Abstract
BACKGROUND
In 2020, SARS-CoV-2 and the COVID-19 pandemic had a huge impact on the access to and provision of ART treatments. Gradually, knowledge of the virus and its transmission has become available, allowing ART activities to resume. Still, questions on the impact of the virus on human gametes and fertility remain.
OBJECTIVE AND RATIONALE
This article summarizes published data, aiming to clarify the impact of SARS-CoV-2 and the COVID-19 disease on human fertility and assisted reproduction, as well as the impact of vaccination, and from this, provide answers to questions that are relevant for people contemplating pregnancy and for health care professionals.
SEARCH METHODS
PUBMED/MEDLINE and the WHO COVID-19 database were searched from inception to 5 October 2022 with search terms focusing on ‘SARS-CoV-2’ and gametes, embryos, reproductive function, fertility and ART. Non-English studies and papers published prior to 2020 were excluded, as well as reviews and non-peer reviewed publications. Full papers were assessed for relevance and quality, where feasible.
OUTCOMES
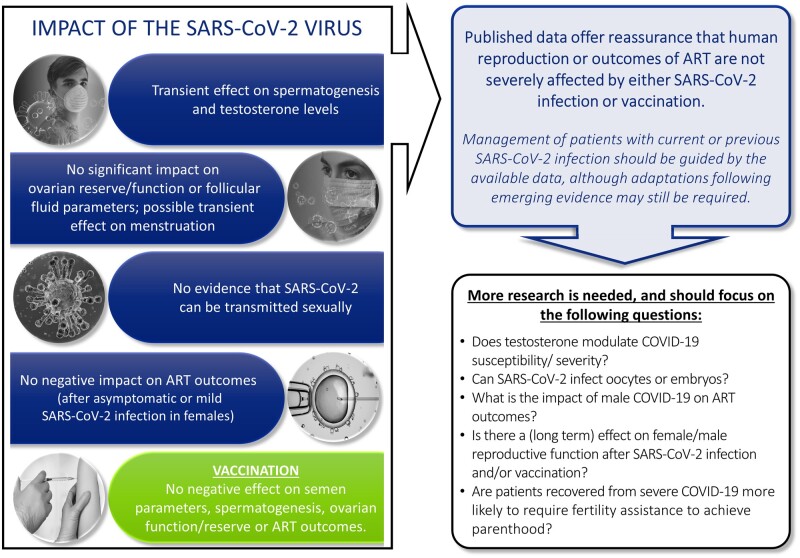
From the 148 papers included, the following observations were made. The SARS-CoV-2-binding proteins, angiotensin-converting enzyme 2 (ACE2) and type II transmembrane serine protease (TMPRSS2), are expressed in the testis, but co-expression remains to be proven. There is some evidence of SARS-CoV-2 RNA in the ejaculate of COVID-19 patients with severe disease, but not in those with mild/moderate disease. SARS-CoV-2 infection can impair spermatogenesis, but this seems to resolve after one spermatogenic cycle. Testosterone levels seem to be lower during and after COVID-19, but long-term data are lacking; disease severity may be associated with testosterone levels. COVID-19 cannot be considered a sexually transmitted disease. There is no co-expression of ACE2 and TMPRSS2 in the myometrium, uterus, ovaries or fallopian tubes. Oocytes seem to have the receptors and protease machinery to be susceptible to SARS-CoV-2 infection; however, viral RNA in oocytes has not been detected so far. Women contemplating pregnancy following COVID-19 may benefit from screening for thyroid dysfunction. There is a possible (transient) impact of COVID-19 on menstrual patterns. Embryos, and particularly late blastocysts, seem to have the machinery to be susceptible to SARS-CoV-2 infection. Most studies have not reported a significant impact of COVID-19 on ovarian reserve, ovarian function or follicular fluid parameters. Previous asymptomatic or mild SARS-CoV-2 infection in females does not seem to negatively affect laboratory and clinical outcomes of ART. There are no data on the minimum required interval, if any, between COVID-19 recovery and ART. There is no evidence of a negative effect of SARS-CoV-2 vaccination on semen parameters or spermatogenesis, ovarian function, ovarian reserve or folliculogenesis. A transient effect on the menstrual cycle has been documented. Despite concerns, cross reactivity between anti-SARS-CoV-2 spike protein antibodies and Syncytin-1, an essential protein in human implantation, is absent. There is no influence of mRNA SARS-CoV-2 vaccine on patients’ performance during their immediate subsequent ART cycle. Pregnancy rates post-vaccination are similar to those in unvaccinated patients.
WIDER IMPLICATIONS
This review highlights existing knowledge on the impact of SARS-CoV-2 infection or COVID-19 on fertility and assisted reproduction, but also identifies gaps and offers suggestions for future research. The knowledge presented should help to provide evidence-based advice for practitioners and couples contemplating pregnancy alike, facilitating informed decision-making in an environment of significant emotional turmoil.
Keywords: COVID-19, embryo, assisted reproduction, infertility, vaccination, clinical practice
Graphical Abstract
Graphical Abstract.
The review summarizes the data concerning the SARS-CoV-2 virus, COVID-19 and SARS-CoV-2 vaccination and its impact on human gametes, endocrinological processes, reproduction and fertility.
Introduction
In March 2020, when SARS-CoV-2 spread across the globe, the World Health Organization (WHO) declared the COVID-19 pandemic, which initiated an abrupt and unexpected disruption of activities, including provision of non-emergency health services. The benefit of continuing fertility treatments did not outweigh the emerging risk of contamination and infection with SARS-CoV-2, and significant unknowns on the SARS-CoV-2 virus, including its mode of transmission, its ability to survive freezing, and its impact on gametes, pregnancy and the health of children born. In consequence, recommendations were made to temporarily suspend fertility services (ESHRE—European Society of Human Reproduction and Embryology, 2020). ART activities were paused for a mean of 7 weeks between March and May 2020 in many ART centres across Europe (ESHRE Covid-19 Working Group et al., 2020) and in other regions (Ory et al., 2020; Tan et al., 2020; Qiao and National Expert Group for Quality Management on Assisted Reproductive Technology, 2021). Urgent fertility preservation treatments, allowing for instance cancer patients to store gametes prior to starting gonadotoxic treatments, were carried out during this period in some centres under specific safety measures to mitigate possible risks (Adiga et al., 2020).
The suspension of fertility services, even for a limited period of time, affected treatment waiting lists, especially in countries where such lists were already long. Two years down the road, the situation does not seem to have resolved. An additional challenge remains the age limit for access to or funding of treatment in public clinics. The COVID-19 pandemic-related delays in treatment caused some patients to reach the age limit strictly followed by funding institutions, acting as a barrier to accessing treatment. Also, the significant economic impact of the pandemic, including loss of jobs or temporary layoffs, made it impossible for some patients to start or continue with their treatments.
As such, the suspension of fertility services had a huge impact for patients and couples in the midst of fertility therapy or on a waiting list to start ART. In addition, fertility treatments are known to be physically and financially challenging and cause a significant psychological burden on patients. The COVID-19 pandemic, as well as the pandemic-related increased waiting time to start fertility treatment, has caused further anxiety, stress and sorrow among infertility patients (Boivin et al., 2020; Esposito et al., 2020; Kaur et al., 2020; Rosielle et al., 2021; Barra et al., 2022; Gurtin et al., 2022). Especially the uncertainty regarding how long ART treatments would be suspended, how the pandemic would evolve, and how it would affect fertility treatment and pregnancy increased the psychological toll on patients. The strict conditions under which clinics operated after resumption of activities, for example partners not being allowed into the clinic in some centres, also contributed to patients’ stress levels.
The impact of the suspension of activities in ART clinics on patients’ chances of achieving a live birth are debated. A study in the UK estimated, based on a population data-based model, that a 6 month delay in starting fertility treatment would reduce the chance of a live birth by 9.8% and 11.8%, in women aged 38–39 and 40–42 years, respectively (Bhattacharya et al., 2021). Apart from age, the cause of infertility was another factor affecting the chance of success after a delay in treatment. Contradicting these results, two retrospective studies reported no reduction in the chance of success with a treatment delay of up to 180 days in women with diminished ovarian reserve or for a delay of up to 1 year in women aged 39 years or above with unexplained infertility (Romanski et al., 2020; Carosso et al., 2022).
On a population level, it was estimated that the COVID-19 pandemic would have a profound impact on the IVF live birth in the USA, with up to 25 143 fewer live births between 2020 and 2023 due to the temporary suspension of ART activities and the economic recession associated with the pandemic (Gromski et al., 2021). The significant impact on cumulative live birth rates due to the delay in fertility treatment was specifically significant in older women (>40 years old) (Smith et al., 2020). In contrast, a study in Italy reported a decrease of 5.1% in the monthly birth rate in 2020 as compared to 2019, but calculated that this could be largely attributed to a decrease in natural conceptions while the closing of ART centres only played a marginal role (Somigliana et al., 2021). Others have suggested that the pandemic may increase the importance of family and increase family building (Lambalk et al., 2020). The prospective annual ESHRE IVF monitoring data collection will in due course provide further data on the specific impact of the pandemic on the number of ART procedures performed and the possible rebound afterwards.
Another aspect is COVID-19 vaccination, which was largely recommended by WHO and national governments to provide strong protection against serious illness, hospitalization and death from COVID-19. The exclusion of pregnant women from the vaccine trials resulted in a lack of data on whether the vaccines were safe to be used before or during fertility treatment or during pregnancy. This uncertainty led to some hesitancy towards vaccination, which lasted even after the publication of the studies showing that the COVID-19 mRNA vaccines do not negatively affect the pregnancy or the new-born baby.
Gradually, knowledge of SARS-CoV-2 and its transmission has become available and has allowed ART activities to resume within certain restrictions. COVID-19 vaccination was shown to be safe, including for pregnant women. Still, questions remained on the impact of the virus and vaccination on gametes and fertility. Answers to such questions are needed to support clinics in proceeding with safe ART treatments in the post-COVID-19 era, both in the short- and the long term. Additionally, the lessons learned from the COVID-19 pandemic should be recorded to allow for more informed guidance and recommendations in future emerging situations. This article aims to elucidate the impact of SARS-CoV-2, COVID-19 disease and vaccination on human reproduction for the purpose of answering questions that are relevant for people contemplating pregnancy and health care professionals.
Methods
A literature review was performed to collect studies and reports focusing on SARS-CoV-2 and human reproduction (gametes, embryos, reproductive function, fertility and ART) as well as the impact of vaccination upon human reproduction and ART. PUBMED/MEDLINE was searched, and studies were included from inception (1966) up to 5 October 2022; all retrieved references were checked for relevance. Similar search terms were applied to the COVID-19 database of the WHO (https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/).
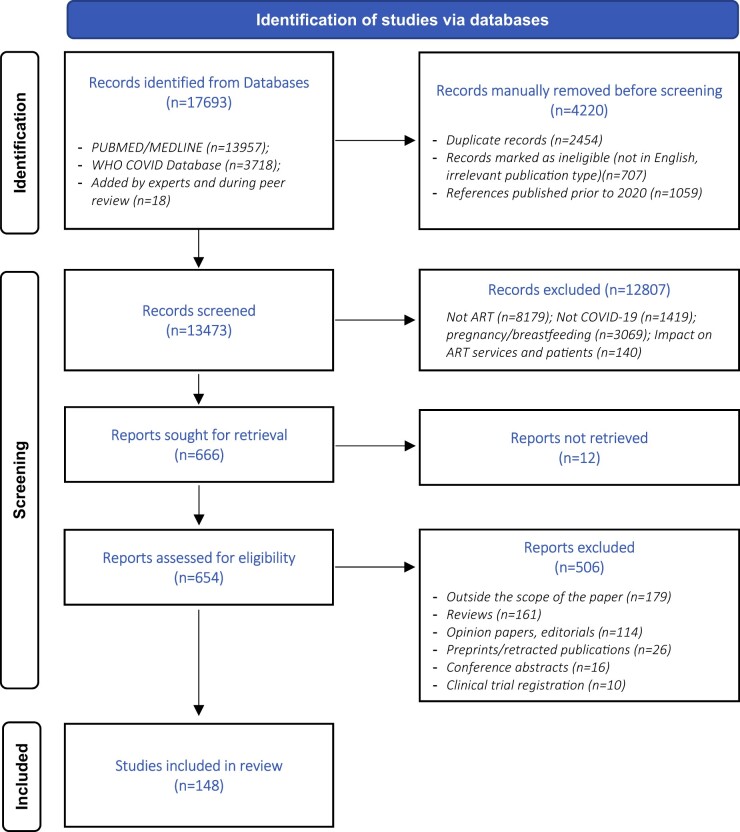
Non-English language studies and papers published before the start of the COVID-19 pandemic (prior to 2020) were excluded from the retrieved references. Titles and abstracts of the remaining references were screened against the inclusion criteria, which were to report on SARS-CoV-2, COVID-19 or COVID-19 vaccination and to describe the impact on reproductive tissues and cells, including gametes (oocytes or sperm) and embryos, gamete development, reproductive function, endocrine function, male and female fertility and ART treatment outcomes. Full-text papers of relevant references were collected and assessed. Papers that did not fit the inclusion criteria upon full-text evaluation, e.g. those focusing on the impact of COVID-19 on ART services and ART patients’ psychosocial factors, were further excluded. With regards to the publication type, original studies were selected, and reviews, opinion papers, editorials and other types of papers were excluded from the evidence. Conference abstract and non-peer reviewed publications were excluded as well. The results of the literature search are summarized in a PRISMA flowchart (Fig. 1) (Page et al., 2021). Full papers were assessed for relevance and quality, where feasible, as some of the published COVID-19 studies have small samples and short durations, which undermines the relevance of performing thorough assessment of quality.
