Abstract
OBJECTIVES
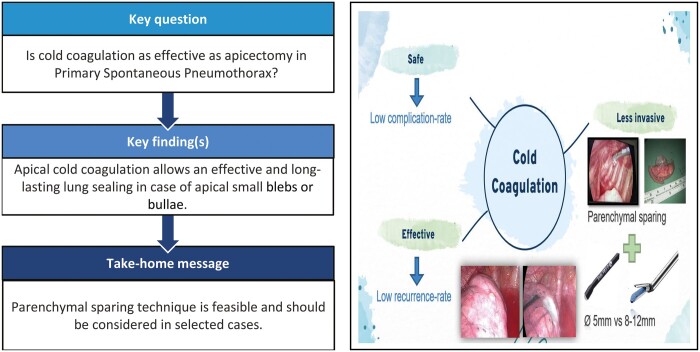
Primary spontaneous pneumothorax is a common disease, whose surgical treatment is still enigmatic in terms of timing and technique. Herein, we reported our experience with the parenchymal-sparing technique via cold coagulation (CC), in comparison to stapler apicectomy (SA).
METHODS
We retrospectively collected data of all patients with apical blebs or <2 cm bullae treated with minimally invasive surgery for recurrent or persistent spontaneous pneumothorax, from 2010 to 2020. Two different surgical techniques were used: SA and the parenchymal-sparing CC of the apex. Perioperative and long-term results were analysed and compared.
RESULTS
Out of 177 patients enrolled, 77 patients (CC group) underwent cold-coagulation of the apex while 100 patients (SA group) were treated with SA. Two groups were comparable in terms of age, surgical indication, intraoperative findings and affected side. CC group had a mean operative time of 43.2 min (standard deviation ± 19.5), shorter than SA group with 49.3 min (standard deviation ± 20.1, P-value: 0.050). Complication rate was significantly different between 2 groups, 5 (7%) and 16 (16%), for the CC and SA groups, respectively (P: 0.048), even if not in terms of prolonged postoperative air leak (P: 0.16). During the follow-up, 13 homolateral recurrences were reported: 2 (3%) in group CC and 11 (11%) in group SA; with a significant difference (P: 0.044). All reinterventions (postoperative prolonged air leak and recurrences) required an SA.
CONCLUSIONS
Parenchymal-sparing technique through CC of apical blebs and bullae is an effective treatment for primary spontaneous pneumothorax and guarantees a good immediate lung sealing, despite stapling still represents the choice treatment in complex cases.
Keywords: Primary pneumothorax, Cold coagulation, Apicectomy, Parenchymal-sparing surgery, Floating ball
Primary spontaneous pneumothorax (PSP) is historically defined as pneumothorax occurring in absence of underlying pulmonary disease, preceding trauma or iatrogenic injury [1].
INTRODUCTION
Primary spontaneous pneumothorax (PSP) is historically defined as pneumothorax occurring in absence of underlying pulmonary disease, preceding trauma or iatrogenic injury [1].
Most patients with PSP, however, present apical blebs, small bullae or emphysema-like changings (ELC), barely detectable by the pre-operative imaging, which make the above-mentioned definition obsolete and inappropriate [2, 3]. Due the high frequency of PSP, most of the main thoracic societies worldwide faced with it; nevertheless, PSP represents an enigmatic condition since little attention has been dedicated to its surgical management [4].
To date, surgery is mostly recommended in pneumothorax that do not improve with conservative management or after pleural drainage procedures, in case of persistent (more than 5–7 days) air leak, as well as for recurrent (homolateral and contralateral) ones [1]. Thanks to the advantages of reduced trauma together with a quick recovery, video-assisted thoracic surgery (VATS) has gradually replaced thoracotomies and is now widely used in clinical practice [5].
Despite the above-mentioned cornerstones, a large consensus on the best technique to settle the air-leak source, to treat the apical blebs or bullae, whereas present, or to induce the pleurodesis is still far from being achieved [6–8].
Most procedures reported in literature consist in the stapler resection of the (usually) affected apices of the lung, followed by a surgical pleurodesis that could be reached mechanically, via pleural abrasion and cauterization or chemically by using sclerosing agents [7].
In this wide scenario, characterized by different reported techniques, instruments and approaches to remove the eventual PSP causes and prevent recurrences, >10 years ago we described a new technique consisting in the apical transcollation through the cold coagulation (CC) of the apical blebs or bullae [9].
