Abstract
The biological definition of Alzheimer’s disease using CSF biomarkers requires abnormal levels of both amyloid (A) and tau (T). However, biomarkers and corresponding cutoffs may not always reflect the presence or absence of pathology. Previous studies suggest that up to 32% of individuals with autopsy-confirmed Alzheimer’s disease show normal CSF p-tau levels in vivo, but these studies are sparse and had small sample sizes. Therefore, in three independent autopsy cohorts, we studied whether or not CSF A+T− excluded Alzheimer’s disease based on autopsy.
We included 215 individuals, for whom ante-mortem CSF collection and autopsy had been performed, from three cohorts: (i) the Amsterdam Dementia Cohort (ADC) [n = 80, 37 (46%) Alzheimer’s disease at autopsy, time between CSF collection and death 4.5 ± 2.9 years]; (ii) the Antwerp Dementia Cohort (DEM) [n = 92, 84 (91%) Alzheimer’s disease at autopsy, time CSF collection to death 1.7 ± 2.3 years]; and (iii) the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [n = 43, 31 (72%) Alzheimer’s disease at autopsy, time CSF collection to death 5.1 ± 2.5 years]. Biomarker profiles were based on dichotomized CSF Aβ1-42 and p-tau levels. The accuracy of CSF AT profiles to detect autopsy-confirmed Alzheimer’s disease was assessed. Lastly, we investigated whether the concordance of AT profiles with autopsy diagnosis improved when CSF was collected closer to death in 9 (10%) DEM and 30 (70%) ADNI individuals with repeated CSF measurements available.
In total, 50–73% of A+T− individuals and 100% of A+T+ individuals had Alzheimer’s disease at autopsy. Amyloid status showed the highest accuracy to detect autopsy-confirmed Alzheimer’s disease (accuracy, sensitivity and specificity in the ADC: 88%, 92% and 84%; in the DEM: 87%, 94% and 12%; and in the ADNI cohort: 86%, 90% and 75%, respectively). The addition of CSF p-tau did not further improve these estimates. We observed no differences in demographics or degree of Alzheimer’s disease neuropathology between A+T− and A+T+ individuals with autopsy-confirmed Alzheimer’s disease. All individuals with repeated CSF measurements remained stable in Aβ1-42 status during follow-up. None of the Alzheimer’s disease individuals with a normal p-tau status changed to abnormal; however, four (44%) DEM individuals and two (7%) ADNI individuals changed from abnormal to normal p-tau status over time, and all had Alzheimer’s disease at autopsy.
In summary, we found that up to 73% of A+T− individuals had Alzheimer’s disease at autopsy. This should be taken into account in both research and clinical settings.
Keywords: Alzheimer’s disease, AT(N), CSF, biomarker, autopsy
The biological definition of Alzheimer’s disease using CSF biomarkers requires both abnormal amyloid (A) and tau (T) levels. However, in a large multicentre cohort, Vromen et al. found that up to 73% of A+T− individuals had Alzheimer’s disease at autopsy, with implications for both research and clinical settings.
Introduction
Recently, the National Institute on Aging and Alzheimer’s Association (NIA-AA) introduced a research framework to biologically define Alzheimer’s disease (AD) based on biomarkers, rather than clinical symptoms.1 This biological definition for AD requires abnormal biomarkers for both amyloid (A) and tau (T), which can be measured in CSF with amyloid-β1-42 (Aβ1-42) and phosphorylated tau (p-tau), by PET imaging or in blood. Previous criteria using biomarker evidence for AD required at least an abnormal amyloid marker, regardless of the tau measure.2 The AT classification system assumes that the combination of both abnormal amyloid and tau biomarkers most accurately reflects the presence of AD pathology, and that individuals who have only abnormal amyloid do not have AD but instead have Alzheimer’s pathological change.
However, this approach is potentially problematic in two ways. First, it requires the dichotomization of amyloid and tau biomarkers. Ideally, cutoff values for dichotomization should be determined against the gold standard of autopsy-proven diagnoses. However, only a few studies have done this, since pathological data are scarce. As an alternative, cutoff points have often been determined based on distinguishing between clinical diagnosis of AD dementia versus controls,3,4 which may not accurately reflect the presence of neuropathological changes.1 This introduces uncertainty in determining the AT status of individuals. Secondly, one previous study reported that up to 32% of individuals with autopsy-confirmed AD can show normal p-tau levels in CSF in vivo,5 which suggests that the criteria requiring both abnormal amyloid and abnormal tau status may be too strict and would miss a substantial number of individuals with AD neuropathology who have only abnormal amyloid. Still, the literature on the association between ante-mortem CSF biomarker status and post-mortem verified AD is sparse. Previous studies investigating biomarker accuracy in the context of autopsy-confirmed AD were often limited in sample size, did not include clinical diagnoses other than AD or only investigated single biomarkers and not combined markers in the context of the AT(N) framework.5–7
In this study, using three independent cohorts, we investigated whether individuals with abnormal CSF amyloid and normal p-tau levels (i.e. A+T− biomarker profile) had AD at autopsy and whether CSF p-tau status improved detection of autopsy-confirmed AD over CSF amyloid status alone. In addition, for individuals with repeated CSF measurements available, we investigated whether the concordance of CSF amyloid and tau status with autopsy diagnosis improved when CSF was collected closer to death.
