Abstract
STUDY QUESTION
What are the chances of achieving a live birth after embryo, oocyte and ovarian tissue cryopreservation (OTC) in female cancer survivors?
SUMMARY ANSWER
The live birth rates (LBRs) following embryo and oocyte cryopreservation are 41% and 32%, respectively, while for IVF and spontaneous LBR after tissue cryopreservation and transplantation, these rates are 21% and 33%, respectively.
WHAT IS KNOWN ALREADY
Currently, fertility preservation (FP) has become a major public health issue as diagnostic and therapeutic progress has made it possible to achieve an 80% survival rate in children, adolescents and young adults with cancer. In the latest ESHRE guidelines, only oocyte and embryo cryopreservation are considered as established options for FP. OTC is still considered to be an innovative method, while it is an acceptable FP technique in the American Society for Reproductive Medicine guidelines. However, given the lack of studies on long-term outcomes after FP, it is still unclear which technique offers the best chance to achieve a live birth.
STUDY DESIGN, SIZE, DURATION
We performed a systematic review and meta-analysis of published controlled studies. Searches were conducted from January 2004 to May 2021 in Medline, Embase and the Cochrane Library using the following search terms: cancer, stem cell transplantation, FP, embryo cryopreservation, oocyte vitrification, OTC and reproductive outcome.
PARTICIPANTS/MATERIALS, SETTING, METHODS
A total of 126 full-text articles were preselected from 1436 references based on the title and abstract and assessed via the Newcastle–Ottawa Quality Assessment Scale. The studies were selected, and their data were extracted by two independent reviewers according to the Cochrane methods. A fixed-effect meta-analysis was performed for outcomes with high heterogeneity.
MAIN RESULTS AND THE ROLE OF CHANCE
Data from 34 studies were used for this meta-analysis. Regarding cryopreserved embryos, the LBR after IVF was 41% (95% CI: 34–48, I2: 0%, fixed effect). Concerning vitrified oocytes, the LBR was 32% (95% CI: 26–39, I2: 0%, fixed effect). Finally, the LBR after IVF and the spontaneous LBR after ovarian tissue transplantation were 21% (95% CI: 15–26, I2: 0%, fixed-effect) and 33% (95% CI: 25–42, I2: 46.1%, random-effect), respectively. For all outcomes, in the sensitivity analyses, the maximum variation in the estimated percentage was 1%.
LIMITATIONS, REASONS FOR CAUTION
The heterogeneity of the literature prevents us from comparing these three techniques. This meta-analysis provides limited data which may help clinicians when counselling patients.
WIDER IMPLICATIONS OF THE FINDINGS
This study highlights the need for long-term follow-up registries to assess return rates, as well as spontaneous pregnancy rates and birth rates after FP.
STUDY FUNDING/COMPETING INTEREST(S)
This work was sponsored by an unrestricted grant from GEDEON RICHTER France. The authors have no competing interests to declare.
REGISTRATION NUMBER
CRD42021264042.
Keywords: fertility preservation, cancer, haematopoietic stem cell transplantation, live birth rate, embryo cryopreservation, oocyte vitrification, ovarian tissue cryopreservation, meta-analysis
Introduction
Currently, fertility preservation (FP) has become a major public health issue in the care of women with oncological pathologies. Indeed, fertility damage has a high impact on the quality of life of adults of childbearing age (The ESHRE Guideline Group on Female Fertility Preservation, 2020). Diagnostic and therapeutic progress has made it possible to achieve an 80% survival rate in children, adolescents and young adults. Even though the main treatments proposed for cancer (chemotherapy and/or radiotherapy) are improving by decreasing the gonadotoxicity, they reduce the chances of live births in women of reproductive age. In addition, certain non-oncological diseases require haematopoietic stem cell transplantation, which also impairs the fertility of young women. In 2020, the ESHRE proposed best practice guidelines for women eligible to receive FP (The ESHRE Guideline Group on Female Fertility Preservation, 2020). Only oocyte vitrification and embryo cryopreservation are regarded as established options for FP, whereas ovarian tissue cryopreservation (OTC) is still considered to be an innovative method, and IVM is regarded as an experimental method (The ESHRE Guideline Group on Female Fertility Preservation, 2020). Conversely, in the American Society for Reproductive Medicine (ASRM) guidelines, OTC is an acceptable FP technique (Practice Committee of the American Society for Reproductive Medicine, 2019). Since the first live birth after OTC and ovarian autografting in 2004, OTC has been widely offered in many centres for FP. In contrast to oocyte or embryo cryopreservation, OTC has the advantage of not only restoring spontaneous fertility, but also endocrine functions (Khattak et al., 2022).
Many children worldwide have been born from frozen embryos, oocytes or OTCs (Noyes et al., 2009; Diesch-Furlanetto et al., 2021; Walker et al., 2022), yet long-term outcome data are still scarce and generally reported for a single FP technique. To date, only Diaz-Garcia et al. (2018) have published live birth rates (LBRs) in two prospective observational cohorts of women undergoing oocyte vitrification or OTC. In that study, they showed a non-significantly higher LBR after oocyte vitrification than with OTC.
Appropriate FP counselling based on the literature on live birth outcomes should be given to women at the time of diagnosis (Zaami et al., 2022). Nevertheless, the small number of scientific publications and the differences in medical practices around the world make this counselling difficult.
The purpose of our study was to provide a systematic review and meta-analysis of reproductive outcomes following the three main techniques of FP: embryo cryopreservation, oocyte vitrification and OTC in female cancer survivors.
Materials and methods
Literature search strategy and eligibility criteria
The search strategy, selection criteria, data extraction, quality assessment and statistical analyses described below were predefined in version-controlled documents (Supplementary Data File S1). The conduct and reporting of this systematic review and meta-analysis was guided by PRISMA guidelines and registered prospectively (PROSPERO CRD42021264042). All studies (randomized controlled trials, cohort studies, case–control studies, case series, case reports, cross-sectional studies and literature reviews containing original data) involving female cancer or women who underwent stem cell transplantation (any age) who had undergone FP, whose cryopreserved oocyte, embryo or tissue had been used and for which the cumulative live birth rate (CLBR) was reported, were included in the initial screening. This review included three independent exposure groups: oocyte vitrification; embryo cryopreservation; and OTC.
The primary outcome was the LBR, as defined by the number of deliveries resulting in at least one live-born baby divided by the initial number of females who requested the return of frozen-thawed oocytes or embryos or who had undergone an ovarian tissue autograft to restore fertility. Secondary outcomes were the percentage of women with at least one live birth, the rate of miscarriages and the rate of ovarian function restoration. This review excluded women lost to follow-up and data from those who had undergone elective FP, IVM of oocytes, oocyte slow-freezing or conception resulting from oocyte donation. Studies on animal models were also excluded.
PubMed, Embase and the Cochrane Library were searched for relevant literature. The search strategy was limited to articles published in English or French between 01 January 2004 (first birth after ovarian tissue transplantation (OTT)) and 30 June 2021. The literature search strategy was performed in association with the referral Inter-University Library of Medicine of Paris Descartes, Paris 5, France. Searches were made using a combination of Medical Subject Headings (MeSH) and free text terms for the following search terms (and their variants): ‘fertility preservation’, ‘oocyte vitrification’, ‘embryo cryopreservation’, ‘ovarian tissue cryopreservation’ and ‘live birth’ (Supplementary Data File S1).