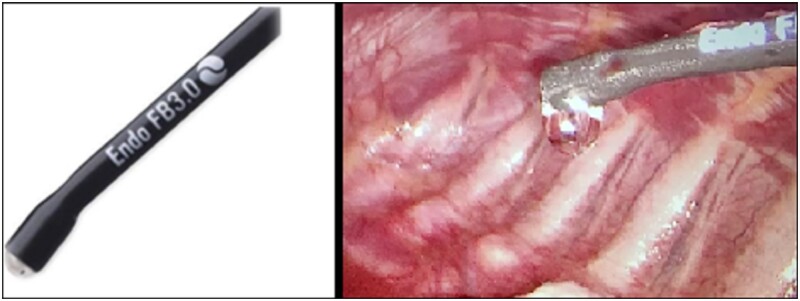
This latter represents a minimally invasive parenchymal-sparing technique to seal the affected lung by inducing a local collapse of the parenchymal air leak source [8]. The dedicated endoscopic instrument called Floating Ball presents a specially designed round tip (Fig. 1) from which it spills a cold saline solution that lowers the temperature of the target parenchyma and avoids any burning or scarring [9, 10]. At the beginning of our experience, we demonstrated the safety and feasibility of this technique; herein, we report results of >10-year experience with this technique in the treatment of PSP. Lastly, we compared these results with those of patients treated with the more common apicectomy.
Figure 1:
The Endo-Floating Ball is an endoscopic 5-mm electrocautery with spherical steel tip from which the cold saline solution spills out drop by drop.
MATERIALS AND METHODS
We retrospectively collected clinical and surgical data of all the patients with diagnosis of PSP addressed at our division of Thoracic Surgery from January 2010 to December 2020.
The Floating Ball instrument (Medtronic Inc., Minneapolis, MN, USA) was available for certain periods at our centre since 2005; however, it was used in the daily practice starting from 2010.
Out of 265 patients operated on for PSP according to the main international guidelines, we selected those with the following criteria: VATS procedures, intraoperative stage III blebs or bullae (according to Vanderschueren’s classification) located exclusively in lung apex; age ≤40 years at the time of surgery [11].
Patients with large apical bullae (>2 cm or Vanderschueren’s stage IV), with anamnesis of previous lung surgery, affected by known or proven underlying pulmonary disease, catamenial or traumatic pneumothorax or those treated merely with pleurodesis (mechanical or chemical) were excluded from this study [12].
Demographic and clinical data including gender, age at diagnosis, surgical indication and side of PSP, as well as surgical and postoperative data, postoperative hospital stay, complications and long-term outcomes were recorded and analysed.
Clinical and radiological surveillance was conducted, after the patient discharge, at our dedicated outpatient clinic, with a 15-, 30- and 60-day chest X-ray; if requested by the clinical condition, surveillance was prolonged until complete recovery. All patients were then systematically phone-called to continue the follow-up and update data collection, so that we are confident that every recurrence case has been addressed to our centre.
Recurrence was considered as radiological appearance of new detectable pneumothorax in the treated fully expanded lung or as radiological worsening of the existing pneumothorax in the slow-to-expandable lungs, regardless the onset timing. Patients previously treated for spontaneous pneumothorax on the contralateral side were considered as new cases.
Patients were divided into 2 groups according to the surgical technique: group CC included all patients underwent CC of the apical blebs or bullae, while group stapler apicectomy (SA) included those underwent to stapler resection of the apex.
Since we selected only patients with comparable clinical features, including the dimensions and the extent of the apical blebs/bullae, the surgical treatment was selected exclusively according to the surgeon’s choice and confidence with the technique.
Lastly, all patients of this series had the same mechanical pleurodesis consisting in a patchy cauterization of parietal pleura along the arcs from 2nd to 8th ribs through the floating ball instrument after the saline-drop stopping or by the electrocautery. Eventual residual air leak sources were searched by the water submersion test, as well as the lung expandability was checked thanks to anaesthesiologist cooperation.
All patients signed a dedicated informed consent to the anonymous use of their clinical, surgical and pathological data for research purposes.
Cold coagulation surgical technique
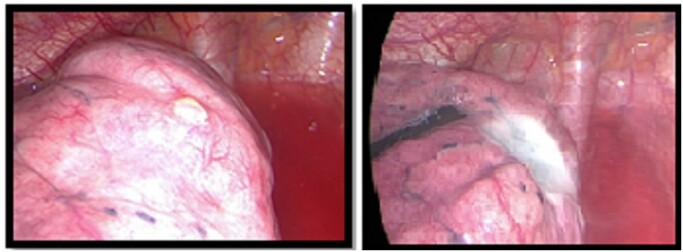
All the procedures were performed under general anaesthesia, single lung ventilation with double-lumen endotracheal tube or spontaneously breathing laryngeal mask anaesthesia and in the lateral decubitus position. Two or 3 sub-centimetric incisions were performed in the axillary triangle. After the chest exploration, apical blebs or small bullae (stage III according to Vanderschueren’s classification) were sealed via the CC as described previously [9, 10]. The endoscopic instrument adopted was the Endo-Floating Ball and consists in a 5-mm monopolar electrocautery with a steel floating sphere on the tip from which it spills saline solution drop by drop (Fig. 1). This instrument allows transcollation of blebs or bullae without charring and burning. Saline irrigation keeps cooled down the temperature of the tip that contacts the lung surface, avoiding eschars formation. Instrument was placed for few seconds over the apical blebs or bulla until they shrink over to their basis by sealing the lung parenchyma (Fig. 2).
