The glutamate modulator, riluzole, extends survival in amyotrophic lateral sclerosis (ALS) and was recently assessed in a clinical trial for Alzheimer’s disease (Ad).1 This trial found that riluzole-treated patients with Ad had a significantly reduced decline of cerebral glucose metabolism in multiple Ad predilection brain regions compared to placebo, which correlated with cognitive performance.1 Importantly however, assessment of amyloid load was not included in the trial and as such, the effect of riluzole treatment on Ad neuropathological changes in patients remains to be clarified. Given that riluzole is a first-line treatment for ALS,2 which is a TDP-43 proteinopathy with concomitant Ad neuropathological changes in a subset of patients, the present study set out to assess the relationship between riluzole and Ad neuropathological changes in a post-mortem series of ALS cases.
All cases with a pathological confirmation of clinically diagnosed ALS and that had complete clinical records including riluzole treatment were selected from a neuropathological series collected by the Sydney Brain Bank through regional brain donor programs in Sydney, Australia. The brain donor programs hold approvals from the Human Research Ethics Committees of the University of Sydney and Macquarie University, and the Sydney Brain Bank holds approval from the University of New South Wales. All studies comply with the statement on human experimentation issued by the National Health and Medical Research Council of Australia. Patients were diagnosed during life by experienced clinicians using standard clinical diagnostic criteria following a medical interview and informant history. Standardized neuropathological characterization was performed on all cases including, but not confined to assessment of TDP-43 proteinopathy and Alzheimer’s pathology.3,4 Thirty-seven cases met these inclusion criteria. The total dose of riluzole taken was calculated by multiplying the daily prescription dosage by the number of days on this dosage. All cases had previously been assessed for genetic mutations in the C9orf72, TARDBP and SOD1 genes and a C9orf72 expansion was identified in eight cases. No other mutation was found. This research project was approved by the Human Research Ethics Committee of the University of Sydney. All ALS cases were screened according to current standardized neuropathological consensus recommendations and staged for topographical progression of TDP-43 pathology3 using an antibody to phosphorylated TDP-43 (1:80 000, TDP, TDP-MO1, Cosmo Bio). The severity of TDP-43 pathology in the motor cortex and entorhinal cortex was graded semi-quantitatively using a 4-point severity scale: 0 = no detectable pathology, 1 = mild, 2 = moderate, 3 = severe, as previously described.5 Ad neuropathological change was assessed according to the National Institute of Ageing and Alzheimer's Disease Association Recommendations which includes an amyloid-β plaque (A) score, Braak neurofibrillary tangle (B) score and neuritic plaque (C) score.4 Statistical analysis was performed using SPSS (Version 25) with a P-value <0.05 taken as significant. Data were assessed for normality of distribution using Kolmogorov-Smirnov tests. Demographic variables showing parametric distribution (age at death and onset, post-mortem delay) were analysed using univariate ANOVA. Non-parametric data (disease duration, pathological measures) were analysed using Kruskall-Wallis tests with post hoc Mann–Whitney U-tests and chi-square (sex, genetic mutations, pathological prevalence). Spearman rank correlations were used to identify any associations between the total dose of riluzole treatment with demographic features and pathological measures.
All ALS cases included in this study had received riluzole. Amyloid-β plaques were identified in 54% (n = 20), neurofibrillary tangles in 51% (n = 19) and neuritic plaques in 30% (n = 11) of cases (Table 1). No significant difference in the presence or stage of plaques and tangles were identified between cases with (22%, n = 8) and without (88%, n = 29) a C9orf72 expansion (P > 0.1 for all). Pathological TDP-43 was observed in all ALS cases and no significant difference in the stage of ALS-TDP nor in regional severities were identified between cases with and without a C9orf72 expansion (P > 0.1 for all). Increasing total dose of riluzole was significantly associated with decreasing severity of neuritic plaques as measured by the neuritic plaque (C) score (r > −0.35; P < 0.04, n = 37) (Fig. 1). Increasing ALS-TDP stage was significantly associated with increasing severity of pathological TDP-43 in the entorhinal cortex (r = 0.68, P < 0.001, n = 37), decreasing amyloid-β plaque (A) score (r = −0.34, P < 0.04, n = 37) and neuritic plaque (C) score (r = −0.44, P = 0.006, n = 37). As expected, a significant positive correlation was observed between age at death and amyloid-β plaque (A) score, neurofibrillary tangle (B) score and neuritic plaque (C) scores (r ≥ 0.4, P < 0.02, n = 37 for all). No significant association was observed between age at death with total riluzole dose, regional severity of TDP-43 pathology or ALS-TDP stage (P > 0.1 n = 37 for all).
