Abstract
Objectives
Preventive measures against COVID-19 are essential for pregnant women. Pregnant women are particularly vulnerable to emerging infectious pathogens due to alterations in their physiology. We aimed to determine the optimum timing of vaccination to protect pregnant women and their neonates from COVID-19.
Methods
A prospective observational longitudinal cohort study in pregnant women who received COVID-19 vaccination. We collected blood samples to evaluate levels of antispike, receptor binding domain and nucleocapsid antibodies against SARS-CoV-2 before vaccination and 15 days after the first and second vaccination. We determined the neutralizing antibodies from mother-infant dyads in maternal and umbilical cord blood at birth. If available, immunoglobulin A was measured in human milk.
Results
We included 178 pregnant women. Median antispike immunoglobulin G levels increased significantly from 1.8 to 5431 binding antibody units/ml and receptor binding domain from 6 to 4466 binding antibody units/ml. Virus neutralization showed similar results between different weeks of gestation at vaccination (P >0.3).
Conclusion
We advise vaccination in the early second trimester of pregnancy for the optimum balance between the maternal antibody response and placental antibody transfer to the neonate.
Keywords: COVID-19, Antispike IgG, Virus neutralization, Pregnancy, Placental antibody transfer, Vaccination
Introduction
Due to the extensive global health and economic impact of COVID-19, vaccines have been developed with unprecedented rapidity [1]. Vaccination data on pregnant women are important because they are particularly vulnerable to emerging infectious pathogens due to alterations in immune, respiratory, and cardiovascular physiology during pregnancy [2]. In addition, pregnant women are more vulnerable to complications of COVID-19, especially during the third trimester [3], [4], [5]. Although severe COVID-19 is uncommon, compared with nonpregnant women, pregnant women show increased rates of intensive care unit admission, invasive ventilation, extracorporeal membrane oxygenation, and higher mortality rates [6], [7], [8], [9]. The Centers for Disease Control and Prevention data indicate that infants aged 0 to 2 months covered 20% of all COVID-19 hospitalizations among children aged below 18 years [10].
A safety study including pregnant women vaccinated by messenger RNA (mRNA) vaccines started in December 2020 in the United States. More than 35,000 participants showed similar side effect patterns compared with the general population [11,12]. Consequently, the American College of Obstetrics and Gynecology, the society of maternal fetal medicine, and European guidelines strongly recommend pregnant and lactating women to get vaccinated [13,14]. Since then, a number of studies have reported on the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating women [15].
In terms of vaccine response, several small studies indicate that the administration of the mRNA vaccines results in a robust maternal humoral response [16,17]. Furthermore, immunoglobulin (Ig) G antibodies efficiently cross the placenta, resulting in relatively high titers in the fetus [14,17], which should attribute to the prevention of neonatal COVID-19. A few smaller cross sectional and case control studies have shown that maternal IgG levels against SARS-CoV-2 were linearly associated with cord blood IgG levels [16]. Furthermore, the placental transfer ratio was positively correlated with the number of weeks elapsed since maternal vaccination [16,18]. COVID-19 vaccination of lactating women has resulted in long-lasting presence of antibodies in breastmilk [19,20].
However, data are still scarce regarding the optimal timing of vaccination in pregnancy in terms of maternal antibody response and placental antibody transfer to the fetus and additional protection of maternal vaccination in breast-fed children.
This study aimed to describe the antibody response during and after COVID-19 vaccination in pregnant women and the antibody transfer to the neonate during pregnancy through umbilical cord blood and human milk. In addition, we aimed to define the optimum timing of vaccination during pregnancy, considering antibody levels and virus neutralization in mothers and their children.
Methods
Setting and sample
We performed a prospective, observational, longitudinal cohort study in pregnant women who received COVID-19 vaccination through the Dutch vaccination program. The approved vaccines for pregnant women included mRNA vaccines (Pfizer BioNTech BNT162b2 and Moderna mRNA-1273).
Pregnant women living in the Netherlands were invited to participate from June 6, 2021 to June 20, 2021 through social media, including Facebook, Instagram, and LinkedIn. The inclusion criteria were age ≥18 years and scheduled for COVID-19 vaccination. The exclusion criteria were COVID-19 vaccination before inclusion, no written informed consent, or no mastery of the Dutch language. Written informed consent was obtained from all participants. We conducted this study in compliance with the principles of the declaration of Helsinki, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guideline for Good Clinical Practice Guidelines, and the Regulation on Medical Research Involving Human Subjects, after approval was acquired from the Independent Ethics Committee on May 26, 2021 (METc 2021.0278).
Data collection
Blood samples were collected at four different time points during home visits. The first blood sample was collected between 1 and 3 days before the first vaccination (t0). A second blood sample was obtained 15 days after the first vaccination (t1) and another sample 15 days after the second vaccination (t2). The second vaccinations were administered around 35 days after the first vaccination. Women who recently had COVID-19 received one vaccination only.
At birth, a sample of cord (venous) blood was collected by the treating physician (t3). An appointment was scheduled within 7 days to pick up the cord blood and to withdraw the t3 serum sample from the participant. Plasma was harvested and samples were stored at the Amsterdam University Medical Center, at -80°C. Breastfeeding women provided a sample of 1-10 ml human milk at t3, after additional consent was obtained. The milk samples were stored at -20°C. A copy of the vaccination certificate was used to confirm the vaccination status, date, type of vaccination, and lot number.
Luminex assays
To assess the IgG levels specific for SARS-CoV-2 antigens, recombinant prefusion stabilized spike protein and monomeric receptor binding domain (RBD) were produced in HEK293F cells, as previously described [21]. The nucleocapsid antigen was provided by Gestur Vidarsson and Federica Linty of Sanquin Research, Amsterdam, the Netherlands. The antigens were covalently coupled to MagPlex beads (Luminex) using a two-step carbodiimide reaction with a ratio of 75 µg protein to 12.5 million beads, as described previously [22]. Antigen-specific IgG levels were assessed using a previously established custom Luminex assay [22]. For human milk, the Luminex assay did not specifically distinguish between subtype IgA with and without a secretory component. In short, 1: 100,000 diluted serum or 1: 100 diluted human milk and 15 of each bead per µl were incubated overnight at 4°C. Goat-Anti-Human IgG-PE (Southern Biotech) were used as the secondary antibody and incubated for 2 hours at room temperature. The PE signal was detected on a MAGPIX machine (Luminex). The resulting median fluorescence intensity values were background-corrected by subtraction of the median fluorescence intensity values of buffer-only controls for each antigen separately. A titration of serum or human milk of an adult convalescent COVID-19 patient was included to compare data between plates. Beads with no antigen coupled were included to confirm the absence of antibodies binding to beads or blocking components for each individual sample. Positive and negative control sera were included on each plate. We ran replicate assays on a selection of samples to assess the robustness of the data.