Materials and methods
Participants and cohorts
We selected individuals from the Amsterdam Dementia Cohort (ADC), the Antwerp Dementia Cohort (DEM) and the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu) who had CSF Aβ1-42and p-tau measurements available at first visit and subsequent post-mortem neuropathological examination. The institutional review boards of all participating institutions approved the procedures for this study. Written informed consent was obtained from all participants and/or their legal representatives. The ADC is comprised of individuals who visited the Alzheimer Center of the Amsterdam UMC, location VUmc.8,9 Individuals visiting the memory clinic receive an extensive diagnostic workup for dementia, of which the results are discussed in weekly multidisciplinary meetings in order to reach a consensus clinical diagnosis according to the diagnostic and research guidelines of all major neurodegenerative diseases.10–21 In the ADC, patients were selected regardless of clinical diagnosis. The DEM was selected from the Institute Born-Bunge Neurobiobank based on a neuropathological diagnosis of AD. Patients were recruited through the Memory Clinic of Hospital Network Antwerp (ZNA) Middelheim and Hoge Beuken and through other centres referring to the Neurobiobank of the Institute Born-Bunge.22,23 ADNI is a public-private partnership led by Principal Investigator Michael W. Weiner MD and was started in 2003. ADNI’s main aim is to validate AD biomarkers and investigate the use of these biomarkers in combination with clinical and neuropsychological examination to assess the clinical progression of AD individuals with mild cognitive impairment or early dementia. For up-to-date information, see www.adni-info.org.
CSF biomarker analyses
Lumbar puncture was performed using a standardized protocol with a small-bore atraumatic needle, and CSF was collected in sterile polypropylene collection tubes.24 The mean time between CSF collection and death was 4.5 ± 2.9 years in the ADC, 1.7 ± 2.3 years in the DEM and 5.1 ± 2.5 years in the ADNI cohort. For both the ADC and DEM, Aβ1-42 and p-tau concentrations were determined using enzyme-linked immunosorbent assays (ELISAs) [Innotest® β-AMYLOID(1-42) and Innotest PHOSPHO-TAU(181P), Fujirebio]. For the ADC, a drift-corrected cutoff of <813 pg/ml was applied to determine Aβ1-42 abnormality,25 and the clinically-validated cutoff of >52 pg/ml was applied to determine p-tau abnormality.4 For the DEM, the cutoffs were <638.5 pg/ml and >56.5 pg/ml, respectively (autopsy-based).26 For the ADNI cohort, Aβ1-42 and p-tau levels were analysed using the Roche Elecsys immunoassay platform as described previously.27,28 A cutoff of <977 pg/ml was applied to determine Aβ1-42 abnormality and >24 pg/ml for p-tau abnormality (clinically-validated cutoffs).29 Biomarker profiles were based on dichotomized CSF Aβ1-42 with p-tau (AT), resulting in four possible combinations for each profile: A−T−, A+T−, A−T+ and A+T+. For the ADC, a cutoff of >0.08 pg/ml was used to determine abnormality of the ratio of Aβ1-42/p-tau,6 and in the ADNI cohort, the cutoff was >0.025 pg/ml.29
Neuropathological examination
Neuropathological assessment of AD was performed according to the NIA-AA guidelines.30,31 The ABC scoring system was used to describe AD neuropathological changes. The A score is based on the location of Aβ deposition (plaques) according to Thal phases 0 to 5,32,33 which are converted to an A score of 0 (phase 0), 1 (phase 1 and 2), 2 (phase 3) or 3 (phase 4 and 5). The B score is based on the location of neurofibrillary tangles according to Braak stages 0–VI,34,35 which are converted to a B score of 0 (phase 0), 1 (phase 1 and 2), 2 (phase 3 and 4) or 3 (phase 5 and 6). The C score is based on the density of neuritic plaques according to the CERAD stages,31,36 which is converted to a C score of 0 (none), 1 (sparse), 2 (moderate) or 3 (frequent). Based on this score a diagnosis was given of ‘no AD’ or ‘low’, ‘intermediate’ or ‘high’ AD neuropathologic change. For a small subset of ADC participants (n = 13, 16%) criteria from the National Institute of Aging and Reagan Institute for the neuropathological assessment of AD were used.37 For our analyses both ‘intermediate’ and ‘high’ AD neuropathologic change scores were considered as pathologically confirmed AD, while ‘low’ AD neuropathologic change scores were considered as absence of pathologically confirmed AD. In the ADC, of all individuals with available neuropathological diagnosis, a subset of 39 (49%) individuals had all A, B and C scores available. In the DEM, eight patients were found to have an ‘ABC’ score that would not be considered sufficient to explain dementia (i.e. seven had a score of ‘low’ AD neuropathologic change and one a score of ‘no AD’) but were diagnosed with definite AD by the exclusion of all other causes based on neuropathology.
