Abstract
Stem cell therapy has been shown to improve stroke outcomes in animal models and is currently advancing towards clinical practice. However, uncertainty remains regarding the optimal route for cell delivery to the injured brain. Local intracerebral injections are effective in precisely delivering cells into the stroke cavity but carry the risk of damaging adjacent healthy tissue. Systemic endovascular injections, meanwhile, are minimally invasive, but most injected cells do not cross CNS barriers and become mechanically trapped in peripheral organs. Although the blood–brain barrier and the blood–CSF barrier tightly limit the entrance of cells and molecules into the brain parenchyma, immune cells can cross these barriers especially under pathological conditions, such as stroke. Deciphering the cell surface signature and the molecular mechanisms underlying this pathophysiological process holds promise for improving the targeted delivery of systemic injected cells to the injured brain. In this review, we describe experimental approaches that have already been developed in which (i) cells are either engineered to express cell surface proteins mimicking infiltrating immune cells; or (ii) cell grafts are preconditioned with hypoxia or incubated with pharmacological agents or cytokines. Modified cell grafts can be complemented with strategies to temporarily increase the permeability of the blood–brain barrier. Although these approaches could significantly enhance homing of stem cells into the injured brain, cell entrapment in off-target organs remains a non-negligible risk. Recent developments in safety-switch systems, which enable the precise elimination of transplanted cells on the administration of a drug, represent a promising strategy for selectively removing stem cells stuck in untargeted organs. In sum, the techniques described in this review hold great potential to substantially improve efficacy and safety of future cell therapies in stroke and may be relevant to other brain diseases.
Keywords: stroke, stem cells, brain shuttle, iPSC, BBB
Achón Buil et al. review current developments in stem cell delivery after stroke with a focus on optimizing application routes. They summarize novel strategies including preconditioning, as well as genetic and pharmacological engineering of the cell graft before transplantation to facilitate crossing of the brain barriers.
Introduction
The average human brain is composed of 86 billion neurons and 85 billion glia—indispensable cells that require a special protective environment to ensure proper brain function.1 This protection is guaranteed by a 650-km (400-mile) network of specialized blood vasculature designed to selectively restrict the entry of potentially toxic substances into the brain.2 This blood–brain barrier (BBB) is fundamental in maintaining homeostasis and physiological brain function, with BBB disruption being a hallmark of most major neurological disorders.3 Unfortunately, the BBB is, at the same time, a barrier for most systemically applied drugs. An intact BBB is impermeable for 98% of all small molecule drugs and for nearly all large substances, such as peptides, antibodies or gene- and cell-based therapeutics.4
Therefore, many research laboratories are developing diverse strategies to improve therapeutic drug delivery to the brain, ranging from targeted ultrasound and alteration of administration routes to biochemical or genetic modifications. For instance, various drug delivery systems have been tested to transport small molecules across the BBB based on nanoparticles, such as liposomes or cyclodextrins. For larger molecules, such as antibodies, the brain shuttle technology has been recently developed and is currently being clinically tested for Alzheimer’s disease to enable efficient crossing of the BBB by binding the transferrin receptor.5 By contrast, the delivery of cells to the brain has been considerably less explored, even though cell therapy holds great promise to restore damaged neural networks and become a promising regenerative strategy for acute neurological injuries, such as stroke. In total, more than 80 clinical trials in the field of cell therapy for stroke have already been conducted or are currently ongoing. Although the initial clinical results have demonstrated the safety of cell therapies, further validating their translational potential, promising results from preclinical animal research have not been confirmed beyond doubt in a clinical setting.
The reasons for this limited translational success are multifaceted. For example, it is poorly understood which is the most promising cell source for transplantation. Some clinical trials have used primary, non-neuronal cells, such as mesenchymal stem cells (MSCs), which can be obtained from various tissues, such as bone marrow (BM, BD-MSCs), adipose tissue (AD-MSCs) or umbilical cord blood (UCB-MSCs), while other experimental settings have favoured the use of neural cells, including neural stem/progenitor cells (NSCs/NPCs). With advances in reprogramming technology, the use of induced pluripotent stem cell (iPSC)–derived NPCs is gaining more popularity, but this approach is not yet in the clinical phase for stroke therapy. An overview of the cell sources used in cell therapies for stroke is provided in Table 1. In addition to the cell source itself, the delivery of transplants to injured brain regions has been recognized as a major hurdle by experts in the field.6 Despite the great promise of cell-based therapies for stroke, there is currently no clinical ‘gold standard’ for how to deliver cells most efficiently to injured brain regions.
Table 1.
Stem cells used for treating stroke
| Cell type | ESC | Primary NSC | iPSC | iPSC- NPC | DPSC | MSC | MNC (=MSC + HSC) |
|---|---|---|---|---|---|---|---|
| Cell source7 | Embryonic tissue | Embryo or foetal tissue | Diverse, often fibroblasts | Diverse, often fibroblasts | Dental Pulp | BM-MSC AD-MSC UCB-MSC |
BM, cord blood, peripheric blood |
| Differentiation potential8 | ++ | + | ++ | + | + | + | + |
| Low risk of oncogenesis7,9,10 | − | + | − | + | + | + | + |
| In vitro expansion9,11 | ++ | + | ++ | + | ++ | + | ++ |
| Immunotolerance7 | − | − | − | − | + | ++ | − |
| Reduction of infarct volume7,11,12 | + | ++ | + | ++ | + | ++ | ++ |
| Improved functional recovery7,11,12 | + | ++ | + | ++ | ++ | ++ | ++ |
| Anti-inflammatory factor secretion7,11,12 | + | + | + | + | ++ | ++ | ++ |
| Trophic factor secretion7,11,12 | + | + | + | ++ | ++ | ++ | ++ |
| Cell replacement7,11,12 | + | ++ | + | ++ | ++ | − | − |
ESC = embryonic stem cell; DPSC = dental pulp stem cells; HSC = haematopoietic stem cells. (++) Advantage described in >60% of the studies; (+) Advantage described in <60% of the studies; (−) Absent advantage or disadvantage.
In this review, we discuss promising approaches by focusing on the newest strategies in cell delivery and cellular engineering that may shape a new generation of cell therapies capable of reaching injured brain regions more efficiently, thus constituting improved future strategies for stroke therapy.
Delivery routes for the cell graft to the injured brain
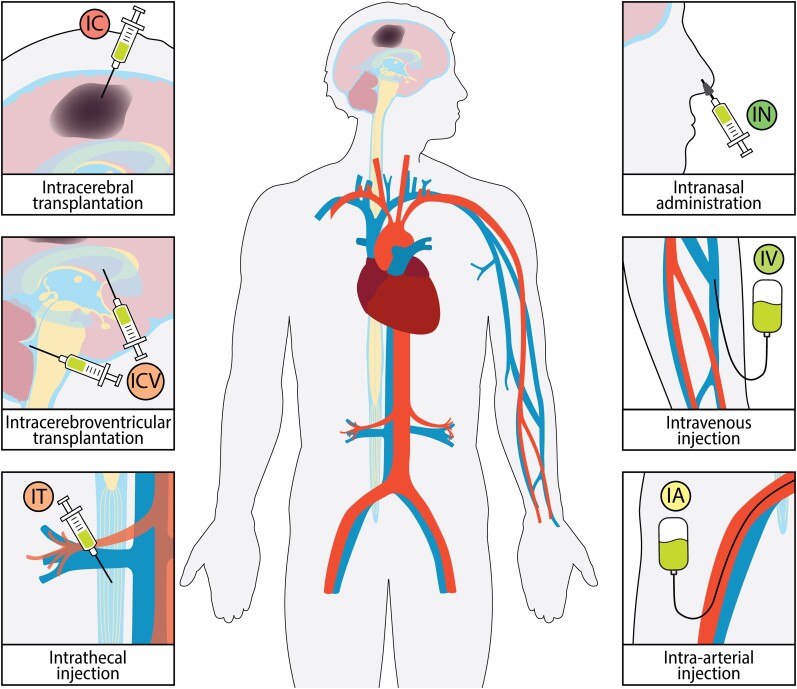
There are several feasible routes for delivering a cellular graft to the recipient of a cell therapy. The most commonly used routes for cell delivery in animal models and in humans are the intracerebral (IC), intracerebroventricular (ICV), intrathecal (IT), intra-arterial (IA), intravenous (IV) and intranasal (IN) deliveries (Fig. 1). Although intraperitoneal and subcutaneous injections are the preferred routes for drug administration in rodent models, their use for cell delivery is not effective enough and is even less so in humans13; therefore, we will not consider these routes in this review. Each route of cell delivery has its advantages and disadvantages in terms of efficacy and safety—a careful weighing of these factors is essential in developing successful cell therapy (Table 2).
Figure 1.
Stem cell administration routes for treating stroke in clinics. Cell grafts can be transplanted into the ischaemic area (IC) or CSF via the ventricles (ICV) or IT injection. Stem cells can also be injected systematically through the IA or IV routes, or cells can be administered IN.
Table 2.
Pros and cons of stem cell delivery routes for treating stroke
| Route of administrtion | Advantages | Disadvantages |
|---|---|---|
| IC | Precise graft placement14 High levels of grafted cells at the lesion15 |
Poor cell distribution throughout the lesion14 Small volume of injected cells16 Adverse events in clinical trials (Supplementary Table 1) |
| Intra-cerebroventricular, ICV, IT | Cell distribution throughout different parts of the CNS14 | Mainly for lesions close to ventricles14 |
| Intravascular IA IV |
Good cell distribution throughout the lesion16,17 Immunomodulation via spleen (IV)17 Big volume of injected cells16 Safe in clinical trials (Supplementary Table 1) |
1–10% of grafted cells in the lesion16,18 Adverse events in preclinical studies (IA)19 Cell entrapment in lungs, liver and spleen17 |
| IN | Bypass BBB20 No entrapment in other organs20 |
Only preclinical trials21 |
Intracerebral transplantation
IC delivery enables the precise injection of cells into the damaged brain parenchyma, which is the main advantage of this route. This route entails the smallest injection volume, which may prompt the need for multiple injections to reach the therapeutic threshold (∼106 cells). A comparative preclinical study showed that an IC NPC injection resulted in a higher number of transplanted cells in the ischaemic brain compared to ICV and IV administration.15 However, it remains unclear to what extent the graft migrates and spreads throughout the whole lesion. Some preclinical studies have shown that ESC, BM-MSC and NSC have the potential to migrate to the damaged brain regions.22–26 At the same time, other authors have suggested that ESC-derived NPCs and oligodendrocyte precursor cells injected IC migrated only short distances27,28 or only towards the peri-infarct region rather than to the stroke core when ESCs, NPCs or BM-MSCs were injected.25,29,30 A large body of research confirms that local IC transplantation of BM-MSCs or NSCs improved functional recovery and decreased infarct volume of stroked rodents.15,23,31,32 However, the stereotaxic IC injection is an invasive procedure that can be challenging or even impossible to perform depending on the stroke location. This technique also carries the risk of damaging adjacent healthy tissue and causing unexpected adverse events. Although some studies have demonstrated a good safety profile,33,34 other clinical trials have revealed adverse effects related to surgical procedures, including headache, partial seizures and subdural haematoma.35–38 Regarding efficacy of cell therapies, some studies demonstrated improved recovery in at least one of the functional scores compared to the baseline when BM-MSCs, NSCs and CD34+ cells were locally transplanted in subacute or chronic stroke patients (Table 3 and Supplementary Table 1).34,36–38 However, a major limitation in reliably assessing efficacy is the absence of a placebo-controlled group in IC cell therapies due to the invasiveness of the procedure. Additionally, IC injections cannot be carried out in acute stroke patients due to the associated risks with the procedure, which limits the therapeutic application of IC cell transplantations to the subacute and chronic stroke phase.
Table 3.
Resume of clinical trials using stem cells for treating stroke in the last 10 years
| Route | Cell type | Controlled study | Stroke phase |
|---|---|---|---|
| IC | BM-MSC33,36,37 | No | Subacute |
| CD34+ cells34 | Yes | Chronic | |
| NSC35,38 | No | Subacute/chronic | |
| ICV, IT | BM-MNC39 | No | Subacute/chronic |
| BM-MSC40 | No | Chronic (haemorrhagic) | |
| CD34+ cells41 | No | Chronic | |
| IA | BM-ALD40142 | Yes | Subacute |
| BM-MNC43–45 | Yes | Acute/subacute | |
| BM-MNC46 | No | Acute | |
| CD34+ HSC47 | No | Acute | |
| IV | AD-MSC48 | Yes | Acute/subacute |
| BM-MNC49–51 | Yes | Subacute/chronic | |
| BM-MNC52,53 | No | Acute/subacute | |
| BM-MSC54–57 | Yes | Acute/subacute | |
| BM-MSC58–60 | No/Yes | Chronic | |
| BM-MSC or EPC61 | Yes | Subacute | |
| BM multipotent cell62 | Yes | Acute | |
| UCB cells63 | No | Acute/subacute |
BM-ALD401 = BM-derived aldehyde dehydrogenase bright stem cells; EPC = endothelial progenitor cells; HSC = haematopoietic stem cells. Stroke phase: acute <7 days; subacute 7 days to 6 months; chronic > 6 months.