SARS-CoV-2 pseudovirus neutralization assay
The neutralizing antibody levels were assessed using a pseudovirus neutralization assay, as described previously [23]. In short, HEK293T cells expressing the SARS-CoV-2 receptor angiotensin converting enzyme-2 (ACE2; HEK293T/ACE2) [24] were seeded in 96-well plates coated with poly-L-lysine. The next day, triplicate serial dilutions of heat inactivated serum were mixed 1: 1 with the pseudovirus and incubated for 1 hour at 37°C before adding to the HEK293T/ACE2 cells in a 1: 1 ratio with the medium. After 48 hours, the cells were lysed and the Luciferase signal was measured using the Nano-Glo Luciferase Assay System (Promega) with a Glomax system (Turner BioSystems). The resulting relative luminescence units were normalized to relative luminescence units from SARS-CoV-2 pseudovirus-infected cells in the absence of serum. The neutralization titers were calculated as the serum dilution where infectivity was inhibited by 50%. The neutralization assays were done in triplicates.
Questionnaires
Baseline characteristics were retrieved at t0 and self-reported side effects after vaccination (t1 and t2) by filling out questionnaires that were sent using the data management system CASTOR. The last questionnaire was sent at birth (t3) for obstetrical and neonatal outcomes. Two additional questions were later added to the last questionnaire regarding having a SARS-CoV-2 infection in the interval between vaccination and birth and regarding booster vaccination during pregnancy. These questions were not initially included in the questionnaires because it was not clear in the design of this study that SARS-CoV-2 infections could occur despite vaccination nor did we know that booster vaccination would be necessary to maintain protective antibody levels.
Statistical analysis
Demographic characteristics were described using descriptive statistics. The antibody levels were log base 10-transformed because the data were skewed. The differences in antibody levels between the different time points were determined by one-way analysis of variance and differences between specific time points were determined by post hoc paired t-tests and Wilcoxon signed rank tests for skewed data. The correlation analyses were performed using Pearson to determine correlations between log-transformed antispike and RBD IgA in human milk in relation to weeks since the last vaccination and antispike IgG and RBD IgG at birth in relation to weeks since the last vaccination. The gestational age at vaccination was categorized into six groups: 11-15 weeks, 16-20 weeks, 21-25 weeks, 26-30 weeks, 31-35 weeks, and above 35 weeks. To determine the optimal timing of vaccination for spike IgG levels and neutralizing antibody levels, we performed a mixed-analysis of variance analyses, with groups of gestational age at vaccination as the between-subject factor and repeated measurements over time as within-subject factor. The data were analyzed using R (version 4.0.3).
Results
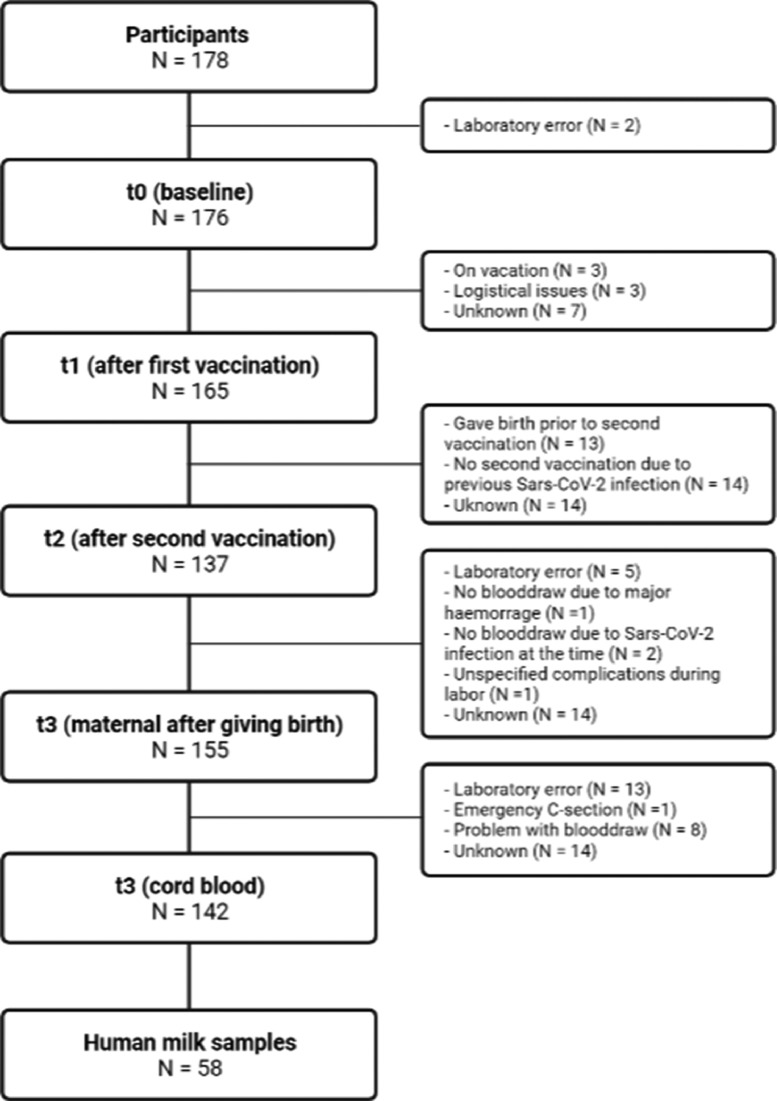
After screening, 178 women were eligible to participate (Figure 1 ). At baseline, 176 women provided a sample before vaccination (t0), 165 women provided a sample 2 weeks after the first vaccination (t1), 137 women provided a sample 2 weeks after the second vaccination (t2), and 155 women provided a sample at birth (t3), with 142 concomitant umbilical cord samples and 58 human milk samples. The baseline characteristics are displayed in Table 1 . The majority of women were multiparous, highly educated, and had few comorbidities [25,26]. The baseline sample (t0) was collected at a mean gestational age of 23.1 ± 7.2 (SD) weeks, t1 at 25.4 ± 7.4 (SD) weeks, and t2 at 29.5 ± 6.3 (SD) weeks pregnancy. A total of 151 women received two vaccinations and 27 women received one vaccination; 14 (7.9%) due to a previous SARS-CoV-2 infection and 13 (7.3%) gave birth before the second vaccination. At baseline, eight women had nucleocapsid IgG above the threshold, indicating a previous SARS-CoV-2 infection. Women with a previously polymerase chain reaction-confirmed SARS-CoV-2 infection had this infection on average 30 weeks before the sample collection. Furthermore, for nine women, an increase of nucleocapsid IgG levels above the threshold was observed, in seven cases at t1 and in two cases at t3, indicative of a SARS-CoV-2 breakthrough infection. Only two of 178 (1.1%) women reported a polymerase chain reaction-confirmed SARS-CoV-2 infection between vaccination and birth and reported only mild symptoms; seven women were not aware of having had a SARS-CoV-2 infection. Four (2%) women received an additional booster vaccination while pregnant.
