Abstract
Alzheimer's disease is a neurodegenerative disorder in which the pathological accumulation of amyloid-β and tau begins years before symptom onset. Emerging evidence suggests that β-blockers (β-adrenergic antagonists) increase brain clearance of these metabolites by enhancing CSF flow. Our objective was to determine whether β-blocker treatments that easily cross the blood–brain barrier reduce the risk of Alzheimer's disease compared to less permeable β-blockers.
Data from the Danish national registers were used to identify a retrospective cohort of individuals with hypertension, and those treated with β-blockers were included in the analysis. People with indications for β-blocker use other than hypertension (e.g. heart failure) were only retained in a sensitivity analysis. β-blockers were divided into three permeability groups: low, moderate and high. We used multivariable cause-specific Cox regression to model the effect of β-blocker blood–brain barrier permeability on time to dementia outcomes, adjusting for baseline comorbidities, demographics and socioeconomic variables. Death was modelled as a competing risk. The 10-year standardized absolute risk was estimated as the averaged person-specific risks per treatment.
In a cohort of 69 081 (median age = 64.4 years, 64.8% female) people treated with β-blockers for hypertension, highly blood–brain barrier-permeable β-blockers were associated with reduced risk of Alzheimer's disease versus low permeability β-blockers (−0.45%, P < 0.036). This effect was specific to Alzheimer's diagnoses and did not extend to dementia in general. Propensity score analysis matching high and low blood–brain barrier-permeable patients also detected a decreased Alzheimer's risk (−0.92%, P < 0.001) in the high permeability group compared to the low, as did a 1-year landmark analysis (−0.57%, P < 0.029) in which events within the first year of follow-up were ignored as likely unrelated to treatment.
Our results suggest that amongst people taking β-blockers for hypertension, treatment with highly blood–brain barrier permeable β-blockers reduces the risk of Alzheimer's disease compared to low permeability drugs. Our findings support the hypothesis that highly permeable β-blockers protect against Alzheimer's disease by promoting waste brain metabolite clearance.
Keywords: Alzheimer's disease, antihypertensive drugs, adrenergic antagonists, glymphatic clearance, blood–brain barrier
Beaman et al. report that among people taking β-blockers for hypertension, treatment with highly blood–brain barrier permeable β-blockers reduces the risk of Alzheimer's disease compared to low permeability drugs, possibly by promoting brain waste clearance via the glymphatic system.
Introduction
Alzheimer's disease is a progressive neurodegenerative condition, characterized by an accumulation of amyloid-β and tau proteins that begins decades before symptoms occur.1,2 While the precise mechanisms are unclear, amyloid-β oligomers contribute to cell damage,3 and levels of pathogenic tau are closely linked to symptom severity.4 Alzheimer's is the most common form of dementia, with similar prevalence in Denmark as other European countries.5 It affects more than 46 million people worldwide and is projected to nearly triple in prevalence by 2050.6
CSF bulk flow propelled by cardiac-arterial pulsations7 may remove amyloid-β8 and tau9 via efflux along perivenous spaces and cranial nerves,10 a mechanism which is notably impaired in murine Alzheimer's models.11 The importance of this CSF-dependent clearance is supported by human imaging,12,13 histopathology14 and genetic studies.15,16
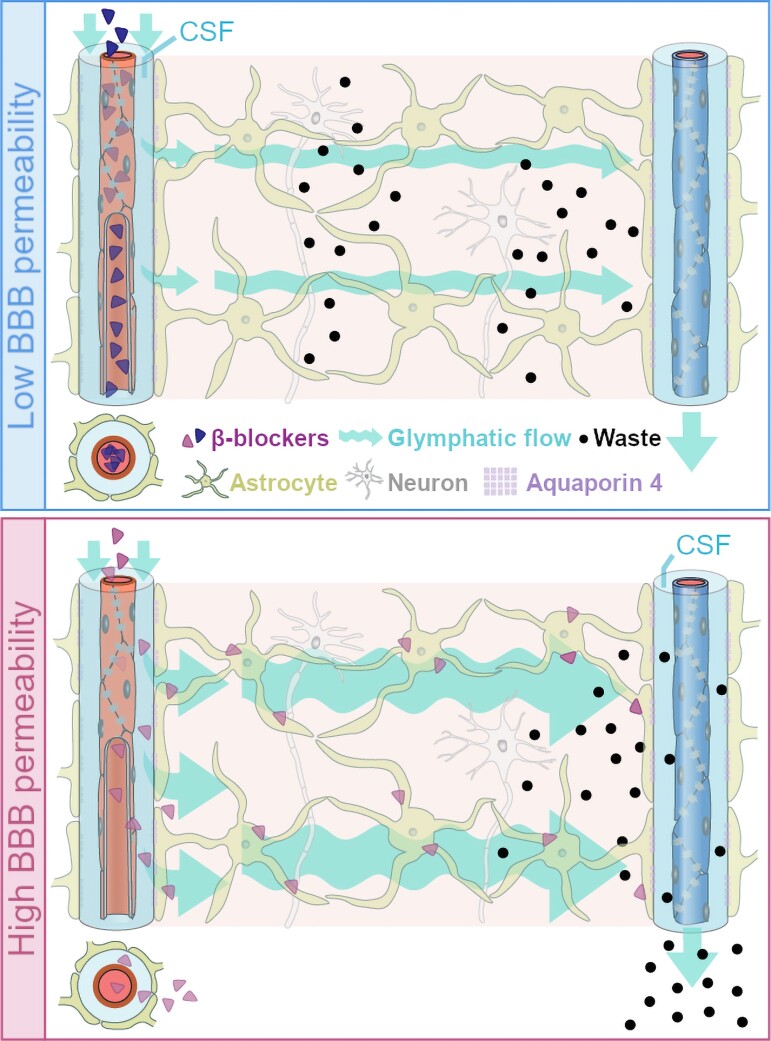
Norepinephrine binds to α- and β-adrenergic receptors (α and βARs) expressed throughout the CNS.17 A key neuromodulator of sleep and arousal,18 norepinephrine also regulates CSF-dependent clearance: reducing norepinephrine signalling improves CNS metabolite clearance,19 likely by diminishing glial cell volume, increasing interstitial space and lowering resistance to parenchymal flow.20 β-Blockers (βBs, i.e. β-adrenergic antagonists), typically used to treat cardiovascular conditions like hypertension by inhibiting cardiac βARs,21 may therefore also promote CSF-dependent clearance if they reach the CNS8 (Fig. 1).
Figure 1.
BBB permeable βBs may reduce the risk of Alzheimer’s disease. Proposed model: βBs increase CSF-dependent brain clearance of soluble neurotoxic proteins, including amyloid-β and tau, which are prominent in Alzheimer's disease pathology.8,22 CSF enters via the periarterial space, facilitated by aquaporin 4 (AQP4) water channels on surrounding astrocytic endfeet and driven by cardio-respiratory pulsations.7 As CSF is propelled into the parenchyma it mixes with solute-laden interstitial fluid. Convective bulk flow carries both towards the perivenous spaces, where fluid drains into meningeal or cervical lymphatic vessels10 (efflux along cranial and spinal nerves not illustrated here). βBs that cross the BBB (pink) may reduce Alzheimer's risk compared to those that do not cross (blue) by binding to astrocytes and decreasing their cell volume, thereby lowering resistance to bulk flow and promoting convection of waste products from the brain's interstitium to the periphery.20
Blood–brain barrier (BBB) permeability can be determined using a variety of methods, including biodistribution and autoradiography in animals, and invasive sampling, PET imaging or post-mortem investigations in humans. Such studies have shown consistent results that allow grouping of βBs by their ability to cross the BBB (Table 1).
Table 1.
