Abstract
Blood-based biomarkers for amyloid beta and phosphorylated tau show good diagnostic accuracies and agreements with their corresponding CSF and neuroimaging biomarkers in the amyloid/tau/neurodegeneration [A/T/(N)] framework for Alzheimer’s disease. However, the blood-based neurodegeneration marker neurofilament light is not specific to Alzheimer’s disease while total-tau shows lack of correlation with CSF total-tau. Recent studies suggest that blood total-tau originates principally from peripheral, non-brain sources.
We sought to address this challenge by generating an anti-tau antibody that selectively binds brain-derived tau and avoids the peripherally expressed ‘big tau’ isoform. We applied this antibody to develop an ultrasensitive blood-based assay for brain-derived tau, and validated it in five independent cohorts (n = 609) including a blood-to-autopsy cohort, CSF biomarker-classified cohorts and memory clinic cohorts.
In paired samples, serum and CSF brain-derived tau were significantly correlated (rho = 0.85, P < 0.0001), while serum and CSF total-tau were not (rho = 0.23, P = 0.3364). Blood-based brain-derived tau showed equivalent diagnostic performance as CSF total-tau and CSF brain-derived tau to separate biomarker-positive Alzheimer’s disease participants from biomarker-negative controls. Furthermore, plasma brain-derived tau accurately distinguished autopsy-confirmed Alzheimer’s disease from other neurodegenerative diseases (area under the curve = 86.4%) while neurofilament light did not (area under the curve = 54.3%). These performances were independent of the presence of concomitant pathologies. Plasma brain-derived tau (rho = 0.52–0.67, P = 0.003), but not neurofilament light (rho = −0.14–0.17, P = 0.501), was associated with global and regional amyloid plaque and neurofibrillary tangle counts. These results were further verified in two memory clinic cohorts where serum brain-derived tau differentiated Alzheimer’s disease from a range of other neurodegenerative disorders, including frontotemporal lobar degeneration and atypical parkinsonian disorders (area under the curve up to 99.6%). Notably, plasma/serum brain-derived tau correlated with neurofilament light only in Alzheimer’s disease but not in the other neurodegenerative diseases. Across cohorts, plasma/serum brain-derived tau was associated with CSF and plasma AT(N) biomarkers and cognitive function.
Brain-derived tau is a new blood-based biomarker that outperforms plasma total-tau and, unlike neurofilament light, shows specificity to Alzheimer’s disease-type neurodegeneration. Thus, brain-derived tau demonstrates potential to complete the AT(N) scheme in blood, and will be useful to evaluate Alzheimer’s disease-dependent neurodegenerative processes for clinical and research purposes.
Keywords: Alzheimer’s disease, neurodegenerative disease, plasma brain-derived-tau, total-tau, neurofilament light
A blood-based biomarker that tracks neurodegeneration in Alzheimer’s disease and differentiates it from other dementias is lacking. Gonzalez et al. report the discovery and clinical validation of plasma brain-derived tau, an improved total-tau assay that specifically targets CNS tau in blood.
Introduction
The AT(N) (amyloid, tau, neurodegeneration) research framework provides a unified scheme that emphasizes on pathophysiological evidence of amyloid beta (A), tau (T) and neurodegeneration (N) for the definition and staging of Alzheimer’s disease.1–3 However, the framework currently relies on established CSF and neuroimaging biomarkers that have major challenges from economical, practical and logistical perspectives that limit their widespread applications, particularly in contexts that require cost-effective and high-throughput assessments for biological evidence of Alzheimer’s disease. For example, given the paucity of dementia specialists in many hospital systems,4 biomarker screening at the primary care level would be beneficial to streamline patient management including referrals to specialist practitioners.5–8 Moreover, as therapeutic trials are now required to show biomarker evidence of candidate drug efficacy,9 it is essential that only participants with confirmed underlying pathology are included and further monitored in the course of trials.6 The Alzheimer’s disease field will therefore benefit from biomarker modalities that have improved simplicity, accessibility, convenience and cost-effectiveness without compromising on performance.5–8
The development of AT(N) blood biomarkers improves the needed scalability for large-scale diagnostic, prognostic and therapeutic trial applications. Plasma amyloid beta (Aβ)42/Aβ40 methods, measured using immunoprecipitation-mass spectrometry methods, have shown good accuracies to detect Aβ abnormalities in Alzheimer’s disease that are absent in neurodegenerative diseases without amyloidosis.10–13 Plasma p-tau, including those targeting p-tau181, p-tau217 and p-tau231, have also demonstrated excellent diagnostic performances to detect and to differentiate Alzheimer’s disease from other neurodegenerative diseases.14–19 Plasma Aβ and p-tau therefore show promise as the A and T biomarkers, respectively, in the AT(N) framework.6,8,20 For neurodegeneration (N), however, plasma neurofilament light chain (NfL) has demonstrated excellent diagnostic performance to identify Alzheimer’s disease compared with controls, but is unable to distinguish it from other neurodegenerative diseases.21–24 For these reasons, plasma NfL may not be optimal for use as an Alzheimer’s disease-specific neurodegeneration marker. Moreover, current plasma total-tau (t-tau) assays do not show good diagnostic utility,24–29 contrary to CSF t-tau that reliably reflects neurodegeneration in Alzheimer’s disease but not in other neurodegenerative diseases like Parkinson’s disease, Lewy body dementia and frontotemporal dementia.30–34 Plasma t-tau concentrations show large overlaps between diagnostic groups and do not correlate with CSF t-tau, suggesting that plasma and CSF t-tau do not originate from the same tissue sources.25,27,29 Indeed, while tau is known to be highly abundant in the CNS, the protein is also present in peripheral tissues (e.g. liver, kidney, heart).35,36 The protein structure of tau in the CNS and PNS has fundamental differences in splice variants: while there are six tau isoforms of varying lengths in the adult human brain, the main form of tau in the PNS is distinguishable from these isoforms by the presence of a large peptide insert resulting from the transcription of an extra exon (exon 4a) of the MAPT gene.37,38 Since CSF t-tau, but not plasma t-tau, agrees with PET and neuropathological evidence of Alzheimer’s disease,30,32–34 it is plausible that the tau forms measured by the blood assay originate, to a large extent, from non-CNS sources. In line with this reasoning, a recent study estimated that only a fifth of the signal measured by plasma t-tau is brain-derived while the remainder originates from peripheral sources.25 As a result, plasma t-tau tends to show promising diagnostic function largely in disorders with acute increases in CNS tau production and release, including traumatic brain injury and acute stroke following cardiac arrest.39–44 Together, a blood-based biomarker for neurodegeneration specific to Alzheimer’s disease is currently lacking. Discovery of such a biomarker would complete the AT(N) system in blood, and enable examination of neurodegenerative process(es) specific to Alzheimer’s disease pathogenesis while differentiating these from neurodegenerative mechanisms common to related dementias.
In this study, we hypothesized that: (i) an immunoassay can be innovated to selectively measure brain-derived tau (BD-tau) in blood by using an antibody engineered to specifically target tau isoforms originating from the brain; (ii) such a novel assay would show strong correlations between plasma and CSF levels; and (iii) the assay would demonstrate specificity to Alzheimer’s disease by showing good performances to differentiate it from non-Alzheimer’s disease neurodegenerative diseases. Here, we report the development, analytical and clinical validation of a novel blood-based biomarker that is specific for BD-tau. In five independent research cohorts (n = 609 participants), we evaluated the capabilities of this new blood biomarker to: (i) differentiate neurochemically defined Alzheimer’s disease from biomarker-negative controls; (ii) distinguish Alzheimer’s disease from other neurodegenerative diseases, including in a cohort with neuropathological confirmation; and (iii) to associate with the severity of plaque and tangle pathologies at autopsy, CSF AT(N) biomarkers (including in paired plasma/serum versus CSF samples), and cognition.
Materials and methods
Development and validation of a BD-tau blood assay
Sheep monoclonal antibodies were generated using a custom-designed synthetic peptide antigen conjugated to KLH at the N terminus (GenScript Biotech). All work was undertaken according to the UK Animal Scientific Procedures Act, and the methodology has been described previously.45 Candidate hybridomas were selected based on binding to an expressed glutathione S-transferase-linked protein construct for exons 4–5 of tau and recombinant full-length tau-441 (rPeptide). To ensure reactivity was solely to the contiguous sequence of exons 4–5, hybridomas were screened against additional peptides and glutathione S-transferase-linked protein constructs representing exons 4–4a, 4a–5 and 4–4a–5. The TauJ.5H3 monoclonal antibody was shown in our validation studies to demonstrate specific reactivity to the junction between MAPT exons 4 and 5, and was therefore selected for use in assay development. Antibody design, generation and validation were performed at Bioventix Plc.
For the BD-tau assay, TauJ.5H3 was used as the capture antibody, thereby precluding the binding of tau isoforms containing the exon 4a insert. A mouse monoclonal antibody raised against the N-terminal region of tau was used for detection. In vitro phosphorylated recombinant full-length tau-441 (#TO8-50FN, SignalChem) was used as the assay calibrator. Blood samples and calibrators were diluted with assay diluent (Homebrew buffer; #101556, Quanterix). Analytical validation followed protocols described previously.14,19,46 Assay development work was conducted at the University of Gothenburg, Sweden.
