ABSTRACT
Background
Common genetic variants of the enzymes and efflux pump involved in tacrolimus disposition have been associated with calcineurin inhibitor nephrotoxicity, but their importance is unclear because of the multifactorial background of renal fibrosis. This study explores the pro-fibrotic response of tacrolimus exposure in relation to the differential capacity for tacrolimus metabolism in proximal tubule cells (PTCs) with a variable (pharmaco)genetic background.
Methods
PTCs were obtained from protocol allograft biopsies with different combinations of CYP3A5 and ABCB1 variants and were incubated with tacrolimus within the concentration range found in vivo. Gene and protein expression, CYP3A5 and P-glycoprotein function, and tacrolimus metabolites were measured in PTC. Connective tissue growth factor (CTGF) expression was assessed in protocol biopsies of kidney allograft recipients.
Results
PTCs produce CTGF in response to escalating tacrolimus exposure, which is approximately 2-fold higher in cells with the CYP3A5*1 and ABCB1 TT combination in vitro. Increasing tacrolimus exposure results in relative higher generation of the main tacrolimus metabolite {13-O-desmethyl tacrolimus [M1]} in cells with this same genetic background. Protocol biopsies show a larger increase in in vivo CTGF tissue expression over time in TT vs. CC/CT but was not affected by the CYP3A5 genotype.
Conclusions
Tacrolimus exposure induces a pro-fibrotic response in a PTC model in function of the donor pharmacogenetic background associated with tacrolimus metabolism. This finding provides a mechanistic insight into the nephrotoxicity associated with tacrolimus treatment and offers opportunities for a tailored immunosuppressive treatment.
Keywords: calcineurin inhibitor toxicity, connective tissue growth factor, genetic-dependent pro-fibrotic response, human donor proximal tubule cells, tacrolimus
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Long-term treatment with calcineurin inhibitors (CNIs) is associated with the development of renal fibrosis and a progressive decline in kidney allograft function.
A direct profibrotic effect of CNIs has never been unequivocally demonstrated because of the multifactorial background of renal fibrosis in solid organ transplantation.
What this study adds?
Production of connective tissue growth factor (CTGF) (a pivotal pro-fibrotic cytokine) in proximal tubule cells is in direct response to tacrolimus exposure at clinically relevant tissue concentrations.
Increased CTGF expression in response to tacrolimus exposure was most pronounced in proximal tubule cells with the combined CYP3A5*1–ABCB1 TT genotype, which also demonstrated the highest formation of the major tacrolimus metabolite {13-O-desmethyl tacrolimus [M1]}.
What impact this may have on practice or policy?
These findings add the donor pharmacogenetic background as a risk factor for developing kidney allograft fibrosis and provide the possibility for (preemptive) personalized immunosuppressive treatment of CNI avoidance or minimization.
INTRODUCTION
The calcineurin inhibitor (CNI) tacrolimus constitutes the basis of the majority of immunosuppressive regimens after solid organ transplantation [1, 2]. One of the most important presumed adverse effects of long-term CNI treatment is nephrotoxicity (CNIT) leading to permanent kidney (allograft) dysfunction [3, 4].
The origin of kidney allograft failure is complex in that multiple donor- and recipient-related factors, such as ischemia reperfusion injury and rejection, can contribute to a cascade of different processes that lead to renal fibrosis. In this final common pathway, increased tubular connective tissue growth factor (CTGF) production acts as a mediator of the pro-fibrotic effects of transforming growth factor β (TGF-β) [5]. To unravel the importance of CNIs for developing renal fibrosis, we must study the different pathophysiologic mechanisms in an isolated setting.
Multiple clinical studies have demonstrated a poorer kidney allograft survival and increased prevalence of CNIT in patients with a fast metabolizer phenotype for tacrolimus (low C0/dose ratio) [6, 7]. Interindividual variation in tacrolimus metabolization is largely determined by variations in the genes encoding the CYP3A5 enzyme and the P-glycoprotein (P-gp) efflux transporter (ABCB1 gene), which play a central role in tacrolimus disposition. High tacrolimus dose requirements are encountered especially in carriers of a single-nucleotide variant (SNV) in CYP3A5, designated *1, who require a 2- to 3-fold higher tacrolimus dose because of a higher enzyme expression vs. homozygous carriers of the *3 variant {*1 allele present in 5%−20% of Caucasians and dominant in individuals of African descent [allele frequency 70%−90%]} [8]. The most commonly studied SNV for P-gp is a C-to-T transition at position 3435 within exon 26 of ABCB1 (3435C>T; T allele frequency 40%−60% in Caucasian individuals and 10%−15% in people of African descent), which is associated with a lower transport capacity for tacrolimus [9, 10]. The vast majority of tacrolimus metabolism occurs in the gut and liver, and the genotype of these organs plays an important role in individual tacrolimus disposition. The specific genotype of the kidney for CYP3A5 and ABCB1, however, has also been associated with a differential potential for developing CNIT [11–15].
Because proximal tubule cells (PTCs) are the only kidney cells metabolizing CNI, a pathophysiologic role for the differential expression of CYP3A5 and/or P-gp in the development of CNIT seems likely. Until now, exploring CNI metabolism and toxicity in an isolated fashion was not possible because of the lack of a human PTC model that expresses the relevant enzymes and transporters [16]. In addition, in vitro studies that investigated CNIT previously used drug concentrations exceeding those measured in tissue in vivo [17, 18]. We developed a novel cell model of conditionally immortalized human PTCs (ciPTC) derived from an allograft kidney biopsy. This cell model includes different selected clones that incorporate different combinations of the aforementioned genetic variants associated with tacrolimus disposition and toxicity in clinical studies (*1 or *3/*3 and ABCB1 CC/CT or TT) [19]. In this study, we explored whether in vitro CTGF expression by PTCs is associated with tacrolimus exposure at levels derived from in vivo tissue measurements and whether this expression is dependent on the functional and genetic background related to tacrolimus disposition in PTCs.
MATERIALS AND METHODS
Cell culture and reagents
We used ciPTC clones derived from protocol kidney biopsies in human kidney allograft recipients, as described previously [19]. Patients with a functioning kidney graft and who were scheduled for protocol biopsy (as part of the standard clinical care program) were asked to participate. All participants provided informed consent before tissue samples were collected and processed for PTC isolation and immortalization. Different ciPTC clones were selected based on the underlying variation in CYP3A5 (rs776746) and ABCB1 (rs1045642) genotype. Four genotype combinations based on the mentioned polymorphisms were chosen, and at least two ciPTC clones were used per genotype combination. A detailed description of reagents and tacrolimus incubations is provided in the Supplementary file.
Quantitative real-time polymerase chain reaction
Approximately 120 000 cells of differentiated ciPTC were used for RNA extraction, complementary DNA synthesis, and real-time polymerase chain reaction (PCR). A detailed description is provided in the Supplementary file.
Western blotting
Approximately 300 000 cells of a specific differentiated ciPTC clone were incubated with tacrolimus-supplemented medium at concentrations of 50 and 300 ng/mL for 72 hours. We used 0.1% DMSO as the vehicle control. A detailed description is provided in the Supplementary file.