Figure 1.
PRISMA flow diagram.
Results
Coronaviruses enter hosts cells by binding of the viral spike (S) protein to cell receptors and, upon S protein priming, by host cell proteases (Laporte and Naesens, 2017; Hoffmann et al., 2020). SARS-CoV-2 uses the angiotensin-converting enzyme 2 (ACE2) receptor and the cell protease type II transmembrane serine protease (TMPRSS2) to bind to the cell and for virus–cell fusion (Hoffmann et al., 2020). This indicates that the co-expression of ACE2 and TMPRSS2 can predict the potential of cells to be prone to infection (Matsuyama et al., 2020; Zang et al., 2020). In addition to ACE2 and TMPRSS2, another possible mechanism suggested for SARS-CoV-2 viral entry, although not considered confirmed by others, is mediated through CD147 or Basigin receptor (BSG) with cysteine protease cathepsin L (CTSL) as the protease (Ulrich and Pillat, 2020; Vilella et al., 2021).
COVID-19 impact on male reproductive organs, gametes and endocrine function
Receptors facilitating SARS-COV-2 entry in testicular tissue, Sertoli cells, Leydig cells and spermatozoa
Studies using public single cell RNA sequencing (scRNA-seq) datasets reported a high expression level of ACE2 and TMPRSS2 in the testis including spermatogonia, peritubular myoid cell, testis somatic cells and spermatogonial stem cells (Qi et al., 2021). The testis has thus been categorized as an organ with a high susceptibility to SARS-CoV-2. This conclusion is controversial as other authors reported that even if ACE2 is highly expressed in the testis (Al-Benna, 2021; Cai et al., 2021), co-expression with TMPRSS2 is not observed, suggesting that testicular cells do not have a high risk of viral entry and infection (Baughn et al., 2020; Pan et al., 2020; Borges et al., 2021).
In comprehensive studies, ACE2 protein expression has been documented in the testis by immunohistochemistry and compared with transcriptomics, scRNA-seq datasets, western blot and mass spectrometry data (Fu et al., 2020; Hikmet et al., 2020).
Studies using scRNA-seq datasets of male embryo primordial germ cells and normal testis cells to assess the possible effects of SARS-CoV-2 demonstrated the expression of ACE2 in all testis cell types and Sertoli cells (Liu et al., 2020; Shen et al., 2020). BSG and CTSL co-expression, at lower levels, has been detected in Leydig cells, myoid cells, endothelial cells and differentiating spermatogonia (Stanley et al., 2020). COVID-19 has been shown to damage both Sertoli and Leydig cells (Yang et al., 2020; Nie et al., 2021).
These studies point to the potential entry of SARS-CoV-2 infection in the male reproductive system.
Histopathology, ultrastructural microscopy and immunofluorescence investigations of prostate tissue, retrieved after surgery for benign indications in a man recovering from COVID-19, showed the presence of viral particles in the prostate 4 months after the infection (Reddy et al., 2022).
Through scRNA-seq datasets analysis, ACE2 expression, but no co-expression of ACE2 and TMPRSS2, was reported in sperm (Stanley et al., 2020), and BSG/CSTL co-expression was reported in early and late primary spermatocytes (Stanley et al., 2020). The ACE2 protein is expressed on mature spermatozoa after ejaculation, as documented from western blot analysis (Ramal-Sanchez et al., 2022).
Presence of SARS-CoV-2 RNA in semen
The presence of SARS-CoV-2 in the sperm of men affected by COVID-19 has been extensively studied at different stages, including after patient recovery, as well as in relation to the severity of the infection.
Some studies have suggested the absence of SARS-CoV-2 RNA in semen samples of asymptomatic COVID-19-positive men or those suffering or recovering from mild COVID-19, even though they were still in the acute phase and positive for the virus in the nasopharyngeal swab (Holtmann et al., 2020; Rawlings et al., 2020; Best et al., 2021; Burke et al., 2021; Guo et al., 2021a; Gupta et al., 2021; Ma et al., 2021; Paoli et al., 2021a; Ruan et al., 2021; Scroppo et al., 2021; SharMa et al., 2021; Temiz et al., 2021; Donders et al., 2022; Fraietta et al., 2022; Huang et al., 2022b; Pavone et al., 2022). Other authors reported the presence of SARS-CoV-2 in the semen and in the seminal plasma fraction (by PCR testing) in the acute phase and after recovery after mild and moderate COVID-19 in a limited percentage of patients, respectively, in 4/15, 1/6, 1/31, 1/19 and 6/38 cases (Li et al., 2020a; Delaroche et al., 2021; Machado et al., 2021; Purpura et al., 2022). One study additionally reported SARS-CoV-2 in 2 of 23 patients (8.7%) who were recovering from COVID-19 (Li et al., 2020a).
In men with severe COVID-19 disease, presence of the virus in the semen was not shown in one study (Kayaaslan et al., 2020), while another study detected SARS-CoV-2 in the semen of 4 out of 30 men, all with acute infection, severe pneumonia and high viral load (Saylam et al., 2021). Scrotal discomfort, orchitis and epididymitis have been described in severe infections (Pan et al., 2020; Achua et al., 2021; Chen et al., 2021; Ruan et al., 2021; Duarte-Neto et al., 2022). The presence of viral particles in the semen due to residual urinary shedding cannot be ruled out (Holtmann et al., 2020; Paoli et al., 2021b; Saylam et al., 2021).
A recent systematic review calculated a detection rate of SARS-CoV-2 mRNA in 7% (range 5–11%) of men with COVID-19, which was independent of patient age, disease severity or associated comorbidities (Corona et al., 2022). The only factor significantly associated with detection rate was found to be period between diagnosis of COVID-19 and semen analysis; SARS-CoV-2 mRNA was more often found if the semen sample was collected within 11 days of COVID-19 diagnosis (Corona et al., 2022).
COVID-19: impact on sperm parameters and spermatogenesis
Most publications have reported alterations in sperm quality when compared to controls both in the number and motility of sperm during the course of COVID-19 disease (Holtmann et al., 2020; Best et al., 2021; Gacci et al., 2021; Gupta et al., 2021; Hajizadeh Maleki and Tartibian, 2021; Ruan et al., 2021; Donders et al., 2022; Enikeev et al., 2022). In a multicentre study, significant differences were reported in sperm motility and vitality for mild symptomatic patients and in all sperm parameters for moderate symptomatic patients after COVID-19 as compared to before infection (Erbay et al., 2021). In contrast, a recent study of 594 patients indicates that the number of patients who presented for infertility in a urology clinic was not increased and sperm quality was similar during the pandemic when compared to pre-pandemic values (Sarier et al., 2022). Others also have reported no significant difference in semen volume, sperm count or motility before versus after COVID-19 (Gul et al., 2021; Pazir et al., 2021; Temiz et al., 2021).
Sperm morphology was shown to be affected during the acute phase of the infection (Holtmann et al., 2020; Hajizadeh Maleki and Tartibian, 2021; Temiz et al., 2021; Fraietta et al., 2022). Data for morphology before and after the infection are available in one publication which reported a significant decrease in normal morphology, as well as sperm count and concentration, after SARS-CoV-2 infection as compared to pre-pandemic samples (Hamarat et al., 2022).
While some authors report no differences in sperm morphology between mild and moderate disease (Scroppo et al., 2021), other publications support evidence for a strong correlation between sperm parameters and the severity of the disease (Holtmann et al., 2020). It seems that the infection affects spermatogenesis and that this effect can last at least for a whole spermatogenic cycle after recovery (Gacci et al., 2021; Guo et al., 2021b; Hajizadeh Maleki and Tartibian, 2021; Donders et al., 2022). Return to normal sperm morphology values is observed with increasing time after recovery (Guo et al., 2021a; Donders et al., 2022; Hu et al., 2022). Emerging evidence showed that key biological pathways relevant to male reproductive function are disrupted in COVID-19 convalescent men (Ghosh et al., 2022).
It remains to be elucidated whether impairment of spermatogenesis is due to direct or indirect effects of the infection. Specific pathogenic mechanisms of the virus including viral replication and dissemination leading to testicular inflammation and damage together with the effect of fever, medication and psychological stress due to the COVID-19 can play complementary roles. Additionally, the immune response in testicular tissues and the epididymis during SARS-CoV-2 infection could result in impaired spermatogenesis (Li et al., 2020b).
Most authors analysing possible transmission routes of SARS-CoV-2 do not consider that COVID-19 is a sexually transmitted disease. However, it has to be considered that the blood–testis barrier can be tampered during high viraemia and fever and the passage of viral particles cannot be completely ignored. Further research in this area is needed.
Conclusion
ACE2 and TMPRSS2 are expressed in the testis, but co-expression remains to be proven.
SARS-CoV-2 RNA is not found in the ejaculate in asymptomatic COVID-19-positive men, but there is some evidence of RNA positivity in symptomatic cases of COVID-19.
SARS-CoV-2 infection can, directly or indirectly, affect spermatogenesis, but the impairment seems to resolve after one complete spermatogenic cycle.
SARS-CoV-2 infection cannot be considered a sexually transmitted disease (male-to-female).
Suggested future research
Which factors determine SARS-CoV-2 presence in the ejaculate of men with COVID-19?
Can SARS-CoV-2 viral particles cross the blood–testis barrier, for example during high viraemia and fever?
COVID-19: impact on testosterone secretion
Sex differences in COVID-19 susceptibility, severity and fatality are widely acknowledged (de Lusignan et al., 2020; Global Health 50/50, 2020; Peckham et al., 2020). Testosterone levels, which are higher in men than women, are suspected to play a role in susceptibility to severe COVID-19 (Coelingh Bennink et al., 2021). It has been suggested that testosterone can upregulate ACE2 and TMPRSS2 expression and thereby facilitate viral entry into host cells (Chen et al., 2020a). Furthermore, testosterone exhibits an immunosuppressive effect that may contribute to the severity of COVID-19 (Ghare Naz et al., 2021). Interestingly, prostate cancer patients on testosterone deprivation therapy were found to be significantly less likely to have SARS-CoV-2 infection, supporting the testosterone theory (Montopoli et al., 2020).
On the other hand, although comprehensive data are lacking, an increasing number of studies suggest that testicular endocrine function, i.e. testosterone secretion, is impaired during acute COVID-19 infection (Çayan et al., 2020; Kadihasanoglu et al., 2021; Papadopoulos et al., 2021; RastrelLi et al., 2021; Moreno-Perez et al., 2022), and more severe disease is associated with lower levels of testosterone (Camici et al., 2021; Dhindsa et al., 2021; Salonia et al., 2021; Cinislioglu et al., 2022). It is unknown whether these men had lower testosterone levels before SARS-CoV-2 infection or whether the virus affects the production of androgens. There are studies suggesting the latter, as they showed that testosterone levels were low during the infection but increased during the healing phase, at least in a proportion of COVID-19-infected men (Apaydin et al., 2022; Salonia et al., 2022; Yamamoto et al., 2022). Still, hypogonadism, defined by decreased testosterone levels, was observed in about half of the patients during and after recovery of COVID-19 (in studies with a follow-up from 71 days to 7 months) (Apaydin et al., 2022; Salonia et al., 2022; Yamamoto et al., 2022).
COVID-19: testosterone levels and disease severity
In a cohort study of 152 COVID-19 patients, including 143 hospitalized patients, testosterone levels at the time of presentation and on Day 3 were inversely related to disease severity and circulating inflammatory cytokines in men but not in women (Dhindsa et al., 2021). Reports have also shown significant associations between serum testosterone levels, markers of inflammation, disease progression and clinical outcome in male patients with COVID-19 irrespective of age and comorbidities (Camici et al., 2021; Dhindsa et al., 2021; Ma et al., 2021; Salciccia et al., 2021). Lower testosterone levels increased the likelihood of having pneumonia (Okcelik, 2021), having a longer duration of hospitalization (Camici et al., 2021; Apaydin et al., 2022), being transferred to intensive care (ICU) or dying (Lanser et al., 2021; Papadopoulos et al., 2021; RastrelLi et al., 2021; Vena et al., 2022). Moreover, in men with COVID-19, normalization of testosterone levels or a failure to normalize were found to be associated with survival or death, respectively (Toscano-Guerra et al., 2022). Of interest, a study comparing 32 men diagnosed with COVID-19 while on testosterone replacement therapy with 63 men with COVID-19 but not on testosterone treatment, found no association between testosterone treatment and the outcome of COVID-19 with regards to hospitalization, ICU admission, complications or death (Rambhatla et al., 2021).
Further studies are needed to determine whether there is a causal relationship between severe acute illness and testosterone levels and to confirm pathophysiological assumptions. As low testosterone levels during COVID-19 appear to be a marker of disease severity, it may be relevant to consider testosterone levels in predictions of prognosis and possibly also in treatment planning.