Statistical analysis
Baseline demographic features were analyzed and compared using χ2 tests, unpaired t-tests and Mann–Whitney U-tests, depending on type and distribution of the variable. We assessed the concordance, diagnostic accuracy, i.e. the proportion of correctly classified subjects [true positive (TP) + true negative (TN)] among all subjects (TP + TN + FP + FN), sensitivity and specificity of AT profiles to detect autopsy-confirmed AD. In order to analyse the additional value of p-tau to CSF Aβ1-42 status, analyses were performed between all A+ and all A− individuals and between A+T+ individuals and individuals with other AT profiles. We used Spearman’s rho (ρ) to determine correlations between CSF amyloid and p-tau levels with pathological scores A, B and C. We investigated differences in clinical diagnosis, demographic characteristics and in degree of AD neuropathology between A+T+ and A+T− individuals with and without autopsy-confirmed AD. Finally, a subset of individuals from the DEM (n = 9) and the ADNI cohort (n = 30) had repeated CSF measurements available (mean time between first and last CSF measurement 2.1 ± 1.8 and 2.9 ± 1.7 years, respectively), and for these we investigated changes in CSF Aβ1-42 and p-tau status over time and whether these were in concordance with their autopsy diagnosis. All analyses were performed using R version 3.6.1—‘Action of the Toes’.38
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data from the ADC and DEM are not publicly available due to privacy restrictions. ADNI data can be requested through their website.
Results
Cohort demographics
In total, we included 215 individuals with CSF collection and autopsy from the ADC [n = 80, median (IQR) age 62 (58–66) years, 29 (36%) female, 37 (46%) with AD at autopsy], the DEM [n = 92, median (IQR) age 76 (71–84) years, 45 (49%) female, 84 (91%) with AD at autopsy] and the ADNI cohort [n = 43, median (IQR) age 78 (73–83) years, 9 (21%) female, 31 (72%) with AD at autopsy] (Tables 1 and 2). The ADC individuals with AD at autopsy were older at time of death [median (IQR): 69 (66–74) years versus 65 (61–69) years, P = 0.004], more likely to be diagnosed with AD dementia (P < 0.001) and had a longer period between CSF collection and death [median (IQR) 5.2 (3.1–7.4) versus 3 (1.8–5.6) years, P = 0.006] compared with ADC individuals without AD at autopsy. The DEM individuals with AD at autopsy did not show differences in these characteristics from those without AD at autopsy. ADNI autopsy-confirmed AD individuals more frequently carried an APOE ε4 allele compared with those without AD at autopsy [21 (68%) versus 0 (0%), P = 0.001].
Table 1.
Cohort demographics
| Total | ADC | DEM | ADNI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ADC | DEM | ADNI | No AD | AD | No AD | AD | No AD | AD | |
| n | 80 | 92 | 43 | 43 | 37 | 8 | 84 | 12 | 31 |
| Demographics | |||||||||
| Sex (female) | 29 (36%) | 45 (49%) | 9 (21%) | 17 (40%) | 12 (32%) | 6 (75%) | 39 (46.4%) | 2 (16.7%) | 7 (23%) |
| Age at baseline, years | 62 (58–66) | 76 (71–84) | 78 (73–83) | 61 (56–65) | 63 (59–68) | 81 (77–83) | 76 (71–84) | 80 (76–83) | 77 (73–83) |
| Age at death, years | 67 (64–70) | 78 (73–86) | 83 (78–86) | 65 (61–69) | 69 (66–74)** | 83 (78–85) | 77 (72–86) | 84 (81–86) | 81 (77–86) |
| Time between CSF collection and death, years | 4 (2–6.1) | 0.5 (0.1–2.8) | 4.9 (3.1–7.1) | 3 (1.8–5.6) | 5.2 (3.1–7.4)** | 0.1 (0.1–1.6) | 0.6 (0.1–2.8) | 4.5 (3.5–6.7) | 5 (2.7–7.1) |
| APOE ε4 carrier | 36 (51%) | 35/64 (55%) | 21 (49%) | 16 (43%) | 20 (61%) | 1/4 (25%) | 34/60 (56.7%) | 0 (0%) | 21 (68%)*** |
| Clinical diagnosis | n = 7 | n = 74 | |||||||
| Cognitively normal | 3 (4%) | 0 | 7 (16%) | 1 (2%) | 2 (5%) | 0 | 0 | 4 (33.3%) | 3 (10%) |
| Mild cognitive impairment | 3 (4%) | 2 (2.5%) | 21 (49%) | 1 (2%) | 2 (5%) | 0 (0%) | 2 (2.7%) | 8 (66.7%) | 13 (42%) |
| AD dementia | 28 (35%) | 53 (65.4%) | 15 (35%) | 3 (7%) | 25 (68%)*** | 2 (28.6%) | 51 (68.9%) | 0 (0%) | 15 (48%)* |
| Non-AD dementia | 35 (44%)a | 14 (17.3%)b | — | 30 (70%) | 5 (14%)*** | 5 (71.4%) | 9 (12.2%)** | — | — |
| Other | 11 (14%)c | 12 (14.8%)d | — | 8 (19%) | 3 (8%) | 0 (0%) | 12 (16.2%) | — | — |
Cohort demographics of the ADC, DEM and ADNI cohort with comparisons made between the three cohorts and stratified for presence of pathological AD at autopsy. Results presented in n (%) or median (IQR).
Including dementia with Lewy bodies (no AD n = 1; AD n = 2), frontotemporal lobar degeneration (no AD n = 19; AD n = 2), primary progressive aphasia (no AD n = 2), vascular dementia (no AD n = 1) and other dementias (no AD n = 7; AD n = 1).
Including dementia with Lewy bodies (AD n = 1), frontotemporal lobar degeneration spectrum (no AD n = 1, AD n = 6), vascular dementia (no AD n = 1), Creutzfeldt-Jakob disease (no AD n = 2, AD n = 1), Parkinson's disease (no AD n = 1) and Charles Bonnet syndrome (AD n = 1).