Study cohorts, biomarker and neuropathological assessments
Discovery and Neurochemical cohorts
The Discovery cohort (n = 20) included paired CSF and serum samples from neurochemically defined Alzheimer’s disease participants (n = 10) and controls (n = 10) selected based on their CSF biomarker profile (CSF Aβ42 < 530 pg/ml, CSF p-tau > 60 pg/ml and CSF t-tau > 350 pg/ml14,31). The Alzheimer’s disease group had no evidence of other neurological conditions based on routine clinical and laboratory assessments. The control group consisted of selected patients with a biomarker-negative profile.
The Neurochemical cohort (n = 60) consisted of serum samples from neurochemically defined Alzheimer’s disease participants (n = 24) and age-matched controls (n = 36). The selection criteria were the same as in the Discovery cohort. CSF biomarkers were measured using the established INNOTEST assays from Fujirebio. Both cohorts were from the Sahlgrenska University Hospital, Sweden.
Neuropathology cohort
The Neuropathology cohort consisted of plasma samples (n = 52) from research participants enrolled in the University of California San Diego (UCSD) Shiley-Marcos Alzheimer’s Disease Research Center. Participants underwent longitudinal annual assessments, which included blood sample collection, received consensus clinical diagnoses and were followed until death. Subsequently, post-mortem neuropathological examination was performed to determine the presence and extent of amyloid and tau pathologies consistent with Alzheimer’s disease, as well as with other neurodegenerative and vascular pathologies. Brains were divided sagittally and the left hemibrain was fixed in 10% buffered formalin while the right hemibrain was sectioned coronally and frozen at −80°C. The formalin-fixed left hemibrain was cut serially into 1-cm slices for paraffin embedding. Sections were taken and stained with haematoxylin and eosin for histopathological examination from: middle frontal cortex (Brodmann areas 8/9), rostral superior temporal cortex, inferior parietal cortex, hippocampus, entorhinal cortex, basal nuclei, midbrain with substantia nigra, pons with locus coeruleus and cerebellar cortex with dentate nucleus. Lesions were evaluated in 10-μm thick sections stained with thioflavin-S or in 5-μm thick sections with immunohistochemical staining.
Neuritic plaques, diffuse plaques and neurofibrillary tangles (NFTs) were identified either with 1% thioflavin-S stains viewed with ultraviolet illumination and a 440-μm bandpass wavelength excitation filter, or with immunohistochemical staining using antibodies to Aβ (rabbit polyclonal antibody 69D, kindly provided by Edward Koo; 1:1200 dilution) or PHF1 tau (courtesy of Peter Davies; 1:600 dilution). Neuritic plaque density was estimated using methods recommended by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)47 while Braak stage for NFT pathology was determined according to Braak et al.48 For more recent cases, pathological diagnosis of Alzheimer’s disease was made using the NIA-AA consensus criteria for the post-mortem diagnosis of Alzheimer’s disease, wherein Thal phase 4–5 (A3), Braak stage V–VI (B3) and moderate-to-severe neuritic plaque density (C2/3) corresponds to high Alzheimer’s disease neuropathological change (ADNC).49 For biomarker–pathology associations, we evaluated comparisons with NIA Reagan,50 Braak stage47 and CERAD48 stages separately. Cerebral amyloid angiopathy was graded from 0 (absent) to 3 (severe) according to procedures described by the NACC Neuropathology Working Group.49 We defined the ‘Low Pathology’ group as Braak 0–II in the absence of significant Lewy body dementia, major vascular pathology, hippocampal sclerosis, limbic-predominant age-related TAR DNA binding protein-43 (TDP-43) encephalopathy or other neurodegenerative pathology.51 The ‘Other Pathology’ group consisted of individuals who had been autopsy-verified to have had non-Alzheimer neurodegenerative diseases.
Memory Clinic Cohorts 1 and 2
The two memory mlinic cohorts included plasma (Cohort 1) or serum (Cohort 2) samples from patients clinically diagnosed with frontotemporal lobar degeneration or Alzheimer’s disease, as well normal control individuals. The frontotemporal lobar degeneration group included behavioural frontotemporal dementia, agrammatic variant primary progressive aphasia, semantic variant primary progressive aphasia, corticobasal syndrome and progressive supranuclear palsy according to current clinical criteria.52–54 The Alzheimer’s disease participants showed clinical profiles of Alzheimer’s disease dementia, in agreement with up-to-date diagnostic recommendations.55 Individuals with suspected neurodegenerative diseases were assessed with the core CSF biomarkers to rule in or rule out Alzheimer’s disease pathophysiology. Normal controls without a clinical presentation of memory problems and with a biomarker-negative profile were recruited among caregivers. Memory Clinic Cohort 1 included n = 375 participants, namely 60 individuals with Alzheimer’s disease, 256 with frontotemporal lobal degeneration and 59 healthy controls from the Center for Neurodegenerative Disorders, University of Brescia, Italy and from the IRCCS Istituto San Giovanni di Dio Fatebenefratelli, Brescia, Italy. Memory Clinic Cohort 2 included n = 102 participants; 19 with Alzheimer’s disease, 70 with frontotemporal lobal degeneration and 13 healthy controls from the Center for Neurodegenerative Disorders, University of Brescia, Italy. Further details about this cohort have been provided previously.56
Measurement of BD-tau in the clinical cohorts
BD-tau was measured blinded on Simoa HD-X using the previously described in-house assay at the University of Gothenburg, Mölndal, Sweden. Biotinylated N-terminal anti-tau mouse monoclonal antibody was used for detection. Full-length recombinant tau 441 phosphorylated in vitro by glycogen synthase kinase 3β (SignalChem) was used as the calibrator. The assay validation focused on dilution linearity, spike recovery, antibody specificity, precision and lower limit of quantification, following published methods.14,19,46 Plasma and CSF samples were diluted 4- and 30-fold, respectively, before analyses.
Signal variations within and between analytical runs were assessed using three internal quality control samples analysed in duplicates at the beginning and the end of each run. Within-run variation for the Discovery, the Neurochemical and the Neuropathology cohorts (each analysed in a single run) were 6.0, 6.9 and 8.7%, respectively. For Memory Clinic Cohort 1, the within- and between-run variations were 5.0 and 5.3%, respectively. The respective within- and between-run variations for Memory Clinic Cohort 2 were 7.9 and 8.0%, respectively. All these values are less than the 20% allowable limit for clinical chemistry purposes.57 The Memory Clinic Cohort 1 results, generated using a different batch of reagents, were adjusted to the other cohorts by multiplying values by three, according to signals for the same quality control samples analysed on all plates.
Other plasma biomarkers
Measurements were performed on the Simoa HD-X platform. Plasma p-tau181 was measured either with a commercial method from Quanterix Inc. (pTau-181 V2 Advantage Kit #103714; Neuropathology cohort) or according to the method by Karikari et al.14 for all other cohorts. Plasma p-tau231 was measured using the University of Gothenburg assay.19 Plasma t-tau was measured with the Quanterix kit (#101552).
Ethical clearance
Participant consent was obtained according to the Declaration of Helsinki. The Discovery and Neurochemistry cohorts that used de-identified leftover clinical samples were both approved by the ethics committees at the University of Gothenburg (#EPN140811). The Neuropathology cohort was reviewed and approved by the human subject review board at UCSD. Informed consent was obtained from all patients or their caregivers consistent with California State law. The Memory Clinic cohorts were approved by the Brescia Ethics Committee (#NP 1965).
Statistical analyses
Statistical analyses were performed with Prism v.9.3.1 (GraphPad, San Diego, CA, USA). Data are shown as mean ± standard deviation unless otherwise stated. The distributions of data sets were examined for normality using the Kolmogorov–Smirnov test. Non-parametric tests were used for non-normally distributed data. Spearman correlation and the chi square test were used for continuous and categorical variables, respectively. Diagnostic performances were evaluated with receiver operating curves (ROC) and area under the curve (AUC) assessments. Fold changes were examined by comparing biomarker values with the mean of the control group.
Group differences were examined using two-tailed Mann–Whitney test (two categories) or the Kruskal–Wallis test with Dunn’s multiple comparisons (three or more groups). Abnormally high BD-tau values beyond 500 pg/ml were excluded from the graphs for clarity of presentation but were included in the statistical analyses. Significance was set at P < 0.05.
Data availability
Anonymized data are available on reasonable request from the corresponding author.
Results
TauJ.5H3 antibody specifically recognizes BD-tau
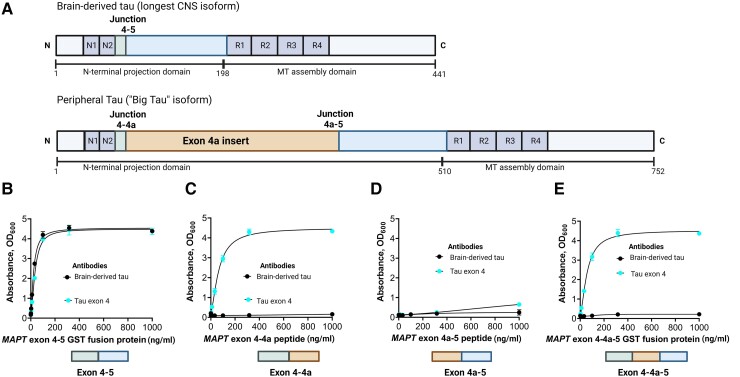
The TauJ.5H3 antibody was designed to selectively bind to continuous exon 4–5 sequences on CNS-derived tau isoforms (Fig. 1A). As expected, the antibody did exclusively bind to recombinant protein constructs representing the exon 4–5 in a concentration-dependent manner. An identical binding pattern was observed for a control antibody selected by affinity purification of polyclonal serum against the exon 4 peptide (Fig. 1B). However, the TauJ.5H3 antibody did not recognize recombinant protein constructs containing the exon 4a insert; this includes constructs for the exons 4–4a (Fig. 1C), 4a–5 (Fig. 1D) and 4–4a–5 regions (Fig. 1E), confirming binding was only observed when the contiguous exon 4–5 sequence was present. The tau exon-4 antibody recognized recombinant constructs that included the exon 4 region (that is, exons 4–4a and 4–4a–5) while both the TauJ.5H3 and the tau exon-4 antibodies did not bind to a protein construct spanning the exon 4a–5 region (Fig. 1D). Additionally, TauJ.5H3 recognized all six tau isoforms abundantly expressed in the adult human CNS (Supplementary Fig. 1). Together, these results indicate that the TauJ.5H3 antibody recognizes tau isoforms that are derived from the CNS but avoids tau forms that include the exon 4a region predominantly expressed in peripheral tissues.
