Abstract
Gemcitabine is a widely used anti-cancer drug of pyrimidine structure, which can exist as a free base molecular form in crystals. Tautomers are structural isomers of molecules, which interconvert via proton transfer. Mechano-chemistry studies reactions of solids under mechanical impact. We investigated gemcitabine free base for the presence of specific molecular tautomers, using ATR-FTIR spectroscopic analysis, powder XRD, optical microscopy and HPLC. The amino-keto tautomer has the characteristic infrared (IR) peak of the amino group at 3390 cm−1. For the first time, the imino-keto tautomer of gemcitabine free base was detected. The imino-keto tautomer has the characteristic IR peak of the =N–H group, and its peak due to the C=O group in pyrimidine ring is shifted vs. that of the amino-keto tautomer. This serves as the unique spectroscopic “fingerprints” of these tautomers. The ATR-FTIR spectroscopic analysis shows that gemcitabine free base can be enriched with the amino-keto or the imino-keto tautomer. Further, we studied the transformation of gemcitabine free base in crystals between its tautomers under conditions of liquid-assisted grinding (LAG). The imino-keto tautomer undergoes tautomerization to the amino-keto tautomer, while the amino-keto tautomer remains stable. No destruction of molecules of gemcitabine free base, when present as either tautomer, occurs during LAG as was verified by the HPLC-UV analysis. LAG is a new, straightforward, facile and fast method to interconvert tautomers in crystals, and ATR-FTIR spectroscopy is a method of choice to study tautomerization reactions of pharmaceuticals. The presented approach is promising for analysis of crystals of drugs containing one or more than one tautomer, and the knowledge-driven design of composite materials, which contain specific tautomeric molecular forms of pyrimidines, purines and other biologically active heterocyclic compounds.
Keywords: gemcitabine, tautomer, tautomerization, mechano-chemistry, ATR-FTIR, spectroscopy
Graphical abstract

1). Introduction
Gemcitabine (Gem) is major anti-cancer antimetabolite drug of pyrimidine structure [1] which can exist in crystals as its free base (molecular) form or as hydrochloride salt. The United States Food and Drug Administration (USDA) has approved gemcitabine hydrochloride (Gemzar®) for chemotherapy of pancreatic and other cancers. Gemcitabine is actively investigated, both in-vitro and in-vivo, as an anti-cancer agent. Further, encapsulation of gemcitabine to suitable matrices is of significant interest for delayed drug release [2] and local drug delivery.
Mechano-chemistry (MC) refers to the branch of chemical science, which studies reactions of solids under mechanical impact. The MC reactions span across organic and inorganic chemistry, and they are widely used in research and industrial processing of pharmaceuticals. In particular, MC processing is used in synthesis of drugs [3] and their complexes with molecules. The MC reaction is conducted by grinding the specimen, usually at ambient temperature, without or with a small amount of grinding fluid. The MC process using grinding fluid is denoted liquid-assisted grinding, LAG [4].
Tautomers are structural isomers which can interconvert in the process of tautomerization – the intramolecular proton transfer. Their most well-known types are the amino-, imino-, keto-, and enol tautomers. The keto-enol tautomers of pyrimidines [5] and purines [6] are of importance to base pairing in DNA and RNA [7]. Further, tautomerization plays major role in mechanism of action of medicinal drugs [8]. Notably, molecules of several major pyrimidine [9] and purine [10] drugs exist in the tautomeric forms. For other drugs, stable tautomers have been found in crystals e.g. [11]. In crystals, proton transfer in the tautomerization reaction can be very slow, so it may be possible to prepare solids containing one specific tautomer [12]. Mechanochemical treatment of crystals could result in transformation of the less thermodynamically stable form to the more stable one. This property has been used in studies of reactions of pharmaceuticals [13]. To our knowledge, tautomerization of crystals of gemcitabine free base has not been investigated under mechano-chemical (MC) treatment.
Vibrational spectroscopy is widely used in analysis of pharmaceuticals [14] and in characterization of active pharmaceutical ingredients prepared by grinding [3]. Recently, Kazarian at al. [15] published a comprehensive review of using ATR-FTIR spectroscopy for analysis of pharmaceuticals. In particular, attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy was used in characterization of grinded medicinal drugs [16] and to study drug polymorphs administered as part of cancer chemotherapy [17].
In this work, we report detection of specific tautomeric molecular forms of Gem by ATR-FTIR spectroscopy and complementary methods. Further, mechano-chemical transformation of Gem results in reaction of its tautomerization. First, identification of two distinct tautomers of Gem has been done, based on their characteristic peaks of specific functional groups in ATR-FTIR spectra. Second, the presence of one or two tautomers in the given specimen of Gem was detected. Third, a study of Gem is conducted, based on complementary XRD and optical microscopy, of the correlation between its “macroscopic” properties (shape and size of crystals) and the “microscopic” property, i.e. the content of molecules present as two distinct tautomers. Fourths, the capability of liquid-assisted grinding (LAG) was tested to allow the controllable conversion between two major tautomers in crystals of Gem. Fifths, an HPLC-UV analysis was used to check for the absence of other reactions of Gem, than tautomerization, occurring during the LAG.
2). Material and Methods
Gemcitabine free base (Gem) was obtained from two suppliers. Form 1 was from ACROS Organics (product AC45689-0050) of ≥97.5 % purity by high-performance liquid chromatography (HPLC). Form 2 was from LC Laboratories (product # G-4199) of >99 % purity. Upon receiving, both samples were kept in a freezer at −20 °C as specified by vendors.
The LAG was conducted using automatic grinder (model Retsch Qiagen TissueLyser, by Retsch GmbH & Co. KG, Haan, Germany) which was equipped with two 10 ml stainless steel grinding vials. In each grinding vial, a sample of 1 mmol Gem was mixed with 0.3 ml n-hexane (anhydrous ≥96 % from TCI). In addition, each vial contained two stainless steel grinding balls 12 mm in diameter. The grinding frequency was set at 30 Hz and grinding time was 30 min.
