Abstract
Hereditary motor neuropathies (HMN) were first defined as a group of neuromuscular disorders characterized by lower motor neuron dysfunction, slowly progressive length-dependent distal muscle weakness and atrophy, without sensory involvement. Their cumulative estimated prevalence is 2.14/100 000 and, to date, around 30 causative genes have been identified with autosomal dominant, recessive,and X-linked inheritance. Despite the advances of next generation sequencing, more than 60% of patients with HMN remain genetically uncharacterized. Of note, we are increasingly aware of the broad range of phenotypes caused by pathogenic variants in the same gene and of the considerable clinical and genetic overlap between HMN and other conditions, such as Charcot-Marie-Tooth type 2 (axonal), spinal muscular atrophy with lower extremities predominance, neurogenic arthrogryposis multiplex congenita and juvenile amyotrophic lateral sclerosis.
Considering that most HMN present during childhood, in this review we primarily aim to summarize key clinical features of paediatric forms, including recent data on novel phenotypes, to help guide differential diagnosis and genetic testing. Second, we describe newly identified causative genes and molecular mechanisms, and discuss how the discovery of these is changing the paradigm through which we approach this group of conditions.
Keywords: dHMN, HMN, paediatric neuronopathies, distal SMA, SMA-LED
Zambon et al. review the expanding phenotypic and genetic spectrum of early onset hereditary motor neuropathies, and highlight the clinical and genetic overlap between these disorders and other conditions such as Charcot-Marie-Tooth type 2 and juvenile ALS. They describe newly identified causative genes and underlying molecular mechanisms.
Introduction
Hereditary motor neuropathies (HMN) are a heterogeneous group of disorders that were first defined by the following characteristics: (i) slowly progressive, length-dependent distal muscle weakness and atrophy; (ii) signs of chronic denervation on needle EMG in presence of normal/slightly reduced motor nerve conduction velocity (NCV) and normal/reduced compound motor action potential (CMAP); and (iii) absence of clinical and/or electrophysiological sensory involvement, which should mark the difference with Charcot-Marie-Tooth (CMT) type 2.1,2
The estimated pooled prevalence of HMNs is 2.14/100 000 and, to date, around 30 causative genes have been identified, including forms inherited in an autosomal dominant (AD), recessive (AR), and X-linked pattern (Table 1).3 Despite the great advances achieved through next-generation sequencing (NGS), the diagnostic yield in patients with a pure HMN phenotype is still ∼32.5%, at latest estimate.3–5
Table 1.
Summary of main early-onset motor neuronopathies
| Gene symbol | OMIM ref | Inheritance | Protein/Function | Associated conditions |
|---|---|---|---|---|
| Conditions with predominant distal involvement | ||||
| HSPB1 | 608634 | AD/AR | UPR; stabilization of microtubules? | HMN2B, CMT2F |
| HSPB8 | 158590 | AD | UPR | HMN2A, CMT2L |
| HSPB3 | 613376 | AD | - | HMN2C, CMT2 |
| ATP7A | 300489 | X-linked | Copper transport | X-linked HMN (SMAX3) |
| WARS | 617721 | AD | Tryptophanyl-tRNA synthetase | HMN9 |
| FBXO38 | 615575 | AD | Transcriptional activator | HMN2D |
| DNAJB2 | 614881 | AR | Co-chaperone | DSMA5 |
| HINT1 | 137200 | AR | Purine phosphoramidase | HMN with neuromyotonia |
| IGHMBP2 | 604320 | AR | Helicase | HMN6/SMARD, CMT2S |
| GARS | 600794 | AD | Aminoacyl-tRNA synthetases. Disruption of the VEGF/Nrp1 signalling pathway? | CMT2D, HMN5A, SMARD like phenotype |
| REEP1 | 614751 | AD | ER-mitochondrial interactions | HMN5B, HSP31 |
| BSCL2 | 619112 | AD | UPR | HMN5C, Silver syndrome |
| TRPV4 | 600175, 181405 | AD | Ion channel—possible disruption of mitochondrial axonal trafficking | HMN7, CMT2C, SPSMA, AMC, skeletal dysplasia |
| SLC5A7 | 158580 | AD | Presynaptic choline transporter | HMN7A, CMS 20 |
| DCTN1 | 607641 | AD | Axonal transport along the microtubules | HMN7B, juvenile ALS, Perry syndrome |
| SIGMAR1 | 605726 | AR | ER chaperone | DSMA2, Juvenile ALS |
| SETX | 606002, 602433 | AR, AD |
RNA and DNA helicase | HMN + pyramidal |
| AAAS | 231550 | AR | Nuclear protein import | Triple A syndrome |
| SORD | 618912 | AR | Oxidation of sorbitol to fructose | N/A |
| COQ7 | *601683 | AR | Coenzyme Q10 biosynthesis | N/A |
| MFN2 | n/a | AD | Mitochondrial fusion | CMT2A |
| SMA-LED or neuronopathies with early proximal weakness | ||||
| DYNC1H1 | 58600 | AD | Axonal retrograde transport + other housekeeping functions | SMALED 1, HMN1, AMC, ID + MCD |
| BICD2 | 615290, 618291 | AD | Cargo adaptor protein, interact with dynein/kinesin complex. Localize Rab6 to Golgi | SMALED2A, SMALED2B, AMC |
| SYT2 | 616040 | AD | Ca2+ sensors for vesicular trafficking and exocytosis | Presynaptic CMS (AR) |
| PLEKHG5 | 611067 | AR | Nuclear factor kappa-B activator | CMT-RIC |
| VWA1 | 619216 | AR | ECM component | N/A |
AMC = arthrogryposis multiplex congenita; CMT = Charcot Marie Tooth; CMS = congenital myasthenic syndrome; HSP = hereditary spastic paraplegia; ID = intellectual disability; LL = lower limbs; MCD = malformation of cortical development; UL = upper limbs; UPR = unfolded protein response.
A more comprehensive table describing salient clinical features and age of onset is provided in the Supplementary material.
The first classification made by Harding and colleagues in 1993 was based on the mode of inheritance, clinical findings, and the age at symptoms onset. This resulted in the definition of seven subtypes (HMN 1–7).6 However, the advent of NGS has progressively revealed the complexity underlying this group of disorders, which cannot be exhaustively captured by the original phenotypical classification.7
There is indeed a considerable clinical and genetic overlap with conditions ranging from axonal CMT (CMT2) to spinal muscular atrophy with lower extremities predominance (SMA-LED), neurogenic arthrogryposis multiplex congenita (AMC), juvenile amyotrophic lateral sclerosis (ALS), hereditary spastic paraplegias (HSP), and even distal myopathies.3,8,9 Moreover, pathogenic variants in the same gene can cause heterogeneous and variable phenotypes.9–13
Most HMNs present during childhood or even at birth. In this review we primarily aim to give a detailed characterization of paediatric HMN, highlighting the clues that may help direct genetic testing (Fig. 1). Second, we aim to report on novel phenotypes and genes, and how the discovery of these is expanding the paradigm through which we approach this group of conditions.
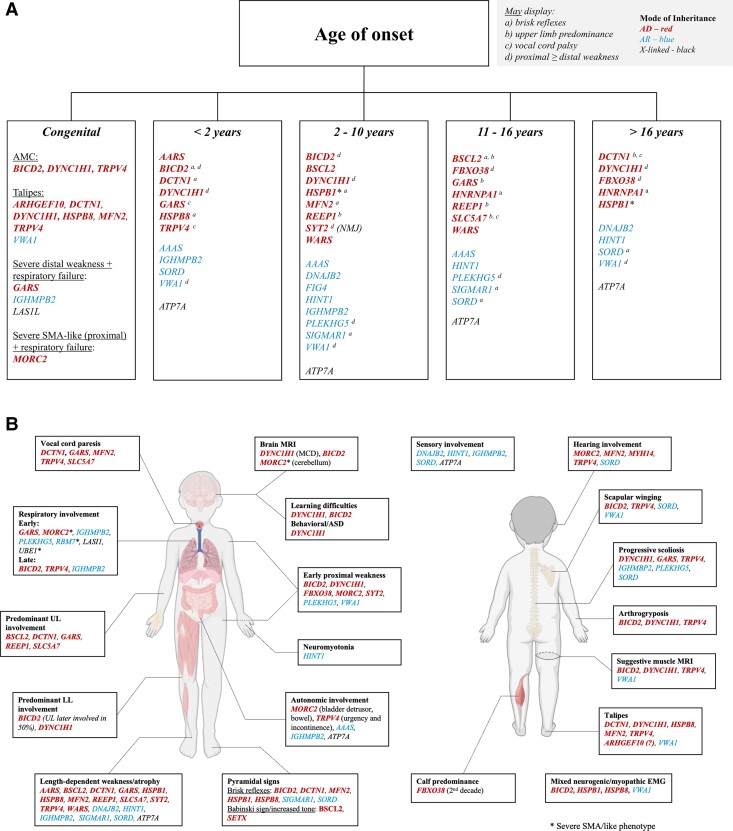
Figure 1.
Diagnostic clues based on age of onset (A) and relevant clinical features (B). Relevant clinical features (B) may or may not be present due to disease spectrum or stage at assessment. Genes are listed in alphabetical order and according to mode of inheritance: autosomal dominant (AD) pattern, bold font and red; autosomal recessive (AR), regular font and blue; X-linked in black (LAS1L and ATP7A). Asterisk indicates when more than one mode of inheritance has been associated with the same gene. EMG = electromyography; MCD = malformation of cortical development.
Importantly, we acknowledge that the terminology commonly used (e.g. HMN, dHMN, distal SMA) may oversimplify pathophysiology as it does not necessarily reflect a biological substrate or clinical phenotype.14,15 Hence, we would rather use the general term ‘motor neuronopathies’, which we have subdivided in the following grouping:
HMN: this group reflects the original classification by Harding et al.,6 in which the key finding is a length-dependent (i.e. distal > proximal) pattern of weakness and atrophy in the absence of sensory involvement. For a matter of clarity, we listed every gene presenting with these features according to its predominant phenotype [e.g. predominant distal lower/upper limb (LL/UL) involvement, presence of significant respiratory involvement, vocal cord paresis, pyramidal signs].
CMT2 overlap: this group includes conditions with a very similar phenotype to HMN, but in which sensory abnormalities are usually found.
SMA-LED: this group was first defined by the congenital or infantile onset of non-length dependent lower limb predominant weakness.16 While the evident discrepancy between UL and LL involvement is suggestive for the diagnosis, exceptions exist. We included in this section those conditions in which distal and proximal weakness can be equally observed, i.e. those in which length dependent weakness is not found.
Neurogenic AMC.
Juvenile ALS: this group of progressive disorders is the differential diagnosis of HMN, mainly defined by the coexistence of lower and upper motor neuron signs and early bulbar involvement.
Motor neuronopathies associated with brain involvement: conditions sometimes termed SMA-plus (e.g. SMA plus pontocerebellar hypoplasia, seizures, severe bulbar involvement), and riboflavin transporter deficiency associated disease,17 will not be covered in this article. A summary is provided in Supplementary Table 1 and detailed review is provided by Teoh et al.18 and Farrar et al.19
To conduct the present review, we searched in PubMed for the following keywords: ‘HMN', ‘dHMN', ‘Distal SMA', ‘SMA-LED', ‘neuronopathies' and ‘CMT2’.
Additionally, we searched for genes listed in papers or book chapters that have been previously associated with motor neuronopathies, in order to retrieve detailed information regarding the spectrum of phenotypes associated with each condition.
Early onset neuronopathies
Hereditary motor neuropathies
HMN usually beginning in the lower limbs
HSPB1 (AD/AR), HSPB8 (AD) and HSPB3 (AD)
The HSPB genes encode for small heat-shock proteins characterized by the highly conserved alpha-crystallin domain, whose complex biological function ranges from cellular housekeeping to unfolded protein response and regulation of microtubule stability. A detailed discussion regarding the molecular function of heat-shock protein 27-kD (HSPB120), heat-shock protein 22-kD (HSPB821) and heat-shock protein B3 (HSPB322) is provided by Rossor et al.9 HSPB1 and HSPB8 related phenotypes are considered similar, but variants in HSPB1 are far more common, accounting for up to 10% of all HMN cases (when considering adult-onset forms).5,23,24 The mode of inheritance is usually autosomal dominant, but recessive cases have been reported as well.23 Interestingly, HSPB1 mutants have also been associated with adult-onset motor neuropathy and distal vacuolar myopathy.25
HSPB1-related disease usually manifests in the fourth decade of life (median 30 years, but up to the seventh decade) with muscle weakness and atrophy in the distal lower limb muscles, and diminished deep tendon reflexes (DTR). Upper limbs involvement occurs many years later. Few cases presenting within the first decade of life and with brisk rather than diminished reflexes have been reported.23,24 While to our best knowledge no HSPB1 patient presented with congenital onset, HSPB8-related HMN may manifest at birth with talipes and congenital hip dysplasia. Delayed acquisition of walking is followed by a mild disease course despite the presence of distal atrophy. DTR can be brisk.
Variants in HSPB3 have been exclusively identified in adult-onset HMN.