Figure 1.
Design and characterization of the TauJ.5H3 sheep monoclonal antibody specific for CNS-derived tau isoforms. (A, top) Schematic illustration of the full-length tau isoform (2N4R) in the adult human brain showing the different regions including the junction between exons 4 and 5, indicating the absence of the exon 4a insert. Note that the organization of the exons 4 and 5 here also applies to the other five major tau isoforms commonly expressed in the adult human CNS. (A, bottom) Schematic illustration of the high molecular weight tau (‘big tau’) isoform, which is the predominant form of tau in the adult human PNS. The exon 4a insert breaks the junction between exons 4 and 5 in the 2N4R isoform into two separate junctions—between exons 4 and 4a and between 4a and 5. The TauJ.5H3 BD-tau antibody was generated against a small contiguous peptide that specifically stretches the junction between exons 4 and 5, making it unique to CNS tau isoforms. The control anti-exon-4 antibody was generated against a recombinant protein form of the exon 4 that is common to all tau isoforms. (B) The TauJ.5H3 antibody did bind in a concentration-dependent manner to a recombinant protein construct corresponding to the exon 4–5 region found in the 2N4R and other CNS tau isoforms but not in the high molecular weight tau isoform abundantly expressed in peripheral tissue. The binding profile was the same as that of a control antibody generated against the exon-4 region. (C) The TauJ.5H3 antibody did not bind to a recombinant protein construct that covers the exons 4–4a region found in the high molecular weight, but not the 2N4R, tau isoform. However, the anti-exon-4 antibody did bind in a concentration-dependent manner as it did against the exon 4–5 region in B above. (D) Both the TauJ.5H3 and anti-exon-4 antibodies did not recognize a recombinant protein construct for the exon 4a–5 region that is found in the high molecular weight tau but not CNS isoforms. (E) TauJ.5H3, but not the anti-exon-4 antibody, gave no signal in the presence of a recombinant fusion construct corresponding to the continuous exon 4–4a–5 region.
BD-tau assay shows good analytical performance in plasma and serum
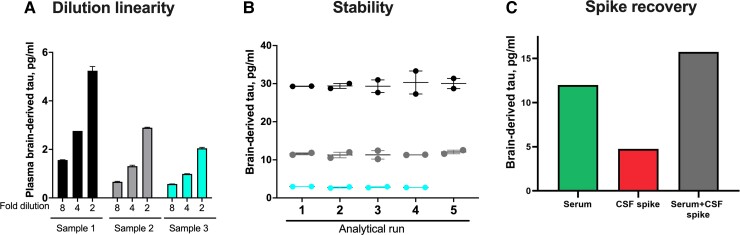
We next developed a novel immunoassay to measure BD-tau in blood, by pairing the TauJ.5H3 antibody with an N-terminal-tau targeting antibody. Following optimization, the assay showed excellent analytical performance in both plasma and serum. Concentrations of the biomarker in endogenous blood samples decreased linearly when measured 2-fold diluted with the assay diluent versus when diluted 4- or 8-fold (Fig. 2A). The assay also showed between-run stability of 92–95% when aliquots of three independent plasma or serum samples were measured in up to five separate analytical runs (Fig. 2B). Moreover, there was a recovery of 93% of the expected analyte signal in exogenous CSF samples when spiked into test blood samples (Fig. 2C). These values were within recommended/acceptable limits as stipulated by an international consortium of clinical chemists57 and were also comparable to those we have reported previously for p-tau assays.14,19,46 The lower limit of quantification for the assay was estimated to be 0.03 pg/ml.
Figure 2.
Technical validation of a novel assay to measure BD-tau in blood. (A) Dilution linearity. The panel shows serial dilutions of three unique plasma samples with the assay diluent. Compared with sample aliquots diluted 2-fold, those diluted 4-fold gave ∼50% less signal for BD-tau. The trend was the same when comparing 4- and 8-fold diluted samples. The bar plots show the mean values and the error bars show the standard error of the mean. (B) Within- and between-run stability. The concentrations for three separate plasma or serum samples were measured in duplicates in up to five independent analytical runs are shown, to depict day-to-day stability of the BD-tau assay. (C) Spike recovery. Serum samples diluted 1:2 as well as the assay diluent were each ‘spiked’ with CSF and levels in each sample were measured with our assay. The plot shows signals for the non-spiked serum sample, the CSF spike sample alone and the serum + CSF spike sample together.
Correlation between BD-tau levels in paired CSF and blood samples suggests lack of interference from peripheral tau
We hypothesized that if BD-tau is not significantly affected by peripheral tau contamination, concentrations in paired CSF and blood samples should show good correlation and similar diagnostic performances. In the Discovery cohort, there was a strong correlation (Spearman’s rho = 0.85, P < 0.0001; Supplementary Table 3) of BD-tau levels in paired serum and CSF. To the contrary, concentrations of the Quanterix t-tau assay (the performance of which is known to be impacted by tau from peripheral tissues25) did not correlate in the same serum versus CSF sample pairs (Spearman’s rho = 0.23, P = 0.3364; Supplementary Table 3). CSF BD-tau correlated strongly with CSF t-tau measured with either the INNOTEST (Spearman’s rho = 0.93, P < 0.0001) or Quanterix (Spearman’s rho = 0.85, P < 0.0001) assays.
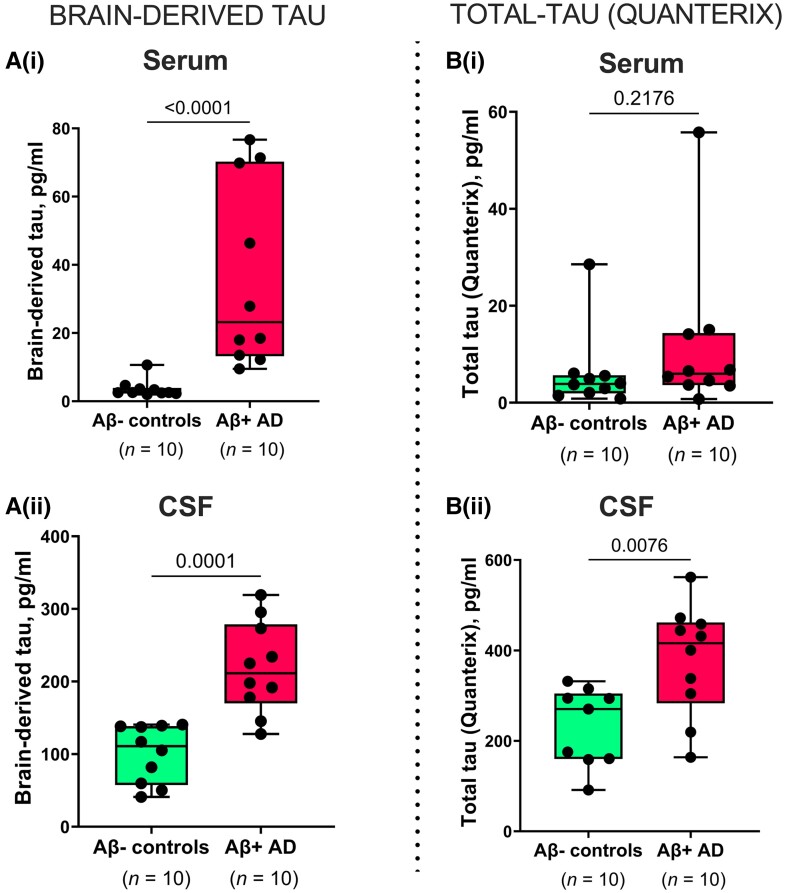
BD-tau levels were increased in Alzheimer’s disease versus controls in paired serum and CSF samples [P ≤ 0.0001; Fig. 3A(i and ii)], with fold changes of 9.5 and 2.2, respectively. However, t-tau was increased in Alzheimer’s disease versus controls in CSF (P = 0.0076) but not in paired serum samples [P = 0.2176; Fig. 3B(i and ii)]. Here, the fold changes were smaller; 1.9 and 1.6 for serum and CSF, respectively. In the same sample pairs, the diagnostic accuracy of serum BD-tau was 100 versus 67.0% [5% confidence interval (CI) = 42.3–91.7%] for serum t-tau.
Figure 3.