Figure 2:
Intraoperative pictures of apical blebs, before and after the treatment.
Eventual residual air leak was tested by inflating the lung under saline solution and then sealed with a floating ball second pass.
Finally, we block the saline solution spilling, and we use the instrument as a common electrocautery to induce a mechanical pleurodesis by patchy cauterization of the parietal pleura until its interruption (Fig. 3). At the end of the procedure 1 or 2 pleural drainages (according to the surgeon’s choice) are placed in the chest through the operative incisions. Chest drains were connected to a suction system at −15 cm H2O for at least 2 days.
Figure 3:
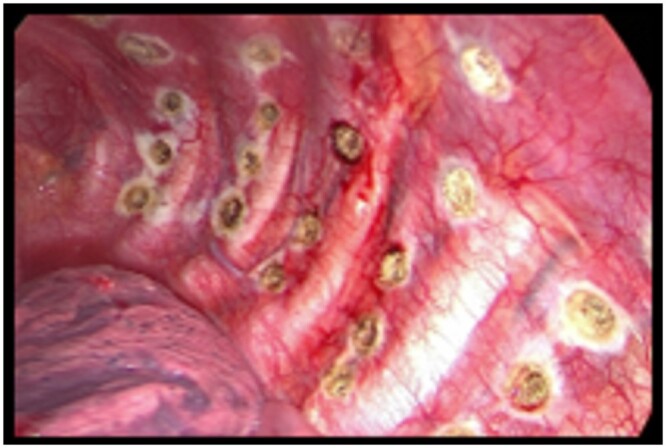
Intraoperative picture of the patchy pleural cauterization performed with the endo-floating ball instrument with the saline solution to achieve a uniform pleurodesis.
Mechanical pleurodesis was similarly induced in all patients of the study. In both groups, pleural irritation was obtained by a patchy cauterization of parietal pleural with Floating Ball (CC group) or long-bladed monopolar electrocautery (SA group).
Statistical analysis
The main aim of the study was to assess the efficacy of CC technique to seal the air-leak source by sparing the lung parenchyma as much as possible. We report short- and long-term results in an over 10-years’ experience; then, to validate this technique, we compared results with those of patients treated for the same pathology with the more common apicectomy with staplers.
Continuous variables were defined as mean and standard deviation or as median, where appropriate, and compared with t-test for normal distribution and Mann–Whitney U-test for non-normal distribution. Categorical variables were defined as absolute numbers and relative percentages and compared with Chi square test or Fisher's exact test, where appropriate. The statistical significance level (alpha) was set at 5%.
The Kaplan–Meier method and the log-rank test were used to estimate time to event-free survival (EFS) and to plot relative curves. The univariable analysis was used to check risk factors that influence postoperative EFS. Variables proved to be significantly associated with outcome at univariate analysis were entered in a multivariable model. A stepwise selection approach was used to limit the number of variables in the final multivariable model to significant independent predictors of EFS. The analysis was performed with IBM SPSS Statistics for Windows, Version 23.0 (Armonk, NY: IBM Corp).
Ethics statement
The institutional review board of all the hospitals waived the requirement for ethics approval due to the nature of the study. This latter was conducted in accordance with the principles of the Declaration of Helsinki. All patients signed an informed consent for the collection, analysis and publication of the retrospectively obtained anonymized data for this non interventional study.
RESULTS
According to inclusion criteria, 177 patients were enrolled (Table 1), 138 were males (78.0%) and 39 females (22.0%), with a median age of 24 years (range 13–40).
Table 1:
Demographics and clinical data of all patients enrolled
| Variables | Number (%) |
|---|---|
| Gender | 138 (78.0%) males |
| 39 (22.0%) females | |
| Age at surgery, median (range) | 24 (13–40) years |
| Number of homolateral PSP episode to surgery | 1: 72 (40.7%) |
| 2: 84 (47.5%) | |
| ≥3: 21 (11.8%) | |
| Side of pneumothorax | 83 (46.9%) right |
| 94 (53.1%) left | |
| Intraoperative findings | 123 (69.5%) apical blebs |
| 54 (30.5%) apical ≤2-cm bullae | |
| Operative time, median (range) | 40 (15–120) min |
| Conversion rate | 0 (0.0%) |
| Postoperative complications | Missing data: 15 (8.4%) |
| No: 141 (79.6%) | |
| Yes: 21 (12.0%) | |
| − Prolonged (>3 days) air leak: 15 (8.4%) | |
| − Haemothorax: 2 (1.1%) | |
| − Others: 4 (2.5%) | |
| Hospital stay, median (range) | 4 (2–23) days |
PSP: primary spontaneous pneumothorax.
Seventy-seven patients (43.5%) underwent CC of the apex followed by mechanical pleurodesis while 100 subjects (56.5%) underwent to SA followed by the same pleurodesis. Clinical and demographics characteristics of 2 groups are presented in Table 2.