Diagnosis either psychiatric disorder, neurologic non-neurodegenerative disorder or postponed diagnosis due to clinical uncertainty.
Diagnosis either mixed dementia (n = 3), unspecified dementia (n = 2) or clinical doubt between AD dementia and non-AD dementia (n = 7).
*** P < 0.001, ** P < 0.01, * P < 0.05 for AD versus no AD within cohort.
Table 2.
Cohort biomarker concentrations
| ADC | DEM | ADNI | ||||
|---|---|---|---|---|---|---|
| no AD | AD | no AD | AD | no AD | AD | |
| n | 43 | 37 | 8 | 84 | 12 | 31 |
| Aβ1-42 pg/ml | 977 (861–1143) | 640 (561–752) | 475 (332–583) | 442 (306–495) | 1275 (1068–1703) | 594 (445–726) |
| ȃabnormal | 7 (16%) | 34 (92%) | 7 (87.5%) | 79 (94%) | 3 (25%) | 28 (90%) |
| P-tau pg/ml | 39 (32–44) | 78 (60–100) | 48 (39–54) | 75 (57–97) | 18 (15–22) | 36 (26–44) |
| ȃabnormal | 6 (14%) | 29 (78%) | 0 (0%) | 63 (75%) | 3 (25%) | 25 (81%) |
| T-tau pg/ml | 340 (258–449) | 594 (453–830) | 427 (302–796) | 584 (375–1003) | 198 (171–252) | 330 (275–453) |
| ȃabnormal | 17 (40%) | 31 (84%) | 6 (75%) | 71 (84.5%) | 2 (17%) | 25 (81%) |
Baseline biomarker concentrations in the ADC, DEM and ADNI cohort, stratified for presence of pathological AD at autopsy. Results presented in median (IQR) or n (%). For ADC and ADNI all P ≤ 0.002. For DEM p-tau all P ≤ 0.001, for DEM Aβ1-42 and t-tau all P not significant.
Concordance of AT and AN with Alzheimer’s disease pathology
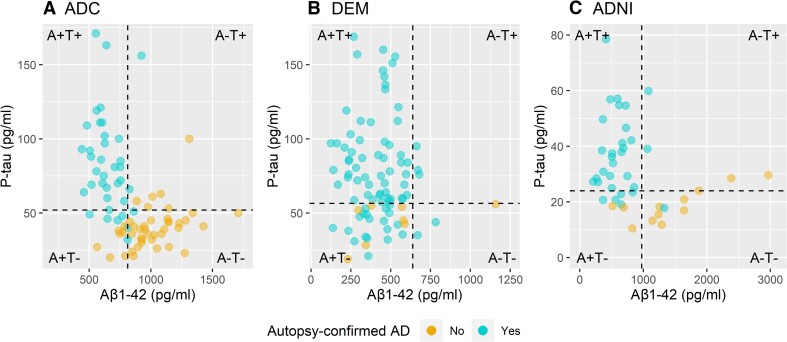
First, we investigated the concordance of the CSF AT profiles with the neuropathological diagnosis of AD. Of individuals with an A+T− profile, in the ADC 7/14 (50%) had AD at autopsy, in the DEM 19/26 (73%) and in the ADNI cohort 5/8 (63%). All individuals with an A+T+ profile had AD at autopsy (n = 27 in the ADC, n = 60 in the DEM, n = 23 in the ADNI cohort) (Fig. 1). Overall, amyloid status by itself showed the highest accuracy to detect the pathological diagnosis of AD (Table 3). When using only p-tau, accuracy decreased compared with using only amyloid status (respectively, 0.77–0.82 compared with 0.86–0.88); however, given the overlapping 95% confidence interval, the superiority of the amyloid biomarker should be taken with caution. Compared with amyloid alone, the combination of amyloid and p-tau did not improve the accuracy to detect the pathological diagnosis of AD. A+T+ status showed a higher specificity but also lower sensitivity compared with amyloid status alone. Using the ratio of p-tau/Aβ1-42in the ADC and the ADNI cohort resulted in the highest accuracy to detect pathological AD.
Figure 1.
Neuropathological confirmation of AD and biomarker AT classification. (A) ADC, (B) DEM and (C) ADNI. The dotted vertical lines correspond to the Aβ1-42 cutoffs of <813 pg/ml for ADC, <638.5 pg/ml for DEM and <977 pg/ml for ADNI. The dotted horizontal lines correspond to the p-tau cutoffs of >52 pg/ml for ADC, >56.5 pg/ml for DEM and >24 pg/ml for ADNI.
Table 3.