Concentrations and correlation of BD-tau in paired serum and CSF samples. [A(i and ii)] Concentrations of BD-tau in paired serum and CSF samples showing significant increases in Aβ+ Alzheimer’s disease and Aβ− control individuals classified according to their neurochemical CSF biomarker profiles. The corresponding levels of t-tau (Quanterix) in the same paired serum and CSF samples are shown in B(i) and B(ii), respectively. For B(ii), one sample in the Aβ− control group returned no measurable signal due to a technical instrument error. Excluding the CSF-serum pair of this sample from the analyses did not change the results. P-values indicate the results of Mann–Whitney tests. In each box plot, the horizontal bar on top of the coloured area shows the 75% percentile, the middle bar depicts the median and the lower bar shows the 25% percentile. Values that are above the 75% percentile and below the 25% percentile are shown outside the coloured areas. Note that there are differences in the absolute concentrations of BD-tau and t-tau in both serum and CSF, which can be explained by the use of different assay designs, analytical technologies, calibrators, and standard curves for each biomarker. This means that the values are a reflection of several factors, including assay sensitivity, and that absolute concentrations are not directly comparable in numerical sense.
Assuming that serum and CSF tau originate from the same BD-tau pool, we estimated that serum BD-tau reflects 4% of the corresponding CSF concentrations in normal ageing (Aβ− controls) and 16% in Alzheimer’s disease. The t-tau assay, on the other hand, showed 3% serum-to-CSF ratios in both Alzheimer’s disease and Aβ− controls.
In the Neurochemical cohort, serum BD-tau was increased in Alzheimer’s disease versus controls (Mann–Whitney U = 0, P < 0.0001; fold change = 3.1) similar to CSF t-tau, with an AUC of 100% (Supplementary Fig. 2).
Plasma BD-tau differentiates Alzheimer’s disease from other neurodegenerative diseases
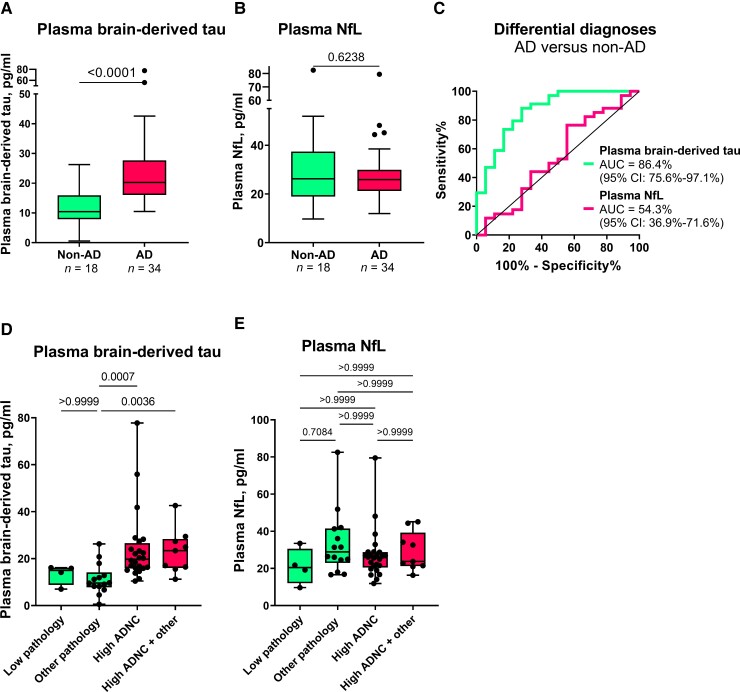
In the Neuropathology cohort, plasma BD-tau was increased in Alzheimer’s disease versus non-Alzheimer’s disease (Mann–Whitney U = 83.5, P < 0.0001) with a diagnostic accuracy of 86.4% (95% CI = 75.6–97.1%; Fig. 4A and C). To the contrary, plasma NfL was not significantly increased in Alzheimer’s disease (Mann–Whitney U = 280, P = 0.6238; AUC = 54.3%, 95% CI = 36.9–71.6%; Fig. 4B and C). Fold changes of 5.0 versus 1.0 were observed for plasma BD-tau and NfL, respectively.
Figure 4.
Plasma BD-tau accurately differentiates autopsy-verified Alzheimer’s disease from other neurodegenerative diseases. (A) and (B) Tukey plots of plasma BD-tau and plasma NfL levels in the Alzheimer’s disease (AD) and the non-Alzheimer’s disease (non-AD) groups in the Neuropathology cohort. The corresponding ROC and AUC values indicating between-group discriminatory accuracies of the biomarkers are shown in C. The diagonal line on the ROC plot shows 50% accuracy meaning no difference from chance events. (D and E) Plasma BD-tau and NfL stratified according to ADNC. The non-AD group was divided into Low Pathology (limited amyloid plaques in the absence of tau tangles) or Other Pathology (non-Alzheimer pathologies). The pathology-confirmed Alzheimer’s disease group was also divided into High ADNC and High ADNC + Other (Alzheimer’s disease in the presence of concomitant pathologies) subgroups. (A) Plasma BD-tau was significantly increased in both the High ADNC and the High ADNC + Other subgroups compared with the Other Pathology group. P-values indicate the results of Mann–Whitney test (for two groups) or Kruskal–Wallis test adjusted for multiple comparisons (three or more groups). In each box plot, the horizontal bar on top of the coloured area shows the 75% percentile, the middle bar depicts the median and the lower bar shows the 25% percentile. Values that are above the 75% percentile and below the 25% percentile are shown outside the coloured areas.
In secondary analyses, we stratified the Alzheimer’s disease group into High ADNC and High ADNC + Other (Alzheimer’s disease with concomitant pathologies such as Lewy body dementia and hippocampal sclerosis) subgroups, and the non-Alzheimer’s disease group into those diagnosed with Low or Other Pathology groups. We found no difference in plasma BD-tau between the High ADNC and the High ADNC + Other subgroups (P > 0.999; Fig. 4D). Similarly, there was no difference between the Low and Other Pathology subgroups (P > 0.999; Fig. 4D). However, plasma BD-tau differentiated the Other Pathology subgroup from both the High ADNC and High ADNC + Other subgroups (P ≤ 0.0036; Fig. 4D). While we observed differences between the Low Pathology versus each of the High ADNC and the High ADNC + Other subgroups at the group-level (Table 1), these did not reach statistical significance probably because of the reduced statistical power. Plasma NfL did not differentiate between any of the subgroups (Fig. 4E).
Table 1.
Demographic characteristics of the Neuropathology cohort
| Variable | Non-Alzheimer’s disease (n = 18) | Alzheimer’s disease (n = 34) | P-value (t-test or chi-squared as appropriate) | ||
|---|---|---|---|---|---|
| Low Pathology | Other Pathology | High ADNC | High ADNC + Other | Alzheimer’s disease versus non-Alzheimer’s disease | |
| n | 4 | 14 | 25 | 9 | |
| Age at plasma | 78.9 ± 4.1 | 74.8 ± 8.2 | 72.5 ± 8.1 | 71.4 ± 9.5 | 0.13 |
| Age at death | 84.3 ± 4.2 | 78.1 ± 8.6 | 75.7 ± 7.8 | 74.8 ± 10.7 | 0.11 |
| Last plasma to death interval | 5.4 ± 3.6 | 3.3 ± 2.4 | 3.2 ± 1.9 | 3.4 ± 1.8 | 0.50 |
| Female | 0 (0%) | 1 (7%) | 10 (40%) | 2 (22%) | 0.04 |
| Hispanic | 2 (50%) | 1 (7%) | 1 (4%) | 0 (0%) | 0.22 |
| Education, years | 13.8 ± 2.9 | 16.6 ± 3.3 | 16.3 ± 2.5 | 15.6 ± 3.1 | 0.88 |
| APOE 0 ε4 alleles | 3 (75%) | 12 (86%) | 9 (36%) | 4 (44%) | *0.007 |
| APOE 1 ε4 allele | 1 (25%) | 2 (14%) | 12 (48%) | 5 (56%) | |
| APOE 2 ε4 alleles | 0 (0%) | 0 (0%) | 4 (16%) | 0 (0%) | |
| MMSE | 29.5 ± 1 | 22.7 ± 5.7 | 15.9 ± 7.1 | 22.1 ± 3.3 | 5.4 × 10−4 |
| DRS | 130.5 ± 11.1 | 108.8 ± 19.5 | 95.1 ± 26.7 | 105.9 ± 22.7 | 0.02 |
| CDR-SOB | 4.8 ± 6.7 | 6.7 ± 4.6 | 9.5 ± 4.1 | 7.7 ± 4.8 | 0.07 |
| Clinical diagnosis | |||||
| ȃNormal | 3 (75%) | 2 (14%) | 0 (0%) | 0 (0%) | **0.006 |
| ȃMCI | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | - |
| ȃAlzheimer’s disease | 1 (25%) | 3 (21%) | 23 (92%) | 9 (100%) | **5.0 × 10−7 |
| ȃDLB/PDD | 0 (0%) | 8 (57%) | 0 (0%) | 0 (0%) | **1.3 × 10−4 |
| ȃFTLD | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) | **1.0 |
| ȃOther | 0 (0%) | 1 (7%) | 1 (4%) | 0 (0%) | **1.0 |
| Plasma Aβ42, pg/ml | 13.5 ± 1.9 | 14.7 ± 4.3 | 10.9 ± 3.6 | 10.6 ± 2.1 | 0.002 |
| Plasma Aβ40, pg/ml | 223.5 ± 30.6 | 214.1 ± 58.4 | 200.5 ± 52.8 | 198.2 ± 29.9 | 0.28 |
| Plasma Aβ42/40 ratio | 0.06 ± 0.002 | 0.069 ± 0.01 | 0.054 ± 0.009 | 0.054 ± 0.008 | 2.7 × 10−5 |
| Plasma t-tau, pg/ml | 1.8 ± 0.5 | 1.7 ± 0.4 | 2.5 ± 1.3 | 2.3 ± 1 | 0.003 |
| Plasma p-tau181, pg/ml | 2.6 ± 1 | 2.8 ± 1.6 | 5.6 ± 2 | 5.8 ± 2 | 4.1 × 10−7 |
| Plasma p-tau231, pg/ml | 7.5 ± 1.6 | 8.3 ± 5.4 | 14.6 ± 6.1 | 15.2 ± 3.9 | 1.1 × 10−4 |
| Plasma NfL, pg/ml | 21 ± 9.8 | 33.6 ± 17.4 | 27.3 ± 13.2 | 29.1 ± 10.5 | 0.50 |
| Plasma BD-tau, pg/ml | 13.3 ± 4.3 | 11.3 ± 6.6 | 72.1 ± 240.8a | 23.1 ± 9.5 | 7.3 × 10−5 |
CDR-SOB = Clinical Dementia Rating Sum of Boxes; DLB = dementia with Lewy bodies; DRS = Dementia Rating Scale; FTLD = frontotemporal lobal degeneration; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination; PDD = Parkinson’s disease with dementia.