ATR-FTIR spectra of samples before and after LAG were collected using infrared spectrometer model Nicolet IS10 (from Thermo Fisher Scientific, Madison, WI, USA). This instrument was equipped with ATR attachment model Golden Gate (from SPECAC INC., Fort Washington, PA, USA). The spectra were collected using OMNIC software, spectral resolution was set at 4 cm−1 and each spectrum was averaged 512 times. When using higher spectroscopic resolution setting at 2 cm−1 was attempted, the result was low absorbance, especially in the high wavenumbers range of ca. 3600-3000 cm−1, and poor quality of spectra. To avoid negative effects of water vapor in air on IR spectra, the FTIR spectrometer was continuously purged with dried air at the flow rate of 40 scfh (standard cubic feet per hour). Dried air was produced by the FT-IR purge gas generator (model 75-45 Whatman - Parker Balston, made by Parker Hannifin Corporation, Haverhill, MA, USA). The inlet of FT-IR purge gas generator at pressure of 60 psi (square pound per inch) was connected to the outlet of a single-stage portable electric hot dog air compressor (model 2-Gallon Kobalt QUIET TECH, made by Kobalt Tools, Mooresville, NC, USA). To continuously monitor the quality of FTIR spectra and remove artifacts due to water vapor, the Atmospheric Correction option was enabled in OMNIC software. The Spectral Quality Results parameter in OMNIC software was set for “H2O level” parameter at ≥ 85 %. ATR-FTIR spectra were plotted in absorbance mode.
Powder X-Ray diffraction (XRD) patterns were collected using diffractometer Rigaku MiniFlex (from Rigaku Corporation, Tokyo, Japan) on a Cu K-alpha line at 0.15418 nm with increments of the 2θ angle at 0.02 deg.
Optical microscopy was performed using microscope Nikon i90 (from Nikon Instruments Inc., Melville, NY, USA) at x40 magnification. Samples were sandwiched between two microscope glass slides with small amount of liquid immersion microscope oil.
HPLC-UV analysis was conducted using Agilent 1100 instrument (from Agilent Technologies Inc., Santa Clara, CA, USA) and Chemstation software. The protocol was similar to the one in [18]; mobile phase was a 25:75 vol/vol mixture of methanol and 1.36 % aqueous solution of potassium dihydrogen phosphate. A reverse phase HPLC column Eclipse XDB-C18 (5 μm) was used in isocratic mode with 0.5 mL/min flow rate at 25 °C. Injection volume was 1 μL and the detection wavelength was 270 nm.
3). Results and Discussion
3.1). Investigation of tautomers in crystals of gemcitabine free base by ATR-FTIR spectroscopic analysis
Figure 1 shows ATR-FTIR spectra of gemcitabine free base (Gem) of Form 1 and Form 2. The high wavenumber range in IR spectra at ca. 3700-3000 cm−1 contains characteristic peaks due to vibrations of N-H and O-H bonds. In this range (Figure 1a) the spectra of Form 1 and Form 2 are distinctly different. In particular, in spectrum of Form 1, a sharp peak at 3475 cm−1 (marked with arrow) is present. This peak is nearly absent in spectrum of Form 2. Further, the peak at 3403 cm−1 of Form 1 is red shifted to 3390 cm−1 for Form 2 (marked with arrow). Next, a wide peak centered at ca. 3200 cm−1 is present for Form 2, but not for Form 1. Such wide peaks are due to hydrogen bonded functional groups; therefore, spectra in Figure 1a reflect different N-H chemical bonds in gemcitabine molecule, when it is present in crystals of Form 1 and Form 2.
Figure 1.

The ATR-FTIR spectra of Gem of Form 1 and Form 2.
In Figure 1a, peaks at ca. 2950 and 2900 cm−1 are due to the C-H stretch vibrations in the sugar (2-deoxy-2,2-difluoro-D-ribose) unit of Gem molecule; there are no peaks within 2800-1750 cm−1.
The low frequency IR range (Figure 1b) also has several major differences for Form 1 and Form 2. However, this spectral range contains group frequencies, which are more difficult to assign to vibrations of specific chemical bonds. Nevertheless, differences in spectra of Form 1 and Form 2 in Figure 1b are significant, and they cannot be explained by minor differences in the purity of two forms of Gem. Chen et al. [19] reported that Gem can exist as distinct crystalline polymorphs with different IR spectra. In particular, the peak of Form 1 at 3403 cm−1 in Figure 1a in this work can be found in the IR spectrum of crystalline “Form” A at 3404.84 cm−1 in [19].
Chen et al. [19] did not assign specific structural formulas to molecules of Gem present in those crystalline polymorphs. This is likely that manufacturers of Gem use various methods of its synthesis [20] and/or purification, which leads to different crystalline polymorphs, while purity of the sample (by chromatographic analysis of solution) is the same. This explains that Form 1 and Form 2 of Gem in this work, which are obtained from two vendors, correspond to different crystalline polymorphs of the same compound.
Figure 2 shows ATR-FTIR spectra of Gem in the mid-IR range, which is often used for detection of molecular groups. The differences in the mid-IR spectra of Form 1 and Form 2 are significant (marked with arrows).
Figure 2.