Figure 1.
Included patients and blood draw at each time point.
Table 1.
Baseline characteristics of the participants.
| Characteristics | Participants N = 178 (%) |
|---|---|
| General | |
| Age (years), mean (SD) | 32.7 (3.3) |
Educational level
|
2 (1.1) 19 (10.7) 156 (87.6) |
| Prepregnancy body mass index >30 | 25 (14.0) |
Maternal comorbidity
|
2 (1.1) 4 (2.2) 5 (2.8) 26 (14.6) |
| Medication | 67 (37.6) |
Previous COVID-19
|
22 (12.4) 14 (7.9) |
| Pregnancy related | |
Parity
|
74 (41.6) 104 (58.4) |
Conception
|
160 (89.9) 18 (10.1) |
Complications current pregnancy
|
5 (2.8) 2 (1.1) 1 (0.6) 13 (7.3) |
Antenatal care
|
157 (88.2) 21 (11.8) |
Obstetric history
|
10 (5.6) 46 (25.8) 9 (5.1) 24 (13.5) |
Side effects and obstetric outcomes
The first and second questionnaire regarding side effects after vaccination were filled in by 171 (96.1%) and 135 (89.4%) women (Table 2 ). The questionnaire at birth was filled in by 130 (73.0%) women.
Table 2.
Reported side effects after the first and second vaccination.
| Side effects | First vaccination (n = 171) | Second vaccination N = 135 (%) |
|---|---|---|
| Injection side pain | 139 (81.3) | 105 (77.8) |
| Injection side swelling | 9 (5.3) | 9 (6.7) |
| Injection side redness | 4 (2.3) | 3 (2.2) |
| Fatigue | 58 (33.9) | 59 (43.7) |
| Headache | 34 (19.9) | 41 (30.4) |
| Nausea | 16 (9.4) | 14 (10.4) |
| Vomiting | 2 (1.2) | 3 (2.2) |
| Fever (≥37.5°C) | 5 (2.9) | 8 (5.9) |
| Myalgia | 59 (34.5) | 57 (42.2) |
Pain at the injection site was the most frequently reported side effect; 81% and 78% after the first and second vaccination, respectively (Table 2). The other frequently reported side effects were myalgia: 35% and 42%, fatigue: 34% and 44%, and headache: 20% and 30%, respectively. Fever was reported by 3% and 6% of the participants, respectively.
Obstetric outcomes are displayed in Table 3 . The infants were born at a mean gestational age of 40.0 ± 1.4 (SD) weeks, with an average birthweight of 3617 grams.
Table 3.
Obstetric outcomes.
| Participants N = 130 (%) | |
|---|---|
| Gestational age at delivery (weeks), median (IQR) | 40 (39-41) |
| Mode of delivery Spontaneous vaginal delivery Assisted vaginal delivery Primary cesarean section Emergency cesarean section |
107 (82.3) 13 (10.0) 4 (3.1) 6 (4.6) |
| Preterm birth | 1 (0.8) |
| Still birth | 0 (0) |
| Gender - Male - Female |
69 (53.1)61 (46.9) |
| APGAR score, median (IQR) - After 1 minute - After 5 minutes |
9 (9-9) 10 (10-10) |
| Birthweight - p < 10 - p10 – p50 - p50 – p90 - p90 – p95 - p > 95 |
12 (9.3) 47 (36.2) 53 (40.8) 9 (6.9) 9 (6.9) |
| Neonatal hospital admission | 11 (8.5) |
IQR, interquartile range.