Table 1.
Demographic, genetic, riluzole and pathological characteristics of ALS cases
| ALS | |
|---|---|
| n (male/female) | 37 (21/16) |
| Mean age at death (years) | 65 ± 2 |
| Mean disease duration (years) | 2.7 ± 0.3 |
| Mean postmortem delay (h) | 29 ± 2 |
| C9ORF72 expansion prevalence (%) | 22 |
| Mean riluzole treatment duration (days) | 881 ± 97 |
| Daily riluzole dosage prevalence (50 mg/100 mg/200 mg) | 1/32/4 |
| Mean riluzole total dose (mg) | 96 289 ± 12 069 |
| Amyloid-β plaque score prevalence (A0/A1/A2/A3)4 | 17/8/9/3 |
| Neurofibrillary tangle score prevalence (B0/B1/B2/B3)4 | 18/14/4/1 |
| Neuritic plaque score prevalence (C0/C1/C2/C3)4 | 26/5/5/1 |
| Ad probability (ABC) prevalence (not/low/intermediate/high) | 17/17/3/0 |
| ALS-TDP stage prevalence (1/2/3/4)3 | 5/9/3/20 |
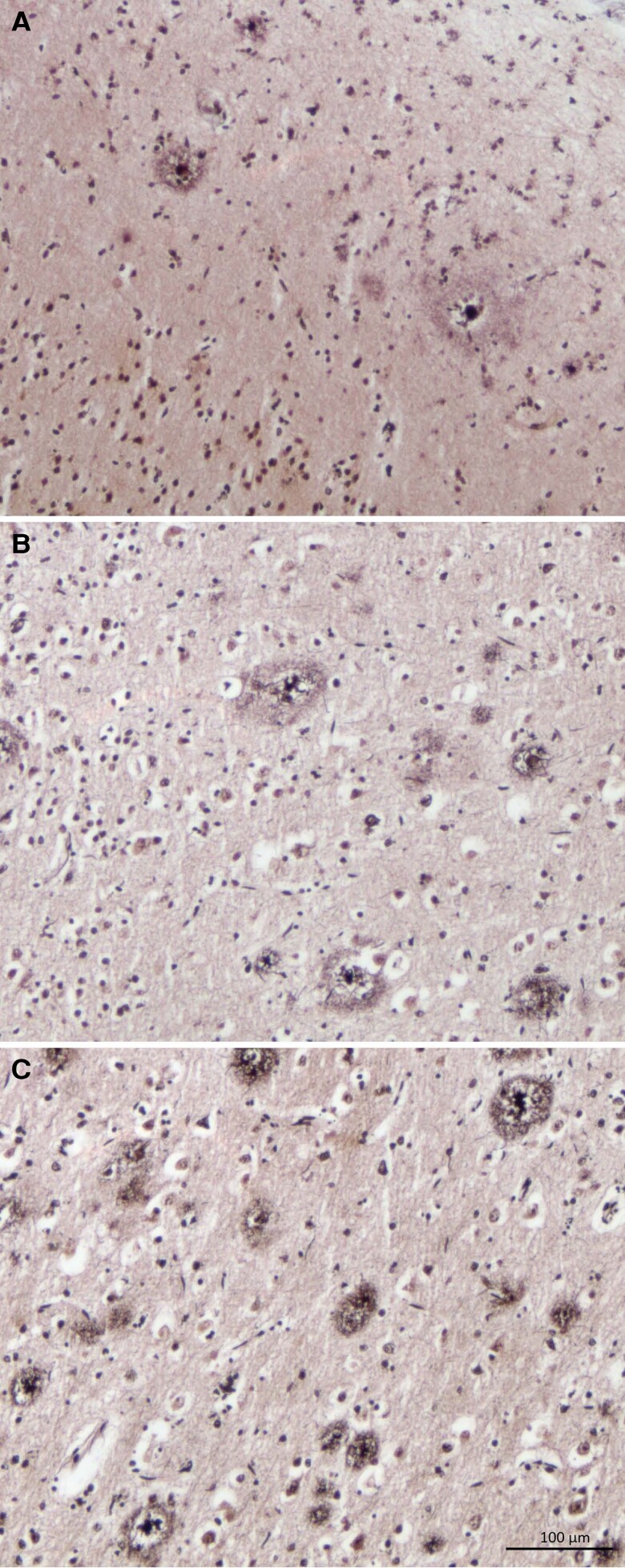
Figure 1.