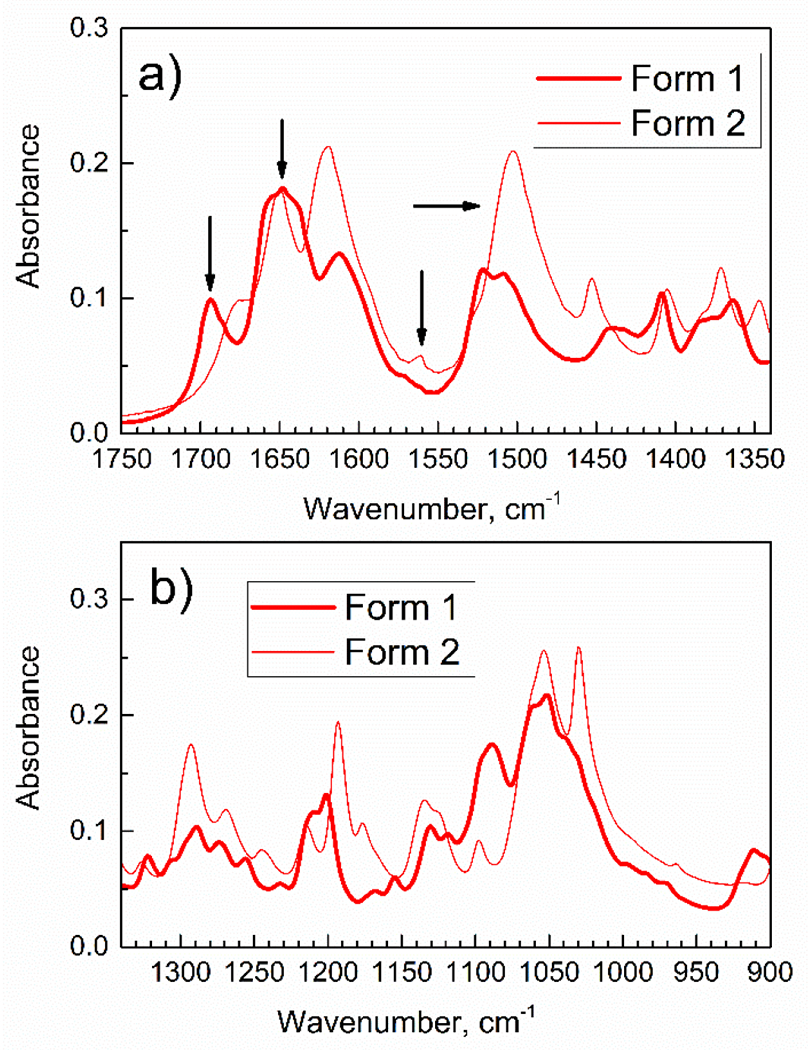
The mid-range ATR-FTIR spectra of Gem of Forms 1 and 2.
Importantly, in Figure 2a the peak at 1694 cm−1 (marked with arrow) is present for Form 1, but not for Form 2.
This is of interest to understand the specific molecular structure of crystalline Gem present as Form 1 and Form 2. Chemical name of gemcitabine free base (Gem) is 2′-deoxy-2′,2′-difluorocytidine, and it contains cytosine ring covalently connected to 2-deoxy-2,2-difluoro-D-ribose. Due to complex structure of Gem molecule, interpretation of its IR spectra relies on model compounds, and one of them is cytosine. For cytosine, keto-enol tautomerism is well-known [6, 21, 22] and it affects the following: the amino group (−NH2), the keto group (−C=O), and nitrogen atom in heterocyclic pyrimidine ring. Scheme 1 shows the reported [23–25] tautomers of cytosine.
Scheme 1.

Molecular structure of tautomers of cytosine.
The alternative names of tautomers of cytosine are as follows [24, 26]. The amino-keto tautomer is also denoted the amino-oxo (AO) tautomer. The amino-enol tautomer is also denoted the amino-hydroxy (AH) tautomer. The imino-keto tautomer is also denoted the imino-oxo (IO) tautomer.
Since molecule of Gem contains the cytosine ring, it is expected to exist in few distinct tautomeric forms, which differ by position of the proton H in cytosine group. However, Gem molecule is able to have not as many tautomers as cytosine molecule since for Gem molecule, one nitrogen atom in cytosine ring is covalently connected to pentose (2-deoxy-2,2-difluoro-D-ribose) unit. The amino-keto tautomer of Gem was predicted by DFT calculations [27] and it is in Scheme 2.
Scheme 2.

Gem molecule in the amino-keto tautomeric form.
Further, the enol tautomer of Gem has been proposed [28], however no spectroscopic study was conducted. This molecular form can be denoted as the imino-hydroxy tautomer, Scheme 3.
Scheme 3.

Gem in the hypothesized imino-hydroxy tautomeric form.
Finally, Gem could potentially exist in the imino-keto tautomeric form, in Scheme 4; to our knowledge, this tautomer has never been reported.
Scheme 4.

Gem in the hypothesized imino-keto tautomeric form.
Major difference between the amino-keto, imino-hydroxy and imino-keto tautomers is localization of proton in the molecule, which should result in the characteristic peaks in IR spectra. Namely, IR peaks would reflect the presence of certain combinations of −NH2, =N–H, − C=O and −O–H groups in the molecule, which structurally define the given tautomer.
FTIR spectra of gemcitabine free base (Gem) were reported in few papers, which describe solid-state nanocomposites for delayed release of this drug [2, 29]. However, these reports assign only few vibrations, mainly the stretching vibration of C=O group [29] and N–H group, and these assignments are contradictory. To comprehensively assign the FTIR peaks of Gem of Form 2 in this work to functional groups in specific tautomer, we used literature assignments of vibrations in the model compounds cytosine and 2-deoxy-2,2-difluoro-D-ribose, see Table 1. Data in Table 1 indicate that Gem of Form 2 in this work exists as the amino-keto tautomer (Scheme 2).
Table 1.
