta, GA. [PubMed] [Google Scholar]
- Baio J, 2014. Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2010. [PubMed] [Google Scholar]
- Ballesteros V, Costa O, Iniguez C, Fletcher T, Ballester F, Lopez-Espinosa M-J, 2017. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: a systematic review of epidemiologic studies. Environment international. 99, 15–28. [DOI] [PubMed] [Google Scholar]
- Bar S, Milanaik R, Adesman A, 2016. Long-term neurodevelopmental benefits of breastfeeding. Current opinion in pediatrics. 28, 559–566. [DOI] [PubMed] [Google Scholar]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K, 2010. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environmental health perspectives. 118, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DH, Busgang SA, Kannan K, Parsons PJ, Takazawa M, Palmer CD, Schmidt RJ, Doucette JT, Schweitzer JB, Gennings C, 2022. Environmental exposures to pesticides, phthalates, phenols and trace elements are associated with neurodevelopment in the CHARGE study. Environment international. 161, 107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg AJ, Shih Y-H, Messerlian C, Jørgensen LH, Weihe P, Grandjean P, 2021. Early-life associations between per-and polyfluoroalkyl substances and serum lipids in a longitudinal birth cohort. Environmental Research. 200, 111400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, 2017. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nature Reviews Endocrinology. 13, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjödin A, Hauser R, Webster GM, Chen A, Lanphear BP, 2014. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4-and 5-year-old children: the HOME study. Environmental health perspectives. 122, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis G, Yeung E, Kannan K, Maisog J, Zhang C, Grantz KL, Sundaram R, 2019. Patterns and variability of endocrine disrupting chemicals during pregnancy: implications for understanding the exposome of normal pregnancy. Epidemiology. 30, S65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, Factor-Litvak P, 2015. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. Journal of agricultural, biological, and environmental statistics. 20, 100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wang Q, Shan G, Zhu L, Yang L, Liu M, 2018. Occurrence, partitioning and bioaccumulation of emerging and legacy per-and polyfluoroalkyl substances in Taihu Lake, China. Science of the Total Environment. 634, 251–259. [DOI] [PubMed] [Google Scholar]
- Duffek A, Conrad A, Kolossa-Gehring M, Lange R, Rucic E, Schulte C, Wellmitz J, 2020. Per-and polyfluoroalkyl substances in blood plasma–Results of the German Environmental Survey for children and adolescents 2014–2017 (GerES V). International Journal of Hygiene and Environmental Health. 228, 113549. [DOI] [PubMed] [Google Scholar]
- Eriksson U, Kärrman A, Rotander A, Mikkelsen B, Dam M, 2013. Perfluoroalkyl substances (PFASs) in food and water from Faroe Islands. Environmental science and pollution research. 20, 7940–7948. [DOI] [PubMed] [Google Scholar]
- Forns J, Verner M-A, Iszatt N, Nowack N, Bach CC, Vrijheid M, Costa O, Andiarena A, Sovcikova E, Høyer BB, 2020. Early life exposure to perfluoroalkyl substances (PFAS) and ADHD: a meta-analysis of nine European population-based studies. Environmental health perspectives. 128, 057002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel-Boroviczény O, Koletzko B, 2010. Pre-and postnatal exposure to perfluorinated compounds (PFCs). Environmental science & technology. 44, 7123–7129. [DOI] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D, 2009. Perfluorinated compounds–exposure assessment for the general population in Western countries. International journal of hygiene and environmental health. 212, 239–270. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Sakurada T, Harada KH, Koizumi A, Kimura O, Endo T, Haraguchi K, 2015. Long-chain perfluoroalkyl carboxylic acids in Pacific cods from coastal areas in northern Japan: a major source of human dietary exposure. Environmental Pollution. 199, 35–41. [DOI] [PubMed] [Google Scholar]
- Graham JW, Olchowski AE, Gilreath TD, 2007. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prevention science. 8, 206–213. [DOI] [PubMed] [Google Scholar]
- Gump BB, Wu Q, Dumas AK, Kannan K, 2011. Perfluorochemical (PFC) exposure in children: associations with impaired response inhibition. Environmental science & technology. 45, 8151–8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllenhammar I, Benskin JP, Sandblom O, Berger U, Ahrens L, Lignell S, Wiberg K, Glynn A, 2018. Perfluoroalkyl acids (PFAAs) in serum from 2–4-month-old infants: Influence of maternal serum concentration, gestational age, breast-feeding, and contaminated drinking water. Environmental science & technology. 52, 7101–7110. [DOI] [PubMed] [Google Scholar]
