Abstract
Despite decades of research, there are no medications approved by the United States Food and Drug Administration to treat stimulant use disorders. Self-administration procedures are widely used to screen candidate medications for stimulant use disorder, although preclinical reductions in stimulant self-administration have not translated to meaningful reductions in stimulant use in humans. One possible reason for this discordance is that most preclinical studies evaluate candidate medications under conditions that promote predictable, and well-regulated patterns of drug-taking rather than the dysregulated and/or compulsive patterns of drug-taking characteristic of a stimulant use disorder. A subset of rats (“high-responders”) that self-administer 3,4-methelyendioxypyrovalerone (MDPV), a monoamine uptake inhibitor, develop high levels of dysregulated drug-taking consistent with behaviors related to stimulant use disorders. Because MDPV acts on dopamine, serotonin (5-HT), and sigma receptor systems, the current studies compared the potency and effectiveness of a dopamine D3 receptor partial agonist (VK4-40) or antagonist (VK4-116), a sigma receptor antagonist (BD1063), a dopamine D2/D3/sigma receptor antagonist (haloperidol), and a 5-HT2C receptor agonist (CP-809,101) to reduce MDPV (0.0032–0.1 mg/kg/infusion) self-administration in high- and low-responding rats as well as rats self-administering cocaine (0.032–1 mg/kg/infusion). VK4-40, VK4-116, haloperidol, and CP-809,101 were equipotent and effective at reducing drug-taking in all three groups of rats, including the high-responders; however, VK4-116 and CP-809,101 were less potent at reducing drug-taking in female compared with male rats. Together, these studies suggest that drugs targeting dopamine D3 or 5-HT2C receptors can effectively reduce dysregulated patterns of stimulant use, highlighting their potential utility for treating stimulant use disorders.
SIGNIFICANCE STATEMENT
There are no United States Food and Drug Administration-approved treatments for stimulant use disorder, perhaps in part because candidate medications are most often evaluated in preclinical models using male subjects with well-regulated drug-taking. In an attempt to better model aberrant drug taking, this study found compounds acting at dopamine D3 or 5-HT2C receptors can attenuate drug-taking in male and female rats that self-administered two different stimulants and exhibited either a high or low substance use disorder-like phenotype.
Introduction
Substance use disorders affect 18 million Americans per year, with ∼3.2 million of those diagnosed with a stimulant use disorder (Substance Abuse and Mental Health Services Administration, 2021: https://www.samhsa.gov/data/report/2020-nsduh-annual-national-report). Yet, despite decades of research, there are currently no pharmacotherapies approved by the United States Food and Drug Administration to treat stimulant use disorder. One potential reason for the failure to identify effective treatments is that the most commonly used models may not represent the problematic drug-taking behavior characteristic of stimulant use disorders. Most rodent studies evaluating candidate medications for stimulant use disorder use relatively short self-administration sessions (e.g., 1–3 hours) in male rats with limited histories of drug taking. Such study designs are favored because they result in highly predictable and homogenous patterns of drug-taking with low inter-subject variability. Other session parameters (e.g., extended-, intermittent-access) can result in subsets of rats developing aberrant patterns of drug-taking, similar to those observed in individuals with a stimulant use disorder. Identifying conditions capable of generating robust stimulant use disorder-like phenotypes might improve the predictive validity of rodent self-administration procedures for evaluating candidate medications for stimulant use disorder (e.g., Lynch 2018).
Recently, we identified a reliable and persistent high-responder phenotype that emerges in ∼30% of male and female rats that self-administer 3,4-methylenedioxypyrovalerone (MDPV) and structurally-related synthetic cathinones, characterized by high levels of dysregulated drug-taking and -seeking that might be related to stimulant use disorder (Gannon et al., 2017, 2018a, 2021; Doyle et al., 2021a,c; Abbott et al., 2022). These high-responder rats earn more infusions across a range of self-administration doses (i.e., upward shift in the dose-response curve), make more responses during signaled periods of drug unavailability, and reach greater breakpoints under progressive ratio schedules of reinforcement (Gannon et al., 2017; Doyle et al., 2021a,c; Abbott et al., 2022). Since high-responder rats exhibit a robust stimulant use disorder-like phenotype, they may provide a translationally-relevant framework for evaluating candidate medications for stimulant use disorder. Because MDPV has been reported to interact with monoamine transporters (i.e., dopamine transporter, norepinephrine transporter, and serotonin transporter), and sigma receptors (Eshleman et al., 2013; Gannon et al., 2018a), potential pharmacotherapies targeting these neurotransmitter systems were viewed as logical starting points.
Antagonism of dopamine D2 receptors is effective at reducing drug-taking in preclinical studies (De Vry et al., 1989; Roberts et al., 1989; Barrett et al., 2004; Oleson et al., 2011), but impractical for use in humans because of extrapyramidal side effects (e.g., acute dyskinesia, akathisia; Blair and Dauner, 1992). In contrast, antagonists or partial agonists that are selective for dopamine D3 receptors can reduce drug-taking at doses that are devoid of extrapyramidal effects (Pilla et al., 1999; Gilbert et al., 2005; Keck et al., 2015). Recently, VK4-40 and VK4-116 were developed as highly-selective partial agonists (261-fold) or antagonists (1709-fold) at the dopamine D3 receptor relative to the dopamine D2 receptor (Kumar et al., 2016; Jordan et al., 2019b) and inhibit drug-taking and drug-seeking in rats self-administering cocaine or oxycodone at doses that do not affect food-maintained responding or produce adverse cardiovascular effects (Jordan et al., 2019a,b; You et al., 2019; de Guglielmo et al., 2020). Importantly, the effects of VK4-116 are dependent on the drug-taking phenotype of the rat, attenuating oxycodone self-administration in rats with high, but not low, levels of intake (de Guglielmo et al., 2020), suggesting it may also be effective at decreasing drug-taking in rats that engage in high levels of stimulant self-administration (i.e., the MDPV high-responders).
Serotonin (5-HT)2C receptors have been investigated as a therapeutic target for stimulant use disorder, in part because of their ability to modulate dopamine neurotransmission (for review, see Howell and Cunningham, 2015). 5-HT2C receptor agonists (e.g., lorcaserin) can decrease stimulant-taking and -seeking in rodents and nonhuman primates (Collins et al., 2016, 2018; Gerak et al., 2016, 2019; Harvey-Lewis et al., 2016; Collins and France, 2018; Gannon et al., 2018b; Anastasio et al., 2020), but also produce adverse effects associated with agonist actions at 5-HT2A receptors in both humans and rodents (Shram et al., 2011; Serafine et al., 2015). CP-809,101 is a 5-HT2C receptor agonist that is >1000-fold selective over 5-HT2A receptors (Siuciak et al., 2007) that can reduce drug-taking or -seeking in rats self-administering cocaine or nicotine (Higgins et al., 2013; Pockros-Burgess et al., 2014). However, it is important to note that most of these studies were conducted under limited-access conditions and in subjects displaying very predictable and homogenous patterns of drug-taking.