Conclusion
While testosterone at the physiological level for males may predispose to COVID-19, testosterone levels seem to be lower during and after COVID-19.
In a significant proportion of male patients, hypogonadism is detected in the mid-term after the infection, but long-term data are lacking.
Data indicate a link between testosterone levels and disease severity.
Suggested future research
Does the testosterone level modulate susceptibility to and the severity of COVID-19?
COVID-19 impact on female reproductive organs, gametes and endocrine function
Several studies indicate that SARS-CoV-2 virus may affect female reproductive functions, and thereby potentially also female fertility. As described above, the virus has been shown to enter the host cells through either the ACE2/TMPRSS2 route or the BSG/CTSL pathway. Both of these mechanisms could play a role in mediating endometrial and ovarian function during follicle development and corpus luteum formation.
Impact on female lower genital tract
An evaluation of vaginal samples from 38 women with a positive nasopharyngeal SARS-CoV-2 PCR test did not detect the virus in any of the samples (Takmaz et al., 2021a). These findings were supported by others evaluating, respectively, vaginal fluid from 10 women with confirmed severe COVID-19 pneumonia, cervico-vaginal secretions from 35 COVID-19-positive women, and vaginal fluid and cervical exfoliated cells retrieved from 35 women, between 8 and 41 days after COVID-19 symptom onset (Cui et al., 2020; Qiu et al., 2020; Agarwal et al., 2021). These publications indicate a low risk of transmission of the virus by vaginal intercourse from women to men.
However, contradictory results were also reported. In two studies, SARS-CoV-2 RT-PCR-positive results were reported for 2 out of 35 (5.7%) vaginal samples from COVID-19-positive patients and for 10 out of 80 vaginal swabs of women with acute COVID-19 (Schwartz et al., 2021; Atarod et al., 2022). A third study used transcription-mediated amplification for SARS-CoV-2 detection, and found viral presence in 5 out of 61 (8.2%) vaginal samples and 4 out of 38 (10.53%) cervical swabs (Khoiwal et al., 2022).
Impact on female upper genital tract and oocytes
From available scRNA-seq datasets, co-expression of ACE2 and TMPRSS2 has been reported in the fallopian tube (Qi et al., 2021). High mRNA expression as well as protein expression of ACE2 has been reported in the placenta (Hikmet et al., 2020). No co-expression of ACE2 with TMPRSS2 nor CTSL expression was found in the myometrium, uterus, ovaries or fallopian tubes when investigating published scRNA-seq datasets and reproductive tissue from patients undergoing hysterectomy (Goad et al., 2020).
Another analysis of scRNA-seq datasets reported low efficiency of SARS-CoV-2 infection for the endometrium based on no significant expression of ACE2 in stromal or unciliated epithelial cells and no co-expression of ACE2 and TMPRSS2 at the time of embryo implantation (Vilella et al., 2021). Combining gene expression data from 5 studies of 112 patients confirmed low expression of ACE2, medium expression of TMPRSS2 and limited co-expression in the endometrium (Henarejos-Castillo et al., 2020). Both studies reported that BSG was highly expressed in the endometrium, although they did not agree on the relevance of the BSG machinery for viral infection (Henarejos-Castillo et al., 2020; Vilella et al., 2021).
Endometrial samples were not found to contain SARS-CoV-2 RNA (Boudry et al., 2022; de Miguel-Gomez et al., 2022). Follicular fluid and cumulus cells from 16 women who had a positive SARS-CoV-2 nasopharyngeal RNA test < 48 h before oocyte retrieval were also investigated, but viral RNA was not detected in any of the samples (Boudry et al., 2022). In addition, endometrial biopsies taken during OPU showed no histopathologic changes. The authors pointed out that their results need to be considered with caution as the study included only a small number of patients, none with severe COVID-19 symptoms, and they all had different viral loads. In addition, the tests were performed with a technique validated for nasopharyngeal samples. Still, other studies confirmed the absence of the virus in follicular fluid from SARS-CoV-2-positive women (Demirel et al., 2021). Based on scRNA-seq dataset research, the uterus is considered to be at low risk of SARS-CoV-2 infection (Qi et al., 2021).
Ovarian follicular cells, i.e. cumulus and granulosa cells, showed BSG and CTSL mRNA expression, and co-expression of both BSG and CTSL proteins in low abundance (Rajput et al., 2021). ACE2 mRNA and protein expression was reported to be upregulated in human ovulatory follicles after administration of hCG (Choi et al., 2021). SARS-CoV-2 infection affecting ovarian follicles and their function seems possible and was recently confirmed, at least in the laboratory, through co-culture of granulosa and cumulus cells with SARS-CoV-2 (Luongo et al., 2022). No SARS-CoV-2 viral RNA was found in cumulus cells from 16 COVID-19-positive women with no or light symptoms undergoing ART (Boudry et al., 2022).
Co-expression of ACE2 and TMPRSS2 was also reported in human ovarian cortex and medulla, and oocytes based on scRNA-seq datasets (Stanley et al., 2020; Rajput et al., 2021; Wu et al., 2021). In oocytes, the degree of ACE2 and TMPRSS2 co-expression increased with maturity (Stanley et al., 2020). Another study using scRNA‐seq datasets reported that ACE2 is mainly expressed during gametogenesis in oocytes of antral follicles, granulosa cells of antral follicles and pre‐ovulatory follicles, while TMPRSS2 has lower expression in oocytes or granulosa cells (Cheng et al., 2021).
Immunohistochemistry and confocal microscopy studies of human primary oocytes donated for research visualized ACE2 and BSG in human oocytes, and revealed the presence of BSG and absence of ACE2 in the oolemma (Essahib et al., 2020). However in another study, the BSG protein was not detected in oocytes (Rajput et al., 2021). Assessment of 16 mature oocytes from two women who underwent ovarian stimulation and oocyte retrieval while SARS-CoV-2-positive (PCR testing) showed no viral RNA in any of the oocytes (Barragan et al., 2021).
Conclusion
The risk of transmission of the virus by vaginal intercourse is low.
There is no co-expression of ACE2 and TMPRSS2 in the myometrium, uterus, ovaries or fallopian tubes, indicating no or very low susceptibility to SARS-CoV-2 infection.
Oocytes seem to have the receptor/protease machinery to be susceptible to SARS-CoV-2 infection, but viral RNA in oocytes has not been detected so far.
Suggested future research
Is the co-expression of entry factors for SARS-CoV-2 as reported based on published scRNA-seq datasets confirmed from evaluation of tissues and cells of infected patients?
Can SARS-CoV-2 infect oocytes?
Impact on endocrine function
SARS-CoV-2 infection can affect endocrine glands through various mechanisms including a direct impact, indirect damage through immune response and/or activation of hypothalamo–pituitary axis by the inflammatory status (Geslot et al., 2022).
The effect of COVID-19 on thyroid function, the endometrium and menstrual cycle has been studied, as well as the effect on female hormones, ovarian reserve and follicular fluid parameters. As the latter parameters are most often evaluated in women starting or undergoing ART treatments, the information is covered in the section ‘Impact of COVID-19 on ART’.
The thyroid gland seems to be particularly susceptible to damage by SARS-CoV-2, with non-thyroidal illness syndrome, characterized by normal thyroid functions, decreased free T3 and thyroiditis as the most common clinical manifestations. Thyroid dysfunction seems to be related to the severity of SARS-CoV-2 infection (Lui et al., 2021).
It is assumed that destruction of thyroid follicular cells during a cytokine storm of the acute phase of SARS-CoV-2 infection can result in thyroiditis. Between 11 and 15% of hospitalized patients have thyrotoxicosis (Lania et al., 2020; Muller et al., 2020). The period between respiratory symptoms and diagnosis of thyroiditis is variable, with average of 26 days (Christensen et al., 2022). Common symptoms include fever, tachycardia and neck pain. Patients often have lymphopenia with negative thyroid antibodies, while thyroid ultrasound findings are non-specific. An increased risk of atrial fibrillation and thromboembolism is reported in patients with thyrotoxicosis during COVID-19. Return to normal thyroid function can take time; one study reported that 87% of patients were still hypothyroid more than 3 months after COVID-19 (Brancatella et al., 2021). It seems prudent to screen COVID-19 patients for early and late thyroid dysfunction (Kazakou et al., 2021).
A number of publications have shown alterations in the menstrual patterns in SARS-CoV-2 IgG-positive women with changes in the cycle length and bleeding volume. In two studies, menstrual irregularities were reported in 16% and 28% of COVID-19 patients, respectively (Li et al., 2021; Khan et al., 2022). An association between menstrual irregularities and severity of COVID-19 disease, with respect to symptoms, was reported only in one study (Khan et al., 2022). Within 1–2 months after discharge, normal menstrual volume and normal cycle duration was reported by the majority of patients (84% and 99%, respectively), suggesting a transient impact (Li et al., 2021). While both studies give the impression that COVID-19 may be associated with oligomenorrhea, possibly due to delayed ovulation or anovulation, they have important limitations, such as small sample sizes, inclusion of only hospitalized women, lack of assessment of either follicle development by ultrasound or sex steroid levels, which prevent establishment of a causal relationship (Li et al., 2021; Khan et al., 2022). An additional aspect to consider is that the menstrual cycle is influenced by stress or anxiety (Demir et al., 2021; Nguyen et al., 2021). Indeed, studies have reported a high prevalence of irregular menstrual cycles in the general population during the pandemic, which were not related to a COVID-19 diagnosis, but rather to levels of anxiety, depression and/or stress (Takmaz et al., 2021b; Aolymat et al., 2022; Medina-Perucha et al., 2022; Ozimek et al., 2022). Indeed, a prospective study including 3858 premenopausal women who were followed up with biannual questionnaires in the Nurses’ Health 3 study reported no change in menstrual cycle length and regularity in analyses once adjusted for cycle characteristics at baseline, healthcare worker status, mental health state and local COVID-19 burden (Wang et al., 2022).
While the data on the impact of COVID-19 on the menstrual cycle are reassuring, amenorrhea and hormone levels consistent with premature ovarian insufficiency have been reported at 7–8 months after SARS-CoV-2 infection in two patients, respectively, aged 27 and 34 years (Wilkins and Al-Inizi, 2021; Puca and Puca, 2022).
Conclusion
Women contemplating pregnancy following COVID-19 may be screened for thyroid dysfunction.
There is a possible (transient) impact of COVID-19 on menstrual pattern.
Suggested future research
Does COVID-19 disease affect ovulation?
Impact of COVID-19 on embryos
A few studies have investigated the presence of the viral cell entry receptors and proteases in embryos and have showed variable levels of co-expression of ACE2/TMPRSS2 and BSG/CTSL.
Based on published scRNA‐seq datasets, ACE2 was reported to be expressed in early embryos while TMPRSS2 expression started in the late blastocyst stage, resulting in significant co‐expression only in trophectoderm of late blastocysts (Cheng et al., 2021). Other studies confirmed a strong co-expression of ACE2 and TMPRSS2 in Day 6 trophectoderm cells in peri-implantation embryos, indicating susceptibility to SARS-CoV-2 infection (Chen et al., 2020b; Weatherbee et al., 2020).
Analysis of zygotes and blastocysts donated for research showed co-expression of ACE2 and TMPRSS2, but not the ACE2-independent receptor system (BSG and CTSL) (Rajput et al., 2021). Co-expression of ACE2 and TMPRSS2 proteins was confirmed in a proportion of epiblast and trophectoderm cells (Colaco et al., 2021). Another study evaluating protein expression, reported that Days 5 and 7 blastocysts exhibit ACE2 and BSG on the membrane of trophectoderm, epiblast and hypoblast cells, which implicates that SARS-CoV-2 is, at least theoretically, able to bind and infect embryos (Essahib et al., 2020).
Using surplus embryos donated for research, Montano and colleagues demonstrated that pseudotyped virus particles expressing S protein robustly infected human trophectoderm cells. Anti-S protein and anti-ACE2 antibodies significantly decreased infection and confirmed viral entry to trophectoderm cells through the S protein—ACE2 path. When human blastocysts were exposed to live SARS-CoV-2 virus, both trophectoderm and inner cell mass cells were infected. Infection could be prevented with neutralizing antibodies against S protein or ACE2. The zona pellucida seemed protective against infection, as only one non-hatching blastocyst demonstrated evidence of infection, while infection was demonstrated on herniated trophectoderm cells or cells closer to the zona opening in partially hatched blastocysts. Infection was most common in totally hatched blastocysts. Infected cells had signs of cell degeneration suggesting cytopathic effects of infection (Montano et al., 2022). Studies evaluating the impact of COVID-19 on embryo developmental potential and ART outcomes are discussed in the next section.
Conclusion
Although data are conflicting, embryos and particularly late blastocysts seem to have the receptor/protease machinery to be susceptible to SARS-CoV-2 infection.
The presence of SARS-CoV-2 viral RNA in embryos has not been studied.
Suggested future research
Are the entry factors for SARS-CoV-2 co-expressed in human embryos at different developmental stages?
Can the SARS-CoV-2 virus infect embryos in vivo?