Assignments of IR peaks of gemcitabine free base (Gem) of Form 2.
| Wave number, cm−1 | Compound / wavenumber, cm−1 / literature peak assignment / ref. | Assignment | Tautomer |
|---|---|---|---|
| 3390 s | Gem / 3400 / νsym(NH2) (100%) / [27] | νsym(NH2) | amino- |
| 3306 s | Gem / 3300 s (wide) / ν(O11H26) (100 %) / [27] | ν(O-H) in pentose | - |
| 3215-3180 s, wide | Cytosine / 3170 s / amino group ν(NH) (60%) + ν(NH) (40 %) / [30] | ν(N–H) in NH2 | amino- |
| 1676 s | Gem / 1682 / ν(C=0) (69%) + ν(N7–C6) (15%) / [27] Cytosine / 1684 / ν(C=O) / [23] |
ν(C=O) mainly | keto- |
| 1650 s | Cytosine / 1645-1672 / C=O stretch, hydrogen bonded / [31] | ν(C=O) | keto- |
| 1619 | Gem / 1602 / ν(C4–C5) (38%) + ν(N1–C6) (14%) / [27] | C–C and C–N in cytosine ring | - |
| 1561 w | Gem / 1540 / δ(NH2) (83%) / [27] | δ(NH2) | amino- |
| 1502 s | Cytosine / 1505.6 / δip(ring) and νC–N(H2) / [32] | νC–N(H2) of amino group and cytosine ring | amino- |
| 1452 | Gem / 1442 / not assigned / [27] | - | - |
| 1405 | Gem / 1410 / δ(H26O11C9) / [27] | pentose | - |
| 1371 | Gem / 1350 / δ(H29O18C10) (10%) + τ(H24C14C9) (35%) + τ(H25C10C9) (18%) / [27] | pentose | - |
| 1327 | Gem / 1323 / δ(H29O18C) (21%) + δ(H24C10O18) (29%) + δ(H25C10O18) (14%) / [27] | pentose | - |
| 1293 | Gem / 1297 / ν(N3C4) (16%)+δ(H19C4C5) (10%) +δ(H20C5C4) (15%) / [27] | cytosine ring | - |
| 1134 | Gem / 1133 / ν(FC) (21%) / [27] | ν(C–F) in pentose | - |
| 1097 s | Gem / 1091 / ν(O11C9) (25%) + δ(H14C9C15) (11%) / [27] 2-deoxy-2,2-difluoro-D-ribose / 1098 / ? / [33] |
group vibration in pentose | - |
Abbreviations: s = strong; w = weak; ν = stretching vibration; sym = symmetric vibration; δ = deformation vibration; δip = in-plane deformation vibration.
Next, it is important to understand if molecules of enol (imino-hydroxy) tautomer of gemcitabine (Scheme 3), which has the O–H group, are present in crystals of Form 1 or Form 2. First, presence of the O–H group can be detected in experimental IR spectrum above 3500 cm−1. Szczesniak et al. [34] reported that the amino-hydroxy (aka enol) tautomer of cytosine has strong O–H stretch vibration at 3553 cm−1. Lapinski et al. [26] reported that the O–H stretch of the amino-hydroxy (aka enol) tautomer of cytosine is at ca. 3601 cm−1 and 3591 cm−1.
However, IR peaks at such high wavenumbers are not present in spectra of either Form of Gem in this work. Second, the enol tautomer with the O–H group (Scheme 3) would not have the characteristic C=O stretch, which is often found in IR spectra of cytosine within ca. 1620-1700 cm−1. An exact position of peak due to the C=O stretch depends on: a) magnitude of hydrogen bonding, and b) proximity to the N atom and N-H group in cytosine ring. Importantly, the presence of at least one peak in the 1620-1680 cm−1 range for crystalline Form 1 and Form 2 in this work indicates, that molecules in both contain the C=O group. Namely, Gem molecules of each Form contain keto tautomer (Scheme 2) of gemcitabine free base; this excludes the presence of imino-hydroxy tautomer (Scheme 3).
The final possibility is that Gem of crystalline Form 1 is present, at least in part, as molecules of the imino-keto tautomer (Scheme 4). To our knowledge, vibrational (IR or Raman) spectra of Gem present as its imino-keto tautomer have not been reported. Therefore, we used model compounds cytosine and 2-deoxy-2,2-difluoro-D-ribose. Cytosine was reported as imino-keto tautomer, and its IR and Raman spectra were interpreted, while 2-deoxy-2,2-difluoro-D-ribose has only few IR peaks, Table 2.
Table 2.
Assignments of IR peaks of gemcitabine free base (Gem) of Form 1.
| Wave number, cm−1 | Compound / wavenumber, cm−1 / literature peak assignment / ref. | Assignment | Tautomer |
|---|---|---|---|
| 3477 s | Cytosine / ca. 3495 / imino-keto tautomer / [25] Cytosine / 3497 / the imino-keto (imino-oxo) tautomer / [24] Cytosine / 3495 / imino-keto tautomer / [26] |
ν(N–H) in imino-keto tautomer | imino-keto |
| 3405 s | Cytosine / 3382 / ν(NH) (60%) – ν(NH) (40%) / [30] | ν(NH) | amino- or imino- |
| 3306 w | Gem / 3300 (wide) / ν(O11H26) (100 %) / [27] | ν(O–H) in pentose | - |
| 1694 | Cytosine / 1700 / ν(C=O) / [31] | ν(C=O) | keto (but not amino-keto) |
| 1657 sh | Cytosine / 1659 vs / multiple vibrations including ν(C=O) stretch / [30] | ν(C=O) | keto (but not amino-keto) |
| 1650 s | Cytosine / 1645-1672 / C=O stretch, hydrogen bonded / [31] | ν(C=O) | Keto |
| 1611 | Gem / 1602 / ν(C4–C5) (38%) + ν(N1–C6) (14%) / [27] | C–C and C–N in cytosine ring | - |
| 1507 | Cytosine / 1504 / ring vibrations / [30] | cytosine ring | - |
| 1409 | Gem / 1410 / δ(H26O11C9) / [27] | pentose | - |
| 1364 | Gem / 1350 / δ(H29O18C10) (10%) + τ(H24C14C9) (35%) + τ(H25C10C9) (18%) / [27] | pentose | - |
| 1321 | Gem / 1323 / δ(H29O18C) (21%) + δ(H24C10O18) (29%) + δ(H25C10O18) (14%) / [27] | pentose | - |
| 1290 | Gem / 1297 / ν(N3C4) (16%)+δ(H19C4C5) (10%) +δ(H20C5C4) (15%) / [27] | cytosine ring | - |
| 1131 | Gem / 1133 / ν(FC) (21%) / [27] | ν(C–F) in pentose | - |
| 1096 | Gem / 1091 / ν(O11C9) (25%) + δ(H14C9C15) (11%) / [27] 2-deoxy-2,2-difluoro-D-ribose / 1098 / ? / [33] |
group vibration in pentose | - |
Abbreviations: s = strong; w = weak; sh = shoulder; ν = stretching vibration; δ = deformation vibration.