Accuracy of CSF biomarkers for each cohort
| ADC | DEM | ADNI | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSF biomarker | TP | FN | FP | TN | A (95% CI) | Se | Sp | TP | FN | FP | TN | A (95% CI) | Se | Sp | TP | FN | FP | TN | A (95% CI) | Se | Sp |
| Aβ1-42 status | 34 | 3 | 7 | 36 | 0.88 (0.78–0.94) | 0.92 | 0.84 | 79 | 5 | 7 | 1 | 0.87 (0.78–0.93) | 0.94 | 0.12 | 28 | 3 | 3 | 9 | 0.86 (0.72–0.95) | 0.9 | 0.75 |
| P-tau status | 29 | 8 | 6 | 37 | 0.82 (0.72–0.9) | 0.78 | 0.86 | 63 | 21 | 0 | 8 | 0.77 (0.67–0.85) | 0.75 | 1 | 25 | 6 | 3 | 9 | 0.79 (0.64–0.9) | 0.81 | 0.75 |
| Aβ1-42 and p-tau status | 27 | 10 | 0 | 43 | 0.88 (0.78–0.94) | 0.73 | 1 | 60 | 24 | 0 | 8 | 0.74 (0.64–0.83) | 0.71 | 1 | 23 | 8 | 0 | 12 | 0.81 (0.67–0.92) | 0.74 | 1 |
| P-tau/Aβ1-42 ratio | 28 | 9 | 0 | 43 | 0.89 (0.8–0.95) | 0.76 | 1 | 30 | 1 | 2 | 10 | 0.93 (0.81–0.99) | 0.97 | 0.83 | |||||||
A = Accuracy; CI = confidence interval; FN = false negative; FP = false positive; Se = sensitivity; Sp = specificity; TN = true negative; TP = true positive.
Next, we studied relationships between CSF Aβ1-42 and p-tau levels with separate pathological scores (ABC). In the subset of the ADC with available pathological scores (n = 39, 49%), lower Aβ1-42 and higher p-tau levels were strongly correlated with higher pathological scores A, B and C (all P < 0.001) (Supplementary Table 1). In the ADNI cohort, the correlations between Aβ1-42 and p-tau markers and pathological scores were similar to those of the ADC, albeit slightly weaker (all P < 0.001), and in the DEM only higher p-tau levels were correlated with higher pathological scores (all P ≤ 0.01).
Comparison of A+T+ and A+T– individuals
We next studied if A+T− individuals with autopsy-confirmed AD differed in their clinical diagnosis, demographic characteristics or degree of AD neuropathology from A+T+ individuals with autopsy-confirmed AD (Supplementary Table 2). We did not find any differences, but this may also reflect that the group sizes were small. On a trend level, A+T− individuals with autopsy-confirmed AD were more often diagnosed with non-AD dementia, including dementia with Lewy bodies and frontotemporal dementia, compared to A+T+ individuals. Within the A+T− group, individuals with autopsy-confirmed AD did not differ from those with another autopsy diagnosis in terms of clinical and demographical information. In the ADC and the ADNI cohort, A+T− individuals without AD at autopsy had lower neuropathological B and C scores, and in the DEM they had lower A and B scores compared with A+T− individuals with AD at autopsy.
Stability over time in AT classification
For a subset of 9 (10%) DEM and 30 (70%) ADNI individuals with repeated CSF measurements (mean ± SD time between first and last measurement 2.1 ± 1.8 and 2.9 ± 1.7 years, respectively), we investigated the stability of AT profiles over time. Over the repeated measurements, all individuals showed a stable Aβ1-42 status. None changed from A+T− to A+T+. In the DEM, four individuals changed from A+T+ to A+T− over time (all pathological diagnosis of AD and clinical diagnosis of dementia at first CSF measurement). In the ADNI cohort, the majority of individuals showed a stable tau status [n = 28 (93%)]. Two individuals changed from A+T+ to A+T− (all pathological diagnosis of AD, one with clinical diagnosis of dementia at first CSF measurements and one with mild cognitive impairment at first measurement and progression to dementia at last CSF measurement).
Discussion
In the current biomarker-based definition of AD, individuals with an A+T− profile are considered to have ‘AD pathologic change’, but not AD, with the suggestion that a T− profile reflects a lack of tangles. However, biomarkers and their corresponding cutoffs may not always accurately reflect the presence or absence of pathology, and here we studied whether a CSF biomarker A+T− profile indeed excluded AD based on autopsy. We found that up to 73% of A+T− individuals did have AD at autopsy. Furthermore, we found that CSF amyloid abnormality (i.e. A+ status), irrespective of p-tau abnormality (i.e. T+ status), showed the highest accuracy to detect a neuropathological diagnosis of AD. Adding p-tau increased the specificity, but at the cost of decreased sensitivity over amyloid status by itself. Our results imply that a biomarker profile with abnormal CSF amyloid and normal p-tau levels does not exclude the presence of AD at autopsy.
In our study, 19 to 25% of all autopsy-confirmed AD individuals showed normal CSF p-tau levels in vivo (while Aβ1-42 was abnormal), which is in line with previously reported sensitivities of p-tau to detect AD pathology, which range between 66 and 85% (see Supplementary material). These previous studies5,6,22,39–44 also show that Aβ1-42by itself is the most sensitive predictor for the post-mortem pathological diagnosis of AD. In the current AD research framework, these A+T− individuals would be considered to show AD pathological change and might be incorrectly labelled as not having AD (at time of measurement). For research purposes, it may be preferable to label these cases as biological AD, since this profile does not exclude pathological AD and may represent a distinct subtype of AD with possibly different biological characteristics. Alternatively, the ratio of p-tau/Aβ1-42 may be used for the diagnosis of AD, since our study showed that the application of this ratio improved accuracy compared with amyloid status alone. Still, in the ADNI cohort, but not in the ADC, this ratio was more likely to be abnormal in A+T− individuals with autopsy-confirmed AD. While using only the amyloid marker resulted in a lower specificity, nearly all A+ cases without pathologically-confirmed AD did show some degree of plaques and/or tangles, indicating that these individuals too could potentially benefit from amyloid and tau modifying treatments. It should be noted that depending on the research goal, sensitivity and specificity should be weighed up, and when the intention is to reduce false positives, A+T+ is preferred over amyloid status alone.