ATP7A (X-linked)
The ATP7A gene encodes a transmembrane copper-transporting P-type ATPase with numerous functions within the cells.26 Its deficiency has been primarily associated with Menkes disease and occipital Horn syndrome (OHS).27 Missense variants causing aminoacidic substitutions within or near the C-term transmembrane segments of the protein have however been described in ATP7A-related distal motor neuropathy, also named X-linked distal spinal muscular atrophy (SMAX3). Preclinical studies on SMAX3-causing variants suggest that mutant ATP7A might be mislocalized within the axon, possibly due to its impaired binding to adaptor proteins 1 and 2 (AP1 and 2). However, the reason why such changes cause axonal damage remains elusive.28
ATP7A-related HMN usually begins with distal muscle weakness and atrophy of the lower extremities, feet deformities (i.e. pes cavus, hammer toes, curled fingers), later followed by involvement of the upper limbs. DTR are usually reduced or absent. A clinical (e.g. reduction in tactile and vibratory sensation) and neurophysiological mild sensory involvement has been documented in some cases. Disease onset is highly variable even within the same family and may occur as early as 1 year of age, but more commonly in the second and third decade.29 Nerve conduction studies show normal conduction velocities and a diffuse reduction of CMAPs.
Of note, while once considered separate clinical entities with different clinical and biochemical profiles (in SMAX3 serum copper and ceruloplasmin levels are expected to be normal), recent reports suggested that OHS and SMAX3 might represent a continuum30: some SMAX3 patients indeed present with features typical of OHS, such as skin abnormalities and dysautonomia.31
WARS (AD)
Autosomal dominant pathogenic variants in the WARS gene, encoding for cytoplasmic tryptophanyl-tRNA synthetase (TrpRS), have been recently found to be the cause of HMN9 and mostly but not exclusively reported in patients of Asian origin.32 The disease is characterized by slowly progressive weakness and atrophy of the feet, legs and subsequently of the hands.33,34 The onset usually occurs at the beginning of the second decade (juvenile onset) and close to all reported patients were able to walk independently in adulthood. Electrophysiological studies demonstrate pure axonal motor neuropathy without sensory involvement.
FBXO38 (AD)
FBXO38 is a known coactivator of the transcription factor Krüppel-like factor 7 (KLF7), which regulates several genes required for axonal outgrowth and repair. A dominant variant leading to the disruption of this transcriptional activity was found in individuals presenting during early adulthood (range 13 to 48 years; more commonly between the second and third decade) with weakness beginning in the calves and subsequently progressing to both distal and proximal leg and arm muscles. Particularly, the triceps and intrinsic hand muscles (i.e. abductor pollicis brevis and first dorsal interosseus) were the most affected muscles in the hands. DTR were absent. Clinical severity ranges from mild weakness in the eighth decade, to inability to walk independently in adulthood. EMG reveals chronic neurogenic changes and active denervation, and nerve conduction studies show reduced cMAP and normal sensory responses. Cognition and bulbar function are normal.35
DNAJB2 (AR)
DNAJB2, also known as HSJ1a (heat-shock protein J1a), encodes a molecular co-chaperone belonging to the HSP40/DNAJ co-chaperone family. Co-chaperones participate in the chaperone systems, which are in turn tightly connected to the protein turnover pathways, the ubiquitin–proteasome system, and the autophagy–lysosome system, thus playing a crucial role in preventing protein aggregation.36
Biallelic pathogenic variants in DNAJB2 are a relatively common cause of HMN, particularly in young adults (onset between 16–23 years).5 The disease usually manifests at the end of the second decade with gait abnormalities (e.g. foot drop, stumbling). Distal weakness and absent reflexes are noted first, then a progression towards severe distal paresis and proximal weakness leading to wheelchair dependency in the 4–5th decade of life is common, but not invariable. Upper limbs are usually spared or only mildly affected.37 Sensory involvement (i.e. a predominant CMT2 phenotype) has been reported in few cases.38 Interestingly, few families also showed early-onset parkinsonism with neuronopathy and pyramidal signs.5,39
HINT1 (AR)
Loss-of-function, biallelic variants in HINT1 (histidine triad nucleotide-binding protein 1) cause axonal neuropathy with neuromyotonia.40 HINT1 primarily hydrolyses nucleoside 5′-phosphoramidates (preferentially purines) with a monophosphate group and acyl-AMP, although additional roles include modulation of transcriptional activation, p53/TP53-mediated apoptosis and proteasomal degradation of target proteins.
Disease onset is typically in the first decade, with severe distal weakness and atrophy of both lower and upper limbs. Most of the reported patients maintained the ability to walk without support in their thirties. Notably, some patients may present with muscle stiffness, myotonia (50%) or cramps in the hands and legs. Patients presenting later in life, with asymmetric weakness and without myotonia, have been reported as well.
Neurophysiological studies show an axonal neuropathy, which can be pure motor or mixed sensorimotor (CMT2 phenotype) in around a half of patients. Needle EMG findings are typical, with high-frequency motor unit action potentials consistent with neuromyotonic or myokymic discharges. Of note, about 20–30% of patients carrying HINT1 mutations lack clinical or electrophysiological signs of neuromyotonia.41,42 CK can be elevated, and skeletal muscle biopsies may reveal signs of chronic denervation. Aspects of axonal damage on sural nerve biopsies even in the absence of clinical sensory abnormalities have been reported.
HMN usually beginning in the upper limbs (HMN5)
GARS (AD)
The GARS gene encodes for a glycyl-tRNA synthetase (GlyRS), an enzyme that covalently attaches glycine to its cognate transfer RNA (tRNA), a necessary step in protein translation. GARS is not only part of the cytosolic translational apparatus but is also involved in the translation of mitochondrial DNA. Dominantly inherited variants have been associated with both CMT2D (onset in the second decade) and with HMN with upper limbs predominance (HMN5).43 Recessive variants have been reported in a mitochondrial disorder with cardiac involvement and in a child with a complex multi-system neurological disease with severe mental retardation and brain structural abnormalities, in both cases without neuropathy.44,45
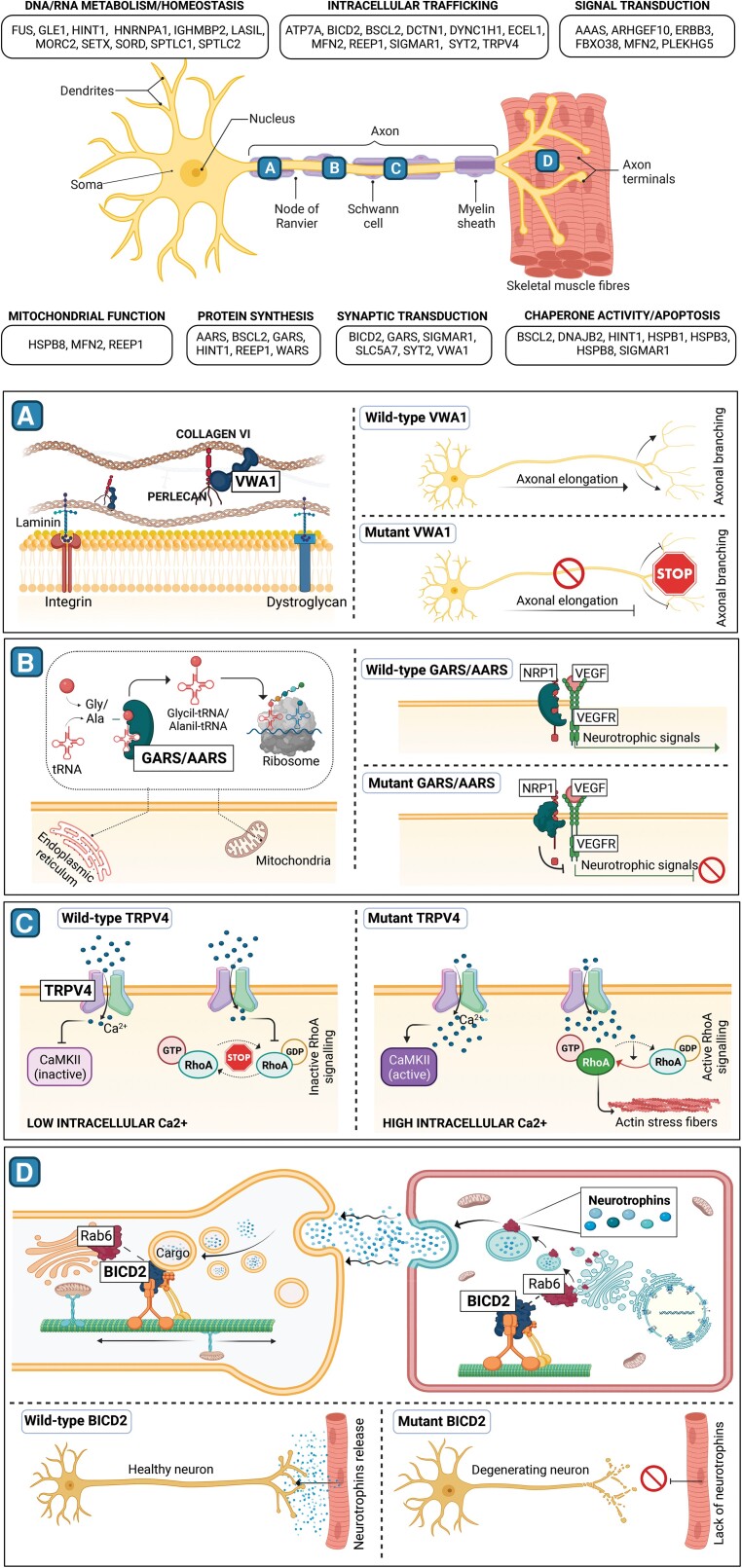
Based on the evidence that dominant variants in five distinct tRNA synthetases caused axonal and intermediate forms of CMTs (i.e. GARS, YARS, AARS, HARS, MARS), it was first hypothesized that disease causing mechanism could be linked to a common function of these enzymes, for instance aminoacylation. However, the evidence that disease-causing mutations did not affect such activity, together with subsequent studies on Drosophila mutants, disputed such theory.46 A recent report showed that dominant variants in the gene cause neomorphic binding of GlyRS that directly antagonizes an essential signalling pathway for motor neuron survival. in fact, GlyRS mutants bind to the neuropilin 1 (NRP1) receptor, thus competing and interfering with the binding of the cognate ligand vascular endothelial growth factor (VEGF)47 (Fig. 2B).
Figure 2.
Schematic representation of molecular mechanism involved in neuronopathies. The top section includes a central schematic of motor nerve structure and its interaction with skeletal muscle fibres. Genes associated with neuronopathies, grouped according to their main function, are mentioned in panels surrounding the schematic. (A–D) Aim to show the physiological role of genes recently implicated in these disorders and their presumed pathological mechanism. (A) Mutations in the extracellular protein VWA, that in its wild-type state connects collagen VI and perlecan, affect both axonal elongation and branching. (B) Mutant GARS and AARS tRNA synthetases are thought to impede the release of neurotrophic signals by binding NRP1 protein and thus altering Nrp1/VEGF signalling. (C) The increase of intracellular calcium resulting from the mutant TRPV4 channel activates CAMKII ad RhoA signalling, leading to the formation of cytoplasmic actin stress fibers. (D) A defective BICD2 protein impairs both the release of muscle neurotrophins and the neuronal vesicles retrograde transport, resulting in abnormal development of the motor unit and neuronal degeneration.
The prototypical phenotype of GARS-associated neuronopathy is characterized by distal muscle weakness and atrophy predominantly affecting upper limbs (Fig. 3E) with onset in the first decades of life (usually starting after 10 years of age). The disorder is slowly progressive, but most patients eventually develop lower limb weakness and foot deformities (pes cavus).48
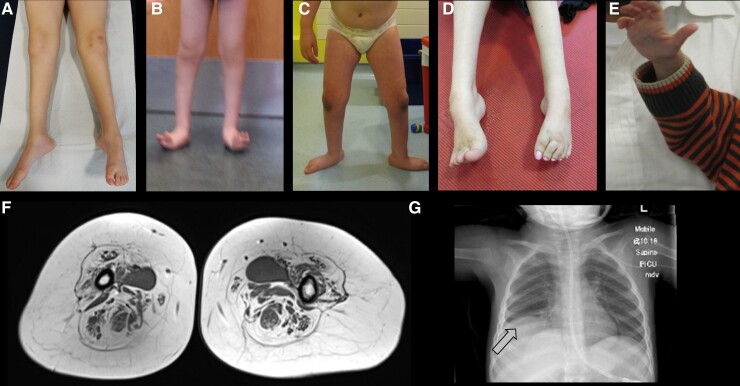
Figure 3.
Clinical and radiological features of neuronopathies. (A) Distal leg thinning in a patient with MFN2 variant; (B) lower leg atrophy and foot deformities in a patient with BICD2 variant; (C) lower leg atrophy and foot posture in a patient with DYNC1H1 variant. (D) Foot posture in a young adult with TRPV4 variant; (E) photo of hand atrophy (split-hand sign) in a patient with GARS variant; (F) thigh muscle MRI showing islands of muscle, a finding strongly suggestive of neuronopathy; (G) X-ray showing diaphragmatic paralysis in SMARD1 (arrow).