- Hallgren S, Fredriksson A, Viberg H, 2015. More signs of neurotoxicity of surfactants and flame retardants–Neonatal PFOS and PBDE 99 cause transcriptional alterations in cholinergic genes in the mouse CNS. Environmental toxicology and pharmacology. 40, 409–416. [DOI] [PubMed] [Google Scholar]
- Harris MH, Oken E, Rifas-Shiman SL, Calafat AM, Bellinger DC, Webster TF, White RF, Sagiv SK, 2021. Prenatal and childhood exposure to per-and polyfluoroalkyl substances (PFAS) and child executive function and behavioral problems. Environmental Research. 202, 111621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MH, Oken E, Rifas-Shiman SL, Calafat AM, Ye X, Bellinger DC, Webster TF, White RF, Sagiv SK, 2018. Prenatal and childhood exposure to per-and polyfluoroalkyl substances (PFASs) and child cognition. Environment international. 115, 358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J-J, Lee J-W, Kim S-K, Oh J-E, 2014. Foodstuff analyses show that seafood and water are major perfluoroalkyl acids (PFAAs) sources to humans in Korea. Journal of hazardous materials. 279, 402–409. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Hernández-Díaz S, Robins JM, 2004. A structural approach to selection bias. Epidemiology. 615–625. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, Van de Water J, Pessah IN, 2006. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environmental health perspectives. 114, 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Green PG, Delwiche L, Hansen R, Walker C, Pessah IN, 2010. Blood mercury concentrations in CHARGE Study children with and without autism. Environmental health perspectives. 118, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Schmidt RJ, Krakowiak P, 2018. Understanding environmental contributions to autism: Causal concepts and the state of science. Autism Research. 11, 554–586. [DOI] [PubMed] [Google Scholar]
- Honda M, Robinson M, Kannan K, 2018. A rapid method for the analysis of perfluorinated alkyl substances in serum by hybrid solid-phase extraction. Environmental Chemistry. 15, 92–99. [Google Scholar]
- Johansson JH, Berger U, Vestergren R, Cousins IT, Bignert A, Glynn A, Darnerud PO, 2014. Temporal trends (1999–2010) of perfluoroalkyl acids in commonly consumed food items. Environmental pollution. 188, 102–108. [DOI] [PubMed] [Google Scholar]
- Johansson N, Eriksson P, Viberg H, 2009. Neonatal exposure to PFOS and PFOA in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain. Toxicological Sciences. 108, 412–418. [DOI] [PubMed] [Google Scholar]
- Johansson N, Fredriksson A, Eriksson P, 2008. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 29, 160–169. [DOI] [PubMed] [Google Scholar]
- Kärrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, Lignell S, Lindström G, 2007. Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environmental health perspectives. 115, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley SL, Eliot MN, Kelsey KT, Calafat AM, Ehrlich S, Lanphear BP, Chen A, Braun JM, 2018. Variability and predictors of serum perfluoroalkyl substance concentrations during pregnancy and early childhood. Environmental research. 165, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen LS, Bonefeld-Jørgensen EC, 2013. Perfluorinated compounds affect the function of sex hormone receptors. Environmental Science and Pollution Research. 20, 8031–8044. [DOI] [PubMed] [Google Scholar]
- Klenow S, Heinemeyer G, Brambilla G, Dellatte E, Herzke D, de Voogt P, 2013. Dietary exposure to selected perfluoroalkyl acids (PFAAs) in four European regions. Food Additives & Contaminants: Part A. 30, 2141–2151. [DOI] [PubMed] [Google Scholar]
- Koponen J, Winkens K, Airaksinen R, Berger U, Vestergren R, Cousins IT, Karvonen AM, Pekkanen J, Kiviranta H, 2018. Longitudinal trends of per-and polyfluoroalkyl substances in children’s serum. Environment international. 121, 591–599. [DOI] [PubMed] [Google Scholar]
- Lazarevic N, Barnett AG, Sly PD, Knibbs LD, 2019. Statistical methodology in studies of prenatal exposure to mixtures of endocrine-disrupting chemicals: a review of existing approaches and new alternatives. Environmental health perspectives. 127, 026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Lord C, Rutter M, 2003. Autism Diagnostic Interview - Revised (ADI-R). Western Psychological Services. [Google Scholar]
- Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, Bossi R, Henriksen TB, Bonefeld-Jørgensen EC, Olsen J, 2014. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: a nested case–control study in the Danish National Birth Cohort. Environmental health perspectives. 123, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao X, Liu Y, Qiao X, Wang X, Ma M, Jin X, Liu C, Zheng B, Shen J, 2019. High contamination, bioaccumulation and risk assessment of perfluoroalkyl substances in multiple environmental media at the Baiyangdian Lake. Ecotoxicology and environmental safety. 182, 109454. [DOI] [PubMed] [Google Scholar]
- Long M, Ghisari M, Kjeldsen L, Wielsøe M, Nørgaard-Pedersen B, Mortensen EL, Abdallah MW, Bonefeld-Jørgensen EC, 2019. Autism spectrum disorders, endocrine disrupting compounds, and heavy metals in amniotic fluid: a case-control study. Molecular autism. 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Espinosa M-J, Mondal D, Armstrong B, Bloom MS, Fletcher T, 2012. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environmental health perspectives. 120, 1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, Fombonne E, Leboyer M, Minshew N, 1997. Diagnosing autism: analyses of data from the Autism Diagnostic Interview. Journal of autism and developmental disorders. 27, 501–517. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M, 2000. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of autism and developmental disorders. 30, 205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A, 1994. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of autism and developmental disorders. 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Lyall K, Schmidt RJ, Hertz-Picciotto I, 2014. Maternal lifestyle and environmental risk factors for autism spectrum disorders. International journal of epidemiology. 43, 443–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Yau VM, Hansen R, Kharrazi M, Yoshida CK, Calafat AM, Windham G, Croen LA, 2018. Prenatal maternal serum concentrations of per-and polyfluoroalkyl substances in association with autism spectrum disorder and intellectual disability. Environmental health perspectives. 126, 017001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MS, Esler A, Furnier SM, Hallas L, Hall-Lande J, Hudson A, 2021. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveillance Summaries. 70, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariussen E, 2012. Neurotoxic effects of perfluoroalkylated compounds: mechanisms of action and environmental relevance. Archives of toxicology. 86, 1349–1367. [DOI] [PubMed] [Google Scholar]
- Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jørgensen E, 2015. Breastfeeding as an exposure pathway for perfluorinated alkylates. Environmental science & technology. 49, 10466–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM, 1997. Mullen scales of early learning. Western Psychological Services. [Google Scholar]
- Oh J, Bennett DH, Calafat AM, Tancredi D, Roa DL, Schmidt RJ, Hertz-Picciotto I, Shin H-M, 2021a. Prenatal exposure to per-and polyfluoroalkyl substances in association with autism spectrum disorder in the MARBLES study. Environment International. 147, 106328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Schmidt RJ, Tancredi D, Calafat AM, Roa DL, Hertz-Picciotto I, Shin H-M, 2021b. Prenatal exposure to per-and polyfluoroalkyl substances and cognitive development in infancy and toddlerhood. Environmental research. 196, 110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR, 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environmental health perspectives. 115, 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Coull B, Bind M-A, Debes F, Nielsen F, Tamayo I, Weihe P, Grandjean P, 2019. Joint and independent neurotoxic effects of early life exposures to a chemical mixture: A multi-pollutant approach combining ensemble learning and g-computation. Environmental Epidemiology. 3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Steuerwald U, Debes F, Weihe P, Grandjean P, 2016. Behavioral difficulties in 7-year old children in relation to developmental exposure to perfluorinated alkyl substances. Environment international. 97, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou E, Sabaredzovic A, Namork E, Nygaard UC, Granum B, Haug LS, 2016. Exposure of Norwegian toddlers to perfluoroalkyl substances (PFAS): the association with breastfeeding and maternal PFAS concentrations. Environment international. 94, 687–694. [DOI] [PubMed] [Google Scholar]
- Renzetti S, Curtin P, Just AC, Bello G, Gennings C, Renzetti MS, Rsolnp I, 2021. Package ‘gWQS’.