Neuritic plaques in ALS. The severity of neuritic plaques were graded according to the neuritic plaque score of (A) C1: sparse (1–5 neuritic plaques per mm2); (B) C2: moderate (≥6 neuritic plaques per mm2); and (C) C3: frequent (≥20 neuritic plaques per mm2).
The present study assessed the relationship between riluzole treatment and Ad neuropathological changes in a series of ALS-TDP cases. Our results identified a significant negative correlation between total riluzole dose and severity of neuritic plaques, as graded by the neuritic plaque (C) score. In contrast to the amyloid-β (A) score, which incorporates both diffuse and neuritic plaques, the C score evaluates the severity of neuritic plaques only, which has been found to be more closely associated with neuronal loss and cognitive decline in Ad.6 The present findings of a significant negative correlation between riluzole and neuritic plaques suggest that the incidence of Ad in ALS may not be solely attributable to age but may also relate to riluzole treatment.
The higher prevalence of TDP-43 pathology in Ad than in tauopathies suggest a synergistic interaction between amyloid-β plaques and TDP-43 deposition, which statistical modelling in large community-based autopsy cohorts corroborate.7 In contrast to this however, the present study found increasing ALS-TDP stage was associated with decreasing amyloid-β (A) and neuritic plaque (C) scores, underscoring how the relationship between amyloid-β plaques and TDP-43 deposition is not well understood. Consistent with the present findings, lower levels of neuritic plaques have been reported in Ad cohorts with neocortical TDP-43 pathology.8 Importantly, most of these studies have focused on limbic-predominant age-related TDP-43 encephalopathy (LATE)9 which has a distinct topographical progression from ALS and may represent a distinct TDP-43 molecular entity.10 No significant associations were identified between regional severities of plaques and TDP-43 pathology in the present series highlighting the need for future studies seeking to understand how these pathologies evolve and propagate over the disease course.
In summary, the significant relationship identified between riluzole treatment and neuritic plaques in the present study converges with the slower decline in cerebral glucose metabolism recently reported in riluzole-treated patients with Ad.1 These findings corroborate the need for further studies and trials seeking to evaluate riluzole as a potential therapy for Ad.
Ethics approval
This research project was approved by the Human Research Ethics Committees of the Universities of Sydney and New South Wales and complies with the statement on human experimentation issued by the National Health and Medical Research Council of Australia. Tissues were selected from a neuropathological series collected by the Sydney Brain Banks through regional brain donor programs in Sydney, Australia. The brain donor programs hold approval from the Human Research Ethics Committees of the University of New South Wales and comply with the statement on human experimentation issued by the National Health and Medical Research Council of Australia.
Acknowledgements
The authors thank the participating patients and families. Tissues were received from the Sydney Brain Bank at Neuroscience Research Australia which is supported by Neuroscience Research Australia. Results of genetic testing were obtained from the Australian Motor Neurone Disease DNA Bank, supported by an Australian NHMRC Enabling Grant. Clinical data from participants of the ForeFront MND clinic at Sydney University and the Neurodegenerative Disease Biobank at Macquarie University was used in this research.