Analysis of FTIR spectra of Gem in crystalline Form 1 reveals the following. First, it has an unusual peak at 3477 cm−1 which was not reported in spectra of amino-keto tautomer [27]. However, peaks at the similar wavenumbers were reported for the N–H stretch in imino tautomer of cytosine [24–26]. Second, in Figure 2a for Form 1, there is a wide peak at ca. 1650 cm−1 which is similar to reported [34] peak at 1642 cm−1, plus two additional shoulders at 1657 cm−1 and 1638 cm−1. Several peaks in this range indicate that Form 1 contains molecules of gemcitabine free base as a mixture of amino-keto tautomer (Scheme 2) and an additional tautomer. Based on data in Table 2, this is the imino-keto tautomer (Scheme 4).
3.2). Characterization of crystalline forms of gemcitabine by complementary methods
Both shape and size of crystals are significantly different for Form 1 and Form 2. Namely, crystals of Form 2 (bottom) are often much larger with largest size of ca. 15 x 50 μm, and of rectangular shape. In contrast, crystals of Form 1 (top) are smaller of mostly <10 μm in size and their shape is irregular. Larger crystals with regular shape usually mean higher purity of the sample. This indicates that crystalline Form 2 contains, predominantly, molecules of the amino-keto tautomer of Gem. This finding is consistent with FTIR data in Table 1. In Figure S3, small dark circles (top) and stripes (bottom) are due to liquid immersion microscope oil, see Experimental.
On the other hand, for Form 1 (top), its smaller and irregularly shaped crystals indicate that they contain mixture of amino-keto tautomer and the other tautomer (the imino-keto tautomer, see Table 2). Data in Figure S3 and Table 2 provide a correlation between the “macroscopic” properties (shape and size of crystals) of the specimen and its “microscopic” properties, i.e. the relative content of molecules present in the two tautomeric forms.
3.3). Transformation of Form 1 of gemcitabine free base upon liquid-assisted grinding (LAG)
Different tautomers have different relative stability, while mechano-chemistry allows activating chemical reactions, including the tautomerization reaction, by supplying energy to molecules via mechanical impact. In crystals, molecules of the given tautomer could remain for significant amount of time. One could expect that mechano-chemical treatment of crystals of Gem may result in conversion of its molecules from one tautomer to another. To our knowledge, mechano-chemical tautomerization of gemcitabine crystals has not been reported.
Figure 3 shows ATR-FTIR spectra of Gem of Form 1, before and after LAG. Interestingly, spectra of Gem of Form 1 after LAG are similar to spectra of Form 2, compare Figure 3 and Figure 1.
Figure 3.

The ATR-FTIR spectra. Form 1 before LAG; Form 1 after LAG; n-hexane as LAG fluid.
Namely, the sample of Form 1 after LAG (dashed line) does not have the characteristic peak at 3477 cm−1 due to the imino group in molecule of the imino-keto tautomer. Instead, it has a wide peak at ca 3200 cm−1 due to the amino group in molecule of the amino-keto tautomer. This indicates that during LAG, tautomerization of Gem of Form 1 takes place. The HPLC-UV analysis of Gem base in Form 1 before and after LAG indicates no chemical decomposition of this compound (data not shown). Figure 3 also shows the IR spectra of n-hexane (blue line) used as “grinding fluid” in the LAG process. Importantly, n-hexane not significantly contribute to IR spectra of samples; this indicates that n-hexane evaporates soon after LAG, the product of LAG contains only Gem molecules.
An interesting question is mechanism of tautomerization of Gem molecules in crystals of Form 1 during LAG. In LAG, “grinding fluid” is not intended to dissolve reactants or products. For example, in LAG “very small amounts of added liquid” [35] are used, which are often present in the microliter quantities. This amount of liquid is not sufficient to dissolve solid reactant or product, but it results in significant reaction progress.
In this work, “grinding fluid” is n-hexane in which gemcitabine free base is insoluble. Therefore, tautomerization of Gem molecules takes place during LAG without dissolution of Gem. Further, n-hexane is a chemically inert fluid with low boiling point of 69 °C, so it easily evaporates after LAG. Low toxicity of n-hexane is essential for potential use of LAG in preparing new pharmaceutical formulations of anti-cancer drugs, which contain gemcitabine.
Data in Figure 3 indicate that during LAG, gemcitabine free base undergoes transformation from mixture of the imino-keto tautomer and the amino-keto tautomer (Form 1) to a nearly pure amino-keto tautomer. The low frequency IR range in Figure 3b also supports this statement, see the non-symmetric doublet peak at ca. 800 cm−1 which is characteristic for the amino-keto tautomer present in crystals of Form 2.
The mid-IR spectra of Gem of Form 1 are shown in Figure 4.
Figure 4.