Intracerebroventricularand intrathecal transplantation
In rodents, cells can enter the CSF through ICV transplantation via the ventricles (fourth and lateral), the subdural space, the cisterna magna (subarachnoid space) or by IT administration.64–68 The most common way of accessing the CSF in humans is through the IT route by lumbar puncture, but ICV administration has also been performed. Compared to direct parenchymal delivery, ICV cell injection is less invasive and allows for a higher cell injection volume. Furthermore, this route entails higher levels of grafted cells being delivered into the ischaemic brain compared to systemic IV engraftment.15,68 Stem cells are widely distributed throughout the brain depending on the fluidity of the CSF.69 NPCs grafted by ICV injections migrated post-stroke to the damaged tissue in a middle cerebral artery occlusion (MCAO) mouse model.15 The injection of UCB-MSCs and iPSCs into the CSF can improve motor functions and reduce lesion size after cerebral ischaemia.66,68 Administered cells also exhibit an indirect effect by activating endogenous NSCs and oligodendrocyte progenitors localized at the subventricular zone.65,70 Conversely, the effect of injected cells is considered insufficient in the damaged areas further away from the ventricles.14 Furthermore, NSC ICV injection showed no beneficial outcomes in improving the sensorimotor function of stroked rats, whereas NSC IC transplantation resulted in a significant improvement.71 A pilot clinical study on treating stroke with IT injection of autologous CD34+ cells improved National Institutes of Health Stroke Scale (NIHSS) and Barthel index from baseline, without reporting adverse events.41 Another clinical trial on ischaemic and haemorrhagic stroke determined that IT injection of bone marrow-mononuclear cells (BM-MNCs) is safe and leads to a better functional recovery in ischaemic stroke patients.39 ICV administration of autologous BM-MSCs improved NIHSS from baseline in some haemorrhagic stroke patients (Table 3 and Supplementary Table 1).40 However, all these studies lack a control cohort which is crucial for determining efficacy.
Intra-arterial injection
Cells can enter arterial circulation through the common carotid artery in mice or the external carotid artery in rats.72 In humans, cells are delivered to the middle cerebral artery through cannulation entering via the femoral artery.47 Endovascular administration allows at least a seven-times higher injection volume compared to IC graft.73 In a rat model, the percentage of NPCs found in the ischaemic brain following IA injection was relatively low (1–10% of injected cells).16 NPCs and UCB-MSCs IA injected into experimentally stroked rats presented a broader distribution throughout the lesion compared to cells grafted into the cisterna magna, IC or IV.16,67 The IA route circumvents the venous circulation that entails mechanical entrapment in peripheric organs74; this advantage is of most interest when stem cells present a substantial adhesion capacity.75 Higher levels of transplanted cells in the ischaemic brain via the IA route compared to IV administration were correlated with better neurological recovery in animal models.76,77 Furthermore, IA Injection of BM-MSCs and BM-MNCs after rodent MCAO led to better outcomes in various behavioural tests, such as the rotarod, a standard test for assessing neuromuscular coordination after stroke in a rodent.16,77–79 In some preclinical studies, IA cell injection has been associated with complications, such as reduction of cerebral blood flow, micro-embolisms or even death,67,80,81 while other studies have not reported any adverse effects.77,78,82 More recently, procedural refinements of IA cell injections, such as replacing microcatheters by microneedles, have been found to improve the safety of the procedure.80 Cell size, cell dose and cell infusion velocity should all be considered for reducing the number of micro-embolisms and Lacunar strokes that may occur when cells are injected via the IA route.19,81,83 Clinical trials using BM-MNCs for treating acute stroke have reported two cases of partial seizures and some moderate adverse events, e.g. urinary infection and pneumonia following IA injection.45,46 Nevertheless, the IA injection of BM-MNCs for treating subacute stroke has been reported to be safe in other clinical trials, which highlights the importance of determining the optimal timing for cell engraftment.43,44 Regarding efficiency, the IA administration of CD34+ HSCs or BM-MNCs significantly improved NIHSS, modified ranking scale and/or Barthel index from baseline.46,47 Nevertheless, the inclusion of a control arm, determined that although there is a trend to better improvement in patients treated with BM-MNCs, there are no significant differences compared to control cohort (Table 3 and Supplementary Table 1).43–45 A biodistribution clinical study determined that IA administration of BM-MNCs resulted in high cell counts in liver and spleen, but relatively low cell number in the brain, which might explain the mixed results in clinical trials.84
Intravenous injection
IV injection in rodents is usually performed via the tail vein, but the femoral vein can also be used.85 In patients, IV administration is usually performed via the three main veins of the antecubital fossa on the anterior surface of the elbow joint. The main drawback in IV delivery is the increased entrapment of injected cells in peripheral organs, mainly in the lungs, liver and spleen.17,86 This downside can also be considered an advantage due to the anti-inflammatory effect observed in the spleen following IV NSC injection.17 Due to cell entrapment and brain-barrier impediment, only 1% of injected cells arrive at the ischaemic brain.18 Therefore, systemic IA and IV administration requires a higher cell dose (∼108 cells)than the IC route to reach the therapeutic threshold, which entails higher costs and potentially more side effects.87 In rodents, the cells grafted using this route are distributed throughout the brain parenchyma and can migrate to the damaged area.88–92 Despite the low number of grafted cells in the lesion, IV injections of BM-MSCs or NSCs have been shown to promote functional recovery post-stroke in rodents,88,92–95 which may be at least partially explained by the secretion of immunomodulatory or trophic factors.18,94 In clinical settings, the IV route is the most preferred one and has been described as a feasible and safe technique in several clinical trials.49–51,55–57,59–63 IV injections of BM-MSCs or BM-MNCs for treating subacute or chronic stroke have shown mixed results.50,54,56,57,59,60 However, in other studies, the IV injection of adipose-derived-MSCs, BM-MSCs or BM-MNCs for treating acute, subacute or chronic stroke have not achieved significant improvement in any of the studied functional outcome scores compared to the control cohort.48,49,51,52,55 A biodistribution clinical study determined that IV administration of BM-MNCs resulted in lung entrapment and low cell counts in brain which might explain the failure observed in clinical trials (Table 3 and Supplementary Table 1).84 However, the determination of the optimal cell type, cell dose and injection timing are also crucial for improving the recovery of stroke patients following endovascular cell administration.
Intranasal administration
The pathways and mechanisms underlying cell migration to the brain parenchyma after IN administration are not fully understood. IN-administered cells migrate from nasal mucosa through the cribriform plate into the olfactory bulb and other brain regions or go into the CSF to eventually enter the brain parenchyma.20 Cells can also enter the peripheral trigeminal system spread throughout the nasal epithelium, then migrate again to the CSF or to the brainstem and the spinal cord.96 IN delivery is the least invasive route, but it entails low injection volumes (<20 µl in mice).73 It has also been demonstrated that IN administration enables a faster homing of BM-MSC (1.5 h) compared to intravascular delivery (from 24 h to 10 days).97 Moreover, IN administration enables the homing of MSCs towards the ischaemic area98 and improves functional recovery after stroke.97,99 However, the IN route has not yet been studied as thoroughly as the other routes and requires further investigation to determine key parameters, such as the optimal dose, timing and adverse events. IN cell grafts have only been performed in rodents, which possess a more developed olfactory system than humans.21 Thus, a possible translation into clinical practice is still far away.
Overall, all current routes of cell delivery to the brain have advantages and disadvantages. Contemporary strategies must balance the potential effectiveness of cell therapy against the procedure’s invasiveness and safety risks for stroke patients. BBB is the major obstacle to non-invasive and endovascular routes of applications, such as IV cell delivery. Recent studies on BBB function and advances in cellular engineering hold significant potential for enabling the modification of graft properties, which would facilitate the development of targeted delivery to injured brain regions, thus improving the effectiveness of minimally invasive cell therapies.
Cell trafficking across the barriers of the CNS
To develop an effective cell therapy capable of overcoming the barriers of the CNS, a promising strategy is to outline the molecular mechanism underlying the physiological cell trafficking of, for example, peripheral immune cells. The cell surface signature and molecular pathways can be used as a template for improving the migratory abilities of therapeutic cells. Depending on the selected route for delivering cells, there are different obstacles to reaching the ischaemic area. IC-injected cells can be delivered directly to the damaged area, whereas ICV and IN require a further step in migration from the CSF towards the injury site. Cells injected into the systemic blood circulation (IA and IV) must overcome the additional hurdle of brain barriers, which include the BBB and the brain–CSF barrier (BCSFB) located at the choroid plexus.100 Although the brain parenchyma was considered to be immune privileged,101 studies have demonstrated that immune cells such as neutrophils, monocytes and T cells can migrate across the vascular endothelial monolayer and, under pathological conditions such as stroke, can enter the CNS parenchyma.102–105
Immune cell trafficking across an intact blood–brain barrier
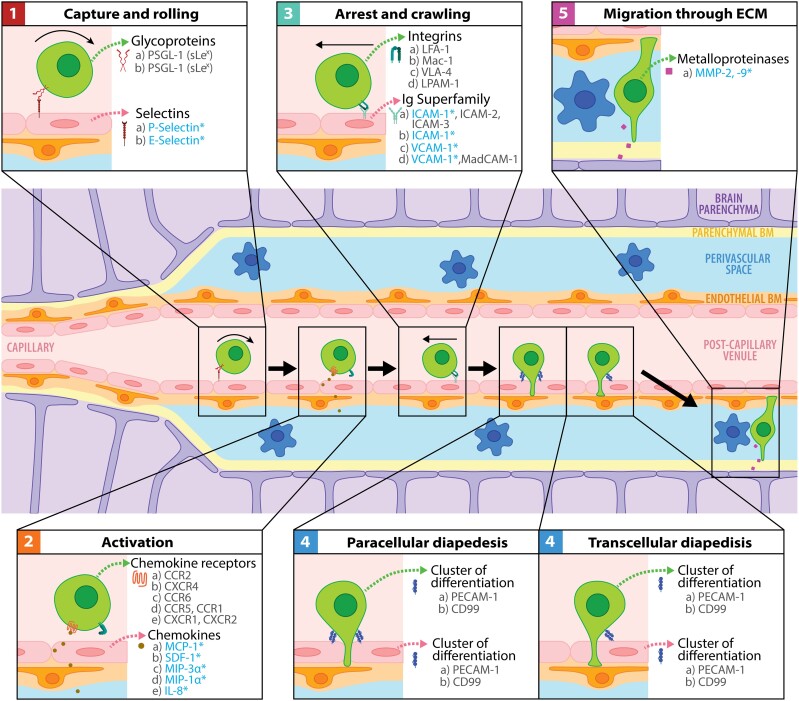
The extravasation of immune cells from the bloodstream into target tissues (including the brain) depends on the orchestrated interaction of circulating leucocytes (e.g. neutrophils and monocytes) with vascular endothelial cells (ECs). This process, first described 30 years ago,106 starts with (i) the capture and rolling of circulating leucocytes along the EC surface and is followed by (ii) the integrin activation in leucocytes necessary for (iii) the subsequent leucocyte arrest and crawling, which leads to (iv) leucocyte diapedesis across the EC monolayer, before the eventual leucocyte migration through the extracellular matrix located between the blood vessels and the parenchyma (Fig. 2).
Figure 2.
Immune cell transmigration across the BBB. The first step during the transmigration process is known as capture and rolling and is mediated by selectins located in ECs. Second, the interaction between the chemokines and their receptors in immune cells activates the integrins necessary for firm adhesion. Next, diapedesis can occur between ECs (paracellular) or through ECs (transcellular). After interacting with antigen-presenting cells (blue flower-shaped cells), immune cells can secrete metalloproteinases to break the components of the endothelial and parenchymal basement membrane (BM) and eventually enter the brain parenchyma. Upregulated molecules in stroke are indicated with an asterisk in blue. The same letter indicates the interaction partners.
The capture and rolling of leucocytes
The first step is regulated by the transient interaction between selectins and glycoproteins. P-selectin (expressed on an activated platelet and EC) and E-selectin (expressed on an activated EC) interact with P-selectin glycoprotein ligand-1 (PSGL-1) containing the tetrasaccharide sialyl Lewis × (sLex), which is the minimal glycan determinant for selectin interaction.107 The molecules involved also depend on cell type and inflammatory status—for example, in a mouse model of multiple sclerosis (a CNS inflammatory disease), leucocyte rolling was also mediated by endothelial α4 integrins.108
Integrin activation in leucocytes
The main actors in the second phase are chemokines, which can be secreted by ECs, especially during the inflammatory response. Damaged parenchymal cells can also secrete chemokines, which can directly diffuse or be transcytosed by ECs into the bloodstream.109 The chemokine family is classified into four subfamilies (CXC, CC, C and CX3C)110 and their receptors are G-coupled protein receptors located at leucocyte surfaces.111 The binding of chemokines to their receptors starts a intracellular signalling cascade in leukocytes that results in the conformational change of integrins.112 The activation of leukocytic integrins via chemokine signalling is necessary for the subsequent step of the extravasation process.