Finally, although MDPV is highly selective for dopamine transporter and norepinephrine transporter, relative to serotonin transporter, it is also known to interact with sigma receptors (Eshleman et al., 2013). Given that stimulant use changes the activity of sigma receptor in ways that are thought to be important for the treatment of stimulant use disorder (Katz et al., 2016), the present studies sought to determine if antagonism of sigma receptors with BD1063 would reduce MDPV self-administration, in low- and/or high-responders, even though previous studies suggest it is ineffective at altering the cocaine self-administration under limited-access conditions (Hiranita et al., 2013). Because haloperidol has antagonist properties at both dopamine D2, D3, and sigma receptors, it was included to determine if this combination of activities was beneficial in reducing the aberrant drug-taking observed in the MDPV high-responder rats.
The present study sought to directly compare the potency and effectiveness of ligands targeting dopamine D2 or D3, 5-HT2C, or sigma receptors to reduce the self-administration of MDPV or cocaine, and to determine whether any of these effects differed as a function of sex and/or drug-taking phenotype (i.e., low- versus high-responders). With the exception of the sigma receptor antagonist, BD1063, all compounds were equipotent and effective at reducing the self-administration of MDPV and cocaine. Although the effects of dopamine D3 and 5-HT2C ligands were comparable in both high- and low-responding rats, VK4-116 and CP-809,101 were less potent at reducing drug-taking in female compared with male rats.
Methods
Subjects
Male (n = 20) and female (n = 19) Sprague Dawley rats were obtained from Envigo (Indianapolis, IN) and weighed 275–299g or 200–224g, respectively, upon arrival. Rats were singly-housed and provided ad libitum access to food and water in their home cages throughout the experiment. The temperature- and humidity-controlled vivarium operated on a 14/10 hour light cycle (lights on at 6 AM), and experiments were conducted while lights were on. A subset of male rats (n = 9) also participated in another self-administration experiment before beginning this study, in which they self-administered MDPV and either cocaine, ketamine, nicotine, or fentanyl for ∼6 weeks (Doyle et al., 2021a). The self-administration behavior of these rats did not differ from the rats with no prior experimental history. All experiments were conducted in accordance with the University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee and the Guide for Use of Laboratory Animals (National Research Council, 2011).
Apparatus
Standard operant chambers (Med Associates, St. Albans, VT) were housed within sound- and light-attenuating chambers. Operant chambers had two levers positioned on the same wall, with a set of LEDs above each lever. A food trough, connected to a 45mg pellet dispenser, was located between the two levers, and a houselight was positioned on the opposite wall. A syringe driver delivered infusions through Tygon tubing, which was connected to a swivel that was held in place with a counterbalance arm and a magnetic tether, which attached to the vascular access button.
Surgery
Rats were anesthetized (2–3% isoflurane) and surgically implanted with a catheter in the left femoral vein, which was connected to a vascular access button placed in the mid-scapular region (Collins et al., 2012a; Doyle et al., 2021a,b,c). If catheter patency was lost (confirmed with 5 mg/kg methohexital), a new catheter was placed in the right femoral, right jugular, or left jugular veins, as needed (n = 18). Rats were treated with a long-acting antibiotic, Excede (20 mg/kg; SC), and an analgesic, meloxicam (5 mg/kg; SC), once, immediately following surgery, and allowed 5–7 days to recover. Rats were flushed daily with 0.5 ml of 100 U/ml of heparinized saline, including after experimental sessions. Once experiments began, rats were also flushed with 0.2 ml of saline before each session.
Training
Rats were initially allowed to respond for drug (0.032 mg/kg MDPV or 0.32 mg/kg cocaine) under a fixed ratio (FR) 1 schedule of reinforcement during ten daily 90-minute sessions. The active lever (counterbalanced across rats) was indicated by a lit yellow LED above the lever, and completion of the response requirement resulted in delivery of an infusion and the initiation of a 5-second post-infusion timeout (TO), which was indicated by a change in stimuli (i.e., all three LEDs above the active lever and the houselight were illuminated). After rats acquired responding (>80% of responses on the active lever and >20 infusions earned), the response requirement was increased to an FR5. Once responding was reliably maintained under an FR5: TO 5-second schedule of reinforcement (>20 infusions per session for at least three sessions), rats were allowed to respond on the alternate lever for delivery of a 45 mg grain-based food pellet (Dustless Precision Pellets Rodent, 45 mg; Bio-Serv, Flemington, NJ). Under these conditions, a new set of stimuli (i.e., red and green LEDs illuminated above the new active lever) were presented to indicate food availability, and completion of the response requirement resulted in initiation of the 5-second TO and delivery of a single food pellet. Once rats responded for >20 pellets on two consecutive days under a FR5: TO 5-second schedule of food delivery, rats were switched to the multiple-component procedure (see Supplemental Fig. 1 for timeline).
The multiple-component procedure was 132 minutes in duration and consisted of six components, each separated by a 2-minute intercomponent interval. The first and last components were 10 minutes in duration, during which the red and green LEDs were illuminated to indicate that food was available for responding under a FR5:TO 10-second schedule of food delivery; responding on the alternate lever was recorded but had no scheduled consequence. Components two through five were 25 minutes in duration and were initiated by the illumination of the yellow LED above the drug-reinforced lever (see Supplemental Fig. 1 for visual representation of schedule). The unit dose of MDPV (0.0032–0.1 mg/kg/infusion) or cocaine (0.032–1.0 mg/kg/infusion) increased in half-log steps as described previously (Collins et al., 2012b; Gannon et al., 2017) and was available under a FR5: TO 10-second schedule of reinforcement. The concentration of the drug remained constant, with different unit-doses administered by adjusting the duration of the infusion (based on body weight) from ∼0.3 seconds for the smallest unit-dose to ∼10 seconds for the largest unit-dose. During the intercomponent intervals, all stimuli were extinguished, and responses were recorded but had no scheduled consequence. After responding stabilized, defined at peak responding occurring in the same component for three consecutive sessions and within 25% of the mean of those three sessions, saline was substituted for drug for 1–5 sessions and until responding was below eight infusions per component. Rats were switched back to drug until stability criteria were met, after which saline was substituted again. This pattern was repeated until rapid extinction occurred, quantified as rats earning ≤8 reinforcers per drug component on the first day of saline substitution to increase the likelihood rats would extinguish responding if a pretreatment reduced the reinforcing effects of the self-administration drug.
Rats were subsequently classified as being a high- or low-responder based upon previously established criteria (Gannon et al., 2017, 2018a; Doyle et al., 2021a,c; Abbott et al., 2022). Briefly, the percentage of responses that were made during the 5-second post-infusion timeout out of total responses on the active lever in drug components was calculated for the three sessions preceding the final saline substitution. Rats that made ≥20% of total active lever responses during the post-infusion timeout periods were classified as high-responder rats, whereas low-responder rats made <20% during the timeouts. See Supplemental Table 1 for the number of subjects in each group.
Testing
Pretreatments were administered after three consecutive sessions of stable responding. Although all doses of a pretreatment drug were generally completed before moving on to the next pretreatment drug, the order of both the treatment drugs and the doses were pseudorandom. All rats received a vehicle injection for each pretreatment drug, as well as three or four doses of a pretreatment drug in half-log steps, with the exception of the largest dose of CP-809,101 that was a quarter-log step. Pretreatment drugs were the dopamine D3 receptor partial agonist, VK4-40 (3.2–32 mg/kg), the dopamine D3 receptor antagonist, VK4-116 (3.2–32 mg/kg), the 5-HT2C receptor agonist, CP-809,101 (0.32–5.6 mg/kg), the sigma1 receptor antagonist, BD1063 (1–10 mg/kg), and the dopamine D2/D3/sigma receptor antagonist, haloperidol (0.01–0.32 mg/kg). Most subjects completed all drugs, although a subset did not. The number of subjects is reported in Supplemental Table 1.