However, more recent finding suggests that forms with earlier onset may also occur. These are characterized by the involvement of both lower and upper limbs and a congenital presentation. Patients can display signs of severe distal weakness as early as 6 months. Although motor milestones up to independent walking may be attained, the disease seems progressive.49 The occurrence of distal muscle wasting, scoliosis, pes planus, vocal cord palsy and even respiratory distress requiring ventilatory support have been variably described.50,51
The most severe end of the spectrum resembles SMA with diaphragmatic involvement (SMARD).52 Remarkably, such severe and early onset phenotypes are due to pathogenic variants located in the anticodon binding and the catalytic domains of GARS. Affected individuals present at birth or within the first few weeks of life with severe respiratory insufficiency (involvement of the diaphragm could help to differentiate from 5q-SMA), inspiratory stridor (vocal fold palsy), feeding difficulties, hypotonia of lower more than upper limbs, and absent DTR. Tongue fasciculation may be present. Muscle weakness is severe and affects both proximal and distal muscles with a distal-proximal gradient. While patients may acquire the ability to sit independently, limb muscle weakness is progressive. Brain MRI is normal and electrophysiological studies show active denervation on EMG and severe axonal neuropathy on electroneuronography (ENG). Sensory action potentials are variably affected, supporting the idea of an overlap with CMT2D.
REEP1 (AD)
Autosomal dominant variants in REEP1, a gene encoding for a protein that regulates endoplasmic reticulum (ER) and mitochondrial interaction, are a well established cause of spastic paraplegia (SPG31). A splice-site alteration in REEP1 (c.304-2A>G) was also associated with HMN.53 Interestingly, while HSP causing variants are thought to derive from haploinsufficiency, HMN phenotype may be generated by a toxic gain-of-function. The conservation of the N-terminal half and the inability to trigger nonsense mediated decay of the mutant RNA was unique of this variant. REEP1-associated HMN may therefore be a rare condition since only a small fraction of genetic changes are expected to exert similar consequences.
Clinically, the disease is characterized by an onset within the first or second decade of life. Selective hand muscle weakness and atrophy confined to the thenar and first dorsalis interosseus muscle (i.e. split hand sign) may be the presenting symptom (HMN5B), but patients can also display lower limb involvement such as pes cavus and peroneal atrophy and weakness causing stepping gate. DTR are absent or diminished in all patients.
BSCL2 (AD)
While recessive variants in the BSCL2 gene were first identified as the cause of congenital generalized lipodystrophy type 2 (Berardinelli-Seip congenital lipodystrophy type 2), heterozygous variants cause a wide spectrum of conditions ranging from HMN5C, Silver syndrome (HSP17, spastic paraparesis plus marked weakness and atrophy of hand muscles), CMT254 and ALS.55 Incomplete penetrance and significant clinical variability have been described even within the same family and the same dominant mutation can manifest with very different phenotypes.56,57 Of note, motor neuron ‘seipinopathies’ are mainly caused by two toxic gain-of-function pathogenic variants (p.N88S; p.S90L) in the BSCL2 gene.
BSCL2 encodes for the ER resident glycoprotein Seipin. Preclinical studies have demonstrated that toxic mutations in the gene induce unfolded protein response (UPR) and subsequent ER-stress mediated cell death.9,58
Seipinopathies are usually characterized by degeneration of both upper and lower motor neurons. Hence, the finding of pyramidal signs (e.g. from isolated brisk reflexes to increased muscle tone and positive Babinski sign) is not uncommon. Most affected individuals present in the second decade with progressive distal muscle weakness and atrophy affecting upper and lower limbs. However, given that BSCL2-related HMN can occasionally first manifests in the upper limbs (‘split hand’-type atrophy, sometimes asymmetrical),59,60 this condition should go in the differential also with HMN5.
The key message is that there can be extreme intrafamilial variability and the suspicion of BSCL2 mutations should also rise in presence of a distal neuronopathy associated with pyramidal signs in patients presenting during the first decade of life,57,61 or even in cases presenting in the first decade of life with distal weakness of the legs, rather than in the hands, and absent DTRs.59
HMN with prominent respiratory impairment
This group includes the recently described phenotype associated with variants in GARS, which was discussed above.
IGHMBP2 (AR)
Autosomal recessive variants in the gene encoding for immunoglobulin μ-binding protein 2 (IGHMBP2) are a well established cause of SMARD1. IGHMBP2 is an ATP-dependent helicase, which binds to ribosomes and unwinds RNA and DNA duplexes. Amino acid substitutions causing HMN seem to affect the ATPase and helicase activity rather than the ribosome-binding ability, a pathophysiological mechanism which is supported by the finding of a similar phenotype associated with another helicase (LAS1L).62,63
Relevant clinical differences with 5q-SMA are the involvement of the diaphragm (Fig. 3F) within the first weeks of life (this is initially spared in SMA) and the distribution of weakness (distal rather than proximal).64,65 A decrease in the number of large myelinated fibres on sural nerve biopsy and slow motor nerve conduction velocities may be observed. The closest phenotypes to SMARD1 are X-linked SMARD2 (LAS1L) and the newly recognized severe phenotype associated with GARS mutations.
Unsurprisingly, the range of possible phenotypes associated with biallelic mutations in IGHMBP2 has increasingly expanded, including sensorimotor polyneuropathy (CMT2S) with onset ranging from <2 years66,67 to late childhood,68,69 with or without respiratory involvement.70 Some cases can even present prenatally with talipes.
Milder cases have been reported also within the HMN spectrum. Specifically, patients may first present with delayed walking evolving into early wheelchair dependency due to progressive severe distal weakness. Respiratory impairment occurs years after the onset of symptoms.71,72 Of note, both phenotypes (classic severe and mild) can co-exist in the same family.73 Autonomic disturbances (bladder dysfunction, acute bowel distension), a feature of the severe SMARD1 form, were also reported in a child presenting with CMT2S and in one with the milder HMN phenotype.67,72
The common finding of abnormal sensory conduction velocities and pathological sural nerve biopsies (reduction of the larger myelinated fibres along different stages of axonal degeneration and atrophy) is in contrast with the definition of a pure motor neuronopathy.
HMN that may be associated with vocal cord paralysis (HMN7)
TRPV4 (AD)
Dominantly inherited pathogenic variants in the TRPV4 gene cause different clinical phenotypes spanning from HMN7 to scapulo-peroneal SMA, congenital SMA with arthrogryposis,74 and autosomal dominant CMT2C.75,76 Mutations causing ‘neuronal’ forms usually localize to the intracellular N-terminal domain while other distinct variants give raise to diseases of bone and connective tissue,77 fuelling the unsolved conundrum of the reason behind selective vulnerability of specific cell types to mutations happening in ubiquitously expressed genes.78
TRPV4 (transient receptor potential vanilloid 4) is a non-selective ion channel preferentially permeable to Ca2+. The mechanisms through which mutations in TRPV4 cause neurodegeneration are still debated but intriguing, given the paucity of ion channels associated with axonal degeneration and the potential for modulatory treatments.
A recent study on Drosophila suggested that the primary defect could be the disruption of mitochondrial axonal transport (which has been linked to neurodegeneration in other CMTs) subsequent to the unexpected activation of CaMKII (Ca2+/calmodulin-dependent protein kinase II) due to the increased calcium influx caused in turn by the abnormal functioning of the TRPV4 ion channel.79 Another study suggested that neuronal phenotype could specifically derive from the selective disruption of a protein domain involved in the interaction with the small RhoA GTPase, thus allowing RhoA activation and the subsequent formation of actin stress fibers in the cytoplasm80,81 (Fig. 2C).
The penetrance of the disease is variable so that not only there are four ‘distinct’ clinical entities, but in the same family affected individuals may present with very different phenotypes. With these premises in mind, the CMT2C phenotype is usually associated with vocal cord paresis, bilateral sensorineural hearing loss, bladder urgency and incontinence, skeletal dysplasia and short stature.82
Vocal cord palsy is not exclusive of CMT2C and has been reported also in 94% of patients presenting with TRPV4-related neuronopathy in one case series (17 individuals), thus representing a strong diagnostic clue.83 Of note, vocal cord paralysis may present with congenital stridor or with subtle hoarseness and should not be overlooked. Foot deformity (pes cavus) and kyphoscoliosis develop with time in most cases. Mildly elevated (<500 U/l) CK levels are also a common finding. Interestingly, although initial reports did not mention respiratory insufficiency as a feature of TRPV4-related disease, this was later described in around a quarter of patients, who required the use of non-invasive nocturnal ventilation (BiPAP).83 Scapular winging was reported in close to a half of patients, including individuals not presenting with the scapulo-peroneal type.83 Hearing loss, which can be progressive,84 skeletal dysplasia and AMC were reported in <25% of cases. In contrast with DYNC1H1 and BICD2 SMALED, talipes at birth is equinovarus rather than calcaneovalgus (Fig. 3D). Distal weakness and wasting are usually seen in both lower and upper limbs, but patients with milder proximal weakness at lower limbs has been also reported. DTR are absent or diminished. Patients usually acquire the ability to walk with support, but not in all cases.
Nerve conduction studies show a slowly progressive, symmetrical, and predominantly distal motor axonal neuropathy (CMAPs are diffusively reduced), sometimes associated with mild sensory involvement. No signs of active denervation are usually seen.
Muscle MRI of lower legs may show initial involvement of the vastus intermedius, vastus lateralis, soleus and medial gastrocnemii muscles. The sparing of biceps femoris and medial gastrocnemius rather than thigh adductors and semitendinosus has been proposed as a differentiating feature compared to SMA-LED.85
SLC5A7 (AD)
Heterozygous pathogenic variants in the SLC5A7 gene on chromosome 2q12 have been associated with HMN7, namely HMN with vocal cord palsy. Interestingly, SLC5A7 encodes a sodium and chloride dependent high-affinity transporter that mediates the presynaptic uptake of choline for acetylcholine synthesis in cholinergic neurons.86
In the original article from Young and Harper,87 affected individuals presented in early teens (age 12–14) with hands weakness and/or selective wasting of the thenar eminence. Lower limb weakness occurred usually later, except for one patient who manifested at the age of 11 years with toe walking, and in whom distal UL involvement occurred 10 years later. Other features include pes cavus, hyporeflexia and hoarseness. Importantly, there was variable involvement of one or both vocal cords (but also one patient without vocal cords involvement was reported), a feature that may require early monitoring and intervention.
DCTN1 (AD)
Autosomal dominant variants in the DCTN1 gene can cause neuronopathy. This encodes p150(Glued), the largest of the 10 different polypeptides that constitute the dynactin complex, a crucial macromolecular complex participating in the dynein-driven movement of organelles along microtubules. The spectrum of neurodegenerative conditions related to DCTN1 spans from HMN to juvenile ALS and Perry syndrome (rapidly evolving parkinsonism, weight loss, depression and central hypoventilation). DCTN1-related HMN was first described in young adults presenting with vocal cord paralysis causing respiratory distress (HMN7B), progressive muscle weakness and atrophy in the hands and facial muscles, and later involvement of LL.88
Very recently, a patient with congenital onset harbouring frameshift variant in DCTN1 gene was reported.89 This variant was shown to cause an abnormal distribution of Dynactin 1 within the motor neuron and loss of colocalization with microtubules. The proband presented with talipes and delayed acquisition of independent walking (achieved at 3 years). There was early involvement of the hands (clumsiness, tremor and muscle twitching appearing at the age of 16 years). Distal muscle weakness and atrophy of legs muscles, Achilles tendon contractures and toe varus were evident at the age of 11 years. DTR were brisk but muscle tone was normal. Nerve conduction studies showed a global reduction of CMAPs with normal sensory responses. Sural nerve biopsy was unremarkable.
HMN associated with pyramidal signs
This group includes conditions in which overt pyramidal signs are commonly observed (namely, not only brisk reflexes but also increased muscle tone and extensor plantar response). BSCL2-associated condition was described above.
SETX (AD)
Autosomal dominant variants in the senataxin (SETX) gene are associated either with HMN with pyramidal signs or with juvenile ALS. Senataxin interacts with several proteins involved in RNA processing, hence suggesting a role in the modulation of transcription.
SETX-related HMN is usually associated with a teen-age onset, and a much slower disease course compared to other forms of juvenile ALS, with survival times extending well into the fourth decade. A predominant upper motor neuron involvement, bulbar sparing and an occasional overlapping sensory ataxia seem to be other characteristic features of this form.90SPG11-related cases are similarly indolent, sometimes associated with parkinsonian features, and clinical courses lasting for as long as two decades.91 Autosomal recessive variants can cause spinocerebellar ataxia with axonal neuropathy.
Other HMN
SIGMAR1 (AR)
The SIGMAR1 gene encodes an ER chaperone assisting lipid transport from the ER either to plasma membrane or to mitochondria-associated membrane. SIGMAR1 is essential for the retrograde mitochondrial axonal transport, controls ER calcium efflux and plays an important role in the regulation of ion channels and neurotransmitters release.