Antibody response
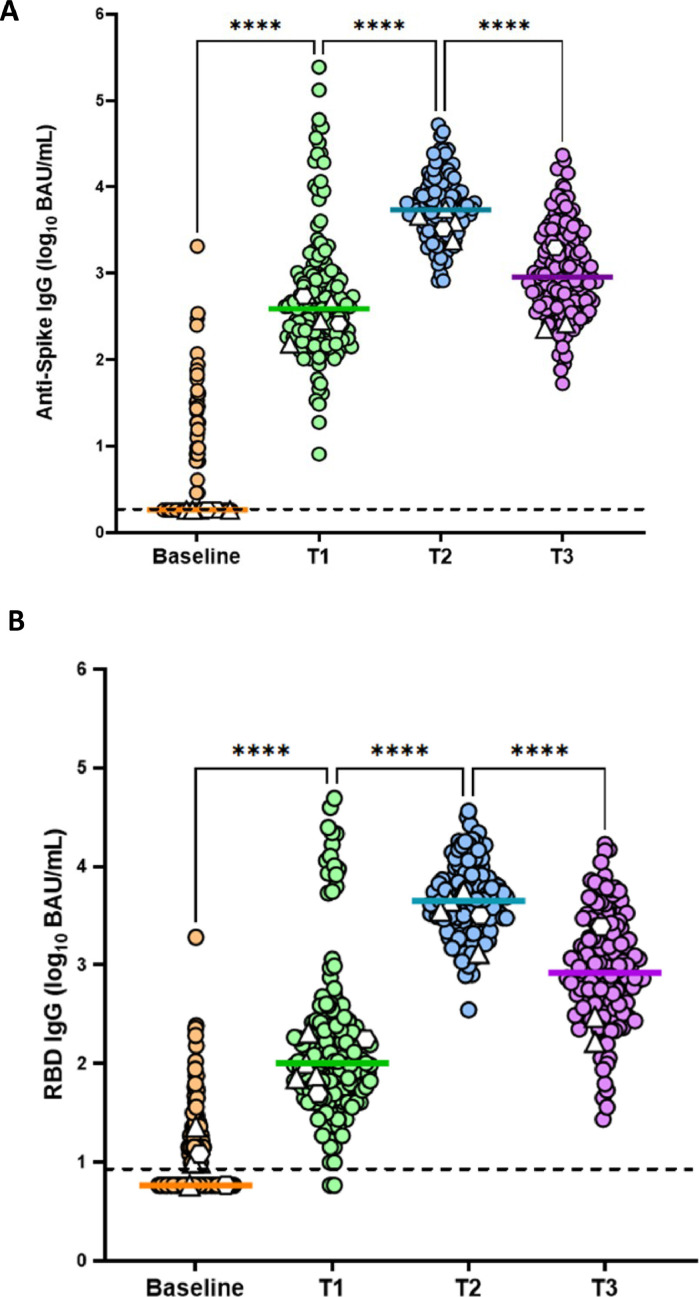
Significant differences in antispike IgG (P <0.001) and RBD IgG (P <0.001) were observed across the different time points. A significant rise from 1.8 (interquartile range [IQR] 1.8-2.9) and 6 (IQR 6-14) binding antibody units (BAU)/ml to 386 (IQR 181-848) and 101 (IQR 54-243) BAU/ml in antispike IgG and RBD IgG, respectively, was observed between baseline and t1 (P <0.001), with a further rise to 5431 (IQR 3248-9222) and 4446 (IQR 2215-7380) BAU/mL at t2 (P ≤0.001, Figure 2 ). The antispike IgG was 5376 (IQR 3130-9222) and the RBD IgG 4406 (IQR 2195-7380) BAU/ml on average when excluding the eight samples with a nucleocapsid IgG above the threshold. Six women displayed antispike IgG and 26 RBD IgG above the threshold at t0 without having nucleocapsid IgG. The results on the antibody titers were similar when leaving out the 26 women with an RBD IgG above the threshold. The mean time between t2 and t3 was 88.6 ± SD 48 days, and a significant decrease was observed in maternal blood from 5431 (IQR 3248-9222) and 4446 (IQR 2215-7380) BAU/ml to 910 (IQR 455-2553) and 795 (IQR 385-2010) BAU/ml of antispike and RBD IgG, respectively (P <0.001).
Figure 2.
Evaluation of Log10 antispike IgG (panel a), RBD IgG (panel b) at baseline (before vaccination), t1 (15 days after the first vaccination) and t2 (15 days after the second vaccination) and t3 (final blood sample from the participant at birth). Colored lines indicate the median. Dashed lines indicate detection limits. Differences in antibody levels between the different time points were determined by one-way analysis of variance and differences between specific time points were determined by post hoc paired t-tests and Wilcoxon signed rank tests for skewed data.
Δ women who received a booster vaccination during pregnancy ◯ women who had a SARS-CoV-2 infection between vaccination and giving birth
BAU, binding antibody unit; Ig, immunoglobulin; RBD, receptor binding domain.
In addition, the neutralizing antibody levels at t3 in maternal blood were 82 (29-218) IU/ml.
Antibody transfer to the neonate
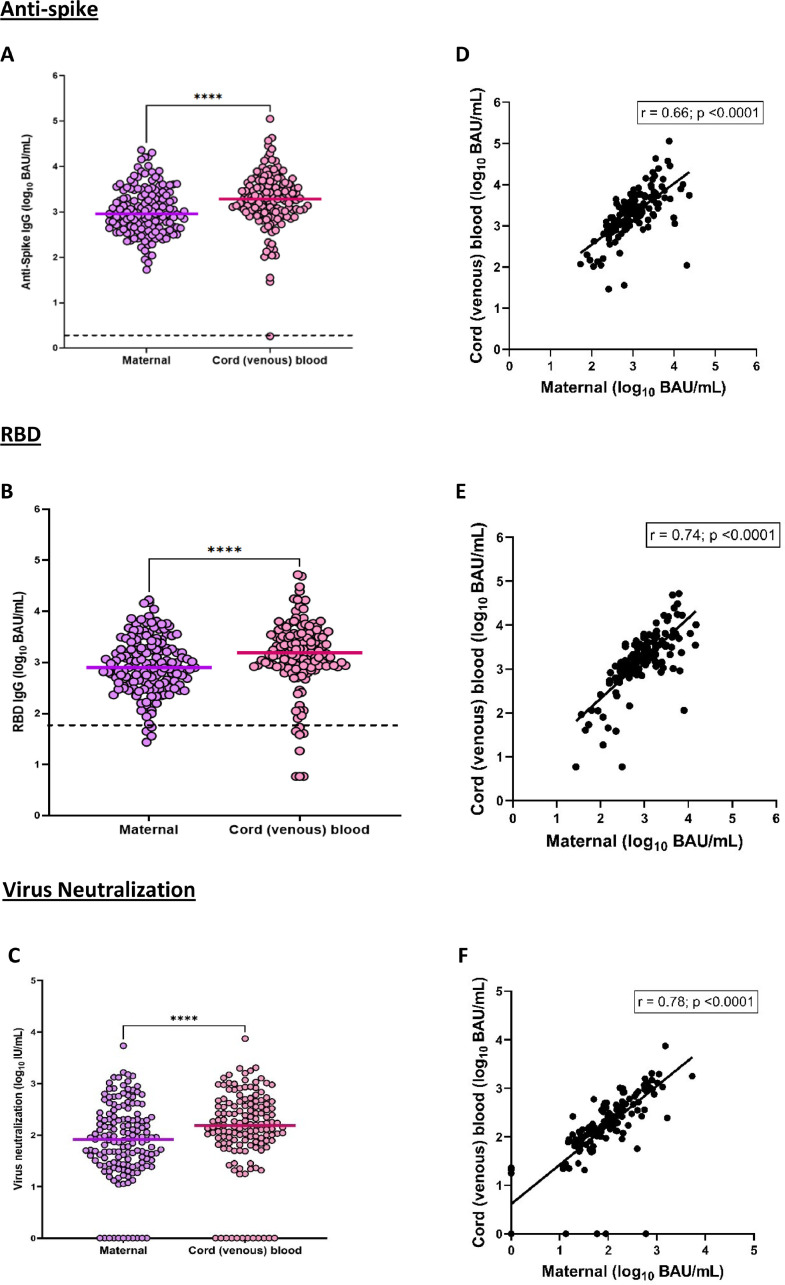
Figure 3, panels a and b, displays the IgG antibody transfer to the neonate in cord blood in comparison to the antibody levels in maternal blood at t3. The level of antispike and RBD IgG antibodies in venous cord blood were 1932 (992-4112) and 1550 (858-3589) BAU/ml, respectively; both were significantly higher than the corresponding maternal antibodies at t3 (P <0.003). The transfer ratio for antispike was 2.3 (IQR 1.4-3.6), RBD 2.3 (IQR 1.4-3.3), and virus neutralization 2.1 (IQR 1.4-3.3). There was a moderate correlation between cord blood and maternal blood for antispike IgG (r = 0.66, P <0.0001) and RBD IgG (r = 0.74, P <0.001).