Leucocyte arrest and crawling
Integrin activation in leucocytes is necessary for their interaction with immunoglobulin gene superfamily (IgSF) molecules present in ECs. There are four main integrins involved in leucocyte arrest: lymphocyte function-associated antigen 1 (LFA-1, CD11a/CD18, αLβ2-integrin), macrophage-1 antigen (Mac1, CD11b/CD18, αMβ2-integrin), very late antigen-4 (VLA-4, CD49d/CD29, α4β1-integrin) and lymphocyte Peyer’s patch adhesion molecule-1 (LPAM-1, α4β7-integrin). LFA-1 interacts with intercellular adhesion molecule-1 (ICAM-1), ICAM-2 and ICAM-3; Mac-1 binds to ICAM-1; VLA-4 interacts with vascular cell adhesion molecule-1 (VCAM-1); and LPAM-1 links VCAM-1 and mucosal addressin cell adhesion molecule-1 (MadCAM-1).113 The link between leucocytic activated integrins and endothelial IgSF molecules results in a firm arrest of immune cells. Following this step, through a process known as crawling, leucocytes can laterally move towards the nearest junction or towards a permissive site of transmigration.114 In the initial characterizations, researchers thought that Mac-1, LFA-1 and their respective counter-receptors (ICAM-1 and ICAM-2) were involved in this process.114 However, a subsequent study suggested that LFA-1 is mainly involved in firm adhesion, whereas crawling mainly depends on the interaction of Mac-1 with ICAM-1.115
Leucocyte diapedesis
Before leucocyte diapedesis, an ICAM-1 cluster forms on the EC membrane surrounding the leukocytic filopodia-like structures.116 Diapedesis can occur in two ways: across ECs (transcellular) or between ECs (paracellular). Transcellular diapedesis is thought to be preferable when cell junctions are very tight—for example, at the BBB.117 This pathway requires the homophilic interaction of platelet EC adhesion molecule-1 (PECAM-1, CD31) and CD99 between leucocytes and ECs.116 Junctional adhesion molecule A (JAM-A, JAM-1) is also necessary for transcellular migration, but its role remains controversial.118 As for the paracellular diapedesis, the IgSF–integrin interaction induces the loosening of the adherent junctions.116 In addition to the already mentioned PECAM-1, CD99 and JAM-A, other molecules, such as ICAM-2, VCAM-1 and JAM-C, are involved in paracellular transmigration.116
Leucocyte migration through the extracellular matrix towards the brain parenchyma
After crossing the EC monolayer, leucocytes must cross the basement membrane, a highly cross-linked sheet of extracellular matrix composed of collagen IV, laminin, nidogen and perlecan, to reach the target tissue. Except for the capillaries, all CNS blood vessels have the following two different basement membranes: (i) the endothelial basement membrane in contact with ECs; and (ii) the parenchymal basement membrane in contact with the endfeet of astrocytes, which delimitates a CSF-drained perivascular space in between. As immune cell trafficking through the BBB occurs at post-capillary venules, following vascular monolayer extravasation, immune cells enter the perivascular space119 where they seek permissive areas. For example, T cells migrate across endothelial BM through areas containing laminin-α4 and low laminin-α5 levels via α6β1-integrin.120 Once in the perivascular space, immune cells interact with resident antigen-presenting cells before traversing the parenchymal basement membrane.121 In physiological conditions, there is no antigen recognition and, thus, immune cells undergo apoptosis or are drained out of the CNS due to the connection of the perivascular and leptomeningeal/subarachnoidal space with the cervical lymph node or via the glymphatic system.122 However, after antigen recognition, leukocytes can secrete matrix metalloproteinases (MMPs) such as MMP2 and MMP-9 to break down the parenchymal basement membrane and enter the CNS parenchyma.123
Much less is known about leukocytic extravasation through the BCSFB, whose structure differs from that of the BBB. BCSFB presents fenestrated capillaries, followed by a monolayer of tightly joined epithelial cells. Choroid plexus vessels constitutively display E- and P-selectin,124 and epithelial cells constitutively express ICAM-1 and VCAM-1, but only on the apical surface125; therefore, the extravasation molecular pathway remains uncertain. For instance, interleukin 17–producing T helper (TH-17) cells can go through the BCSFB via the interaction of CCR6 with CCL20, which is constitutively expressed in choroid plexus epithelial cells.126 This pathway allows the entrance of TH-17 cells into the uninflamed brain, which eventually leads to a pro-inflammatory state and the entrance of more immune cells via the activation of different pathways.126 Severe dysregulation of leukocyte migration and brain barriers occurs after major brain injuries, such as stroke. In the acute phase, immune cell extravasation from blood vessels exacerbates the pro-inflammatory environment and increases BBB permeability. This pathological condition could be used to improve the migratory abilities of therapeutic cells.
Immune cell trafficking across barriers in the ischaemic brain
Important hallmarks of ischaemic stroke include a local and systemic inflammatory response and a temporal disruption of the BBB that eventually leads to increased permeability for molecules and immune cells.127 However, it remains unclear whether the infiltration of immune cells is due to the pro-inflammatory state or the absence of brain barriers, or both.
Post-ischaemic neurons and glial cells secrete damage-associated molecular patterns that activate astrocytes and microglia, which, in turn, secrete cytokines, chemokines and metalloproteinases,128 similar to the molecule secretion following traumatic brain injury.129 The main pro-inflammatory cytokines secreted in the acute phase are interleukin-1beta (IL-1β) and tumour necrosis factor-alpha (TNF-α).130 Another two released pro-inflammatory cytokines are interferon-gamma (IFN-γ) and IL-6, but IL-6 can also present a neuroprotective effect.128 In addition, high levels of IL-23 activate γδ T cells (an ‘unconventional’ subtype of T cells) that produce IL-17, which results in increased neutrophil infiltration.131 At the same time, the secretion of anti-inflammatory cytokines such as IL-4, IL-10, TGF-β and IFN-β by immune cells and microglia may exert a protective effect.128,130 The classification of cytokines in pro- or anti-inflammatory mediators is a quite simplistic vision for determining their aversive/protective function, as for example, TNF-α present a detrimental role in the acute phase but it is necessary for long-term recovery.132 The inflammatory environment generated by the microglia, astrocytes and leukocytes can, in turn, activate endothelial and immune cells, increasing the levels of the molecules involved in transmigration.133
The capture and rolling of leucocytes after an experimental stroke
After MCAO, E-selectin and P-selectin expression levels are increased in the brain vasculature adjacent to the ischaemic area.134–139 Following inflammation, P-selectin is rapidly localized at the surface of ECs containing Weibel–Palade bodies. In addition to P-selectin, these granules contain von Willebrand factor, which interacts with PSGL-1 and β2-integrins, triggering further extravasation steps.140 Similar pathways are involved in neuroinflammatory diseases such as multiple sclerosis, as in an experimental autoimmune encephalomyelitis mouse model it was demonstrated that interaction between E-/P-selectin and PSGL-1 is crucial for T-cell rolling in inflamed spinal cord, but not for the onset of the disease.141
Integrin activation in leucocytes in the ischaemic brain
Chemokines play a key role in the second step (activation) and in the directed migration of cells to the lesion. Monocyte chemoattractant protein-1 (MCP-1, CCL2) is one of the most studied chemokines whose levels increase after ischaemic brain injury.142–147 The interaction between MCP-1 and its receptor CCR2 leads to a higher permeability of the BBB and a higher leukocyte infiltration,148–151 which also have an essential role in multiple sclerosis.152 Other post-ischaemic upregulated molecules are stromal cell-derived factor 1 (SDF-1, CXCL12),153,154 macrophage inflammatory protein 3α (MIP-3α, CCL20) and MIP-1α (CCL3),142,143,155,156 whose receptors are CXCR4, CCR6 and CCR5 and CCR1, respectively. After reperfusion, levels of cytokine-induced neutrophil chemoattractant (CINC) were increased, which was associated with neutrophil infiltration.147,157 CINC is thought to be part of the IL-8 (CXCL8) family, which binds leukocytic CXCR1 and CXCR2158 and whose mRNA and serum protein levels are higher in patients suffering from ischaemic stroke.157,159
Leucocyte arrest and crawling after an experimental stroke
The integrin activation on the leucocyte surface enables their interaction with IgSF proteins, whose expression by ECs is also modified after ischaemic injury. Cytokines, including TNF-α and IL-1, increase the ICAM-1 and VCAM-1 levels.160 Following a stroke, the ICAM-1 mRNA and protein levels are increased not only in rodent models but also in primates.139,161,162 Regarding VCAM-1, after MCAO in rodents, mRNA levels remain unmodified, but protein levels increase, mainly in the ischaemic microvasculature.135,163 The interaction of integrins with IgSF proteins is an indispensable step, as the blockade of CD49d with a monoclonal antibody (MAb) (Natalizumab) is an approved treatment that prevents the extravasation of immune cells into the CNS in multiple sclerosis.164
Leucocyte diapedesis in the ischaemic brain
Little is known about the upregulation of molecules involved in diapedesis following ischaemic brain damage. CD99, PECAM-1 and ICAM-1 were significantly upregulated in an immortalized cell line of endothelial brain cells after oxygen–glucose deprivation.165 However, depending on the animal strain or the inflammatory model, leukocyte transmigration can occur in a PECAM-1-dependent or a PECAM-1-independent manner (e.g. in the presence of IL-1β or TNF-α, respectively).166
Leucocyte migration through the extracellular matrix towards the parenchyma post-stroke
After ischaemic damage, MMP-2 and MMP-9 activation results in increased migration through the basement membranes and in BBB disruption.167 MMP-9 can be upregulated not only by cytokines (TNF-α and IL-6)168 but also by a tissue plasminogen activator (the main treatment for removing the blood clot in acute stroke), which has been associated with several complications, such as cerebral oedema and haemorrhagic transformation.169 Regarding MMP-2, it may play a role in the early opening of the BBB or in a later stage concerning the glial scar formation.168,170
Leucocyte migration through the brain parenchyma under pathological conditions
After entering the brain parenchyma, stem cells still have to migrate to the injured area. This process is regulated by cytokines. For instance, the MCP-1/CCR-2 axis may play a crucial role in the macrophage and, to a lesser extent, in neutrophil migration following transient cerebral ischaemia.171 Astrocytic and endothelial SDF-1α expression following ischaemic brain injury is involved in NSC migration towards the lesion.154 Another receptor expressed in NSC/NPCs is c-kit, which can direct their migration towards the stem cell factor whose expression is increased in neurons within the injured brain area.172
The complex transmigration mechanism is carried out not only by immune cells but also by cancer cells that can escape from the primary tumour and metastasize to the CNS. In addition to the upregulation of certain molecules involved in immune cell extravasation (e.g. VLA-4, MMP-2 and MMP-9),173,174 three specific proteins, namely cyclooxygenase-2, epidermal growth factor receptor (EGFR) ligand and α2,6-sialyltransferase (ST6GALNAC5), were identified as mediators of BBB transmigration.175 Deciphering the molecular pathways of cell extravasation into the CNS can be used as a template for improving the migration of stem cells.
Approaches to improving stem cell migration
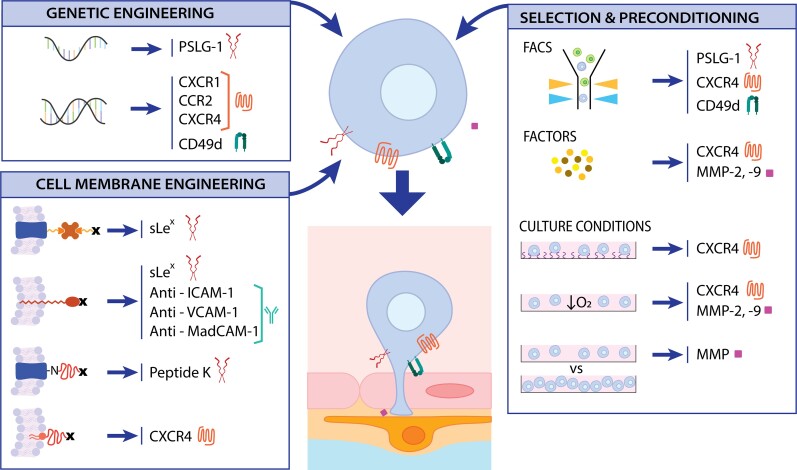
Stem cells can also pass through brain barriers, but only 1–10% of systemically injected stem cells migrate to the ischaemic brain.16 Differences in MSC and leukocyte migration have been extensively reviewed.176 In short, rolling is not required by MSC, crawling patterns are different compared to leucocytes (absence of lateral migration) and the duration of the process is longer in MSC (1–2 h versus 20 min for leucocyte).176 Depending on the source, type and handling, the stem cells exhibit different sizes and surface-protein patterns, which can influence their migration capacities. For example, BM-MSCs cultured for 24 h in vitro and IV injected into sub-lethally irradiated mice presented lower migration capacity to BM and the spleen compared to primary MSCs.177 Several approaches have been developed to enhance stem cell homing by focusing on increasing the levels of molecules involved in post-ischaemic extravasation through genetic modifications, surface cell engineering, preconditioning or cell selection (Fig. 3). A different approach that does not involve the modification of the transplanted cells is to increase the permeability of the BBB.