β-B classification
| Drug name | Codes | Number of people | BBB permeability | |
|---|---|---|---|---|
| Before exclusion criteria | After exclusion criteria | |||
| β1 selective antagonists | ||||
| Metoprolol | C07AB02 C07BB02 C07FB02 |
298 032 | 43 055 | Moderate23–26 |
| Atenolol | C07AB03 C07CB03 |
57 483 | 12 883 | Low23–27 |
| Bisoprolol | C07AB07 | 26 516 | 3647 | Low23 |
| Non-selective β antagonist | ||||
| Propranolol | C07AA05 | 31 867 | 6974 | High23–26,28,29 |
| Sotalol | C07AA07 | 4066 | — | Low28 |
| α and β antagonist | ||||
| Carvedilol | C07AG02 | 36 548 | 2520 | High30,31 |
Name, activity, anatomical therapeutic chemical system (ATC) code,6 and number of people treated for each βB considered in the study. First generation βBs (propranolol, sotalol) target both the β1-adrenergic receptor and the β2-adrenergic receptor, whereas second generation βBs (metoprolol, atenolol, bisoprolol) only bind the β2-adrenergic receptor at higher doses. Carvedilol, a third generation βB, is additionally active at the α1-adrenergic receptor.32 The number of people treated is reported before and after applying exclusion criteria. Classification of βBs as low, moderate or high BBB permeability was based on available literature reporting distribution in human or animal tissue.23,25,28,30 Sotalol is used primarily as an anti-arrhythmic33; persons with this prescription were excluded from the primary cohort since arrhythmia is a competing indication for βB use.
Hypertension remains a main indication for βB use, although they are no longer recommended as one of the starting treatment options.34 It affects an estimated 1.13 billion people globally, is treated with interchangeable βBs of varying BBB permeability,34 but similar ability to control blood pressure,35,36 and often warrants treatment decades before typical Alzheimer's onset.37 These features make hypertension an ideal backdrop for investigating the impact of βB use and BBB permeability on Alzheimer's risk.
Here we used an epidemiological approach, interrogating data from the Danish national registry to test the hypothesis that treatment with highly BBB-permeable βBs reduces the risk of Alzheimer's disease compared to βBs with low BBB permeability.
Materials and methods
Registry data
Data were retrieved from the Danish Civil Registration System, the Danish National Patient Registry, National Prescription Registry, the Death Register, the National Migration Register and the Family Income Register. Diagnoses since 1994 are recorded using the Danish edition of the ICD-10 (International Classification of Diseases, 10th Revision), and prescriptions are registered by their ATC (Anatomical Therapeutic Chemical) code. Data were anonymized without possibility for identification of individuals. Ethical approval is not required for registry-based studies in Denmark.
The Danish national registers are centrally maintained and contain information on nearly all residents of Denmark.38 Any prescription drugs dispensed are legally required to be recorded. Hospital reporting to the Danish National Patient Registry is mandatory for public institutions, and psychiatric and outpatient clinic visits have been included since 1995. We had access to data for more than 7 million current and former residents of Denmark.
Study population
We identified people with hypertension based on their use of two different antihypertensive drug classes in two consecutive quarters.39 Individuals were included at the end date of the second quarter and retained if either of the drug classes was βB. We considered the most common βB drugs available from 1995–2017: metoprolol, atenolol, bisoprolol, propranolol, carvedilol and sotalol (Table 1). We established a relatively homogenous and comparable primary cohort by excluding those with evidence of competing indications for βB treatment. Individuals were followed from inclusion until an event (death or diagnosis of dementia), emigration or the end of the study period on 31 December 2017 (Supplementary Fig. 1). Diagnosis and medication codes are listed in Supplementary Table 1.
Exclusion criteria
People with competing indications for βB treatment (heart failure, arrhythmia or ischaemic heart disease) were excluded, along with those diagnosed with hypertension as a complication of a more serious underlying condition (secondary hypertension) or with concomitant intake of >2 antihypertensive drug classes. Persons treated with βBs for liver disease (almost always using propranolol) were excluded to avoid violating model assumptions.
We retained individuals between 50 and 99 years of age at inclusion, so as to capture the onset of most Alzheimer's disease cases.40 Those with prior diagnoses of Alzheimer's or other types of dementia were removed from the analysis. Individuals were required to have a minimum of 5 years intact data prior to inclusion. Therefore, the earliest possible inclusion date for our study was 1 January 2000. Persons included before 2000, and those who lived outside Denmark within 5 years prior to inclusion, were removed due to incomplete data.
Outcomes
Our main outcome was diagnosis of Alzheimer's disease, and for comparison, a diagnosis of any dementia (including Alzheimer's, unspecified dementia, and other causes). Both registry diagnoses have previously been validated, with positive predictive values of 81.0% and 86.8%, respectively.41
Measures
Individuals with hypertension were divided into groups based on which βB they were taking at baseline. Atenolol,23–27 bisoprolol23 and sotalol28 were classified as low BBB permeability; metoprolol23–26 as moderate BBB permeability; and carvedilol30,31 and propranolol23–26,28,29 as high BBB permeability (Table 1). Our analyses defined exposure by prescriptions at inclusion, regardless of discontinuation. This framework avoided bias due to non-random discontinuation, including probable confounding by unobservable features. Unlike a traditional prospective intention-to-treat framework, however, participants were not randomized to a treatment group.
Covariates
Each individual’s medical history, socioeconomic group and living situation were determined from the years prior to inclusion. Covariates relating to prescriptions pertained to medications received in the 6 months preceding inclusion (Supplementary Fig. 1 and Supplementary Table 1).
Covariates were based on established risk factors for Alzheimer's disease.42,43 We adjusted for the following: age; sex; whether the subject was living alone or with others; socioeconomic quartile based on equivalized household income; area of residence as a proxy for provider density, environmental exposures and regional affluence; diabetes; stroke; head trauma; hyperlipidaemia; atherosclerosis; chronic obstructive pulmonary disease; and depression and anxiety.
Diagnostic criteria and treatment of both hypertension and dementia, as well as βB usage in general, changed over the timeframe of our study. In particular, prescriptions declined after hypertension treatment guidelines were revised in 2009.44 We corrected for the relative time of inclusion to accommodate this potential confounder.
Lastly, we corrected for the second antihypertensive used alongside βB at inclusion, grouped as: calcium channel blockers, angiotensin-II receptor blockers, other renin-angiotensin system-acting agents, potassium-sparing diuretics, loop diuretics, and thiazides and other diuretics. This allowed for appropriate adjustment given possible unrelated protective effects of calcium channel blockers,45 angiotensin-II receptor blockers46 and potassium-sparing diuretics47 that have been described previously.48
Statistical analysis
Baseline characteristics for each group were compared using the Chi-squared or Kruskal–Wallis H-tests as appropriate. Unless otherwise specified, numbers are presented as the median and interquartile range (IQR).
Non-standardized absolute risk was calculated using the Aalen Johansen estimator.49 When adjusting for covariates, cause-specific Cox regression was used to model the treatment effect and covariates on time to outcome. Death was considered as a competing risk, such that in the case of two events (e.g. dementia, then death) the first to occur was considered the outcome for that individual, and death without a dementia diagnosis was not equivalent to survival without dementia. The standardized absolute risk of an outcome was estimated as the averaged person-specific absolute risk over each cohort under a given treatment modality. Risks were calculated at 10 years from the date of inclusion and are shown as a percentage alongside 95% confidence intervals (CIs).
Because age and relative time of inclusion did not meet the linearity assumption of Cox models, their effects were modelled using B-splines (basis regression splines).50
Standardized absolute risk was calculated every 6 months up to 10 years in each group. The differences in outcome risk were then computed between low and high BBB permeability using the average treatment effect.51 Adjustment for multiple comparisons over time was performed using the quantile of the confidence bands.52
Data were processed using SAS (version 9.4, SAS Institute, Cary, NC, USA) and R statistical software (version 4.0.3, R development core team), including the R package riskRegression (version 2020.12.08) to compute the standardized absolute risks and confidence bands.
Sensitivity analyses
In order to investigate our hypothesis in a less homogenous but more representative sample, we repeated the analysis in a secondary cohort where competing diagnoses for βB use (arrhythmia, ischaemic heart disease and heart failure) were corrected for instead of excluded.
To compare our populations with previous investigations, we evaluated the use of any βB together with a second antihypertensive drug class versus treatment with two non-βB antihypertensive drug classes. Exclusion criteria were otherwise the same as in the main analysis.