Figure 3.
Evaluation of Log10 antispike and RBD IgG at t3 (maternal and cord blood, a and b) and virus neutralization at t3 (maternal and cord blood, c). d, e, and f show the maternal and cord blood correlation for antispike and RBD IgG as well as virus neutralization. Panels a, b and c: paired t-test. Panels d, e and f: Pearson correlation BAU, binding antibody unit; Ig, immunoglobulin; RBD, receptor binding domain.
The neutralizing antibody levels in cord blood were 153 (71-400) IU/ml, which were significantly higher than maternal blood at t3 (P >0.001), as shown in Figure 3, panel c. There was a strong correlation between maternal and cord blood neutralizing antibody levels (r = 0.78, P ≤0.0001). In 15 mother-infant dyads, no neutralizing antibodies were detected.
Specific IgA antibodies were measured in 58 human milk samples (collected before day 10 after birth). The level of antispike IgA in human milk was 466.5 (IQR 182-1428) BAU/ml, and the level of RBD IgA in human milk was 1663 (IQR 663-3805) BAU/ml.
Relationship between gestational age at vaccination and antibody levels at and after birth
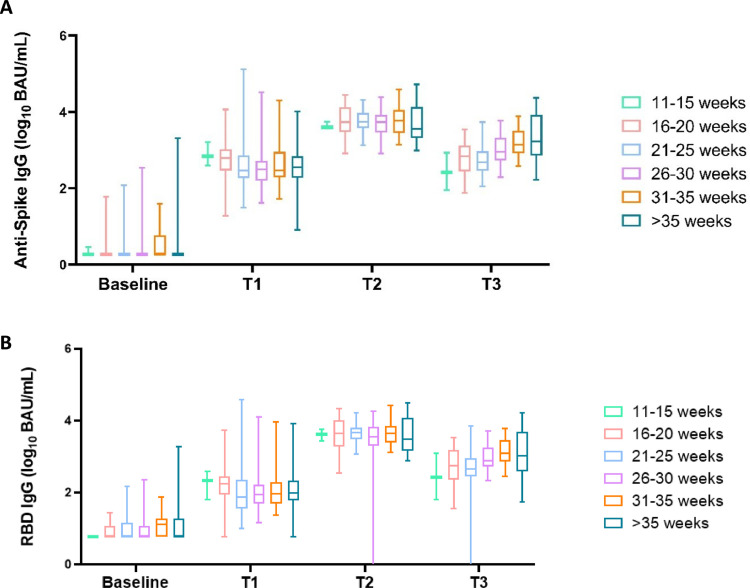
Figure 4 displays the relationship between the gestational age at the last vaccination and maternal SARS-CoV-2 IgG levels at birth. Women were appointed to six groups in correspondence with their gestational age at the last vaccination. Women vaccinated in the third trimester (groups 5 and 6 in Figure 4) had similar antibody levels of SARS-CoV-2 antispike IgG (P = 0.39) and RBD IgG (P = 0.27) on average in comparison to other first and second trimester gestational age groups, that is 1427 (IQR 823-3847) and 1221 (IQR 698-3868) BAU/ml, respectively at t3.
Figure 4.
Antispike and RBD IgG maternal antibodies in relation to gestational age at the time of the last vaccination before delivery was administered, divided into six groups (11-15 weeks N = 4, 16-20 weeks N = 24, 21-25 weeks N = 27, 26-30 weeks N = 35, 31-35 weeks, N = 33, >35 weeks N = 20). BAU, binding antibody unit; Ig, immunoglobulin; RBD, receptor binding domain.
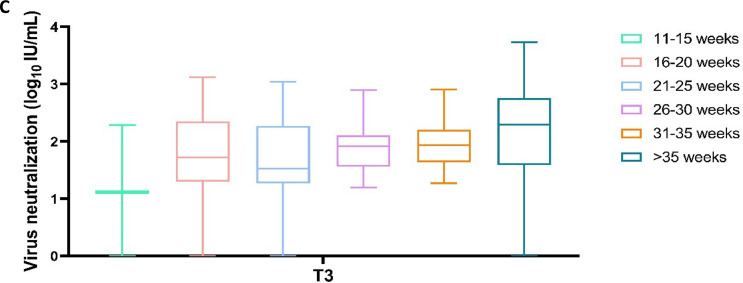
The virus neutralization showed similar results between different weeks of gestation at vaccination (P >0.3; Figure 5 ).