Impact of COVID-19 on ART outcomes
Early publications such as a large market research-based study reported an association between severe illness in the male and/or female partner within 3 months prior to natural conception and complications like preterm birth and early pregnancy loss (Kasman et al., 2020). Initial publications also raised concerns about the impact of the SARS-CoV-2 virus upon reproductive function, by interrogating databases in relation to cell entry pathways. However, further data provided reassurance for resumption of ART services with specific safeguards such as the acknowledged need for patients to be tested and COVID-19 free at the start of therapy and for staff to follow strict protocols (ESHRE Covid-19 Working Group et al., 2021).
COVID-19: impact on ovarian reserve and follicular function
Alterations of ovarian reserve and sex-related hormones could theoretically result from viral infection, such as SARS-CoV-2, and have a potential impact on fertility and fecundity. In this context, studies have evaluated anti-Mullerian hormone (AMH), antral follicle count (AFC), follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, prolactin and testosterone levels in women with COVID-19.
Early follicular-phase serum levels of FSH, LH, estradiol and AMH were similar between women hospitalized for COVID-19 and controls (Li et al., 2021). Similarly, the difference between AMH levels measured within 12 months before and those measured while starting an ART cycle was similar in asymptomatic women who tested positive or negative for COVID-19 at the beginning of ART cycle (Kolanska et al., 2021).
In contrast, significantly lower serum AMH levels were reported in 78 women with COVID-19 as compared with 151 healthy age-matched controls (Ding et al., 2021). This study also reported higher FSH, prolactin and testosterone levels in COVID-19 patients. This suggested pituitary and ovarian dysfunction associated with viral infection, but the validity of this conclusion remains to be confirmed as the results of the study could have been impacted by a difference in the time of sample collection (i.e. different phase of menstrual cycle) between patients and controls (Ding et al., 2021).
With regards to ovarian function, a small study compared women who were IgG-positive from past COVID-19 with COVID-19-naïve controls, all undergoing ovarian stimulation for ART (Bentov et al., 2021). No immunological studies were carried out for all these patients. In fact, the authors re-allocated two patients from COVID-19-naïve group to the recovered group after selective serology testing showed the presence of anti-SARS-CoV-2 IgG. Serum estradiol level on the day of ovulation trigger, ratio of estradiol/oocyte, serum progesterone levels on day of oocyte retrieval, follicular fluid estradiol and progesterone concentrations, ratio of oocytes/follicles aspirated, and oocyte maturation rates were similar between the groups. The levels of follicular fluid HSPG2 (pearlecan), an oestrogen-binding glycoprotein produced by granulosa cells and considered a marker of follicular function, were also similar between the groups (Bentov et al., 2021). An observational study evaluating ovarian parameters in 132 women with unexplained infertility before and after COVID-19 reported no statistically significant differences in terms of serum levels of AMH, FSH, LH, FSH/LH ratio or estradiol levels (Madendag et al., 2022). In this study, there was an average of nine months between the two assessments.
While reassuring, these results were only partly confirmed by a study comparing 34 women who never tested positive or reported any symptoms of COVID-19 and 46 women who had COVID-19 symptoms and tested positive at least once (Herrero et al., 2022). As in the other studies, there were no differences between the groups in serum estradiol levels on the day of ovulation trigger, basal serum estradiol, basal serum progesterone, AMH or AFC. However, the study also reported that women in the COVID-19 group had significantly lower follicular fluid interleukine-1 beta and vascular endothelial growth factor (VEGF) levels. Follicular fluid from COVID-19 patients showed decreased steroidogenic acute regulatory protein, oestrogen receptor beta and VEGF expression and increased expression of γH2AX, a marker of DNA damage, from cultured granulosa cells. Likewise, treatment of endothelial cell cultures with follicular fluid from COVID-19 patients caused significantly decreased cell migration and increased γH2AX expression, but VEGF expression or cell proliferation was not affected. Women with medium or high SARS-CoV-2 IgG levels in follicular fluid had significantly fewer (mature) oocytes collected compared to women with low antibody levels. However, the authors did not report whether women with different follicular fluid antibody levels were similar for baseline characteristics such as age, BMI, AFC and AMH. Compared to controls, significantly fewer oocytes were collected from women over 35 years old in the COVID-19 group, but the difference was not found in the younger age group (<35 years old). While these molecular findings are intriguing, embryological and clinical outcomes, such as fertilization, cleavage, blastulation, euploidy, pregnancy and live birth rates, which are more relevant, were not reported.
Other studies confirmed the presence of SARS-CoV-2 IgG antibodies, but not viral antigens, in the follicular fluid of all women who were going through ART post-COVID-19 (Bentov et al., 2021), but no effect on follicular function, measured as steroidogenesis or follicular response to the LH/hCG trigger (Bentov et al., 2021).
COVID-19: impact on laboratory and clinical outcomes in ART
The impact of COVID-19 on ART treatment has been assessed through evaluation of oocyte number and quality, embryo parameters and, in a few studies, pregnancy rates after fresh or frozen embryo transfers (ETs).
In a small case series including nine couples, no difference in ovarian stimulation outcomes (length of stimulation, total dose of gonadotropins, peak estradiol and progesterone) and embryological variables (number of oocytes and mature oocytes, fertilization rate) was reported when comparing treatment of the same couples before and after (confirmed PCR negative) SARS-CoV-2 infection (Orvieto et al., 2021b). The interval between disease recovery and ART treatment varied between 8 and 92 days. The percentage of top-quality (≥7 equally-sized blastomeres and ≤10% fragmentation) Day 3 embryos per fertilized oocyte was significantly lower after COVID-19, irrespective of whether the male or female partner was previously infected. The authors suggested avoidance of ART within the first 3 months after COVID-19, but such a recommendation requires cautious appraisal due to the nature and size of the study, lack of details about the laboratory procedures performed (IVF or ICSI) and absence of information on whether the embryologist assessing the embryos was blinded for the patients’ COVID-19 status, a lack of which could have introduced bias in assessment.
In a retrospective study, a group from Wuhan analysed patients attending for IVF during a 10-month interval, and compared 65 SARS-CoV-2 seropositive (IgM or IgG) women to 195 controls (Wang et al., 2021). The minimum interval between infection and ART treatments was 4 months and none of the patients had active COVID-19 when treated. The number of mature oocytes and damaged oocytes, rates of fertilization, cleavage, high-quality embryos and available blastocysts, i.e. with expansion stage ≥ 3 and inner cell mass grade ≥ B, were similar in both groups, as well as the implantation, biochemical and clinical pregnancy, and early miscarriage rates. Only Day 3 embryos were transferred, and the remaining embryos were cultured to Day 5 or 6. Intriguingly, despite the ‘available blastocyst rate’ (number of good quality blastocysts available for cryopreservation divided by the number of blastocysts formed) being higher in the disease group (72.9% vs 70%), the authors reported a lower ‘blastulation rate per day 3 embryos’. This is likely a random finding due to multiple comparisons by hypothesis tests, which needs to be confirmed. Reassuringly, there was no difference in high quality embryo rates between the past COVID-19 patients and the controls, 45.2% and 48.1%, respectively.
The outcomes of the first fresh ART cycle in 121 patients with previous COVID-19 (within 12 months from diagnosis) were retrospectively compared with 121 patients that did not have COVID-19 previously (Youngster et al., 2022a). From this largest study to date, recent past SARS-CoV-2 infection was found to have no impact on oocyte yield, maturation rate, fertilization rate, number of vitrified embryos and clinical pregnancy rates. In a subgroup analysis, a negative effect on oocyte yield was observed in patients who had the oocyte retrieval more than 180 days after infection.
In line with recommendations to proceed to cryopreserving all embryos (‘freeze all’) in women testing positive for COVID-19 at oocyte retrieval, there are no data on IVF outcomes in patients who were diagnosed with COVID-19 during ART treatment and continued to ET.
Fertilization rates and the rates of excellent and good embryo quality were comparable between 16 women testing COVID-19 positive (nasopharyngeal swab PCR test) with no or light symptoms while undergoing ART and uninfected patients treated in the same clinic (Boudry et al., 2022). All embryos were vitrified and used for frozen ET after patients recovered from COVID-19. A total of 11 embryos were transferred resulting in three live births, two miscarriages and one ongoing pregnancy.
The outcomes of frozen ET cycles were also evaluated in 41 patients who recovered from COVID-19, with embryos generated and frozen prior to infection, and 41 controls. Pregnancy rates were not different between patients and controls (29% and 49% respectively), but lower pregnancy rates were recorded in women who underwent frozen ET < 60 days after infection as compared to those with a longer interval between COVID-19 and ET (21% and 55%, respectively) (Youngster et al., 2022b).
Another question is whether the presence of the SARS-CoV-2 S protein impacts on the outcome of ART treatments. To answer this, a single author publication compared frozen ET outcomes in SARS-CoV-2 vaccine seropositive, infection seropositive, and seronegative women (Morris, 2021). Both implantation and clinical pregnancy rates were similar between groups.
Similarly, immune patients (recovered from COVID-19 infection or having received the mRNA SARS-CoV-2 vaccine) were compared to patients considered non-immune and those treated before the pandemic. Similar implantation rates and clinical and ongoing pregnancy rates per transfer between the groups were reported (Aizer et al., 2022). Of note, the authors did not report how immunity was assessed.
Studies on the impact of COVID-19 on ART outcomes focus almost exclusively on COVID-19 in the female partner. To the best of our knowledge, there are no studies specifically evaluating reproductive outcomes after COVID-19 in the male partner, although, as noted above, SARS-CoV-2 infection affects spermatogenesis with an effect that can last for an extended period after recovery. Some authors and guidelines have therefore suggested an interval of 3 months between COVID-19 recovery and ART treatment to allow for a new cycle of spermatogenesis (Agence de la biomédecine, 2021; Orvieto et al., 2021a).
Conclusions
Most studies did not report a significant impact of COVID-19 on ovarian reserve, ovarian function or follicular fluid parameters.
A history of asymptomatic or mild SARS-CoV-2 infection in females does not seem to negatively affect laboratory and clinical outcomes in fresh and frozen ET cycles.
There are no data on the minimum required interval, if any, between COVID-19 recovery (considering also the male partner) and ART treatment. Clinical and laboratory assessment should guide practice.
Suggested future research
What is the impact of COVID-19 in the male partner on ART outcomes?
Is there a minimum interval between COVID-19 and ART treatment to ensure optimal outcomes?
COVID-19 vaccination and human reproduction
After initial hesitancy regarding vaccination against COVID-19 in pregnant women or those wanting to conceive, based on the complete absence of data, widespread campaigns started to recommend vaccination for all. Still, questions remain as to whether mRNA vaccines should be used in couples who wish to conceive, and whether vaccination has any potentially deleterious effects upon reproduction in humans. This section will try to bring answers to these questions by perusing the published evidence.
COVID-19 vaccination and spermatogenesis
Semen parameters of samples collected from 75 fertile men, on average 37 days after vaccination, were predominantly within the normal reference ranges as set by the WHO, with no indication of any causative detrimental effect from COVID-19 vaccination (Lifshitz et al., 2022).
From prospective evaluation of semen parameters in sperm donors before and after mRNA vaccination, no deleterious effect on total sperm count or motility was reported (Barda et al., 2022). The study included 425 samples before and 473 samples after vaccination collected from 33 donors. In a similar study in 106 patients, COVID-19 vaccination had no impact on sperm parameters or fertilization rates, even after considering different types of vaccines (mRNA and viral vector) (Reschini et al., 2022). The lack of a significant impact of mRNA vaccination on sperm parameters was further confirmed in three studies which examined semen samples of respectively 45, 72 and 41 men at an interval of on average 75, 71 and 30 days, respectively, after vaccination (Gonzalez et al., 2021; Safrai et al., 2022; ZHu et al., 2022). One study including 37 men who provided one pre- and three post-vaccination samples in different periods after vaccination, reported a temporary significant decline in sperm concentration, which also reflected on total motile sperm count, despite unchanged motility, in the samples provided between 75 and 125 days after vaccination (Gat et al., 2022). Samples provided over 145 days after vaccination had recovered to pre-vaccination levels, without a permanent effect (Gat et al., 2022). No man became azoospermic after vaccination (Gonzalez et al., 2021).
COVID-19 vaccination and ovarian reserve
By comparing AMH levels before vaccination and 3 months after administration of the second dose in 129 patients aged 18–42 years, the impact of mRNA COVID-19 vaccination on ovarian reserve was evaluated (Mohr-Sasson et al., 2022). Mean AMH levels were similar at the two timepoints, indicating no impact of mRNA vaccination on AMH levels. Similarly, no difference in AFC between 142 vaccinated and 138 unvaccinated women results was reported (Jacobs et al., 2022).