Selection process and data collection
Two reviewers (E.F. and S.H.) independently screened the titles and abstracts of all articles to determine which studies should be further assessed and excluded any citations deemed irrelevant by both observers. This first screening was made regardless of authors, institutions, journal titles or study results. Any disagreement or uncertainty was resolved by discussion with a third reviewer. Based on the pre-established inclusion criteria, the full texts of potentially relevant articles were retrieved and assessed for inclusion by six reviewers (E.F., S.H., E.L., M.C., M.M. and F.B.). Methodological validity was also assessed prior to inclusion in the review. Any disagreement or uncertainty was resolved by discussion among reviewers to reach a consensus. Six independent reviewers (E.F., S.H., E.L., M.C., M.M. and F.B.) selected the studies, evaluated the biases and extracted the data.
Data were extracted from the included articles via a data extraction form designed by the authors. To characterize the studies included, the following details were collected: study characteristics (country, study design and type, inclusion and exclusion criteria, study period); baseline characteristics of the females (number, age, anti-Müllerian hormone, FSH, oestradiol, indication for FP); FP technique; for oocyte/embryo vitrification, the type of protocol for controlled ovarian stimulation, number of retrieved oocytes, number of mature oocytes, number of oocytes vitrified, insemination method, embryo stage, number of embryos cryopreserved, survival rate after thawing, number of transferred embryos and the number of cycles. For ovarian tissue, we collected the following data: surgical techniques, ovarian transplant site, number of ovarian transplants, number of autografts, duration of ovarian endocrine function; age at retrieval, age at transfer/transplantation, storage duration, time from transfer/transplantation to pregnancy, duration of follow-up, maternal age at delivery, gestational age at delivery, baby’s weight, sex ratio, primary and secondary outcomes following spontaneous conception and after IVF.
The original author was contacted by e-mail when it was necessary to complete information related to oocyte or embryo FP. If no reply was received from the study authors, articles with outcomes expressed only as percentages were excluded. When data were distributed by subgroups in the article, the data extracted were pooled for the overall meta-analysis.
Risk of bias and assessment of study quality
The methodological quality of the selected studies was assessed by six independent reviewers (E.F., S.H., E.L., M.C., M.M. and F.B.) using the Cochrane Handbook methods and the adapted Newcastle–Ottawa Quality Assessment Scale for cohort and case–control studies (Stang, 2010). This system evaluates studies based on three criteria: participant selection, comparability and ascertainment of outcomes. Risks of bias were assessed using ROBINS-I tools: confounding, selection of participants, intervention classification, intervention deviations, missing data, outcome measurement and selection of reported results. Each criterion with a risk of bias was judged as a ‘low’, ‘high’ or ‘unclear’ risk (Sterne et al., 2016).
Data analysis
Data from cohort and case–control studies were included in the meta-analyses only if more than one woman used cryopreserved oocytes, embryos or ovarian tissue. Each outcome was analysed independently. All qualifying articles with quantitative data for CLBR were included in the meta-analysis. Meta-analysis was performed only if three or more studies could be included. No missing data were replaced.
The measurement for the treatment effect was the overall proportion of females with events. When an article reported zero events, a value of 0.5 was added to allow the estimation. The estimation was made using a generalized linear mixed model with binomial likelihood and using logit transformed proportions (Lin and Chu, 2020). The median of the rate was obtained by the back-transformed value. Forest plots were used to describe point estimates (95% CI) and between-study variability. The Q chi-squared test was used to test the heterogeneity between studies. Inconsistency across studies was quantified using the I2 statistic and interpreted according to the Cochrane Collaboration guide (Deeks et al., 2021). Funnel plots were visually assessed to evaluate reporting biases in the analyses with at least 10 studies.
Sensitivity and subgroup analyses
Several prespecified sensitivity analyses were performed by excluding certain studies: outliers; studies with at least one high-risk bias; the leave-one-out method. To explore statistical heterogeneity and the possible influence of low-weight studies (small sample size), a meta-analysis was performed using a fixed-effects model to compare estimates of fixed- and random-effects models. In the present article, only fixed-effect models were presented when I2 was 0%. All analyses were performed with R statistical software, v 4.1.2, using the Metafor package, version 3.0.2 (Viechtbauer, 2010).
Results
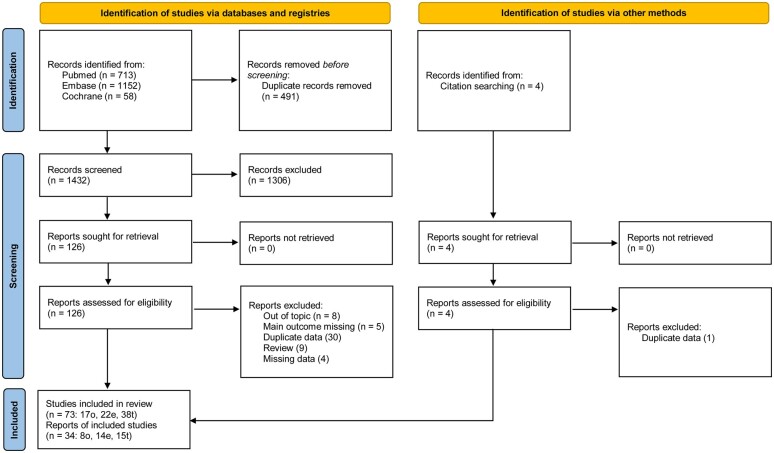
The study flow diagram is presented in Fig. 1. Our search revealed 1927 reports, of which 491 were duplicates. After screening for titles and abstracts, 126 reports were potentially eligible and retrieved in full text. Seventy-three studies were included in the review, and 34 were included in the meta-analysis. Five articles presented more than one FP technique.
Figure 1.
PRISMA flow chart for a systematic review and meta-analysis of live birth rate after female fertility preservation for cancer or haematopoietic stem cells transplantation.
Embryo cryopreservation
Fourteen studies (2 prospective (Marklund et al., 2020; Vriens et al., 2020) and 12 retrospective (Robertson et al., 2011; Babb et al., 2012; Lee and Oktay, 2012; Barcroft et al., 2013; Courbiere et al., 2013; Dolmans et al., 2015; Oktay et al., 2015; Luke et al., 2016; Chien et al., 2017; Alvarez and Ramanathan, 2018; Moravek et al., 2018; Mayeur et al., 2021)) were included, allowing the analysis of 1779 women, including 160 who had returned to use frozen-thawed embryos. The age at embryo cryopreservation ranged from 25.4 to 37.5 years. The average number of cryopreserved embryos ranged from 4.1 to 11.3. Only one study presented details in terms of birthweight (Alvarez and Ramanathan, 2018). Many of the studies concerned women with gynaecological or haematological oncological diseases. ICSI was performed in three studies (Oktay et al., 2015; Alvarez and Ramanathan, 2018; Mayeur et al., 2021). In the other studies, either the method was not specified or both IVF and ICSI were used for fertilization. Regarding the embryonic stage at the time of transfer, three studies specified transfer at the two pronuclei stage (Robertson et al., 2011; Oktay et al., 2015; Mayeur et al., 2021), and one study specified transfer at the cleavage stage (Dolmans et al., 2015). Seven studies did not specify the embryonic stage (Babb et al., 2012; Lee and Oktay, 2012; Barcroft et al., 2013; Luke et al., 2016; Alvarez and Ramanathan, 2018; Moravek et al., 2018; Vriens et al., 2020), and two studies concerned any stage of development (2PN, cleavage and blastocyst) (Courbiere et al., 2013; Marklund et al., 2020). The characteristics of the embryo cryopreservation studies included are presented in Table I.