Table 2:
Comparison of clinical and surgical features between group cold coagulation and stapler apicectomy
| Variables | Group cold coagulation | Group stapler resection | P-Value |
|---|---|---|---|
| 77 patients (43.5%) | 100 patients (56.5%) | ||
| Gender (%) | M: 67 (87.0%) | M: 71 (71.0%) | 0.012 |
| F: 10 (13.0%) | F: 29 (29.0%) | ||
| Age at surgery,a mean (±SD) | 24.0 (±6.7) | 25.8 (±7.2) | 0.085 |
| First episode (persistent PSP) | 31 (40.2%) | 41 (41.0%) | 0.92 |
| Number of episode (%) | 1: 31 (40.2%) | 1: 41 (41.0%) | 0.56 |
| 2: 37 (48.0%) | 2: 47 (47.0%) | ||
| ≥3: 9 (11.8%) | ≥3: 12 (12.0%) | ||
| Side of pneumothorax (%) | Right: 34 (44.2%) | Right: 49 (49.0%) | 0.52 |
| Left: 43 (55.8%) | Left: 51 (51.0%) | ||
| Intraoperative findings (%) | Blebs: 58 (75.3%) | Blebs: 65 (65.0%) | 0.14 |
| Bullae: 19 (24.7%) | Bullae: 35 (35.0%) | ||
| Operative time,a mean (±SD) | 43.2 (±19.5) | 49.3 (±20.1) | 0.050 |
| Postoperative complications (%) | Missing data: 6 (8.0%) | Missing data: 9 (9.0%) | |
| No: 66 (85.0%) | No: 75 (75.0%) | 0.048 | |
| Yes: 5 (7.0%) | Yes: 16 (16.0%) | ||
| Prolonged air leak: 4 (5.0%) | Prolonged air leak: 11 (11.0%) | 0.16 | |
| Bleeding: 1 (2.0%) | Bleeding: 3 (3.0%) | ||
| Fever: 2 (1.0%) | |||
| Hospital stay, mean (±SD) | 4.01 (±1.67) days | 5.2 (±3.55) days | 0.029 |
Group A: patients treated by cold coagulation; group B: patients treated by apicectomy.
Continuous variable.
F: females; M: males; PSP: primary spontaneous pneumothorax; SD: standard deviation.
The 2 groups were comparable in terms of age, surgery indication (persistent first episode or recurrent pneumothorax), side and intraoperative findings in terms of apical blebs or bullae.
Group CC showed a mean operative time of 43.2 min (±19.5), while in Group SA, it was 49.3 min (±20.1) with a statistically significant difference (P: 0.050). No conversion to thoracotomy was observed in both groups. In all cases, no air leak was observed at the end of surgery either intraoperatively or through the closed water-seal drains.
Complications rate was totally 12.0% (n = 21) with a statistically significant difference (P: 0.048) between 2 groups: 5 (7.0%) and 16 (16.0%) for group CC and SA, respectively. Postoperative air leak was observed in 15 cases, despite the intraoperative tests, with no differences between the 2 groups (P: 0.16). Three of those patients (1 of group CC and 2 of group SA) required a second operation after a median of 7 days performed via VATS or thoracotomy in 1 and 2 cases respectively; while 2 patients out of 15 were treated with a new large-bore chest tube usually positioned in the second intercostal space along the emiclavear line.
In all cases of second surgery for prolonged air leak, the leak source was detected in ECL areas close to the stapler lines or near the CC-treated area. After its identification also by under-water test, the affected part was removed by a stapler resection. Redo-pleurodesis was never required.
The median postoperative hospital stay was 4 days for both groups (range: 2–23 days) with a significant difference between groups since the mean post-operative stay for group CC was shorter than that of group SA: 4.01 (±1.67) and 5.2 (±3.55) days, respectively (P: 0.029).
After a median follow-up of 83 months (range 24–144), 13 homolateral late recurrences were reported (7.3%), 2 out of 77 cases in the group CC (2.6%), 11 out of 100 cases in the group SA (11.0%), with statistically significant difference between groups (P = 0.044) as reported in Fig. 4.
Figure 4:
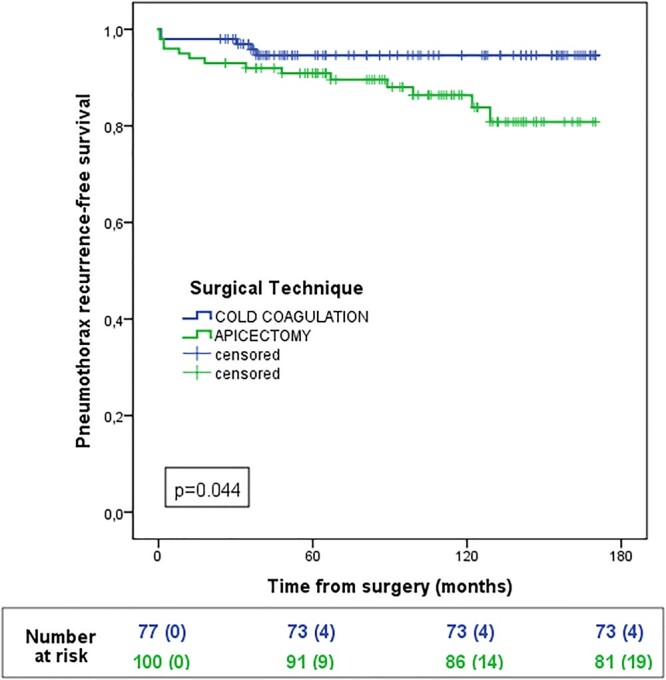
Event-free survival for cold coagulation and stapler apicectomy groups.