One explanation of negative tau markers in individuals with pathologically-confirmed AD could be that CSF p-tau levels may rise later. However, our comparison of last known AT profiles before death to first AT profile measurements showed that the majority of individuals remained stable, and those who changed, changed from T+ to T−, even though they had AD at autopsy. This suggests that the diagnostic value of CSF tau status varies depending on the clinical stage of AD, with lower accuracy later in the disease.45 Possibly, the change from T+ to T− closer to autopsy may reflect a change in tau metabolism, as Mattsson-Carlgren et al.46 showed that altered tau metabolism and subsequent CSF p-tau increases occur early in AD pathogenesis, preceding tau deposition. Together with our findings, this suggests that in vivo CSF p-tau measures may reflect alterations in other processes in response to amyloid and in addition to the presence of insoluble tau aggregates. Recent research has shown that tau is physiologically secreted47 and that tau production is enhanced by amyloid pathology.48 Possibly, individuals with A+T− profiles have physiologically low tau processing, which may result in low CSF p-tau levels despite the presence of amyloid and tangle pathology. A recent CSF proteomics study identified different AD subtypes of which one, characterized by blood–brain barrier dysfunction and hypoplasticity, showed lower p-tau and t-tau levels.49 This has also been reported in a combined proteomics and genetic study by Visser.50 For individuals with an A+T− profiles but without AD at autopsy, it could be possible that all biomarkers are lower due to a CSF flow issue, as is frequently seen in individuals with hydrocephalus.51 However, these studies49,50 also noted specific proteins to be increased, including e.g. neurofilament light.
Our results showing normal tau profiles in AD urge us to re-examine the definition of T status when based on CSF in AD. Possibly, CSF p-tau cutoffs need recalibration, as in our study some AD individuals with A+T− profiles had CSF p-tau concentrations close to the cutoffs, and not all cutoffs were validated on autopsy-confirmed cases. Still, in general dichotomous cutoffs will never be 100% accurate, and a recent study showed that CSF tau shows four subgroups rather than a bimodal distribution.52 Cutoffs allowing for a grey zone may therefore have added value for the diagnosis of AD.
A potential limitation of this study is that sample sizes remained relatively small per cohort, which may have resulted in limited statistical power for subgroup comparisons on clinical and demographical characteristics. Furthermore, the small sample sizes may have attributed to overlapping 95% confidence intervals of biomarker accuracies, and so superiority of the CSF amyloid biomarker over CSF p-tau should be interpreted with caution. Regardless, we were able to replicate our main results in three independent cohorts. Future studies with larger samples of both in vivo AD biomarkers and post-mortem autopsy are needed in order to better investigate differences between A+T+ and A+T− AD subgroups. Another potential limitation is that the DEM was selected based on a neuropathological diagnosis of AD according to the pathologists’ conclusion and contained only a limited number of individuals without AD according to our criteria, thus evoking the risk of selection bias. Out of eight DEM individuals that did not fulfill our criteria for pathological AD, seven had ‘low’ AD pathological change in combination with an abnormal CSF amyloid marker and no other neuropathological diagnosis, which might explain the low specificity found for CSF amyloid in this cohort. Clinically these individuals were often diagnosed with non-AD dementia, and so these individuals may represent atypical AD cases or clinically mild AD cases with comorbidities affecting cognition. Finally, we studied AT definitions based on CSF markers only, and so it remains unclear whether these results generalize to other methods to measure amyloid and tau, including plasma p-tau markers and tau PET. Studies have shown that both CSF and plasma p-tau markers become abnormal earlier than tau PET53,54 and are rather markers in response to early amyloidosis and/or altered tau metabolism,45 while tau PET correlates better with tangle pathology. On the other hand, tau PET becomes abnormal only in more advanced disease stages when more widespread tau pathology is present55,56 and may therefore be less sensitive to earlier pathological changes. Different T markers seem to (partially) provide independent information, and so in order to choose the best fitting definition for T for each study, these biomarker properties need to be taken into account. Future studies should further aim to include all biomarker modalities to investigate and directly compare their accuracy to detect pathology in autopsy-confirmed studies. A strength of this study is that this is one of few studies that also had autopsy confirmation for individuals with intact cognition as well as with other clinical non-AD dementia diagnoses including dementia with Lewy bodies and frontotemporal dementia. Furthermore, since the cohorts used two different assay platforms, we have been able to determine that our results reflect general processes, rather than peculiarities of a specific assay.
In conclusion, our results indicate that normal CSF p-tau levels in combination with an abnormal amyloid marker do not exclude the presence of AD at autopsy. This should be taken into account in both research and clinical settings.
Supplementary Material
Acknowledgements
Data used in preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf. This research was performed at the Amsterdam UMC Alzheimer Center, which is part of the neurodegeneration research program of Amsterdam Neuroscience (www.amsterdamresearch.org). The Alzheimer Center Amsterdam is supported by Stichting Alzheimer Nederland and Stichting VUmc fonds. The clinical database structure was developed with funding from Stichting Dioraphte. The Antwerp Dementia Cohort was supported by Institute Born-Bunge and the Special Research Fund of UAntwerp. Data collection and sharing for this project was funded by the ADNI (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson; Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Contributor Information
Eleonora M Vromen, Alzheimer Center Amsterdam, Amsterdam Neuroscience, VUMC, Amsterdam, The Netherlands; Department of Neurodegeneration, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Sterre C M de Boer, Alzheimer Center Amsterdam, Amsterdam Neuroscience, VUMC, Amsterdam, The Netherlands; Department of Neurodegeneration, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Charlotte E Teunissen, Department of Clinical Chemistry, Neurochemistry Lab and Biobank, Amsterdam Neuroscience, VUMC, Amsterdam, The Netherlands.