Figure 5.
Virus neutralization antibodies in maternal blood in relation to gestational age at the time of the last vaccination before delivery, divided into six groups (11-15 weeks N = 4, 16-20 weeks N = 24, 21-25 weeks N = 27, 26-30 weeks N = 35, 31-35 weeks, N = 33, >35 weeks N = 20).
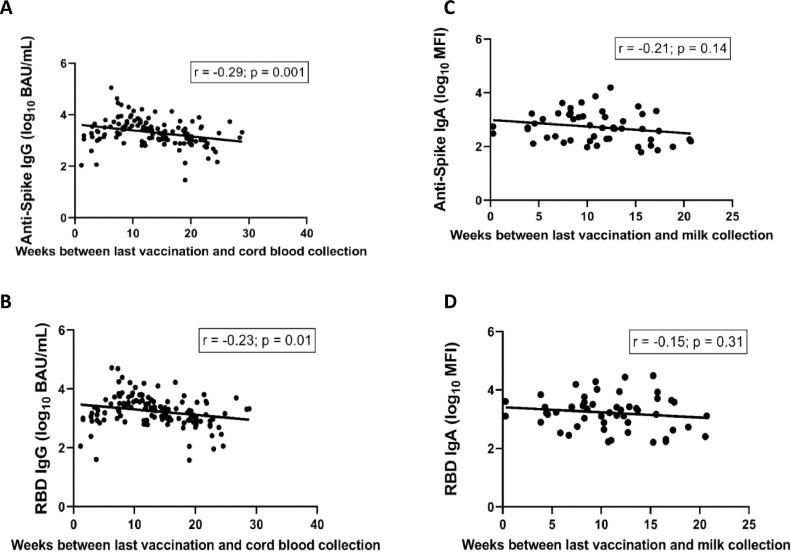
The timing of vaccination, however, was negatively associated with cord blood IgG at birth (antispike IgG: -0.029 [-0.037, -0.01]; P = 0.001, RBD IgG: -0.019 [-0.023, -0.004]; P = 0.01).
Antibody transfer after birth through human milk showed no significant association with gestational age at vaccination (antispike IgA: -0.021 [95% confidence interval: -0.057; 0.008]; P = 0.14, RBD IgA: -0.015 [95% confidence interval: -0.052; 0.017]; P = 0.31, Figure 6 ).
Figure 6.
Linear regression of weeks between last vaccination and cord blood collection (a and b) and weeks between last vaccination and human milk collection (c and d).
BAU, binding antibody unit; Ig, immunoglobulin; RBD, receptor binding domain.
Similar results were obtained when we analyzed the samples from only women who received the BNT162b2 vaccine by excluding the women vaccinated with mRNA-1273 (n = 9; data not shown).
Discussion
In our study, maternal antenatal vaccination led to high titers of antispike IgG and RBD IgG antibodies against COVID-19, with a decline in maternal and cord blood antibody titers with an increasing last vaccination-birth interval. Similar antibody levels were observed when excluding samples from women with positive nucleocapsid IgG at t0, indicative of a previous SARS-CoV-2 infection. Maternal antibody levels at birth did not differ significantly between the different vaccination-gestational age intervals. High virus neutralization titers were observed in both maternal serum and in cord blood samples. Virus neutralization was absent in 15 mother-infant dyads. SARS-CoV-2-specific IgA antibodies were also present in human milk but were not associated with gestational age at vaccination. None of the pregnant women experienced severe COVID-19 infection, and the pregnancy outcomes in this cohort were good. Only mild side effects of vaccination were observed.
Our study design allowed us to follow the vaccine responses in pregnant women of different gestational ages and included data on antibody levels in cord blood and human milk. In addition, virus neutralization provides a robust estimation of the humoral vaccine response. We gathered vaccination data and adverse effects and had a high response rate for the questionnaires and a low number of women lost to follow-up. Our study population was quite homogeneous. We performed separate analyses for women who received the BNT162b2 vaccine to make sure that the data were not influenced by women using mRNA-1273 and were generalizable for both mRNA vaccines. We also analyzed our data without the 26 women with an RBD antibody level above the threshold at T0, with similar results.
A single analysis was done for the samples, but replicate assays were done on a selection of samples with the same results. Neutralizing assays were performed in triplicates. Therefore, we are confident about the robustness of our data.
From our study, we are unable to identify the differential population response and subgroup analysis due to the over-representation of highly educated Caucasian women. Only four of 178 women received a booster vaccination during the study period; so, we are unable to define the impact of booster vaccination during pregnancy. During the study, we specifically looked at the humoral response but not the cellular B and T cell responses and are therefore unable to comment on the attribution of the cellular response to vaccine efficacy and timing of vaccination. We did not compare maternal serum SARS-CoV-2 IgG responses with IgA responses in human milk because the assays were not comparable. Lastly, the outcomes of this study are limited by the fact that only a few women in the first trimester were included. However, it is expected that an increased time lapse between the last vaccination and birth results in lower virus neutralization at birth.
An RBD antibody level above the threshold at t0 was observed in 26 women, of whom only six also had antispike antibody levels above the threshold at t0, without having nucleocapsid IgG, indicative of a previous SARS-CoV-2 infection. An RBD antibody level above the threshold without antispike antibodies may be explained by inaccuracy of the RBD assay rather than a previous SARS-CoV-2 infection because RBD is a part of the spike protein. Probably 20 women were thus RBD-positive without having had a SARS-CoV-2 infection and six may have had a SARS-CoV-2 infection a long time ago, of which they were not aware. The RBD antibody levels in these 26 women were similar at t1 in comparison to women without detectable RBD antibodies at t0. An enormous increase in antibody levels after vaccination after a recent SARS-CoV-2 infection was observed in several studies [27]. Because we did not observe such an antibody response, these women were not excluded from the overall analysis. False-positive baseline antibody results from immunoassay interference have been described previously [28].