Recessively inherited variants in the gene have been first identified as the cause of AR HMN in a cluster of consanguineous families in the Jerash region of Jordan (Rossor et al.9). The disease manifests between 6 and 12 years of age and is characterized by slowly progressive distal muscle weakness and wasting of lower limbs associated with brisk patellar reflexes (ankle reflexes can be absent) and feet deformities (pes varus). UL involvement usually occurs later and most individuals are independent in everyday life activities in mid-adulthood.92,93 Nerve conduction studies show normal conduction velocities, reduced cMAP amplitudes, normal sensory nerve action potentials, and chronic neurogenic changes on needle EMG. Both neurophysiological and pathological (sural nerve biopsy) sensory investigations are unremarkable.
AAAS (AR)
Recessive variants in the ubiquitously expressed AAAS (Aladin WD Repeat Nucleoporin) gene are a cause of Triple A or Allgrove syndrome, defined by the presence achalasia, alacrimia, adrenal insufficiency and neurological syndrome. AAAS localizes at the cytoplasmic side of the nuclear pore complex and supports selective nuclear protein import.
Of note, the triad may not be evident in all patients and a progressive neurological syndrome, including predominant motor neuropathy (although sensory-motor are also reported)94 with onset in the first decade of life (as early as 1 year).5 Other neurological features may include cognitive decline, progressive spasticity, dysautonomia and/or cranial neuropathies.
SORD (AR)
Biallelic pathogenic variants in SORD have been recently identified as a common cause of HMN/CMT2 neuropathy, possibly accounting for ∼10% of undiagnosed HMN/CMT2 cases.95,96 Close to 70% of described individuals had no familiar history.
SORD encodes the second enzyme of the two-step polyol pathway, in which glucose is converted into sorbitol by aldose reductase, and then oxidized to fructose by SORD. The lack of SORD leads to marked intracellular sorbitol concentrations elevation, with putatively reversible toxic consequences on motor neurons.
The onset is usually in the second/third decade, and the disease manifests as slowly progressive distal weakness and atrophy of both lower and upper limbs, often accompanied by foot deformities (e.g. pes cavus). Proximal strength is preserved. Nerve conduction studies revealed an intermediate reduction of NCV in a quarter of patients and decreased or absent sensory amplitudes in another quarter (reduced vibratory sense was also documented). After two decades from disease onset, the severity of this condition was mild in the vast majority of affected individuals.
A variety of associated signs and symptoms have been reported in some patients, including hand tremor, scoliosis, hearing loss, scapular winging, brisk reflexes, and elevated CK levels. Only two patients presenting within the first decade have been reported thus far.95,97
CMT2 overlap (forms with prevalent CMT2 phenotype)
In this section we will expand the description of those conditions commonly observed in paediatric patients thar are usually classified as CMT2, with the caveat that the distinction between HMN and CMT2 is at least blurred.7
MFN2 (AD)
Dominantly inherited pathogenic variants in MFN2 are the most common cause of axonal inherited neuropathies (CMT2). Mitofusin2 (MFN2) is a GTPase dynamin-like mitochondrial membrane proteins implicated in mitochondrial dynamics, calcium signalling and axonal trafficking. The phenotypic spectrum is wide but usually characterized by a bimodal age of onset (childhood or early adulthood), slowly progressive gait difficulties with foot drop (in those with onset <10 years, 36% lost ambulation by the fourth decade), and sensory abnormalities of lower limbs (Fig. 3A). In rare cases, neuropathy can be associated with optic atrophy, auditory impairment, vocal cord palsy or CNS abnormalities.98 Sometimes, the disease manifests in the first decade with isolated motor involvement, with progression of distal weakness occurring rapidly within 3–5 years.99,100 Talipes at birth has been reported and a distinctive feature that might be observed in these cases is the presence of brisk reflexes.
MORC2 (AD)
Autosomal dominant, missense mutations in MORC2 have been identified as the cause of CMT2Z, an axonal sensorimotor neuropathy with moderately to severely progressive course, characterized by onset in the first two decades of life with typical CMT signs (e.g. gait abnormalities, feet deformity, distal weakness) variably associated with hyporeflexia, hyperreflexia and spasticity, asymmetric weakness, scoliosis, hearing loss (60%), and pigmentary retinopathy.101 In contrast with most CMTs, proximal weakness is commonly seen,102 a peculiar finding recently described also in CMT2CC (NEFH).103 MORC2 is an effector of the epigenetic silencing mediated by the human silencing hub (HUSH). MORC2 mutants have been shown to cause a change in the dimerization dynamics leading to abnormal modulation of HUSH function.104 Dominant variants have been reported also in a complex neurological disorder characterized by neuropathy, developmental delay, intellectual disability, growth retardation, microcephaly and variable craniofacial dysmorphism.105 Most importantly for the purpose of this review, MORC2 pathogenic variants were also identified in one patient developing severely (SMA-like) progressive proximal weakness starting from the age of 10 months and leading to complete tetraplegia followed by respiratory insufficiency due to diaphragmatic involvement by the age of 3 years. Electrophysiological studies revealed pure motor neuropathy without sensory involvement. Cranial MRI at 13 months and 4 years demonstrated progressive cerebellar atrophy; cognitive function was abnormal.106
AARS (AD)
Autosomal dominant pathogenic variants in AARS, a gene encoding for alanyl-tRNA synthetase (AlaRS), are a well established cause of CMT2. Like what has been recently demonstrated in GARS, axonal damage seems not to be caused by the loss of enzymatic function, rather from the aberrant interaction between AlaRS and Nrp1,107 a possible shared disease mechanism among different tRNA synthetases. A single patient presenting at the age of 2 years with frequent falls and a very slow-progressing distal weakness of upper and lower limbs was found to have a phenotype consistent with HMN.108
Other CMT
In a recent study, we found heterozygous variants in ARHGEF28 (p.Arg475Thr and p.Cys1458Trp) and AGRN causing slowly-progressive distal weakness and atrophy in patients who started to have difficulties in walking between the age of 2 and 5 years. In both patients though, electrophysiology revealed the presence of sensory impairment in addition to neurogenic changes on EMG needle examination.8
Dominantly inherited pathogenic variants in GBF1 (golgi brefeldin A-resistant guanine nucleotide exchange factor) have been recently identified as causative of a CMT2/HMN phenotype. Whilst the single patient reported with paediatric onset had sensory impairment, we cannot exclude that the clinical spectrum will expand in the forthcoming years.109
Bansagi and colleagues identified a new heterozygous c.1949G.A, p.(Tyr650Cys) sequence change in ARHGEF10, a gene encoding for a member of the family of Rho guanine nucleotide exchange factors that are implicated in neural morphogenesis and connectivity and are regulator of small Rho GTPases. The associated phenotype was characterized by talipes and pure motor neuronopathy.3 However, no other cases have been reported thus far and in the lack of functional studies there is limited evidence of the causative effect of such variant. Another variant [c.1013G > C;p.(Arg338Thr)] has been associated with a CMT2 phenotype but with decreased conduction velocities; its pathogenicity is also not clearly established.110
A heterozygous variant in MYH14 was reported as causative of a complex phenotype comprising peripheral neuropathy, myopathy, hoarseness (but no vocal cord palsy) and hearing loss in a single large autosomal dominant Korean family. Onset occurred at a median of 10.6 years of age with distal weakness and histopathological and electrodiagnostic studies revealed both chronic neuropathic and myopathic features in the affected patients.111 Intriguingly, this study suggested a potential role of myosin heavy chains isoforms also in motor neurons.
Frasquet and colleagues5 recently reported on a large cohort of patients presenting with a phenotype consistent with HMN/CMT2 who were evaluated in two tertiary centres in Spain and underwent comprehensive genetic studies. Although limited clinical data are provided, they described two siblings harbouring compound heterozygous mutations (AR) in the FIG4 gene, with onset in childhood and a pure motor, slowly progressive phenotype. FIG4 encodes an enzyme involved in phosphoinositide phospholipid metabolism and has been extensively associated CMT4J, hence a mixed demyelinating/axonal neuropathy. The phenotype of CMT4J varies from severe to milder forms, with both proximal and distal weakness and asymmetric involvement, which may point towards this genetic diagnosis. Lastly, Jacquier and colleagues112 recently reported on a juvenile-onset, pure motor neuronopathy associated with pyrdamidal signs and caused by homozygous variant c.3G>T (p.1Met?) in the COQ7 gene. This finding is intriguing given the potential for treatment with coenzyme Q10.
SMA-LED and HMN with proximal weakness
DYNC1H1 (AD)
Heterozygous mutations in the cytoplasmic dynein heavy chain 1 (DYNC1H1) gene cause SMA-LED type 1 and HMN type 1. DYNC1H1 encodes for the heavy chain 1 of the dynein complex, a ubiquitously expressed motor complex that consists of two heavy chains, two intermediate chains, four light intermediate chains and several light chains. DYNC1H1 is primary involved in the retrograde axonal transport from the cell terminal to the soma.113,114 Other functions include intracellular cargo transport, organelle motility, regulation of the Golgi apparatus, spindle-pole organization, and nuclear migration during mitosis.115
The prototypical phenotype is characterized by congenital or early childhood onset muscle weakness predominantly affecting lower limbs (proximal ≥ distal, especially the hip extensors), distal muscle wasting and feet deformities (Fig. 3C). Non-evolving, proximal (hip/knees) and distal (Achilles’ tendon) contractures can be observed in approximately half of patients and scoliosis may develop with time. Brisk DTR can be present in some patients, but these are more often reduced/absent.10 Around 37% of individuals present at birth with lower limbs malformations (calcaneus valgus talipes; hip dysplasia), 23% present in infancy (motor delay and abnormal gait), and 17% in childhood (gross motor difficulties or frequent falls). Twenty percent of individuals present in adulthood.
With few exceptions, weakness is non-progressing, with most individuals reaching the ability to walk with or without support. Malformation of cortical development (MCD) is seen in >20% of cases (e.g. polymicrogyria, cortical nodularity or gyral over convolution). Cognitive impairment or attention deficit hyperactivity disorder (ADHD) can be observed in up to one third of cases, usually but not exclusively in association with cortical abnormalities.
The spectrum of DYNC1H1-related disorders is expanding and the role of this protein in multiple steps of neurodevelopment is emerging.116 The former includes severe arthrogryposis, isolated intellectual disability, autism, seizures, spastic tetraplegia, MCD plus cataracts and gut dysmotility.117 Although pathogenic variants in specific domains have been associated with distinct phenotypes (e.g. variants in the dynein motor domain with MCD), genotype-phenotype correlation is limited.115,118
Diagnosis of DYNC1H1-related disease is suspected on the basis of clinical presentation and EMG showing motor neuropathy/neuronopathy without sensory abnormalities. Muscle MRI may reveal a distinctive pattern of muscle involvement characterized by diffuse alteration of quadriceps muscles associated with the selective sparing and relative hypertrophy of the adductor magnus and/or longus and of the semitendinosus muscles in the thigh, and diffuse lower leg involvement with relative sparing of the anterior-medial muscles.
Muscle biopsy usually demonstrates features compatible with chronic denervation (e.g. fibre-type grouping) but can be occasionally suggestive of congenital myopathy due to the presence of core-like areas, increased internal nuclei and rimmed vacuoles.10 For this reason, clinical gestalt, neurophysiology and muscle imaging are very useful in differentiating this condition from congenital myopathies.
BICD2 (AD)
Heterozygous pathogenic variants in the bicaudal D cargo adaptor 2 (BICD2) gene cause SMA-LED type 2, which is often clinically indistinguishable from SMA-LED type 1.11 Disease-causing variants map to mutational hot spots in the three coiled-coil domain regions of BICD2, corresponding to crucial interacting regions. BICD2 regulates the dynein-dynactin complex in different cellular processes (N-terminal domain) and functions as cargo adaptor protein (C-terminal domain). In particular, it interacts with dynein and kinesin motors, RANBP2 and RAB6. The latter is an important tissue specific small GTPase that regulates transport and fusion of secretory vesicles.119 The reason why mutations in BICD2 cause neuronal loss is not clear. One hypothesis suggests that the mechanism is neuronal specific and linked to detrimental increased stability of microtubules.120 However, a recent work on the Bicd2−/− mouse suggested an intriguing alternative explanation: mutations in BICD2 may in fact cause loss of protein function. In normal muscle, BICD2 ensures the correct localization of RAB6 at the trans Golgi surface, which is required for the efficient trafficking of secretory vesicles to the plasma membrane (Fig. 2D). According to the neurotrophin hypothesis,119 during early neurodevelopment a surplus of motor neurons reach the skeletal muscle and compete for muscle-secreted neurotrophic factors for survival. Subsequently, programmed cell-death regulated by neurotrophins follows. Therefore, a reduction in the secretion rate of neurotrophin-containing vesicles (due to RAB6 abnormal localization) may cause an excess of motor neuron death during these early phases of neuronal development.121 This hypothesis would explain why no progressive motor neuron degeneration occurs, at least in some patients, and opens to a series of possible novel pathogenic mechanisms involved in congenital motor neuronopathies.
Patients harbouring BICD2 mutations typically present with delayed motor milestones and contractures, with a subset of individuals also exhibiting arthrogryposis and hip dislocation from birth. While weakness is predominantly observed in lower extremities, upper limb involvement (proximal and/or distal) occurs in ∼40–50% of patients, usually later than lower limbs weakness. Up to one-third of patients never achieve ambulation and orthopaedic complications such as joint abnormalities (Fig. 3B), congenital hip dislocation, scoliosis and kyphosis, are frequent.