Contributor Information
Srestha Mazumder, Brain and Mind Centre, University of Sydney, Sydney, NSW 2050, Australia.
Heather McCann, Neuroscience Research Australia, Randwick, NSW 2031, Australia.
Susan D’Silva, Faculty of Medicine, Health and Human Sciences, Macquarie University Centre for Motor Neuron Disease Research, Macquarie University, Sydney, NSW 2109, Australia.
Sarah Furlong, Faculty of Medicine, Health and Human Sciences, Macquarie University Centre for Motor Neuron Disease Research, Macquarie University, Sydney, NSW 2109, Australia.
Claire E Shepherd, Neuroscience Research Australia, Randwick, NSW 2031, Australia.
Jillian J Kril, Faculty of Medicine and Health, School of Medical Sciences, University of Sydney, Camperdown, NSW 2006, Australia; Dementia Research Centre, Macquarie Medical School, Macquarie University, Sydney, NSW 2109, Australia.
Glenda M Halliday, Brain and Mind Centre, University of Sydney, Sydney, NSW 2050, Australia; Faculty of Medicine and Health, School of Medical Sciences, University of Sydney, Camperdown, NSW 2006, Australia.
Dominic B Rowe, Faculty of Medicine, Health and Human Sciences, Macquarie University Centre for Motor Neuron Disease Research, Macquarie University, Sydney, NSW 2109, Australia.
Matthew C Kiernan, Brain and Mind Centre, University of Sydney, Sydney, NSW 2050, Australia; Institute of Clinical Neurosciences, Royal Prince Alfred Hospital, Sydney, NSW 2050, Australia.
Rachel H Tan, Brain and Mind Centre, University of Sydney, Sydney, NSW 2050, Australia; Faculty of Medicine and Health, School of Medical Sciences, University of Sydney, Camperdown, NSW 2006, Australia.
Data availability
Anonymized data will be shared by request from a qualified academic investigator for the sole purpose of replicating results presented in this article.
Funding
This work was supported by funding to ForeFront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease, from the National Health and Medical Research Council (NHMRC) (1132524, 1095127, 1060992). M.C.K. is supported by an NHMRC Practitioner Fellowship (1156093). G.M.H. is supported by an NHMRC Senior Leadership Fellowship (1176607). R.H.T is supported by a FightMND Mid-Career Research Fellowship.
Competing interests
The authors report no competing interests.
References
- 1. Matthews DC, Mao X, Dowd K, et al. Riluzole, a glutamate modulator, slows cerebral glucose metabolism decline in patients with Alzheimer's disease. Brain. 2021;144:3742–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vucic S, Lin CSY, Cheah BC, et al. Riluzole exerts central and peripheral modulating effects in amyotrophic lateral sclerosis. Brain. 2013;136:1361–1370. [DOI] [PubMed] [Google Scholar]
- 3. Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74:20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montine TJ, Phelps CH, Beach TG, et al. National institute on aging-Alzheimer's association guidelines for the neuropathologic assessment of Alzheimer's disease: A practical approach. Acta Neuropathol. 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan RH, Kril JJ, Fatima M, et al. TDP-43 proteinopathies: Pathological identification of brain regions differentiating clinical phenotypes. Brain. 2015;138:3110–3122. [DOI] [PubMed] [Google Scholar]
- 6. Malek-Ahmadi M, Perez SE, Chen K, et al. Neuritic and diffuse plaque associations with memory in non-cognitively impaired elderly. J Alzheimers Dis. 2016;53:1641–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Power MC, Mormino E, Soldan A, et al. Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol. 2018;84:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katsumata Y, Abner EL, Karanth S, et al. Distinct clinicopathologic clusters of persons with TDP-43 proteinopathy. Acta Neuropathol. 2020;140:659–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain. 2019;142:1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tome SO, Vandenberghe R, Ospitalieri S, et al. Distinct molecular patterns of TDP-43 pathology in Alzheimer's disease: Relationship with clinical phenotypes. Acta Neuropathol Commun. 2020;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from a qualified academic investigator for the sole purpose of replicating results presented in this article.