The mean ± SD for plasma BD-tau becomes 24.1 ± 15.1 pg/ml when excluding an outlier BD-tau value of 1225.8 pg/ml.
Calculated as chi-squared test for overall number of e4 alleles (0, 1 or 2).
P-value for each diagnosis tested separately. The P-value for the overall chi-squared with all diagnostic possibilities is 7.2 × 10−7.
Profile of BD-tau in different neurodegenerative diseases
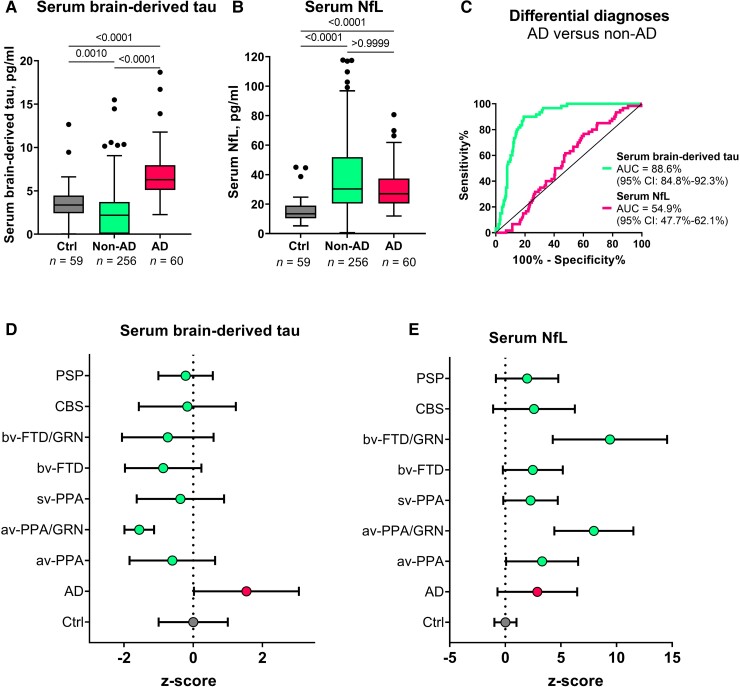
In Memory Clinic Cohort 1, serum BD-tau was significantly increased in Alzheimer’s disease versus non-Alzheimer’s disease (P < 0.0001; AUC = 88.6%, 95% CI = 84.8–92.3%; Fig. 5A and C). To the contrary, plasma NfL showed no differential diagnostic utility (AUC = 54.9%, 95% CI = 47.7–62.1%; Fig. 5B and C). The fold changes (versus controls) in Alzheimer’s disease were 8.9 for BD-tau and 2.6 for NfL. In non-Alzheimer’s disease, serum BD-tau had a fold change of 1.3 while for NfL this was 2.2.
Figure 5.
Serum BD-tau profile in Alzheimer’s disease versus several other neurodegenerative diseases in Memory Clinic Cohort 1. The Tukey box plots in A and B show serum BD-tau and serum NfL respectively in the control, non-Alzheimer’s disease (non-AD) and Alzheimer’s disease (AD) groups. (C) ROC and AUC values for the differential diagnostic function of serum BD-tau and NfL. (D and E) Z-score transformed plots of serum BD-tau and NfL in the control (Ctrl), AD and specific non-AD groups. AUC comparisons of serum BD-tau and NfL to differentiate each group from Alzheimer’s disease is shown in Table 2. In each box plot, the horizontal bar on top of the coloured area shows the 75% percentile, the middle bar depicts the median and the lower bar shows the 25% percentile. Values that are above the 75% percentile and below the 25% percentile are shown outside the coloured areas. Note that the tendency of serum BD-tau concentrations to be lower than in the frontotemporal lobal degeneration groups especially in GRN mutation carriers has also been shown for serum p-tau181 and NfL in this same population.56 Similarly, the highly increased levels of serum NfL in GRN mutation carriers has also been reported before.58 AD = Alzheimer’s disease; avPPA = agrammatic variant primary progressive aphasia; avPPA/GRN = agrammatic variant primary progressive aphasia with progranulin mutation; bvFTD = behavioural frontotemporal dementia; bvFTD/GRN = behavioural frontotemporal dementia with progranulin mutation; CBS = corticobasal syndrome; PSP = progressive supranuclear palsy; svPPA = semantic variant primary progressive aphasia.
When the non-Alzheimer’s disease group in Memory Clinic Cohort 1 was divided into specific diagnostic groups, the differential diagnostic accuracy of serum BD-tau versus Alzheimer’s disease remained high (up to 99.6%) and outperformed serum NfL in each group except in those with GRN mutations where NfL is known to be highly increased58 (Table 2). In agreement, concentrations of serum BD-tau were significantly higher in the Alzheimer’s disease group versus each other diagnostic group (Fig. 5D and E).
Table 2.
Discriminatory accuracy of serum BD-tau and other serum biomarkers to separate Alzheimer’s disease from specific non-Alzheimer’s disease disorders
| Serum BD-tau | Serum NfL | |
|---|---|---|
| Group comparison | AUC (95% confidence interval) | |
| AD versus avPPA | 88.9% (81.1–96.6%) | 62.2% (50.5–74%) |
| AD versus avPPA/GRN | 99.6% (98.7–100%) | 94% (88.2–99.7%) |
| AD versus svPPA | 87.3% (77.4–97.1%) | 50.9% (36.5–65.3%) |
| AD versus bvFTD | 93.2% (89.4–97%) | 51.2% (42.2–60.25%) |
| AD versus bvFTD/GRN | 81.6% (63.7–99.5%) | 94.4% (89–99.8%) |
| AD versus CBS | 83.8% (75.2–92.4%) | 51.5% (39.4–63.6%) |
| AD versus PSP | 77.8% (65.8–90.5%) | 58.9% (45.4–72.3%) |
AD = Alzheimer’s disease; avPPA = agrammatic variant primary progressive aphasia; avPPA/GRN = agrammatic variant primary progressive aphasia with progranulin mutation; bvFTD = behavioural frontotemporal dementia; bvFTD/GRN = behavioural frontotemporal dementia with progranulin mutation; CBS = corticobasal syndrome; PSP = progressive supranuclear palsy; svPPA = semantic variant primary progressive aphasia.
In Memory Clinic Cohort 2, plasma BD-tau concentrations were higher in Alzheimer’s disease versus non-Alzheimer’s disease (P < 0.0001; AUC = 80.0%, 95% CI = 69.1–91.0%), (Supplementary Fig. 3).
BD-tau in blood associates with neurodegeneration markers in Alzheimer’s disease but not in other neurodegenerative diseases
Serum BD-tau correlated with CSF t-tau in the Discovery and Neurochemical cohorts (Spearman’s rho = 0.65–0.83, P < 0.0001). These associations existed in the entire cohorts and in the Alzheimer’s disease group (Supplementary Table 3).
In the Neuropathology cohort, plasma BD-tau correlated with NfL only in the Alzheimer’s disease group but not in the non-Alzheimer’s disease group (Supplementary Table 4). Similarly, serum BD-tau correlated with NfL in Memory Clinic Cohort 1 only in the Alzheimer’s disease group (Spearman’s rho = 0.63, P < 0.0001) and controls (Spearman’s rho = 0.33, P = 0.0115), but not in the non-Alzheimer’s disease group (Spearman’s rho = −0.12, P = 0.0658; Supplementary Table 5). In agreement, serum NfL but not BD-tau correlated with cortical thickness in the non-Alzheimer’s disease group (data not shown). This is in line with our hypothesis because an association of BD-tau with cortical thickness in non-Alzheimer's disease was not expected, given increases in the biomarker levels in Alzheimer's disease but not in non-Alzheimer's disease (Figs 4 and 5). Cortical thickness data were not available to perform a similar analysis for the Alzheimer's disease group in this cohort.