The mid-range ATR-FTIR spectra. Form 1 before LAG; Form 1 after LAG; n-hexane as LAG fluid.
Before LAG, see Figure 4a at ca. 1700 cm−1, the characteristic peak of the imino-keto tautomer (crystals of Form 1) is present. After LAG, this peak is absent. In addition, in Figure 4a after LAG a single peak at 1642 cm−1 is observed, instead of a wide IR band with two shoulders typical for spectrum of Form 1 (mixture of tautomers). Further, a strong peak at 1485 cm−1 is formed after LAG as “fingerprint” of the amino-keto tautomer. The spectra in Figure 4b are consistent with finding that the imino-keto tautomer is absent after LAG.
Further, in Figure 4b one can see yet another characteristic change of Form 1 upon LAG: it is a strong enhancement of peak at 1200 cm−1 versus a doublet before LAG. The same conclusion is obtained: mechano-chemical transformation of Gem molecules occurs during LAG, from the imino-keto tautomer to the amino-keto tautomer, Scheme 5.
Scheme 5.

Reaction of tautomerization of Gem molecule during LAG from the imino-keto tautomer to the amino-keto tautomer.
3.4). The behavior of crystals of gemcitabine free base in Form 2 under LAG
It is of interest to investigate if the amino-keto tautomer present in Form 2 is stable in LAG, Figure 5. Spectra of Form 2 before and after LAG are very similar, and n-hexane does not significantly contribute to spectra. This confirms that the amino-keto tautomer present in crystals of Form 2 remains stable under conditions of LAG, which include mechano-chemical force on reactant and potential action of n-hexane as the LAG fluid.
Figure 5.

The ATR-FTIR spectra. Form 2 before LAG; Form 2 after LAG; n-hexane as LAG fluid.
Figure 6 shows the mid-range ATR-FTIR spectra of Form 2 before and after LAG. There are no major changes, meaning that in crystals of Form 2 no tautomerization of Gem molecules occurred after LAG. Findings from spectra in Figure 6 are consistent with findings in Figure 5. This supports the conclusion that after LAG, crystalline Form 2 retains Gem molecules as the amino-keto tautomer.
Figure 6.

The mid-range ATR-FTIR spectra. Form 2 before LAG; Form 2 after LAG; n-hexane as LAG fluid.
Gemcitabine free base is one of major and the archetypal anti-cancer drugs, and structural derivative of pyrimidine. As pertinent to fundamental chemical science, this study contributes to understanding molecules present in crystals of gemcitabine free base, and specifically its tautomeric forms. Beyond gemcitabine, this work opens the path to controlled transformation of tautomers of molecules of substituted pyrimidines, conducted by facile mechano-chemical route. For example, tautomerization affects hydrogen bonding in complexes of small molecules [6], and inter-molecular “stacking” [22] as one of major mechanisms of bonding.
From the standpoint of applied science, results of this research can be used in controlled, knowledge-driven encapsulation of gemcitabine and other medicinal drugs to solid-state nanocomposite materials, using facile and non-destructive mechano-chemical approach. Specifically, due to different chemical bonds in molecules present as the amino-keto and the imino-keto tautomer, gemcitabine molecule encapsulated on a “matrix” can form stronger or weaker bonds with functional groups of the matrix. Then, stronger or weaker bonding of Gem molecules to the “matrix” is anticipated to result in controlling the rate of delayed release of this drug. Studies are in progress to encapsulate Gem to coordination polymers metal-organic frameworks (MOFs), where Gem molecules are present as specific tautomers, and then study delayed release of Gem from these materials.
4). Conclusions
Major anti-cancer drug gemcitabine free base (Gem) is found to exist as the two distinct crystalline forms, with the characteristic infrared spectra, XRD patterns and crystal sizes and shapes. These forms denoted Form 1 and Form 2 are assigned to the mixture of the imino-keto and amino-keto tautomers of Gem, and to the amino-keto tautomer, respectively. For the first time, the IR spectra of the imino-keto tautomer of Gem are reported, and its unique spectral “fingerprint” peaks are identified. The comprehensive assignments are presented for the IR peaks of Gem molecules present as each tautomer to vibrations of chemical specific bonds.
Further, we investigate liquid-assisted grinding (LAG) of Gem of each Form, using chemically inert, non-toxic and volatile n-hexane as a “grinding fluid”. During the LAG, Gem molecules present as the imino-keto tautomer are less stable, than molecules present as the amino-keto tautomer, and they are converted to the latter. In contrast, molecules of Gem present as the amino-keto tautomer are stable during the LAG. At the same time, during LAG no chemical decomposition of Gem molecules present as the either tautomer occurs, as found by the HPLC-UV analysis. The described method of LAG is new, facile and effective for the controlled, knowledge-driven interconversion of tautomers of gemcitabine. This method can find applications in synthesis of composite nanomaterials containing the specific molecular forms of gemcitabine, and other small molecule drugs of pyrimidine structure.
Beyond this, the described studies establish an approach to controlling and understanding tautomerization of pyrimidines, purines and related biologically active heterocyclic compounds. This opens the road to new advanced functional composite nanomaterials prepared by mechano-chemical route, while maintaining the control of molecular structure and bonding between drug molecule (active ingredient) and the encapsulation “matrix”, and the underlying molecular mechanism of bonding.
Supplementary Material
Highlights.
Gemcitabine (Gem) crystals contain the amino-keto and the imino-keto tautomers.
ATR-FTIR spectra allow to detect tautomers of Gem and their “fingerprints”.
Liquid-assisted grinding (LAG) changes Gem from imino-keto to amino-keto tautomer.
HPLC-UV analysis shows tautomerization, but not decomposition, of Gem in LAG.
LAG and ATR-FTIR spectra allow to control and understand tautomerization of drugs.