Figure 3.
Approaches to enhancing stem cell transmigration to the brain. Stem cells can be modified via genetic or cell membrane engineering to increase the levels of selectin ligands, chemokine receptors, integrins and metalloproteins. These molecules can also be upregulated by preconditioning cells with specific factors or by adjusting culture conditions. These modifications may enable a more efficient migration of cell grafts from the blood circulation to the brain.
Genetic engineering
One of the most widespread techniques for overexpressing proteins is genetic engineering, which enables the introduction of nucleic acids into the cell. Starting with the proteins involved in capture and rolling, primary MSCs have been transfected with a mRNA containing PSGL-1, sLex and IL-10.178 This construct resulted in not only higher cell homing to the inflamed ear but also to immunomodulation via the anti-inflammatory cytokine IL-10.178 Second, several chemokine receptors have been overexpressed in UCB- and BM-MSC, including CXCR1, CCR2 and CXCR4, using a non-viral, lentiviral or adenoviral vector, respectively.179–182 Regarding the firm adhesion step, CD49d was cloned into a recombinant adeno-associated virus for achieving a transient expression in BM-MSCs.183 CD49d dimerized with endogenous CD29 to form VLA-4, resulting in enhanced BM-MSC homing to BM.183 Furthermore, transfection of VLA-4 into human glial precursor cells induced higher homing to an inflamed brain following IA injection.184
Cell membrane engineering
Different techniques have enabled the attachment of extravasation-involved molecules to the surface of stem cells. One approach for enhancing MSC rolling involved the development of biotin-streptavidin-biotin bridges for anchoring selectin ligands to the cell membrane. Free amine groups on the MSC surface were biotinylated with sulfonated biotinyl-N-hydroxy-succinimide; afterwards, the cells were incubated with streptavidin and finally with biotinylated sLex.185 This technique was further developed by fusing biotinylated lipid vesicles with MSCs instead of using BNHS186 or by designing a nanoscale polymer to link biotin to sLex, which promoted robust rolling in vitro and in vivo.187 Another approach consisted of transfecting the first 19 amino acids of PSGL-1 and the tail of IgG1 into HEK cells overexpressing α1,3-fucosyltransferase (FUT7) to obtain a fusion protein correctly glycosylated with sLex. Then, palmitated protein G (PPG) was incorporated into the MSC membrane, which enabled the non-covalent coupling of the glycosylated fusion protein, which, in turn, enhanced rolling via selectin interaction.188 Another technique required reacting NHS-PEG2-maleimide with amine residues at the MSC surface for anchoring peptides containing N-terminal thiols; this approach was used to anchor peptide K, an E-selectin ligand involved in rolling and capture.189 Cell membrane engineering was also used to increase these levels of chemokine receptors, which play a key role in integrin activation. Dimyristoyl-sn-glycero-3-phosphoethanolamine was chemically conjugated to CXCR4 to subsequently coat the MSC membrane.190 PPG was also used for coating an immortalized MSC line (BMC-9) with antibodies against ICAM-1, VCAM-1 and MadCAM-1, which promoted the adhesion and delivery of these modified cells to inflamed tissues.191,192 Similar to PPG conjugation use to improve the rolling and adhesion steps, all the described techniques can be used to increase the levels of other molecules involved in the extravasation process.
Stem cell selection and cell culture preconditioning
Instead of directly modifying stem cells via genetic or cell surface engineering, stem cells can also be selected according to the presence of molecules involved in the transmigration process. The selection of NSCs expressing sLex and CXCR4 via fluorescence activated cell-sorting increased their migration towards the brain previously injected with SDF-1 after IV administration.193 Furthermore, fluorescence activated cell-sorting selection of CD94+ NSCs allowed higher numbers of NSCs into the ischaemic hemisphere compared to CD94− NSCs following IA injection, which improved stroke outcomes.75
Another alternative is adjusting cell culture conditions or preconditioning stem cells with molecules that activate the intracellular pathways involved in promoting migration. These molecules, such as Toll-like receptor ligands194 or IL-1β that upregulate CXCR4 in BM-MSCs or UCB-MSCs, respectively, can be involved in the inflammatory response, resulting in an enhanced migration to the inflamed tissue.195 BM-MSC exposure to a cytokine cocktail composed of stem cell factor, IL-3, IL-6, Flt-3 ligand and hepatocyte growth factor increased CXCR4 expression and thus enhanced their migration to BM after IV injection in a sub-lethally irradiated mouse.196 Preconditioning or co-injection of Insulin-like growth factor 1 (IGF-1) promoted BM-MSCs homing to injured sites197,198 and towards SDF-1 via CXCR4 upregulation.199 BM-MSC exposure to Glycogen synthase kinase 3 beta inhibitors resulted in the upregulation of CXCR4, MMP2 and MT1-MMP.200 Complement component 1 subcomponent q also promoted SDF-1-directed migration in UCB-MSCs by increasing the expression of CXCR4 and MMP-2.201 Pre-incubation of MSCs with valproate or lithium upregulated CXCR4 or MMP9, respectively, and resulted in enhanced graft migration to the ischaemic brain following IV injection in an MCAO rat model.202 A screening method was developed to identify molecules that upregulate the expression of CD11a in MSC to increase the interaction with IgSF molecules in ECs. Ro-31-8425 was chosen as the lead candidate, and when it was used to precondition MSCs, it enhanced their homing to inflamed tissues.203 NPCs cultured using a hyaluronic acid and laminin hydrogel presented upregulated CXCR4 and an increased in vivo migration rate towards exogenous SDF-1α.204,205 Acidic preconditioning of BM c-kit+ also increased CXCR4 levels and chemotaxis towards SDF-1.206 Hypoxic preconditioning of BM-MSCs not only increased their survival, proliferative and differentiation rates but also upregulated CXCR4, leading to higher homing to the ischaemic brain.207 By contrast, the high culture confluency of BM-MSCs increased tissue inhibitor of metalloproteinase-3 (TIMP-3), which reduces MMP activity and thus transendothelial migration.208 Most of the studies are focused on increasing CXCR4 levels, which can be explained by the pleiotropic involvement of SDF-1/CXCR4 axis in development, metastasis of cancer cells and NSC migration towards damaged areas.209
Increasing blood–brain barrier permeability
Increasing BBB permeability may be an alternative means of promoting cell homing to the brain without having to modify the transplanted cells. Administration of cyclophilin A triggers the transient opening of the BBB by reducing the levels of the vascular tight junction protein Claudin-5.210 Accordingly, IV injection of cyclophilin A enabled a higher incorporation of the hydrophilic molecule doxorubicin into the brain parenchyma.210 Mannitol has been proven to open the BBB in a safe way, and when it was co-injected with adipose-derived-MSCs, behavioural functions improved following experimental stroke. However, mannitol administration did not increase the number of cells entering the brain, which suggests a higher penetration of trophic factors instead of cells.211 Focused ultrasound has also been shown to transiently open the BBB, and it has been demonstrated that this process enabled NSC entrance to the brain parenchyma.212 Nevertheless, the transient opening of the BBB may entail several side effects, especially in pathological conditions, as in the case of stroke, when the BBB is already impaired. In fact, BBB disruption leads to higher levels of inflammatory factors, vasogenic oedema and a higher risk of haemorrhagic transformation, which worsen stroke outcomes and increase the mortality rate.213
Reduction of stem cell off-target entrapment following systemic injection
Systemic cell injection poses the risk of cells clogging vessels or becoming trapped in peripheral organs. The pulmonary first-pass effect refers to the mechanical entrapment in lungs of objects displaying a larger diameter than lung capillaries (ø = 8 µm in humans and ø = 4 µm in mice).214–216 As the average diameter of stem cells ranges from 6 to 20 µm (e.g. NPC ø = 16 µm),7 they are likely to become trapped in the lungs following vascular infusion. Ex vivo expansion can influence cell morphology and physiology, giving rise to even larger diameters (MSC diameter can reach up to 53 µm).217 Furthermore, these techniques for improving stem cell migration can also increase the cellular adherence properties, leading to a higher entrapment rate.218 In addition to the first uptake by the lungs, stem cells can undergo secondary redistribution to other organs, including the liver, kidneys and the spleen,219 which may entail further adverse effects. Endovascular administration of MSCs can lead to pulmonary embolism in small animal models,217 and can facilitate the tumour growth and promote metastasis.220,221 Therefore, although systemically injected cells can also exhibit a beneficial effect without entering the CNS,222 the potential adverse events related to peripheral entrapment is still considered as a non-negligeable risk. It can be addressed by reducing relative cell diameters and thus the probability of cell entrapment or by selectively removing cells that have become trapped in organs other than the brain.
Decreasing the probability of cell entrapment
The diameter of cells and blood vessels is a key factor in the occurrence of mechanical entrapment. On the one hand, increasing blood vessel diameter with the vasodilator sodium nitroprusside decreased cell entrapment in pulmonary microvasculature.215,219 On the other hand, smaller cells are likelier to avoid the pulmonary first-pass effect. For instance, a comparison between BM-MSCs and BM-MNCs revealed that BM-MNCs (ø = 7 µm) exhibited a 30-fold higher pulmonary passage than BM-MSCs (ø = 18 µm).216 Culture conditions can also be optimized to decrease cell volume—for example, by adapting cell confluency or growing cells in suspension.223–226 However, cell entrapment is determined not only by cell and vasculature size but also by cell deformability.227 Preconditioning haematopoietic stem cells with SDF-1a or H2O2 can increase their deformability, which may result in better properties for passing even thinner blood vessels.228
Safety-switch systems for selective specific ablation of cells
Reducing cell entrapment by modifying cell size or deformability properties does not guarantee the absence of cells in off-target organs. Therefore, the selective removal of cells trapped in organs other than the brain is required. Suicide gene therapy, first developed for selectively removing cancer cells,229–231 has great potential for accomplishing this goal. The application of genes encoding proteins that induce cell death following the administration of a specific molecule has enabled the development of ‘safety switches’. This technology has already been applied to reduce graft-versus-host disease after allogenic transplantation of BM cells.232,233 The impermeability of brain barriers could be considered an advantage because it can prevent the passage of the safety switches to the brain parenchyma, leading to a selective removal of transplanted cells in the periphery and not in the brain.
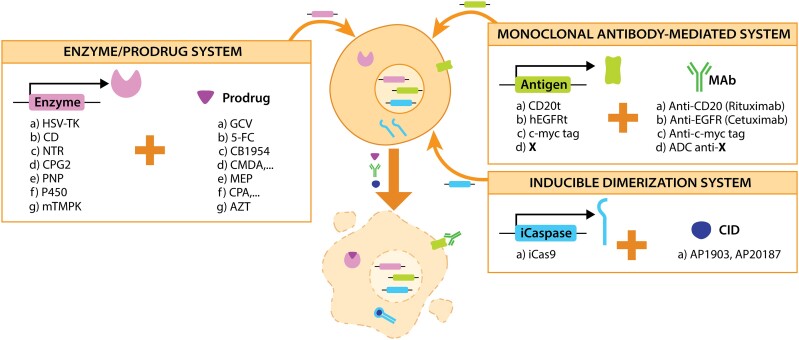
There are three main techniques for the selective ablation of cells via transgene insertion: enzyme/prodrug system, MAb-mediated system and inducible dimerization system234 (Fig. 4).
Figure 4.
Genetic safety systems for eliminating cell grafts in untargeted organs. After cloning a gene encoding for a suicide enzyme, a specific antigen or inducible caspases, cells can be eliminated by adding the corresponding prodrug, MAb or a CID, respectively.
Enzyme–prodrug system
The enzyme–prodrug system enabled the development of gene-directed enzyme–prodrug therapy. The system consists of delivering suicide genes to cancer cells for their subsequent ablation by prodrug administration, the prodrug is then converted into a toxic metabolite. The most studied suicide genes are herpes simplex virus thymidine kinase (HSV-TK), cytosine deaminase (CD), nitroreductase (NTR), carboxypeptidase G2, purine nucleoside phosphorylase, Cytochrome P450 (P450) and a mutated version of thymidylate monophosphate kinase (TMPK). These enzymes can metabolize Ganciclovir (GCV), 5-fluorocytosine, CB1954, a nitrogen mustard-based prodrug (e.g. CMDA), 6-methylpurine deoxyriboside, Oxazaphosphorine drugs (e.g. cyclophosphamide) and azidothymidine, respectively, leading to cell death.235,236
One of the major concerns of the enzyme/prodrug system is the source of the suicide gene. Exogenous sequences coming from a virus (HSV-TK), bacteria (NTR, carboxypeptidase G2, purine nucleoside phosphorylase, CD) or yeast (CD) are more immunogenic than endogenous genes, which could lead to a premature elimination of the administered cells by the recipient’s immune system.237 GCV is already clinically approved for the treatment of cytomegalovirus infections in immunocompromised individuals, which can also result in an undesired ablation of transgenic cells.238 At the same time, although the human enzyme P450 is less immunogenic, it is mainly expressed in the liver, which may result in liver toxicity. Catalytically improved variants of the enzymes, as is the case with human TMPK, may help to overcome this issue.236 Another point to consider is cell cycle dependency. HSV-TK and P450 are only active in dividing cells, while the other enzymes are also functional in non-dividing cells.235,236 A noteworthy feature of the enzyme–prodrug system is the bystander effect, which refers to the ability to induce apoptosis in cells lacking the suicide gene by the diffusion of the converted toxic metabolite. This effect is favourable for oncotherapy but is less applicable for the selective removal of entrapped stem cells, as it can result in the death of endogenous healthy cells. In general terms, bacterial systems exhibit a stronger bystander effect compared to P450, but the result depends on the chosen prodrug.239 Regarding BBB passage, GCV and 5-fluorocytosine have been widely used to treat brain tumours,240,241 which shows their ability to enter the brain parenchyma and potentially ablate therapeutic cells in the CNS. Therefore, the obtention of BBB-impermeable prodrugs would be favourable for the removal of transgenic cells exclusively in peripheric organs.