Annemieke Rozemuller, Department of Pathology, Amsterdam Neuroscience, Amsterdam UMC, Amsterdam, The Netherlands.
Anne Sieben, Institute Born-Bunge, University of Antwerp, Wilrijk, Belgium.
Maria Bjerke, Clinical Neurochemistry Laboratory, Department of Clinical Biology, Universitair Ziekenhuis Brussel and Center for Neurosciences (C4N), Vrije Universiteit Brussel (VUB), Brussels, Belgium; Department of Biomedical Sciences, University of Antwerp, Antwerp, Belgium.
Pieter Jelle Visser, Alzheimer Center Amsterdam, Amsterdam Neuroscience, VUMC, Amsterdam, The Netherlands; Department of Neurodegeneration, Amsterdam Neuroscience, Amsterdam, The Netherlands; Department of Psychiatry, Maastricht University, Maastricht, The Netherlands; Department of Neurobiology, Care Sciences and Society, Division of Neurogeriatrics, Karolinska Institutet, Stockholm, Sweden.
Femke H Bouwman, Alzheimer Center Amsterdam, Amsterdam Neuroscience, VUMC, Amsterdam, The Netherlands; Department of Neurodegeneration, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Sebastiaan Engelborghs, Department of Biomedical Sciences, University of Antwerp, Antwerp, Belgium; Department of Neurology, Universitair Ziekenhuis Brussel and Center for Neurosciences (C4N), Vrije Universiteit Brussel (VUB), Brussels, Belgium.
Betty M Tijms, Alzheimer Center Amsterdam, Amsterdam Neuroscience, VUMC, Amsterdam, The Netherlands; Department of Neurodegeneration, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Funding
P.J.V. and B.M.T receive funding from ZonMw Memorabel Grant no 733050824.
Competing interests
C.T. received grants from the European Commission, the Dutch Research Council (ZonMW), Association of Frontotemporal Dementia/Alzheimer’s Drug Discovery Foundation, The Weston Brain Institute, Alzheimer Netherlands. C.T. has served on advisory boards of Roche, received non-financial support in the form of research consumables from ADxNeurosciences and Euroimmun, and performed contract research or received grants from Probiodrug, Biogen, Esai, Toyama, Janssen Prevention Center, Boehringer, AxonNeurosciences, EIP farma, PeopleBio and Roche. P.J.V. received grant support from ZonMW, IMI and Biogen. All payments were made to the institution. He also received funding from Synapsis for a workshop on grant writing. F.B. performs contract research for Optina Dx and Optos; she has been an invited speaker at Roche and has been invited for expert testimony at Biogen. All funding is paid to her institution. S.E. received unrestricted research funding from Janssen Pharmaceutica and ADx Neurosciences and served as a consultant for Biogen, Danone, Eisai, icometrix, Novartis, Nutricia and Roche. P.J.V. and B.M.T. have a patent on CSF proteomic subtypes in AD (No. PCT/NL2020/050216, owner Stichting VUmc). The other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Jr JC, Bennett DA, Blennow K, et al. NIA-AA research framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG;2 criteria. Lancet Neurol. 2014;13:614–629. [DOI] [PubMed] [Google Scholar]
- 3. Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. [DOI] [PubMed] [Google Scholar]
- 4. Mulder C, Verwey NA, van der Flier WM, et al. Amyloid-beta(1-42), total tau, and phosphorylated tau as cerebrospinal fluid biomarkers for the diagnosis of Alzheimer disease. Clin Chem. 2010;56:248–253. [DOI] [PubMed] [Google Scholar]
- 5. Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duits FH, Teunissen CE, Bouwman FH, et al. The cerebrospinal fluid ‘Alzheimer profile’: easily said, but what does it mean? Alzheimers Dement. 2014;10:713–723.e2. [DOI] [PubMed] [Google Scholar]
- 7. Clark CM, Xie S, Chittams J, et al. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol. 2003;60:1696–1702. [DOI] [PubMed] [Google Scholar]
- 8. van der Flier WM, Scheltens P. Amsterdam dementia cohort: performing research to optimize care. J Alzheimers Dis. 2018;62:1091–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Flier WM, Pijnenburg YA, Prins N, et al. Optimizing patient care and research: the Amsterdam dementia cohort. J Alzheimers Dis. 2014;41:313–327. [DOI] [PubMed] [Google Scholar]
- 10. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017;32:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sachdev P, Kalaria R, O'Brien J, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28:206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain. 2009;132:2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 20. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 21. Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 22. Somers C, Struyfs H, Goossens J, et al. A decade of cerebrospinal fluid biomarkers for Alzheimer's disease in Belgium. J Alzheimers Dis. 2016;54:383–395. [DOI] [PubMed] [Google Scholar]
- 23. Willemse EAJ, Sieben A, Somers C, et al. Neurogranin as biomarker in CSF is non-specific to Alzheimer's disease dementia. Neurobiol Aging. 2021;108:99–109. [DOI] [PubMed] [Google Scholar]
- 24. Engelborghs S, Niemantsverdriet E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement (Amst). 2017;8:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tijms BM, Willemse EAJ, Zwan MD, et al. Unbiased approach to counteract upward drift in cerebrospinal fluid amyloid-β 1-42 analysis results. Clin Chem. 2018;64:576–585. [DOI] [PubMed] [Google Scholar]