5). Acknowledgement
Research reported in this publication was supported by the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number SC3GM136647. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
A. S. thanks Prof. Ashkan Emadi from University of Maryland School of Medicine for critical reading of this manuscript and helpful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authorship contribution statement
Dr. Alexander Samokhvalov: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing - original draft; Writing - review & editing, Barrington Henry: Data curation; Formal analysis; Methodology; Software.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Noble S, Goa KL, Gemcitabine. A review of its pharmacology and clinical potential in non-small cell lung cancer and pancreatic cancer, Drugs 54(3) (1997) 447–472. 10.2165/00003495-199754030-00009. [DOI] [PubMed] [Google Scholar]
- [2].Kush P, Kaur M, Sharma M, Madan J, Kumar P, Deep A, Kim K-H, Investigations of potent biocompatible metal-organic framework for efficient encapsulation and delivery of gemcitabine: Biodistribution, pharmacokinetic and cytotoxicity study, Biomed. Phys. Eng. Express 6(2) (2020) Article 025014. 10.1088/2057-1976/ab73f7. [DOI] [PubMed] [Google Scholar]
- [3].Sović I, Lukin S, Meštrović E, Halasz I, Porcheddu A, Delogu F, Ricci PC, Caron F, Perilli T, Dogan A, Colacino E, Mechanochemical preparation of active pharmaceutical ingredients monitored by in situ Raman spectroscopy, ACS Omega 5(44) (2020) 28663–28672. 10.1021/acsomega.0c03756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Denlinger KL, Ortiz-Trankina L, Carr P, Benson K, Waddell DC, Mack WJ, Liquid-assisted grinding and ion pairing regulates percentage conversion and diastereoselectivity of the Wittig reaction under mechanochemical conditions, Beilstein J. Org. Chem 14 (2018) 688–696. 10.3762/bjoc.14.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Balachandran V, Parimala K, Molecular structures, FT-IR and FT-Raman spectra, NBO analysis, NLO properties, reactive sites and quantum chemical calculations of keto–enol tautomerism (2-amino-4-pyrimidinol and 2-amino-pyrimidine-4(1H)-one), Spectrochim. Acta A 102 (2013) 30–51. 10.1016/j.saa.2012.10.011. [DOI] [PubMed] [Google Scholar]
- [6].Colominas C, Luque FJ, Orozco M, Tautomerism and protonation of guanine and cytosine. Implications in the formation of hydrogen-bonded complexes, J. Am. Chem. Soc 118(29) (1996) 6811–6821. 10.1021/ja9542931. [DOI] [Google Scholar]
- [7].Singh V, Fedeles BI, Essigmann JM, Role of tautomerism in RNA biochemistry, RNA 21(1) (2015) 1–13. http://www.rnajournal.org/cgi/doi/10.1261/rna.048371.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sanna N, Chillemi G, Grandi A, Castelli S, Desideri A, Barone V, New hints on the pH-driven tautomeric equilibria of the topotecan anticancer drug in aqueous solutions from an integrated spectroscopic and quantum-mechanical approach, J. Am. Chem. Soc 127(44) (2005) 15429–15436. 10.1021/ja052637u. [DOI] [PubMed] [Google Scholar]
- [9].Rastogi VK, Palafox MA, Vibrational spectra, tautomerism and thermodynamics of anticarcinogenic drug: 5-fluorouracil, Spectrochim. Acta A 79(5) (2011) 970–977. 10.1016/j.saa.2011.04.008. [DOI] [PubMed] [Google Scholar]
- [10].Leszczynski J, Tautomers of 6-thioguanine: Structures and properties, J. Phys. Chem 97(14) (1993) 3520–3524. 10.1021/j100116a014. [DOI] [Google Scholar]
- [11].Stephenson GA, Pfeiffer RR, Byrn SR, Solid-state investigation of the tautomerism of acetohexamide, Int. J. Pharm 146(1) (1997) 93–99. 10.1016/S0378-5173(96)04775-8. [DOI] [Google Scholar]
- [12].Centore R, Manfredi C, Capobianco A, Volino S, Ferrara MV, Carella A, Fusco S, Peluso A, Solid state separation and isolation of tautomers of fused-ring triazolotriazoles, J. Org. Chem 82(10) (2017) 5155–5161. 10.1021/acs.joc.7b00380. [DOI] [PubMed] [Google Scholar]
- [13].Kaneva N, Bojinova A, Papazova K, Dimitrov D, Zaharieva K, Cherkezova-Zheleva Z, Eliyas A, Effect of thermal and mechano-chemical activation on the photocatalytic efficiency of ZnO for drugs degradation, Arch. Pharm. Res 39(10) (2016) 1418–1425. 10.1007/s12272-016-0789-6. [DOI] [PubMed] [Google Scholar]
- [14].Mishra S, Mishra AK, Raman microspectroscopic and quantum chemical investigations of neuroleptic drugs interactions with dipalmitoylphosphatidylcholine (DPPC) lipid, Vib. Spectrosc 114 (2021) Article 103242. 10.1016/j.vibspec.2021.103242. [DOI] [Google Scholar]
- [15].Tiernan H, Byrne B, Kazarian SG, ATR-FTIR spectroscopy and spectroscopic imaging for the analysis of biopharmaceuticals, Spectrochim. Acta A 241 (2020) 118636. 10.1016/j.saa.2020.118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Grisedale LC, Jamieson MJ, Belton P, Barker SA, Craig DQM, Characterization and quantification of amorphous material in milled and spray-dried salbutamol sulfate: A comparison of thermal, spectroscopic, and water vapor sorption approaches, J. Pharm. Sci 100(8) (2011) 3114–3129. 10.1002/jps.22484. [DOI] [PubMed] [Google Scholar]
- [17].Helmy R, Zhou GX, Chen YW, Crocker L, Wang T, Wenslow RM Jr., Vailaya A, Characterization and quantitation of aprepitant drug substance polymorphs by attenuated total reflectance Fourier transform infrared spectroscopy, Anal. Chem 75(3) (2003) 605–611. 10.1021/ac020538i. [DOI] [PubMed] [Google Scholar]
- [18].Mastanamma S, Ramkumar G, Kumar DA, Seshagiri Rao JVLN, A stability indicating RP-HPLC method for the estimation of gemcitabine HCl in injectable dosage forms, E.-J. Chem 7 (2010) Article 724915. 10.1155/2010/724915. [DOI] [Google Scholar]
- [19].Chen S-P, Shieh C-L, Crystalline polymorphs of gemcitabine base, Patent EP 2 303 906 B1 (2016).