Monoclonal antibody-mediated system
Rituximab is a MAb against CD20 that has been widely used to treat B-cell lymphoma. Rituximab can lead to cell death via complement activation and antibody-dependent cell cytotoxicity.242 The latter is caused by the interaction between the Fc region of the MAb attached to the target cell with the Fc receptor of an effector cell, such as NK cells.243 Rituximab’s mechanism of action has been used to develop safety switches by adding the CD20 truncated gene to T cells to avoid the graft-versus-host disease after transplantation.244–246 This approach was further developed by combining the epitopes of CD34 and CD20 antigens (RQR8) to obtain T cells containing a selection marker and a suicide gene.247 However, as CD20 is present in endogenous B cells, a similar system was developed by introducing a truncated human EGFR gene into chimeric antigen receptor T cells, followed by Cetuximab (anti-EGFR) administration.248,249 Another strategy consists of adding a 10 amino acid tag of c-myc to the chimeric antigen receptor followed by the administration of a MAb against c-myc.250
An antibody–drug conjugate refers to a MAb that is chemically linked to a toxic payload, which was initially developed to reduce off-target toxicities in cancer treatment.251 In 2017, the Food and Drug Administration had approved only three antibody–drug conjugates for the treatment of different cancer types, but the number of approved treatments has now increased to 11.252 Therefore, another strategy involves developing antibody–drug conjugates against specific molecules present in the surface of therapeutic stem cells. Antibody–drug conjugates can be engineered to specifically recognize the previously described proteins for enhancing migration, which might improve the efficacy and safety of cell therapies at the same time. Furthermore, antibody–drug conjugates have a limited ability to cross the BBB, which can be considered a downside for treating glioblastomas253 but an advantage for the selective removal of entrapped cells outside the brain.
Inducible dimerization system
Human caspase-9 was fused to an FK506 binding protein to enable conditional dimerization in the presence of a small molecule known as the chemical inducer of dimerization (CID).254 The administration of CIDs (AP1903 or AP20187)255 leads to the activation of the inducible caspase-9 (iCas9), resulting in the rapid induction of apoptosis. The main advantages of this approach are its low immunogenicity (human gene) and the use of an inert small molecule. Although this system is gaining popularity, the available clinical data are still limited. However, CIDs are lipid-permeable synthetic ligands that are likely to cross the BBB. Accordingly, AP20187 was used to selectively ablate macrophages in an intracranial glioma mouse model.256
To conclude, features such as the bystander effect, efficacy, immunogenicity and suicide molecule hydrophobicity should be considered when choosing the ideal ablation system for a specific disease. A comparative study analysed some of these characteristics in HSV-TK, mTMPK–azidothymidine, CD20–MAb and iCas9–CID systems. The results showed that mTMPK was the least effective system, while for the other three systems, the main difference was that HSV-TK required 3 days of constant GCV administration instead of having an immediate effect, as was the case with CD20 and iCas9.257
Conclusion
Both preclinical data and initial clinical trials using cell therapy for brain regeneration show highly promising results. Importantly, the transplantation of stem cells has proven to be safe, without major adverse events. Nevertheless, there are still some hurdles to overcome before cell therapy can be established as a standard clinical strategy for treating brain diseases. In addition to the choice of the ideal cell source, which may differ depending on the disease in question, the administration route is of utmost importance. Systemic injection of cells represents the method with the highest translational potential because it is the least invasive and highly practicable in everyday clinical practice. However, the low permeability of the BBB and BCSFB prevents the entry of systemically applied cells into the brain. Therefore, genetic engineering and cell surface modification of transplanted cells will be necessary to increase the BBB/BCSFB penetration and homing to the injury site using similar mechanisms as observed in different types of immune cells. In parallel, it is essential to prevent unwanted entrapment of transplanted cells in peripheral organs. Safety switches, such as the incorporation of suicide genes into cell grafts, can be applied to specifically remove cells that did not reach their target region.
The techniques described in this review have great potential to increase the efficacy and safety of a cell therapy and to establish next-generation cell therapies as standard treatments for treating other brain diseases in the future.
Supplementary Material
Acknowledgements
The human body and brain visualization in Fig. 1 was modified from Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License. http://smart.servier.com/.
Contributor Information
Beatriz Achón Buil, Institute for Regenerative Medicine, University of Zurich, 8952 Schlieren, Switzerland; Neuroscience Center Zurich, University of Zurich and ETH Zurich, Zurich, Switzerland.
Christian Tackenberg, Institute for Regenerative Medicine, University of Zurich, 8952 Schlieren, Switzerland; Neuroscience Center Zurich, University of Zurich and ETH Zurich, Zurich, Switzerland.
Ruslan Rust, Institute for Regenerative Medicine, University of Zurich, 8952 Schlieren, Switzerland; Neuroscience Center Zurich, University of Zurich and ETH Zurich, Zurich, Switzerland.
Funding
The authors acknowledge funding from Mäxi Foundation, Swiss 3R Competence Center (OC-2020-002) and the Swiss National Science Foundation (CRSK-3_195902).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Herculano-Houzel S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc Natl Acad Sci U S A. 2012;109(Suppl 1):10661–10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res. 2003;61:39–78. [DOI] [PubMed] [Google Scholar]
- 3. Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: From physiology to disease and back. Physiol Rev. 2018;99:21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:54–61. [DOI] [PubMed] [Google Scholar]
- 5. Weber F, Bohrmann B, Niewoehner J, et al. Brain shuttle antibody for Alzheimer’s disease with attenuated peripheral effector function due to an inverted binding mode. Cell Rep. 2018;22:149–162. [DOI] [PubMed] [Google Scholar]
- 6. Rodríguez-Frutos B, Otero-Ortega L, Gutiérrez-Fernández M, Fuentes B, Ramos-Cejudo J, Díez-Tejedor E. Stem cell therapy and administration routes after stroke. Transl Stroke Res. 2016;7:378–387. [DOI] [PubMed] [Google Scholar]
- 7. Boltze J, Arnold A, Walczak P, Jolkkonen J, Cui L, Wagner DC. The dark side of the force – constraints and complications of cell therapies for stroke. Front Neurol. 2015;6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alessandrini M, Preynat-Seauve O, de Bruin K, Pepper MS. Stem cell therapy for neurological disorders. S Afr Med J. 2019;109:70–77. [DOI] [PubMed] [Google Scholar]
- 9. Zhang S, Lachance BB, Moiz B, Jia X. Optimizing stem cell therapy after ischemic brain injury. J Stroke. 2020;22:286–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown C, McKee C, Bakshi S, et al. Mesenchymal stem cells: Cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13:1738–1755. [DOI] [PubMed] [Google Scholar]
- 11. Suda S, Nito C, Yokobori S, et al. Recent advances in cell-based therapies for ischemic stroke. Int J Mol Sci. 2020;21:6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ford E, Pearlman J, Ruan T, et al. Human pluripotent stem cells-based therapies for neurodegenerative diseases: Current status and challenges. Cells. 2020;9:2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ohshima M, Taguchi A, Tsuda H, et al. Intraperitoneal and intravenous deliveries are not comparable in terms of drug efficacy and cell distribution in neonatal mice with hypoxia-ischemia. Brain Dev. 2015;37:376–386. [DOI] [PubMed] [Google Scholar]
- 14. Walczak P, Zhang J, Gilad AA, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jin K, Sun Y, Xie L, et al. Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol Dis. 2005;18:366–374. [DOI] [PubMed] [Google Scholar]
- 16. Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–1672. [DOI] [PubMed] [Google Scholar]
- 17. Lee S-T, Chu K, Jung K-H, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. [DOI] [PubMed] [Google Scholar]
- 18. Chen J, Sanberg PR, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. [DOI] [PubMed] [Google Scholar]
- 19. Janowski M, Lyczek A, Engels C, et al. Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J Cereb Blood Flow Metab. 2013;33:921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Danielyan L, Schäfer R, von Ameln-Mayerhofer A, et al. Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88:315–324. [DOI] [PubMed] [Google Scholar]
- 21. Jiang Y, Zhu J, Xu G, Liu X. Intranasal delivery of stem cells to the brain. Expert Opin Drug Deliv. 2011;8:623–632. [DOI] [PubMed] [Google Scholar]
- 22. Dinh AT, Kubis N, Tomita Y, et al. In vivo imaging with cellular resolution of bone marrow cells transplanted into the ischemic brain of a mouse. NeuroImage. 2006;31:958–967. [DOI] [PubMed] [Google Scholar]
- 23. Zhao L-R, Duan W-M, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174:11–20. [DOI] [PubMed] [Google Scholar]
- 24. Modo M, Mellodew K, Cash D, et al. Mapping transplanted stem cell migration after a stroke: A serial, in vivo magnetic resonance imaging study. NeuroImage. 2004;21:311–317. [DOI] [PubMed] [Google Scholar]
- 25. Hoehn M, Küstermann E, Blunk J, et al. Monitoring of implanted stem cell migration in vivo: A highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci U S A. 2002;99:16267–16272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen J-R, Cheng G-Y, Sheu C-C, Tseng G-F, Wang T-J, Huang Y-S. Transplanted bone marrow stromal cells migrate, differentiate and improve motor function in rats with experimentally induced cerebral stroke. J Anat. 2008;213:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bühnemann C, Scholz A, Bernreuther C, et al. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain. 2006;129:3238–3248. [DOI] [PubMed] [Google Scholar]
- 28. O’Leary MT, Blakemore WF. Oligodendrocyte precursors survive poorly and do not migrate following transplantation into the normal adult central nervous system. J Neurosci Res. 1997;48:159–167. [PubMed] [Google Scholar]
- 29. Ishibashi S, Sakaguchi M, Kuroiwa T, et al. Human neural stem/progenitor cells, expanded in long-term neurosphere culture, promote functional recovery after focal ischemia in Mongolian gerbils. J Neurosci Res. 2004;78:215–223. [DOI] [PubMed] [Google Scholar]
- 30. Li Y, Chopp M, Chen J, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. [DOI] [PubMed] [Google Scholar]
- 31. Huang H, Qian K, Han X, et al. Intraparenchymal neural stem/progenitor cell transplantation for ischemic stroke animals: A meta-analysis and systematic review. Stem Cells Int. 2018;2018:e4826407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Veizovic T, Beech JS, Stroemer RP, Watson WP, Hodges H. Resolution of stroke deficits following contralateral grafts of conditionally immortal neuroepithelial stem cells. Stroke. 2001;32:1012–1019. [DOI] [PubMed] [Google Scholar]
- 33. Kawabori M, Shichinohe H, Kuroda S, Houkin K. Abstract 23: Intracerebral transplantation of autologous bone marrow stem cell (BMSC) for subacute ischemic stroke, phase 1 clinical trial (RAINBOW trial). Stroke. 2021;52(Suppl_1):A23. [Google Scholar]
- 34. Chen D-C, Lin S-Z, Fan J-R, et al. Intracerebral implantation of autologous peripheral blood stem cells in stroke patients: A randomized phase II study. Cell Transplant. 2014;23:1599–1612. [DOI] [PubMed] [Google Scholar]
- 35. Muir KW, Bulters D, Willmot M, et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: Multicentre prospective single-arm study (PISCES-2). J Neurol Neurosurg Psychiatry. 2020;91:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Steinberg GK, Kondziolka D, Wechsler LR, et al. Two-year safety and clinical outcomes in chronic ischaemic stroke patients after implantation of modified bone marrow-derived mesenchymal stem cells (SB623): A phase 1/2a study. J Neurosurg. Published online 23 November2018:1–11. 10.3171/2018.5.JNS173147 [DOI] [PubMed] [Google Scholar]
- 37. Steinberg GK, Kondziolka D, Wechsler LR, et al. Clinical outcomes of transplanted modified bone marrow–derived mesenchymal stem cells in stroke. Stroke. 2016;47:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kalladka D, Sinden J, Pollock K, et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. The Lancet. 2016;388:787–796. [DOI] [PubMed] [Google Scholar]