Drugs
BD1063 dihydrochloride was purchased from Tocris (Ballwin, MO) and dissolved in sterile saline. Haloperidol was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in saline with 1N HCL added dropwise until solution was clear. CP-809,101 was synthesized by the Center for Innovative Drug Discovery (San Antonio, TX) and dissolved in 5% Tween-80 in sterile saline. Both VK4-40 and VK4-116 were synthesized by Jianjing Cao (Medicinal Chemistry Section, NIDA-IRP) according to published procedures (Kumar et al., 2016) and dissolved in a solution of 10% DMSO, 15% Tween-80, and sterile saline. Pretreatment drugs were administered intraperitoneally at a volume of 1 ml/kg, except the largest dose of both VK4-40 and VK4-116, which were given at 1.78 ml/kg. BD1063 and CP-809,101 were administered 15 minutes prior to the session, and VK4-40, VK4-116, and haloperidol were administered 30 minutes prior to the session. The half-lives of these drugs are sufficient to allow for adequate blood levels of each drug for the duration of the 134-minute session (Watanabe et al., 1999; Hiranita et al., 2013; Higgins et al., 2017; Jordan et al., 2019a, You et al., 2019). Cocaine hydrochloride was provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD), and MDPV was synthesized by Kenner Rice (Bethesda, MD); both were dissolved in sterile saline and administered intravenously at volumes ranging from 0.044 to 1.4 ml/kg.
Data Analysis
A two-factor (group and dose) repeated measures ANOVA was used to compare the number of baseline infusions earned by MDPV high-responders, low-responders, and rats self-administering cocaine, and Tukey’s post-hoc comparisons were used to compare number of infusions earned at each dose by group. Two-factor (drug and component) repeated measures ANOVAs were run within each of the three self-administration groups to compare number of infusions earned when drug compared with saline was available. Two-factor (sex and dose) repeated measures ANOVAs were run within each of the three self-administration groups to compare number of infusions earned by male and female rats, and Sidak’s multiple comparison test was used when needed. Baseline was the three-day average immediately preceding the manipulation (i.e., saline substitution or pretreatment). Area under the curve (AUC) was calculated using the number of infusions earned at each dose in individual subjects. Percent change from baseline AUC was calculated in individual subjects using the AUC of the mean number of infusions earned during the three sessions prior to the pretreatment and were analyzed using two-factor (group x pretreatment dose) repeated measures ANOVA or mixed-effects analysis (if data set was incomplete) for each pretreatment dose and sex. Dunnett’s multiple comparisons was used to compare pretreatment dose to vehicle. ID50 was calculated in individual subjects using a linear regression with a single point >80% and <20%. In cases were responding was not decreased by >50%, a value of 0% was filled in at a half log higher dose range for a conservative estimate of potency. Drug ID50s were calculated using the percent change from baseline AUC and food ID50s were used calculated using percent change from baseline number of food pellets in the first food component. Potency ratios were calculated by dividing the group food ID50 by the group drug ID50. BD1063 was excluded from ID50 calculations because it did not decrease responding by 50% in any subjects.
Results
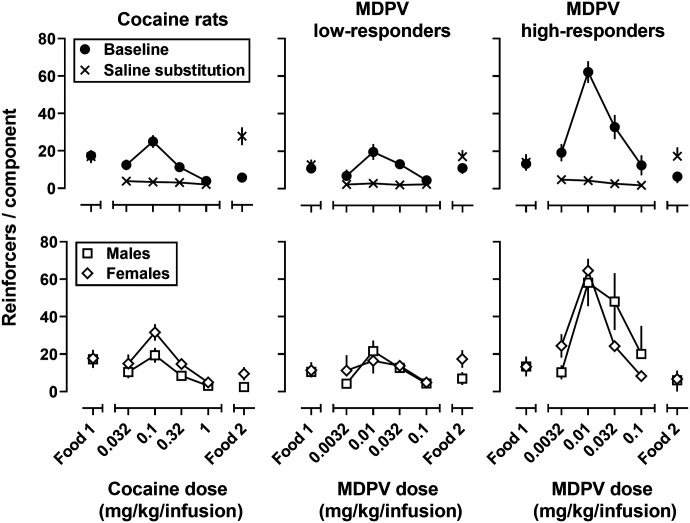
Under the multiple-component schedule of reinforcement, the dose-response curves for cocaine and MDPV self-administration were inverted U-shaped in nature, with the peak number of infusions earned at doses of 0.1 mg/kg/infusion of cocaine or 0.01 mg/kg/infusion of MDPV (Fig. 1). MDPV was ∼10-fold more potent than cocaine in both high- and low-responder rats, but equipotent in high- and low-responders. When compared with the peak number of infusions for all rats self-administering cocaine, the peak number of MDPV infusions was comparable for low-responder rats (∼20–25 infusions), but ∼2–3 times greater in the high-responder rats self-administering MDPV (∼40–75 infusions); a similar difference was also observed for the 2 larger unit-doses of MDPV. A 2-factor (group x dose) indicated main effect of group (F(2, 37) = 14.01; P < 0.0001), main effect of dose (F(2.08, 77.12) = 49.56; P < 0.0001), and an interaction (F(6, 111) = 6.82; P < 0.0001), where high-responders earned significantly more infusions than low-responders or rats self-administering cocaine at the two intermediate doses (ps ≤ 0.05). There was no difference between low-responders and rats self-administering cocaine. When saline was substituted for drug, rats earned significantly fewer infusions than when drug was available (high-responders: F (1, 24) = 22.77; P < 0.0001; low-responders: F (1, 24) = 5.39; P = 0.03; cocaine: F (1, 26) = 6.48; P = 0.02). When the dose-response curves were analyzed by sex, a two-factor ANOVA revealed that female rats self-administered significantly more cocaine than male rats (F (1, 12) = 5.68; P = 0.04). In contrast, there was no significant main effect of sex in either of the groups that self-administered MDPV (high responders: F (1, 11) = 0.11; P = 0.75); low-responders: F (1, 11) = 0.051; P = 0.82).
Fig. 1.
Self-administration dose-response curves obtained under a multicomponent FR5 schedule of reinforcement for cocaine (left), MDPV, separated by low- (center) and high-responder phenotype. Top row: number of infusions earned under baseline (drug) conditions (filled circles) and when saline was substituted for cocaine or MDPV during a single session (x). Bottom row: baseline conditions split by male (squares) and female rats (diamonds). Ordinate: reinforcers obtained during each component. Abscissa: Food 1 and Food 2 refer to components when grain-based pellets were available. The numbers refer to dose of cocaine (left) and MDPV (center and right) expressed as mg/kg/infusion on a log scale. Error bars represent ± S.E.M.