Defining exposure by baseline prescriptions does not factor in possible discontinuation of the drug assigned at the start of the study or switching between groups. We estimated the effect of the intention to treat rather than the treatment effect; this definition avoided bias due to non-random discontinuation likely related to features unavailable in the registers. Therefore, to check how well the designated group represented actual treatment, we calculated coverage by the BBB permeability group assigned at inclusion (on-target) and by non-assigned groups (off-target) using dispensation records during the follow-up period. We also performed a sensitivity analysis in which subjects were censored upon switching groups or discontinuing βB treatment, forcing perfect compliance.
To reduce any overt selection bias, we compared a subset of highly BBB-permeable βB users and low BBB permeability users with similar likelihoods of taking highly BBB-permeable βBs. This was achieved by using logistic regression models53 based on the same covariates described below to calculate each subject's propensity for taking highly BBB-permeable βBs.54 For each high BBB permeability individual, we then selected (without replacement) the closest propensity low BBB permeability individual.55
Finally, to reduce the impact of latency between dementia onset and diagnosis, we ran landmark analyses in which follow-up began at 1, 3 or 5 years after inclusion and any person who had started βB, stopped βB, had an event (death or dementia) or been censored (e.g. emigrated) prior to that point was excluded.
Data availability
Access to anonymized Danish National Registry data for research purposes is controlled by Statistics Denmark.
Results
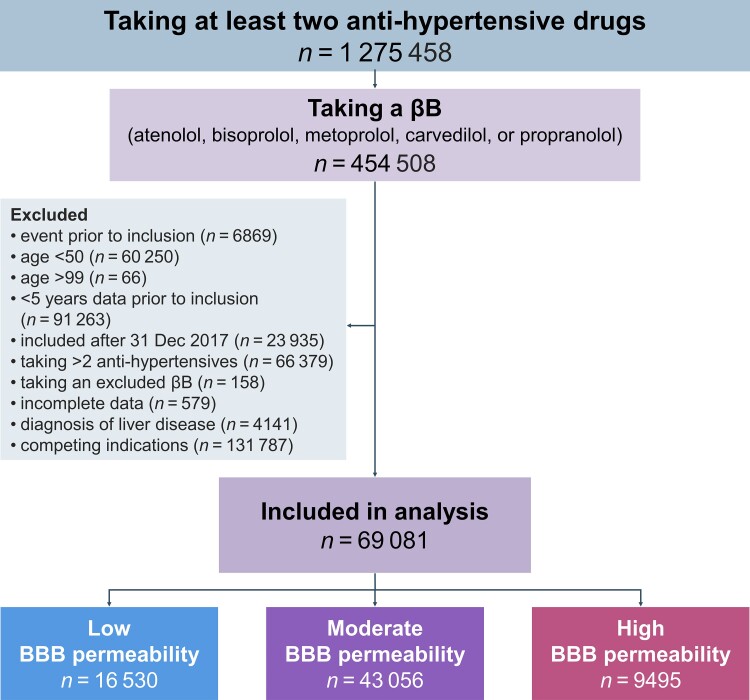
We labelled 1 275 458 individuals taking one or more antihypertensive drug from two different classes as having hypertension,39 454 508 (35.6%) of whom were prescribed βBs. After exclusion criteria (Fig. 2), we retained a primary cohort of 69 081 people with a total follow-up of more than 522 065 person-years, a median follow-up time of 9.8 (IQR: 5.3 to 10.0) years and a median age of 64.4 years (IQR: 57.7 to 72.3 years; 64.8% female). The cohort was divided into low, moderate and high BBB permeability βB groups (see the ‘Materials and methods’ section) as shown in Fig. 2. Baseline demographics are summarized in Supplementary Table 2.
Figure 2.
Flow chart describing cohort selection. A total of 1 275 458 individuals took at least two different antihypertensive drug classes between 1995 and 2017. For 454 508 (35.6%) of them, βBs were one of the drug classes. Following exclusion criteria, we included 69 081 people in the primary cohort, from which people with indications for βBs other than hypertension were excluded. Individuals were further divided into groups based on the BBB permeability of the βB prescribed at baseline, with atenolol25 and bisoprolol23 considered low; metoprolol moderate25; and carvedilol30 and propranolol25 high BBB permeability. Altogether, 23.9% of people were grouped as taking low BBB permeability βBs, 62.3% as moderate, and 13.7% as high.
Highly BBB-permeable β-blockers are associated with reduced risk of Alzheimer's disease
At the end of follow-up, there were 837 Alzheimer's events, 13 505 deaths and 2075 cases of any type of dementia (including Alzheimer's, unspecified dementia or other causes).
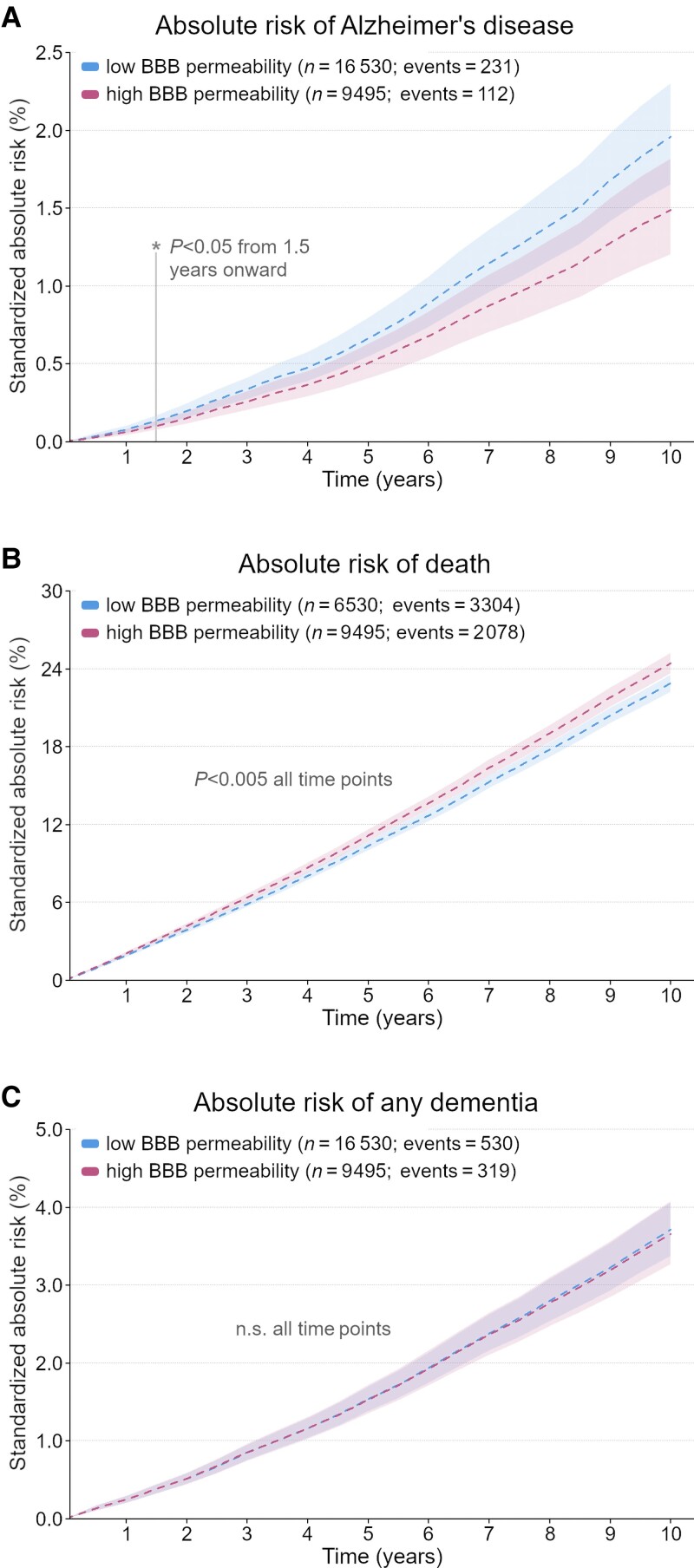
The 10-year standardized absolute risk of Alzheimer’s disease was reduced by 24% in the high versus low BBB permeability group, with a risk ratio of 0.76 (CI: 0.61 to 0.95, P < 0.040; Table 2, Fig. 3). Assessment of risk over time showed a significant effect beginning at 1.5 years (Fig. 3A). The observed risk modulation associated with BBB permeability followed the hypothesized dose-response relationship, decreasing from low (1.96%) to moderate (1.63%) to high (1.49%) permeability groups (Table 2), with significant differences between the low and high groups.