Table I.
Characteristics of studies on live birth rate after embryo cryopreservation for fertility preservation included in a systematic review and meta-analysis.
| Author, year of publication, country | Study design and type | Study period | Females before treatment for FP (n) | Age, years (mean/median/min–max) | Number of embryos cryopreserved (mean) | Age at retrieval, years (mean ± SD) | Age retrieval, years (min–max) | Females with embryo reimplantation (n) |
|---|---|---|---|---|---|---|---|---|
| Robertson, 2011, USA | Retrospective cohort | January 2001–October 2007 | 38 | 34/–/– | 6 | |||
| Babb, 2012, UK | Retrospective cohort | 2009 | 12 | –/–/34–41 | 37.5 | 34–41 | 7 | |
| Lee, 2012, USA | Retrospective cohort | 151 | 43.3/–/– | 6.10 | 21 | |||
| Barcroft, 2013, UK | Retrospective cohort | July 1996–December 2010 | 42 | 31.9/–/25–41 | 6.70 | 31.9 ± 3.9 | 5 | |
| Courbiere, 2013, France | Retrospective cohort | January 1999–July 2011 | 52 | 28.9/–/– | 4.20 | 11 | ||
| Dolmans, 2015, Belgium | Retrospective cohort | January 1997–June 2014 | 52 | 30/–/21–41 | 4.06 | 30.0 ± 4.6 | 21–41 | 9 |
| Oktay, 2015, USA | Retrospective cohort | 131 | 35.8/–/– | 6.50 | 36.6 ± 4 | 18 | ||
| Luke, 2016, USA | Retrospective cohort | 2004–2009 | 270 | 33.25/–/– | 22 | |||
| Chien, 2017, USA | Retrospective cohort | April 2010–February 2017 | 34 | 35/35.5/25–42 | 8.7 | 3 | ||
| Alvarez, 2018, UK | Retrospective cohort | 2000–2014 | 306 | 30.3/–/17–43 | 7.50 | 2 | ||
| Moravek, 2018, USA | Retrospective cohort | January 2005–January 2016 | 204 | –/31/15–42 | 6.00 | 33.8 | 14 | |
| Marklund, 2020, Sweden | Prospective cohort | January 1995–June 2017 | 468 | 32.5/–/21–42 | 4.55 | 32 | ||
| Vriens, 2020, Netherlands | Prospective cohort | 2008–2015 | 34 | 31/–/23–40 | 31.0 | 23–40 | 3 | |
| Nordan, 2020, USA | Retrospective case control | 2007–2018 | 10 | 30.4/–/– | 11.30 | 1 | ||
| Mayeur, 2021, France | Retrospective cohort | January 2009–December 2019 | 9 | 36/–/– | 36 | 9 |
FP, fertility preservation.
Primary outcome
Only one study had a high risk of bias for missing data (Babb et al., 2012).
LBR after frozen-thawed embryo transfer
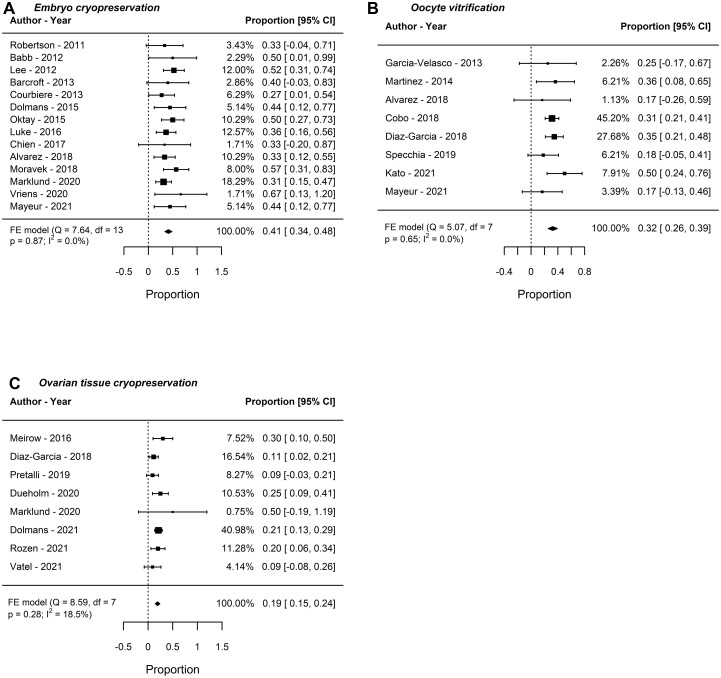
Fourteen studies (175 women) were included in the meta-analysis for LBR after frozen-thawed embryo transfer. The LBR was 41% (95% CI: 34–48, I2: 0%, fixed-effect). In the sensitivity analyses, the estimated percentage varied by <0.5% (Fig. 2A).
Figure 2.
Live birth rate after female fertility preservation for cancer or haematopoietic stem cells transplantation. Analysis of live birth rate after (A) embryo cryopreservation, (B) oocyte vitrification and (C) IVF after ovarian tissue cryopreservation. FE, fixed effect.
Secondary outcome
Percentage of women with at least one livebirth after frozen-thawed embryo transfer
Fourteen studies (175 women) reporting the percentage of women with at least one livebirth after frozen-thawed embryo transfer were included. The percentage of women with at least one livebirth was 43% (95% CI: 36–50, I2: 0%, fixed-effect). In the sensitivity analyses, the estimated percentage varied by <5%.
Miscarriage
Ten studies (101 women) were included in the meta-analysis for miscarriage after frozen-thawed embryo transfer. The percentage of women with miscarriage was 22% (95% CI: 14–30, I2: 0%, fixed-effect).
Oocyte vitrification
Eight studies were included (one prospective (Diaz-Garcia et al., 2018) and seven retrospective (Garcia-Velasco et al., 2013; Martinez et al., 2014; Alvarez and Ramanathan, 2018; Cobo et al., 2018; Specchia et al., 2019; Kato et al., 2021; Mayeur et al., 2021)). A total of 3851 women were analysed, of whom 178 were chosen to return their vitrified oocytes. The age at oocyte vitrification ranged from 15 to 45 years. The main indications for FP were haematological and gynaecological diseases (including breast cancer). The mean number of vitrified oocytes ranged from 5.9 to 9.5. Only three studies reported information on birthweights (Garcia-Velasco et al., 2013; Martinez et al., 2014; Specchia et al., 2019). The study from Moravek et al. was not included here because only one woman returned to use her vitrified oocytes. The characteristics of the included studies on oocyte vitrification are presented in Table II.
Table II.