- 26. Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer's disease: a longitudinal study. J Alzheimers Dis. 2014;42:1239–1250. [DOI] [PubMed] [Google Scholar]
- 27. Alzheimer’s Disease Neuroimaging Initiative. Accessed 11 November 2020. http://adni.loni.usc.edu/methods/research-tools/
- 28. Shaw LM, Hansson O, Manuilova E, et al. Method comparison study of the Elecsys® β-Amyloid (1-42) CSF assay versus comparator assays and LC-MS/MS. Clin Biochem. 2019;72:7–14. [DOI] [PubMed] [Google Scholar]
- 29. Blennow K, Shaw LM, Stomrud E, et al. Predicting clinical decline and conversion to Alzheimer's disease or dementia using novel Elecsys Aβ(1-42), pTau and tTau CSF immunoassays. Sci Rep. 2019;9:19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alafuzoff I, Thal DR, Arzberger T, et al. Assessment of beta-amyloid deposits in human brain: a study of the BrainNet Europe Consortium. Acta Neuropathol. 2009;117:309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. [DOI] [PubMed] [Google Scholar]
- 34. Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 36. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 37. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 38. R Core Team . A language and environment for statistical computing. R Foundation for Statistical Computing; 2019. https://www.R-project.org/ [Google Scholar]
- 39. Le Bastard N, Coart E, Vanderstichele H, Vanmechelen E, Martin JJ, Engelborghs S. Comparison of two analytical platforms for the clinical qualification of Alzheimer's disease biomarkers in pathologically-confirmed dementia. J Alzheimers Dis. 2013;33:117–131. [DOI] [PubMed] [Google Scholar]
- 40. Korecka M, Waligorska T, Figurski M, et al. Qualification of a surrogate matrix-based absolute quantification method for amyloid-β42 in human cerebrospinal fluid using 2D UPLC-tandem mass spectrometry. J Alzheimers Dis. 2014;41:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seeburger JL, Holder DJ, Combrinck M, et al. Cerebrospinal fluid biomarkers distinguish postmortem-confirmed Alzheimer's disease from other dementias and healthy controls in the OPTIMA cohort. J Alzheimers Dis. 2015;44:525–539. [DOI] [PubMed] [Google Scholar]
- 42. de Jager CA, Honey TE, Birks J, Wilcock GK. Retrospective evaluation of revised criteria for the diagnosis of Alzheimer's disease using a cohort with post-mortem diagnosis. Int J Geriatr Psychiatry. 2010;25:988–997. [DOI] [PubMed] [Google Scholar]
- 43. Koopman K, Le Bastard N, Martin JJ, Nagels G, De Deyn PP, Engelborghs S. Improved discrimination of autopsy-confirmed Alzheimer's disease (AD) from non-AD dementias using CSF P-tau(181P). Neurochem Int. 2009;55:214–218. [DOI] [PubMed] [Google Scholar]
- 44. Struyfs H, Niemantsverdriet E, Goossens J, et al. Cerebrospinal fluid P-Tau181P: Biomarker for improved differential dementia diagnosis. Front Neurol. 2015;6:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sutphen CL, McCue L, Herries EM, et al. Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer's disease. Alzheimers Dement. 2018;14:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mattsson-Carlgren N, Andersson E, Janelidze S, et al. Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer's disease. Sci Adv. 2020;6:eaaz2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pooler AM, Phillips EC, Lau DH, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013;14:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sato C, Barthélemy NR, Mawuenyega KG, et al. Tau kinetics in neurons and the human central nervous system. Neuron. 2018;97:1284–1298.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tijms BM, Gobom J, Reus L, et al. Pathophysiological subtypes of Alzheimer's disease based on cerebrospinal fluid proteomics. Brain. 2020;143:3776–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Visser PJ, Reus LM, Gobom J, et al. Cerebrospinal fluid tau levels are associated with abnormal neuronal plasticity markers in Alzheimer's disease. Mol Neurodegener. 2022;17:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Graff-Radford NR. Alzheimer CSF biomarkers may be misleading in normal-pressure hydrocephalus. Neurology. 2014;83:1573–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Duits FH, Wesenhagen KEJ, Ekblad L, et al. Four subgroups based on tau levels in Alzheimer's disease observed in two independent cohorts. Alzheimers Res Ther. 2021;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meyer PF, Pichet Binette A, Gonneaud J, Breitner JCS, Villeneuve S. Characterization of Alzheimer disease biomarker discrepancies using cerebrospinal fluid phosphorylated Tau and AV1451 positron emission tomography. JAMA Neurol. 2020;77:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mattsson N, Schöll M, Strandberg O, et al. 18F-AV-1451 and CSF T-tau and P-tau as biomarkers in Alzheimer's disease. EMBO Mol Med. 2017;9:1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ossenkoppele R, Rabinovici GD, Smith R, et al. Discriminative accuracy of [18F]flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2018;320:1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fleisher AS, Pontecorvo MJ, Devous MD Sr, et al. Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. 2020;77:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data from the ADC and DEM are not publicly available due to privacy restrictions. ADNI data can be requested through their website.