Table 2.
Ten-year outcomes
| Events, n (% of population) | Non-standardized absolute risk, % (95% CI) | Standardizeda absolute risk, % (95% CI) | Standardizeda absolute risk difference, % (95% CI) | P-value | |
|---|---|---|---|---|---|
| Alzheimer's disease | |||||
| Low BBB permeability | 231 (1.4%) | 1.54 (1.34 to 1.74) | 1.96 (1.65 to 2.30) | Reference | |
| Moderate BBB permeability | 494 (1.1%) | 1.44 (1.32 to 1.57) | 1.63 (1.43 to 1.85) | −0.33 (−0.63 to −0.03) | 0.074 |
| High BBB permeability | 112 (1.2%) | 1.54 (1.25 to 1.83) | 1.49 (1.20 to 1.82) | −0.47 (−0.85 to −0.10) | <0.036 |
| Death (as competing risk with Alzheimer's disease) | |||||
| Low BBB permeability | 3304 (20.0%) | 21.9 (21.2 to 22.5) | 22.9 (22.2 to 23.5) | Reference | |
| Moderate BBB permeability | 8123 (18.9%) | 23.1 (22.6 to 23.5) | 23.8 (23.3 to 24.3) | 0.91 (0.24 to 1.58) | <0.022 |
| High BBB permeability | 2078 (21.9%) | 27.2 (26.2 to 28.3) | 24.4 (23.6 to 25.2) | 1.55 (0.63 to 2.47) | <0.003 |
| Any dementia | |||||
| Low BBB permeability | 530 (3.2%) | 3.52 (3.22 to 3.81) | 3.71 (3.38 to 4.06) | Reference | |
| Moderate BBB permeability | 1226 (2.8%) | 3.53 (3.34 to 3.73) | 3.54 (3.31 to 3.78) | −0.17 (−0.54 to 0.20) | 0.641 |
| High BBB permeability | 319 (3.4%) | 4.28 (3.81 to 4.74) | 3.66 (3.27 to 4.08) | −0.05 (−0.55 to 0.44) | 0.973 |
Number of Alzheimer's disease, death and any dementia (including Alzheimer's, unspecified dementia, and other causes) outcomes in the primary cohort, and risk at 10 years. Death was treated as a competing risk (see the ‘Materials and methods’ section). Non-standardized risk was uncorrected, while standardized absolute risk was modelled using the covariates listed below. P-values refer to standardized risk differences. There were 16 530 people taking low, 43 056 taking moderate and 9495 taking high BBB-permeable βBs. People taking βBs with high BBB permeability had decreased risk of Alzheimer's disease compared to low permeability βB users.
Models were corrected for sex, age (B-splines), relative time of inclusion (B-splines), socioeconomic group, municipality, living alone, loop diuretic use, diabetes mellitus, hyperlipidaemia, depression, stroke, head trauma, atherosclerosis, chronic obstructive pulmonary disease and second antihypertensive drug class.
Figure 3.
Standardized absolute risk over time. Standardized absolute risk over 10 years for the primary cohort, where subjects with indications other than hypertension for treatment with β-adrenergic antagonists were excluded. 95% CIs are shaded. Models were corrected and P-values adjusted for multiple comparisons as described in the ‘Materials and methods’ section. (A) Absolute risk of Alzheimer's with death as a competing risk, showing significantly reduced risk for those taking high BBB permeability βBs (pink) compared to low BBB permeability βBs (blue) from 1.5 years onward. (B) Absolute risk of death with Alzheimer's disease as a competing risk. (C) Absolute risk of any dementia (including Alzheimer's, unspecified dementia and other causes) with death as a competing risk. n.s. = not significant.
Standardized absolute risk of death (as a competing risk with Alzheimer's disease) was significantly higher in both moderate [+0.91% (CI: 0.24% to 1.58%), P < 0.022] and high [+1.55% (CI: 0.63% to 2.47%), P < 0.003] groups compared to the low BBB permeability group across the follow-up period (Fig. 3B).
We also calculated the standardized absolute risk for any dementia to investigate whether our findings may be specific to Alzheimer's disease; we did not find significant differences between the groups taking low, moderate or high BBB permeability βBs (Fig. 3C).
Sensitivity analyses
To verify the biological relevance of the risk reduction associated with highly BBB-permeable βBs, we performed several additional analyses (see the ‘Materials and methods’ section).
In the secondary cohort, where competing βB indications of heart failure, ischaemic heart disease and arrhythmia were retained, there were 200 868 people, 2920 Alzheimer's events, 56 081 deaths and 7532 cases of any dementia. After correction, we observed a numerically lower Alzheimer's risk in the high BBB permeability group versus the low BBB permeability group, although the difference was not significant [−0.23% (CI: −0.55% to 0.09%), P = 0.334, n = 200 868]. In keeping with the primary cohort, Alzheimer's risk decreased numerically along the gradient from low to moderate to high BBB permeability, without reaching statistical significance. Absolute risk of death was slightly higher in both moderate [+0.71% (CI: 0.21% to 1.21%), P < 0.012] and high [+1.86% (CI: 1.18% to 2.53%), P < 0.001] BBB permeability groups compared to the low BBB permeability group. As with the primary cohort, we did not observe significant differences for risk of a diagnosis of any dementia (including Alzheimer's, unspecified and other dementia) between groups (Supplementary Table 3).
To validate grouping by prescriptions at baseline, we measured individuals' coverage by the BBB permeability group identified at inclusion (i.e. how many daily doses of medication were dispensed for an individual relative to their total days follow-up). We found median coverage of the BBB permeability groups identified at baseline to be above 74% in each group, with minimal off-target coverage of non-assigned drugs (data not shown). Results from a sensitivity analysis forcing perfect drug compliance by censoring subjects if they discontinued βB use or switched BBB permeability groups followed a similar trend to the results of the main analysis but did not reach significance (data not shown).
Comparing βB use as a group against alternative hypertensive drug classes in the wider hypertension population, we did not find any significant difference in terms of either Alzheimer's risk or risk of any dementia (Supplementary Table 4). Risk of death was increased from 21.1% for non-βB users to 22.4% in βB users [+1.36% (CI: 1.02% to 1.70%), P < 0.001, n = 368 892].
When using a propensity score approach, Alzheimer's risk was decreased by 0.92% (CI: −1.42% to −0.42%; P < 0.001; n = 16 166) for highly BBB-permeable βB users as compared to propensity-matched low BBB permeability users, and risk of death was increased by 1.82% (CI: 0.69% to 2.94%; P < 0.002; n = 16 166) (Table 3).
Table 3.