- Richardson DB, Ciampi A, 2003. Effects of exposure measurement error when an exposure variable is constrained by a lower limit. American journal of epidemiology. 157, 355–363. [DOI] [PubMed] [Google Scholar]
- Ripley B, Venables W, Ripley MB, 2016. Package ‘nnet’. R package version. 7, 700. [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, Cook EH Jr, Leventhal BL, Pickles A, 2006. Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 45, 1094–1103. [DOI] [PubMed] [Google Scholar]
- Rubin DB, 2004. Multiple imputation for nonresponse in surveys. John Wiley & Sons. [Google Scholar]
- Rutter M, Bailey A, Lord C, 2003. The social communication questionnaire: Manual. Western Psychological Services. [Google Scholar]
- Schecter A, Malik-Bass N, Calafat AM, Kato K, Colacino JA, Gent TL, Hynan LS, Harris TR, Malla S, Birnbaum L, 2012. Polyfluoroalkyl compounds in Texas children from birth through 12 years of age. Environmental health perspectives. 120, 590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Vexler A, Whitcomb BW, Liu A, 2006. The limitations due to exposure detection limits for regression models. American journal of epidemiology. 163, 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P, 2000. Identifying critical windows of exposure for children’s health. Environmental health perspectives. 108, 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H-M, Bennett DH, Calafat AM, Tancredi D, Hertz-Picciotto I, 2020. Modeled prenatal exposure to per-and polyfluoroalkyl substances in association with child autism spectrum disorder: A case-control study. Environmental Research. 109514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair E, Kim SK, Akinleye HB, Kannan K, 2007. Quantitation of gas-phase perfluoroalkyl surfactants and fluorotelomer alcohols released from nonstick cookware and microwave popcorn bags. Environmental science & technology. 41, 1180–1185. [DOI] [PubMed] [Google Scholar]
- Skogheim TS, Weyde KVF, Aase H, Engel SM, Surén P, Øie MG, Biele G, Reichborn-Kjennerud T, Brantsæter AL, Haug LS, 2021. Prenatal exposure to per-and polyfluoroalkyl substances (PFAS) and associations with attention-deficit/hyperactivity disorder and autism spectrum disorder in children. Environmental Research. 202, 111692. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV, 1984. Vineland Adaptive Behavior Scales. [DOI] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Bellinger DC, 2013. Perfluorooctanoate (PFOA) and neuropsychological outcomes in children. Epidemiology (Cambridge, Mass). 24, 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Bellinger DC, 2014. Perfluorooctanoate exposure in a highly exposed community and parent and teacher reports of behaviour in 6–12-year-old children. Paediatric and perinatal epidemiology. 28, 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbühler K, 2008. Estimating consumer exposure to PFOS and PFOA. Risk Analysis: An International Journal. 28, 251–269. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt IA, van Zelst BD, van den Berg SA, de Fluiter KS, van der Steen M, Hokken-Koelega AC, 2022. Longitudinal poly-and perfluoroalkyl substances (PFAS) levels in Dutch infants. Environment international. 160, 107068. [DOI] [PubMed] [Google Scholar]
- Van Buuren S, Groothuis-Oudshoorn K, 2011. mice: Multivariate imputation by chained equations in R. Journal of statistical software. 45, 1–67. [Google Scholar]