In all those cases, a new large-bore chest tube was placed, while in 5 cases a new operation was required due to air leak persistence, by stapling the sources of air leak that were located distantly from the previously treated area (3 cases), with no evidence of dehiscence of this latter.
Cox proportional hazard model
Concerning possible prognostic factors influencing recurrence rate (RR) (EFS, in the analysis), gender (P: 0.18), age (P: 0.72), persistent first PSP episode (P: 0.07), side (P: 0 0.87), intraoperative anatomical findings (P: 0.78), number of chest tubes (P: 0.57) and the occurrence of postoperative complications (P: 0.77) did not show any significant impact. Conversely, previous homolateral PSP episodes ≥3 (P: 0.005) and the surgical technique (P: 0.042) were significantly related to EFS. At multivariable analysis, both number of PSP episodes (P: 0.005; hazard ratio: 3.3, 95% CI: 1.4–7.8) and surgical technique (P: 0.042; hazard ratio: 0.1, 95% CI: 0.0–9.2) confirmed their prognostic role as reported in Table 3.
Table 3:
Statistical analysis of prognostic factors affecting event-free survival; Cox proportional hazard model, stepwise regression
| Variable | Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|---|
| HR | P-Value | HR | 95% CI | P-Value | |
| Gender (M versus F) | 2.1 | 0.18 | |||
| Age (continuous variable) | 1.1 | 0.72 | |||
| Persistent first PSP episode (Y versus N) | 0.2 | 0.07 | |||
| Number of episode (≤2 versus ≥3) | 3.2 | 0.005 | 3.3 | 1.4–7.8 | 0.005 |
| Side (R versus L) | 0.9 | 0.87 | |||
| Intraoperative findings (blebs versus ≤2-cm bullae) | 0.9 | 0.78 | |||
| Number of chest tube (1 versus 2) | 0.6 | 0.57 | |||
| Postoperative complications (Y versus N) | 0.7 | 0.77 | |||
| Cold coagulation versus apicectomy | 0.1 | 0.043 | 0.1 | 0.0–9.2 | 0.042 |
CI: confidence interval; F: female; HR: hazard ratio; L: left; M: male; N: not; PSP: primary spontaneous pneumothorax; R: right; Y: yes.
DISCUSSION
Is there anything new to say on primary pneumothorax that has not already been said?
Much has been written on PSP, which represents a very common pathology with high social costs, since it usually affects working-age population [1, 6].
Its management involves different physicians, mainly pneumologists and thoracic surgeons; so that, in literature exists many papers on different management strategies that range from the more conservative approaches to the more aggressive ones [4, 13]. Much of the debate, however, is focused mainly on the clinical and radiological work-up, and on the best strategy to treat the first PSP episode; while, regarding surgery, only the indication for persistent (>5–7 days) or recurrent pneumothorax could be considered well established [8].
The principal international guidelines, in fact, do not explicitly define or suggest any surgical procedure [4, 11].
Surgical technique usually consists in a two-step’s procedure: removing the potential cause of air leak and inducing an effective pleurodesis; actually, the choice of 1 or another technique depends entirely on the single centre’s experience and to the surgeons’ habits rather than on real evidence [14].
Minimally invasive stapler resection of the bullae (also called bullectomy) and stapler resection of the apex (apicectomy) are the most common techniques used worldwide. These techniques are safe and effective, with complication rates about 10% and RRs of almost 10–15% in most cases, although the resection usually requires the sacrifice of a variable portion of healthy lung together with the affected by ELC one [15, 16].
Conversely, with the introduction of minimally invasive approaches, at the beginning of 1990, much attention has been paid also to less-aggressive techniques in order the improve the quality of life and a rapid recovery. Pioneers as Torre and Belloni [17] reported their first successfully experience with thoracoscopic Nd:Yag laser, a parenchymal-sparing technique applied in 13 PSP patients that developed no recurrences during the surveillance.
With the aim to reduce as much as possible the physiological and economic impact, Liu et al. [18] reported their experience with the thoracoscopic loop ligation of parenchymal blebs and bullae with excellent short- and long-term results (14.8% of postoperative air leak and 2.1% of RR).