Respiratory involvement is a feature of severe disease, with 27% of families reported to require some respiratory assistance in a recent review.122 In contrast with what initially believed, roughly half of patients showed signs of CNS involvement including brisk reflexes, brain abnormalities, or cognitive impairment. Seizures are infrequent.
Interestingly, although EMG findings are usually neurogenic, in some individuals myopathic features in the context of chronic myopathy have been reported.123
As previously mentioned, pathogenic variants map to all three coiled-coil domains, with no strong genotype-phenotype correlation. An exception is the HSP-like phenotype observed in a family harbouring the p.(R501P) aminoacidic change, which is putatively disrupting the interaction with kinesin-1 (KIF5A), a protein which has been involved in in HSP (HSP10) and CMT.124 Lastly, de novo variants have been found to be more likely associated with severe disease in terms of congenital onset, respiratory impairment, severity of contractures, brain abnormalities, and lethal outcome, compared to familial cases.
SYT2 (AD)
Dominant missense variants in Synaptotagmin 2 (SYT2) have been reported as a rare cause of distal motor neuropathy and myasthenic syndrome, manifesting with stable or slowly progressive distal weakness of variable severity affecting both upper and lower limbs.
SYT2 are integral membrane proteins of synaptic vesicles thought to serve as Ca2+ sensors regulating vesicular trafficking and exocytosis. Variants in SYT2 are thought to have a dominant-negative effect on these processes, although the precise pathophysiological mechanism remains unclear.
After a normal development, the disease manifests during childhood (first decade) with foot deformities (pes cavus and hammer toes) and a variable degree of proximal and distal limb weakness, distal muscle atrophy, muscle fatigue, mild gait difficulties, and reduced or absent DTR. Clinically, the differential diagnosis would include CMT, but sensory studies are normal.125
The key feature of this disease is the co-occurrence of impaired presynaptic neuromuscular transmission (Lambert-Eaton like) and a distal motor neuropathy. As such, repetitive stimulation or single fibre EMG should be performed, as patients may benefit by treatment with 3,4-diaminopyridine.126
PLEKHG5 (AR)
Autosomal recessive variants in the Pleckstrin homology domain containing family G member 5 gene (PLEKHG5) have been reported in a Malian family with a SMA-like phenotype.127 PLEKHG5 is an activator of the nuclear factor kappa-B1 but the pathogenesis has not been elucidated. In the few reported cases the onset was around 2–3 years of age (one sibling with a milder form presented at 11 years and was still ambulant aged 20). The disease course is characterized by progressive proximal weakness, scoliosis, contractures and respiratory impairment.
VWA1 (AR)
The recent discovery of an autosomal recessive, slowly progressive, non-length dependent, HMN caused by biallelic pathogenic variants in the von Willebrand factor A domain containing 1 (VWA1) gene opened up new avenues for investigating the role of extracellular matrix (ECM) components in cell growth and differentiation, particularly with regards to muscle and nerve embryonic development.128,129 VWA1 is a protein interacting with perlecan and Collagen VI and whose putative function is to stabilize ECM structures (Fig. 2A). However, the disease-causing mechanism is not clear.
Disease onset is usually around the age of 2 years, but antenatal abnormalities can be present and adult-onset cases have been reported as well. Congenital or early-onset foot deformities (e.g. talipes, pes cavus, pes equinovarus) are common. With the caveat that only 32 cases have been reported thus far, the first manifestation of this disorder is usually distal (anterior > posterior) LL weakness variably leading to delayed acquisition of independent ambulation, tip-toe walking, Achilles tendon contractures and feet deformities. In contrast with other forms of HMN, atrophy of the distal muscles is not common at the time of diagnosis. Interestingly, recurrent hip and patellar dislocations, which we observed also in other undiagnosed HMN patients and other HMSN, have been reported.129 These presenting symptoms slowly evolve in the development of symmetric proximal weakness of lower (but also upper) limbs. Proximal weakness can be either equal, worse or milder than distal involvement. While UL involvement remains mild in the course of the disease, LL involvement can be severe (3/17 individuals lost ambulation in one series), but overall, the progression of the disease was very slow or even non-evolving.
Nerve conduction studies reveal a motor axonal neuropathy (reduction of cMAP) in most cases, usually in presence of normal sensory examination (although clinical sensory disturbances have also been reported). Needle EMG is usually neurogenic but can show a mixed picture of proximal myopathic changes with distal neurogenic abnormalities.
Muscle biopsy results are also variable, with both myopathic and neurogenic changes. One individual presented with paracrystalline inclusions in the mitochondria. CK have been found to be elevated in a minority of individuals (369–1628 IU/l). Muscle MRI of lower limbs showed a rather uniform pattern with predominant affection of the vasti, gastrocnemius and peroneal muscles, with relative sparing of the sartorius, gracilis and soleous muscle.128 In those individuals in which brain MRI was performed, this was normal.
In one of the two published series, a founder allele causing a 10-bp expansion at the end of exon 1 (c.62_71dup10;p.G25Rfs*74, NM_022834.5) was found in several families. Notably, patients harbouring a compound heterozygous variants seemed milder than individuals homozygous for the founder mutation. In the broad CMT spectrum, pathogenic variants in VWA1 may account for up to 1% of cases and new phenotypes are likely to be reported in the future.
Neurogenic arthrogryposis multiplex congenita
AMC is defined by the presence of joint contractures in two or more districts of the body (including jaw, neck and spine) associated with foetal akinesia (reduced foetal movements). The estimated incidence is 1 in 3000–5000 newborn children130 and the underlying acquired or genetic causes are numerous and heterogeneous,131 with more than 150 causative genes identified thus far.131 Common AMC causes include maternal events (e.g. myasthenia gravis), amyoplasia (sporadic congenital defect in the development of muscle132), conditions in which we observe a primary central nervous system involvement (e.g. congenital infections, mitochondrial disorders, metabolic disorders) and disorders in which a component of the peripheral nervous system is affected.
Neurogenic arthrogryposis is defined by the presence of AMC and neurogenic changes on EMG. Importantly, such ‘peripheral’ phenotype should include in the differential diagnosis affections of the skeletal muscle (40% of cases are due to congenital myopathies), congenital myasthenic syndromes, severe genetic neuropathies (e.g. new genes such as CNTNAP1), congenital muscular dystrophies (e.g. FKRP, FKTN), and genes causing altered mechanical transduction (e.g. PIEZO2). Isolated motor neuron involvement was found to be the cause of 9% of genetically confirmed cases in one large study on >300 individuals presenting with AMC.133
With the caveat that most of case series in the past lacked genetic confirmation, neurogenic AMC without severe foetal phenotype is usually considered a non-progressive condition, and close to half of the patients with LL AMC will acquire independent ambulation despite contractures.134
The boundary between neuronopathies with congenital onset and a non-progressive course (e.g. DYNC1H1, BICD2)119 and neurogenic AMC is blurred. The defect that leads to foetal akinesia involves considering a variety of developmental pathways, and complex and yet-to be elucidated molecular mechanisms. Both in diagnosed and undiagnosed individuals features suggestive of reduced foetal movements such as talipes, hip dislocation, breech presentation and polyhydramnios are common.
Several genes implicated in the embryonic development of central and/or peripheral nervous system (including lower motor neurons, nodes of Ranvier, and myelin) have been associated with lethal congenital contracture syndrome (LCCS) or lethal arthrogryposis with anterior horn cell disease (LAAHD).135 The description of such conditions goes beyond our scope (see Beecroft et al.135 for a thorough review), but genes such as GLE1 suggests that the phenotypic spectrum of these very severe forms is ever expanding, with the possibility that milder ‘HMN-like’ phenotypes might be reported in the future.136 The peculiar combination of AMC and visceral involvement (gut dysmotility spectrum) could represent a diagnostic clue pointing to known or still unidentified genes (e.g. ERBB3).
Of note, neurogenic EMG changes can be also observed in patients presenting with a phenotype consistent with sporadic amyoplasia (see Hall et al.132,137 for an extensive review). A hypothesis is that anterior horn damage could be the consequence of vascular compromise happening between the 8th and 12th week of gestation. Hence, the possibility of an ‘acquired’ insult happening at specific stages of uterine life (which could also explain the different involvement of upper and lower limbs) should also be considered.132
Juvenile ALS
ALS is a relentlessly progressive neurological disease belonging to the broader spectrum of motor neuron diseases. It is characterized by a progressive degeneration of upper and lower motor neurons, leading to muscle weakness, respiratory failure and ultimately death, typically occurring 3–5 years after symptoms onset. Despite a classical onset in middle to late life, juvenile forms exist, defined by the occurrence of motor symptoms before the 25th year of age,138 while still respecting the diagnostic criteria for the adult-onset form.139
The state-of-the-art working view panel of ALS genes suggest that they cluster in three categories, involving protein homeostasis, RNA homeostasis and trafficking, and cytoskeletal dynamics.140
Usually, the first symptoms of juvenile ALS manifests in late childhood to adolescence, characterized by progressive muscle weakness, often with an asymmetric distribution, accompanied by atrophy, fasciculations and cramps, pointing to lower motor neurons and their axons as the prime site of pathology. Pyramidal tract signs, such as brisk tendon reflexes and a pathological plantar reflex, are usually present, indicating a concomitant involvement of the central motor tracts. Bulbar involvement may occur as well, including dysarthria, dysphagia, tongue atrophy and hyperactive gag reflex. Emotional lability and inappropriate crying or laughing (pseudobulbar signs) may be present. Cognitive and behavioral functions are seldom involved at the beginning of the disease, but phenotypic variability is almost paradigmatic in this class of disorders, further complicating the already challenging diagnostic process. Neurophysiology can assist the diagnosis, providing evidence of a diffuse lower motor neuron involvement with electromyographic signs of active and chronic denervation.141
Mutations in several genes have been classically associated with juvenile ALS, with both an autosomal dominant pattern of inheritance (SETX, SPTLC1, SPTLC2), and with autosomal recessive inheritance (ALS2, SPG11/spatacsin, FUS, SIGMAR1). The presence of overt pyramidal signs, tongue fasciculations and early bulbar involvement should guide the diagnosis in favour of juvenile ALS versus HMN.
FUS (AR/AD)
FUS, encoding for a nuclear-cytosolic shuttling protein with both DNA and RNA binding properties and various functions in RNA metabolism, represents the most frequently mutated gene,142 accounting for more than 30% of juvenile forms.143
As a rule, FUS mutated forms tend to be more aggressive, representing the juvenile phenotype closer to the adult form of the disease. These patients have an earlier onset, prominent bulbar involvement, and a premature need for a continuous ventilatory support, usually occurring within 2 years from symptom onset.144 Some cases may be accompanied by learning difficulties and mental retardation. Atypical presentations may include myoclonus with generalized epileptic discharges,145 and postural tremor.
Others
Serine palmitoyltransferase, long-chain base subunit 1 (SPTLC1)146,147 and 2 (SPTLC2)148 and sigma-1 receptor (SIGMAR1)149 mutations have been described in both familiar and sporadic forms, characteristically associated with onset from neonatal period to early-childhood, spasticity involving predominantly the lower limbs, followed by diffuse lower motor neurons involvement, and bulbar involvement. Interestingly, SPTLC1 and SPTLC2 belong to the serine palmitoyltransferase complex (SPT) and both have been associated with hereditary sensory autonomic neuropathy type 1 (HSAN1). However, in contrast with HSAN, L-serine supplementation might be detrimental in these forms of juvenile ALS due to the unregulated overactivity of SPT.147
One Japanese case has been associated with a spectrin repeat containing nuclear envelope protein 1 (SYNE1) gene mutation, in the context of a complex phenotype accompanied by cognitive decline and cerebellar ataxia.150
Lastly, mutations in RNA binding proteins HNRNPA1 and HNRNPA2B1,151 encoding for the heterogeneous nuclear ribonucleoprotein (hnRNP) A1 and B1, have been associated with a spectrum of disorders ranging from ALS, multisystem proteinopathy (MSP),152 to phenotypes consistent with HMN and/or distal myopathies.153 The phenotype is determined by the type of mutation i.e. missense, frameshift.
Discussion
The aim of this review is to convey the biological and clinical complexity underlying a heterogeneous group of conditions that together constitute early onset motor neuronopathies. Over the last decade, several novel genes have been discovered and, intriguingly, phenotypic diversity and variability continue to expand. Most importantly, more than 60% of patients presenting with an HMN-like phenotype still lack a genetic diagnosis. Given the low prevalence of these disorders taken separately, collaborations to gather and deep phenotype similar cases are crucial for an improved and comprehensive understanding of the pathogenesis of these disorders and discovery of novel gene candidates. Interestingly, genes that are thought to be relatively ‘common’ causes of HMN such as SORD or VWA1 have eluded genetic investigations for years.