The current vaccination study showed a robust antibody response and protection from severe COVID-19 at the cost of only mild side effects from vaccination. A recent paper by Halasa et al. showed that maternal vaccination protects both mother and infants aged younger than 6 months from severe COVID-19 disease [29]. In line with the general population, all women had good obstetric outcomes. As expected from other studies [30], fever was reported in only a low percentage of women. This is especially important because pregnant women are sometimes hesitant to take a vaccine out of fear of fever and its association with contractions and premature birth. Overall, pregnant women showed comparable antibody levels compared with nonpregnant women [30,31]. After the second vaccination, antibody titers were comparable for all the pregnancy intervals. Women vaccinated in the second and third trimester had similar antibody levels of SARS-CoV-2 antispike IgG and RBD IgG on average at birth, despite a longer time lapse between the last vaccination and birth for the second trimester vaccination. Probably the vaccination-birth time interval was too short during pregnancy for antibodies to decline significantly over time [32]. Our results are in line with the paper of Atyeo et al. who studied SARS-CoV-2 antibody response in 158 pregnant individuals and neutralizing antibodies in 175 mother-infant dyads after vaccination. They concluded that the trimester of vaccination did not have a significant impact on the proportion of individuals with neutralizing antibodies against Omicron pseudovirus [17].
Our human milk data are in line with a previous study in 84 pregnant, 31 lactating, and 16 nonpregnant women, in whom a robust antibody response after vaccination was observed [33]. Vaccines in pregnancy are known to protect the newborn infant in a period when their humoral response is still inefficient. In addition, human milk helps provide antibodies to the neonate to protect against various infectious diseases. Previous research has shown that milk antibodies are still present in the first months after birth after maternal vaccination during pregnancy, which is in accordance with our findings [34,35].
With increasing time lapse between the last vaccination and birth, we observed lower antibody levels and an expected decline in transplacental transfer. This is in conflict with a study of 171 pregnant women showing an increased transplacental antibody transfer after vaccination early in the third trimester compared with late in the third trimester [36]. This may be due to the short interval between vaccination in the late third trimester and birth when the vaccine response is ongoing and antibodies have not reached their maximum levels. IgG is the only antibody class that significantly crosses the human placenta [37].
Because COVID-19 vaccines do not contain a live attenuated virus, animal reproductive toxicology and postvaccination studies suggest that COVID-19 vaccines are safe for use in pregnancy [38]. Another study showed no traces of mRNA vaccine products in placenta tissue and cord blood after birth [39]. The transfer of immune antibodies from the mother to the fetus after COVID-19 vaccination in pregnancy is estimated at 94-100% [40]. There was a strong correlation between maternal and cord blood neutralizing antibody levels (r = 0.78, P ≤0.0001). Although the correlation coefficient r was 0.78, we considered the correlation strong because this is not a standard diagnostic assay. Living cells in general display various responses and a great variation within the assays.
The level of antispike and RBD IgG antibodies in cord blood at birth were significantly higher than the corresponding maternal antibodies. Umbilical cord levels to maternal serum ratios of 1.5-2 have been described previously, especially after the second trimester vaccination, with higher ratios after vaccination than after natural infection [41,42]. The fact that umbilical cord blood was collected 5 days before maternal blood cannot explain the measured difference in antibody titers.
Maternal antibody response was good throughout all the gestational intervals. The timing of vaccination was thus negatively associated with cord blood IgG at birth because of the expected decline in antibodies between the last vaccination and birth. Based on these results, we conclude that the optimal timing of vaccination is right after 16 weeks of pregnancy because this offers maternal protection throughout pregnancy, specifically in the third trimester, when the risks and complications rates are higher for pregnant women. In addition, it establishes specific antibody transfer to the neonate through the cord blood and human milk. Due to the small number of women in the week 11-15 interval, we were not able to give strong recommendations for the first trimester. However, the vaccination advice of pregnant women should also consider the number of ongoing COVID-19 infections in the community, the virulence of the SARS-CoV-2 variant, and disease burden.
In conclusion, pregnant women show a robust immune response after vaccination in the second and third trimester, with virus neutralization that lasts until birth and antibody transfer to the neonate during and after birth. The early second trimester of pregnancy can be considered the optimal window of COVID-19 vaccination that balances maternal and fetal protection. No serious side effects nor adverse pregnancy outcomes occurred.
The outcomes of this study will hopefully assist pregnant women and clinicians to balance the benefits of vaccination and risks of COVID-19 for pregnant women and their children.
Declaration of Competing Interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This study received funding from Amsterdam University Medical Centre internal reproduction and development institute research grant.
Ethical approval
Name of the ethics committee: Medisch Ethische Toetsingscommissie – AMC. Date of approval: 05-26-2021. Reference number: 2021.0278.
Acknowledgments
The authors would like to thank the laboratory technicians, Judith A. Burger, Joey H. Bouhuijs, Jacqueline van Rijswijk, and Khadija Tejjani, from the microbiology department for analyzing the samples.
Author contributions
EvL, CJMdG, and CRS contributed to the design of the study. After helping with the design of the study, CRS helped throughout the whole study on a number of different tasks. The logistics to make sure all the blood samples were tested, the rules and regulations were followed. Provided information for the biobank. She gave expert knowledge about the possibilities for the statistical analyses and so on. RadL built the site for the social media campaign. SJMZ, BvK, HJ, and CJMdG reviewed the questionnaires that were included for this study. SJMZ, RAdL, EvL, JvG, and CJMdG helped raise awareness for the study through online content. EB and DNV included patients for the study. BvK, JvG, and HJ helped with the logistical aspect of the study. EB and DNV participated in home visits and blood sample collection. MG and MvG were responsible for the laboratory testing of all the samples. SJMZ, SR, MG, EvL, and CJMdG analyzed and interpreted the data. SR, MG, MvG, EvL, and CJMdG provided background knowledge to the data analysis and interpretation. SJMZ, EvL, and CJMdG drafted the manuscript. All authors reviewed the manuscript. All authors have seen and approved the final version of the manuscript.