CYP3A5 activity measurement
Cells were grown for a minimum of 10 days at 37°C in a 12-well plate. Standard medium was replaced by 500 μL medium with 50 μM midazolam (MDZ) hydrochloride (Roche: RO-21–3981/000) and incubated at 37°C for 30 minutes in 4-fold per condition. Supernatant was removed and frozen at −20°C. MDZ and its major metabolites, 1-hydroxy MDZ (1-OH MDZ) were measured using a previously described method [20].
The same sample preparation with liquid–liquid extraction {ethyl acetate-heptane [1:4]} was used. The calibration standards were prepared by spiking appropriate amounts of diluted stock solutions into blank supernatant. The samples were injected on an Alliance 2695 high-performance liquid chromatograph (Waters, Zellik, Belgium), and we performed the detection using a Quattro micro API tandem mass spectrometer (Waters, Zellik, Belgium).
P-gp activity measurement
We assessed P-gp transport activity by measuring the accumulation of calcein in cell lysates [21]. A detailed description is provided in the Supplementary file.
Tacrolimus and tacrolimus metabolites measurement
We used 11 ciPTC clones and at least 2 clones per genetic combination. Approximately 90 000 cells of a specific ciPTC clone were seeded and kept for at least 10 days at 37°C before incubation with tacrolimus-supplemented medium at concentrations of 0 (vehicle), 50, and 300 ng/mL tacrolimus for 72 hours. Next to tacrolimus itself, we initially focused on the three most common first-generation metabolites: 13-O-desmethyl tacrolimus (M1), 31-O-desmethyl tacrolimus (M2), and 15-O-desmethyl tacrolimus (M3) (in order of abundance). Measurement of tacrolimus and its metabolites were performed as previously described [22],
Quantification of CTGF expression in protocol biopsies
The pharmacogenetic background of the kidney allograft, together with histologic and functional follow-up data, were available from 28 patients participating in our hospital's protocol biopsy program who had also been included in our previous study establishing the ciPTC cell model [19]. The allograft genotype for CYP3A5 (rs776746) and ABCB1 (rs1045642) was determined on primary cells, cultured following the protocol biopsy previously described [19]. A detailed description is provided in the Supplementary file.
Statistical analyses
The obtained values are representative of three independent experiments and, in the case of different donors, representative of at least two donors per genetic combination. For the quantitative analysis of tacrolimus and M1, every value is an average of two technical replicates, and the values below the limit of detection or quantification were replaced with ‘half of threshold of the detection or quantification.’ A detailed description of the statistical methods is presented in Table 1 and the Supplementary file.
Table 1:
Detailed description of statistical methods used in experiments.
| Experimental series | Statistical method |
|---|---|
|
Effect of the increasing concentration of tacrolimus (50 and 300 ng/mL) for the following: -CYP3A5 and P-gp protein -CYP3A5 function (MDZ hydroxylation) -P-gp transport (calcein assay) -Tacrolimus disposition -M1 production and disposition -CTGF protein |
Repeated measures ANOVA with Huynh-Feldt correction |
|
Differences of the following between two independent genetic groups: -Tacrolimus disposition -M1 production and disposition |
Mann-Whitney U test |
|
Correlation analyses between: -Total M1 and CTGF mRNA -Intracellular M1 and CTGF mRNA -Intracellular M1/tacrolimus and CTGF mRNA |
Pearson correlation |
|
Correlation analyses between: -Intracellular tacrolimus and CTGF mRNA -Intracellular tacrolimus and CTGF protein -Cleared tacrolimusa and CTGF mRNA -Cleared tacrolimusa and CTGF protein -Total M1 and CTGF protein -Intracellular M1 and CTGF protein -M1/cleared tacrolimus and CTGF mRNA -M1/cleared tacrolimusa and CTGF protein -Intracellular M1/tacrolimus and CTGF protein |
Spearman correlation |
aCleared tacrolimus is the total amount of tacrolimus retrieved extra- and intracellularly after 72 hours subtracted from the actual amount of tacrolimus at the time of incubation. ANOVA, analysis of variance.
Ethics
The study protocol was approved by the institutional ethical board (B32220109632) in Leuven University Hospital.
RESULTS
Tacrolimus increases pro-fibrotic CTGF in PTCs
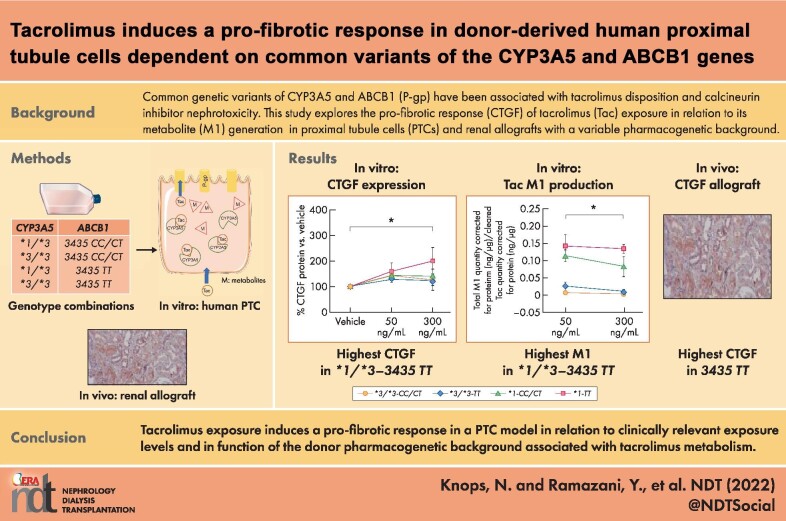
First, exposure of ciPTC clones to increasing concentrations of tacrolimus results in a significant 1.5-fold increase relative to CTGF mRNA and protein expression (Fig. 1a, mean mRNA vs. vehicle in 50 and 300 ng/mL, respectively: 155% and 158%; Fig. 1b, mean protein expression vs. vehicle in 50 and 300 ng/mL, respectively: 143% and 150%). The presence of a pro-fibrotic signature resulting from tacrolimus exposure was confirmed by increased mRNA expression of an additional set of genes (HMOX1, PA1-1, ACTA2, and SMAD6) associated with renal fibrosis (Supplementary Fig. 1). No difference in CTGF expression was detected in 50 to 300 ng/mL tacrolimus for all ciPTC clones combined, but subgroup analysis according to genotype (Fig. 1c) shows that clones with a *1 allele combined with the TT genotype have a significant 2- to 3-times higher increase in CTGF protein expression at 300 ng/mL tacrolimus compared with clones with three other combinations (mean CTGF protein relative to vehicle in *1-TT vs. other genotypes: at 50 ng/mL tacrolimus, 161% vs. 133% and at 300 ng/mL tacrolimus, 201% vs. 132%).