In 1989, Wakabayashi [19] reported the use of electrocautery in thoracoscopic ablation of blebs as an alternative to lung resection in PSP patients with a 90% of success and no recurrences. but his experience has not been followed for a long time.
In the wave of the fascinating parenchymal-sparing technique, in 2005, we first described a new device for the coagulation of the apical blebs and bullae as an alternative to stapler resection, that allowed good results by sparing as much lung parenchyma as possible [10].
In our experience, the CC of the apical blebs/bullae may be considered a safe procedure with a lower complication rate compared to the most common apicectomy (16% vs 7%, P: 0.048).
Possible explanations lie in the small dimension of the floating ball (5 mm), significantly smaller than the common endostapler devices (usually larger than 10 mm) and on the gentle effect that the CC exerts on a fragile apical lung tissue due to the ECL. Combination of these factors reduces the trauma on the intercostal spaces and on the lung, parenchyma compared to the relatively small jaw opening of the modern endostapler devices, that increase the friction and the tearing on the lung with the risk of creating visceral pleura disruption [20]. Notwithstanding, both the techniques have an excellent sealing capacity that could explain the comparable low rate of postoperative air leak of the 2 groups.
Regarding the RR, a fundamental role is played by the pleurodesis induction.
Pleurodesis between visceral and parietal pleura is the consequence of the pleura irritation that could be reached mechanically (pleurectomy, pleural abrasion or cauterization) or chemically (talc and other sclerosing agents) [21].
In a systematic review published in 2016 by Sudduth et al. on the utopistic research of optimal surgical technique in PSP, authors selected 51 studies with >6000 patients, highlighting the lack of high-quality evidence and randomized trials. Their conclusion was that VATS wedge resection of the affected lung (blebs, bullae or ELC) reach the highest effectiveness when followed by chemical pleurodesis with an RR of 1.7%, while apicectomy alone leads to a highest risk with a 9.7% RR [14].
Czerny et al. [22], as long ago as 2004, demonstrated that wedge resection of the apex followed by mechanic pleurodesis through apical pleurectomy is more effective than pleurectomy alone in reducing recurrences (P: 0.009) even in patients with no detectable blebs or adhesions during VATS (Vanderschueren’s stage I).
To date, there is a large and heated debated on the best pleurodesis technique able to reduce the pneumothorax RR with different studies reporting fluctuating results [21].
According to the literature, pleural abrasion allows good long-term results in respect of low complication rates compared to potentially more effective procedures as pleurectomy and talc poudrage, characterized by a high complication rate such as bleeding, chest pain, fever and respiratory distress [7, 23].
Park et al. [24], however, analysed the role of mechanical pleurodesis via pleural abrasion after wedge resection of bullae for PSP and they found no significant differences in the RR (P: 0.94), and duration of chest tube drainage stay (P: 0.52) between the patients treated with and without pleural abrasion.
In our experience, all patients of both groups underwent the same mechanical pleurodesis technique that represent a good compromise between the pleurectomy and the pleural abrasion. We exclusively reserve chemical pleurodesis for secondary pneumothorax, in case of redo-surgery for pneumothorax, pathological proven catamenial pneumothorax and in case of malignant pleural effusion.
Since 2005, in PSP patients who underwent surgery, we usually perform a patchy pleural electrocauterization to discontinue the parietal pleural surface along the first 8 costal arcs, inducing a homogeneous irritation of the pleural without increasing the bleeding risk (Fig. 3). In patients who underwent apicectomy, pleurodesis was obtained thanks to a common endoscopic electrocautery, while in patients of group CC the same floating ball device was used.
This latter instrument, in fact, once stopped the saline irrigation, represents a spherical electrocautery that allows pleural disruption without sinking in the deeper layers; moreover, it is a long and curved device enabling surgeons to reach any area of the pulmonary parenchyma as well as all the parietal pleura surface.
The pleurodesis achieved in this way may be considered as safe and effective, considering the low morbidity and complications rate reported in our experience, that substantially do not differ from results reported in literature that range from 0% (in selected single bulla cases) to 30% for high-risk patients [25].
To date, the present study represents the only one that compares a parenchymal-sparing technique with the widely used apicectomy in the PSP subjects addressed to surgical treatment. Strong points of this study are in the large numbe of patients with comparable selected features, the long follow-up and the single-centre experience; in addition to the fact that is one of the first studies on this technique with a control group; that allows to support, with more evidence, our conclusions.
Limitations
On the other hand, there are some limitations that should be reported, most of them are related to the retrospective nature of the study as well as the potential selection bias and the non-randomized dichotomy of the groups.
This latter in fact depended exclusively on the surgeon habit and experience, even though patients presented comparable anatomical intraoperative findings in terms of ELC of the apex.
Moreover, although we strongly recommend this technique to reduce the surgical detrimental impact on lung parenchyma, no analysis on the residual postoperative pulmonary function has been conducted due to the benign nature of the disease and the young age of the patients, as well as no cost–benefit analysis has been performed, mainly due to the fact that the stapler devices and floating ball instrument changed their cost many times over the last 10 years.