- 39. Sharma A, Sane H, Gokulchandran N, et al. Autologous bone marrow mononuclear cells intrathecal transplantation in chronic stroke. Stroke Res Treat. 2014;2014:e234095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Al Fauzi A, Sumorejo P, Suroto NS, et al. Clinical outcomes of repeated intraventricular transplantation of autologous bone marrow mesenchymal stem cells in chronic haemorrhagic stroke. A one-year follow up. Open Neurol J. 2017;11:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang L, Ji H, Li M, et al. Intrathecal administration of autologous CD34 positive cells in patients with past cerebral infarction: A safety study. ISRN Neurol. 2013;2013:e128591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Savitz SI, Yavagal D, Rappard G, et al. A phase 2 randomized, sham-controlled trial of internal carotid artery infusion of autologous bone marrow–derived ALD-401 cells in patients with recent stable ischemic stroke (RECOVER-stroke). Circulation. 2019;139:192–205. [DOI] [PubMed] [Google Scholar]
- 43. Bhatia V, Gupta V, Khurana D, Sharma RR, Khandelwal N. Randomized assessment of the safety and efficacy of intra-arterial infusion of autologous stem cells in subacute ischemic stroke. Am J Neuroradiol. 2018;39:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghali AA, Yousef MK, Ragab OA, ElZamarany EA. Intra-arterial infusion of autologous bone marrow mononuclear stem cells in subacute ischemic stroke patients. Front Neurol. 2016;7:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moniche F, Gonzalez A, Gonzalez-Marcos JR, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke. Stroke. 2012;43:2242–2244. [DOI] [PubMed] [Google Scholar]
- 46. Friedrich MAG, Martins MP, Araújo MD, et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012; 21(1_suppl):13–21. [DOI] [PubMed] [Google Scholar]
- 47. Banerjee S, Bentley P, Hamady M, et al. Intra-arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Transl Med. 2014;3:1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Celis-Ruiz E, Fuentes B, Alonso de Leciñana M, et al. Final results of allogeneic adipose tissue–derived mesenchymal stem cells in acute ischemic stroke (AMASCIS): A phase II, randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. Cell Transplant. 2022;31:09636897221083863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bhasin A, Srivastava MVP, Mohanty S, et al. Paracrine mechanisms of intravenous bone marrow-derived mononuclear stem cells in chronic ischemic stroke. Cerebrovasc Dis Extra. 2016;6:107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bhasin A, Srivastava M, Bhatia R, Mohanty S, Kumaran S, Bose S. Autologous intravenous mononuclear stem cell therapy in chronic ischemic stroke. J Stem Cells Regen Med. 2012;8:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Prasad K, Sharma A, Garg A, et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke. Stroke. 2014;45:3618–3624. [DOI] [PubMed] [Google Scholar]
- 52. Taguchi A, Sakai C, Soma T, et al. Intravenous autologous bone marrow mononuclear cell transplantation for stroke: Phase1/2a clinical trial in a homogeneous group of stroke patients. Stem Cells Dev. 2015;24:2207–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Prasad K, Mohanty S, Bhatia R, et al. Autologous intravenous bone marrow mononuclear cell therapy for patients with subacute ischaemic stroke: A pilot study. Indian J Med Res. 2012;136:221–228. [PMC free article] [PubMed] [Google Scholar]
- 54. Lee J, Chang WH, Chung J-W, et al. Efficacy of intravenous mesenchymal stem cells for motor recovery after ischemic stroke: A neuroimaging study. Stroke. 2022;53:20–28. [DOI] [PubMed] [Google Scholar]
- 55. Law ZK, Tan HJ, Chin SP, et al. The effects of intravenous infusion of autologous mesenchymal stromal cells in patients with subacute middle cerebral artery infarct: A phase 2 randomized controlled trial on safety, tolerability and efficacy. Cytotherapy. 2021;23:833–840. [DOI] [PubMed] [Google Scholar]
- 56. Chung J-W, Chang WH, Bang OY, et al. Efficacy and safety of intravenous mesenchymal stem cells for ischemic stroke. Neurology. 2021;96:e1012–e1023. [DOI] [PubMed] [Google Scholar]
- 57. Jaillard A, Hommel M, Moisan A, et al. Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: A randomized clinical trial. Transl Stroke Res. 2020;11:910–923. [DOI] [PubMed] [Google Scholar]
- 58. Levy ML, Crawford JR, Dib N, Verkh L, Tankovich N, Cramer SC. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke. 2019;50:2835–2841. [DOI] [PubMed] [Google Scholar]
- 59. Bhasin A, Kumaran SS, Bhatia R, Mohanty S, Srivastava MVP. Safety and feasibility of autologous mesenchymal stem cell transplantation in chronic stroke in Indian patients. A four-year follow up. J Stem Cells Regen Med. 2017;13:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bhasin A, Padma Srivastava MV, Mohanty S, Bhatia R, Kumaran SS, Bose S. Stem cell therapy: A clinical trial of stroke. Clin Neurol Neurosurg. 2013;115:1003–1008. [DOI] [PubMed] [Google Scholar]
- 61. Fang J, Guo Y, Tan S, et al. Autologous endothelial progenitor cells transplantation for acute ischemic stroke: A 4-year follow-up study. Stem Cells Transl Med. 2019;8:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hess DC, Wechsler LR, Clark WM, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:360–368. [DOI] [PubMed] [Google Scholar]
- 63. Laskowitz DT, Bennett ER, Durham RJ, et al. Allogeneic umbilical cord blood infusion for adults with ischemic stroke: Clinical outcomes from a phase I safety study. Stem Cells Transl Med. 2018;7:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ohta M, Suzuki Y, Noda T, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004;187:266–278. [DOI] [PubMed] [Google Scholar]
- 65. Cruz-Martinez P, González-Granero S, Molina-Navarro MM, et al. Intraventricular injections of mesenchymal stem cells activate endogenous functional remyelination in a chronic demyelinating murine model. Cell Death Dis. 2016;7:e2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen S-J, Chang C-M, Tsai S-K, et al. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev. 2010;19:1757–1767. [DOI] [PubMed] [Google Scholar]
- 67. Li L, Jiang Q, Ding G, et al. Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an MRI study. J Cereb Blood Flow Metab. 2010;30:653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lim JY, Jeong CH, Jun JA, et al. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia. Stem Cell Res Ther. 2011;2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang Z, Yang X, He J, Du J, Liu S, Jia X. Intracerebroventricular administration of neural stem cells after cardiac arrest. In: 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE; 2019:p 4213–4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Oh SH, Kim HN, Park H-J, Shin JY, Lee PH. Mesenchymal stem cells increase hippocampal neurogenesis and neuronal differentiation by enhancing the Wnt signaling pathway in an Alzheimer’s disease model. Cell Transplant. 2015;24:1097–1109. [DOI] [PubMed] [Google Scholar]
- 71. Smith EJ, Stroemer RP, Gorenkova N, et al. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells. 2012;30:785–796. [DOI] [PubMed] [Google Scholar]
- 72. Zhang B, Joseph B, Saatman KE, Chen L. Intra-Arterial delivery of neural stem cells to the rat and mouse brain: Application to cerebral ischemia. JoVE J Vis Exp. 2020;160:e61119. [DOI] [PubMed] [Google Scholar]
- 73. Shimizu S. Hedrich HJ, Bullock GR, eds. The laboratory mouse. Elsevier Academic Press; 2004. [Google Scholar]
- 74. Krueger TEG, Thorek DLJ, Denmeade SR, Isaacs JT, Brennen WN. Concise review: Mesenchymal stem cell-based drug delivery: The good, the bad, the ugly, and the promise. Stem Cells Transl Med. 2018;7:651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guzman R, De Los Angeles A, Cheshier S, et al. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke. 2008;39:1300–1306. [DOI] [PubMed] [Google Scholar]
- 76. Pendharkar AV, Chua JY, Andres RH, et al. Biodistribution of neural stem cells after intravascular therapy for hypoxic–ischemia. Stroke. 2010;41:2064–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gutiérrez-Fernández M, Rodríguez-Frutos B, Álvarez-Grech J, et al. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience. 2011;175:394–405. [DOI] [PubMed] [Google Scholar]
- 78. Shen LH, Li Y, Chen J, et al. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–399. [DOI] [PubMed] [Google Scholar]
- 79. Kamiya N, Ueda M, Igarashi H, et al. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci. 2008;83:433–437. [DOI] [PubMed] [Google Scholar]
- 80. Chua JY, Pendharkar AV, Wang N, et al. Intra-arterial injection of neural stem cells using a microneedle technique does not cause microembolic strokes. J Cereb Blood Flow Metab. 2011;31:1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cui L-L, Kerkelä E, Bakreen A, et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;18:813–819. [DOI] [PubMed] [Google Scholar]
- 83. Yavagal DR, Lin B, Raval AP, et al. Efficacy and dose-dependent safety of intra-arterial delivery of mesenchymal stem cells in a rodent stroke model. PloS ONE. 2014;9:e93735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rosado-de-Castro PH, Schmidt FR, Battistella V, et al. Biodistribution of bone marrow mononuclear cells after intra-arterial or intravenous transplantation in subacute stroke patients. Regen Med. 2013;8:145–155. [DOI] [PubMed] [Google Scholar]
- 85. Willing AE, Lixian J, Milliken M, et al. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73:296–307. [DOI] [PubMed] [Google Scholar]
- 86. Kraitchman DL, Tatsumi M, Gilson WD, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20:531–536. [DOI] [PubMed] [Google Scholar]
- 88. Jeong S-W, Chu K, Jung K-H, Kim SU, Kim M, Roh J-K. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003;34:2258–2263. [DOI] [PubMed] [Google Scholar]
- 89. Chu K, Kim M, Chae S-H, et al. Distribution and in situ proliferation patterns of intravenously injected immortalized human neural stem-like cells in rats with focal cerebral ischemia. Neurosci Res. 2004;50:459–465. [DOI] [PubMed] [Google Scholar]
- 90. Chu K, Kim M, Jeong S-W, Kim SU, Yoon B-W. Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci Lett. 2003;343:129–133. [DOI] [PubMed] [Google Scholar]
- 91. Vendrame M, Cassady J, Newcomb J, et al. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. [DOI] [PubMed] [Google Scholar]
- 92. Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–703. [DOI] [PubMed] [Google Scholar]
- 93. Chu K, Kim M, Park K-I, et al. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004;1016:145–153. [DOI] [PubMed] [Google Scholar]
- 94. Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. [DOI] [PubMed] [Google Scholar]
- 95. Chen J, Li Y, Katakowski M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–786. [DOI] [PubMed] [Google Scholar]
- 96. Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628. [DOI] [PubMed] [Google Scholar]
- 97. Wei N, Yu SP, Gu X, et al. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant. 2013;22:977–991. [DOI] [PubMed] [Google Scholar]
- 98. van Velthoven CTJ, Kavelaars A, van Bel F, Heijnen CJ. Nasal administration of stem cells: A promising novel route to treat neonatal ischemic brain damage. Pediatr Res. 2010;68:419–422. [DOI] [PubMed] [Google Scholar]
- 99. van Velthoven CTJ, Sheldon RA, Kavelaars A, et al. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke. 2013;44:1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Engelhardt B, Sorokin L. The blood–brain and the blood–cerebrospinal fluid barriers: Function and dysfunction. Semin Immunopathol. 2009;31:497–511. [DOI] [PubMed] [Google Scholar]
- 101. Forrester JV, McMenamin PG, Dando SJ. CNS infection and immune privilege. Nat Rev Neurosci. 2018;19:655.- . [DOI] [PubMed] [Google Scholar]
- 102. Iadecola C, Buckwalter MS, Anrather J. Immune responses to stroke: Mechanisms, modulation, and therapeutic potential. J Clin Invest. 2020;130:2777–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhou W, Liesz A, Bauer H, et al. Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol. 2013;23:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55:195–203. [DOI] [PubMed] [Google Scholar]
- 105. Easton AS. Neutrophils and stroke – Can neutrophils mitigate disease in the central nervous system? Int Immunopharmacol. 2013;17:1218–1225. [DOI] [PubMed] [Google Scholar]
- 106. Butcher EC. Leukocyte-endothelial cell recognition: Three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. [DOI] [PubMed] [Google Scholar]
- 107. Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: Specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118:6743–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kerfoot SM, Kubes P. Overlapping roles of P-selectin and α4 integrin to recruit leukocytes to the central nervous system in experimental autoimmune encephalomyelitis. J Immunol. 2002;169:1000–1006. [DOI] [PubMed] [Google Scholar]
- 109. Middleton J, Neil S, Wintle J, et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. [DOI] [PubMed] [Google Scholar]
- 110. Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 111. Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Herter J, Zarbock A. Integrin regulation during leukocyte recruitment. J Immunol. 2013;190:4451–4457. [DOI] [PubMed] [Google Scholar]
- 113. Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. [DOI] [PubMed] [Google Scholar]
- 114. Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. [DOI] [PubMed] [Google Scholar]