Figure 1:
CTGF under tacrolimus. Results in ciPTC clones with a different genetic background after 72-hour incubation with vehicle (0.1% DMSO) or tacrolimus at 50 and 300 ng/mL. (a), Quantitative real-time PCR analysis of CTGF mRNA in all ciPTC clones. Mean mRNA vs. vehicle in 50 and 300 ng/mL tacrolimus, respectively: 155% and 158%. Repeated measures analysis of variance (ANOVA), **P < .01, n = 16. (b), Relative CTGF protein expression in all ciPTC clones. Mean protein expression vs. vehicle in 50 and 300 ng/mL, respectively: 143% and 150%. Repeated measures ANOVA, ***P < .001, n = 20. (c), Subgroup analysis of relative CTGF protein expression in ciPTC according to pharmacogenetic background. *1-TT combination vs. other genotype combinations (*1-CC/CT, *3/*3-CC/CT, and *3/*3-TT). Mean CTGF protein relative to vehicle in *1-TT vs. other genotypes: at 50 ng/mL tacrolimus, 161% vs. 133%; at 300 ng/mL tacrolimus, 201% vs. 132%. Repeated measures ANOVA, *P < .05, n = 6 and n = 14, respectively. CTGF protein expression is normalized to β-actin protein expression in all conditions. Plots depict the mean of individual values, and error bars represent the standard deviation in 1a and 1b and standard error of the mean in 1c. *1: CYP3A5 *1, *3/*3: CYP3A5*3; CC/CT: ABCB1 3435CC/CT and TT: ABCB1 3435TT.
Tacrolimus decreases the function of CYP3A5 and P-gp without affecting their expression
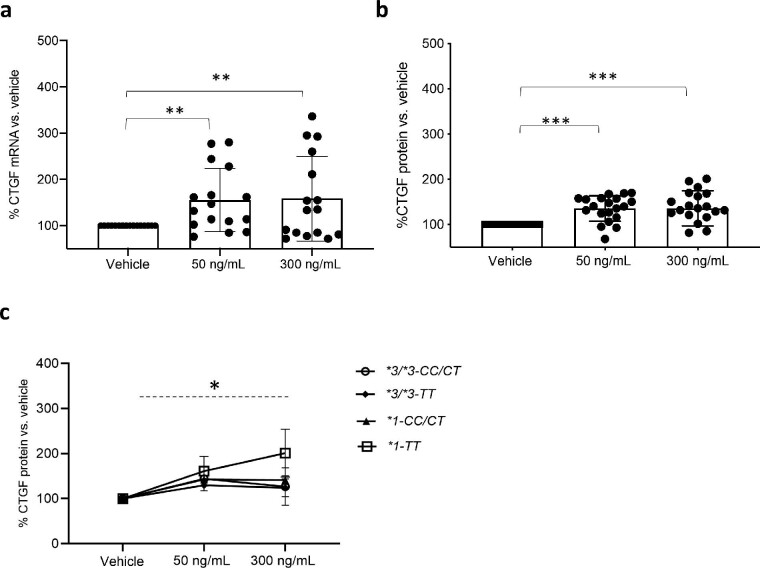
To further elucidate the underlying reasons for the relative higher CTGF expression in ciPTC clones with the *1-TT combination, we examined the effect of tacrolimus on the absolute and functional expression of CYP3A5 and P-gp in relation to the genetic background and the level of tacrolimus exposure. In our previous paper, we confirmed higher intrinsic CYP3A5 protein expression and demonstrated a significantly increased capacity for 1-OH MDZ generation and tacrolimus clearance in ciPTC of the *1 allele vs. the *3/*3 allele [19]. In Fig 2a, we illustrate the effect of increasing tacrolimus concentration on 1-OH MDZ generation in *1 allele carriers, which suggests a significant decreasing capacity for 1-OH MDZ formation under increasing tacrolimus exposure (mean 1-OH MDZ: 1.87 and 1.19 ng/mL at tacrolimus 50 and 300 ng/mL, respectively, compared with 2.3 ng/mL vehicle; P < .05). We further showed that this effect of tacrolimus on MDZ hydroxylation was not associated with a difference in quantitative CYP3A5 protein expression (Supplementary Fig. 2).
Figure 2:
Quantitative CYP3A5 and P-gp function under tacrolimus. Results in ciPTC clones after incubation with vehicle (0.1% DMSO) or tacrolimus at 50 and 300 ng/mL. (a), CYP3A5 activity expressed as 1-OH MDZ concentrations (ng/mL) after 30-minute incubation with 50 μM MDZ hydrochloride only in *1 allele carriers (nonsignificant 1-OH MDZ generation in *3/*3 allele carriers). Mean 1-OH MDZ: 1.87 and 1.19 ng/mL at tacrolimus 50 and 300 ng/mL, respectively, compared with 2.3 ng/mL vehicle. Repeated measures analysis of variance (ANOVA), *P < .05, n = 8. (b), P-gp activity expressed as relative calcein accumulation in all ciPTC clones exposed to vehicle with and without tacrolimus for 72 hours. Mean Δ fluorescence in vehicle was 39.2% compared with 34.8% and 30.7% at tacrolimus 50 and 300 ng/mL, respectively. Repeated measures ANOVA, ***P < .001, n = 10. Plots depict the mean of individual values, and error bars represent the standard error of the mean.
Moreover, we found that increasing tacrolimus exposure significantly inhibits P-gp's efflux capacity for calcein irrespective of the genetic background (Fig. 2b, mean Δ fluorescence in vehicle, 39.2% compared with 34.8% and 30.7% at tacrolimus 50 and 300 ng/mL, respectively; P < .01). This effect was not associated with a decrease in quantitative P-gp expression (Supplementary Fig. 3).
Collectively, these data show that in addition to the underlying differences in protein function in relation to the genetic background, increasing tacrolimus exposure decreases the function of CYP3A5 and P-gp without affecting their quantitative expression levels in ciPTC.
PTCs with the *1 allele have a higher preferential generation of M1 compared with cells homozygous for the *3 allele
To assess the functional differences of CYP3A5 in relation to the genetic variations, we assessed tacrolimus metabolism in ciPTC by measuring tacrolimus clearance and the generation of M1, its primary metabolite (M2 and M3 concentrations were below the limit of quantification/detection).
In our previous experiments, we had already established a significantly higher tacrolimus disappearance rate in *1 allele carriers within the first 24 hours, which was most pronounced (1.7-fold) in cells with the *1-TT combination [19]. Based on pilot experiments for detecting quantifiable amounts of CTGF and M1 (data not shown), we prolonged our incubation time to 72 hours. After 72 hours, we did not find a significant difference in total tacrolimus clearance for the CYP3A5 genotype anymore, most probably because the majority of the tacrolimus available for metabolism had been metabolized after this period (Supplementary Fig. 4).