- Vestergren R, Berger U, Glynn A, Cousins IT, 2012. Dietary exposure to perfluoroalkyl acids for the Swedish population in 1999, 2005 and 2010. Environment international. 49, 120–127. [DOI] [PubMed] [Google Scholar]
- Viberg H, Lee I, Eriksson P, 2013. Adult dose-dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology. 304, 185–191. [DOI] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R, 2013. Traffic-related air pollution, particulate matter, and autism. JAMA psychiatry. 70, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Xie C, Dietrich KN, Braun JM, Webster GM, Calafat AM, Lanphear BP, Chen A, 2019. Prenatal and childhood exposure to poly-and perfluoroalkyl substances (PFAS) and cognitive development in children at age 8 years. Environmental research. 172, 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Xie C, Dietrich KN, Braun JM, Webster GM, Calafat AM, Lanphear BP, Chen A, 2021. Childhood exposure to per-and polyfluoroalkyl substances (PFAS) and neurobehavioral domains in children at age 8 years. Neurotoxicology and teratology. 88, 107022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM, 2011. Multiple imputation using chained equations: issues and guidance for practice. Statistics in medicine. 30, 377–399. [DOI] [PubMed] [Google Scholar]
- Williams GR, 2008. Neurodevelopmental and neurophysiological actions of thyroid hormone. Journal of neuroendocrinology. 20, 784–794. [DOI] [PubMed] [Google Scholar]
- Winkens K, Vestergren R, Berger U, Cousins IT, 2017. Early life exposure to per-and polyfluoroalkyl substances (PFASs): A critical review. Emerging Contaminants. 3, 55–68. [Google Scholar]
- Wong F, MacLeod M, Mueller JF, Cousins IT, 2014. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: evidence from population-based pharmacokinetic modeling. Environmental science & technology. 48, 8807–8814. [DOI] [PubMed] [Google Scholar]
- Wu Q, Kannan K Perfluoroalkyl substances (PFASs) in foodstuffs and human dietary exposure. Advances in the Determination of Xenobiotics in Foods: Bentham Science Publishers; 2019 [Google Scholar]
- Ye X, Kato K, Wong L-Y, Jia T, Kalathil A, Latremouille J, Calafat AM, 2018. Per-and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. International journal of hygiene and environmental health. 221, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yolton K, Webster GM, Ye X, Calafat AM, Dietrich KN, Xu Y, Xie C, Braun JM, Lanphear BP, 2018a. Prenatal and childhood perfluoroalkyl substances exposures and children’s reading skills at ages 5 and 8 years. Environment international. 111, 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Ye J, Wei Q, Li M, Xu K, Li Z, Lin W, Liu P, Chen R, Ma A, 2018b. Plasma concentration of 14 perfluoroalkyl acids (PFAAs) among children from seven cities in Guangdong, China. Science of the Total Environment. 616, 1469–1476. [DOI] [PubMed] [Google Scholar]
- Zheng G, Schreder E, Dempsey JC, Uding N, Chu V, Andres G, Sathyanarayana S, Salamova A, 2021. Per-and polyfluoroalkyl substances (PFAS) in breast milk: Concerning trends for current-use PFAS. Environmental Science & Technology. 55, 7510–7520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Lab and epidemiological data are hosted at the Human Health Exposure Analysis Resources (HHEAR) Data Center Repository (https://hheardatacenter.mssm.edu/).