CONCLUSIONS
Surgical options for PSP treatment represent a wide ocean with very few tools to navigate in it. Surgery, whereby there is an indication, should provide both the 2 cornerstone steps: the lung sealing and the pleurodesis to reduce the RR, which is the most common short- and long-term complication for this kind of benign disease. To date, apicectomy followed by pleural abrasion is the most common combination used worldwide and provides excellent results.
Anyway, times are changing, and an increasing attention is daily paid to the quality of life and towards less invasive surgical strategies. In this view, CC technique represents an excellent alternative to stapler resection, with minimal morbidity rate in respect of high effectiveness.
Conflict of interest: none declared.
Glossary
ABBREVIATIONS
- CC
Cold coagulation
- EFS
Event-free survival
- ELC
Emphysema-like changing
- PSP
Primary spontaneous pneumothorax
- RR
Recurrence rate
- SA
Stapler apicectomy
- VATS
Video-assisted thoracic surgery
Contributor Information
Vittorio Aprile, Department of Surgical, Medical and Molecular Pathology and Critical Care Medicine, University of Pisa, Pisa, Italy.
Diana Bacchin, Department of Surgical, Medical and Molecular Pathology and Critical Care Medicine, University of Pisa, Pisa, Italy.
Elena Marrama, Department of Surgical, Medical and Molecular Pathology and Critical Care Medicine, University of Pisa, Pisa, Italy.
Stylianos Korasidis, Unit of Thoracic Surgery, University Hospital of Pisa, Pisa, Italy.
Maria Giovanna Mastromarino, Unit of Thoracic Surgery, University Hospital of Pisa, Pisa, Italy.
Gerardo Palmiero, Unit of Pneumology, Versilia Hospital, Lido di Camaiore, Italy.
Marcello Carlo Ambrogi, Department of Surgical, Medical and Molecular Pathology and Critical Care Medicine, University of Pisa, Pisa, Italy; Unit of Thoracic Surgery, University Hospital of Pisa, Pisa, Italy.
Marco Lucchi, Department of Surgical, Medical and Molecular Pathology and Critical Care Medicine, University of Pisa, Pisa, Italy; Unit of Thoracic Surgery, University Hospital of Pisa, Pisa, Italy.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Vittorio Aprile: Conceptualization; Data curation; Formal analysis; Software; Writing—original draft. Diana Bacchin: Conceptualization; Data curation; Formal analysis. Elena Marrama: Data curation. Stylianos Korasidis: Conceptualization; Supervision; Validation; Writing—review & editing. Maria Giovanna Mastromarino: Supervision; Validation; Visualization. Gerardo Palmiero: Validation; Visualization. Marcello Carlo Ambrogi: Conceptualization; Supervision; Validation; Visualization. Marco Lucchi: Conceptualization; Supervision; Validation; Visualization; Writing—review & editing.
Reviewer information
Interdisciplinary CardioVascular and Thoracic Surgery thanks Charalambos Zisis, Andrea Zuin and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
Presented at the 30th ESTS annual meeting, Hague, Netherlands, 19–21 June 2022.
REFERENCES
- 1. Tschopp JM, Bintcliffe O, Astoul P, Canalis E, Driesen P, Janssen J. et al. ERS task force statement: diagnosis and treatment of primary spontaneous pneumothorax. Eur Respir J 2015;46:321–35. [DOI] [PubMed] [Google Scholar]
- 2. Porcel JM, Lee P.. Thoracoscopy for spontaneous pneumothorax. J Clin Med 2021;10:3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inderbitzi RG, Leiser A, Furrer M, Althaus U.. Three years' experience in video-assisted thoracic surgery (VATS) for spontaneous pneumothorax. J Thorac Cardiovasc Surg 1994;107:1410–5. [PubMed] [Google Scholar]
- 4. Giles AE, Kidane B, Schellenberg M, Ball CG, Brown C, Dixon E. et al. ; Evidence Based Reviews in Surgery (EBRS) Group. The Primary Spontaneous Pneumothorax trial: a critical appraisal from the surgeon's perspective. J Thorac Cardiovasc Surg 2021;162:1428–32. [DOI] [PubMed] [Google Scholar]
- 5. Lin Z, Zhang Z, Wang Q, Li J, Peng W, Ge G.. A systematic review and meta-analysis of video-assisted thoracoscopic surgery treating spontaneous pneumothorax. J Thorac Dis 2021;13:3093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J. et al. ; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590–602. [DOI] [PubMed] [Google Scholar]