The small study by Bentov and colleagues mentioned previously, included a subgroup of nine COVID-19 vaccinated women and compared these with seven women who had recovered from COVID-19 and 16 women considered COVID-19 naïve (Bentov et al., 2021). The intervals between either infection or vaccination and fertility treatment varied between 8 days (single dose vaccine) and 169 days (recovery after COVID-19). The patients with positive serum anti-SARS-CoV-2 IgG had detectable levels of anti-SARS-CoV-2 IgG in their follicular fluid, with a linear association between both parameters, which was suggested to be indicative of a non-regulated passage of the anti-SARS-CoV-2 IgG across the follicular membrane. Interestingly, albeit not significant, was the observation that women who had recovered from COVID-19 had 10-fold lower IgG levels than those who were vaccinated. Oestrogen and progesterone levels in follicular fluid and serum, response to the LH/hCG trigger and an oocyte quality biomarker (HSPG2) in the aspirated follicular fluid were assessed. With regards to follicular function, no difference was observed between the vaccinated patients and other patients for any of the parameters measured. Interestingly, the progesterone levels on day of the ovulation trigger were significantly lower in the naïve patients, but the values were similar on day of oocyte collection.
Multiple studies have evaluated the impact of COVID-19 vaccination on the menstrual cycle and symptoms. From an analysis of self-entered data to menstrual cycle tracking apps, including 23 754 cycles from 2403 vaccinated and 1556 unvaccinated women (Edelman et al., 2022), a temporary change of < 1 day in the duration of the cycle was reported following vaccination (Edelman et al., 2022). Menstrual irregularities were reported in three studies using online questionnaires, included a total of 37 905 women and reported that 40–66% reported some change, mostly heavier periods (Lagana et al., 2022; Lee et al., 2022; Muhaidat et al., 2022). However, another study with an online questionnaire, which selectively included 76 North American women who were prospectively monitoring their menstrual cycle parameters daily including bleeding patterns, urinary hormone levels or cervical mucus observations, reported no change in objective parameters including length and duration of the cycle, estimated day of ovulation and luteal phase between pre-vaccine, vaccine and post-vaccine cycles, yet 22% of respondents reported some subjective changes (Bouchard et al., 2022). There were no significant differences between vaccine types in these studies. Overall, we consider the observations on the data that were spontaneously logged in menstrual cycle tracking apps, and the questionnaire on women who were prospectively tracking their cycle seem more reliable than other studies, since online surveys are more likely to be completed by and shared among people affected by the condition being studied (Sharp et al., 2022). COVID-19 vaccination does not seem to have a quantitative effect on menstruation and women seem to perceive that their cycles are back to normal in as few as two cycles.
COVID-19 vaccination and natural conception
Through a questionnaire, the link between male and female COVID-19 vaccination, SARS-CoV-2 infection and fecundability, reported as the per-cycle probability of conception, was interrogated in a North American prospective cohort study of 2126 couples trying to conceive (Wesselink et al., 2022). Data for the male partner were available in only 1369 couples, and there was no request for confirmation of a positive test or vaccination. COVID-19 vaccination in either partner was not appreciably associated with fecundability (female fecundability ratio (FR) = 1.08, 95% CI: 0.95–1.23; male FR = 0.95, 95% CI: 0.83–1.10).
COVID-19 vaccination and ART
Vaccination appears not to affect the outcome of ART. In a retrospective cohort study, the stimulation outcomes of fresh cycles were analysed in 222 fully vaccinated and 983 unvaccinated patients (Aharon et al., 2022). The oocytes retrieved, mature oocytes retrieved, mature/total oocytes ratio, and blastulation, ICSI fertilization and euploidy rates were similar between the vaccinated and the unvaccinated patients after multivariate linear regression analysis. Also, the type of vaccine used did not alter the results. The same study reported the treatment outcomes from 947 cycles of frozen–thawed euploid ETs in 214 vaccinated and 733 unvaccinated patients. After multivariate linear regression analysis, no significant difference was observed in clinical and ongoing pregnancy rate per transfer, nor in biochemical or clinical pregnancy loss rate. The clinical pregnancy rates were 59.5% and 63.7% in vaccinated and unvaccinated patients, respectively, and the authors concluded that COVID-19 (mRNA) vaccination does not seem to have an adverse effect on stimulation or early pregnancy outcomes after ART.
Similar findings were published from an observational study reporting the outcomes of fresh ET in 36 patients that had their first IVF cycle before the COVID-19 pandemic and a second cycle after full vaccination (mRNA vaccine) (Orvieto et al., 2021b). The interval between the time of the second vaccine to the date of the subsequent IVF treatment cycle was 7–85 days. No differences were observed in stimulation outcomes or embryological variables such as number of oocytes and mature oocytes retrieved, nor in the proportion of top-quality embryos. Three pregnancies were recorded from 10 ETs performed in the cycle following SARS-CoV-2 vaccination. Further studies with a similar approach reported no differences in the mean number of oocytes retrieved, fertilization rates, embryo quality or clinical pregnancy rate after fresh ET in vaccinated versus unvaccinated patients (Avraham et al., 2022; Huang et al., 2022a; Jacobs et al., 2022).
An initial concern regarding vaccination and human reproduction was related to the homology between the SARS-CoV-2 S protein and Syncytin-1, an essential element in human implantation. As the available SARS-CoV-2 vaccines target the S protein, the question of interference with Syncytin-1 function arose. To clarify, the cross reactivity of anti-S protein antibodies with Synctin-1 was investigated in two clinical circumstances: SARS-CoV-2 disease and vaccination (Prasad et al., 2021). In the first experiments, no anti-Syncytin-1 antibodies were detected in the plasma of 37 patients, analysed 28 days after infection. Similarly, a group of 66 vaccinated patients had their plasma assessed for S protein binding with plasma components at pre-vaccination, at 21 days after the first dose and 2 months after the second dose. The analysis showed no cross-reactivity with Syncytin-1 in either the pre- or post-vaccination samples, despite a measured increase in anti-S protein titres.
Conclusions
There is no evidence of a negative effect of SARS-CoV-2 vaccination on semen parameters or spermatogenesis.
There is no evidence of a negative effect of SARS-CoV-2 vaccination on ovarian function, ovarian reserve or folliculogenesis. A transient effect on the menstrual cycle has been documented.
Cross reactivity between anti-SARS-CoV-2 S protein antibodies and Syncytin-1 was shown not to be present.
There is no influence of mRNA SARS-CoV-2 vaccine on the performance of patients during their immediate subsequent ART cycle. Pregnancy rates in post-vaccination ART cycles are similar to those in unvaccinated patients.
Suggested future research
Is there a long-term impact of COVID-19 vaccination on female/male reproductive function, including ovarian reserve?
Conclusion
The current article summarizes published data on the theoretical impact of SARS-CoV-2 upon human fertility and assisted reproduction based on cell entry mechanisms, and explores the interaction of SARS-CoV-2 viral RNA with reproductive tissues, gametes and embryos, as well as the impact of SARS-CoV-2-infection, COVID-19 or COVID-19 vaccination upon human reproductive potential and ART outcomes. It offers reassurance that human fertility or outcomes of ART are not severely affected by either SARS-CoV-2 infection or vaccination.
Since the start of the pandemic, ESHRE and other professional societies have recommended a precautionary approach to assisted reproduction to avoid additional stress to overloaded healthcare systems, but also based on uncertainty on the effect of the SARS-CoV-2 virus on reproduction and pregnancy (ESHRE—European Society of Human Reproduction and Embryology, 2020). The paucity of data, and call for more research was echoed by others (Lambalk et al., 2020; Veiga et al., 2020).
The current article documents and presents in an organized manner the scientific knowledge and facilitates evidence-based management of patients with current or previous SARS-CoV-2 infection, although further data may still require adaptations. Additionally, as the long-term impact of COVID-19 remains unclear, future studies should investigate whether any long-term effects on human reproduction present and, in particular, if these patients are more likely to require fertility assistance in their desire to achieve parenthood.
Apart from the information gathered on the virus itself, the COVID-19 pandemic has presented the ART sector with significant challenges, some of which resulted in approaches which are and should be maintained. Such novel approaches include the wider application of telemedicine in the fertility clinic, new modes of communication to patients and fast-track publication in research.
Acknowledgements
The ESHRE COVID-19 Working Group would like to acknowledge the support of ESHRE for its activities.
Authors’ roles
All authors equally contributed to the conception or design of the work, and shared the work of drafting of the sections. All authors critically revised the paper and approved it’s final version for publication.
Funding
There was no funding for the current paper, apart from technical support from ESHRE.
Conflict of interest
The authors have no conflicts of interest to declare.
Contributor Information
Baris Ata, Obstetrics and Gynecology Department, Koc University, Istanbul, Turkey; ART Fertility Clinics, Dubai, United Arab Emirates.
Nathalie Vermeulen, ESHRE Central Office, Strombeek-Bever, Belgium.
Edgar Mocanu, Department of Reproductive Medicine, Rotunda Hospital and Royal College of Surgeons in Ireland, Dublin, Ireland.
Luca Gianaroli, Società Italiana Studi di Medicina della Riproduzione, S.I.S.Me.R. Reproductive Medicine Institute, Bologna, Emilia-Romagna, Italy.
Kersti Lundin, Reproductive Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden.
Satu Rautakallio-Hokkanen, Fertility Europe (No Dept), Evere, Belgium.
Juha S Tapanainen, Department of Obstetrics and Gynaecology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland; Department of Obstetrics and Gynaecology, Oulu University Hospital and Medical Research Centre PEDEGO Research Unit, Oulu, Finland.
Anna Veiga, Barcelona Stem Cell Bank, IDIBELL Programme for Regenerative Medicine, Barcelona, Spain.
Data availability
The data underlying this article are available in the article. Further information can be shared on reasonable request to the corresponding author.
References
- Achua JK, Chu KY, Ibrahim E, Khodamoradi K, Delma KS, Iakymenko OA, Kryvenko ON, Arora H, Ramasamy R.. Histopathology and ultrastructural findings of fatal COVID-19 infections on testis. World J Mens Health 2021;39:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adiga SK, Tholeti P, Uppangala S, Kalthur G, Gualtieri R, Talevi R.. Fertility preservation during the COVID-19 pandemic: mitigating the viral contamination risk to reproductive cells in cryostorage. Reprod Biomed Online 2020;41:991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal M, Basumatary S, Bhusan D, Pati BK.. Detection of severe acute respiratory syndrome corona virus 2 in cervico-vaginal secretion of COVID-19-affected female: a prospective observational study from India. SAGE Open Med 2021;9:20503121211022993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agence de la biomédecine. Recommandations sur les activités d'assistance médicale à la procréation en contexte de ciculation du SARS-CoV-2. 2021. https://www.procreation-medicale.fr/reprise-de-lactivite-dassistance-medicale-a-la-procreation-amp/ (7 November 2022, date last accessed).