Characteristics of studies on live birth rate after oocyte vitrification for fertility preservation included in a systematic review and meta-analysis.
| Author, year of publication, country | Study design and type | Study period | Females before treatment for FP (n) | Age, years (mean/median/min–max) | Number vitrified oocytes (mean) | Age at retrieval, years (mean ± SD) | Age retrieval, years (min–max) | Females with oocyte reutilization (n) |
|---|---|---|---|---|---|---|---|---|
| Garcia-Velasco, 2013, Spain | Retrospective cohort | March 2007–June 2012 | 475 | 31.9/–/– | 8.5 | 31.9 ± 5.1 | 4 | |
| Martinez, 2014, Spain | Retrospective cohort | May 2007–November 2012 | 357 | 31.9/–/15–43 | 5.9 | 35.6 ± 3.4 | 30–41 | 11 |
| Alvarez, 2018, UK | Retrospective cohort | January 2000–December 2014 | 306 | 30.3/–/17–43 | 2 | |||
| Cobo, 2018, Spain | Retrospective cohort | January 2007–May 2018 | 1073 | 32.3/–/– | 9.5 | 34.8 ± 2.1 | 80 | |
| Diaz-Garcia, 2018, Spain | Prospective cohort | January 2005–December 2015 | 1024 | 31.7/–/– | 35.2 ± 3.1 | 49 | ||
| Moravek, 2018, USA | Retrospective cohort | January 2005–January 2016 | 204 | –/31/15–42 | 41.1 | 1 | ||
| Specchia, 2019, Italy | Retrospective cohort | January 2001–March 2019 | 244 | 31.3/–/16–45 | 9.5 | 35.2 ± 4.1 | 25–41 | 11 |
| Kato, 2021, Japan | Retrospective cohort | February 2007–December 2019 | 162 | 26.2/–/16–40 | 6.3 | 29.5 ± 1.1 | 14 | |
| Mayeur, 2021, France | Retrospective cohort | January2009–December 2019 | 6 | –/35.5/– | 6 |
FP, fertility preservation.
Primary outcome
The risk of bias was low for all articles included in the meta-analysis for oocyte vitrification.
LBR after oocyte vitrification
Eight studies (177 women) were included in the meta-analysis of LBR after IVF with vitrified oocytes. The LBR was 32% (95% CI: 26–39, I2: 0%, fixed-effect). In the sensitivity analyses, the overall estimated percentage varied by <1% (Fig. 2B).
Secondary outcome
Percentage of women with at least one livebirth after oocyte vitrification
Eight studies (177 women) reporting the percentage of women with at least one livebirth after IVF with vitrified oocytes were included in the meta-analysis. The percentage of women with at least one livebirth was 32% (95% CI: 25–39, I2: 0%, fixed-effect). In sensitivity analyses, the estimated percentage varied by <1%.
Miscarriage
Eight studies (177 women) were included in the meta-analysis for miscarriage after IVF with vitrified oocytes. Using a random-effect model, the percentage of women with miscarriage was 11% (95% CI: 6–19, I2: 0%, fixed-effect). The estimated rates reported by the individual studies ranged from 0% to 50%. In the sensitivity analyses, the overall estimated percentage varied from 10% to 15%.
Ovarian tissue cryopreservation
Fifteen studies were included for OTC (2 prospective (Oktay and Oktem, 2010; Diaz-Garcia et al., 2018) and 13 retrospective (Imbert et al., 2014; Meirow et al., 2016; Lambertini et al., 2018; Silber et al., 2018; Liebenthron et al., 2019; Poirot et al., 2019; Pretalli et al., 2019; Dueholm Hjorth et al., 2020; Marklund et al., 2020; Dolmans et al., 2021; Karavani et al., 2021; Rozen et al., 2021; Vatel et al., 2021)). Seven studies reported orthotopic transplantation (Meirow et al., 2016; Lambertini et al., 2018; Silber et al., 2018; Liebenthron et al., 2019; Pretalli et al., 2019; Dueholm Hjorth et al., 2020; Rozen et al., 2021), one reported heterotopic transplantation, four reported both (Imbert et al., 2014; Poirot et al., 2019; Dolmans et al., 2021; Vatel et al., 2021) and three did not provide this information (Diaz-Garcia et al., 2018; Marklund et al., 2020; Karavani et al., 2021). The time between OTT and first menstruation varied from 3.9 to 94.3 months. Only one study presented details in terms of birthweight (Lambertini et al., 2018). The characteristics of the included studies for OTC are presented in Table III.
Table III.
Characteristics of studies on live birth rate after ovarian tissue cryopreservation for fertility preservation included in a systematic review and meta-analysis.
| Author, year of publication, country | Study design and type | Study period | Females before treatment for FP (n) | Age, years (mean/median/min–max) | Age at retrieval, years |
Age at transplantation, years |
||
|---|---|---|---|---|---|---|---|---|
| (mean ± SD) | (min–max) | (mean ± SD) | (min–max) | |||||
| Oktay, 2010, USA | Prospective cohort | May 1999–March 2008 | 59 | 26.7/–/4–44 | 29.5 | 29–30 | 33.5 | 31–36 |
| Imbert, 2014, Belgium | Retrospective cohort | March 1999–June 2011 | 225 | 24.6/–/0–37 | 25.3 ± 7.6 | 30.4 ± 6.3 | ||
| Meirow, 2016, Israel | Retrospective cohort | January 2004–March 2015 | 20 | 28.7/–/14–39 | 28.7 ± 7.5 | 14–39 | 34.0 | 21–45 |
| Silber, 2018, USA* | Retrospective cohort | 1997–2017 | 66 | –/–/– | 23.7 ± 4.6 | 31.9 ± 3.98 | ||
| Diaz-Garcia, 2018, Spain | Prospective cohort | January 2005–December 2015 | 800 | 28.2/–/– | 34.3 ± 3.8 | 38.9 ± 4.1 | ||
| Lambertini, 2018, Belgium | Retrospective cohort | January 2006–December 2016 | 72 | –/–/– | 31.5 | 30–33 | 35.5 | |
| Liebenthron, 2019, Germany | Retrospective cohort | 2000–2017 | 30 | 31.1/–/– | 31.1 ± 5.0 | 34.8 ± 4.3 | ||
| Poirot, 2019, France | Retrospective cohort | 2005–2015 | 31 | 26.2/27.1/16–37 | 26.21 | 16–37 | 33.47 | 24–42 |
| Pretalli, 2019, France | Retrospective cohort | 2013–2021 | 22 | –/–/– | 32.61 | 22–43 | ||
| Dueholm Hjorth, 2020, Denmark | Retrospective cohort | 2008–2013 (up to 2017) | 28 | 29.8/–/15–39 | 29.8 ± 5.2 | 15–39 | 34 ± 5.1 | 24–42 |
| Marklund, 2020, Sweden | Retrospective cohort | January 1995–June 2017 | 468 | 32.5/–/21–42 | ||||
| Dolmans, 2021, Multinational** | Retrospective cohort | up to October 2020 | 285 | 29.3/–/9–44 | 29.3 ± 6.2 | 9–44 | 34.6 ± 5.5 | |
| Karavani, 2021, Israel | Retrospective cohort | 1997–2017 | 7 | 23.7/–/13–40 | 23.7 | |||
| Rozen, 2021, Australia | Retrospective cohort | 2006–2019 | 30 | 29.4/31/18–39 | 29.4 | 18–39 | 34.9 | |
| Vatel, 2021, France | Retrospective cohort | 2005–2017 | 11 | 26.3/27.1/18–35 | 26.3 ± 6.0 | 18–35 | 33.53 | 24–42 |
Not included in the meta-analysis for live birth rate, FP, fertility preservation.