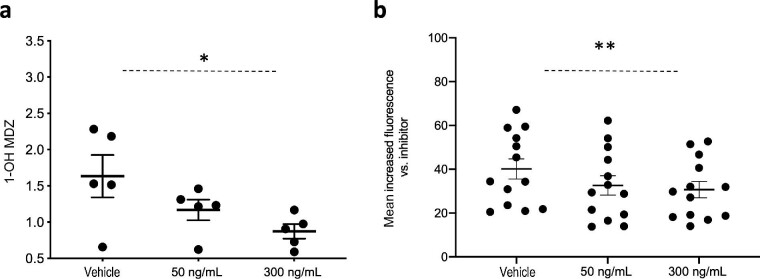
The generation of the total M1 metabolite was measured in intra- and extracellular fractions, and then normalized to the total cleared tacrolimus. We could not detect any differences in total (intra- and extracellular) M1 amount between the CC/CT and TT variants (P = .6 and P = .5 at 50 and 300 ng/mL tacrolimus, respectively; Fig. 3a). Similarly, there were no significant differences in M1 generation corrected for the clearance of tacrolimus based on the genetic background for ABCB1 (P = .7 and P = .9 for 50 and 300 ng/mM tacrolimus, respectively; Fig. 3b). Because the intracellular M1 concentration in *3/*3 was below the limit of quantification/detection, we compared the differences in the intracellular M1 disposition in relation to the ABCB1 genotype only within the *1 allele carriers but found no significant difference (P = .9 at 50 ng/mL tacrolimus in CC/CT vs. TT 0.06 vs. 0.08, and P = .7 at 300 ng/mL tacrolimus 0.08 vs. 0.10; Fig. 3c).
Figure 3:
Differential tacrolimus (tac) M1 generation in ciPTC by genetic background. Results after 72-hour incubation with tacrolimus at 50 or 300 ng/mL depicted according to the genetic background. (a), Quantity of the intracellular and extracellular M1 (ng), corrected for protein amount in the lysate (μg) in CC/CT vs. TT. Mann Whitney U test: P = .6 and P = .5 at 50 and 300 ng/mL tacrolimus, respectively, n = 5 and n = 6. (b), Relative M1 metabolite generation corrected for tacrolimus clearance {ratio of ‘total amount of M1 in the intra- and extracellular compartments [ng] corrected for total protein amount in the lysate [μg]’ to ‘cleared tacrolimus [ng] corrected for total protein amount in the lysate [μg]’}. Mann-Whitney U test: P = .7 and P = .9 for 50 and 300 ng/mL tacrolimus, respectively, n = 5 and n = 6 in CC/CT vs. TT. (c), Intracellular M1 quantity (ng) corrected for total protein amount in the lysate (μg) within the *1 allele carriers {mean intracellular M1 [ng/μg protein lysate] at 50 ng/mL tacrolimus in CC/CT vs. TT, 0.06 vs. 0.08, P = .9, and at 300 ng/mL tacrolimus 0.08 vs. 0.10, P = .7 [Mann-Whitney U test, n = 3 CC/CT and TT}. (d), Quantity of the intracellular and extracellular M1 (ng), corrected for protein amount in the lysate (μg) in *1 vs. *3/*3: at 50 ng/mL tacrolimus, 1.42 vs. 0.82, and at 300 ng/mL tacrolimus, 6.90 vs. 0.73. Mann Whitney U test, **P < .01, n = 6 and n = 5, respectively. (e), Relative M1 metabolite generation, corrected for tacrolimus clearance {ratio of ‘total amount of M1 in the intra- and extracellular compartments [ng]’ to ‘cleared tacrolimus [ng],’ corrected for total protein amount in the lysate [μg]} in *1 vs. *3/*3: at 50 ng/mL, 0.12 vs. 0.01, and at 300 ng/mL, 0.10 vs. 0.007. Mann-Whitney U test: **P < .01, n = 6 and n = 5, respectively. (f), Relative M1 metabolite generation, corrected for tacrolimus clearance {ratio of ‘total amount of M1 in the intra- and extracellular compartments [ng] corrected for total protein amount in the lysate [μg]’ to ‘cleared tacrolimus [ng] corrected for total protein amount in the lysate [μg]’} in ciPTC with the specific combination of *1-TT vs. other genetic combinations: at 50 ng/mL tacrolimus, 0.14 vs. 0.05, and at 300 ng/mL tacrolimus, 0.13 vs. 0.03. Repeated measures analysis of variance: *P < .05, n = 3 and n = 8, respectively. Tacrolimus clearance: total amount of tacrolimus retrieved extra- and intracellularly after 72 hours subtracted from the actual amount of tacrolimus at the time of incubation. Plots depict the mean of individual values, and error bars represent the standard deviation in all panels except in (f), where error bars represent a 95% confidence interval. *1: CYP3A5*1, *3/*3: CYP3A5*3, CC/CT: ABCB1 3435CC/CT and TT: ABCB1 3435TT.
We did, however, find a significant 1.7- to 9.5-fold higher total M1 in *1 allele carriers compared with *3/*3 allele carriers dependent on tacrolimus exposure (in *1 vs. *3/*3: at 50 ng/mL tacrolimus 1.42 vs. 0.82 and at tacrolimus 300 ng/mL 6.90 vs. 0.73, P < .01; Fig. 3d). The relative generation of M1 from cleared tacrolimus (M1/cleared tacrolimus) was on average 13-fold higher in *1 vs. the *3/*3 (at 50 ng/mL 0.12 vs. 0.01 and at 300 ng/mL 0.10 vs. 0.007, P < .01; Fig. 3e). Interestingly, the M1/cleared tacrolimus shows a significant trend toward lower values at 300 vs. 50 ng/mL tacrolimus in both genetic variants.
Analysis of the subgroups revealed a significant 4-fold-higher relative generation of M1 in relation to tacrolimus clearance in cells with the *1-TT genotype compared with other genotype combinations (at 50 ng/mL tacrolimus 0.14 vs. 0.05 and at 300 ng/mL tacrolimus 0.13 vs. 0.03, P < .05; Fig. 3f). In accordance with the data presented in Fig 3a and Fig 3d, absolute M1 concentration was highest under 300 ng/mL tacrolimus vs. 50 ng/mL tacrolimus (*1-TT: 7.7 ng/μg vs. 2.9 ng/μg, respectively; P = .02; not depicted).
In summary, this section describes a higher capacity for M1 generation from tacrolimus in *1 vs. *3/*3. The M1 production in ciPTC with *1-TT is considerably higher than other genetic combinations.
Higher M1 production in *1-TT overlaps with higher CTGF synthesis in these cells
We performed correlation analyses between different parameters of in vitro tacrolimus disposition (intracellular tacrolimus, cleared tacrolimus, total M1, intracellular M1, M1/cleared tacrolimus, and intracellular M1/tacrolimus) with CTGF mRNA and protein expression values. These analyses reveal a moderately strong positive correlation between intracellular M1 and the intracellular ‘M1/tacrolimus’ with CTGF mRNA (correlation co-efficient r > 0.4; P = .004 and P = .07, respectively, n = 10). We found no significant correlation with CTGF protein expression and tacrolimus metabolism, however. Detailed specifications of the analyses are outlined in Supplementary Table 2.