- Aharon D, Lederman M, Ghofranian A, Hernandez-Nieto C, Canon C, Hanley W, Gounko D, Lee JA, Stein D, Buyuk E et al In vitro fertilization and early pregnancy outcomes after coronavirus disease 2019 (COVID-19) vaccination. Obstet Gynecol 2022;139:490–497. [DOI] [PubMed] [Google Scholar]
- Aizer A, Noach-Hirsh M, Dratviman-Storobinsky O, Nahum R, Machtinger R, Yung Y, Haas J, Orvieto R.. The effect of coronavirus disease 2019 immunity on frozen-thawed embryo transfer cycles outcome. Fertil Steril 2022;117:974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Benna S. Angiotensin-converting enzyme 2 gene expression in human male urological tissues: implications for pathogenesis and virus transmission pathways. Af J Urol 2021;27:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aolymat I, Khasawneh AI, Al-Tamimi M.. COVID-19-Associated Mental Health Impact on Menstrual Function Aspects: Dysmenorrhea and Premenstrual Syndrome, and Genitourinary Tract Health: A Cross Sectional Study among Jordanian Medical Students. Int J Environ Res Public Health 2022;19:1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apaydin T, Sahin B, Dashdamirova S, Dincer Yazan C, Elbasan O, Ilgin C, Bilgin H, Cam HK, Bahramzada G, Kucuk A et al The association of free testosterone levels with coronavirus disease 2019. Andrology 2022;10:1038–1046. [DOI] [PubMed] [Google Scholar]
- Atarod Z, Zamaniyan M, Moosazadeh M, Valadan R, Soleimanirad SM, Gordani N.. Investigation of vaginal and rectal swabs of women infected with COVID-19 in two hospitals covered by Mazandaran University of Medical Sciences, 2020. J Obstet Gynaecol 2022;42:2225–2229. [DOI] [PubMed] [Google Scholar]
- Avraham S, Kedem A, Zur H, Youngster M, Yaakov O, Yerushalmi GM, Gat I, Gidoni Y, Hochberg A, Baum M et al Coronavirus disease 2019 vaccination and infertility treatment outcomes. Fertil Steril 2022;117:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barda S, Laskov I, Grisaru D, Lehavi O, Kleiman S, Wenkert A, Azem F, Hauser R, Michaan N.. The impact of COVID-19 vaccine on sperm quality. Int J Gynaecol Obstet 2022;158:116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra F, La Rosa VL, Vitale SG, Commodari E, Altieri M, Scala C, Ferrero S.. Psychological status of infertile patients who had in vitro fertilization treatment interrupted or postponed due to COVID-19 pandemic: a cross-sectional study. J Psychosom Obstet Gynaecol 2022;43:145–152. [DOI] [PubMed] [Google Scholar]
- Barragan M, Guillen JJ, Martin-Palomino N, Rodriguez A, Vassena R.. Undetectable viral RNA in oocytes from SARS-CoV-2 positive women. Hum Reprod 2021;36:390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn LB, Sharma N, Elhaik E, Sekulic A, Bryce AH, Fonseca R.. Targeting TMPRSS2 in SARS-CoV-2 infection. Mayo Clin Proc 2020;95:1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentov Y, Beharier O, Moav-Zafrir A, Kabessa M, Godin M, Greenfield CS, Ketzinel-Gilad M, Ash Broder E, Holzer HEG, Wolf D et al Ovarian follicular function is not altered by SARS-CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum Reprod 2021;36:2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JC, Kuchakulla M, Khodamoradi K, Lima TFN, Frech FS, Achua J, Rosete O, Mora B, Arora H, Ibrahim E et al Evaluation of SARS-CoV-2 in human semen and effect on total sperm number: a prospective observational study. World J Mens Health 2021;39:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Maheshwari A, Ratna MB, van Eekelen R, Mol BW, McLernon DJ.. Prioritizing IVF treatment in the post-COVID 19 era: a predictive modelling study based on UK national data. Hum Reprod 2021;36:666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin J, Harrison C, Mathur R, Burns G, Pericleous-Smith A, Gameiro S.. Patient experiences of fertility clinic closure during the COVID-19 pandemic: appraisals, coping and emotions. Hum Reprod 2020;35:2556–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges E Jr, Setti AS, Iaconelli A Jr, Braga D.. Current status of the COVID-19 and male reproduction: a review of the literature. Andrology 2021;9:1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard TP, Schneider M, Schmidt M, Manhart M, Fehring RJ.. Menstrual cycle parameters are not significantly different after COVID-19 vaccination. J Womens Health (Larchmt) 2022;31:1097–1102. [DOI] [PubMed] [Google Scholar]
- Boudry L, Essahib W, Mateizel I, Van de Velde H, De Geyter D, Pierard D, Waelput W, Uvin V, Tournaye H, De Vos M et al Undetectable viral RNA in follicular fluid, cumulus cells, and endometrial tissue samples in SARS-CoV-2-positive women. Fertil Steril 2022;117:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancatella A, Viola N, Rutigliano G, Sgro D, Santini F, Latrofa F.. Subacute thyroiditis during the SARS-CoV-2 pandemic. J Endocr Soc 2021;5:bvab130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CA, Skytte AB, Kasiri S, Howell D, Patel ZP, Trolice MP, Parekattil SJ, Michael SF, Paul LM.. A cohort study of men infected with COVID-19 for presence of SARS-CoV-2 virus in their semen. J Assist Reprod Genet 2021;38:785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Mao G, Zheng R, Fang M, Yang X, Wang L, Qi C.. Testicular injury during SARS-CoV-2 infection may be neglected: an assessment from scRNA-seq profiling and protein detection of angiotensin-converting enzyme II. Exp Ther Med 2021;22:1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camici M, Zuppi P, Lorenzini P, Scarnecchia L, Pinnetti C, Cicalini S, Nicastri E, Petrosillo N, Palmieri F, D’Offizi G et al; ReCoVeRi Study Group. Role of testosterone in SARS-CoV-2 infection: a key pathogenic factor and a biomarker for severe pneumonia. Int J Infect Dis 2021;108:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carosso AR, van Eekelen R, Revelli A, Canosa S, Mercaldo N, Stura I, Cosma S, Scarafia C, Benedetto C, Gennarelli G.. Expectant management before in vitro fertilization in women aged 39 or above and unexplained infertility does not decrease live birth rates compared to immediate treatment. Reprod Sci 2022;29:1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çayan S, Uğuz M, Saylam B, Akbay E.. Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID-19) in SARS-CoV-2 infected male patients: a cohort study. Aging Male 2020;23:1493–1503. [DOI] [PubMed] [Google Scholar]
- Chen J, Jiang Q, Xia X, Liu K, Yu Z, Tao W, Gong W, Han JJ.. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 2020a;19:e13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Huang X, Yi Z, Deng Q, Jiang N, Feng C, Zhou Q, Sun B, Chen W, Guo R.. Ultrasound imaging findings of acute testicular infection in patients with coronavirus disease 2019: a single-center-based study in Wuhan, China. J Ultrasound Med 2021;40:1787–1794. [DOI] [PubMed] [Google Scholar]
- Chen W, Yuan P, Yang M, Yan Z, Kong S, Yan J, Liu X, Chen Y, Qiao J, Yan L.. SARS-CoV-2 entry factors: ACE2 and TMPRSS2 are expressed in peri-implantation embryos and the maternal–fetal interface. Engineering (Beijing) 2020b;6:1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng GP, Guo SM, Zhou LQ.. Suggestions on cleavage embryo and blastocyst vitrification/transfer based on expression profile of ACE2 and TMPRSS2 in current COVID-19 pandemic. Mol Reprod Dev 2021;88:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Jeon H, Brannstrom M, Akin JW, Curry TE Jr, Jo M.. Ovulatory upregulation of angiotensin-converting enzyme 2, a receptor for SARS-CoV-2, in dominant follicles of the human ovary. Fertil Steril 2021;116:1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, O’Callaghan K, Sinclair H, Hawke K, Love A, Hajkowicz K, Stewart AG.. Risk factors, treatment and outcomes of subacute thyroiditis secondary to COVID-19: a systematic review. Intern Med J 2022;52:522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinislioglu AE, Cinislioglu N, Demirdogen SO, Sam E, Akkas F, Altay MS, Utlu M, Sen IA, Yildirim F, Kartal S et al The relationship of serum testosterone levels with the clinical course and prognosis of COVID-19 disease in male patients: a prospective study. Andrology 2022;10:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelingh Bennink HJT, Foidart JM, Debruyne FMJ.. Treatment of serious COVID-19 with testosterone suppression and high-dose estrogen therapy. Eur Urol 2021;80:523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaco S, Chhabria K, Singh D, Bhide A, Singh N, Singh A, Husein A, Mishra A, Sharma R, Ashary N et al Expression map of entry receptors and infectivity factors for pan-coronaviruses in preimplantation and implantation stage human embryos. J Assist Reprod Genet 2021;38:1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona G, Vena W, Pizzocaro A, Pallotti F, Paoli D, Rastrelli G, Baldi E, Cilloni N, Gacci M, Semeraro F et al Andrological effects of SARS-Cov-2 infection: a systematic review and meta-analysis. J Endocrinol Invest 2022;45:2207–2219. doi: 10.1007/s40618-022-01801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui P, Chen Z, Wang T, Dai J, Zhang J, Ding T, Jiang J, Liu J, Zhang C, Shan W et al Severe acute respiratory syndrome coronavirus 2 detection in the female lower genital tract. Am J Obstet Gynecol 2020;223:131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lusignan S, Dorward J, Correa A, Jones N, Akinyemi O, Amirthalingam G, Andrews N, Byford R, Dabrera G, Elliot A et al Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis 2020;20:1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel-Gomez L, Romeu M, Castells-Ballester J, Pellicer N, Faus A, Mullor JL, Pellicer A, Cervello I.. Undetectable viral RNA from SARS-CoV-2 in endometrial biopsies from women with COVID-19: a preliminary study. Am J Obstet Gynecol 2022;226:434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaroche L, Bertine M, Oger P, Descamps D, Damond F, Genauzeau E, Meicler P, Le Hingrat Q, Lamazou F, Gschwind R et al Evaluation of SARS-CoV-2 in semen, seminal plasma, and spermatozoa pellet of COVID-19 patients in the acute stage of infection. PLoS One 2021;16:e0260187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir O, Sal H, Comba C.. Triangle of COVID, anxiety and menstrual cycle. J Obstet Gynaecol 2021;41:1257–1261. [DOI] [PubMed] [Google Scholar]
- Demirel C, Tulek F, Celik HG, Donmez E, Tuysuz G, Gokcan B.. Failure to detect viral RNA in follicular fluid aspirates from a SARS-CoV-2-positive woman. Reprod Sci 2021;28:2144–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhindsa S, Zhang N, McPhaul MJ, Wu Z, Ghoshal AK, Erlich EC, Mani K, Randolph GJ, Edwards JR, Mudd PA et al Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19. JAMA Netw Open 2021;4:e2111398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding T, Wang T, Zhang J, Cui P, Chen Z, Zhou S, Yuan S, Ma W, Zhang M, Rong Y et al Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne) 2021;8:635255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders GGG, Bosmans E, Reumers J, Donders F, Jonckheere J, Salembier G, Stern N, Jacquemyn Y, Ombelet W, Depuydt CE.. Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: a prospective, observational study and validation of the SpermCOVID test. Fertil Steril 2022;117:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Neto AN, Teixeira TA, Caldini EG, Kanamura CT, Gomes-Gouvea MS, Dos Santos ABG, Monteiro RAA, Pinho JRR, Mauad T, da Silva LFF et al Testicular pathology in fatal COVID-19: a descriptive autopsy study. Andrology 2022;10:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman A, Boniface ER, Benhar E, Han L, Matteson KA, Favaro C, Pearson JT, Darney BG.. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: A U.S. cohort. Obstet Gynecol 2022;139:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enikeev D, Taratkin M, Morozov A, Petov V, Korolev D, Shpikina A, Spivak L, Kharlamova S, Shchedrina I, Mestnikov O et al Prospective two-arm study of the testicular function in patients with COVID-19. Andrology 2022;10:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbay G, Sanli A, Turel H, Yavuz U, Erdogan A, Karabakan M, Yaris M, Gultekin MH.. Short-term effects of COVID-19 on semen parameters: a multicenter study of 69 cases. Andrology 2021;9:1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESHRE—European Society of Human Reproduction and Embryology. Assisted Reproduction and COVID-19: A Statement From ESHRE for Phase 1-Guidance on Fertility Services During Pandemic. 2020. https://wwweshreeu/Europe/Position-statements/COVID19/#phase1 (3 June 2022, date last accessed).
- ESHRE COVID-19 Working Group, Gianaroli L, Ata B, Lundin K, Rautakallio-Hokkanen S, Tapanainen JS, Vermeulen N, Veiga A, Mocanu E.. The calm after the storm: re-starting ART treatments safely in the wake of the COVID-19 pandemic. Human Reproduction (Oxford, England) 2021;36:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESHRE Covid-19 Working Group, Vermeulen N, Ata B, Gianaroli L, Lundin K, Mocanu E, Rautakallio-Hokkanen S, Tapanainen JS, Veiga A.. A picture of medically assisted reproduction activities during the COVID-19 pandemic in Europe. Hum Reprod Open 2020;2020:hoaa035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito V, Rania E, Lico D, Pedri S, Fiorenza A, Strati MF, Conforti A, Marrone V, Carosso A, Revelli A et al Influence of COVID-19 pandemic on the psychological status of infertile couples. Eur J Obstet Gynecol Reprod Biol 2020;253:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essahib W, Verheyen G, Tournaye H, Van de Velde H.. SARS-CoV-2 host receptors ACE2 and CD147 (BSG) are present on human oocytes and blastocysts. J Assist Reprod Genet 2020;37:2657–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraietta R, de Carvalho RC, Camillo J, Groner MF, Truzzi J, Petkov CN, Barradas V, Homsi C, Bellei N, Oehninger S.. SARS-CoV-2 is not found in human semen during mild COVID-19 acute stage. Andrologia 2022;54:e14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Zhou B, Zhang L, Balaji KS, Wei C, Liu X, Chen H, Peng J, Fu J.. Expressions and significances of the angiotensin-converting enzyme 2 gene, the receptor of SARS-CoV-2 for COVID-19. Mol Biol Rep 2020;47:4383–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacci M, Coppi M, Baldi E, Sebastianelli A, Zaccaro C, Morselli S, Pecoraro A, Manera A, Nicoletti R, Liaci A et al Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum Reprod 2021;36:1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat I, Kedem A, Dviri M, Umanski A, Levi M, Hourvitz A, Baum M.. Covid-19 vaccination BNT162b2 temporarily impairs semen concentration and total motile count among semen donors. Andrology 2022;10:1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geslot A, Chanson P, Caron P.. Covid-19, the thyroid and the pituitary – the real state of play. Ann Endocrinol (Paris) 2022;83:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghare Naz MS, Banaei M, Dashti S, Tehrani FR.. An overview of sex hormones in relation to SARS-CoV-2 infection. Future Virol 2021;16:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Parikh S, Nissa MU, Acharjee A, Singh A, Patwa D, Makwana P, Athalye A, Barpanda A, Laloraya M et al Semen proteomics of COVID-19 convalescent men reveals disruption of key biological pathways relevant to male reproductive function. ACS Omega 2022;7:8601–8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Health 50/50. COVID-19 sex-disaggregated data tracker. Sex, gender, and COVID‐19 Project2020. https://globalhealth5050.org/the-sex-gender-and-covid-19-project/the-data-tracker/ (7 November 2022, date last accessed).