Belgium, Switzerland, Denmark, Germany, Austria, France, Spain.
Primary outcome
Two studies had a high risk of bias for the selection of participants (Silber et al., 2018; Liebenthron et al., 2019).
LBR after OTT and IVF
Eight studies (266 women) were included in the meta-analysis for LBR following IVF after OTC. The LBR was 19% (95% CI: 15–24, I2: 18.5%, fixed-effect). In sensitivity analyses, the estimated percentage varied by <0.5% (Fig. 2C).
Spontaneous LBR
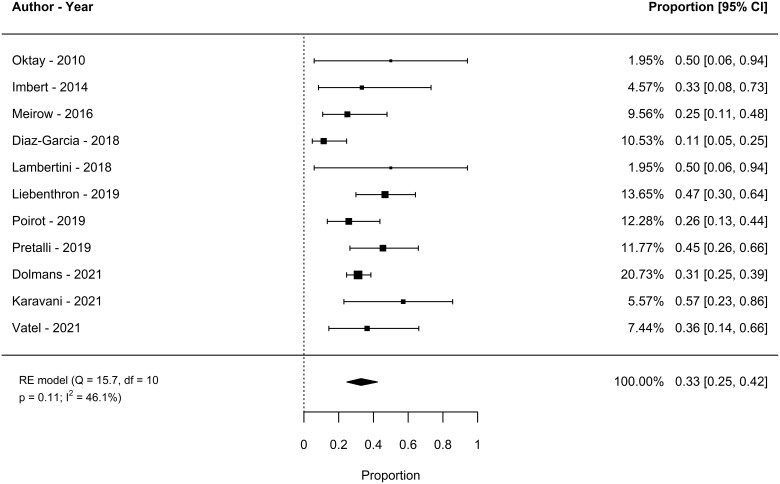
Eleven studies (342 women) were included in the meta-analysis for spontaneous LBR after OTT. The spontaneous LBR was 33% (95% CI: 25–42, I2: 46.1%, random-effect). In sensitivity analyses, the estimated percentage varied by <1% (Fig. 3).
Figure 3.
Spontaneous live birth rate after ovarian tissue cryopreservation and transplantation in women undergoing fertility preservation for cancer or haematopoietic stem cells transplantation. RE, random effect.
Secondary outcome
Percentage of women with at least one livebirth after OTT and IVF
Eight studies (266 women) were included in the meta-analysis for LBR after OTT and IVF. The LBR was 17% (95% CI: 13–22, I2: 0%, fixed-effect). In sensitivity analyses, the estimated percentage varied by <2%.
Percentage of women with at least one spontaneous livebirth
Twelve studies (352 women) reporting the percentage of women with at least one spontaneous livebirth after OTT were included. The percentage of women with at least one spontaneous livebirth was 32% (95% CI: 23–41, I2: 51%, random-effect). In sensitivity analyses, the estimated percentage varied by <2%.
Miscarriage
Ten studies (436 women) were included in the meta-analysis for miscarriage after OTT. The percentage of women with miscarriage was 14% (95% CI: 9–21, I2: 33%, random-effect).
Ovarian function restoration rate
Twelve studies (499 women) were included in the meta-analysis for ovarian function restoration rate. Seven articles reported a 100% restoration rate. To allow the estimation, analysis was performed on the outcome ‘no ovarian function restoration’ and was 6% (95% CI: 3–12%, I2: 40%, random-effect).
Table IV summarizes fertility outcomes according to each FP technique.
Table IV.
Female fertility preservation for cancer or haematopoietic stem cells transplantation.
| Embryo cryopreservation | Oocyte vitrification | Ovarian tissue cryopreservation | |
|---|---|---|---|
| LBR after IVF | 41 (34–48, I2: 0%)* | 32 (26–39, I2: 0%)* | 19 (15–24, I2: 18.5%)* |
| Women with at least one live birth after IVF | 43 (36–50, I2: 0%)* | 32 (25–39, I2: 0%)* | 17 (13–22, I2: 0%)* |
| Miscarriage | 22 (14–30, I2: 0%)* | 11 (6–19, I2: 0%)* | 14 (9–21, I2: 33%)** |
| Spontaneous LBR | 33 (25–42, I2: 46.1%)** | ||
| Women with at least one spontaneous live birth | 32 (23–41, I2: 51%)** |
Live birth rate (LBR as %) and percentage of miscarriage estimated for each fertility preservation method.
Percentage of events with two-sided CI estimated over all the publications; heterogeneity = I2.
Fixed effect model.
Random effect model.
Discussion
Our meta-analysis estimated LBR after embryo cryopreservation and oocyte vitrification of 41% (95% CI: 34–48, I2: 0%) and 32% (95% CI: 26–39, I2: 0%), respectively. Regarding OTC, the IVF and spontaneous estimated LBR were 19% (95% CI: 15–24, I2: 18.5%) and 33% (95% CI: 25–42, I2: 46.1%), respectively. The miscarriage rate varied from 22% (95% CI: 14–30, I2: 0%) for embryos, 11% (95% CI: 6–19, I2: 0%) for oocytes and 14% (95% CI: 9–21, I2: 33%) for OTCs. These rates are similar to the miscarriage rate in the general population (Linnakaari et al., 2019). Only 6% (95% CI: 3–12%, I2: 40%) of women did not have a return of ovarian function after ovarian transplantation.
In the latest ESHRE guidelines, published in 2020, only oocyte and embryo cryopreservation were considered as established options for FP after puberty, whereas OTC was an innovative method. However, OTC was regarded as an acceptable FP technique in the ASRM guidelines in 2019.
For ESHRE, OTC could be offered in situations where oocyte or embryo cryopreservation cannot be performed or according to the woman’s request (The ESHRE Guideline Group on Female Fertility Preservation, 2020). Regarding the ASRM guidelines, OTC is the only option to preserve fertility for prepubertal girls (Practice Committee of the American Society for Reproductive Medicine, 2019). Given the lack of studies on long-term outcomes after FP, it is still unclear which technique offers the best chance of achieving a live birth. The three techniques cannot be compared, and the literature remains difficult to interpret owing to heterogeneous methodologies and ways of reporting results. Moreover, the age and clinical situation of women at the time of diagnosis may influence the type of FP chosen, affecting the age of return and, inevitably, the LBR. Therefore, concerning our results, it is important to consider not only the estimated percentage but also the CI when providing the information to women. Appropriate and realistic counselling remains a challenge in this field.