Ten-year outcomes for propensity-scored analysis
| Events, n (% of population) | Non-standardized absolute risk, % (95% CI) | Standardizeda absolute risk, % (95% CI) | Standardizeda absolute risk difference, % (95% CI) | P-value | |
|---|---|---|---|---|---|
| Alzheimer's disease | |||||
| Low BBB permeability | 157 (1.9%) | 2.35 (1.98 to 2.71) | 2.46 (1.95 to 3.1) | Reference | |
| High BBB permeability | 96 (1.2%) | 1.45 (1.16 to 1.74) | 1.54 (1.16 to 2.01) | −0.92 (−1.42 to −0.42) | <0.001 |
| Death (as competing risk with Alzheimer's disease) | |||||
| Low BBB permeability | 1665 (20.6%) | 24.4 (23.3 to 25.4) | 24.2 (23.2 to 25.2) | Reference | |
| High BBB permeability | 1816 (22.5%) | 26.4 (25.4 to 27.5) | 26.0 (25.0 to 27.1) | 1.82 (0.69 to 2.94) | <0.002 |
| Any dementia | |||||
| Low BBB permeability | 315 (3.9%) | 4.65 (4.14 to 5.15) | 4.51 (4.00 to 5.07) | Reference | |
| High BBB permeability | 282 (3.5%) | 4.18 (3.70 to 4.66) | 4.05 (3.55 to 4.59) | −0.47 (−1.12 to 0.19) | 0.159 |
Number of Alzheimer's disease, death and any dementia (including Alzheimer's, unspecified dementia, and other causes) outcomes in the propensity-scored primary cohort, and risk at 10 years. Individuals were assigned a propensity score for their likeliness to take high BBB permeability βBs, based on the below covariates. ‘Cases’ who took high BBB permeability βBs were then propensity-matched to ‘controls’ who did not. Death was treated as a competing risk (see the ‘Materials and methods’ section). Non-standardized risk was uncorrected, while standardized absolute risk was modelled using the covariates listed below. P-values refer to standardized risk differences. There were 8083 people taking high BBB permeability Bβs that were able to be matched to a person taking low permeability βBs. The risk of Alzheimer's disease was reduced for those taking high BBB permeability βBs. However, risk of death was increased for high BBB permeability βB users.
Models were corrected for sex, age (B-splines), relative time of inclusion (B-splines), socioeconomic group, municipality, living alone, loop diuretic use, diabetes mellitus, hyperlipidaemia, depression, stroke, head trauma, atherosclerosis, chronic obstructive pulmonary disease and second antihypertensive drug class.
Standardized absolute risk of Alzheimer's disease was also reduced in the moderate and high compared to low BBB permeability group in a 1-year landmark analysis [moderate: −0.46% (CI: −0.81% to −0.12%), P < 0.020; high: −0.57% (CI: −1.02% to −0.12%), P < 0.029, n = 54 769]. Here, any events (i.e. death or dementia) within the first year were excluded to offset the effect of lag between dementia onset and diagnosis (Table 4). Absolute risk was not significantly different between high and low groups for death [+1.07% (CI: 0.02% to 2.12%), P = 0.108] or any dementia [−0.27% (CI: −0.85% to 0.31%), P = 0.623]. The direction of these results was similar in 3- and 5-year landmark analyses, but differences were not significant (data not shown).
Table 4.
Ten-year outcomes for 1-year landmark analysis
| Events, n (% of population) | Non-standardized absolute risk, % (95% CI) | Standardizeda absolute risk, % (95% CI) | Standardizeda absolute risk difference, % (95% CI) | P-value | |
|---|---|---|---|---|---|
| Alzheimer's disease | |||||
| Low BBB permeability | 211 (1.6%) | 1.75 (1.51 to 1.98) | 2.16 (1.81 to 2.56) | Reference | |
| Moderate BBB permeability | 412 (1.2%) | 1.54 (1.39 to 1.69) | 1.69 (1.47 to 1.95) | −0.46 (−0.81 to −0.12) | <0.020 |
| High BBB permeability | 86 (1.3%) | 1.68 (1.32 to 2.04) | 1.59 (1.25 to 1.99) | −0.57 (−1.02 to −0.12) | <0.029 |
| Death (as competing risk with Alzheimer's disease) | |||||
| Low BBB permeability | 2782 (20.6%) | 22.8 (22.1 to 23.6) | 23.8 (23.1 to 24.6) | Reference | |
| Moderate BBB permeability | 6601 (19.1%) | 23.8 (23.3 to 24.3) | 24.3 (23.7 to 24.8) | 0.45 (−0.29 to 1.20) | 0.449 |
| High BBB permeability | 1549 (22.9%) | 28.6 (27.4 to 29.9) | 24.9 (24.0 to 25.8) | 1.07 (0.02 to 2.12) | 0.108 |
| Any dementia | |||||
| Low BBB permeability | 461 (3.4%) | 3.79 (3.45 to 4.13) | 3.99 (3.60 to 4.40) | Reference | |
| Moderate BBB permeability | 977 (2.8%) | 3.58 (3.35 to 3.80) | 3.56 (3.31 to 3.82) | −0.43 (−0.85 to −0.01) | 0.102 |
| High BBB permeability | 233 (3.4%) | 4.40 (3.84 to 4.96) | 3.72 (3.26 to 4.23) | −0.27 (−0.85 to 0.31) | 0.623 |
Number of Alzheimer's disease, death and any dementia (including Alzheimer's, unspecified dementia and other causes) outcomes in the primary 1-year landmark cohort, and risk at 10 years. For the landmark analyses, any person with an event in the first year was excluded, and inclusion +1 year was used as the start point. Death was treated as a competing risk (see the ‘Materials and methods’ section). Non-standardized risk was uncorrected, while standardized absolute risk was modelled using the covariates listed below. P-values refer to standardized risk differences. There were 13 500 people taking low, 34 495 taking moderate and 6774 taking high BBB-permeable βBs. People taking βBs with high BBB permeability had decreased risk of Alzheimer's disease. Risk of death was increased for both moderate and high BBB permeability βB users.
Models were corrected for the baseline covariates sex, age (B-splines), relative time of inclusion (B-splines), and second antihypertensive drug class, as well as 1-year values for socioeconomic group, municipality, living alone, loop diuretic use, diabetes mellitus, hyperlipidaemia, depression, stroke, head trauma, atherosclerosis, chronic obstructive pulmonary disease and liver disease.
Discussion
In this nation-wide retrospective cohort study of Danish residents with hypertension, we demonstrated that treatment with βBs that readily cross the BBB is associated with a reduced Alzheimer's risk (−0.47%; risk ratio: 75.9%) compared to treatment with less BBB-permeable βBs. The reduction in Alzheimer's risk for the highly BBB-permeable βBs was significant after 1.5 years (Fig. 3A), a delay which is consistent with the gradual progression of Alzheimer's disease. Our conclusions were supported by a landmark analysis, where events within the first year were ignored as likely unrelated to βB treatment (Table 4). Our cohort was carefully curated for increased rigor at the expense of generalizability; however, we had almost no selection or attrition bias thanks to the integrity of the registry data. Alzheimer's risk was also numerically lower for the high BBB-permeability βB group versus the low BBB-permeability βB group in a larger cohort with fewer exclusions (Supplementary Table 3), suggesting a degree of generalizability.
We found no significant change in risk for any dementia (including Alzheimer's disease, unspecified dementia and other causes) between BBB permeability groups, either in the main analysis (Fig. 3C) or any of the sensitivity measures. This alluded to an Alzheimer's-specific process, tallying with the hypothesis of enhanced glymphatic clearance of amyloid-β and tau. This supported the comparability between groups, as this was an outcome we did not expect to be affected by βB treatment.
βBs treat cardiovascular disease via inhibition of cardiac and renal adrenergic receptors21 and are about as effective as alternatives in treating hypertension.56 Relative to other βBs, Cochrane reports show little difference between the drugs of interest in terms of diastolic or systolic blood pressure control.35,36 However, those βBs that cross the BBB have an additional impact on central ARs. Within the CNS, norepinephrine and βARs are involved in a wide range of functions central to Alzheimer's disease pathology, including memory consolidation, synaptic plasticity, glial function and sleep.57 Non-REM sleep is believed to be the main regulator of glymphatic clearance, where central adrenergic signalling and locus coeruleus activity play an important inhibitory role.8,58 Specifically, α1-antagonists and βBs inhibit norepinephrine release in a similar yet less potent manner than α2AR agonists59 (e.g. dexmedetomidine) to ultimately reduce arousal, promote sleep and improve glymphatic function.8,60 While BBB-permeable βBs in particular are associated with fatigue33 and subjective changes in sleep,61 these side effects cannot easily be separated from the impact of βBs on peripheral blood pressure,62 and objective effects of non-REM sleep in a hypertensive population have not yet been investigated. Therefore, further studies are needed to clarify the impact of βB BBB permeability on blood pressure control, sleep quality and Alzheimer's risk.