- 115. Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: A molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Filippi MD. Neutrophil transendothelial migration: Updates and new perspectives. Blood. 2019;133:2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Muller WA. Transendothelial migration: Unifying principles from the endothelial perspective. Immunol Rev. 2016;273:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Muller WA. Migration of leukocytes across endothelial junctions: Some concepts and controversies. Microcirculation. 2001;8:181–193. [DOI] [PubMed] [Google Scholar]
- 119. Mapunda JA, Tibar H, Regragui W, Engelhardt B. How does the immune system enter the brain? Front Immunol. 2022;13:805657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wu C, Ivars F, Anderson P, et al. Endothelial basement membrane laminin α5 selectively inhibits T lymphocyte extravasation into the brain. Nat Med. 2009;15:519–527. [DOI] [PubMed] [Google Scholar]
- 121. Engelhardt B, Ransohoff RM. Capture, crawl, cross: The T cell code to breach the blood–brain barriers. Trends Immunol. 2012;33:579–589. [DOI] [PubMed] [Google Scholar]
- 122. Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18:123–131. [DOI] [PubMed] [Google Scholar]
- 123. Agrawal S, Anderson P, Durbeej M, et al. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kivisäkk P, Mahad DJ, Callahan MK, et al. Human cerebrospinal fluid central memory CD4+ T cells: Evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wolburg K, Gerhardt H, Schulz M, Wolburg H, Engelhardt B. Ultrastructural localization of adhesion molecules in the healthy. Cell Tissue Res. 1999;296:259–269. [DOI] [PubMed] [Google Scholar]
- 126. Reboldi A, Coisne C, Baumjohann D, et al. C-C chemokine receptor 6–regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. [DOI] [PubMed] [Google Scholar]
- 127. Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory disequilibrium in stroke. Circ Res. 2016;119:142–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. 2019;16:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Alam A, Thelin EP, Tajsic T, et al. Cellular infiltration in traumatic brain injury. J Neuroinflammation. 2020;17:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yang C, Hawkins KE, Doré S, Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol - Cell Physiol. 2019;316:C135–C153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Gelderblom M, Gallizioli M, Ludewig P, et al. IL-23 (interleukin-23)–producing conventional dendritic cells control the detrimental IL-17 (interleukin-17) response in stroke. Stroke. 2018;49:155–164. [DOI] [PubMed] [Google Scholar]
- 132. Scherbel U, Raghupathi R, Nakamura M, et al. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc Natl Acad Sci U S A. 1999;96:8721–8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Clark WM. Cytokines and reperfusion injury. Neurology. 1997;49(Suppl 4):S10–S14. [DOI] [PubMed] [Google Scholar]
- 134. Wang X, Yue TL, Barone FC, Feuerstein GZ. Demonstration of increased endothelial-leukocyte adhesion molecule–1 mRNA expression in rat ischemic cortex. Stroke. 1995;26:1665–1669. [DOI] [PubMed] [Google Scholar]
- 135. Berti R, Williams AJ, Moffett JR, et al. Quantitative real-time RT—PCR analysis of inflammatory gene expression associated with ischemia—Reperfusion brain injury. J Cereb Blood Flow Metab. 2002;22:1068–1079. [DOI] [PubMed] [Google Scholar]
- 136. Zhang R, Chopp M, Zhang Z, Jiang N, Powers C. The expression of P- and E-selectins in three models of middle cerebral artery occlusion. Brain Res. 1998;785:207–214. [DOI] [PubMed] [Google Scholar]
- 137. Connolly ES, Winfree CJ, Prestigiacomo CJ, et al. Exacerbation of cerebral injury in mice that express the P-selectin gene. Circ Res. 1997;81:304–310. [DOI] [PubMed] [Google Scholar]
- 138. Suzuki H, Abe K, Tojo S, et al. Expressions of P-selectin- and HSP72-like immunoreactivities in rat brain after transient middle cerebral artery occlusion. Brain Res. 1997;759:321–329. [DOI] [PubMed] [Google Scholar]
- 139. Okada Y, Copeland BR, Mori E, Tung MM, Thomas WS, del Zoppo GJ. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke. 1994;25:202–211. [DOI] [PubMed] [Google Scholar]
- 140. Kawecki C, Lenting PJ, Denis CV. von Willebrand factor and inflammation. J Thromb Haemost. 2017;15:1285–1294. [DOI] [PubMed] [Google Scholar]
- 141. Sathiyanadan K, Coisne C, Enzmann G, Deutsch U, Engelhardt B. PSGL-1 and E/P-selectins are essential for T-cell rolling in inflamed CNS microvessels but dispensable for initiation of EAE. Eur J Immunol. 2014;44:2287–2294. [DOI] [PubMed] [Google Scholar]
- 142. Minami M, Satoh M. Chemokines and their receptors in the brain: Pathophysiological roles in ischemic brain injury. Life Sci. 2003;74:321–327. [DOI] [PubMed] [Google Scholar]
- 143. Kim JS, Gautam SC, Chopp M, et al. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. J Neuroimmunol. 1995;56:127–134. [DOI] [PubMed] [Google Scholar]
- 144. Gourmala NG, Buttini M, Limonta S, Sauter A, Boddeke HWGM. Differential and time-dependent expression of monocyte chemoattractant protein-1 mRNA by astrocytes and macrophages in rat brain: Effects of ischemia and peripheral lipopolysaccharide administration. J Neuroimmunol. 1997;74:35–44. [DOI] [PubMed] [Google Scholar]
- 145. Wang X, Yue T-L, Barone FC, Feuerstein GZ. Monocyte chemoattractant protein–1 messenger RNA expression in rat ischemic cortex. Stroke. 1995;26:661–666. [DOI] [PubMed] [Google Scholar]
- 146. Che X, Ye W, Panga L, Wu D-C, Yang G-Y. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res. 2001;902:171–177. [DOI] [PubMed] [Google Scholar]
- 147. Yamagami S, Tamura M, Hayashi M, et al. Differential production of MCP-1 and cytokine-induced neutrophil chemoattractant in the ischemic brain after transient focal ischemia in rats. J Leukoc Biol. 1999;65:744–749. [DOI] [PubMed] [Google Scholar]
- 148. Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. [DOI] [PubMed] [Google Scholar]
- 149. Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood–brain barrier permeability during ischemia–Reperfusion injury. J Cereb Blood Flow Metab. 2006;26:797–810. [DOI] [PubMed] [Google Scholar]
- 150. Chen Y, Hallenbeck JM, Ruetzler C, et al. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003;23:748–755. [DOI] [PubMed] [Google Scholar]
- 151. Li L, Lou W, Li H, Zhu Y, Huang X. Upregulated C-C motif chemokine ligand 2 promotes ischemic stroke via chemokine signaling pathway. Ann Vasc Surg. 2020;68:476–486. [DOI] [PubMed] [Google Scholar]
- 152. Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Semin Immunol. 2003;15:23–32. [DOI] [PubMed] [Google Scholar]
- 153. Hill WD, Hess DC, Martin-Studdard A, et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: Association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. [DOI] [PubMed] [Google Scholar]
- 154. Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Cowell RM, Xu H, Galasso JM, Silverstein FS. Hypoxic-Ischemic injury induces macrophage inflammatory protein-1α expression in immature rat brain. Stroke. 2002;33:795–801. [DOI] [PubMed] [Google Scholar]
- 156. Terao Y, Ohta H, Oda A, Nakagaito Y, Kiyota Y, Shintani Y. Macrophage inflammatory protein-3alpha plays a key role in the inflammatory cascade in rat focal cerebral ischemia. Neurosci Res. 2009;64:75–82. [DOI] [PubMed] [Google Scholar]
- 157. Yamasaki Y, Matsuo Y, Matsuura N, Onodera H, Itoyama Y, Kogure K. Transient increase of cytokine-induced neutrophil chemoattractant, a member of the interleukin-8 family, in ischemic brain areas after focal ischemia in rats. Stroke. 1995;26:318–323. [DOI] [PubMed] [Google Scholar]
- 158. Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–3043. [PubMed] [Google Scholar]
- 159. Kostulas N, Kivisäkk P, Huang Y, Matusevicius D, Kostulas V, Link H. Ischemic stroke is associated with a systemic increase of blood mononuclear cells expressing interleukin-8 mRNA. Stroke. 1998;29:462–466. [DOI] [PubMed] [Google Scholar]
- 160. Carlos T, Harlan J. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 161. Zhang R-L, Chopp M, Zaloga C, et al. The temporal profiles of ICAM-1 protein and mRNA expression after transient MCA occlusion in the rat. Brain Res. 1995;682:182–188. [DOI] [PubMed] [Google Scholar]
- 162. Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab. 1995;15:42–51. [DOI] [PubMed] [Google Scholar]
- 163. Justicia C, Martín A, Rojas S, et al. Anti-VCAM-1 antibodies did not protect against ischemic damage either in rats or in mice. J Cereb Blood Flow Metab. 2006;26:421–432. [DOI] [PubMed] [Google Scholar]
- 164. Natalizumab: AN 100226, anti-4alpha integrin monoclonal antibody. Drugs RD. 2004;5:102–107. [DOI] [PubMed] [Google Scholar]
- 165. Hu T, Sun R, Huang F, et al. CD99 Mediates neutrophil transmigration through the bEnd.3 monolayer via the induction of oxygen-glucose deprivation. Biochem Biophys Res Commun. 2020;526:799–804. [DOI] [PubMed] [Google Scholar]
- 166. Nourshargh S, Krombach F, Dejana E. The role of JAM-A and PECAM-1 in modulating leukocyte infiltration in inflamed and ischemic tissues. J Leukoc Biol. 2006;80:714–718. [DOI] [PubMed] [Google Scholar]
- 167. Amantea D, Russo R, Gliozzi M, et al. Early upregulation of matrix metalloproteinases following reperfusion triggers neuroinflammatory mediators in brain ischemia in rat. In: International review of neurobiology. Vol. 82. Neuroinflammation in neuronal death and repair. Academic Press; 2007:149–169. [DOI] [PubMed] [Google Scholar]
- 168. Kurzepa J, Kurzepa J, Golab P, Czerska S, Bielewicz J. The significance of matrix metalloproteinase (MMP)-2 and MMP-9 in the ischemic stroke. Int J Neurosci. 2014;124:707–716. [DOI] [PubMed] [Google Scholar]
- 169. Chaturvedi M, Kaczmarek L. MMP-9 inhibition: A therapeutic strategy in ischemic stroke. Mol Neurobiol. 2014;49:563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kumar G, Patnaik R. Inhibition of gelatinases (MMP-2 and MMP-9) by Withania somnifera phytochemicals confers neuroprotection in stroke: An in silico analysis. Interdiscip Sci. 2018;10:722–733. [DOI] [PubMed] [Google Scholar]
- 171. Schuette-Nuetgen K, Strecker JK, Minnerup J, Ringelstein EB, Schilling M. MCP-1/CCR-2-double-deficiency severely impairs the migration of hematogenous inflammatory cells following transient cerebral ischemia in mice. Exp Neurol. 2012;233:849–858. [DOI] [PubMed] [Google Scholar]
- 172. Sun L, Lee J, Fine HA. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest. 2004;113:1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Soto MS, Serres S, Anthony DC, Sibson NR. Functional role of endothelial adhesion molecules in the early stages of brain metastasis. Neuro-Oncol. 2014;16:540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Pedrosa RMSM, Mustafa DA, Soffietti R, Kros JM. Breast cancer brain metastasis: Molecular mechanisms and directions for treatment. Neuro-Oncol. 2018;20:1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Bos PD, Zhang XH-F, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Nitzsche F, Müller C, Lukomska B, Jolkkonen J, Deten A, Boltze J. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells Dayt Ohio. 2017;35:1446–1460. [DOI] [PubMed] [Google Scholar]
- 177. Rombouts WJC, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–170. [DOI] [PubMed] [Google Scholar]
- 178. Levy O, Zhao W, Mortensen LJ, et al. mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood. 2013;122:e23–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kim SM, Kim D-S, Jeong CH, et al. CXC Chemokine receptor 1 enhances the ability of human umbilical cord blood-derived mesenchymal stem cells to migrate toward gliomas. Biochem Biophys Res Commun. 2011;407:741–746. [DOI] [PubMed] [Google Scholar]
- 180. Yang J-X, Zhang N, Wang H-W, Gao P, Yang Q-P, Wen Q-P. CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats. J Biol Chem. 2015;290:1994–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Huang Y, Wang J, Cai J, et al. Targeted homing of CCR2-overexpressing mesenchymal stromal cells to ischemic brain enhances post-stroke recovery partially through PRDX4-mediated blood-brain barrier preservation. Theranostics. 2018;8:5929–5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Bang OY, Jin KS, Hwang MN, et al. The effect of CXCR4 overexpression on mesenchymal stem cell transplantation in ischemic stroke. Cell Med. 2012;4:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic α4 integrin expression. FASEB J. 2007;21:3917–3927. [DOI] [PubMed] [Google Scholar]
- 184. Gorelik M, Orukari I, Wang J, et al. Use of MR cell tracking to evaluate targeting of glial precursor cells to inflammatory tissue by exploiting the very late antigen-4 docking receptor. Radiology. 2012;265:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sarkar D, Vemula PK, Teo GSL, et al. Chemical engineering of mesenchymal stem cells to induce a cell rolling response. Bioconjug Chem. 2008;19:2105–2109. [DOI] [PubMed] [Google Scholar]