Historically, embryo cryopreservation was the first technique developed for preserving female fertility and, for many years, the only one. Our meta-analysis suggests that embryo cryopreservation might be associated with the highest chances of achieving a live birth, considering the 22% miscarriage rate. This rate of miscarriage cannot be explained. The protocol for frozen embryo transfer has never been described; therefore, we did not know whether progesterone levels were monitored at the time of transfer, which is a recent concept (Labarta et al., 2022). This could be an explanation. Above all, we must specify that the results might be underestimated because slow freezing and cleavage-stage embryos were used in some of the studies included. In fact, embryo vitrification and culture to blastocyst stage have improved IVF outcomes (Glujovsky et al., 2012; Nagy et al., 2020). Nevertheless, the main issue with embryo cryopreservation in FP is the loss of autonomy for women who wish to preserve their fertility. Indeed, a high percentage of couples may break up, not to mention the possible death of a partner, raising ethical and legal concerns about the remaining embryos (Nalbant et al., 2021). In a recent prospective study, women were more likely to vitrify oocytes or split their oocytes and only fertilize half of them after receiving clear information on legal aspects. It is therefore recommended to counsel women to perform both embryo cryopreservation and oocyte vitrification (Practice Committee of the American Society for Reproductive Medicine, 2019; The ESHRE Guideline Group on Female Fertility Preservation, 2020).
Oocyte vitrification is a more recent FP technique than embryo cryopreservation and has revolutionized reproductive biology to the point where it has become the main FP technique used after puberty (Kuleshova et al., 1999). Data concerning long-term outcomes after oocyte vitrification in the event of FP for cancer are still scarce. As the published data are only from experienced teams with a high volume of procedures, a clear CLBR is difficult to estimate (Cobo et al., 2021). Cobo et al.’s study reported a CLBR of 41.1% in women who cryopreserved oocytes for oncological reasons (Cobo et al., 2021). In this multicentre retrospective study, only 7.4% of women returned their own oocytes. It is likely that there is a technical learning curve for oocyte vitrification, although this is not well known. The success rate of oocyte vitrification is probably related to the experience of the embryology team. Therefore, the true results of each centre in the FP field should be provided to women (Lussig et al., 2019; Practice Committee of the American Society for Reproductive Medicine, 2019; Dolmans and Donnez, 2021).
Regarding OTC and according to the literature, the spontaneous pregnancy rate is better than IVF after OTT (Garcia-Velasco et al., 2013; Khattak et al., 2022). The ESHRE and ASRM guidelines recommend performing OTCs before the ages of 36 and 40 years, respectively. The slow freezing technique is the most commonly used, as vitrification protocols for the ovarian cortex are still experimental. Orthotopic ovarian autografts, either onto the remaining ovary or the pelvic sidewall, appear to be the best option (The ESHRE Guideline Group on Female Fertility Preservation, 2020). Reports of the amount of transplanted tissue or the follicular density are often lacking in our included studies and should always be given in future publications. The ovarian tissue must always be checked for malignant cells before grafting. Before performing the graft, the patient must be informed of the necessity to have patent tubes, and a semen analysis should be performed for the partner. The main advantage of OTC compared to oocyte and embryo cryopreservation is the restoration of endocrine function. In our studies, more than 90% of women recovered ovarian function, which is concordant with the literature (Khattak et al., 2022). Another possible advantage with tissue could be the possibility of achieving more pregnancies. Indeed, compared to the tissue, the number of oocytes and embryos is limited.
Finally, a combination of the various FP techniques, especially in women with a high risk of premature ovarian insufficiency, could be the best future option. Indeed, in 2021, Dolmans and Donnez reported a LBR of 50–60% in women surviving cancer who immediately vitrified oocytes after OTC. The combined technique could be offered to postpubertal patients when chemotherapy can be postponed without jeopardizing cancer therapy (Dolmans et al., 2014; Dolmans and Donnez, 2021).
Strengths
To the best of our knowledge, this meta-analysis is the first to compile data on all FP techniques. The risk of including the same population twice was carefully evaluated, and sensitivity analyses were performed when needed. Even though the three techniques cannot be compared, the study provides detailed information about LBRs and could help professionals to counsel women. Another strength is the use of strict methodology using the Prisma guidelines and the Cochrane handbook. The quality of the included studies was assessed by the Newcastle–Ottawa Quality Assessment Scale, and many of them had a low risk of bias.
Limitations
The quality of our meta-analysis depends on the quality of the included studies. Only observational studies were available, and randomized controlled trials cannot be conducted in this field. Most of our included studies had a small number of participants. LBRs were therefore probably underestimated, especially in women followed after embryo cryopreservation and oocyte vitrification, for whom spontaneous pregnancies are never reported. This is emphasized by the current use of vitrification and improvements in laboratory techniques. However, missing data on spontaneous pregnancies for all techniques is also an important issue. There is a crucial lack of information regarding patients who did not come back to use their gamete or ovarian tissue. Follow-up of these cohorts should be the main concern in the future. Another piece of missing information is the evaluation of the efficiency of IVF protocols, and information on the number of cycles required to achieve pregnancy should be collected. The time taken to achieve a live birth should also be gathered. Furthermore, regarding OTC, various protocols are used worldwide, underlining the heterogeneity of this procedure. Finally, as FP is a hot topic, some new studies were published during the writing of our meta-analysis and could therefore not be included.
This meta-analysis highlights the necessity for an international register with a long follow-up of cohorts. Longitudinal studies could be the first step in improving the quality of the literature, with international guidelines to always report the same variables. A consensus should be reached for the OTC procedure with clear steps to follow. Eventually, the vitrification protocol for embryos and especially for oocytes should be homogenized to facilitate comparison between centres.
Conclusion
To conclude, the results from this systematic review and meta-analysis are useful for helping practitioners counsel women about FP techniques. A combination of different techniques could be the best option but requires further investigation. An international registry should be developed with clear guidelines for future publications in this field.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Karin Hammarberg for providing additional information about her work ‘Survey of Reproductive Experiences and Outcomes of Cancer Survivors Who Stored Reproductive Material Before Treatment’ Hammarberg K, Kirkman M, Stern C, McLachlan RI, Clarke G, Agresta F, Gook D, Rombaults L, Vollenhoven B, Fisher JRW, Human Reprod. 2018;33(1):179.
The authors also thank Solène Languille (Monitoring Force Group, Maisons-Laffitte), who developed the search strategy, and Dr Bernadette Darné (Monitoring Force Group, Maisons-Laffitte), who provided statistical expertise.
Authors’ roles
B.C. is the guarantor. B.C., E.F. and S.H. drafted the manuscript. All authors read, provided feedback (particularly on selection criteria, risk of bias assessment strategy, data extraction criteria) and approved the final manuscript.
Funding
This work was sponsored by an unrestricted grant from GEDEON RICHTER France.
Conflict of interest
The authors have no competing interests to declare.
Contributor Information
E Fraison, Service de Médecine de la Reproduction, Hospices Civils de Lyon, Hôpital Mère Enfant, Bron, France; Université Claude Bernard, Faculté de Médecine Laennec, Lyon, France; INSERM Unité 1208, Bron, France.
S Huberlant, Service de Gynécologie Obstétrique et Médecine de la Reproduction, CHU Carémeau, Nîmes, France; Université de Montpellier-Nîmes, Nîmes Cedex 2, France.
E Labrune, Service de Médecine de la Reproduction, Hospices Civils de Lyon, Hôpital Mère Enfant, Bron, France; Université Claude Bernard, Faculté de Médecine Laennec, Lyon, France; INSERM Unité 1208, Bron, France.
M Cavalieri, Service de Gynécologie-Obstétrique et Médecine de la Reproduction, CHU François Mitterrand, Dijon, France.
M Montagut, Service de Médecine de la Reproduction, Clinique Croix du Sud, Quint-Fonsegrives, France.