Pretreatments were evaluated once self-administration stabilized and single session saline substitutions resulted in a rapid extinction of responding (Fig. 2). All test compounds produced qualitatively similar effects in the three groups (MDPV-high, MDPV-low, and cocaine), with haloperidol (Fig. 2, A–C) shifting dose-response curves to the right, whereas VK4-40 (Fig. 2, D–F), VK4-116 (Fig. 2, G–I), and CP-809,101 (Fig. 2, J–L) shifted dose-response curves downward. BD1063 did not reliably alter dose-response curves for either MDPV or cocaine self-administration (Fig. 2, M–O). A larger dose of BD1063 (32 mg/kg) was tested in a subset of rats and produced disruptions in responding maintained by food, but not self-administration (data not shown).
Fig. 2.
Effects of pretreatments on self-administration dose-response curves rats that self-administered cocaine (A, D, G, J, M) or MDPV, separated by low- (B, E, H, K, N) and high-responder phenotype (C, F, I, L, O). A–C: pretreatments of the dopamine D2/D3/sigma receptor antagonist haloperidol (0.01–0.32 mg/kg). D–F: pretreatments of the dopamine D3 receptor partial agonist VK4-40 (3.2–32 mg/kg). G–I: pretreatments of the dopamine D3 receptor antagonist VK4-116 (3.2–32 mg/kg). J–L: pretreatments of the 5-HT2C receptor agonist CP-809,101 (0.32-5.6 mg/kg). M–O: pretreatments of the sigma receptor antagonist BD1063 (1–10 mg/kg). Ordinate: reinforcers obtained during each component. Abscissa: Food 1 and Food 2 refer to components when grain-based pellets were available. The numbers refer to dose of cocaine (left) and MDPV (center and right) expressed as mg/kg/infusion on a log scale. The shaded gray region represents mean ± S.E.M. responding under baseline conditions. Error bars represent ± S.E.M.
The effects of pretreatment drugs were further quantified by generating dose-response curves for changes in the AUC ([pretreatment AUC/baseline AUC]*100) (Fig. 3). Linear regression was used to estimate the dose required to decrease AUC by 50% (ID50; Table 1). Haloperidol produced significant, dose-dependent decreases in AUC in both male and female rats (males: F (3.31, 51.27) = 15.1; P < 0.001; females: F (2.21, 31.43) = 12.65; P < 0.0001); however, these effects did not vary as a function of sex or self-administration group. VK4-40 produced significant, dose-dependent decreases in AUC in both male and female rats (males: F (2.63, 40.36) = 5.83; P = 0.003; females: F (2.49, 37.27) = 7.32; P = 0.0011); however, did not differ as a function of self-administration group. VK4-116 produced significant, dose-dependent decreases in AUC for male, but not female rats (males: F (2.22, 44.44) = 14.58; P < 0.0001; females: F (1.90, 26.56) = 1.57; P = 0.23); these effects or lack thereof did not differ as a function of self-administration group. CP-809,101 produced significant, dose-dependent decreases in AUC in both male and female rats (males: F (3.16, 53.69) = 23.55; P < 0.0001; females: F (2.46, 39.34) = 18.63; P < 0.0001); however, there was no effect of self-administration group. BD1063 did not significantly alter AUCs in males or females, regardless of self-administration group (males: F (1.51, 19.13) = 0.48; P = 0.58; females: F (2.11, 38.76) = 0.22; P = 0.81).
Fig. 3.
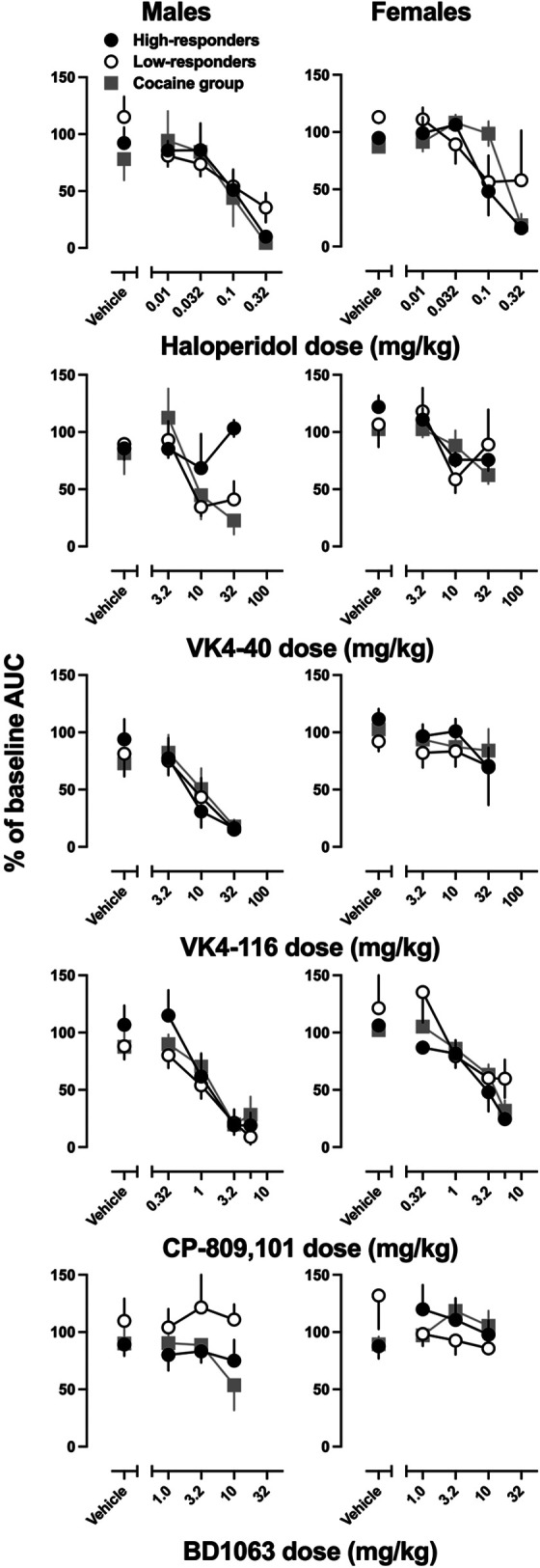
Percent of baseline area under the curve (AUC) for haloperidol (top row), VK4-40 (second row), VK4-116 (third row), CP-809,101 (fourth row), and BD1063 (bottom row) in male (left column) and female rats (right column). Ordinate: % of baseline AUC, where baseline in the three sessions prior to pretreatment administration. Abscissa: Vehicle refers to the vehicle for each pretreatment. The numbers refer to the dose of the pretreatment, expressed as mg/kg on a log scale. Error bars represent ± S.E.M.
TABLE 1.