Plasma BD-tau associates with plaque and tangle pathologies at post-mortem
Plasma BD-tau was increased in individuals with frequent versus sparse neuritic plaques according to the CERAD scale (P < 0.0001) and in those with High ADNC versus Low ADNC in line with the NIA Reagan criteria50 (data not shown). Plasma BD-tau correlated with global neuritic plaque counts (Spearman’s rho = 0.58, P < 0.0001), and with diffuse plaque count (Spearman’s rho = 0.56, P < 0.0001; Table 3). In addition, plasma BD-tau correlated with regional measures of neuritic and diffuse plaque pathologies in the hippocampus, superior temporal gyrus and inferior parietal lobe (Spearman’s rho = 0.53–0.68, P ≤ 0.0079; Table 3). Plasma BD-tau did not correlate with cerebral amyloid angiopathy (data not shown). To the contrary, plasma NfL was neither increased according to, nor correlated with, any of the neuropathological measures of plaque pathology (Table 3).
Table 3.
Spearman correlation of plasma BD-tau versus NfL with regional plaque and tangle pathologies biomarkers in the Neuropathology cohort
| Plasma BD-tau | Plasma NfL | |
|---|---|---|
| Global measures | ||
| Neuritic plaques | rho = 0.58 (P < 0.0001) | rho = −0.06 (P = 0.6708) |
| Diffuse plaques | rho = 0.56 (P < 0.0001) | rho = −0.06 (P = 0.6686) |
| Regional measures | ||
| Neuritic plaques | ||
| ȃHippocampal | rho = 0.53 (P = 0.0066) | rho = −0.14 (P = 0.5009) |
| ȃSuperior temporal | rho = 0.52 (P = 0.0079) | rho = −0.13 (P = 0.5312) |
| ȃInferior parietal | rho = 0.68 (P = 0.0002) | rho = 0.17 (P = 0.4262) |
| Diffuse plaques | ||
| ȃHippocampal | rho = 0.55 (P = 0.0066) | rho = −0.13 (P = 0.5251) |
| ȃSuperior temporal | rho = 0.65 (P = 0.0004) | rho = 0.02 (P = 0.9382) |
| ȃInferior parietal | rho = 0.64 (P = 0.0005) | rho = 0.02 (P = 0.8994) |
| NFTs | ||
| ȃHippocampal | rho = 0.56 (P = 0.0033) | rho = 0.09 (P = 0.6848) |
| ȃSuperior temporal | rho = 0.55 (P = 0.0048) | rho = −0.05 (P = 0.8090) |
| ȃInferior parietal | rho = 0.55 (P = 0.0042) | rho = 0.01 (P = 0.9710) |
| ȃMiddle frontal | rho = 0.67 (P = 0.0003) | rho = 0.03 (P = 0.8720) |
Plasma BD-tau correlated with tangle pathology in the hippocampal, superior temporal and inferior parietal, and middle frontal regions (Spearman’s rho ≥ 0.54, P ≤ 0.0048; Table 3). On the other hand, plasma NfL did not correlate with tangle pathology (Table 3).
Serum BD-tau correlated inversely with CSF Aβ42 in the Discovery and Neurochemical cohorts (Spearman’s rho = −0.59–−0.73, P ≤ 0.0003; Supplementary Table 3). Plasma/serum BD-tau correlated significantly with plasma and CSF p-tau across cohorts (Supplementary Tables 3 and 4). However, serum BD-tau did not correlate with serum t-tau (Spearman’s rho = 0.26, P = 0.2738; Supplementary Table 3).
BD-tau associates with cognitive performance in Alzheimer’s disease
Plasma BD-tau correlated inversely with MMSE (Spearman’s rho = −0.34, P = 0.0184) and Clinical Dementia Rating global scores (Spearman’s rho = −0.30, P = 0.0352) in the Neuropathology cohort. Plasma BD-tau correlated positively with age of disease onset in Memory Clinic Cohort 1 (Spearman’s rho = 0.33, P < 0.0001).
Discussion
In this study, we present the development and validation of an ultrasensitive immunoassay, and report clinical performance results in five independent cohorts for an improved blood-based t-tau biomarker, BD-tau. In short, plasma BD-tau was shown to be an Alzheimer’s disease-type neurodegeneration biomarker that can discriminate between autopsy-verified Alzheimer’s disease from other neurodegenerative diseases, and in addition is associated with clinical severity of disease in the Neuropathology cohort. The significance of this biomarker, which also explains these findings, is that blood and CSF levels correlate strongly in paired samples. The assay was developed using a monoclonal antibody that selectively binds to CNS tau isoforms (hence the name BD-tau). This property makes it superior to the current plasma t-tau biomarker that does not correlate with CSF t-tau, probably because it also captures tau from peripheral sources.25,27,29 Furthermore, BD-tau in blood, like NfL, has high diagnostic accuracy to detect neurodegeneration in Alzheimer’s disease. However, plasma BD-tau demonstrated the novel finding of being able to accurately distinguish pathologically confirmed Alzheimer’s disease from several other neurodegenerative diseases while plasma NfL did not. These performances were specific to the neuropathological diagnosis of Alzheimer’s disease, and were unaffected by mixed pathologies. Furthermore, plasma BD-tau, but not plasma NfL, was associated with global and regional amyloid-plaque and NFT counts in the Neuropathology cohort. Moreover, correlations between plasma/serum BD-tau and NfL were observed only in individuals with Alzheimer’s disease but not those with other neurodegenerative diseases.
Attempts to develop a blood-based t-tau biomarker with similar performance as CSF t-tau have been challenging. Plasma t-tau does not correlate with CSF t-tau when measured in paired samples.25,27,29 In agreement, several studies have reported poor diagnostic performances of plasma t-tau for Alzheimer’s disease and for differential diagnosis of Alzheimer’s disease versus other neurodegenerative diseases.24–29,59,60 Since tau protein is expressed in several peripheral sources in addition to the CNS,35,36 we hypothesized that plasma t-tau is significantly affected by tau from peripheral sources. An estimated 80% of the blood t-tau signal originates from peripheral tissues, meaning that the remaining 20% contribution from the CNS is unlikely to result in significant overall differences even when assuming typical fold increases of two to three in Alzheimer’s disease participants.25 Plasma t-tau rather shows diagnostic and prognostic utility in acute neurological disorders where CNS-derived tau levels in blood increase exponentially over a short duration presumably due to blood–brain barrier impairment.40,42,60,61
To address this problem, we aimed to develop a novel blood biomarker that selectively recognizes tau derived from the brain and avoids tau from peripheral sources. We took advantage of the fact that the MAPT gene has multiple splice variants expressed in a tissue-dependent pattern.36,37 Tau in the adult human brain has six isoforms between 352 and 441 amino acids long.36,62 However, tau in peripheral tissues—including the liver, kidney, heart and pancreas—is predominantly of the high molecular weight (‘big tau’) isoform with the exon 4a insert (Fig. 1A).36 Big tau is preferentially localized in peripheral tissues where it is the main form of tau expressed in the adult PNS.38,63 We hypothesized that by generating an antibody specifically against the junction between exons 4 and 5, we could develop a novel immunoassay that selectively targets BD-tau in blood. Biochemical characterization of the resulting monoclonal antibody, TauJ.5H3, the sequence of which was verified by epitope mapping (data not shown) showed that it only bound to recombinant protein constructs that had the exon 4–5 junction intact, and not those that stretched over exons 4–4a, 4a–5 and 4–4a–5 (Fig. 1). In contrast, the tau exon-4 antibody recognized all constructs that included the exon 4, including those lacking the exon 4–5 junction. The ultrasensitive immunoassay we developed using the TauJ.5H3 antibody showed strong dilution linearity, within- and between-run stability, and suitability for use in both plasma and serum. Importantly, the strong correlation between BD-tau measured in serum/plasma and paired CSF samples is an indication that it targets brain-originating tau forms just like CSF t-tau and CSF BD-tau. This finding is highly significant given that several independent studies have reported that plasma t-tau does not correlate with CSF t-tau27–29 (as also demonstrated herein), which may be partly to blame for its poor diagnostic performance.
The most well-validated blood biomarker for neurodegeneration, NfL, is unable to differentiate between Alzheimer’s disease and other dementias due to its increases in a wide range of neurodegenerative disorders.21,56,64 Consequently, the dementia research field currently lacks a blood biomarker that is specifically altered as a result of Alzheimer-type neurodegenerative changes, such as how plasma p-tau is to tau phosphorylation/pathology in the AT(N) framework.6,8,65 Our findings indicate that plasma BD-tau might be a biomarker that is specific for Alzheimer’s disease-type neurodegeneration and can discriminate Alzheimer’s disease from other neurodegenerative diseases, as shown previously for CSF t-tau.30–34 This conclusion is supported by our findings that plasma BD-tau was increased to the same extent in individuals diagnosed with Alzheimer’s disease at autopsy irrespective of whether or not they had mixed pathologies (Figs 4 and 5). In comparison, the biomarker levels were significantly lower in the non-Alzheimer group. However, plasma NfL failed to distinguish Alzheimer’s disease from other diseases, affirming its limitations for differential diagnosis.
If BD-tau in blood is an Alzheimer’s disease-specific neurodegeneration biomarker, it should be associated with the intensity of the key pathological features of Alzheimer’s disease—plaques and tangles, as demonstrated for blood p-tau.14,15,19,66 Plasma BD-tau correlated with neuritic and diffuse amyloid-plaque and NFT counts, and additionally with CSF and plasma Aβ42 and p-tau. Moreover, plasma BD-tau correlated with NfL only in Alzheimer’s disease but not in other neurodegenerative diseases, further supporting its Alzheimer’s disease specificity. Together, the results demonstrate that plasma/serum BD-tau is an Alzheimer’s disease-type neurodegeneration biomarker that associates with principal pathological features of the disease. Future studies will aim to elucidate what neurodegenerative process(es) in Alzheimer’s disease that plasma BD-tau reflects—for e.g. neuronal injury intensity (such as CSF t-tau), loss and shrinkage of the neuropil (such as by using structural MRI) or functional neuronal impairment including glucose hypometabolism (such as by using FDG PET).