References
- 1.Blumberg D, Sridhar A, Lakshminrusimha S, Higgins RD, Saade G. COVID-19 vaccine considerations during pregnancy and lactation. Am J Perinatol. 2021;38:523–528. doi: 10.1055/s-0041-1726390. [DOI] [PubMed] [Google Scholar]
- 2.Whitehead CL, Walker SP. Consider pregnancy in COVID-19 therapeutic drug and vaccine trials. Lancet. 2020;395:e92. doi: 10.1016/S0140-6736(20)31029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collin J, Byström E, Carnahan A, Ahrne M. Public Health Agency of Sweden's Brief Report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99:819–822. doi: 10.1111/aogs.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17:469–482. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- 5.Wise RA, Polito AJ, Krishnan V. Respiratory physiologic changes in pregnancy. Immunol Allergy Clin North Am. 2006;26:1–12. doi: 10.1016/j.iac.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Portilla RJ, et al. Pregnant women with SARS-CoV-2 infection are at higher risk of death and pneumonia: propensity score matched analysis of a nationwide prospective cohort (COV19Mx) Ultrasound Obstet Gynecol. 2021;57:224–231. doi: 10.1002/uog.23575. [DOI] [PubMed] [Google Scholar]
- 7.Chinn J, et al. Characteristics and outcomes of women with COVID-19 giving birth at US academic centers during the COVID-19 pandemic. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBolt CA, et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2021;224 doi: 10.1016/j.ajog.2020.11.022. 510.e1–510.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zambrano LD, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim L, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 - COVID-NET, 14 states, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1081–1088. doi: 10.15585/mmwr.mm6932e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falsaperla R, Leone G, Familiari M, Ruggieri M. COVID-19 vaccination in pregnant and lactating women: a systematic review. Expert Rev Vaccines. 2021;20:1619–1628. doi: 10.1080/14760584.2021.1986390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimabukuro TT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NVOG. Standpunt: Vaccinatie tegen COVID-19 rondom kinderwens, zwangerschap en kraambed, https://www.nvog.nl/wp-content/uploads/2021/04/Standpunt-Vaccinatie-tegen-COVID-19-rondom-zwangerschap-en-kraambed-versie-22-april-2021-def.pdf. 2021 [accessed 1 June 2021].
- 14.Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol. 2022;226:177–186. doi: 10.1016/j.ajog.2021.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu W, et al. Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int J Gynaecol Obstet. 2022;156:406–417. doi: 10.1002/ijgo.14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prabhu M, et al. Antibody response to coronavirus disease 2019 (COVID-19) messenger RNA vaccination in pregnant women and transplacental passage into cord blood. Obstet Gynecol. 2021;138:278–280. doi: 10.1097/AOG.0000000000004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atyeo CG, et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat Commun. 2022;13:3571. doi: 10.1038/s41467-022-31169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rottenstreich A, Zarbiv G, Oiknine-Djian E, Zigron R, Wolf DG, Porat S. Efficient maternofetal transplacental transfer of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike antibodies after antenatal SARS-CoV-2 BNT162b2 messenger RNA vaccination. Clin Infect Dis. 2021;73:1909–1912. doi: 10.1093/cid/ciab266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juncker HG, Mulleners SJ, Coenen ERM, van Goudoever JB, van Gils MJ, van Keulen BJ. Comparing human milk antibody response after 4 different vaccines for COVID-19. JAMA Pediatr. 2022;176:611–612. doi: 10.1001/jamapediatrics.2022.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juncker HG, et al. Human milk antibodies against SARS-CoV-2: A longitudinal follow-up study. J Hum Lact. 2021;37:485–491. doi: 10.1177/08903344211030171. [DOI] [PubMed] [Google Scholar]
- 21.Brouwer PJM, et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369:643–650. doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grobben M, et al. Cross-reactive antibodies after SARS-CoV-2 infection and vaccination. eLife. 2021;10 doi: 10.7554/eLife.70330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouwer PJM, et al. Two-component spike nanoparticle vaccine protects macaques from SARS-CoV-2 infection. Cell. 2021;184 doi: 10.1016/j.cell.2021.01.035. 1188–1200.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt F, et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J Exp Med. 2020;217 doi: 10.1084/jem.20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zwart JJ, Richters JM, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population-based study of 371,000 pregnancies. BJOG. 2008;115:842–850. doi: 10.1111/j.1471-0528.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 26.Perined. Jaarboek Zorg Perinatale zorg in Nederland anno 2021, https://www.perined.nl/onderwerpen/publicaties-perined/jaarboek-zorg; 2021 [accessed May 14, 2022].
- 27.Appelman B, et al. Time since SARS-CoV-2 infection and humoral immune response following BNT162b2 mRNA vaccination. EBiomedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward G, Simpson A, Boscato L, Hickman PE. The investigation of interferences in immunoassay. Clin Biochem. 2017;50:1306–1311. doi: 10.1016/j.clinbiochem.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Halasa NB, et al. Maternal vaccination and risk of hospitalization for Covid-19 among infants. N Engl J Med. 2022;387:109–119. doi: 10.1056/NEJMoa2204399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collier AY, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atyeo C, et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci Transl Med. 2021;13:eabi8631. doi: 10.1126/scitranslmed.abi8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin EG, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray KJ, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225 doi: 10.1016/j.ajog.2021.03.023. 303.e1–303.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xanthou M. Immune protection of human milk. Biol Neonate. 1998;74:121–133. doi: 10.1159/000014018. [DOI] [PubMed] [Google Scholar]
- 35.Romero Ramírez DS, et al. SARS-CoV-2 antibodies in breast milk after vaccination. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052286. [DOI] [PubMed] [Google Scholar]
- 36.Rottenstreich A, et al. Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: a prospective cohort study. Clin Microbiol Infect. 2022;28:419–425. doi: 10.1016/j.cmi.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012 doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blakeway H, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226 doi: 10.1016/j.ajog.2021.08.007. 236.e1–236.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prahl M, et al. Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and early infancy. Nat Commun. 2021;13:4422. doi: 10.1038/s41467-022-32188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zdanowski W, Waśniewski T. Evaluation of SARS-CoV-2 spike protein antibody titers in cord blood after COVID-19 vaccination during pregnancy in polish healthcare workers: preliminary results. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beharier O, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131 doi: 10.1172/JCI150319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edlow AG, et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]