- Goad J, Rudolph J, Rajkovic A.. Female reproductive tract has low concentration of SARS-CoV2 receptors. PLoS One 2020;15:e0243959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez DC, Nassau DE, Khodamoradi K, Ibrahim E, Blachman-Braun R, Ory J, Ramasamy R.. Sperm parameters before and after COVID-19 mRNA vaccination. JAMA 2021;326:273–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromski PS, Smith A, Lawlor DA, Sharara FI, Nelson SM.. 2008 financial crisis versus 2020 economic fallout: how COVID-19 might influence fertility treatment and live births. Reprod Biomed Online 2021;42:1087–1096. [DOI] [PubMed] [Google Scholar]
- Gul A, Zengin S, Dundar G, Ozturk M.. Do SARS-CoV-2 infection (COVID-19) and the medications administered for its treatment impair testicular functions? Urol Int 2021;105:944–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhao S, Li W, Wang Y, Li L, Jiang S, Ren W, Yuan Q, Zhang F, Kong F et al Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology 2021a;9:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TH, Sang MY, Bai S, Ma H, Wan YY, Jiang XH, Zhang YW, Xu B, Chen H, Zheng XY et al Semen parameters in men recovered from COVID-19. Asian J Androl 2021b;23:479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Choudhary A, Gopal G, Kumar R, Kumar A, Tiwari P, Malhotra N.. Detection of SARS-CoV2 virus using the real-time reverse transcriptase polymerase chain reaction in semen and seminal plasma from men with active COVID-19 infection—a pilot study. Indian J Urol 2021;37:331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtin ZB, Jasmin E, Da Silva P, Dennehy C, Harper J, Kanjani S.. Fertility treatment delays during COVID-19: profiles, feelings and concerns of impacted patients. Reprod Biomed Soc Online 2022;14:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajizadeh Maleki B, Tartibian B.. COVID-19 and male reproductive function: a prospective, longitudinal cohort study. Reproduction 2021;161:319–331. [DOI] [PubMed] [Google Scholar]
- Hamarat MB, Ozkent MS, Yilmaz B, Aksanyar SY, Karabacak K.. Effect of SARS-CoV-2 infection on semen parameters. Can Urol Assoc J 2022;16:E173–E177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henarejos-Castillo I, Sebastian-Leon P, Devesa-Peiro A, Pellicer A, Diaz-Gimeno P.. SARS-CoV-2 infection risk assessment in the endometrium: viral infection-related gene expression across the menstrual cycle. Fertil Steril 2020;114:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero Y, Pascuali N, Velazquez C, Oubina G, Hauk V, de Zuniga I, Pena MG, Martinez G, Lavolpe M, Veiga F et al SARS-CoV-2 infection negatively affects ovarian function in ART patients. Biochim Biophys Acta Mol Basis Dis 2022;1868:166295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikmet F, Mear L, Edvinsson A, Micke P, Uhlen M, Lindskog C.. The protein expression profile of ACE2 in human tissues. Mol Syst Biol 2020;16:e9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A et al SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, Kruessel JS, Bielfeld AP.. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril 2020;114:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Liu K, Ruan Y, Wei X, Wu Y, Feng H, Deng Z, Liu J, Wang T.. Evaluation of mid- and long-term impact of COVID-19 on male fertility through evaluating semen parameters. Transl Androl Urol 2022;11:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Xia L, Lin J, Liu B, Zhao Y, Xin C, Ai X, Cao W, Zhang X, Tian L et al No effect of inactivated SARS-CoV-2 vaccination on in vitro fertilization outcomes: a propensity score-matched study. J Inflamm Res 2022a;15:839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Do DV, Beh D, Lee CK, Yan B, Foo R, Tambyah PA.. Effects of acute severe acute respiratory syndrome coronavirus 2 infection on male hormone profile, ACE2 and TMPRSS2 expression, and potential for transmission of severe acute respiratory syndrome coronavirus 2 in semen of Asian men. F S Sci 2022b;3:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E, Summers K, Sparks A, Mejia R.. Fresh embryo transfer cycle characteristics and outcomes following in vitro fertilization via intracytoplasmic sperm injection among patients with and without COVID-19 vaccination. JAMA Netw Open 2022;5:e228625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadihasanoglu M, Aktas S, Yardimci E, Aral H, Kadioglu A.. SARS-CoV-2 pneumonia affects male reproductive hormone levels: a prospective, cohort study. J Sex Med 2021;18:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasman AM, Bhambhvani HP, Li S, Zhang CA, Stevenson DK, Shaw GM, Simard JF, Eisenberg ML.. Reproductive sequelae of parental severe illness before the pandemic: implications for the COVID-19 pandemic. Fertil Steril 2020;114:1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Pranesh GT, Rao KA.. Emotional impact of delay in fertility treatment due to COVID-19 pandemic. J Hum Reprod Sci 2020;13:317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaaslan B, Korukluoglu G, Hasanoglu I, Kalem AK, Eser F, Akinci E, Guner R.. Investigation of SARS-CoV-2 in semen of patients in the acute stage of COVID-19 infection. Urol Int 2020;104:678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakou P, Paschou SA, Psaltopoulou T, Gavriatopoulou M, Korompoki E, Stefanaki K, Kanouta F, Kassi GN, Dimopoulos MA, Mitrakou A.. Early and late endocrine complications of COVID-19. Endocr Connect 2021;10:R229–R239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Shilen A, Heslin KM, Ishimwe P, Allen AM, Jacobs ET, Farland LV.. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol 2022;226:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoiwal K, Kalita D, Kumari R, Dhundi D, Shankar R, Kumari R, Gaurav A, Bahadur A, Panda PK, Tomy A et al Presence of SARS-CoV-2 in the lower genital tract of women with active COVID-19 infection: a prospective study. Int J Gynaecol Obstet 2022;157:744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanska K, Hours A, Jonquière L, Mathieu d'Argent E, Dabi Y, Dupont C, Touboul C, Antoine J-M, Chabbert-Buffet N, Daraï E.. Mild COVID-19 infection does not alter the ovarian reserve in women treated with ART. Reprod Biomed Online 2021;43:1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagana AS, Veronesi G, Ghezzi F, Ferrario MM, Cromi A, Bizzarri M, Garzon S, Cosentino M.. Evaluation of menstrual irregularities after COVID-19 vaccination: results of the MECOVAC survey. Open Med (Wars) 2022;17:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambalk CB, van Wely M, Kirkegaard K, Williams AC, de Geyter C.. Safety first – assisted human reproduction second. Hum Reprod 2020;35:741–742. [DOI] [PubMed] [Google Scholar]
- Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G.. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol 2020;183:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanser L, Burkert FR, Thommes L, Egger A, Hoermann G, Kaser S, Pinggera GM, Anliker M, Griesmacher A, Weiss G et al Testosterone deficiency is a risk factor for severe COVID-19. Front Endocrinol (Lausanne) 2021;12:694083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte M, Naesens L.. Airway proteases: an emerging drug target for influenza and other respiratory virus infections. Curr Opin Virol 2017;24:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KMN, Junkins EJ, Luo C, Fatima UA, Cox ML, Clancy KBH.. Investigating trends in those who experience menstrual bleeding changes after SARS-CoV-2 vaccination. Sci Adv 2022;8:eabm7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Jin M, Bao P, Zhao W, Zhang S.. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open 2020a;3:e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, Lu W, Pan F, Meng T, Zhao K et al Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 2020b;28:100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Chen G, Hou H, Liao Q, Chen J, Bai H, Lee S, Wang C, Li H, Cheng L et al Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online 2021;42:260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifshitz D, Haas J, Lebovitz O, Raviv G, Orvieto R, Aizer A.. Does mRNA SARS-CoV-2 vaccine detrimentally affect male fertility, as reflected by semen analysis? Reprod Biomed Online 2022;44:145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen Y, Tang W, Zhang L, Chen W, Yan Z, Yuan P, Yang M, Kong S, Yan L et al Single-cell transcriptome analysis of the novel coronavirus (SARS-CoV-2) associated gene ACE2 expression in normal and non-obstructive azoospermia (NOA) human male testes. Sci China Life Sci 2020;63:1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY, Law CY, Leung EKH, To KKW, Tan KCB et al Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab 2021;106:e926–e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo FP, Dragoni F, Boccuto A, Paccagnini E, Gentile M, Canosi T, Morgante G, Luddi A, Zazzi M, Vicenti I et al SARS-CoV-2 infection of human ovarian cells: a potential negative impact on female fertility. Cells 2022;11:1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Xie W, Li D, Shi L, Ye G, Mao Y, Xiong Y, Sun H, Zheng F, Chen Z et al Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol 2021;93:456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado B, Barcelos Barra G, Scherzer N, Massey J, Dos Santos Luz H, Henrique Jacomo R, Herinques Santa Rita T, Davis R.. Presence of SARS-CoV-2 RNA in semen – cohort study in the United States COVID-19 positive patients. Infect Dis Rep 2021;13:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madendag IC, Madendag Y, Ozdemir AT.. COVID-19 disease does not cause ovarian injury in women of reproductive age: an observational before-and-after COVID-19 study. Reprod Biomed Online 2022;45:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S, Nao N, Shirato K, Kawase M, Saito S, Takayama I, Nagata N, Sekizuka T, Katoh H, Kato F et al Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A 2020;117:7001–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Perucha L, López-Jiménez T, Holst AS, Jacques-Aviñó C, Munrós-Feliu J, Martínez-Bueno C, Valls-Llobet C, Pinzón-Sanabria D, Vicente-Hernández MM, Berenguera A.. Self-reported menstrual alterations during the COVID-19 syndemic in Spain: a cross-sectional study. Int J Womens Health 2022;14:529–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr-Sasson A, Haas J, Abuhasira S, Sivan M, Doitch Amdurski H, Dadon T, Blumenfeld S, Derazne E, Hemi R, Orvieto R et al The effect of Covid-19 mRNA vaccine on serum anti-Müllerian hormone levels. Hum Reprod 2022;37:534–541. [DOI] [PubMed] [Google Scholar]
- Montano M, Victor AR, Griffin DK, Duong T, Bolduc N, Farmer A, Garg V, Hadjantonakis A-K, Coates A, Barnes FL et al SARS-CoV-2 can infect human embryos. Sci Rep 2022;12:15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, Carbone GM, Cavalli A, Pagano F, Ragazzi E et al Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol 2020;31:1040–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Perez O, Merino E, Alfayate R, Torregrosa ME, Andres M, Leon-Ramirez JM, Boix V, Gil J, Pico A, Group CAR;. COVID19-ALC Research group. Male pituitary-gonadal axis dysfunction in post-acute COVID-19 syndrome-Prevalence and associated factors: A Mediterranean case series. Clin Endocrinol (Oxf) 2022;96:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RS. SARS-CoV-2 spike protein seropositivity from vaccination or infection does not cause sterility. F S Rep 2021;2:253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhaidat N, Alshrouf MA, Azzam MI, Karam AM, Al-Nazer MW, Al-Ani A.. Menstrual symptoms after COVID-19 vaccine: a cross-sectional investigation in the MENA region. Int J Womens Health 2022;14:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, Ferrante E, Orsi E, Resi V, Longari V et al SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol 2020;8:739–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BT, Pang RD, Nelson AL, Pearson JT, Benhar Noccioli E, Reissner HR, Kraker von Schwarzenfeld A, Acuna J.. Detecting variations in ovulation and menstruation during the COVID-19 pandemic, using real-world mobile app data. PLoS One 2021;16:e0258314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X, Qian L, Sun R, Huang B, Dong X, Xiao Q, Zhang Q, Lu T, Yue L, Chen S et al Multi-organ proteomic landscape of COVID-19 autopsies. Cell 2021;184:775–791.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okcelik S. COVID-19 pneumonia causes lower testosterone levels. Andrologia 2021;53:e13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvieto R, Noach-Hirsh M, Segev-Zahav A, Haas J, Nahum R, Aizer A.. Does mRNA SARS-CoV-2 vaccine influence patients' performance during IVF-ET cycle? Reprod Biol Endocrinol 2021a;19:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvieto R, Segev-Zahav A, Aizer A.. Does COVID-19 infection influence patients' performance during IVF-ET cycle?: an observational study. Gynecol Endocrinol 2021b;37:895–897. [DOI] [PubMed] [Google Scholar]