The role of βARs in Alzheimer's pathogenesis remains controversial.63 Several preclinical studies suggest that βBs can increase Alzheimer's risk64 and that βAR activation can be protective against Alzheimer's disease,65,66 effects that seem contradictory to our findings. However, other data show that βBs can decrease Aβ accumulation and reduce cognitive deficits in mice models67 and slow cognitive decline in people with Alzheimer's disease.68 Genetic studies have found that βAR gene variants associated with decreased Alzheimer's risk also make the βAR receptors less responsive to norepinephrine.69 Finally, a case control study of recently diagnosed Alzheimer's patients showed that cases were significantly less likely to have been using βBs across a 3-year period.70 Taken together with our findings, these results suggest that central βARs may modulate Alzheimer's disease risk and development.
Highly BBB-permeable βBs are less selective for β1ARs (Table 1), which raises the possibility that the observed Alzheimer's risk reduction is driven by increased β2AR inhibition rather than higher BBB permeability. While both types of drug are comparably effective at controlling blood pressure,35,36 β1-selective βBs mainly act by reducing heart rate and contractility to lower cardiac output, whereas non-selective βBs additionally induce slight peripheral vasocontraction.71 However, the decreasing trend in risk from low to moderate to high BBB permeability argues against βB selectivity as the defining feature. Furthermore, β1-selective βBs inhibit both β1 and β2ARs at higher doses.71,72 To account for differences between drugs, such as the α1AR antagonist activity of carvedilol, we ran sensitivity analyses with each drug removed and found similar risks (data not shown), suggesting that our results cannot be attributed to any single compound.
We considered two statistical approaches to estimate risk; the cause-specific Cox model, which relies upon correct specification of the model for risk of Alzheimer's disease and death, and propensity score, which relies upon correct specification of the model for treatment allocation. The propensity score analysis mirrored the main results, showing a 0.92% decrease in Alzheimer's disease risk for the high BBB-permeability group compared to the low (Table 3). However, we also saw an elevated risk of death in the high (1.82%) and moderate (1.55%) BBB-permeability groups compared to the low (Table 2). The high permeability group risk increase was observed throughout the follow-up period, including at the earliest timepoints (Fig. 3B), and was not related to dementia (as follow-up ends at a dementia diagnosis). The effect is likely explained by an increased unmeasured comorbidity burden in the high BBB-permeability group, which should in principle also raise the Alzheimer's risk,73 thereby reducing our effect size. However, when we removed events within the first year after inclusion as unrelated to recently initiated βB treatment, Alzheimer's risk was still reduced for the high BBB permeability group compared to the low, but risk of death was no longer significantly increased (Table 4). A contributing factor could be the poor tolerability of BBB-permeable compounds relative to newer non-permeable options, and their resulting low popularity.74 Although those taking more than two antihypertensives at a time were excluded, individuals receiving high BBB-permeability βBs may still have been on their third or fourth treatment option for more severe or difficult to control hypertension, suggesting they were more ill and at risk for both death and Alzheimer's disease. Moreover, the well-known CNS side effects of the high BBB-permeability drugs could have led prescribers to avoid these options for people with pre-existing cognitive problems (i.e. prodromal dementia), introducing a possible selection bias. Randomized prospective clinical trials are needed to establish a causal link between βB BBB permeability and Alzheimer's disease risk.
Previous epidemiological investigations of the impact of βB treatment on dementia outcomes, which typically compared βBs against other antihypertensives or no treatment, have failed to show a consistent protective effect.75 However, such reports have generally considered βBs as a single group47,70,76–78 and found modest or insignificant effects on Alzheimer's risk. When we combined βBs into a single group and compared it to alternative hypertension treatments without accounting for BBB permeability, we likewise did not detect any significant difference for risk of Alzheimer's disease or any type of dementia (Supplementary Table 4). Our results thereby highlighted that the ability of βBs to access the CNS is relevant to Alzheimer's risk. It is unfortunate that prior investigations have failed to account for this feature. Any central βAR-mediated impact on Alzheimer's risk and pathogenesis requires that βBs pass through the BBB. Our study was specifically designed to address this important gap in the literature and investigate the role of BBB permeability on βB-mediated modulation of Alzheimer's risk using real-world clinical data. The finding that higher BBB permeability is associated with reduced Alzheimer's risk for those taking βBs favours the hypothesis that these medications confer a protective effect that may be independent of their capacity as antihypertensives, if they are able to cross the BBB and reach the site of Alzheimer's disease pathogenesis. Further investigations are warranted to determine the underlying mechanism behind our findings.
Strengths and limitations
This study benefitted from negligible loss to follow-up, near complete population coverage and comprehensive prescription drug information, as is characteristic of the Danish national registry system. The relative racial homogeneity of the Danish population limited interpretation for non-Scandinavian individuals but improved comparability between groups. However, relying on registry data meant that we could not take additional variables that may have influenced the outcomes of interest into account, e.g. body mass index, APOE genotype and tobacco use.
We corrected for the baseline demographics and diagnoses known to influence Alzheimer's risk to accommodate for variability between groups. People taking highly BBB-permeable drugs have notably higher levels of diabetes mellitus, depression and anxiety, stroke and chronic obstructive pulmonary disease compared to those taking low permeability βBs (Supplementary Table 2). This suggests a generally increased comorbidity burden in the high permeability group, and additional studies are needed to clarify the cause of this baseline imbalance. Because risk of death is further elevated when models are not adjusted, confounding may explain the increase.
Our results were robust to different statistical approaches and across sensitivity measures, but the observed increased risk of death in the high BBB permeability was unexpected. Despite careful consideration, we cannot rule out a possible violation of the positivity assumption of the framework used to assess average treatment effects and determine standardized absolute risk. Although our models could not account for switching between or discontinuing treatments, consistent results in our landmark (Table 4) and censor-at-the-switch sensitivity analyses, as well as good βB coverage during follow-up, alleviated concern that the groups based on baseline prescriptions did not represent true exposure.
Finally, while collective reports allowed us to establish a BBB permeability ranking (Table 1), these reports varied in methodology. Future trials investigating the protective effects of βB against Alzheimer's should evaluate the BBB permeability of each compound using consistent methods.
Interpretations
Our results highlight the impact of BBB permeability of commonly prescribed βBs on the risk of Alzheimer's disease. Future studies are warranted to show if these effects can be ascribed to enhanced CSF-dependent clearance of amyloid-β and tau, decreased amyloid-β aggregation, reduced tau phosphorylation or a combination of these.
Supplementary Material
Acknowledgements
We would like to thank Dan Xue from the Center for Translational Neuromedicine and Niels Haraldsted for assistance with illustrations. We thank Dr Poul Jørgen Jennum for initial discussions of the study initiation.
Contributor Information
Emily Eufaula Beaman, Neurobiology Research Unit, Copenhagen University Hospital Rigshospitalet, 2100 Copenhagen Ø, Denmark; Institute of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, 2200 Copenhagen N, Denmark.
Anders Nissen Bonde, Department of Cardiology, Copenhagen University Hospital Herlev-Gentofte, 2900 Hellerup, Denmark.
Sara Marie Ulv Larsen, Neurobiology Research Unit, Copenhagen University Hospital Rigshospitalet, 2100 Copenhagen Ø, Denmark.
Brice Ozenne, Neurobiology Research Unit, Copenhagen University Hospital Rigshospitalet, 2100 Copenhagen Ø, Denmark; Department of Public Health, Section of Biostatistics, University of Copenhagen, 1353 Copenhagen K, Denmark.
Terhi Johanna Lohela, Center for Translational Neuromedicine, Faculty of Health and Medical Sciences (SUND), University of Copenhagen, 2200 Copenhagen N, Denmark.
Maiken Nedergaard, Institute of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, 2200 Copenhagen N, Denmark; Center for Translational Neuromedicine, Faculty of Health and Medical Sciences (SUND), University of Copenhagen, 2200 Copenhagen N, Denmark; Center for Translational Neuromedicine, Department of Neurosurgery, University of Rochester Medical Center, Rochester, NY 14642, USA.
Gunnar Hilmar Gíslason, Department of Cardiology, Copenhagen University Hospital Herlev-Gentofte, 2900 Hellerup, Denmark; Department of Cardiovascular Epidemiology and Research, The Danish Heart Foundation, 1120 Copenhagen K, Denmark.