ID50, as measured by percent change in area under the curve. Doses reported in mg/kg (95% confidence intervals).
| Haloperidol | VK4-40 | VK4-116 | CP-809,101 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug ID50 | Food ID50 | Potency ratio | Drug ID50 | Food ID50 | Potency ratio | Drug ID50 | Food ID50 | Potency ratio | Drug ID50 | Food ID50 | Potency ratio | |
| High-responders | ||||||||||||
| All | 0.08 (0.06, 0.12) |
0.07 (0.05, 0.10) |
0.85 (0.77, 0.93) |
30.6 (21.4, 43.8) |
6.6 (5.1, 8.5) |
0.21 (0.19, 0.24) |
17.3 (9.3, 32) |
7.3 (3.9, 13.6) |
0.42 (0.42, 0.42) |
1.61 (0.99, 2.63) |
1.19 (0.88, 1.60) |
0.73 (0.60, 0.88) |
| Male | 0.06 (0.03, 0.12) |
0.04 (0.04, 0.05) |
0.71 (0.42, 1.19) |
25.7 (12.8, 51.6) |
4.3 (3.8, 4.9) |
0.17 (0.10, 0.30) |
6.2 (3.2, 12.2) |
2.6 (1.9, 3.7) |
0.42 (0.30, 0.59) |
1.04 (0.47, 2.28) |
1.42 (0.88, 2.28) |
1.45 (0.94, 2.25) |
| Female | 0.11 (0.08, 0.15) |
0.10 (0.08, 0.13) |
0.90 (0.84, 0.96) |
34.6 (24.9, 48.2) |
8.1 (6.4, 10.2)* |
0.23 (0.21, 0.26) |
35.9 (23.1, 55.9) * |
14.3 (8.2, 24.8)* |
0.40 (0.36, 0.44) |
2.35 (1.50, 3.68) |
1.00 (0.75, 1.33) |
0.42 (0.38, 0.45) |
| Low-responders | ||||||||||||
| All | 0.08 (0.05, 0.14) |
0.07 (0.03, 0.12) |
0.78 (0.69, 0.88) |
12.4 (7.0, 22.0) |
6.2 (0.37, 10.4) |
0.50 (0.47, 0.53) |
13.5 (8.1, 22.5) |
10.4 (6.6, 16.4) |
0.77 (0.73, 0.82) |
1.78 (1.09, 2.91) |
0.95 (0.64, 1.41) |
0.53 (0.48, 0.58) |
| Male | 0.09 (0.05, 0.19) |
0.05 (0.02, 0.14) |
0.55 (0.40, 0.77) |
8.3 (4.4, 15.9) |
4.9 (2.3, 10.8) |
0.59 (0.52, 0.68) |
8.1 (5.1, 12.9) |
7.2 (4.0, 12.7) |
0.88 (0.70, 0.99) |
1.16 (0.74, 1.83) |
0.81 (0.50, 1.30) |
0.63 (0.57, 0.69) |
| Female | 0.07 (0.04, 0.12) |
0.09 (0.07, 0.12) |
1.32 (0.97, 1.79) |
27.5 (14.4, 52.2) |
8.3 (4.9, 13.9) |
0.30 (0.31, 0.38) |
27.6 (14.8, 51.7) * |
18.3 (16.0, 21.0) |
0.66 (0.41, 1.08) |
3.53 (1.72, 7.25) |
1.22 (0.66, 2.24) |
0.34 (0.31, 0.38) |
| Cocaine | ||||||||||||
| All | 0.12 (0.08, 0.19) |
0.06 (0.03, 0.10) |
0.48 (0.45, 0.51) |
17.5 (11.7, 26.3) |
4.4 (2.7, 7.2) |
0.25 (0.23, 0.27) |
16.4 (10.4, 25.9) |
10.1 (0.65, 15.6) |
0.61 (0.60, 0.63) |
2.54 (1.81, 3.57) |
0.90 (0.61, 1.35) |
0.33 (0.31, 0.35) |
| Male | 0.07 (0.03, 0.13) |
0.03 (0.01, 0.08) |
0.50 (0.40, 0.62) |
9.6 (6.3, 14.6) |
6.8 (5.3, 8.8) |
0.71 (0.60, 0.84) |
10.7 (6.7, 17.1) |
9.0 (0.59, 13.6) |
0.84 (0.80, 0.88) |
1.53 (1.02, 2.29) |
0.89 (0.40, 1.98) |
0.58 (0.39, 0.86) |
| Female | 0.20 (0.15, 0.26) * |
0.09 (0.08, 0.11) |
0.46 (0.41, 0.51) |
28.8 (21.7, 38.4) * |
3.2 (1.6, 6.3) |
0.11 (0.08, 0.16) |
27.4 (15.5, 48.6) |
11.6 (5.2, 25.8) |
0.42 (0.34, 0.53) |
3.94 (3.14, 4.93) * |
0.92 (0.63, 1.33) |
0.20 (0.19, 0.21) |
| All groups | ||||||||||||
| Male | 0.07 (0.05, 0.11) |
0.04 (0.03, 0.04) |
0.56 (0.32, 0.70) |
11.9 (7.7, 18.2) |
5.2 (3.6, 7.6) |
0.44 (0.42, 0.46) |
8.3 (6.0, 11.4) |
6.1 (4.2, 8.8) |
0.73 (0.70, 0.77) |
1.22 (0.88, 1.69) |
0.99 (0.69, 1.42) |
0.78 (0.73, 0.84) |
| Female | 0.12 (0.09, 0.16) |
0.09 (0.08, 0.11) |
0.78 (0.67, 0.90) |
30.7 (24.4, 38.8) * |
6.2 (4.4, 8.8) |
0.20 (0.18, 0.23) |
30.7 (22.4, 42.1) * |
14.2 (9.9, 20.4)* |
0.46 (0.44, 0.48) |
3.16 (2.38, 4.20) * |
1.01 (0.79, 1.29) |
0.30 (0.29, 0.31) |
* Indicates non-overlapping confidence intervals compared with male rats in the same group.
Comparisons of the ID50s across groups (Table 1) revealed that pretreatments were equipotent in high-responder rats compared with low-responder rats or rats that self-administered cocaine. However, significant differences in the ID50s of VK4-40, VK4-116, CP-809,101, and haloperidol were observed across sex, with larger doses of each compound required to reduce self-administration in female compared with male rats. For VK4-40, CP-809,101, and haloperidol, sex-related differences were observed with cocaine, whereas for VK4-116, sex-related differences were observed with both high- and low-responding rats from the MDPV conditions. For VK4-40, VK4-116, and CP-809101, significant sex-related differences were also observed when data were collapsed across the three self-administration groups (MDPV-high, MDPV-low, and cocaine).
The pretreatment dose required to decrease responding maintained by food pellets in the first component was calculated for each of the drugs (Table 1). Generally, similar doses were required to disrupt responding for food in male and female rats, with the exception that larger doses of VK4-40 and VK4-116 were needed to disrupt responding in MDPV high-responder females compared with high-responder males. Potency ratios (food ID50/drug ID50) were calculated to compare doses that decreased responding maintained by food compared with drug. With few exceptions (haloperidol in high-responder males and low-responder females and CP-809,101 in high-responder males), the pretreatments were more potent at decreasing responding for food compared with drug (Table 1).