Despite serum BD-tau levels being significantly lower in non-Alzheimer’s disease versus Alzheimer’s disease, the concentrations in individuals with frontotemporal lobal degeneration, particularly those carrying GRN mutations, tended to be further lower than those of control participants and the other non-Alzheimer’s disease groups (including progressive supranuclear palsy and corticobasal syndrome; Fig. 5). While this observation might be unexpected, highly comparable results have been reported for serum p-tau181 in the same population.56 Together, the findings may suggest a remarkable decrease in the secretion of CNS-specific biomarkers into the bloodstream in frontotemporal lobal degeneration. Conversely, the high increases in serum NfL for the GRN mutation carriers is corroborated in previous independent studies.58 These results deserve further investigation in other cohorts.
What is the value of plasma BD-tau since plasma p-tau also differentiates Alzheimer’s disease from other neurodegenerative diseases? First, we need separate biomarkers of amyloid, tau and neurodegeneration as stipulated in the AT(N) and the International Working Group frameworks.1–3 Biomarkers of neurodegeneration are not interchangeable with those that reflect amyloidosis or tau phosphorylation. The frameworks also allow for flexibility to include novel biomarkers including those identified in other biofluids. More recently, plasma Aβ42/Aβ40 and p-tau biomarkers have shown great potential to substitute for CSF A and T biomarkers in the AT(N) scheme.67 However, unlike in CSF where t-tau shows specificity to Alzheimer’s disease, there is currently no blood biomarker that reflects neurodegeneration specific to Alzheimer’s disease. NfL in blood does not meet this requirement because it reflects neurodegeneration shared among multiple neurodegenerative diseases.21,64 Plasma BD-tau shows high potential as a neurodegeneration biomarker of the Alzheimer's type. We anticipate that combining plasma BD-tau with p-tau and possibly Aβ42/Aβ40 will increase accuracy of a blood biomarker-based diagnosis of Alzheimer’s disease by increasing its agreement with results obtained at autopsy or by using CSF or neuroimaging biomarkers.
In the present study, we report a novel blood biomarker specific for BD-tau. Validation showed strong correlations between paired blood and CSF measures, and we verified a high performance to specifically identify Alzheimer’s disease-type neurodegeneration. Future studies will further address the characteristics of this novel biomarker, including exploring its longitudinal changes across the Alzheimer’s disease continuum in both sporadic and familial Alzheimer’s disease, associations with neuroimaging AT(N) biomarkers and the influence of genetic risks (e.g. APOE ε4). Additionally, we will verify the generalizability of the biomarker in diverse, multi-ethnic cohorts from a variety of populations. Furthermore, we will characterize BD-tau in disorders that CSF t-tau is known to be increased in, including acute traumatic brain injury and Creutzfeldt–Jakob disease.
Supplementary Material
Acknowledgements
The authors thank all participants of the research cohorts studied here and their caregivers for their invaluable time, support and biospecimen donations. We also thank research and medical staff at the University of Gothenburg, the Sahlgrenska University Hospital, the UCSD Shiley-Marcos ADRC, San Diego, and the University of Brescia for their collaborative support. Furthermore, we are grateful to Celia Hök Fröhlander for her immense support with biofluid sample processing and aliquoting.
Contributor Information
Fernando Gonzalez-Ortiz, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, University of Gothenburg, Gothenburg 405 30, Sweden.
Michael Turton, Bioventix Plc, Romans Business Park, Farnham, Surrey GU9 7SX, UK.
Przemysław R Kac, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, University of Gothenburg, Gothenburg 405 30, Sweden.
Denis Smirnov, University of California, San Diego and Shiely-Marcos Alzheimer’s Disease Research Center, La Jolla, CA 92037, USA.
Enrico Premi, Neurology Unit, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, BS 25121, Italy.
Roberta Ghidoni, Molecular Markers Laboratory, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia 25121, Italy.
Luisa Benussi, Molecular Markers Laboratory, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia 25121, Italy.
Valentina Cantoni, Neurology Unit, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, BS 25121, Italy.
Claudia Saraceno, Molecular Markers Laboratory, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia 25121, Italy.
Jasmine Rivolta, Neurology Unit, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, BS 25121, Italy.
Nicholas J Ashton, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, University of Gothenburg, Gothenburg 405 30, Sweden; Wallenberg Centre for Molecular and Translational Medicine, University of Gothenburg, Gothenburg 405 30, Sweden; King’s College London, Institute of Psychiatry, Psychology and Neuroscience, Maurice Wohl Clinical Neuroscience Institute, London, SE5 8AF, UK; NIHR Biomedical Research Centre for Mental Health and Biomedical Research Unit for Dementia at South London and Maudsley NHS Foundation, London, SE5 8AF, UK.
Barbara Borroni, Neurology Unit, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, BS 25121, Italy.
Douglas Galasko, University of California, San Diego and Shiely-Marcos Alzheimer’s Disease Research Center, La Jolla, CA 92037, USA.
Peter Harrison, Bioventix Plc, Romans Business Park, Farnham, Surrey GU9 7SX, UK.
Henrik Zetterberg, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, University of Gothenburg, Gothenburg 405 30, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, 431 80, Sweden; Department of Neurodegenerative Disease, UCL Institute of Neurology, London, WC1N 3BG, UK; UK Dementia Research Institute at UCL, London, WC1E 6BT, UK; Hong Kong Center for Neurodegenerative Diseases, Shatin, N.T., Hong Kong, China.
Kaj Blennow, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, University of Gothenburg, Gothenburg 405 30, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, 431 80, Sweden.
Thomas K Karikari, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, University of Gothenburg, Gothenburg 405 30, Sweden; Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA 15213, USA.
Funding
F.G.-O. was funded by the Anna Lisa and Brother Björnsson’s Foundation and Emil och Maria Palms Foundation. B.B., R.G., L.B. and C.S. were funded by the Italian Ministry of Health, Ricerca Corrente. P.R.K. was funded by Demensförbundet. H.Z. is a Wallenberg Scholar and was additionally funded by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712 and #101053962), Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the Alzheimer’s disease Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C and #ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 860197 (MIRIADE), the European Union Joint Programme—Neurodegenerative Disease Research (JPND2021-00694) and the UK Dementia Research Institute at UCL (UKDRI-1003). K.B. is supported by the Swedish Research Council (#2017-00915), the Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (ADDF), USA (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation (#AF-930351, #AF-939721 and #AF-968270), Hjärnfonden, Sweden (#FO2017-0243 and #ALZ2022-0006), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986 and #ALFGBG-965240), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), the National Institute of Health (NIH), USA (grant #1R01AG068398-01) and the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495). T.K.K. was funded by the Swedish Research Council (Vetenskåpradet; #2021-03244), the Alzheimer’s Association (#AARF-21-850325), the BrightFocus Foundation (#A2020812F), the International Society for Neurochemistry’s Career Development Grant, the Swedish Alzheimer Foundation (Alzheimerfonden; #AF-930627), the Swedish Brain Foundation (Hjärnfonden; #FO2020-0240), the Swedish Dementia Foundation (Demensförbundet), the Swedish Parkinson Foundation (Parkinsonfonden), Gamla Tjänarinnor Foundation, the Aina (Ann) Wallströms and Mary-Ann Sjöbloms Foundation, the Agneta Prytz-Folkes & Gösta Folkes Foundation (#2020-00124), the Gun and Bertil Stohnes Foundation and the Anna Lisa and Brother Björnsson’s Foundation.