A key role in determining the success of the diagnostic genetic screening is the choice of the technology used to support the investigation; variants located in complex genomic regions (including genomic rearrangement and triplicated regions) are often missed when relying on the conventional short-read whole genome sequencing, whereas copy number variants in these genomic regions are nowadays more accessible through the advent of the recent long-read whole genome sequencing technology. An example is represented by sorbitol dehydrogenase deficiency (SORD). The nonsense SORD c.757del variant (rs1042079), which is commonly found either in homozygosity or compound heterozygosity in affected patients, has previously likely escaped short-read analysis due to the presence of the SORD2P pseudogene harbouring the same genomic variant. Long-read WGS can distinguish between genes and pseudogenes, hence overcoming limitations of older genetic diagnostics.154
At the present time, it is difficult to establish a simple diagnostic algorithm for this group of conditions other than based on frequency of individual genetic defects. This should in theory take into consideration the multiple phenotypes associated with the same gene and should also correctly weight the presence or absence of specific findings. Of note, it is also difficult to capture the timing of onset of some peculiar features (Fig. 1B), the absence of which should not exclude a specific diagnosis (for example, respiratory impairment can occur many years after onset in both IGHMBP2 and BICD2-related neuronopathies).
A practical approach could consider the age at which the first signs of the disease manifest (Fig. 1A). This would also reflect the timing at which the noxa patogena (e.g. defective embryonic development/axonal pathfinding, response to ageing, bioenergetics failure etc.) intervenes.
The cornerstone upon which a diagnosis of neuronopathy is pursued is the finding of chronic neurogenic changes on EMG, namely high-amplitude polyphasic motor units of long duration. The distribution of neurogenic changes (distal versus proximal), the unexpected presence of active denervation (positive sharp waves and fibrillation potentials), the finding of mixed neurogenic and myopathic EMG pattern (documented in BICD2, HSPB1, HSPB8, VWA1, GNE, AGRN and ACTA1), or the involvement of sensory fibres (SAPs) could all add to the deep phenotyping of these patients. Muscle MRI might represent a supportive tool to guide the differential diagnosis between distal myopathies and neuronopathies (Fig. 3F)155 and muscle ultrasound can be used in clinics to detect fasciculations. Of note, CK can be elevated.
The molecular mechanisms underlying neuronopathies are heterogeneous and in part still poorly understood. The discovery of VWA1 and insights on the role played by BICD2 in mice (specifically its interaction with RAB6) highlight the importance of further characterizing axonal pathfinding and the maturation of the motor unit, including the role of ECM components.156,157
While we can group pathomechanisms in broad categories such as disturbed mitochondrial function, impaired axonal transport/intracellular trafficking, abnormal protein synthesis, abnormal RNA/DNA metabolism, abnormal cellular signalling etc. (Table 1 and Fig. 2), there are some cautionary tales. As an example, autosomal dominant variants in both GARS and AARS, both belonging to the aminoacyl tRNA synthetases family, do not disrupt the enzymatic activity, rather there is increasing evidence suggesting that neuronal damage is caused by the aberrant activation of the Nrp1/VEGF signalling pathway.
The precise definition of pathogenic mechanisms, such as the elucidation of the role played by TRPV4 mutants, should pave the way towards the unmet need for new treatments. Indeed, while several of these conditions have been considered slowly progressive, they can still be severely disabling for patients and disease-related complications have only recently emerged from larger case-series. Longitudinal, multi-centre, natural history data are crucial to anticipate care and identify the rates of disease progression in preparation of future therapeutic trials.
In conclusion, paediatric onset motor neuronopathies are a wide and extremely complex group of disorders, with several genes and phenotypes yet to be uncovered. High phenotypic variability, overlap between different conditions and elusive molecular mechanisms justify caution when establishing fixed categories and should be considered when approaching patients with a consistent clinical presentation.
Supplementary Material
Acknowledgements
Special thanks and appreciation to the neuromuscular team at the DNC. Figures 1 and 2 were created with BioRender.com.
Contributor Information
Alberto A Zambon, Neuromuscular Repair Unit, Institute of Experimental Neurology (InSpe), Division of Neuroscience, IRCCS Ospedale San Raffaele, 20132 Milan, Italy; Dubowitz Neuromuscular Centre, UCL Great Ormond Street Institute of Child Health and Great Ormond Street Hospital, London, WC1N 1EH, UK.
Veronica Pini, Dubowitz Neuromuscular Centre, UCL Great Ormond Street Institute of Child Health and Great Ormond Street Hospital, London, WC1N 1EH, UK.
Luca Bosco, Neuromuscular Repair Unit, Institute of Experimental Neurology (InSpe), Division of Neuroscience, IRCCS Ospedale San Raffaele, 20132 Milan, Italy.
Yuri M Falzone, Neuromuscular Repair Unit, Institute of Experimental Neurology (InSpe), Division of Neuroscience, IRCCS Ospedale San Raffaele, 20132 Milan, Italy.
Pinki Munot, NIHR Great Ormond Street Hospital Biomedical Research Centre, London, WC1N 1EH, UK.
Francesco Muntoni, Dubowitz Neuromuscular Centre, UCL Great Ormond Street Institute of Child Health and Great Ormond Street Hospital, London, WC1N 1EH, UK; NIHR Great Ormond Street Hospital Biomedical Research Centre, London, WC1N 1EH, UK.
Stefano C Previtali, Neuromuscular Repair Unit, Institute of Experimental Neurology (InSpe), Division of Neuroscience, IRCCS Ospedale San Raffaele, 20132 Milan, Italy.
Funding
No funding was received towards this work.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Harding AE, Thomas PK. Hereditary distal spinal muscular atrophy: A report on 34 cases and a review of the literature. J Neurol Sci. 1980;45:337–348. [DOI] [PubMed] [Google Scholar]
- 2. De Jonghe P, Timmerman V, Van Broeckhoven C. 2nd Workshop of the European CMT consortium: 53rd ENMC international workshop on classification and diagnostic guidelines for Charcot-Marie-Tooth Type 2 (CMT2–HMSN II) and distal hereditary motor neuropathy (distal HMN–spinal CMT): 26–28 September 1997, Naarden, The Netherlands. Neuromuscul Disord. 1998;8:426–431. [PubMed] [Google Scholar]
- 3. Bansagi B, Griffin H, Whittaker RG, et al. Genetic heterogeneity of motor neuropathies. Neurology. 2017;88:1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rossor AM, Evans MRB, Reilly MM. A practical approach to the genetic neuropathies. Pract Neurol. 2015;15:187–198. [DOI] [PubMed] [Google Scholar]
- 5. Frasquet M, Rojas-García R, Argente-Escrig H, et al. Distal hereditary motor neuropathies: Mutation spectrum and genotype-phenotype correlation. Eur J Neurol. 2021;28:1334–1343. [DOI] [PubMed] [Google Scholar]
- 6. Harding AE. Inherited neuronal atrophy and degeneration predominantly of lower motor neurons. In: Dyck PJThomas PKGriffin JWLow PA and Poduslo JF, editors. Peripheral neuropathy. 3rd ed. W. B. Saunders; 1993. p 1051–1064. [Google Scholar]
- 7. Pipis M, Rossor AM, Laura M, Reilly MM. Next-generation sequencing in charcot-marie-tooth disease: Opportunities and challenges. Nat Rev Neurol. 2019;15:644–656. [DOI] [PubMed] [Google Scholar]
- 8. Previtali SC, Zhao E, Lazarevic D, et al. Expanding the spectrum of genes responsible for hereditary motor neuropathies. J Neurol Neurosurg Psychiatry. 2019;90:1171–1179. [DOI] [PubMed] [Google Scholar]
- 9. Rossor AM, Kalmar B, Greensmith L, Reilly MM. The distal hereditary motor neuropathies. J Neurol Neurosurg Psychiatry. 2012;83:6–14. [DOI] [PubMed] [Google Scholar]
- 10. Scoto M, Rossor AM, Harms MB, et al. Novel mutations expand the clinical spectrum of DYNC1H1-associated spinal muscular atrophy. Neurology. 2015;84:668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rossor AM, Oates EC, Salter HK, et al. Phenotypic and molecular insights into spinal muscular atrophy due to mutations in BICD2. Brain. 2015;138:293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Storbeck M, Horsberg Eriksen B, Unger A, et al. Phenotypic extremes of BICD2-opathies: From lethal, congenital muscular atrophy with arthrogryposis to asymptomatic with subclinical features. Eur J Hum Genet. 2017;25:1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dubourg O, Azzedine H, Yaou RB, et al. The G526R glycyl-tRNA synthetase gene mutation in distal hereditary motor neuropathy type V. Neurology. 2006;66:1721–1726. [DOI] [PubMed] [Google Scholar]
- 14. Garg N, Park SB, Vucic S, et al. Differentiating lower motor neuron syndromes. J Neurol Neurosurg Psychiatry. 2017;88:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Irobi J, De Jonghe P, Timmerman V. Molecular genetics of distal hereditary motor neuropathies. Hum Mol Genet. 2004;13:R195–R202. [DOI] [PubMed] [Google Scholar]
- 16. Irobi J, Dierick I, Jordanova A, Claeys KG, De Jonghe P, Timmerman V. Unraveling the genetics of distal hereditary motor neuronopathies. Neuromolecular Med. 2006;8:131–146. [DOI] [PubMed] [Google Scholar]
- 17. Carreau C, Lenglet T, Mosnier I, et al. A juvenile ALS-like phenotype dramatically improved after high-dose riboflavin treatment. Ann Clin Transl Neurol. 2020;7:250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teoh HL, Carey K, Sampaio H, Mowat D, Roscioli T, Farrar M. Inherited paediatric motor neuron disorders: Beyond spinal muscular atrophy. Neural Plast. 2017;2017:6509493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farrar MA, Kiernan MC. The genetics of spinal muscular atrophy: Progress and challenges. Neurotherapeutics. 2015;12:290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evgrafov OV, Mersiyanova I, Irobi J, et al. Mutant small heat-shock protein 27 causes axonal charcot-marie-tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004;36:602–606. [DOI] [PubMed] [Google Scholar]
- 21. Irobi J, Van Impe K, Seeman P, et al. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat Genet. 2004;36:597–601. [DOI] [PubMed] [Google Scholar]
- 22. Kolb SJ, Snyder PJ, Poi EJ, et al. Mutant small heat shock protein B3 causes motor neuropathy: Utility of a candidate gene approach. Neurology. 2010;74:502–506. [DOI] [PubMed] [Google Scholar]
- 23. Houlden H, Laura M, Wavrant-De Vrièze F, Blake J, Wood N, Reilly MM. Mutations in the HSP27 (HSPB1) gene cause dominant, recessive, and sporadic distal HMN/CMT type 2. Neurology. 2008;71:1660–1668. [DOI] [PubMed] [Google Scholar]
- 24. Capponi S, Geroldi A, Fossa P, et al. HSPB1 and HSPB8 in inherited neuropathies: Study of an Italian cohort of dHMN and CMT2 patients. J Peripher Nerv Syst. 2011;16:287–294. [DOI] [PubMed] [Google Scholar]
- 25. Bugiardini E, Rossor AM, Lynch DS, et al. Homozygous mutation in HSPB1 causing distal vacuolar myopathy and motor neuropathy. Neurol Genet. 2017;3:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaler SG. ATP7A-related Copper transport diseases-emerging concepts and future trends. Nat Rev Neurol. 2011;7:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaler SG. Inborn errors of copper metabolism. Handb Clin Neurol. 2013;113:1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yi L, Kaler SG. Direct interactions of adaptor protein complexes 1 and 2 with the copper transporter ATP7A mediate its anterograde and retrograde trafficking. Hum Mol Genet. 2015;24:2411–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kennerson ML, Nicholson GA, Kaler SG, et al. Missense mutations in the copper transporter gene ATP7A cause X-linked distal hereditary motor neuropathy. Am J Hum Genet. 2010;86:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fradin M, Lavillaureix A, Jaillard S, et al. ATP7A Mutation with occipital horns and distal motor neuropathy: A continuum. Eur J Med Genet. 2020;63:104087. [DOI] [PubMed] [Google Scholar]
- 31. Gualandi F, Sette E, Fortunato F, et al. Report of a novel ATP7A mutation causing distal motor neuropathy. Neuromuscul Disord. 2019;29:776–785. [DOI] [PubMed] [Google Scholar]
- 32. Tsai PC, Soong BW, Mademan I, et al. A recurrent WARS mutation is a novel cause of autosomal dominant distal hereditary motor neuropathy. Brain. 2017;140:1252–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li JQ, Dong HL, Chen CX, Wu ZY. A novel WARS mutation causes distal hereditary motor neuropathy in a Chinese family. Brain. 2019;142:e49. [DOI] [PubMed] [Google Scholar]