Discussion
When allowed to self-administer the stimulant drug MDPV, a subset rats rapidly develop patterns of responding that result in significantly greater levels of drug-intake (high-responders) than low-responder rats self-administering MDPV or rats that self-administer cocaine. These high-responder rats exhibit behaviors thought to be consistent with stimulant use disorder in humans, including high levels of drug-taking and -seeking, and reach higher final ratios under progressive ratio schedules of reinforcement (Gannon et al., 2017; Doyle et al., 2021a,c; Abbott et al., 2022). The primary goal of this study was to evaluate the effects of several drugs that interact with receptors that have been targeted for the development of medications for stimulant use disorder to determine if their potency and effectiveness to reduce drug-taking differed as a function of: (1) the self-administered drug (MDPV versus cocaine), (2) the behavioral phenotype (high-responder versus low-responder), or (3) sex (male versus female). These studies were conducted with the ultimate goal of identifying pharmacotherapies capable of reducing aberrant patterns of drug-taking that may be more representative of the patterns of drug-taking observed in individuals with a stimulant use disorder. Pretreatment drugs included a partial agonist and an antagonist of dopamine D3 receptors (VK4-40 and VK4-116, respectively), a 5-HT2C receptor agonist (CP-809,101), a sigma1 receptor antagonist (BD1063), and a non-selective dopamine D2/D3/sigma1 receptor antagonist (haloperidol). Together, this study not only replicates previous literature showing that drugs targeting dopamine D3 and 5-HT2C receptors can reduce well-regulated stimulant self-administration but extends these basic findings by directly comparing the potency and effectiveness of a panel of dopamine D3 and 5-HT2C receptor ligands to reduce unusually high levels of MDPV self-administration and to reduce stimulant self-administration more generally in male and female rats. There were three main findings: (1) each pretreatment drug, except BD1063, produced dose dependent reductions in MDPV and cocaine self-administration; (2) all pretreatment drugs were equipotent at reducing MDPV self-administration in the high- and low-responding groups of rats; and (3) sex differences were observed, in which VK4-116, CP-809,101, and haloperidol were less potent at reducing cocaine self-administration in female compared with male rats, and VK4-116 was less potent at reducing MDPV self-administration in female high- and low-responders relative to male high- and low-responders; for VK4-40, VK4-116, and CP-809,101 sex-related differences were also observed when data were collapsed across MDPV and cocaine.
As expected, all test compounds (except the sigma receptor antagonist BD1063) dose-dependently decreased self-administration in all groups. These findings are consistent with previous reports suggesting that dopamine D3 receptors and 5-HT2C receptors may be viable targets for treating stimulant use disorders (Higgins et al., 2013; Pockros-Burgess et al., 2014; Collins et al., 2016; Gerak et al., 2016; Harvey-Lewis et al., 2016; Collins and France, 2018; You et al., 2019; Jordan et al., 2019a, 2020; Anastasio et al., 2020; de Guglielmo et al., 2020; for reviews, see: Howell and Cunningham, 2015; Collins et al., 2018; Newman et al., 2021), as well as previous findings with haloperidol (De Vry et al., 1989; Roberts et al., 1989; Oleson et al., 2011). VK4-116 was previously shown to be equipotent at reducing drug-taking in male and female rats, but more effective in rats with high, but not low, levels of opioid self-administration (de Guglielmo et al., 2020). Although these findings suggested that VK4-116 might be more potent and/or effective at reducing MDPV self-administration in high- relative to low-responding rats, in the current studies, VK4-116 (and VK4-40) were equipotent at reducing drug-taking in rats with high and low levels of intake, but less potent in female rats compared with male rats. VK4-40 was also less effective at reducing stimulant self-administration in female as compared with male rats, although these evaluations were somewhat limited by issues with solubility, and the sex-related outcomes were not always consistent across self-administered drug. To our knowledge, this is the first time that the effects of VK4-40 on drug self-administration have been evaluated in female rats, so it is unclear whether these differences are specific to stimulants, or whether similar sex-related differences would also be observed with drugs from other classes (e.g., alcohol, nicotine).
Similar to the effects of the dopamine D3 receptor ligands, the 5-HT2C receptor agonist, CP-809,101, dose-dependently decreased self-administration in all rats, regardless of drug, or behavioral phenotype. CP-809,101 was also less potent at reducing stimulant self-administration in female compared with male rats, although these differences were larger for cocaine relative to MDPV self-administration. Sex-related differences in the potency of 5-HT2C receptor agonists to reduce cocaine have been reported (Collins and France, 2018); however, those studies showed that lorcaserin, a 5-HT2C receptor agonist, was more potent in female compared with male rhesus monkeys. The reason(s) underlying this discrepancy in the potential sex-related effects of 5-HT2C receptor agonists might be related to species or procedural differences, but warrants further exploration.
When comparing the effects of the pretreatment drugs on responding maintained by food and drug, all test compounds tended to be more potent at reducing responding reinforced by food compared with drug. This is consistent with the non-selective effect of dopamine D3 and 5-HT2C drugs that have been reported in studies using food-versus-drug choice procedures (John et al., 2015; Banks and Negus, 2017; Thomsen et al., 2017; Townsend et al., 2020). The lack of selectivity in the current study may have also been because the rats were not food restricted, the choice of reinforcer, and/or the choice of test compounds. Greater selectivity for decreasing responding for drug over food might be observed if a different motivational state (e.g., food restriction) or a more palatable food reinforcer, such as sucrose pellets, was used instead. Given that the 5-HT2C agonist lorcaserin was approved by the United States Food and Drug Administration to treat obesity, including assessments of responding maintained by non-food reinforcers (e.g., social interaction) is important when drawing conclusions about the selectivity (or lack thereof) of the effects of these compounds to decrease drug-taking. It should also be noted that the components when food was available were shorter than those when drug was available, which could have contributed to the lower number of food reinforcers earned. However, the decision to limit the food components to 10 minutes was based on preliminary data that suggested rats only responded for the first 5–10 minutes of a 20-minute component, which speaks to the weak reinforcing effects of grain-based pellets in these subjects.
Traditional, short-access stimulant self-administration sessions result in stable levels of drug intake that likely do not capture the problematic use in people with stimulant use disorder. Although no single animal model can capture the multi-symptomatic complexities of stimulant use disorder, high-responder rats exhibit several behaviors thought to be related to stimulant use disorder, even when self-administering drug under short-access conditions. Thus, we chose to directly compare the effects of various candidate medications in high-responder rats, and rats with more regulated drug-taking (i.e., low-responders, rats self-administering cocaine), to determine whether the candidate medications are effective in rats with dysregulated behavior. All the compounds that reduced drug-taking in rats with well-regulated behavior also reduced drug-taking in rats with a more robust stimulant use disorder-like phenotype. Others have employed similar strategies to evaluate candidate medications, such as comparing short- or extended-access procedures, in which the latter procedure was used to model a more robust stimulant use disorder-like phenotype (e.g., Ramôa et al., 2014; Lynch et al., 2021). Another strategy to evaluate candidate medications in a choice procedure in which a drug and an alternative reinforcer (e.g., food, social partner) are available simultaneously to provide a more real-world scenario that is sensitive to selective reductions in drug-taking while also allowing for detection of a general rate decreasing effect (for review, see Banks and Negus, 2017). Candidate medications (e.g., buspirone, lorcaserin) have successfully reduced drug-taking in a subset of preclinical procedures in the past (e.g., Bergman et al., 2013; Czoty and Nader, 2015; Collins et al., 2016; Harvey-Lewis et al., 2016; Neelakantan et al., 2017; Collins and France, 2018), but have been unsuccessful in clinical trials, which suggests alternative procedures for preclinical evaluation are necessary. Perhaps combining models, such as the high-responder, substance use disorder-like phenotype, with a choice procedure would increase the probability of identifying effective candidate medications while also reducing the likelihood of false positives. Although more sophisticated models are needed, identification of the core features of these models is complicated by absence of a positive control (i.e., there are no United States Food and Drug Administration-approved pharmacotherapies for stimulant disorder), and it is likely that convergent evidence obtained from multiple, complementary procedures will be the most fruitful path forward (e.g., Venniro et al., 2020).