F Brugnon, Assistance Médicale à la Procréation, CECOS, CHU Clermont Ferrand, CHU Estaing, Clermont-Ferrand, France; Université Clermont Auvergne, IMoST, INSERM 1240, Faculté de Médecine, Clermont-Ferrand, France.
B Courbiere, Service d’Assistance Médicale à la Procréation, Plateforme Cancer & Fertilité OncoPACA-Corse, AP-HM, Hôpital La Conception, Marseille, France; Aix-Marseille Université, IMBE, CNRS, IRD, Avignon Université, Marseille, France.
Data Availability
Data are available on request.
References
- Alvarez RM, Ramanathan P.. Fertility preservation in female oncology patients: the influence of the type of cancer on ovarian stimulation response. Hum Reprod 2018;33:2051–2059. [DOI] [PubMed] [Google Scholar]
- Babb A, Farah N, Lyons C, Lindsay K, Reddy N, Goldman J, Apperley JF, Salooja N.. Uptake and outcome of assisted reproductive techniques in long-term survivors of SCT. Bone Marrow Transplant 2012;47:568–573. [DOI] [PubMed] [Google Scholar]
- Barcroft J, Dayoub N, Thong KJ.. Fifteen-year follow-up of embryos cryopreserved in cancer patients for fertility preservation. J Assist Reprod Genet 2013;30:1407–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Chambers J, Mcauley F, Kaplan T, Letourneau J, Hwang J, Kim M-O, Melisko ME, Rugo HS, Esserman LJ. et al. Fertility preservation with ovarian stimulation and time to treatment in women with stage II–III breast cancer receiving neoadjuvant therapy. Breast Cancer Res Treat 2017;165:151–159. [DOI] [PubMed] [Google Scholar]
- Cobo A, García-Velasco J, Domingo J, Pellicer A, Remohí J.. Elective and onco-fertility preservation: factors related to IVF outcomes. Hum Reprod 2018;33:2222–2231. [DOI] [PubMed] [Google Scholar]
- Cobo A, García-Velasco JA, Remohí J, Pellicer A.. Oocyte vitrification for fertility preservation for both medical and nonmedical reasons. Fertil Steril 2021;115:1091–1101. [DOI] [PubMed] [Google Scholar]
- Courbiere B, Decanter C, Bringer-Deutsch S, Rives N, Mirallié S, Pech JC, De Ziegler D, Carré-Pigeon F, May-Panloup P, Sifer C. et al. ; French Study Group for Ovarian and Testicular Cryopreservation (GRECOT). Emergency IVF for embryo freezing to preserve female fertility: a French multicentre cohort study. Hum Reprod 2013;28:2381–2388. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins J, Altman D. Chapter 10: analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane, 2021. www.training.cochrane.org/handbook (December 2021, date last accessed).
- Diaz-Garcia C, Domingo J, Garcia-Velasco JA, Herraiz S, Mirabet V, Iniesta I, Cobo A, Remohí J, Pellicer A.. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: a prospective cohort study. Fertil Steril 2018;109:478–485.e2. [DOI] [PubMed] [Google Scholar]
- Diesch-Furlanetto T, Rovó A, Galimard JE, Szinnai G, Dalissier A, Sedlacek P, Bodova I, Roussou VK, Gibson BE, Poiré X. et al. Pregnancy and pregnancy outcomes after hematopoietic stem cell transplantation in childhood: a cross-sectional survey of the EBMT Pediatric Diseases Working Party. Hum Reprod 2021;36:2871–2882. [DOI] [PubMed] [Google Scholar]
- Dolmans M-M, Donnez J.. Fertility preservation in women for medical and social reasons: oocytes vs ovarian tissue. Best Pract Res Clin Obstet Gynaecol 2021;70:63–80. [DOI] [PubMed] [Google Scholar]
- Dolmans M-M, Hollanders de Ouderaen S, Demylle D, Pirard C.. Utilization rates and results of long-term embryo cryopreservation before gonadotoxic treatment. J Assist Reprod Genet 2015;32:1233–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmans M-M, Marotta M-L, Pirard C, Donnez J, Donnez O.. Ovarian tissue cryopreservation followed by controlled ovarian stimulation and pick-up of mature oocytes does not impair the number or quality of retrieved oocytes. J Ovarian Res 2014;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmans M-M, von Wolff M, Poirot C, Diaz-Garcia C, Cacciottola L, Boissel N, Liebenthron J, Pellicer A, Donnez J, Andersen CY.. Transplantation of cryopreserved ovarian tissue in a series of 285 women: a review of five leading European centers. Fertil Steril 2021;115:1102–1115. [DOI] [PubMed] [Google Scholar]
- Dueholm Hjorth IM, Kristensen SG, Dueholm M, Humaidan P.. Reproductive outcomes after in vitro fertilization treatment in a cohort of Danish women transplanted with cryopreserved ovarian tissue. Fertil Steril 2020;114:379–387. [DOI] [PubMed] [Google Scholar]
- Garcia-Velasco JA, Domingo J, Cobo A, Martínez M, Carmona L, Pellicer A.. Five years’ experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil Steril 2013;99:1994–1999. [DOI] [PubMed] [Google Scholar]
- Glujovsky D, Blake D, Bardach A, Farquhar C.. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. In: The Cochrane Collaboration (ed). Cochrane Database of Systematic Reviews. Cochrane Library: John Wiley & Sons, Ltd., 2012, CD002118.pub4. [DOI] [PubMed] [Google Scholar]
- Imbert R, Moffa F, Tsepelidis S, Simon P, Delbaere A, Devreker F, Dechene J, Ferster A, Veys I, Fastrez M. et al. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Hum Reprod 2014;29:1931–1940. [DOI] [PubMed] [Google Scholar]
- Iussig B, Maggiulli R, Fabozzi G, Bertelle S, Vaiarelli A, Cimadomo D, Ubaldi FM, Rienzi L.. A brief history of oocyte cryopreservation: arguments and facts. Acta Obstet Gynecol Scand 2019;98:550–558. [DOI] [PubMed] [Google Scholar]
- Karavani G, Rottenstreich A, Schachter-Safrai N, Cohen A, Weintraub M, Imbar T, Revel A.. Chemotherapy-based gonadotoxicity risk evaluation as a predictor of reproductive outcomes in post-pubertal patients following ovarian tissue cryopreservation. BMC Womens Health 2021;21:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Ochi M, Nakamura Y, Kamiya H, Utsunomiya T, Yano K, Michikura Y, Hara T, Kyono K, Takeuchi K. et al. A multi-centre, retrospective case series of oocyte cryopreservation in unmarried women diagnosed with haematological malignancies. Hum Reprod Open 2021;2021:hoaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak H, Malhas R, Craciunas L, Afifi Y, Amorim CA, Fishel S, Silber S, Gook D, Demeestere I, Bystrova O. et al. Fresh and cryopreserved ovarian tissue transplantation for preserving reproductive and endocrine function: a systematic review and individual patient data meta-analysis. Hum Reprod Update 2022;28:400–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuleshova L, Gianaroli L, Magli C, Ferraretti A, Trounson A.. Birth following vitrification of a small number of human oocytes. Hum Reprod 1999;14:3077–3079. [DOI] [PubMed] [Google Scholar]
- Labarta E, Mariani G, Rodríguez-Varela C, Bosch E.. Individualized luteal phase support normalizes live birth rate in women with low progesterone levels on the day of embryo transfer in artificial endometrial preparation cycles. Fertil Steril 2022;117:96–103. [DOI] [PubMed] [Google Scholar]