Protocol biopsies demonstrate progressive increase in CTGF expression in relation to ABCB1 genotype
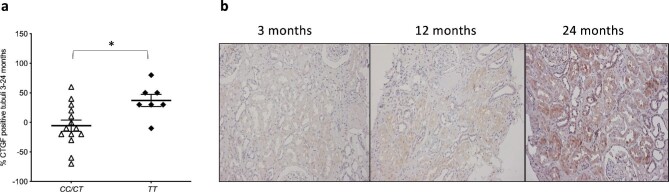
Seventy protocol biopsies were available for CTGF staining derived from 28 allograft–recipient pairs obtained at 3, 12, and 24 months of follow-up after kidney transplantation. All patients were on immunosuppressive therapy with tacrolimus according to the same clinical protocol. We found no significant differences for transplantation-associated background or outcome parameters between donor kidneys of a different genotype (data not shown). In 21 allograft–recipient pairs, we had sufficient data to calculate the change in CTGF immunohistochemistry from 3 to 24 months. The characteristics of these donor–recipient pairs according to their pharmacogenetic background are described in Supplementary Table 3. We found no significant differential effect of the CYP3A5 genotype on CTGF expression in protocol biopsies within this time frame, but we found a significant increase in the percentage of CTGF-positive tubuli over a 2-year follow-up for kidneys with the TT genotype vs. the CC/CT genotype (mean CTGF-positive tubuli: +37% vs. −5%, P = .016; Fig. 4a). The values for CTGF-positive tubuli categorized by genetic background of the donors are outlined in Table 2. A representative series of CTGF staining in the protocol biopsies is depicted in Fig 4b.
Figure 4:
Tubular CTGF expression and representative CTGF biopsy staining. (a), Tubular CTGF increase (%) in protocol biopsies obtained at 3 and 24 months after transplantation. Mann-Whitney U test, *P < .05, n = 21. (b), Light microscopic images (×100) of CTGF staining in sequential protocol biopsies from the same kidney allograft with the TT genotype obtained at 3, 12, and 24 months after transplantation. Plots depict the mean of individual values, and error bars represent the standard error of the mean. CC/CT: ABCB1 3435CC/CT and TT: ABCB1 3435TT.
Table 2:
Percentage CTGF-positive tubuli over a 2-year follow-up in different genotypes.
| Month 3, % | Month 12, % | Month 24, % | |
|---|---|---|---|
| (SD) | (SD) | (SD) | |
| Total | 54.8 (±37.5) | 67.6 (±28.1) | 63.3 (±37.5) |
| CYP3A5*1 | 52.5 (±38.5) | 65.0 (±28.3) | 61.3 (±19.6) |
| CYP3A5*3/*3 | 56.2 (±38.4) | 69.2 (±29.0) | 64.6 (±28.2) |
| ABCB1 3435CC/CT | 65.0 (±37.2) | 64.3 (±33.2) | 59.3 (±27.9) |
| ABCB1 3435TT | 34.3 (±31.0) | 74.3 (±12.7) | 71.4 (±15.7) # |
P = .027 (TT: 3 months vs. 24 months), n = 21.
The increase in CTGF-positive tubuli in protocol biopsies of patients receiving tacrolimus suggested a role for the ABCB1 genotype variant but not the CYP3A5 for the generation of renal fibrosis.
DISCUSSION
The association between CNI therapy and chronic nephrotoxicity was first introduced in heart transplant recipients in 1984 [23] and later propagated for kidney allograft recipients [24], but a direct causal relationship between CNI exposure and renal fibrosis has never been established. We are the first to demonstrate a pro-fibrotic response in human PTC as a direct result of in vitro tacrolimus exposure at concentrations similar to tissue concentrations in allograft recipients [25]. This finding confirms the long-existing paradigm on the toxicity of the drug and offers new incentives to explore this phenomenon.
Others looked at the effects of CNI exposure in in vitro models and found disturbances in energy metabolism, cell cycle regulation, cell integrity, and cell death [26, 27], but the range of CNI exposure levels were greater than 10 000 ng/mL and therefore far above tissue concentrations measured in organ transplant recipients [25]. Another complicating factor is that the applied cell models do not express all the relevant enzymes and transporters involved in CNI disposition [16].
In our validated model of tacrolimus metabolism in human ciPTC, CTGF expression was 1.5-fold increased within only 72 hours of incubation with clinically relevant levels of tacrolimus. This finding is in line with previous studies that showed an increased expression of TGF-β (upstream of CTGF), albeit at much higher tacrolimus concentrations than in allograft tissue [17, 18]. The mechanism of action of CTGF in renal fibrosis has been widely reviewed by others [5]. In short, damaged PTC produce pro-inflammatory and pro-fibrotic factors such as CTGF (a co-factor for TGF-β that in turn affects signal transduction, cytokine function, and extracellular matrix turnover). The relevance of CTGF for the development of kidney allograft fibrosis has been confirmed in multiple clinical studies [28, 29].
Importantly, we observed that this pro-fibrotic response was most pronounced (2- to 3-fold) in ciPTC clones carrying the combined *1-TT genotype. This observation was partially confirmed in the sequential allograft protocol biopsies demonstrating a significantly higher increase of interstitial CTGF expression over time in donor kidneys with the TT genotype compared with the CC/CT genotype. We could not detect any differences between the *1 and *3/*3 allele carriers, however, let alone the effect of the *1-TT genotype combination. We also did not observe a difference in estimated glomerular filtration rate and interstitial fibrosis and tubular atrophy (IFTA) score between genotypes. This finding may have resulted from the insufficient power of the analysis based on a relatively small data set of available protocol biopsies, which was not collected for this purpose. In contrast, Vanhove et al. [29] did demonstrate a correlation between interstitial CTGF expression and IFTA, but 5 years after kidney transplantation in contrast to the 24 months in our study. Interestingly, a larger retrospective study in 50 patients suggested an increased risk (hazard ratio, 3.18; P = .026) for developing CNIT in *1 expressors in whose who received a *3/*3 kidney allograft [15]. Alternatively, the effect of the CYP3A5 PTC genotype may be of lesser importance in the in vivo situation, in which the potential effects of higher metabolite generation is attenuated by a continuous process of removal via the urine vs. the enclosed in vitro situation, where metabolites remain in situ and the effect of the pump is less important. Ideally, a large clinical histologic study with a long follow-up period is required to confirm the importance of the pharmacogenetic background of the allograft as a risk factor for developing fibrosis in addition to more established risk factors. Nevertheless, our in vitro and in vivo data add to the previous evidence on the important role of P-gp expression and specific ABCB1 polymorphism in developing IFTA and kidney failure [12, 30–32].