- [20].Brown K, Dixey M, Weymouth-Wilson A, Linclau B, The synthesis of gemcitabine, Carbohydr. Res 387 (2014) 59–73. 10.1016/j.carres.2014.01.024. [DOI] [PubMed] [Google Scholar]
- [21].Kwiatkowski JS, Person WB, Szczepaniak K, Szczesniak M, On tautomerism of the cytosine molecule, Acta Biochim. Pol 34(2) (1987) 165–181. [PubMed] [Google Scholar]
- [22].Rueda M, Luque FJ, López JM, Orozco M, Amino–imino tautomerism in derivatives of cytosine: Effect on hydrogen-bonding and stacking properties, J. Phys. Chem. A 105(26) (2001) 6575–6580. 10.1021/jp010838o. [DOI] [Google Scholar]
- [23].Alvarez-Malmagro J, Prieto F, Rueda M, In situ surface enhanced infrared absorption spectroscopy study of the adsorption of cytosine on gold electrodes, J. Electroanal. Chem 849 (2019) 113362. 10.1016/j.jelechem.2019.113362. [DOI] [Google Scholar]
- [24].Lapinski L, Nowak MJ, Reva I, Rostkowska H, Fausto R, NIR-laser-induced selective rotamerization of hydroxy conformers of cytosine, Phys. Chem. Chem. Phys 12(33) (2010) 9615–9618. 10.1039/C0CP00177E. [DOI] [PubMed] [Google Scholar]
- [25].Bazsó G, Tarczay G, Fogarasi G, Szalay PG, Tautomers of cytosine and their excited electronic states: A matrix isolation spectroscopic and quantum chemical study, Phys. Chem. Chem. Phys 13(15) (2011) 6799–6807. 10.1039/C0CP02354J. [DOI] [PubMed] [Google Scholar]
- [26].Lapinski L, Reva I, Nowak MJ, Fausto R, Five isomers of monomeric cytosine and their interconversions induced by tunable UV laser light, Phys. Chem. Chem. Phys 13(20) (2011) 9676–9684. 10.1039/C0CP02812F. [DOI] [PubMed] [Google Scholar]
- [27].Rezkallah E, Ibrahim A, Dahy A, Hakiem Ahmed A, Mahfouz R, DFT and thermal decomposition studies on gemcitabine, Z. Phys. Chem 233(10) (2019) 1503–1507. 10.1515/zpch-2018-1304. [DOI] [Google Scholar]
- [28].Wu C, You J, Wang X, Thermal decomposition mechanism and kinetics of gemcitabine, J. Anal. Appl. Pyrolysis 130 (2018) 118–126. 10.1016/j.jaap.2018.01.019. [DOI] [Google Scholar]
- [29].Khare V, Sakarchi WA, Gupta PN, Curtis ADM, Hoskins C, Synthesis and characterization of TPGS-gemcitabine prodrug micelles for pancreatic cancer therapy, RSC Adv. 6(65) (2016) 60126–60137. 10.1039/C6RA09347G. [DOI] [Google Scholar]
- [30].Florián J, Baumruk V, Leszczyński J, IR and Raman spectra, tautomeric stabilities, and scaled quantum mechanical force fields of protonated cytosine, J. Phys. Chem 100(13) (1996) 5578–5589. 10.1021/jp953284w. [DOI] [Google Scholar]
- [31].Ten GN, Baranov VI, Calculation and analysis of the IR spectra of cytosine in various phase states, J. Appl. Spectrosc 72(2) (2005) 155–163. 10.1007/s10812-005-0048-y. [DOI] [Google Scholar]
- [32].Beć KB, Grabska J, Czarnecki MA, Huck CW, Wójcik MJ, Nakajima T, Ozaki Y, IR spectra of crystalline nucleobases: Combination of periodic harmonic calculations with anharmonic corrections based on finite models, J. Phys. Chem. B 123(47) (2019) 10001–10013. 10.1021/acs.jpcb.9b06285. [DOI] [PubMed] [Google Scholar]
- [33].Hertel LW, Kroin JS, Misner JW, Tustin JM, Synthesis of 2-deoxy-2,2-difluoro-D-ribose and 2-deoxy-2,2’-difluoro-D-ribofuranosyl nucleosides, J. Org. Chem 53(11) (1988) 2406–2409. 10.1021/jo00246a002. [DOI] [Google Scholar]
- [34].Szczesniak M, Szczepaniak K, Kwiatkowski JS, KuBulat K, Person WB, Matrix isolation infrared studies of nucleic acid constituents. 5. Experimental matrix-isolation and theoretical ab initio SCF molecular orbital studies of the infrared spectra of cytosine monomers, J. Am. Chem. Soc 110(25) (1988) 8319–8330. 10.1021/ja00233a006. [DOI] [Google Scholar]
- [35].Belenguer AM, Lampronti GI, Sanders JKM, Reliable mechanochemistry: Protocols for reproducible outcomes of neat and liquid assisted ball-mill grinding experiments, JoVE (131) (2018) Article e56824. 10.3791/56824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