- Ory SJ, Miller KA, Horton M, Giudice L.. The global impact of COVID-19 on infertility services. Global Reproductive Health 2020;5:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozimek N, Velez K, Anvari H, Butler L, Goldman KN, Woitowich NC.. Impact of stress on menstrual cyclicity during the coronavirus disease 2019 pandemic: a survey study. J Women’s Health (Larchmt) 2022;31:84–90. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE et al The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, Spivak AM, Alukal JP, Zhang X, Xiong C et al No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril 2020;113:1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli D, Pallotti F, Nigro G, Mazzuti L, Hirsch MN, Valli MB, Colangelo S, Mastroianni CM, Antonelli G, Lenzi A et al Molecular diagnosis of SARS-CoV-2 in seminal fluid. J Endocrinol Invest 2021a;44:2675–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli D, Pallotti F, Turriziani O, Mazzuti L, Antonelli G, Lenzi A, Lombardo F.. SARS-CoV-2 presence in seminal fluid: myth or reality. Andrology 2021b;9:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Li L, Samplaski M.. Why does COVID-19 kill more elderly men than women? Is there a role for testosterone? Andrology 2021;9:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone C, Giammanco GM, Cascino AP, Baiamonte D, Pinelli M, Cangelosi E, Filizzolo C, Sciortino G, Grazia S, Bonura F.. Assessment of SARS-CoV-2 RNA shedding in semen of 36 males with symptomatic, asymptomatic, and convalescent infection during the first and second wave of COVID-19 pandemic in Italy. Asian J Androl 2022;24:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazir Y, Eroglu T, Kose A, Bulut TB, Genc C, Kadihasanoglu M.. Impaired semen parameters in patients with confirmed SARS-CoV-2 infection: a prospective cohort study. Andrologia 2021;53:e14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, Rosser EC, Webb K, Deakin CT.. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 2020;11:6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M, Lin JL, Gu Y, Gupta R, Macary P, Schwarz H.. No crossreactivity of anti-SARS-CoV-2 spike protein antibodies with Syncytin-1. Cell Mol Immunol 2021;18:2566–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puca E, Puca E.. Premature ovarian failure related to SARS-CoV-2 infection. J Med Cases 2022;13:155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura LJ, Alukal J, Chong AM, Liu L, Cantos A, Shah J, Medrano N, Chang JY, Tsuji M, Mohri H et al SARS-CoV-2 RNA shedding in semen and oligozoospermia of patient with severe coronavirus disease 11 weeks after infection. Emerg Infect Dis 2022;28:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Zhou Y, Hua J, Zhang L, Bian J, Liu B, Zhao Z, Jin S.. The scRNA-seq expression profiling of the receptor ACE2 and the cellular protease TMPRSS2 reveals human organs susceptible to SARS-CoV-2 infection. Int J Environ Res Public Health 2021;18:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J; National Expert Group for Quality Management on Assisted Reproductive Technology. Impacts of COVID-19 pandemics on the services of assisted reproductive technology in Chinese mainland: a national cross-sectional survey. Chin J Reprod Contracept 2021;41:7. 11. [Google Scholar]
- Qiu L, Liu X, Xiao M, Xie J, Cao W, Liu Z, Morse A, Xie Y, Li T, Zhu L.. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis 2020;71:813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput SK, Logsdon DM, Kile B, Engelhorn HJ, Goheen B, Khan S, Swain J, McCormick S, Schoolcraft WB, Yuan Y et al Human eggs, zygotes, and embryos express the receptor angiotensin 1-converting enzyme 2 and transmembrane serine protease 2 protein necessary for severe acute respiratory syndrome coronavirus 2 infection. F S Sci 2021;2:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramal-Sanchez M, Castellini C, Cimini C, Taraschi A, Valbonetti L, Barbonetti A, Bernabo N, Barboni B.. ACE2 receptor and its isoform short – ACE2 are expressed on human spermatozoa. Int J Mol Sci 2022;23:3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambhatla A, Bronkema CJ, Corsi N, Keeley J, Sood A, Affas Z, Dabaja AA, Rogers CG, Liroff SA, Abdollah F.. COVID-19 infection in men on testosterone replacement therapy. J Sex Med 2021;18:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, Spreafico F, Greco GF, Cervi G, Pecoriello A et al Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology 2021;9:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings SA, Ignacio C, Porrachia M, Du P, Smith DM, Chaillon A.. No evidence of SARS-CoV-2 seminal shedding despite SARS-CoV-2 persistence in the upper respiratory tract. Open Forum Infect Dis 2020;7:ofaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R, Farber N, Kresch E, Seetharam D, Diaz P, Ramasamy R.. SARS-CoV-2 in the prostate: immunohistochemical and ultrastructural studies. World J Mens Health 2022;40:340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschini M, Pagliardini L, Boeri L, Piazzini F, Bandini V, Fornelli G, Dolci C, Cermisoni GC, Vigano P, Somigliana E et al COVID-19 vaccination does not affect reproductive health parameters in men. Front Public Health 2022;10:839967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski PA, Bortoletto P, Rosenwaks Z, Schattman GL.. Delay in IVF treatment up to 180 days does not affect pregnancy outcomes in women with diminished ovarian reserve. Hum Reprod 2020;35:1630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosielle K, Bergwerff J, Schreurs AMF, Knijnenburg J, De Bie B, Maas JWM, Nap AW, van Wely M, Lambalk CB, Goddijn M et al The impact of the COVID-19 pandemic on infertility patients and endometriosis patients in the Netherlands. Reprod Biomed Online 2021;43:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y, Hu B, Liu Z, Liu K, Jiang H, Li H, Li R, Luan Y, Liu X, Yu G et al No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: a perspective and urogenital evaluation. Andrology 2021;9:99–106. [DOI] [PubMed] [Google Scholar]
- Safrai M, Herzberg S, Imbar T, Reubinoff B, Dior U, Ben-Meir A.. The BNT162b2 mRNA covid-19 vaccine does not impair sperm parameters. Reprod Biomed Online 2022;44:685–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salciccia S, Eisenberg ML, Maggi M, Lai S, Mastroianni CM, Pasculli P, Ciardi MR, Canale V, Ferro M, Busetto GM et al Modeling the contribution of male testosterone levels to the duration of positive COVID testing among hospitalized male COVID-19 patients. Diagnostics (Basel) 2021;11:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonia A, Pontillo M, Capogrosso P, Gregori S, Carenzi C, Ferrara AM, Rowe I, Boeri L, Larcher A, Ramirez GA et al Testosterone in males with COVID-19: a 7-month cohort study. Andrology 2022;10:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonia A, Pontillo M, Capogrosso P, Gregori S, Tassara M, Boeri L, Carenzi C, Abbate C, Cignoli D, Ferrara AM et al Severely low testosterone in males with COVID-19: a case-control study. Andrology 2021;9:1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarier M, Demir M, Emek M, Usta SS, Soylu A, Konuk EY, Turgut H.. Comparison of spermiograms of infertile men before and during the COVID-19 pandemic. Rev Assoc Med Bras (1992) 2022;68:191–195. [DOI] [PubMed] [Google Scholar]
- Saylam B, Uguz M, Yarpuzlu M, Efesoy O, Akbay E, Cayan S.. The presence of SARS-CoV-2 virus in semen samples of patients with COVID-19 pneumonia. Andrologia 2021;53:e14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Yogev Y, Zilberman A, Alpern S, Many A, Yousovich R, Gamzu R.. Detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vaginal swabs of women with acute SARS-CoV-2 infection: a prospective study. BJOG 2021;128:97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroppo FI, Costantini E, Zucchi A, Illiano E, Trama F, Brancorsini S, Crocetto F, Gismondo MR, Deho F, Mercuriali A et al COVID-19 disease in clinical setting: impact on gonadal function, transmission risk, and sperm quality in young males. J Basic Clin Physiol Pharmacol 2021;33:97–102. [DOI] [PubMed] [Google Scholar]
- Sharma AP, Sahoo S, Goyal K, Chandna A, Kirubanandhan S, Sharma V, Grover S, Singh MP, Bhalla A, Singh SK.. Absence of SARS-CoV-2 infection in the semen of men recovering from COVID-19 infection: an exploratory study and review of literature. Andrologia 2021;53:e14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp GC, Fraser A, Sawyer G, Kountourides G, Easey KE, Ford G, Olszewska Z, Howe LD, Lawlor DA, Alvergne A et al The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol 2022;51:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Xiao X, Aierken A, Yue W, Wu X, Liao M, Hua J.. The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J Cell Mol Med 2020;24:9472–9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Gromski PS, Rashid KA, Tilling K, Lawlor DA, Nelson SM.. Population implications of cessation of IVF during the COVID-19 pandemic. Reprod Biomed Online 2020;41:428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somigliana E, Esposito G, Vigano P, Franchi M, Corrao G, Parazzini F.. Effects of the early phase of the COVID-19 pandemic on natural and ART-mediated birth rates in Lombardy Region, Northern Italy. Reprod Biomed Online 2021;43:765–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley KE, Thomas E, Leaver M, Wells D.. Coronavirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril 2020;114:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takmaz O, Kaya E, Erdi B, Unsal G, Sharifli P, Agaoglu NB, Ozbasli E, Gencer S, Gungor M.. Severe acute respiratory syndrome coronavirus (SARS-CoV-2) is not detected in the vagina: a prospective study. PLoS One 2021a;16:e0253072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takmaz T, Gundogmus I, Okten SB, Gunduz A.. The impact of COVID-19-related mental health issues on menstrual cycle characteristics of female healthcare providers. J Obstet Gynaecol Res 2021b;47:3241–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Dahan MH, Baris Ata M, Nair S, Shoham Z, Tan SL.. Trends in fertility practice during the covid-19 pandemic: a global survey of 299 clinics representing 228,500 IVF cycles. Fertil Steril 2020;114:e528. [Google Scholar]
- Temiz MZ, Dincer MM, Hacibey I, Yazar RO, Celik C, Kucuk SH, Alkurt G, Doganay L, Yuruk E, Muslumanoglu AY.. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: a cross-sectional, pilot study. Andrologia 2021;53:e13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano-Guerra E, Martinez-Gallo M, Arrese-Munoz I, Gine A, Diaz-Troyano N, Gabriel-Medina P, Riveiro-Barciela M, Labrador-Horrillo M, Martinez-Valle F, Montalva AS et al Recovery of serum testosterone levels is an accurate predictor of survival from COVID-19 in male patients. BMC Med 2022;20:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich H, Pillat MM.. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep 2020;16:434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga A, Gianaroli L, Ory S, Horton M, Feinberg E, Penzias A.. Assisted reproduction and COVID-19: a joint statement of ASRM, ESHRE and IFFS. Hum Reprod Open 2020;2020:hoaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vena W, Pizzocaro A, Maida G, Amer M, Voza A, Di Pasquale A, Reggiani F, Ciccarelli M, Fedeli C, Santi D, Humanitas et al; COVID19 Task Force. Low testosterone predicts hypoxemic respiratory insufficiency and mortality in patients with COVID-19 disease: another piece in the COVID puzzle. J Endocrinol Invest 2022;45:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilella F, Wang W, Moreno I, Roson B, Quake SR, Simon C.. Single-cell RNA sequencing of SARS-CoV-2 cell entry factors in the preconceptional human endometrium. Hum Reprod 2021;36:2709–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Q, Ren X, Hu J, Li Z, Long R, Xi Q, Zhu L, Jin L.. Investigating the impact of asymptomatic or mild SARS-CoV-2 infection on female fertility and in vitro fertilization outcomes: a retrospective cohort study. EClinicalMedicine 2021;38:101013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Mortazavi J, Hart JE, Hankins JA, Katuska LM, Farland LV, Gaskins AJ, Wang Y-X, Tamimi RM, Terry KL. et al. A prospective study of the association between SARS-CoV-2 infection and COVID-19 vaccination with changes in usual menstrual cycle characteristics. American Journal of Obstetrics and Gynecology 2022;227:739.e1–739.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherbee BAT, Glover DM, Zernicka-Goetz M.. Expression of SARS-CoV-2 receptor ACE2 and the protease TMPRSS2 suggests susceptibility of the human embryo in the first trimester. Open Biol 2020;10:200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselink AK, Hatch EE, Rothman KJ, Wang TR, Willis MD, Yland J, Crowe HM, Geller RJ, Willis SK, Perkins RB et al A prospective cohort study of COVID-19 vaccination, SARS-CoV-2 infection, and fertility. Am J Epidemiol 2022;191:1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins J, Al-Inizi S.. Premature ovarian insufficiency secondary to COVID-19 infection: an original case report. Int J Gynaecol Obstet 2021;154:179–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Ma L, Xue L, Zhu Q, Zhou S, Dai J, Yan W, Zhang J, Wang S.. Co-expression of the SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in human ovaries: identification of cell types and trends with age. Genomics 2021;113:3449–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Otsuka Y, Sunada N, Tokumasu K, Nakano Y, Honda H, Sakurada Y, Hagiya H, Hanayama Y, Otsuka F.. Detection of male hypogonadism in patients with post COVID-19 condition. J Clin Med 2022;11:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Chen S, Huang B, Zhong JM, Su H, Chen YJ, Cao Q, Ma L, He J, Li XF et al Pathological findings in the testes of COVID-19 patients: clinical implications. Eur Urol Focus 2020;6:1124–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngster M, Avraham S, Yaakov O, Landau Rabbi M, Gat I, Yerushalmi G, Baum M, Maman E, Hourvitz A, Kedem A.. The impact of past COVID-19 infection on pregnancy rates in frozen embryo transfer cycles. J Assist Reprod Genet 2022a;39:1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngster M, Avraham S, Yaakov O, Landau Rabbi M, Gat I, Yerushalmi G, Sverdlove R, Baum M, Maman E, Hourvitz A et al IVF under COVID-19: treatment outcomes of fresh ART cycles. Hum Reprod 2022b;37:947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang R, Castro G, McCune MF, Zeng BT, Rothlauf Q, Sonnek PW, Liu NM, Brulois Z, Wang KF, Greenberg X et al TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol 2020;5:eabc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang X, Zhang F, Zhu Y, Du MR, Tao ZW, Sun C, Ma HT, Li YD, Liang GQ et al Evaluation of inactivated COVID-19 vaccine on semen parameters in reproductive-age males: a retrospective cohort study. Asian J Androl 2022;24:441–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article. Further information can be shared on reasonable request to the corresponding author.