- 34. Wang B, Li X, Huang S, et al. A novel WARS mutation (p.Asp314Gly) identified in a Chinese distal hereditary motor neuropathy family. Clin Genet. 2019;96:176–182. [DOI] [PubMed] [Google Scholar]
- 35. Sumner CJ, d'Ydewalle C, Wooley J, et al. A dominant mutation in FBXO38 causes distal spinal muscular atrophy with calf predominance. Am J Hum Genet. 2013;93:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sarparanta J, Jonson PH, Kawan S, Udd B. Neuromuscular diseases due to chaperone mutations: A review and some new results. Int J Mol Sci. 2020;21:1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blumen SC, Astord S, Robin V, et al. A rare recessive distal hereditary motor neuropathy with HSJ1 chaperone mutation. Ann Neurol. 2012;71:509–519. [DOI] [PubMed] [Google Scholar]
- 38. Gess B, Auer-Grumbach M, Schirmacher A, et al. HSJ1-related Hereditary neuropathies: Novel mutations and extended clinical spectrum. Neurology. 2014;83:1726–1732. [DOI] [PubMed] [Google Scholar]
- 39. Sanchez E, Darvish H, Mesias R, et al. Identification of a large DNAJB2 deletion in a family with spinal muscular atrophy and parkinsonism. Hum Mutat. 2016;37:1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zimoń M, Baets J, Almeida-Souza L, et al. Loss-of-function mutations in HINT1 cause axonal neuropathy with neuromyotonia. Nat Genet. 2012;44:1080–1083. [DOI] [PubMed] [Google Scholar]
- 41. Boaretto F, Cacciavillani M, Mostacciuolo ML, et al. Novel loss-of-function mutation of the HINT1 gene in a patient with distal motor axonal neuropathy without neuromyotonia. Muscle Nerve. 2015;52:688–689. [DOI] [PubMed] [Google Scholar]
- 42. Zhao H, Race V, Matthijs G, et al. Exome sequencing reveals HINT1 mutations as a cause of distal hereditary motor neuropathy. Eur J Hum Genet. 2014;22:847–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McMacken G, Whittaker RG, Charlton R, Barresi R, Lochmüller H, Horvath R. Inherited neuropathies with predominant upper limb involvement: Genetic heterogeneity and overlapping pathologies. Eur J Neurol. 2021;28:297–304. [DOI] [PubMed] [Google Scholar]
- 44. Oprescu SN, Chepa-Lotrea X, Takase R, et al. Compound heterozygosity for loss-of-function GARS variants results in a multisystem developmental syndrome that includes severe growth retardation. Hum Mutat. 2017;38:1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boczonadi V, Meyer K, Gonczarowska-Jorge H, et al. Mutations in glycyl-tRNA synthetase impair mitochondrial metabolism in neurons. Hum Mol Genet. 2018;27:2187–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Niehues S, Bussmann J, Steffes G, et al. Impaired protein translation in drosophila models for charcot-marie-tooth neuropathy caused by mutant tRNA synthetases. Nat Commun. 2015;6:7520–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He W, Bai G, Zhou H, et al. CMT2D Neuropathy is linked to the neomorphic binding activity of glycyl-tRNA synthetase. Nature. 2015;526:710–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sivakumar K, Kyriakides T, Puls I, et al. Phenotypic spectrum of disorders associated with glycyl-tRNA synthetase mutations. Brain. 2005;128:2304–2314. [DOI] [PubMed] [Google Scholar]
- 49. James PA, Cader MZ, Muntoni F, Childs AM, Crow YJ, Talbot K. Severe childhood SMA and axonal CMT due to anticodon binding domain mutations in the GARS gene. Neurology. 2006;67:1710–1712. [DOI] [PubMed] [Google Scholar]
- 50. Eskuri JM, Stanley CM, Moore SA, Mathews KD. Infantile onset CMT2D/dSMA V in monozygotic twins due to a mutation in the anticodon-binding domain of GARS. J Peripher Nerv Syst. 2012;17:132–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Forrester N, Rattihalli R, Horvath R, et al. Clinical and genetic features in a series of eight unrelated patients with neuropathy due to Glycyl-tRNA Synthetase (GARS) variants. J Neuromuscul Dis. 2020;7:137–143. [DOI] [PubMed] [Google Scholar]
- 52. Markovitz R, Ghosh R, Kuo ME, et al. GARS-related disease in infantile spinal muscular atrophy: Implications for diagnosis and treatment. Am J Med Genet A. 2020;182:1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Beetz C, Pieber TR, Hertel N, et al. Exome sequencing identifies a REEP1 mutation involved in distal hereditary motor neuropathy type V. Am J Hum Genet. 2012;91:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Windpassinger C, Auer-Grumbach M, Irobi J, et al. Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and silver syndrome. Nat Genet. 2004;36:271–276. [DOI] [PubMed] [Google Scholar]
- 55. Musacchio T, Zaum AK, Üçeyler N, et al. ALS And MMN mimics in patients with BSCL2 mutations: The expanding clinical spectrum of SPG17 hereditary spastic paraplegia. J Neurol. 2017;264:11–20. [DOI] [PubMed] [Google Scholar]
- 56. Auer-Grumbach M, Schlotter-Weigel B, Lochmüller H, et al. Phenotypes of the N88S berardinelli-seip congenital lipodystrophy 2 mutation. Ann Neurol. 2005;57:415–424. [DOI] [PubMed] [Google Scholar]
- 57. Luigetti M, Fabrizi GM, Madia F, et al. Seipin S90L mutation in an Italian family with CMT2/dHMN and pyramidal signs. Muscle Nerve. 2010;42:448–451. [DOI] [PubMed] [Google Scholar]
- 58. Ito D, Suzuki N. Seipinopathy: A novel endoplasmic reticulum stress-associated disease. Brain. 2009;132:8–15. [DOI] [PubMed] [Google Scholar]
- 59. van de Warrenburg BP, Scheffer H, van Eijk JJ, et al. BSCL2 Mutations in two Dutch families with overlapping silver syndrome-distal hereditary motor neuropathy. Neuromuscul Disord. 2006;16:122–125. [DOI] [PubMed] [Google Scholar]
- 60. Auer-Grumbach M, Löscher WN, Wagner K, et al. Phenotypic and genotypic heterogeneity in hereditary motor neuronopathy type V: A clinical, electrophysiological and genetic study. Brain. 2000;123:1612–1623. [DOI] [PubMed] [Google Scholar]
- 61. Choi BO, Park MH, Chung KW, et al. Clinical and histopathological study of charcot-marie-tooth neuropathy with a novel S90W mutation in BSCL2. Neurogenetics. 2013;14:35–42. [DOI] [PubMed] [Google Scholar]
- 62. Guenther UP, Handoko L, Laggerbauer B, et al. IGHMBP2 Is a ribosome-associated helicase inactive in the neuromuscular disorder distal SMA type 1 (DSMA1). Hum Mol Genet. 2009;18:1288–1300. [DOI] [PubMed] [Google Scholar]
- 63. Butterfield RJ, Stevenson TJ, Xing L, et al. Congenital lethal motor neuron disease with a novel defect in ribosome biogenesis. Neurology. 2014;82:1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grohmann K, Varon R, Stolz P, et al. Infantile spinal muscular atrophy with respiratory distress type 1 (SMARD1). Ann Neurol. 2003;54:719–724. [DOI] [PubMed] [Google Scholar]
- 65. Pitt M, Houlden H, Jacobs J, et al. Severe infantile neuropathy with diaphragmatic weakness and its relationship to SMARD1. Brain. 2003;126:2682–2692. [DOI] [PubMed] [Google Scholar]
- 66. Shi CH, Song B, Luo HY, et al. Recessive hereditary motor and sensory neuropathy caused by IGHMBP2 gene mutation. Neurology. 2015;85:383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Blaschek A, Gläser D, Kuhn M, et al. Early infantile sensory-motor neuropathy with late onset respiratory distress. Neuromuscul Disord. 2014;24:269–271. [DOI] [PubMed] [Google Scholar]
- 68. Yuan JH, Hashiguchi A, Yoshimura A, et al. Clinical diversity caused by novel IGHMBP2 variants. J Hum Genet. 2017;62:599–604. [DOI] [PubMed] [Google Scholar]
- 69. Cottenie E, Kochanski A, Jordanova A, et al. Truncating and missense mutations in IGHMBP2 cause charcot-marie tooth disease type 2. Am J Hum Genet. 2014;95:590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Luan X, Huang X, Liu X, Zhou H, Chen S, Cao L. Infantile spinal muscular atrophy with respiratory distress type I presenting without respiratory involvement: Novel mutations and review of the literature. Brain Dev. 2016;38:685–689. [DOI] [PubMed] [Google Scholar]
- 71. Messina MF, Messina S, Gaeta M, et al. Infantile spinal muscular atrophy with respiratory distress type I (SMARD 1): An atypical phenotype and review of the literature. Eur J Paediatr Neurol. 2012;16:90–94. [DOI] [PubMed] [Google Scholar]
- 72. Tomaselli PJ, Horga A, Rossor AM, et al. IGHMBP2 Mutation associated with organ-specific autonomic dysfunction. Neuromuscul Disord. 2018;28:1012–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Joseph S, Robb SA, Mohammed S, et al. Interfamilial phenotypic heterogeneity in SMARD1. Neuromuscul Disord. 2009;19:193–195. [DOI] [PubMed] [Google Scholar]
- 74. Velilla J, Marchetti MM, Toth-Petroczy A, et al. Homozygous TRPV4 mutation causes congenital distal spinal muscular atrophy and arthrogryposis. Neurol Genet. 2019;5:e312–e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Landouré G, Zdebik AA, Martinez TL, et al. Mutations in TRPV4 cause charcot-marie-tooth disease type 2C. Nat Genet. 2010;42:170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Deng HX, Klein CJ, Yan J, et al. Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat Genet. 2010;42:165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nilius B, Voets T. The puzzle of TRPV4 channelopathies. EMBO Rep. 2013;14:152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nishimura G, Lausch E, Savarirayan R, et al. TRPV4-associated Skeletal dysplasias. Am J Med Genet C Semin Med Genet. 2012;160C:190–204. [DOI] [PubMed] [Google Scholar]
- 79. Woolums BM, McCray BA, Sung H, et al. TRPV4 Disrupts mitochondrial transport and causes axonal degeneration via a CaMKII-dependent elevation of intracellular Ca(2). Nat Commun. 2020;11:2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sullivan JM, Zimanyi CM, Aisenberg W, et al. Novel mutations highlight the key role of the ankyrin repeat domain in TRPV4-mediated neuropathy. Neurol Genet. 2015;1:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McCray BA, Diehl E, Sullivan JM, et al. Neuropathy-causing TRPV4 mutations disrupt TRPV4-RhoA interactions and impair neurite extension. Nat Commun. 2021;12:1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen DH, Sul Y, Weiss M, et al. CMT2C With vocal cord paresis associated with short stature and mutations in the TRPV4 gene. Neurology. 2010;75:1968–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Echaniz-Laguna A, Dubourg O, Carlier P, et al. Phenotypic spectrum and incidence of TRPV4 mutations in patients with inherited axonal neuropathy. Neurology. 2014;82:1919–1926. [DOI] [PubMed] [Google Scholar]
- 84. Oonk AM, Ekker MS, Huygen PL, et al. Intrafamilial variable hearing loss in TRPV4 induced spinal muscular atrophy. Ann Otol Rhinol Laryngol. 2014;123:859–865. [DOI] [PubMed] [Google Scholar]
- 85. Astrea G, Brisca G, Fiorillo C, et al. Muscle MRI in TRPV4-related congenital distal SMA. Neurology. 2012;78:364–365. [DOI] [PubMed] [Google Scholar]
- 86. Barwick KE, Wright J, Al-Turki S, et al. Defective presynaptic choline transport underlies hereditary motor neuropathy. Am J Hum Genet. 2012;91:1103–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Young ID, Harper PS. Hereditary distal spinal muscular atrophy with vocal cord paralysis. J Neurol Neurosurg Psychiatr. 1980;43:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Puls I, Jonnakuty C, LaMonte BH, et al. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. [DOI] [PubMed] [Google Scholar]
- 89. Tian W-T, Liu L-H, Zhou H-Y, et al. New phenotype of DCTN1-related spectrum: Early-onset dHMN plus congenital foot deformity. Ann Clin Transl Neurol. 2020;7:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Grunseich C, Patankar A, Amaya J, et al. Clinical and molecular aspects of senataxin mutations in amyotrophic lateral sclerosis 4. Ann Neurol. 2020;87:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Daoud H, Zhou S, Noreau A, et al. Exome sequencing reveals SPG11 mutations causing juvenile ALS. Neurobiol Aging. 2012;33:839.e5–9. [DOI] [PubMed] [Google Scholar]
- 92. Christodoulou K, Zamba E, Tsingis M, et al. A novel form of distal hereditary motor neuronopathy maps to chromosome 9p21.1-p12. Ann Neurol. 2000;48:877–884. [PubMed] [Google Scholar]
- 93. Li X, Hu Z, Liu L, et al. A SIGMAR1 splice-site mutation causes distal hereditary motor neuropathy. Neurology. 2015;84:2430–2437. [DOI] [PubMed] [Google Scholar]
- 94. Weiman DI, Gillespie MK, Hartley T, et al. Neurophysiological characteristics of allgrove (triple A) syndrome: Case report and literature review. Child Neurol Open. 2021;8:2329048X211031059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cortese A, Zhu Y, Rebelo AP, et al. Biallelic mutations in SORD cause a common and potentially treatable hereditary neuropathy with implications for diabetes. Nat Genet. 2020;52:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kramarz C, Rossor AM. Neurological update: Hereditary neuropathies. J Neurol. 2022;269:5187–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yuan RY, Ye ZL, Zhang XR, Xu LQ, He J. Evaluation of SORD mutations as a novel cause of charcot-marie-tooth disease. Ann Clin Transl Neurol. 2021;8:266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bombelli F, Stojkovic T, Dubourg O, et al. Charcot-Marie-Tooth disease type 2A: From typical to rare phenotypic and genotypic features. JAMA Neurol. 2014;71:1036–1042. [DOI] [PubMed] [Google Scholar]