- 186. Sarkar D, Vemula PK, Zhao W, Gupta A, Karnik R, Karp JM. Engineered mesenchymal stem cells with self-assembled vesicles for systemic cell targeting. Biomaterials. 2010;31:5266–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Sarkar D, Spencer JA, Phillips JA, et al. Engineered cell homing. Blood. 2011;118:e184–e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Lo CY, Antonopoulos A, Dell A, Haslam SM, Lee T, Neelamegham S. The use of surface immobilization of P-selectin glycoprotein ligand-1 on mesenchymal stem cells to facilitate selectin mediated cell tethering and rolling. Biomaterials. 2013;34:8213–8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Cheng H, Byrska-Bishop M, Zhang CT, et al. Stem cell membrane engineering for cell rolling using peptide conjugation and tuning of cell–selectin interaction kinetics. Biomaterials. 2012;33:5004–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Won Y-W, Patel AN, Bull DA. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials. 2014;35:5627–5635. [DOI] [PubMed] [Google Scholar]
- 191. Ko IK, Kean TJ, Dennis JE. Targeting mesenchymal stem cells to activated endothelial cells. Biomaterials. 2009;30:3702–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ko IK, Kim BG, Awadallah A, et al. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010;18:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Corti S, Locatelli F, Papadimitriou D, et al. Multipotentiality, homing properties, and pyramidal neurogenesis of CNS-derived LeX(ssea-1)+/CXCR4+ stem cells. FASEB J. 2005;19:1860–1862. [DOI] [PubMed] [Google Scholar]
- 194. Tomchuck SL, Zwezdaryk KJ, Coffelt SB, Waterman RS, Danka ES, Scandurro AB. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Fan H, Zhao G, Liu L, et al. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Shi M, Li J, Liao L, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: Role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897–904. [DOI] [PubMed] [Google Scholar]
- 197. Xinaris C, Morigi M, Benedetti V, et al. A novel strategy to enhance mesenchymal stem cell migration capacity and promote tissue repair in an injury specific fashion. Cell Transplant. 2013;22:423–436. [DOI] [PubMed] [Google Scholar]
- 198. Enoki C, Otani H, Sato D, Okada T, Hattori R, Imamura H. Enhanced mesenchymal cell engraftment by IGF-1 improves left ventricular function in rats undergoing myocardial infarction. Int J Cardiol. 2010;138:9–18. [DOI] [PubMed] [Google Scholar]
- 199. Li Y, Yu X, Lin S, Li X, Zhang S, Song Y-H. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356:780–784. [DOI] [PubMed] [Google Scholar]
- 200. Kim YS, Noh MY, Kim JY, et al. Direct GSK-3β inhibition enhances mesenchymal stromal cell migration by increasing expression of Beta-PIX and CXCR4. Mol Neurobiol. 2013;47:811–820. [DOI] [PubMed] [Google Scholar]
- 201. Qiu Y, Marquez-Curtis LA, Janowska-Wieczorek A. Mesenchymal stromal cells derived from umbilical cord blood migrate in response to complement C1q. Cytotherapy. 2012;14:285–295. [DOI] [PubMed] [Google Scholar]
- 202. Tsai LK, Wang Z, Munasinghe J, Leng Y, Leeds P, Chuang DM. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke. 2011;42:2932–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Levy O, Mortensen LJ, Boquet G, et al. A small-molecule screen for enhanced homing of systemically infused cells. Cell Rep. 2015;10:1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Addington CP, Heffernan JM, Millar-Haskell CS, Tucker EW, Sirianni RW, Stabenfeldt SE. Enhancing neural stem cell response to SDF-1α gradients through hyaluronic acid-laminin hydrogels. Biomaterials. 2015;72:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Addington CP, Dharmawaj S, Heffernan JM, Sirianni RW, Stabenfeldt SE. Hyaluronic acid-laminin hydrogels increase neural stem cell transplant retention and migratory response to SDF-1α. Matrix Biol. 2017;60-61:206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Cencioni C, Melchionna R, Straino S, et al. Ex vivo acidic preconditioning enhances bone marrow ckit+ cell therapeutic potential via increased CXCR4 expression. Eur Heart J. 2013;34:2007–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Wang Y, Deng Y, Zhou GQ. SDF-1α/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–112. [DOI] [PubMed] [Google Scholar]
- 208. Becker AD, Hummelen PV, Bakkus M, et al. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92:440–449. [DOI] [PubMed] [Google Scholar]
- 209. Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1–CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. [DOI] [PubMed] [Google Scholar]
- 210. Nakada-Honda N, Cui D, Matsuda S, Ikeda E. Intravenous injection of cyclophilin A realizes the transient and reversible opening of barrier of neural vasculature through basigin in endothelial cells. Sci Rep. 2021;11:19391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Lee S-H, Kang H-Y, Kim J-H, Park D-H. Mannitol augments the effects of systemical stem cell transplantation without increasing cell migration in a stroke animal model. Tissue Eng Regen Med. 2020;17:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I, Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PloS ONE. 2011;6:e27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Sarvari S, Moakedi F, Hone E, Simpkins JW, Ren X. Mechanisms in blood-brain barrier opening and metabolism-challenged cerebrovascular ischemia with emphasis on ischemic stroke. Metab Brain Dis. 2020;35:851–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Müller B, Lang S, Dominietto M, et al. High-resolution tomographic imaging of microvessels. In: Developments in X-ray tomography VI. Vol. 7078. SPIE; 2008:89–98. [Google Scholar]
- 215. Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: The lung barrier. Transplant Proc. 2007;39:573–576. [DOI] [PubMed] [Google Scholar]
- 216. Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Furlani D, Ugurlucan M, Ong L, et al. Is the intravascular administration of mesenchymal stem cells safe?: Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77:370–376. [DOI] [PubMed] [Google Scholar]
- 218. Wang S, Guo L, Ge J, et al. Excess integrins cause lung entrapment of mesenchymal stem cells. Stem Cells. 2015;33:3315–3326. [DOI] [PubMed] [Google Scholar]
- 219. Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. [DOI] [PubMed] [Google Scholar]
- 220. Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. [DOI] [PubMed] [Google Scholar]
- 221. Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. [DOI] [PubMed] [Google Scholar]
- 222. Borlongan CV, Hadman M, Davis Sanberg C, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–2389. [DOI] [PubMed] [Google Scholar]
- 223. Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: Conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. [DOI] [PubMed] [Google Scholar]
- 224. Baksh D, Zandstra PW, Davies JE. A non-contact suspension culture approach to the culture of osteogenic cells derived from a CD49elow subpopulation of human bone marrow-derived cells. Biotechnol Bioeng. 2007;98:1195–1208. [DOI] [PubMed] [Google Scholar]
- 225. Alimperti S, Lei P, Wen Y, Tian J, Campbell AM, Andreadis ST. Serum-free spheroid suspension culture maintains mesenchymal stem cell proliferation and differentiation potential. Biotechnol Prog. 2014;30:974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Zanetti A, Grata M, Etling EB, Panday R, Villanueva FS, Toma C. Suspension-expansion of bone marrow results in small mesenchymal stem cells exhibiting increased transpulmonary passage following intravenous administration. Tissue Eng Part C Methods. 2015;21:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Lipowsky HH, Bowers DT, Banik BL, Brown JL. Mesenchymal stem cell deformability and implications for microvascular sequestration. Ann Biomed Eng. 2018;46:640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Du M, Kavanagh D, Kalia N, Zhang Z. Characterising the mechanical properties of haematopoietic and mesenchymal stem cells using micromanipulation and atomic force microscopy. Med Eng Phys. 2019;73:18–29. [DOI] [PubMed] [Google Scholar]
- 229. Furman PA, McGuirt PV, Keller PM, Fyfe JA, Elion GB. Inhibition by acyclovir of cell growth and DNA synthesis of cells biochemically transformed with herpesvirus genetic information. Virology. 1980;102:420–430. [DOI] [PubMed] [Google Scholar]
- 230. Nishiyama T, Kawamura Y, Kawamoto K, et al. Antineoplastic effects in rats of 5-fluorocytosine in combination with cytosine deaminase capsules. Cancer Res. 1985;45:1753–1761. [PubMed] [Google Scholar]
- 231. Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: Paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. [PubMed] [Google Scholar]
- 232. Bonini C, Ferrari G, Verzeletti S, et al. HSV-TK Gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. [DOI] [PubMed] [Google Scholar]
- 233. Gallot G, Hallet MM, Gaschet J, et al. Human HLA-specific T-cell clones with stable expression of a suicide gene: A possible tool to drive and control a graft-versus-host-graft-versus-leukemia reaction? Blood. 1996;88:1098–1103. [PubMed] [Google Scholar]
- 234. Sheikh S, Ernst D, Keating A. Prodrugs and prodrug-activated systems in gene therapy. Mol Ther. 2021;29:1716–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Karjoo Z, Chen X, Hatefi A. Progress and problems with the use of suicide genes for targeted cancer therapy. Adv Drug Deliv Rev. 2016;99(Pt A):113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Sato T, Neschadim A, Konrad M, Fowler DH, Lavie A, Medin JA. Engineered human tmpk/AZT as a novel enzyme/prodrug axis for suicide gene therapy. Mol Ther. 2007;15:962–970. [DOI] [PubMed] [Google Scholar]
- 237. Berger C, Flowers ME, Warren EH, Riddell SR. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK–modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107:2294–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Baker R. FDA approves oral ganciclovir as first drug to prevent CMV disease. Food and Drug Administration. BETA. 1995;8. [PubMed] [Google Scholar]
- 239. Dachs GU, Hunt MA, Syddall S, Singleton DC, Patterson AV. Bystander or no bystander for gene directed enzyme prodrug therapy. Molecules. 2009;14:4517–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Hossain JA, Marchini A, Fehse B, Bjerkvig R, Miletic H. Suicide gene therapy for the treatment of high-grade glioma: Past lessons, present trends, and future prospects. Neuro-Oncol Adv. 2020;2:vdaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Tamura R, Miyoshi H, Yoshida K, Okano H, Toda M. Recent progress in the research of suicide gene therapy for malignant glioma. Neurosurg Rev. 2021;44:29–49. [DOI] [PubMed] [Google Scholar]
- 242. Weiner GJ. Rituximab: Mechanism of action. Semin Hematol. 2010;47:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. [DOI] [PubMed] [Google Scholar]
- 244. Introna M, Barbui AM, Bambacioni F, et al. Genetic modification of human T cells with CD20: A strategy to purify and lyse transduced cells with anti-CD20 antibodies. Hum Gene Ther. 2000;11:611–620. [DOI] [PubMed] [Google Scholar]
- 245. Serafini M, Manganini M, Borleri G, et al. Characterization of CD20-transduced T lymphocytes as an alternative suicide gene therapy approach for the treatment of graft-versus-host disease. Hum Gene Ther. 2004;15:63–76. [DOI] [PubMed] [Google Scholar]
- 246. Griffioen M, van Egmond EHM, Kester MGD, Willemze R, Falkenburg JHF, Heemskerk MHM. Retroviral transfer of human CD20 as a suicide gene for adoptive T-cell therapy. Haematologica. 2009;94:1316–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Philip B, Kokalaki E, Mekkaoui L, et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood. 2014;124:1277–1287. [DOI] [PubMed] [Google Scholar]
- 248. Wang X, Chang W-C, Wong CW, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Paszkiewicz PJ, Fräßle SP, Srivastava S, et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J Clin Invest. 2016;126:4262–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Kieback E, Charo J, Sommermeyer D, Blankenstein T, Uckert W. A safeguard eliminates T cell receptor gene-modified autoreactive T cells after adoptive transfer. Proc Natl Acad Sci U S A. 2008;105:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-drug conjugate-based therapeutics: State of the science. JNCI J Natl Cancer Inst. 2019;111:538–549. [DOI] [PubMed] [Google Scholar]
- 252. Tong JTW, Harris PWR, Brimble MA, Kavianinia I. An insight into FDA approved antibody-drug conjugates for cancer therapy. Molecules. 2021;26:5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Marin B-M, Porath KA, Jain S, et al. Heterogeneous delivery across the blood-brain barrier limits the efficacy of an EGFR-targeting antibody drug conjugate in glioblastoma. Neuro-Oncol. 2021;23:2042–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Straathof KC, Pulè MA, Yotnda P, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Miller CP, Blau CA. Using gene transfer to circumvent off-target effects. Gene Ther. 2008;15:759–764. [DOI] [PubMed] [Google Scholar]
- 256. Gabrusiewicz K, Hossain MB, Cortes-Santiago N, et al. Macrophage ablation reduces M2-like populations and jeopardizes tumor growth in a MAFIA-based glioma model. Neoplasia. 2015;17:374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Marin V, Cribioli E, Philip B, et al. Comparison of different suicide-gene strategies for the safety improvement of genetically manipulated T cells. Hum Gene Ther Methods. 2012;23:376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.