Competing interests
M.T. and P.H. are employees of Bioventix Plc. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave, and has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen and Roche. K.B. has served as a consultant or at advisory boards for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics and Siemens Healthineers. H.Z. and K.B. are co-founders of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. The other authors declare no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Jack CR, Bennett DA, Blennow K, et al. . NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jack CR, Bennett DA, Blennow K, et al. . A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dubois B, Villain N, Frisoni GB, et al. . Clinical diagnosis of Alzheimer’s disease: Recommendations of the international working group. Lancet Neurol. 2021;20:484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alzheimer’s Association . 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16:391–460. [Google Scholar]
- 5. Ashton NJ, Hye A, Rajkumar AP, et al. . An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat Rev Neurol. 2020;16:265–284. [DOI] [PubMed] [Google Scholar]
- 6. Karikari TK, Ashton NJ, Brinkmalm G, et al. . Blood phospho-tau in Alzheimer’s disease: Analysis, interpretation, and clinical utility. Nat Rev Neurol. 2022;18:400–418. [DOI] [PubMed] [Google Scholar]
- 7. Teunissen CE, Verberk IMW, Thijssen EH, et al. . Blood-based biomarkers for Alzheimer’s disease: Towards clinical implementation. Lancet Neurol. 2021;21:66–77. [DOI] [PubMed] [Google Scholar]
- 8. Ashton NJ, Leuzy A, Karikari TK, et al. . The validation status of blood biomarkers of amyloid and phospho-tau assessed with the 5-phase development framework for AD biomarkers. Eur J Nucl Med Mol Imaging. 2021;48:2140–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sabbagh MN, Hendrix S, Harrison JE. FDA position statement “Early Alzheimer’s disease: Developing drugs for treatment, guidance for industry”. Alzheimers Dement (N Y). 2019;5:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keshavan A, Pannee J, Karikari TK, et al. . Population-based blood screening for preclinical Alzheimer’s disease in a British birth cohort at age 70. Brain. 2021;144:434–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janelidze S, Teunissen CE, Zetterberg H, et al. . Head-to-head comparison of 8 plasma amyloid-β 42/40 assays in Alzheimer disease. JAMA Neurol. 2021;78:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakamura A, Kaneko N, Villemagne VL, et al. . High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018;554:249–254. [DOI] [PubMed] [Google Scholar]
- 13. Schindler SE, Bollinger JG, Ovod V, et al. . High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93:e1647–e1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karikari TK, Pascoal TA, Ashton NJ, et al. . Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19:422–433. [DOI] [PubMed] [Google Scholar]
- 15. Palmqvist S, Janelidze S, Quiroz YT, et al. . Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Janelidze S, Mattsson N, Palmqvist S, et al. . Plasma p-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020;26:379–386. [DOI] [PubMed] [Google Scholar]
- 17. Thijssen EH, La Joie R, Wolf A, et al. . Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thijssen EH, Joie RL, Strom A, et al. . Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: A retrospective diagnostic performance study. Lancet Neurol. 2021;20:739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ashton NJ, Pascoal TA, Karikari TK, et al. . Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021;141:709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mattsson-Carlgren N, Janelidze S, Bateman RJ, et al. . Soluble p-tau217 reflects amyloid and tau pathology and mediates the association of amyloid with tau. EMBO Mol Med. 2021;13:e14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ashton NJ, Janelidze S, Al Khleifat A, et al. . A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021;12:3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bridel C, van Wieringen WN, Zetterberg H, et al. . Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: A systematic review and meta-analysis. JAMA Neurol. 2019;17:1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benedet AL, Leuzy A, Pascoal TA, et al. . Stage-specific links between plasma neurofilament light and imaging biomarkers of Alzheimer’s disease. Brain. 2020;143:3793–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clark C, Lewczuk P, Kornhuber J, et al. . Plasma neurofilament light and phosphorylated tau 181 as biomarkers of Alzheimer’s disease pathology and clinical disease progression. Alzheimers Res Ther. 2021;13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barthélemy NR, Horie K, Sato C, Bateman RJ. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J Exp Med. 2020;217:e20200861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frank B, Ally M, Brekke B, et al. . Plasma p-tau181 shows stronger network association to Alzheimer’s disease dementia than neurofilament light and total tau. Alzheimers Dement. 2022;18:1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mattsson N, Zetterberg H, Janelidze S, et al. . Plasma tau in Alzheimer disease. Neurology. 2016;87:1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Müller S, Preische O, Göpfert JC, et al. . Tau plasma levels in subjective cognitive decline: Results from the DELCODE study. Sci Rep. 2017;7:9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zetterberg H, Wilson D, Andreasson U, et al. . Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther. 2013;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grothe MJ, Moscoso A, Ashton NJ, et al. . Associations of fully automated CSF and novel plasma biomarkers with Alzheimer disease neuropathology at autopsy. Neurology. 2021;97:e1229–e1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006;5:228–234. [DOI] [PubMed] [Google Scholar]
- 32. Skillbäck T, Farahmand BY, Rosén C, et al. . Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain. 2015;138:2716–2731. [DOI] [PubMed] [Google Scholar]
- 33. Sjögren M, Davidsson P, Tullberg M, et al. . Both total and phosphorylated tau are increased in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;70:624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomann PA, Kaiser E, Schönknecht P, Pantel J, Essig M, Schröder J. Association of total tau and phosphorylated tau 181 protein levels in cerebrospinal fluid with cerebral atrophy in mild cognitive impairment and Alzheimer disease. J Psychiatry Neurosci. 2009;34:136–142. [PMC free article] [PubMed] [Google Scholar]
- 35. Dugger BN, Whiteside CM, Maarouf CL, et al. . The presence of select tau species in human peripheral tissues and their relation to Alzheimer’s disease. J Alzheimers Dis. 2016;51:345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fischer I, Baas PW. Resurrecting the mysteries of big tau. Trends Neurosci. 2020;43:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Couchie D, Mavilia C, Georgieff IS, Liem RK, Shelanski ML, Nunez J. Primary structure of high molecular weight tau present in the peripheral nervous system. Proc Natl Acad Sci U S A. 1992;89:4378–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Georgieff IS, Liem RK, Mellado W, Nunez J, Shelanski ML. High molecular weight tau: Preferential localization in the peripheral nervous system. J Cell Sci. 1991;100(Pt 1):55–60. [DOI] [PubMed] [Google Scholar]
- 39. Mielke MM, Hagen CE, Wennberg AMV, et al. . Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the Mayo Clinic study on aging. JAMA Neurol. 2017;74:1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neselius S, Zetterberg H, Blennow K, et al. . Olympic boxing is associated with elevated levels of the neuronal protein tau in plasma. Brain Inj. 2013;27:425–433. [DOI] [PubMed] [Google Scholar]
- 41. Olivera A, Lejbman N, Jeromin A, et al. . Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 2015;72:1109–1116. [DOI] [PubMed] [Google Scholar]
- 42. Pase MP, Himali JJ, Aparicio HJ, et al. . Plasma total-tau as a biomarker of stroke risk in the community. Ann Neurol. 2019;86:463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rubenstein R, Chang B, Yue JK, et al. . Comparing plasma phospho tau, total tau, and phospho tau–total tau ratio as acute and chronic traumatic brain injury biomarkers. JAMA Neurol. 2017;74:1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shahim P, Tegner Y, Wilson DH, et al. . Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 2014;71:684–692. [DOI] [PubMed] [Google Scholar]
- 45. Osborne J, Harrison P, Butcher R, Ebsworth N, Tan K. Novel super-high affinity sheep monoclonal antibodies against CEA bind colon and lung adenocarcinoma. Hybridoma. 1999;18:183–191. [DOI] [PubMed] [Google Scholar]
- 46. Karikari TK, Emeršič A, Vrillon A, et al. . Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer’s disease diagnosis. Alzheimers Dement. 2021;17:755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 48. Mirra SS, Heyman A, McKeel D, et al. . The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–479. [DOI] [PubMed] [Google Scholar]
- 49. Montine TJ, Phelps CH, Beach TG, et al. . National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hyman BT, Trojanowski JQ. Editorial on consensus recommendations for the postmortem diagnosis of Alzheimer Disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. [DOI] [PubMed] [Google Scholar]
- 51. Smirnov DS, Ashton NJ, Blennow K, et al. . Plasma biomarkers for Alzheimer’s disease in relation to neuropathology and cognitive change. Acta Neuropathol. 2022;143:487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. . Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Höglinger GU, Respondek G, Stamelou M, et al. . Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017;32:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Armstrong MJ, Litvan I, Lang AE, et al. . Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McKhann GM, Knopman DS, Chertkow H, et al. . The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Benussi A, Karikari TK, Ashton N, et al. . Diagnostic and prognostic value of serum NfL and p-Tau181 in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2020;91:960–967. [DOI] [PubMed] [Google Scholar]
- 57. Andreasson U, Perret-Liaudet A, van Waalwijk van Doorn LJC, et al. . A practical guide to immunoassay method validation. Front Neurol. 2015;6:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saracino D, Dorgham K, Camuzat A, et al. . Plasma NfL levels and longitudinal change rates in C9orf72 and GRN-associated diseases: From tailored references to clinical applications. J Neurol Neurosurg Psychiatry. 2021;92:1278–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hesse C, Rosengren L, Andreasen N, et al. . Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297:187–190. [DOI] [PubMed] [Google Scholar]
- 60. Feinstein I, Wilson EN, Swarovski MS, Andreasson KI, Angst MS, Greicius MD. Plasma biomarkers of tau and neurodegeneration during major cardiac and noncardiac surgery. JAMA Neurol. 2021;78:1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bulut M, Koksal O, Dogan S, et al. . Tau protein as a serum marker of brain damage in mild traumatic brain injury: Preliminary results. Adv Ther. 2006;23:12–22. [DOI] [PubMed] [Google Scholar]
- 62. Goedert M, Spillantini MG, Cairns NJ, Crowther RA. Tau proteins of Alzheimer paired helical filaments: Abnormal phosphorylation of all six brain isoforms. Neuron. 1992;8:159–168. [DOI] [PubMed] [Google Scholar]
- 63. Georgieff IS, Liem RK, Couchie D, Mavilia C, Nunez J, Shelanski ML. Expression of high molecular weight tau in the central and peripheral nervous systems. J Cell Sci. 1993;105:729–737. [DOI] [PubMed] [Google Scholar]
- 64. Hansson O, Janelidze S, Hall S, et al. . Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chong JR, Ashton NJ, Karikari TK, et al. . Blood-based high sensitivity measurements of beta-amyloid and phosphorylated tau as biomarkers of Alzheimer’s disease: A focused review on recent advances. J Neurol Neurosurg Psychiatry. 2021;92:1231–1241. [DOI] [PubMed] [Google Scholar]
- 66. Karikari TK, Benedet AL, Ashton NJ, et al. . Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s Disease Neuroimaging Initiative. Mol Psychiatry. 2021;26:429–442. [DOI] [PubMed] [Google Scholar]
- 67. Alcolea D, Delaby C, Muñoz L, et al. . Use of plasma biomarkers for AT(N) classification of neurodegenerative dementias. J Neurol Neurosurg Psychiatry. 2021;92:1206–1214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data are available on reasonable request from the corresponding author.