- Lambertini M, Goldrat O, Ferreira AR, Dechene J, Azim HA Jr, Desir J, Delbaere A, t’Kint D, Roodenbeke M-D, de Azambuja E. et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol 2018;29:237–243. [DOI] [PubMed] [Google Scholar]
- Lee S, Oktay K.. Does higher starting dose of FSH stimulation with letrozole improve fertility preservation outcomes in women with breast cancer? Fertil Steril 2012;98:961–964.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebenthron J, Montag M, Reinsberg J, Köster M, Isachenko V, van der Ven K, van der Ven H, Krüssel J-S, von Wolff M.. Overnight ovarian tissue transportation for centralized cryobanking: a feasible option. Reprod Biomed Online 2019;38:740–749. [DOI] [PubMed] [Google Scholar]
- Lin L, Chu H.. Meta-analysis of proportions using generalized linear mixed models. Epidemiology 2020;31:713–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnakaari R, Helle N, Mentula M, Bloigu A, Gissler M, Heikinheimo O, Niinimäki M.. Trends in the incidence, rate and treatment of miscarriage—nationwide register-study in Finland, 1998–2016. Hum Reprod 2019;34:2120–2128. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Spector LG, Stern JE, Smith YR, Williams M, Koch L, Schymura MJ.. Embryo banking among women diagnosed with cancer: a pilot population-based study in New York, Texas, and Illinois. J Assist Reprod Genet 2016;33:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund A, Eloranta S, Wikander I, Kitlinski ML, Lood M, Nedstrand E, Thurin-Kjellberg A, Zhang P, Bergh J, Rodriguez-Wallberg KA.. Efficacy and safety of controlled ovarian stimulation using GnRH antagonist protocols for emergency fertility preservation in young women with breast cancer—a prospective nationwide Swedish multicenter study. Hum Reprod 2020;35:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Rabadan S, Domingo J, Cobo A, Pellicer A, Garcia-Velasco JA.. Obstetric outcome after oocyte vitrification and warming for fertility preservation in women with cancer. Reprod Biomed Online 2014;29:722–728. [DOI] [PubMed] [Google Scholar]
- Mayeur A, Puy V, Windal V, Hesters L, Gallot V, Benoit A, Grynberg M, Sonigo C, Frydman N.. Live birth rate after use of cryopreserved oocytes or embryos at the time of cancer diagnosis in female survivors: a retrospective study of ten years of experience. J Assist Reprod Genet 2021;38:1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirow D, Ra'anani H, Shapira M, Brenghausen M, Derech Chaim S, Aviel-Ronen S, Amariglio N, Schiff E, Orvieto R, Dor J.. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril 2016;106:467–474. [DOI] [PubMed] [Google Scholar]
- Moravek MB, Confino R, Smith KN, Kazer RR, Klock SC, Lawson AK, Gradishar WJ, Pavone ME.. Long-term outcomes in cancer patients who did or did not pursue fertility preservation. Fertil Steril 2018;109:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy ZP, Shapiro D, Chang C-C.. Vitrification of the human embryo: a more efficient and safer in vitro fertilization treatment. Fertil Steril 2020;113:241–247. [DOI] [PubMed] [Google Scholar]
- Nalbant B, Karger A, Zimmermann T.. Cancer and relationship dissolution: perspective of partners of cancer patients. Front Psychol 2021;12:624902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes N, Porcu E, Borini A.. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online 2009;18:769–776. [DOI] [PubMed] [Google Scholar]
- Oktay K, Oktem O.. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: Report of an ongoing experience. Fertil Steril 2010;93:762–768. [DOI] [PubMed] [Google Scholar]
- Oktay K, Turan V, Bedoschi G, Pacheco FS, Moy F.. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J Clin Oncol 2015;33:2424–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirot C, Fortin A, Lacorte JM, Akakpo JP, Genestie C, Vernant JP, Brice P, Morice P, Leblanc T, Gabarre J. et al. ; CAROLéLISA Cooperative Group. Impact of cancer chemotherapy before ovarian cortex cryopreservation on ovarian tissue transplantation. Hum Reprod 2019;34:1083–1094. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril 2019;112:1022–1033. [DOI] [PubMed] [Google Scholar]
- Pretalli J-B, Frontczak Franck S, Pazart L, Roux C, Amiot C; DATOR Group. Development of ovarian tissue autograft to restore ovarian function: protocol for a French multicenter cohort study. JMIR Res Protoc 2019;8:e12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AD, Missmer SA, Ginsburg ES.. Embryo yield after in vitro fertilization in women undergoing embryo banking for fertility preservation before chemotherapy. Fertil Steril 2011;95:588–591. [DOI] [PubMed] [Google Scholar]
- Rozen G, Sii S, Agresta F, Gook D, Polyakov A, Stern C.. Ovarian tissue grafting: lessons learnt from our experience with 55 grafts. Reprod Med Biol 2021;20:277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber SJ, DeRosa M, Goldsmith S, Fan Y, Castleman L, Melnick J.. Cryopreservation and transplantation of ovarian tissue: results from one center in the USA. J Assist Reprod Genet 2018;35:2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specchia C, Baggiani A, Immediata V, Ronchetti C, Cesana A, Smeraldi A, Scaravelli G, Levi-Setti PE.. Oocyte cryopreservation in oncological patients: eighteen years’ experience of a tertiary care referral center. Front Endocrinol (Lausanne) 2019;10:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ESHRE Guideline Group on Female Fertility Preservation, Anderson RA, Amant F, Braat D, D'Angelo A, Chuva de Sousa Lopes SM, Demeestere I, Dwek S, Frith L, Lambertini M, Maslin C, et al. ESHRE guideline: female fertility preservation. Hum Reprod Open 2020;2020:hoaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatel M, Torre A, Paillusson B, Scheffler F, Bergere M, Benkhalifa M, Le Martelot M-T, Leperlier F, Mirallié S, Selleret L. et al. Efficacy of assisted reproductive technology after ovarian tissue transplantation in a cohort of 11 patients with or without associated infertility factors. J Assist Reprod Genet 2021;38:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft 2010;36:1–48. [Google Scholar]
- Vriens IJH, Ter Welle-Butalid EM, de Boer M, de Die-Smulders CEM, Derhaag JG, Geurts SME, van Hellemond IEG, Luiten EJT, Dercksen MW, Lemaire BMD. et al. Preserving fertility in young women undergoing chemotherapy for early breast cancer; the Maastricht experience. Breast Cancer Res Treat 2020;181:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker Z, Lanes A, Ginsburg E.. Oocyte cryopreservation review: outcomes of medical oocyte cryopreservation and planned oocyte cryopreservation. Reprod Biol Endocrinol 2022;20:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaami S, Melcarne R, Patrone R, Gullo G, Negro F, Napoletano G, Monti M, Aceti V, Panarese A, Borcea MC. et al. Oncofertility and reproductive counseling in patients with breast cancer: a retrospective study. JCM 2022;11:1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request.