To understand the underlying reasons for the differential pro-fibrotic profile in the aforementioned genetic variants, we explored the functional consequences of tacrolimus exposure by measuring protein function and the capacity for tacrolimus metabolism. Higher CYP3A5 expression in *1 allele carriers is well established [33] and was previously confirmed in this cell model [19]. Despite the relatively stable CYP3A5 protein expression under tacrolimus exposure, we found a tacrolimus concentration-dependent decrease in the enzyme's function. These findings are in line with pharmacologic inhibition of the CYP3A5 enzyme by tacrolimus and with in vitro and clinical studies identifying tacrolimus as a competitive inhibitor of CYP3A4/5 enzymes [34, 35]. Similarly, our data demonstrate that increasing tacrolimus concentrations decreases P-gp function in PTCs without altering quantitative P-gp protein expression. This means that under increasing tacrolimus exposure, CYP3A5 *1 allele carriers could have a decreasing potential to generate tacrolimus metabolites. As a result of higher tacrolimus exposure, however, intracellular concentrations of tacrolimus and its metabolites could nevertheless be augmented because of inhibition of the P-gp transporter, thus increasing the risk of drug-induced toxicity. In contrast to the CYP3A5 SNV and in accordance with previous papers, our data do not support an additional effect of intrinsically differential quantitative expression caused by the 3435C>T SNV on P-gp function, but other studies have demonstrated that homozygosity for the T allele results in a substrate-dependent effect on P-gp activity [36–38]. Different models of transfected cell lines with an overexpression of recombinant ABCB1 variants have shown that specific SNVs, including 3435C>T, result in impaired P-gp transport activity for tacrolimus [9, 37]. This finding is in line with clinical studies performed in kidney transplantation that showed increased intracellular tacrolimus accumulation in peripheral blood mononuclear cells of patients with a TT genotype [10, 39].
Our study shows that tacrolimus exposure in ciPTC at clinically relevant concentrations has no major effect on quantitative expression of the main genes involved in its intracellular disposition. It does, however, have a significant effect on CYP3A5 and P-gp functional phenotypes in a concentration-dependent fashion, indicating pharmacologic inhibition. This inhibition could affect the risk of nephrotoxicity and potential drug–drug interactions in concomitant treatment with other CYP3A5/P-gp substrates.
Our previous data on the metabolism of tacrolimus in ciPTC confirmed a substantially higher rate of tacrolimus metabolism in *1 allele carriers, in line with findings in human liver microsome [40] and clinical data [19, 37, 41]. In this study, ciPTC with this genetic background also demonstrated a higher absolute and relative propensity for the formation of the M1 metabolite, which was most pronounced in cells with the additional TT genotype. This finding is in accordance with clinical studies that demonstrated increased M1 production in healthy volunteers with the *1 and TT alleles [41, 42]. Interestingly, one of these studies also showed a potentiation of tacrolimus metabolite production in plasma after administration of a CYP3A4-P-gp inhibitor, which could point to either an effect of preferential metabolite generation by CYP3A5 or the consequences of a decreased P-gp–mediated efflux of tacrolimus [41]. However, no study has directly investigated the importance of P-gp for tacrolimus or tacrolimus metabolite efflux. Nevertheless, we did observe a slightly higher intracellular M1 in ciPTC with the TT background at both tacrolimus concentrations (Fig. 3c), but this was not significant and will be difficult to confirm in our in vitro model with ongoing substrate metabolism and without overexpression of the relevant drug transporter (in contrast to in vitro studies specifically aimed at studying drug transporters). Taken together, these findings support the importance of the interplay between enzyme and pump for tacrolimus disposition and demonstrate an increased capacity for tacrolimus metabolization and M1 generation caused by increased intracellular exposure of tacrolimus in PTCs with intrinsically high CYP3A5 activity in combination with an impaired P-gp–mediated efflux of tacrolimus (and possibly M1). Increasing tacrolimus exposure can further affect the character of this interplay by introducing the effect of inhibition of P-gp and CYP3A5 activity.
There is an apparent overlap in the extent of the pro-fibrotic response and metabolite generation in function of the genetic background for ciPTC. With the combined *1-TT genotype, ciPTC had distinctively higher CTGF and M1 production compared with other genotype combinations. This finding was confirmed by a positive correlation between intracellular M1/tacrolimus and CTGF mRNA expression in ciPTC. Whether there is direct causality between M1 generation and the pro-fibrotic response remains speculative, but this question could be further explored by performing isolated incubations with the separate tacrolimus metabolites or by manipulating metabolite exposure with selective CYP3A5 and P-gp inhibitors. Tacrolimus metabolites are not readily available, however, and commonly used inhibitors such as PSC833 and voriconazole are independently associated with nephrotoxicity. As mentioned earlier, clinical studies have demonstrated more fibrosis in patients with a higher relative tacrolimus dose requirement, indicative of a higher capacity for tacrolimus metabolism and absolute metabolite generation [14, 43]. Higher tacrolimus dosing (per protocol) in the early phase after transplantation is an additional reason for increased exposure to its metabolites, which is more pronounced in patients with an intrinsic (genetic) increased capacity for tacrolimus metabolism.
Although the toxicity of M1 is unknown, the nephrotoxicity of several metabolites of the CNI cyclosporin has been established [44, 45]. There is no evidence, however, that the intracellular concentration of the parent compound is directly associated with CTGF expression. Ideally, the exact disposition and idiosyncratic toxicity of M1 (in function of ABCB1 polymorphisms) could further be explored by separate incubations with only purified M1.
CONCLUSION
Exposing PTCs to tacrolimus within a concentration range found in tissue of solid organ transplant recipients results in a direct response associated with the development of renal fibrosis in general and supports the validity of CNIT as a distinct entity within nephropathology. This response is most pronounced in cells with a common genetic predisposition for increased enzymatic activity and altered efflux capacity related to tacrolimus disposition, resulting in more M1 generation and suggestive of a detrimental role for increased exposure to this metabolite. Further studies are required to confirm the potential clinical relevance of our findings, which could be used to establish donor-derived genetic risk profiles for the selection of CNI-sparing immunosuppressive regimes. Some other clinical perspectives of this study are the addition of pharmacologic interventions (CYP3A5 inhibitors) minimizing the toxic metabolites or the in situ inhibition of CTGF (FG-3109/pamrevlumab) [46].
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Ahmed Reda, former colleague at the Laboratory of Pediatric Nephrology, for kindly providing technical and intellectual guidance. We thank Mrs. Inge Bongaers, Laboratory of Pediatric Nephrology, for her technical assistance.
Contributor Information
Noël Knops, Department of Pediatric Nephrology and Solid Organ Transplantation, UZ Leuven, University Hospitals Leuven, Leuven, Belgium; Laboratory of Pediatric Nephrology, Department of Growth and Regeneration, University of Leuven, Leuven, Belgium.
Yasaman Ramazani, Laboratory of Pediatric Nephrology, Department of Growth and Regeneration, University of Leuven, Leuven, Belgium.
Henriëtte De Loor, Department of Nephrology and Renal Transplantation, University Hospitals Leuven, Leuven, Belgium.
Roel Goldschmeding, Department of Pathology, University Medical Center Utrecht, Utrecht, The Netherlands.
Tri Q Nguyen, Department of Pathology, University Medical Center Utrecht, Utrecht, The Netherlands.
Lambert P van den Heuvel, Laboratory of Pediatric Nephrology, Department of Growth and Regeneration, University of Leuven, Leuven, Belgium; Translational Metabolic Laboratory and Department of Pediatric Nephrology, Radboud University Medical Center, Nijmegen, The Netherlands.
Elena Levtchenko, Department of Pediatric Nephrology and Solid Organ Transplantation, UZ Leuven, University Hospitals Leuven, Leuven, Belgium; Laboratory of Pediatric Nephrology, Department of Growth and Regeneration, University of Leuven, Leuven, Belgium.