- 99. Ma Y, Sun A, Zhang Y, Fan D, Liu X. The genotype and phenotype features in a large Chinese MFN2 mutation cohort. Front Neurol. 2021;12:757518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Robinson PN. Deep phenotyping for precision medicine. Hum Mutat. 2012;33:777–780. [DOI] [PubMed] [Google Scholar]
- 101. Sevilla T, Lupo V, Martínez-Rubio D, et al. Mutations in the MORC2 gene cause axonal charcot-marie-tooth disease. Brain. 2016;139:62–72. [DOI] [PubMed] [Google Scholar]
- 102. Semplicini C, Ollagnon-Roman E, Leonard-Louis S, et al. High intra-familiar clinical variability in MORC2 mutated CMT2 patients. Brain. 2017;140:e21. [DOI] [PubMed] [Google Scholar]
- 103. Pipis M, Cortese A, Polke JM, et al. Charcot-Marie-Tooth disease type 2CC due to NEFH variants causes a progressive, non-length-dependent, motor-predominant phenotype. J Neurol Neurosurg Psychiatr. 2021;93:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Douse CH, Bloor S, Liu Y, et al. Neuropathic MORC2 mutations perturb GHKL ATPase dimerization dynamics and epigenetic silencing by multiple structural mechanisms. Nat Commun. 2018;9:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Guillen Sacoto MJ, Tchasovnikarova IA, Torti E, et al. De Novo variants in the ATPase module of MORC2 cause a neurodevelopmental disorder with growth retardation and Variable craniofacial dysmorphism. Am J Hum Genet. 2020;107:352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Schottmann G, Wagner C, Seifert F, Stenzel W, Schuelke M. MORC2 Mutation causes severe spinal muscular atrophy-phenotype, cerebellar atrophy, and diaphragmatic paralysis. Brain. 2016;139:e70. [DOI] [PubMed] [Google Scholar]
- 107. Sun L, Wei N, Kuhle B, et al. CMT2N-causing Aminoacylation domain mutants enable Nrp1 interaction with AlaRS. Proc Natl Acad Sci USA. 2021;118:e2012898118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhao Z, Hashiguchi A, Hu J, et al. Alanyl-tRNA synthetase mutation in a family with dominant distal hereditary motor neuropathy. Neurology. 2012;78:1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mendoza-Ferreira N, Karakaya M, Cengiz N, et al. De Novo and inherited variants in GBF1 are associated with axonal neuropathy caused by Golgi fragmentation. Am J Hum Genet. 2020;107:763–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Høyer H, Braathen GJ, Busk ØL, et al. Genetic diagnosis of charcot-marie-tooth disease in a population by next-generation sequencing. Biomed Res Int. 2014;2014:210401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Choi BO, Kang SH, Hyun YS, et al. A complex phenotype of peripheral neuropathy, myopathy, hoarseness, and hearing loss is linked to an autosomal dominant mutation in MYH14. Hum Mutat. 2011;32:669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jacquier A, Theuriet J, Fontaine Fet al. Homozygous COQ7 mutation: a new cause of potentially treatable distal hereditary motor neuropathy. Brain. Published online 1 December 2022. 10.1093/brain/awac453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Urnavicius L, Zhang K, Diamant AG, et al. The structure of the dynactin complex and its interaction with dynein. Science. 2015;347:1441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sleigh JN, Rossor AM, Fellows AD, Tosolini AP, Schiavo G. Axonal transport and neurological disease. Nat Rev Neurol. 2019;15:691–703. [DOI] [PubMed] [Google Scholar]
- 115. Schiavo G, Greensmith L, Hafezparast M, Fisher EM. Cytoplasmic dynein heavy chain: The servant of many masters. Trends Neurosci. 2013;36:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Holzbaur EL, Scherer SS. Microtubules, axonal transport, and neuropathy. N Engl J Med. 2011;365:2330–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Amabile S, Jeffries L, McGrath JM, et al. DYNC1H1-related Disorders: A description of four new unrelated patients and a comprehensive review of previously reported variants. Am J Med Genet A. 2020;182:2049–2057. [DOI] [PubMed] [Google Scholar]
- 118. Willemsen MH, Vissers LE, Willemsen MA, et al. Mutations in DYNC1H1 cause severe intellectual disability with neuronal migration defects. J Med Genet. 2012;49:179–183. [DOI] [PubMed] [Google Scholar]
- 119. Rossor AM, Reilly MM. Neurogenic arthrogryposis and the power of phenotyping. Neuromuscul Disord. 2021;31:1062–1069. [DOI] [PubMed] [Google Scholar]
- 120. Martinez Carrera LA, Gabriel E, Donohoe CD, et al. Novel insights into SMALED2: BICD2 mutations increase microtubule stability and cause defects in axonal and NMJ development. Hum Mol Genet. 2018;27:1772–1784. [DOI] [PubMed] [Google Scholar]
- 121. Rossor AM, Sleigh JN, Groves M, et al. Loss of BICD2 in muscle drives motor neuron loss in a developmental form of spinal muscular atrophy. Acta Neuropathol Commun. 2020;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Koboldt DC, Waldrop MA, Wilson RK, Flanigan KM. The genotypic and phenotypic Spectrum of BICD2 variants in spinal muscular atrophy. Ann Neurol. 2020;87:487–496. [DOI] [PubMed] [Google Scholar]
- 123. Unger A, Dekomien G, Güttsches A, et al. Expanding the phenotype of BICD2 mutations toward skeletal muscle involvement. Neurology. 2016;87:2235–2243. [DOI] [PubMed] [Google Scholar]
- 124. Nam DE, Yoo DH, Choi SS, Choi BO, Chung KW. Wide phenotypic spectrum in axonal charcot-marie-tooth neuropathy type 2 patients with KIF5A mutations. Genes Genomics. 2018;40:77–84. [DOI] [PubMed] [Google Scholar]
- 125. Herrmann DN, Horvath R, Sowden JE, et al. Synaptotagmin 2 mutations cause an autosomal-dominant form of lambert-eaton myasthenic syndrome and nonprogressive motor neuropathy. Am J Hum Genet. 2014;95:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Montes-Chinea NI, Guan Z, Coutts M, et al. Identification of a new SYT2 variant validates an unusual distal motor neuropathy phenotype. Neurol Genet. 2018;4:e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Maystadt I, Rezsöhazy R, Barkats M, et al. The nuclear factor κB-activator gene PLEKHG5 is mutated in a form of autosomal recessive lower motor neuron disease with childhood onset. Am J Hum Genet. 2007;81:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Deschauer M, Hengel H, Rupprich K, et al. Bi-allelic truncating mutations in VWA1 cause neuromyopathy. Brain. 2021;144:574–583. [DOI] [PubMed] [Google Scholar]
- 129. Pagnamenta AT, Kaiyrzhanov R, Zou Y, et al. An ancestral 10-bp repeat expansion in VWA1 causes recessive hereditary motor neuropathy. Brain. 2021;144:584–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lowry RB, Sibbald B, Bedard T, Hall JG. Prevalence of multiple congenital contractures including arthrogryposis multiplex congenita in Alberta, Canada, and a strategy for classification and coding. Birth Defects Res A Clin Mol Teratol. 2010;88:1057–1061. [DOI] [PubMed] [Google Scholar]
- 131. Hall JG. Arthrogryposis (multiple congenital contractures): Diagnostic approach to etiology, classification, genetics, and general principles. Eur J Med Genet. 2014;57:464–472. [DOI] [PubMed] [Google Scholar]
- 132. Hall JG, Aldinger KA, Tanaka KI. Amyoplasia revisited. Am J Med Genet A. 2014;164A:700–730. [DOI] [PubMed] [Google Scholar]
- 133. Laquerriere A, Jaber D, Abiusi E, et al. Phenotypic spectrum and genomics of undiagnosed arthrogryposis multiplex congenita. J Med Genet. 2021;59:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ambegaonkar G, Manzur AY, Robb SA, Kinali M, Muntoni F. The multiple phenotypes of arthrogryposis multiplex congenita with reference to the neurogenic variant. Eur J Paediatr Neurol. 2011;15:316–319. [DOI] [PubMed] [Google Scholar]
- 135. Beecroft SJ, Lombard M, Mowat D, et al. Genetics of neuromuscular fetal akinesia in the genomics era. J Med Genet. 2018;55:505–514. [DOI] [PubMed] [Google Scholar]
- 136. Tan QK, McConkie-Rosell A, Juusola J, et al. The importance of managing the patient and not the gene: Expanded phenotype of GLE1-associated arthrogryposis. Cold Spring Harb Mol Case Stud. 2017;3:a002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hall JG. Amyoplasia involving only the upper limbs or only involving the lower limbs with review of the relevant differential diagnoses. Am J Med Genet A. 2014;164A:859–873. [DOI] [PubMed] [Google Scholar]
- 138. Orban P, Devon RS, Hayden MR, Leavitt BR. Chapter 15 juvenile amyotrophic lateral sclerosis. In: Eisen AA and Shaw PJ, editors. Handbook of clinical neurology: Elsevier; 2007. p 301–312. [DOI] [PubMed] [Google Scholar]
- 139. Brooks BR, Miller RG, Swash M, Munsat TL. El escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 140. Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162–172. [DOI] [PubMed] [Google Scholar]
- 141. Boekestein WA, Kleine BU, Hageman G, Schelhaas HJ, Zwarts MJ. Sensitivity and specificity of the ‘Awaji’ electrodiagnostic criteria for amyotrophic lateral sclerosis: Retrospective comparison of the awaji and revised el escorial criteria for ALS. Amyotroph Lateral Scler. 2010;11:497–501. [DOI] [PubMed] [Google Scholar]
- 142. Bäumer D, Hilton D, Paine SM, et al. Juvenile ALS with basophilic inclusions is a FUS proteinopathy with FUS mutations. Neurology. 2010;75:611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zou Z-Y, Liu M-S, Li X-G, Cui L-Y. Mutations in FUS are the most frequent genetic cause in juvenile sporadic ALS patients of Chinese origin. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:249–252. [DOI] [PubMed] [Google Scholar]
- 144. Picher-Martel V, Brunet F, Dupré N, Chrestian N. The occurrence of FUS mutations in pediatric amyotrophic lateral sclerosis: A case report and review of the literature. J Child Neurol. 2020;35:556–562. [DOI] [PubMed] [Google Scholar]
- 145. Dodd KC, Power R, Ealing J, Hamdalla H. FUS-ALS presenting with myoclonic jerks in a 17-year-old man. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:278–280. [DOI] [PubMed] [Google Scholar]
- 146. Johnson JO, Chia R, Miller DE, et al. Association of variants in the SPTLC1 gene with juvenile amyotrophic lateral sclerosis. JAMA Neurol. 2021;78:1236–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Mohassel P, Donkervoort S, Lone MA, et al. Childhood amyotrophic lateral sclerosis caused by excess sphingolipid synthesis. Nat Med. 2021;27:1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Syeda S, Donkervoort S, Mohassel P, et al. SPTLC2 as a cause of childhood-onset amyotrophic lateral sclerosis. In: WMS2021 Virtual Congress. Neuromuscul Disord. 2021;31(suppl 1):LBP.25. 10.1016/S0960-8966(21)00642-8 [DOI]
- 149. Al-Saif A, Al-Mohanna F, Bohlega S. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol. 2011;70:913–919. [DOI] [PubMed] [Google Scholar]
- 150. Naruse H, Ishiura H, Mitsui J, et al. Juvenile amyotrophic lateral sclerosis with complex phenotypes associated with novel SYNE1 mutations. Amyotroph Lateral Scler Frontotemporal Degener. 2021;22(7–8):576–578. [DOI] [PubMed] [Google Scholar]
- 151. Kim HJ, Mohassel P, Donkervoort S, et al. Heterozygous frameshift variants in HNRNPA2B1 cause early-onset oculopharyngeal muscular dystrophy. Nat Commun. 2022;13:2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kim HJ, Kim NC, Wang YD, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Beijer D, Kim HJ, Guo L, et al. Characterization of HNRNPA1 mutations defines diversity in pathogenic mechanisms and clinical presentation. JCI Insight. 2021;6:e148363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Grosz BR, Stevanovski I, Negri S, et al. Long read sequencing overcomes challenges in the diagnosis of SORD neuropathy. J Peripher Nerv Syst. 2022;27:120–126. [DOI] [PubMed] [Google Scholar]
- 155. Astrea G, Morrow JM, Manzur A, et al. Muscle “islands”: An MRI signature distinguishing neurogenic from myopathic causes of early onset distal weakness. Neuromuscul Disord. 2021;32:142–149. [DOI] [PubMed] [Google Scholar]
- 156. Shinwari JM, Khan A, Awad S, et al. Recessive mutations in COL25A1 are a cause of congenital cranial dysinnervation disorder. Am J Hum Genet. 2015;96:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Tanaka T, Wakabayashi T, Oizumi H, et al. CLAC-P/collagen type XXV is required for the intramuscular innervation of motoneurons during neuromuscular development. J Neurosci. 2014;34:1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.