In summary, these studies provide additional support for the effectiveness of dopamine D3 receptor antagonists/partial agonists and 5-HT2C receptor agonists to dose-dependently reduce stimulant self-administration, including in subjects that exhibit aberrant patterns of drug-taking similar to those observed in humans with a stimulant use disorder. Although one of these targets (i.e., the dopamine D3 receptor) was included in the National Institute on Drug Abuse’s Division of Therapeutics and Medical Consequences’ top ten pharmacological targets for the treatment of opioid use disorder (Rasmussen et al., 2019), many of the other targets also show promise for treating stimulant use disorders. Indeed, preclinical data suggest that orexin receptors, muscarinic M5 receptors, and metabotropic glutamate receptors all have the potential to yield broad spectrum pharmacotherapies for substance use disorders more generally and should be evaluated in a similar manner to determine whether other pharmacological mechanisms are also effective at decreasing drug-taking in subjects exhibiting a substance use disorder-like phenotype, such as the MDPV high-responder rats. By continuing to evaluate candidate medications in animal models that incorporate various aspects of the human condition it is possible to not only gain important new information about the factors underlying stimulant use disorders, but also advance efforts to develop novel and effective therapies to assist those with a stimulant use disorder.
Acknowledgments
The authors would like to thank Ayon Bhattacharya for his technical support.
Abbreviations
- 5-HT
5-hydroxytryptophan/serotonin
- AUC
area under the curve
- FR
fixed ratio
- MDPV
4-methylenedioxypyrovalerone
- TO
timeout
Authorship Contributions
Participated in research design: Doyle, Collins.
Conducted experiments: Doyle, Peng.
Contributed new reagents or analytic tools: Cao, Rice, Newman.
Performed data analysis: Doyle, Peng.
Wrote or contributed to the writing of the manuscript: Doyle, Newman, Collins.
Footnotes
This work was supported by the National Institutes of Health: National Institute of Drug Abuse [Grants R01-DA039146 and R36-DA050955], the jointly-sponsored National Institutes of Health Predoctoral Training Program in the Neurosciences [Grant T32-NS082145], and the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism NIDA Intramural Research Program [Grants Z1A-DA000424 and Z1A-DA000527].
No author has an actual or perceived conflict of interest with the contents of this article.
1Current affiliation: Department of Psychiatry, University of California San Diego, La Jolla, CA, USA and The Scripps Research Institute, La Jolla, CA, USA.
Part of this work was presented as a poster presentation at the 2021 Experimental Biology/ASPET Annual Meeting as Doyle MR, Bhattacharya A, Rice KC, Collins GT (2021) MDPV high-responder phenotype as a tool to evaluate candidate medications for stimulant use disorder.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Abbott MS, Seaman RW Jr, Doyle MR, Maguire DR, Rice KC, Collins GT (2022) Interactions between impulsivity and MDPV self-administration in rats. Addict Biol 27:e13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Sholler DJ, Fox RG, Stutz SJ, Merritt CR, Bjork JM, Moeller FG, Cunningham KA (2020) Suppression of cocaine relapse-like behaviors upon pimavanserin and lorcaserin co-administration. Neuropharmacology 168:108009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS (2017) Insights from Preclinical Choice Models on Treating Drug Addiction. Trends Pharmacol Sci 38:181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB (2004) Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology 47 (Suppl 1):256–273. [DOI] [PubMed] [Google Scholar]
- Bergman J, Roof RA, Furman CA, Conroy JL, Mello NK, Sibley DR, Skolnick P (2013) Modification of cocaine self-administration by buspirone (buspar®): potential involvement of D3 and D4 dopamine receptors. Int J Neuropsychopharmacol 16:445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair DT, Dauner A (1992) Extrapyramidal symptoms are serious side-effects of antipsychotic and other drugs. Nurse Pract 17:56–67, 62–64, 67. [DOI] [PubMed] [Google Scholar]
- Collins GT, Cunningham AR, Chen J, Wang S, Newman AH, Woods JH (2012a) Effects of pramipexole on the reinforcing effectiveness of stimuli that were previously paired with cocaine reinforcement in rats. Psychopharmacology (Berl) 219:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, France CP (2018) Effects of lorcaserin and buspirone, administered alone and as a mixture, on cocaine self-administration in male and female rhesus monkeys. Exp Clin Psychopharmacol 26:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Gerak LR, France CP (2018) The behavioral pharmacology and therapeutic potential of lorcaserin for substance use disorders. Neuropharmacology 142:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Gerak LR, Javors MA, France CP (2016) Lorcaserin Reduces the Discriminative Stimulus and Reinforcing Effects of Cocaine in Rhesus Monkeys. J Pharmacol Exp Ther 356:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Narasimhan D, Cunningham AR, Zaks ME, Nichols J, Ko MC, Sunahara RK, Woods JH (2012b) Long-lasting effects of a PEGylated mutant cocaine esterase (CocE) on the reinforcing and discriminative stimulus effects of cocaine in rats. Neuropsychopharmacology 37:1092–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Nader MA (2015) Effects of oral and intravenous administration of buspirone on food-cocaine choice in socially housed male cynomolgus monkeys. Neuropsychopharmacology 40:1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Sedighim S, Newman AH, George O (2020) Dopamine D3 Receptor Antagonism Reverses the Escalation of Oxycodone Self-Administration and Decreases Withdrawal-Induced Hyperalgesia and Irritability-Like Behavior in Oxycodone-Dependent Heterogeneous Stock Rats. Front Behav Neurosci 13:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vry J, Donselaar I, Van Ree JM (1989) Food deprivation and acquisition of intravenous cocaine self-administration in rats: effect of naltrexone and haloperidol. J Pharmacol Exp Ther 251:735–740. [PubMed] [Google Scholar]
- Doyle MR, Sulima A, Rice KC, Collins GT (2021a) Influence of Contingent and Noncontingent Drug Histories on the Development of High Levels of MDPV Self-Administration. J Pharmacol Exp Ther 379:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MR, Sulima A, Rice KC, Collins GT (2021b) Interactions between reinforcement history and drug-primed reinstatement: Studies with MDPV and mixtures of MDPV and caffeine. Addict Biol 26:e12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MR, Sulima A, Rice KC, Collins GT (2021c) MDPV self-administration in female rats: influence of reinforcement history. Psychopharmacology (Berl) 238:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A (2013) Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol 85:1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Baumann MH, Walther D, Jimenez-Morigosa C, Sulima A, Rice KC, Collins GT (2018a) The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology 43:2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Rice KC, Collins GT (2017) Individual Differences in the Relative Reinforcing Effects of 3, 4-Methylenedioxypyrovalerone under Fixed and Progressive Ratio Schedules of Reinforcement in Rats. J Pharmacol Exp Ther 361:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Rice KC, Murnane KS (2021) MDPV “high-responder” rats also self-administer more oxycodone than their “low-responder” counterparts under a fixed ratio schedule of reinforcement. Psychopharmacology (Berl) 238:1183–1192. [DOI] [PubMed] [Google Scholar]