Gitte Moos Knudsen, Neurobiology Research Unit, Copenhagen University Hospital Rigshospitalet, 2100 Copenhagen Ø, Denmark; Institute of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, 2200 Copenhagen N, Denmark.
Sebastian Camillo Holst, Neurobiology Research Unit, Copenhagen University Hospital Rigshospitalet, 2100 Copenhagen Ø, Denmark.
Funding
This project was funded by the H2020 Marie Skłodowska-Curie Actions (H2020-MSCA-IF-2017-798131) (to S.C.H.); by a grant from Savværksejer Jeppe Juhls og hustru Ovita Juhls Mindelegat (to E.E.B.); the Independent Research Fund Denmark, grant ID: 0134-00454B (G.M.K.); The Lundbeck Foundation grant ID: R279-2018-1145 (G.M.K.); and by the Lundbeck Foundation (to M.N.).
Competing interests
S.C.H. is now a full-time employee of Roche Pharmaceuticals. All other authors report no disclosures.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Jack CR, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132(5):1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. [DOI] [PubMed] [Google Scholar]
- 3. Reiss AB, Arain HA, Stecker MM, Siegart NM, Kasselman LJ. Amyloid toxicity in Alzheimer’s disease. Rev Neurosci. 2018;29(6):613–627. [DOI] [PubMed] [Google Scholar]
- 4. Malpas CB, Sharmin S, Kalincik T. The histopathological staging of Tau, but not amyloid, corresponds to antemortem cognitive status, dementia stage, functional abilities and neuropsychiatric symptoms. Int J Neurosci. 2021;131(8):800–809. [DOI] [PubMed] [Google Scholar]
- 5. Nichols E, Szoeke CEI, Vollset SE, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer report 2015: the global impact of dementia. Alzheimer’s Disease International; 2015. Accessed 7 March 2019. https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf [Google Scholar]
- 7. Mestre H, Tithof J, Du T, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9(1):4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harrison IF, Ismail O, Machhada A, et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain. 2020;143(8):2576–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nedergaard M, Goldman SA. Brain drain. Sci Am. 2016;314(3):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng W, Achariyar TM, Li B, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han F, Chen J, Belkin-Rosen A, et al. The coupling of global brain activity and cerebrospinal fluid inflow is correlated with Alzheimer’s disease related pathology. bioRxiv. [Preprint] 10.1101/2020.06.04.134726 [DOI] [Google Scholar]
- 13. Eide PK, Vinje V, Pripp AH, Mardal K-A, Ringstad G. Sleep deprivation impairs molecular clearance from the human brain. Brain. 2021;144(3):863–874. [DOI] [PubMed] [Google Scholar]
- 14. Zeppenfeld DM, Simon M, Haswell JD, et al. Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 2017;74(1):91. [DOI] [PubMed] [Google Scholar]
- 15. Larsen SMU, Landolt H-P, Berger W, Nedergaard M, Knudsen GM, Holst SC. Haplotype of the astrocytic water channel AQP4 is associated with slow wave energy regulation in human NREM sleep. PLoS Biol. 2020;18(5):e3000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burfeind KG, Murchison CF, Westaway SK, et al. The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer’s disease. Alzheimers Dement Transl Res Clin Interv. 2017;3(3):348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pupo AS, Minneman KP. Adrenergic pharmacology: focus on the central nervous system. CNS Spectr. 2001;6(8):656–662. [DOI] [PubMed] [Google Scholar]
- 18. Kjaerby C, Andersen M, Hauglund N, et al. Dynamic fluctuations of the locus coeruleus-norepinephrine system underlie sleep state transitions. bioRxiv. [Preprint] 10.1101/2020.09.01.274977 [DOI] [Google Scholar]
- 19. Benveniste H, Lee H, Ding F, et al. Anesthesia with dexmedetomidine and low-dose isoflurane increases solute transport via the glymphatic pathway in rat brain when compared with high-dose isoflurane. Anesthesiology. 2017;127(6):976–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sherpa AD, Aoki C, Hrabetova S. Noradrenaline drives structural changes in astrocytes and brain extracellular space. In: Vardjan N and Zorec R, eds. Noradrenergic Signaling and Astroglia. Elsevier; 2017:241–255. [Google Scholar]
- 21. Oliver E, Mayor F Jr, D’Ocon P. Beta-blockers: historical perspective and mechanisms of action. Rev Esp Cardiol. 2019;72(10):853–862. [DOI] [PubMed] [Google Scholar]
- 22. Alzheimer’s Association . 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16(3):391–460. [Google Scholar]
- 23. Bühring KU, Sailer H, Faro H-P, Leopold G, Pabst J, Garbe A. Pharmacokinetics and metabolism of bisoprolol-14C in three animal species and in humans. J Cardiovasc Pharmacol. 1985;8:S21–S28. [DOI] [PubMed] [Google Scholar]
- 24. Cruickshank JM, Neil-Dwyer G. β-blocker brain concentrations in man. Eur J Clin Pharmacol. 1985;28(S1):21–23. [DOI] [PubMed] [Google Scholar]
- 25. McAinsh J, Cruickshank JM. Beta-blockers and central nervous system side effects. Pharmacol Ther. 1990;46(2):163–197. [DOI] [PubMed] [Google Scholar]
- 26. van Bree JB, de Boer AG, Danhof M, Ginsel LA, Breimer DD. Characterization of an in vitro blood-brain barrier: effects of molecular size and lipophilicity on cerebrovascular endothelial transport rates of drugs. J Pharmacol Exp Ther. 1988;247(3):1233–1239. [PubMed] [Google Scholar]
- 27. Agon P, Goethals P, Van Haver D, Kaufman J-M. Permeability of the blood-brain barrier for atenolol studied by positron emission tomography. J Pharm Pharmacol. 1991;43(8):597–600. [DOI] [PubMed] [Google Scholar]
- 28. Arendt RM, Greenblatt DJ, deJong RH, Bonin JD, Abernethy DR. Pharmacokinetics, central nervous system uptake, and lipid solubility of propranolol, acebutolol, and sotalol. Cardiology. 1984;71(6):307–314. [DOI] [PubMed] [Google Scholar]
- 29. Olesen J, Hougård K, Hertz M. Isoproterenol and propranolol: ability to cross the blood-brain barrier and effects on cerebral circulation in man. Stroke. 1978;9(4):344–349. [DOI] [PubMed] [Google Scholar]
- 30. Bart J, Dijkers ECF, Wegman TD, et al. New positron emission tomography tracer [11C]carvedilol reveals P-glycoprotein modulation kinetics. Br J Pharmacol. 2005;145(8):1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elsinga P, Hendrikse N, Bart J, Vaalburg W, Waarde A. PET studies on P-Glycoprotein function in the blood-brain barrier: how it affects uptake and binding of drugs within the CNS. Curr Pharm Des. 2004;10(13):1493–1503. [DOI] [PubMed] [Google Scholar]
- 32. do Vale GT, Ceron S, Gonzaga NA, Simplicio JA., Padovan JC. Three generations of β-blockers: History, class differences and clinical applicability. Curr Hypertens Rev. 2019;15(1):22–31. [DOI] [PubMed] [Google Scholar]
- 33. López-Sendón J, Swedberg K, Mcmurray Jet al. Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J 2004;25:1341–1362. [DOI] [PubMed] [Google Scholar]
- 34. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. [DOI] [PubMed] [Google Scholar]
- 35. Wong GW, Boyda HN, Wright JM. Blood pressure lowering efficacy of beta-1 selective beta blockers for primary hypertension. Cochrane Database Syst Rev. 2016;3(3):CD007451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wong GW, Wright JM. Blood pressure lowering efficacy of nonselective beta-blockers for primary hypertension. Cochrane Database Syst Rev. 2014;(2):CD007452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shang X, Hill E, Zhu Z, et al. The association of age at diagnosis of hypertension with brain structure and incident dementia in the UK biobank. Hypertension. 2021;78(5):1463–1474. [DOI] [PubMed] [Google Scholar]
- 38. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mendez MF, et al. Early-ons zheimer disease. Neurol Clin. 2017;35(2):263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Phung TKT, Andersen BB, Høgh P, Kessing LV, Mortensen PB, Waldemar G. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord. 2007;24(3):220–228. [DOI] [PubMed] [Google Scholar]