Dirk J Kuypers, Department of Nephrology and Renal Transplantation, University Hospitals Leuven, Leuven, Belgium.
FUNDING
We acknowledge the following funding: Y.R. was supported by a doctoral fellowship of the Research Foundation Flanders (F.W.O. Vlaanderen), grant number 1S24417N. E.L. is funded by Clinical Investigator grant of the Research Foundation Flanders (F.W.O. Vlaanderen), grant number 1 801 110 N. This project was supported by an IDS grant from Astellas Pharmaceuticals. Astellas was not involved in the experimental setup, data analyses, or writing of this manuscript.
AUTHORS’ CONTRIBUTIONS
N.K. and Y.R. contributed to the study design, performing the majority of the experiments, data analyses, data interpretation, and writing of the manuscript. H.DL. performed the mass spectrometry and assisted in data interpretation of this series of experiments. R.G. and T.N. performed the scoring of CTGF expression in protocol biopsies and provided comments on the manuscript text. L.vdH., E.L., and D.K. contributed to the study design and data interpretation and provided critical comments on the manuscript. All authors approved the final version of the manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared upon reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Gardiner KM, Tett SE, Staatz CE.. Multinational evaluation of mycophenolic acid, tacrolimus, cyclosporin, sirolimus, and everolimus utilization. Ann Transplant 2016;21:1–11. 10.12659/AOT.895664 [DOI] [PubMed] [Google Scholar]
- 2. Axelrod DA, Naik AS, Schnitzler MAet al. National variation in use of immunosuppression for kidney transplantation: a call for evidence-based regimen selection. Am J Transplant 2016;16:2453–62. 10.1111/ajt.13758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nankivell BJ, P'Ng CH, O'Connell PJet al. Calcineurin inhibitor nephrotoxicity through the lens of longitudinal histology: comparison of cyclosporine and tacrolimus eras. Transplantation 2016;100:1723–31. 10.1097/TP.0000000000001243 [DOI] [PubMed] [Google Scholar]
- 4. Nankivell BJ, Borrows RJ, Fung CLet al. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation 2004;78:557–65. 10.1097/01.TP.0000128636.70499.6E [DOI] [PubMed] [Google Scholar]
- 5. Ramazani Y, Knops N, Elmonem MAet al. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol 2018;68-69:44–66. 10.1016/j.matbio.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 6. Jouve T, Fonrose X, Noble Jet al. The TOMATO study (tacrolimus metabolization in kidney transplantation): impact of the concentration-dose ratio on death-censored graft survival. Transplantation 2020;104:1263–71. 10.1097/TP.0000000000002920 [DOI] [PubMed] [Google Scholar]
- 7. Kwiatkowska E, Kwiatkowski S, Wahler Fet al. C/D ratio in long-term renal function. Transplant Proc 2019;51:3265–70. 10.1016/j.transproceed.2019.08.030 [DOI] [PubMed] [Google Scholar]
- 8. Macphee IA, Fredericks S, Mohamed Met al. Tacrolimus pharmacogenetics: the CYP3A5*1 allele predicts low dose-normalized tacrolimus blood concentrations in whites and south Asians. Transplantation 2005;79:499–502. 10.1097/01.TP.0000151766.73249.12 [DOI] [PubMed] [Google Scholar]
- 9. Wang R, Sun X, Deng YSet al. Effects of MDR1 1236C > T-2677G > T-3435C > T polymorphisms on the intracellular accumulation of tacrolimus, cyclosporine A, sirolimus and everolimus. Xenobiotica 2019;49:1373–8. 10.1080/00498254.2018.1563732 [DOI] [PubMed] [Google Scholar]
- 10. Han SS, Yang SH, Kim MCet al. Monitoring the intracellular tacrolimus concentration in kidney transplant recipients with stable graft function. PLoS One 2016;11:e0153491. 10.1371/journal.pone.0153491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore J, McKnight AJ, Dohler Bet al. Donor ABCB1 variant associates with increased risk for kidney allograft failure. J Am Soc Nephrol 2012;23:1891–9. 10.1681/ASN.2012030260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woillard JB, Rerolle JP, Picard Net al. Donor P-gp polymorphisms strongly influence renal function and graft loss in a cohort of renal transplant recipients on cyclosporine therapy in a long-term follow-up. Clin Pharmacol Ther 2010;88:95–100. 10.1038/clpt.2010.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Metalidis C, Lerut E, Naesens Met al. Expression of CYP3A5 and P-glycoprotein in renal allografts with histological signs of calcineurin inhibitor nephrotoxicity. Transplantation 2011;91:1098–102. 10.1097/TP.0b013e3182177502 [DOI] [PubMed] [Google Scholar]
- 14. Kuypers DR, Naesens M, de Jonge Het al. Tacrolimus dose requirements and CYP3A5 genotype and the development of calcineurin inhibitor-associated nephrotoxicity in renal allograft recipients. Ther Drug Monit 2010;32:394–404. 10.1097/FTD.0b013e3181e06818 [DOI] [PubMed] [Google Scholar]
- 15. Udomkarnjananun S, Townamchai N, Chariyavilaskul Pet al. The cytochrome P450 3A5 non-expressor kidney allograft as a risk factor for calcineurin inhibitor nephrotoxicity. Am J Nephrol 2018;47:182–90. 10.1159/000487857 [DOI] [PubMed] [Google Scholar]
- 16. Jenkinson SE, Chung GW, van Loon Eet al. The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflugers Arch 2012;464:601–11. 10.1007/s00424-012-1163-2 [DOI] [PubMed] [Google Scholar]
- 17. Shihab FS, Bennett WM, Tanner AMet al. Mechanism of fibrosis in experimental tacrolimus nephrotoxicity. Transplantation 1997;64:1829–37. 10.1097/00007890-199712270-00034 [DOI] [PubMed] [Google Scholar]
- 18. Bennett J, Cassidy H, Slattery Cet al. Tacrolimus modulates TGF-β signaling to induce epithelial-mesenchymal transition in human renal proximal tubule epithelial cells. J Clin Med 2016;5:50. 10.3390/jcm5050050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knops N, van den Heuvel LP, Masereeuw Ret al. The functional implications of common genetic variation in CYP3A5 and ABCB1 in human proximal tubule cells. Mol Pharmaceutics 2015;12:758–68. 10.1021/mp500590s [DOI] [PubMed] [Google Scholar]
- 20. de Loor H, de Jonge H, Verbeke Ket al. A highly sensitive liquid chromatography tandem mass spectrometry method for simultaneous quantification of midazolam, 1'-hydroxymidazolam and 4-hydroxymidazolam in human plasma. Biomed Chromatogr 2011;25:1091–8. 10.1002/bmc.1576 [DOI] [PubMed] [Google Scholar]