- 7. Rena O, Massera F, Papalia E, Della Pona C, Robustellini M, Casadio C.. Surgical pleurodesis for Vanderschueren's stage III primary spontaneous pneumothorax. Eur Respir J 2008;31:837–41. [DOI] [PubMed] [Google Scholar]
- 8. Bertoglio P, Viti A, Lomangino I, Terzi CA, Minervini F.. Surgical management of pneumothorax: still sailing with no compass. J Thorac Dis 2020;12:3007–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ambrogi MC, Zirafa CC, Davini F, Giarratana S, Lucchi M, Fanucchi O. et al. Transcollation® technique in the thoracoscopic treatment of primary spontaneous pneumothorax. Interact CardioVasc Thorac Surg 2015;20:445–8. [DOI] [PubMed] [Google Scholar]
- 10. Ambrogi MC, Melfi F, Duranti L, Mussi A.. Cold coagulation of blebs and bullae in the spontaneous pneumothorax: a new procedure alternative to endostapler resection. Eur J Cardiothorac Surg 2008;34:911–3. [DOI] [PubMed] [Google Scholar]
- 11. Mendogni P, Vannucci J, Ghisalberti M, Anile M, Aramini B, Congedo MT. et al. ; Collaborators of the Pneumothorax Working Group, on behalf of the Italian Society for Thoracic Surgery (endorsed by the Italian Ministry of Health) Collaborators of the Pneumothorax Working Group. Epidemiology and management of primary spontaneous pneumothorax: a systematic review. Interact CardioVasc Thorac Surg 2020;30:337–45. [DOI] [PubMed] [Google Scholar]
- 12. Vanderschueren RG. The role of thoracoscopy in the evaluation and management of pneumothorax. Lung 1990;168:627–30. [DOI] [PubMed] [Google Scholar]
- 13. Tsuboshima K, Kurihara M, Nonaka Y, Ochi T.. Is conventional management of primary spontaneous pneumothorax appropriate? Gen Thorac Cardiovasc Surg 2021;69:716–21. [DOI] [PubMed] [Google Scholar]
- 14. Sudduth CL, Shinnick JK, Geng Z, McCracken CE, Clifton MS, Raval MV.. Optimal surgical technique in spontaneous pneumothorax: a systematic review and meta-analysis. J Surg Res 2017;210:32–46. [DOI] [PubMed] [Google Scholar]
- 15. Tsuboshima K, Matoba Y, Wakahara T.. Optimal margin distance of bullectomy for primary spontaneous pneumothorax reduces postoperative recurrence. J Thorac Dis 2019;11:5115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrmann D, Klapdor B, Ewig S, Hecker E.. Initial management of primary spontaneous pneumothorax with video-assisted thoracoscopic surgery: a 10-year experience. Eur J Cardiothorac Surg 2016;49:854–9. [DOI] [PubMed] [Google Scholar]
- 17. Torre M, Belloni P.. Nd:YAG laser pleurodesis through thoracoscopy: new curative therapy in spontaneous pneumothorax. Ann Thorac Surg 1989;47:887–9. [DOI] [PubMed] [Google Scholar]
- 18. Liu HP, Chang CH, Lin PJ, Hsieh MJ.. Thoracoscopic loop ligation of parenchymal blebs and bullae: is it effective and safe? J Thorac Cardiovasc Surg 1997;113:50–4. [DOI] [PubMed] [Google Scholar]
- 19. Wakabayashi A. Thoracoscopic ablation of blebs in the treatment of recurrent or persistent spontaneous pneumothorax. Ann Thorac Surg 1989;48:651–3. [DOI] [PubMed] [Google Scholar]
- 20. Subotic D, Hojski A, Wiese M, Lardinois D.. Use of staplers and adverse events in thoracic surgery. J Thorac Dis 2019;11:S1216–S1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Min X, Huang Y, Yang Y, Chen Y, Cui J, Wang C. et al. Mechanical pleurodesis does not reduce recurrence of spontaneous pneumothorax: a randomized trial. Ann Thorac Surg 2014;98:1790–6. [DOI] [PubMed] [Google Scholar]
- 22. Czerny M, Salat A, Fleck T, Hofmann W, Zimpfer D, Eckersberger F. et al. Lung wedge resection improves outcome in stage I primary spontaneous pneumothorax. Ann Thorac Surg 2004;77:1802–5. [DOI] [PubMed] [Google Scholar]
- 23. Sim SKR, Nah SA, Loh AHP, Ong LY, Chen Y.. Mechanical versus chemical pleurodesis after bullectomy for primary spontaneous pneumothorax: a systemic review and meta-analysis. Eur J Pediatr Surg 2020;30:490–6. [DOI] [PubMed] [Google Scholar]
- 24. Park JS, Han WS, Kim HK, Choi YS.. Pleural abrasion for mechanical pleurodesis in surgery for primary spontaneous pneumothorax: is it effective? Surg Laparosc Endosc Percutan Tech 2012;22:62–4. [DOI] [PubMed] [Google Scholar]
- 25. Asban A, Raza SS, McLeod C, Donahue J, Wei B.. Mechanical or chemical and mechanical pleurodesis for spontaneous pneumothorax: what is the most effective approach in preventing recurrence? A systematic review and meta-analysis. Eur J Cardiothorac Surg 2020;58:682–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.