- Gannon BM, Sulima A, Rice KC, Collins GT (2018b) Inhibition of Cocaine and 3,4-Methylenedioxypyrovalerone (MDPV) Self-Administration by Lorcaserin is Mediated by 5-HT2C Receptors in Rats. J Pharmacol Exp Ther 364:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Collins GT, France CP (2016) Effects of Lorcaserin on Cocaine and Methamphetamine Self-Administration and Reinstatement of Responding Previously Maintained by Cocaine in Rhesus Monkeys. J Pharmacol Exp Ther 359:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Collins GT, Maguire DR, France CP (2019) Effects of lorcaserin on reinstatement of responding previously maintained by cocaine or remifentanil in rhesus monkeys. Exp Clin Psychopharmacol 27:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, Ashby CR Jr, Heidbreder CA, Pak AC, Peng XQ, Xi ZX (2005) Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse 57:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey-Lewis C, Li Z, Higgins GA, Fletcher PJ (2016) The 5-HT(2C) receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology 101:237–245. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Lau W, de Lannoy IAM, Lee DKH, Izhakova J, Coen K, Le AD, Fletcher PJ (2013) Evaluation of chemically diverse 5-HT2c receptor agonists on behaviours motivated by food and nicotine and on side effect profiles. Psychopharmacology (Berl) 226:475–490. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Patrick A, De Lannoy IAM, Fletcher PJ, Parker LA, MacLusky NJ, Sullivan LC, Chavera TA, Berg KA (2017) Studies To Examine Potential Tolerability Differences between the 5-HT2C Receptor Selective Agonists Lorcaserin and CP-809101. ACS Chem Neurosci 8:1074–1084. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Mereu M, Soto PL, Tanda G, Katz JL (2013) Self-administration of cocaine induces dopamine-independent self-administration of sigma agonists. Neuropsychopharmacology 38:605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Cunningham KA (2015) Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev 67:176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, Banala AK, Newman AH, Nader MA (2015) Effects of buspirone and the dopamine D3 receptor compound PG619 on cocaine and methamphetamine self-administration in rhesus monkeys using a food-drug choice paradigm. Psychopharmacology (Berl) 232:1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, He Y, Bi GH, You ZB, Cao J, Xi ZX, Newman AH (2020) (±)VK4-40, a novel dopamine D3 receptor partial agonist, attenuates cocaine reward and relapse in rodents. Br J Pharmacol 177:4796–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Humburg B, Rice M, Bi GH, You ZB, Shaik AB, Cao J, Bonifazi A, Gadiano A, Rais R, et al. (2019a) The highly selective dopamine D3R antagonist, R-VK4-40 attenuates oxycodone reward and augments analgesia in rodents. Neuropharmacology 158:107597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Humburg BA, Thorndike EB, Shaik AB, Xi ZX, Baumann MH, Newman AH, Schindler CW (2019b) Newly Developed Dopamine D3 Receptor Antagonists, R-VK4-40 and R-VK4-116, Do Not Potentiate Cardiovascular Effects of Cocaine or Oxycodone in Rats. J Pharmacol Exp Ther 371:602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Hong WC, Hiranita T, Su T-PP (2016) A role for sigma receptors in stimulant self-administration and addiction. Behav Pharmacol 27:100–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck TM, John WS, Czoty PW, Nader MA, Newman AH (2015) Identifying Medication Targets for Psychostimulant Addiction: Unraveling the Dopamine D3 receptor Hypothesis. J Med Chem 58:5361–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Bonifazi A, Ellenberger MP, Keck TM, Pommier E, Rais R, Slusher BS, Gardner E, You ZB, Xi ZX, et al. (2016) Highly Selective Dopamine D3 Receptor (D3R) Antagonists and Partial Agonists Based on Eticlopride and the D3R Crystal Structure: New Leads for Opioid Dependence Treatment. J Med Chem 59:7634–7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ (2018) Modeling the development of drug addiction in male and female animals. Pharmacol Biochem Behav 164:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Bakhti-Suroosh A, Abel JM, Davis C (2021) Shifts in the neurobiological mechanisms motivating cocaine use with the development of an addiction-like phenotype in male rats. Psychopharmacology (Berl) 238:811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals, 8th ed, National Academy Press, Washington, DC. [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M, Anastasio NC, Moeller FG, Cunningham KA (2017) Lorcaserin Suppresses Oxycodone Self-Administration and Relapse Vulnerability in Rats. ACS Chem Neurosci 8:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Ku T, Jordan CJ, Bonifazi A, Xi ZX (2021) New Drugs, Old Targets: Tweaking the Dopamine System to Treat Psychostimulant Use Disorders. Annu Rev Pharmacol Toxicol 61:609–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Richardson JM, Roberts DCS (2011) A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology (Berl) 214:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P (1999) Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature 400:371–375. [DOI] [PubMed] [Google Scholar]
- Pockros-Burgess LA, Pentkowski NS, Der-Ghazarian T, Neisewander JL (2014) Effects of the 5-HT2C receptor agonist CP809101 in the amygdala on reinstatement of cocaine-seeking behavior and anxiety-like behavior. Int J Neuropsychopharmacol 17:1751–1762. [DOI] [PubMed] [Google Scholar]
- Ramôa CP, Doyle SE, Lycas MD, Chernau AK, Lynch WJ (2014) Diminished role of dopamine D1-receptor signaling with the development of an addicted phenotype in rats. Biol Psychiatry 76:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, White DA, Acri JB (2019) NIDA’s medication development priorities in response to the Opioid Crisis: ten most wanted. Neuropsychopharmacology 44:657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Loh EA, Vickers G (1989) Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 97:535–538. [DOI] [PubMed] [Google Scholar]
- Serafine KM, Rice KC, France CP (2015) Directly Observable Behavioral Effects of Lorcaserin in Rats. J Pharmacol Exp Ther 355:381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Schoedel KA, Bartlett C, Shazer RL, Anderson CM, Sellers EM (2011) Evaluation of the abuse potential of lorcaserin, a serotonin 2C (5-HT2C) receptor agonist, in recreational polydrug users. Clin Pharmacol Ther 89:683–692. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P, Marala R, Patterson T, Seymour PA, Swick A, et al. (2007) CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology 52:279–290. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Butler P, Negus SS, Caine SB (2017) Effects of Acute and Chronic Treatments with Dopamine D2 and D3 Receptor Ligands on Cocaine Versus Food Choice in Rats. J Pharmacol Exp Ther 362:161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Poklis JL, Banks ML (2020) Lorcaserin maintenance fails to attenuate heroin vs. food choice in rhesus monkeys. Drug Alcohol Depend 208:107848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Banks ML, Heilig M, Epstein DH, Shaham Y (2020) Improving translation of animal models of addiction and relapse by reverse translation. Nat Rev Neurosci 21:625–643. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Tateishi T, Asoh M, Nakura H, Tanaka M, Kumai T, Kobayashi S (1999) Role of CYP3A in haloperidol N-dealkylation and pharmacokinetics in rats. Fundam Clin Pharmacol 13:337–342. [DOI] [PubMed] [Google Scholar]
- You ZB, Bi GH, Galaj E, Kumar V, Cao J, Gadiano A, Rais R, Slusher BS, Gardner EL, Xi ZX, et al. (2019) Dopamine D3R antagonist VK4-116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects. Neuropsychopharmacology 44:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]