- 21. van de Water FM, Boleij JM, Peters JGet al. Characterization of P-glycoprotein and multidrug resistance proteins in rat kidney and intestinal cell lines. Eur J Pharm Sci 2007;30:36–44. 10.1016/j.ejps.2006.09.008 [DOI] [PubMed] [Google Scholar]
- 22. de Loor H, Vanhove T, Annaert Pet al. Determination of tacrolimus, three mono-demethylated metabolites and a M1 tautomer in human whole blood by liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal 2021;205:114296. 10.1016/j.jpba.2021.114296 [DOI] [PubMed] [Google Scholar]
- 23. Myers BD, Ross J, Newton Let al. Cyclosporine-associated chronic nephropathy. N Engl J Med 1984;311:699–705. 10.1056/NEJM198409133111103 [DOI] [PubMed] [Google Scholar]
- 24. Nankivell BJ, Borrows RJ, Fung CLet al. The natural history of chronic allograft nephropathy. N Engl J Med 2003;349:2326–33. 10.1056/NEJMoa020009 [DOI] [PubMed] [Google Scholar]
- 25. Noll BD, Coller JK, Somogyi AAet al. Validation of an LC-MS/MS method to measure tacrolimus in rat kidney and liver tissue and its application to human kidney biopsies. Ther Drug Monit 2013;35:617–23. 10.1097/FTD.0b013e31828e8162 [DOI] [PubMed] [Google Scholar]
- 26. Massicot F, Martin C, Dutertre-Catella Het al. Modulation of energy status and cytotoxicity induced by FK506 and cyclosporin A in a renal epithelial cell line. Arch Toxicol 1997;71:529–31. 10.1007/s002040050423 [DOI] [PubMed] [Google Scholar]
- 27. Zhou X, Yang G, Davis CAet al. Hydrogen peroxide mediates FK506-induced cytotoxicity in renal cells. Kidney Int 2004;65:139–47. 10.1111/j.1523-1755.2004.00380.x [DOI] [PubMed] [Google Scholar]
- 28. Nogare AL, Dalpiaz T, Pedroso JAet al. Expression of fibrosis-related genes in human renal allografts with interstitial fibrosis and tubular atrophy. J Nephrol 2013;26:1179–87. 10.5301/jn.5000274 [DOI] [PubMed] [Google Scholar]
- 29. Vanhove T, Kinashi H, Nguyen TQet al. Tubulointerstitial expression and urinary excretion of connective tissue growth factor 3 months after renal transplantation predict interstitial fibrosis and tubular atrophy at 5 years in a retrospective cohort analysis. Transpl Int 2017;30:695–705. 10.1111/tri.12960 [DOI] [PubMed] [Google Scholar]
- 30. Naesens M, Lerut E, de Jonge Het al. Donor age and renal P-glycoprotein expression associate with chronic histological damage in renal allografts. J Am Soc Nephrol 2009;20:2468–80. 10.1681/ASN.2009020192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bloch J, Hazzan M, Van der Hauwaert Cet al. Donor ABCB1 genetic polymorphisms influence epithelial-to-mesenchyme transition in tacrolimus-treated kidney recipients. Pharmacogenomics 2014;15:2011–24. 10.2217/pgs.14.146 [DOI] [PubMed] [Google Scholar]
- 32. Tavira B, Gómez J, Díaz-Corte Cet al. The donor ABCB1 (MDR-1) C3435T polymorphism is a determinant of the graft glomerular filtration rate among tacrolimus treated kidney transplanted patients. J Hum Genet 2015;60:273–6. 10.1038/jhg.2015.12 [DOI] [PubMed] [Google Scholar]
- 33. Kuehl P, Zhang J, Lin Yet al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 2001;27:383–91. 10.1038/86882 [DOI] [PubMed] [Google Scholar]
- 34. Amundsen R, Asberg A, Ohm IKet al. Cyclosporine A- and tacrolimus-mediated inhibition of CYP3A4 and CYP3A5 in vitro. Drug Metab Dispos 2012;40:655–61. 10.1124/dmd.111.043018 [DOI] [PubMed] [Google Scholar]
- 35. de Jonge H, de Loor H, Verbeke Ket al. In vivo CYP3A activity is significantly lower in cyclosporine-treated as compared with tacrolimus-treated renal allograft recipients. Clin Pharmacol Ther 2011;90:414–22. 10.1038/clpt.2011.130 [DOI] [PubMed] [Google Scholar]
- 36. Fung KL, Pan J, Ohnuma Set al. MDR1 synonymous polymorphisms alter transporter specificity and protein stability in a stable epithelial monolayer. Cancer Res 2014;74:598–608. 10.1158/0008-5472.CAN-13-2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kimchi-Sarfaty C, Oh JM, Kim IWet al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 2007;315:525–8. 10.1126/science.1135308 [DOI] [PubMed] [Google Scholar]
- 38. Gow JM, Hodges LM, Chinn LWet al. Substrate-dependent effects of human ABCB1 coding polymorphisms. J Pharmacol Exp Ther 2008;325:435–42. 10.1124/jpet.107.135194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Capron A, Mourad M, De Meyer Met al. CYP3A5 and ABCB1 polymorphisms influence tacrolimus concentrations in peripheral blood mononuclear cells after renal transplantation. Pharmacogenomics 2010;11:703–14. 10.2217/pgs.10.43 [DOI] [PubMed] [Google Scholar]
- 40. Dai Y, Hebert MF, Isoherranen Net al. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos 2006;34:836–47. 10.1124/dmd.105.008680 [DOI] [PubMed] [Google Scholar]
- 41. Vanhove T, de Jonge H, de Loor Het al. Relationship between in vivo CYP3A4 activity, CYP3A5 genotype, and systemic tacrolimus metabolite/parent drug ratio in renal transplant recipients and healthy volunteers. Drug Metab Dispos 2018;46:1507–13. 10.1124/dmd.118.081935 [DOI] [PubMed] [Google Scholar]
- 42. Yoon SH, Cho JH, Kwon Oet al. CYP3A and ABCB1 genetic polymorphisms on the pharmacokinetics and pharmacodynamics of tacrolimus and its metabolites (M-I and M-III). Transplantation 2013;95:828–34. 10.1097/TP.0b013e31827eef57 [DOI] [PubMed] [Google Scholar]
- 43. Chamoun B, Torres IB, Gabaldón Aet al. Progression of interstitial fibrosis and tubular atrophy in low immunological risk renal transplants monitored by sequential surveillance biopsies: the influence of tac exposure and metabolism. J Clin Med 2021;10:141. 10.3390/jcm10010141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Radeke HH, Christians U, Bleck JSet al. Additive and synergistic effects of cyclosporine metabolites on glomerular mesangial cells. Kidney Int 1991;39:1255–66. 10.1038/ki.1991.159 [DOI] [PubMed] [Google Scholar]
- 45. Stephens E, Bolderson I, Clark Bet al. The measurement of whole blood pre-treatment cyclosporine A: metabolite ratios predicts the onset of renal dysfunction in recipients of allogeneic stem cell transplantation. Ann Clin Biochem 2006;43:382–8. 10.1258/000456306778520160 [DOI] [PubMed] [Google Scholar]
- 46. Raghu G, Scholand MB, de Andrade Jet al. FG-3019 anti-connective tissue growth factor monoclonal antibody: results of an open-label clinical trial in idiopathic pulmonary fibrosis. Eur Respir J 2016;47:1481–91. 10.1183/13993003.01030-2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.