- 42. Prince M, Albanese E, Guerchet M, Prina M. World Alzheimer Report 2014: Dementia and Risk Reduction. Alzheimer’s Disease International; 2014. Accessed 7 March 2019. https://www.alz.co.uk/research/WorldAlzheimerReport2014.pdf [Google Scholar]
- 43. Silva MVF, Loures CMG, Alves LCV, de Souza LC, Borges KBG, Carvalho MG. Alzheimer’s disease: risk factors and potentially protective measures. J Biomed Sci. 2019;26(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bang LE, Bruun NE, Christensen KL, Ibsen H, Svendsen TL. Hypertensio arterialis - behandlingsvejledning. Dansk Hypertensionsselskab; 2009. Accessed 21 August 2019.https://www.dahs.dk/guidelines/ [Google Scholar]
- 45. Trompet S, Westendorp RGJ, Kamper AM, de Craen AJM. Use of calcium antagonists and cognitive decline in old age. Neurobiol Aging. 2008;29(2):306–308. [DOI] [PubMed] [Google Scholar]
- 46. Barthold D, Joyce G, Wharton W, Kehoe P, Zissimopoulos J. The association of multiple anti-hypertensive medication classes with Alzheimer’s disease incidence across sex, race, and ethnicity. PLoS One. 2018;13(11):e0206705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Khachaturian AS. Antihypertensive medication use and incident Alzheimer disease: the cache county study. Arch Neurol. 2006;63(5):686. [DOI] [PubMed] [Google Scholar]
- 48. Tadic M, Cuspidi C, Hering D. Hypertension and cognitive dysfunction in elderly: blood pressure management for this global burden. BMC Cardiovasc Disord. 2016;16(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Borgan Ø. Aalen-Johansen estimator. In: Armitage P, Colton T, eds. Encyclopedia of biostatistics. John Wiley & Sons, Ltd; 2005:b2a11001. [Google Scholar]
- 50. Therneau TM, Grambsch PM. 5.4 Regression splines. In: Modeling survival data: extending the cox model. 1st ed. Statistics for Biology and Health. Springer; 2000. Accessed 8 June 2020. https://public.ebookcentral.proquest.com/choice/publicfullrecord.aspx?p=5575752 [Google Scholar]
- 51. Ozenne BMH, Scheike TH, Stærk L, Gerds TA. On the estimation of average treatment effects with right-censored time to event outcome and competing risks. Biom J. 2020;62(3):751–763. [DOI] [PubMed] [Google Scholar]
- 52. Ozenne B, Sørensen AL, Scheike T, Torp-Pedersen C, Gerds TA. riskRegression: predicting the risk of an event using cox regression models. R J. 2017;9(2):440–460. [Google Scholar]
- 53. Gant T, Crowland K. A practical guide to getting started with propensity scores. Paper 689-2017. 2017. Accessed 10 August 2020.https://support.sas.com/resources/papers/proceedings17/0689-2017.pdf
- 54. Pan W, Bai H. Propensity score analysis: fundamentals and developments. The Guilford Press; 2015. [Google Scholar]
- 55. Kosanke J, Bergstralh E. Gmatch Macro. Mayo Clinic Research, Division of Biomedical Statistics and Informatics; 2004. http://bioinformaticstools.mayo.edu/research/gmatch/ [Google Scholar]
- 56. Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2017;1(1):CD002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Foote SL, Berridge CW. New developments and future directions in understanding locus coeruleus–Norepinephrine (LC-NE) function. Brain Res. 2019;1709:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hablitz LM, Vinitsky HS, Sun Q, et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv. 2019;5(2):eaav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Broese M, Riemann D, Hein L, Nissen C. α-Adrenergic receptor function, arousal and sleep: mechanisms and therapeutic implications. Pharmacopsychiatry. 2012;45(06):209–216. [DOI] [PubMed] [Google Scholar]
- 60. Lilius TO, Blomqvist K, Hauglund NL, et al. Dexmedetomidine enhances glymphatic brain delivery of intrathecally administered drugs. J Controlled Release. 2019;304:29–38. [DOI] [PubMed] [Google Scholar]
- 61. Ko DT, Herbert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. β-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA. 2002;288(3):351. [DOI] [PubMed] [Google Scholar]
- 62. Hecht K, Vogt WF, Wachtel E, Fietze I. [Relationship between insomnia and arterial hypotension]. Pneumologie. 1991;45(Suppl 1):196–199. [PubMed] [Google Scholar]
- 63. Mather M. Noradrenaline in the aging brain: Promoting cognitive reserve or accelerating Alzheimer’s disease? Semin Cell Dev Biol. 2021;116:108–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Evans AK, Ardestani PM, Yi B, Park HH, Lam RK, Shamloo M. Beta-adrenergic receptor antagonism is proinflammatory and exacerbates neuroinflammation in a mouse model of Alzheimer’s Disease. Neurobiol Dis. 2020;146:105089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chai G-S, Wang Y-Y, Yasheng A, Zhao P. Beta 2-adrenergic receptor activation enhances neurogenesis in Alzheimer’s disease mice. Neural Regen Res. 2016;11(10):1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Branca C, Wisely EV, Hartman LK, Caccamo A, Oddo S. Administration of a selective β2 adrenergic receptor antagonist exacerbates neuropathology and cognitive deficits in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2014;35(12):2726–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dobarro M, Gerenu G, Ramírez MJ. Propranolol reduces cognitive deficits, amyloid and tau pathology in Alzheimer’s transgenic mice. Int J Neuropsychopharmacol. 2013;16(10):2245–2257. [DOI] [PubMed] [Google Scholar]
- 68. Rosenberg PB, Mielke MM, Tschanz J, et al. Effects of cardiovascular medications on rate of functional decline in Alzheimer disease. Am J Geriatr Psychiatry. 2008;16(11):883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yu J-T, Tan L, Ou J-R, et al. Polymorphisms at the β2-adrenergic receptor gene influence Alzheimer’s disease susceptibility. Brain Res. 2008;1210:216–222. [DOI] [PubMed] [Google Scholar]
- 70. Wagner G, Icks A, Abholz H-H, Schröder-Bernhardi D, Rathmann W, Kostev K. Antihypertensive treatment and risk of dementia: a retrospective database study. Int J Clin Pharmacol Ther. 2012;50(03):195–201. [DOI] [PubMed] [Google Scholar]
- 71. McDevitt DG. Pharmacologic aspects of cardioselectivity in a beta-blocking drug. Am J Cardiol. 1987;59(13):F10–F12. [DOI] [PubMed] [Google Scholar]
- 72. Baker JG. The selectivity of β-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br J Pharmacol. 2005;144(3):317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang J-H, Wu Y-J, Tee BL, Lo RY. Medical comorbidity in Alzheimer’s disease: a nested case-control study. J Alzheimers Dis. 2018;63(2):773–781. [DOI] [PubMed] [Google Scholar]
- 74. Manrique C, Giles TD, Ferdinand KC, Sowers JR. Realities of newer β-blockers for the management of hypertension. J Clin Hypertens. 2009;11(7):369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rouch L, Cestac P, Hanon O, et al. Antihypertensive drugs, prevention of cognitive decline and dementia: a systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms. CNS Drugs. 2015;29(2):113–130. [DOI] [PubMed] [Google Scholar]
- 76. Holm H, Ricci F, Di Martino G, et al. Beta-blocker therapy and risk of vascular dementia: A population-based prospective study. Vascul Pharmacol. 2020;125–126:106649. [DOI] [PubMed] [Google Scholar]
- 77. Walker VM, Davies NM, Martin RM, Kehoe PG. Comparison of antihypertensive drug classes for dementia prevention. Epidemiology. 2020;31(6):852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yasar S, Xia J, Yao W, et al. Antihypertensive drugs decrease risk of Alzheimer disease: Ginkgo evaluation of memory study. Neurology. 2013;81(10):896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to anonymized Danish National Registry data for research purposes is controlled by Statistics Denmark.