Abstract
STUDY QUESTION
Are ovarian antral follicle dynamics altered in women with obesity and regular ovulatory cycles?
SUMMARY ANSWER
Eumenorrheic women with obesity display evidence of suppressed antral follicle dynamics as judged by fewer recruitment events, selectable follicles, and anovulatory dominant follicles, as well as lower anti-Müllerian hormone (AMH) concentrations and an increased prevalence of luteal phase defects.
WHAT IS KNOWN ALREADY
Ovarian antral follicle development is a dynamic process involving distinct follicular and endocrine events that are critical for the occurrence of regular monthly ovulations. Follicle dynamics have not been prospectively evaluated in eumenorrheic women with obesity despite the known impact of obesity on gonadotropin production, ovarian steroid hormone concentrations, and fecundity.
STUDY DESIGN, SIZE, DURATION
This was a prospective, longitudinal study of 42 women conducted over one inter-ovulatory interval (IOI).
PARTICIPANTS/MATERIALS, SETTING, METHODS
A group of 21 women with obesity (total percent body fat ≥35%) and a group of 21 women without obesity (total percent body fat <35%) underwent transvaginal ultrasonography and venipuncture every-other-day for one IOI at an academic clinical research unit. Participants were aged 19–38 years and had a history of self-reported regular menstrual cycles (21–35 days). Follicle number and diameter (≥2 mm) were quantified at each visit. Individual growth profiles for all follicles that grew to ≥7 mm were assessed. Blood samples were assayed for gonadotropins, AMH, estradiol, and progesterone.
MAIN RESULTS AND THE ROLE OF CHANCE
Women with obesity exhibited fewer recruitment events (mean ± SD, 1 ± 1 vs 2 ± 1 events; P = 0.010) and fewer selectable follicles (4 ± 3 vs 8 ± 6 follicles per participant; P = 0.022) during an IOI compared to women without obesity. AMH levels were lower in women with obesity (4.40 ± 3.01 vs 5.94 ± 2.49 ng/ml; P = 0.023), while gonadotropin profiles were similar between groups, across the IOI. Of the individual follicles tracked, fewer follicles progressed to >10 mm in the cohort with obesity (30 vs 40 follicles; P = 0.04) and fewer anovulatory follicles achieved dominance (9 vs 18 follicles; P = 0.041). Ovulatory follicles were selected at smaller diameters in women with compared to those without obesity (7.5 ± 1.6 vs 9.5 ± 1.9 mm; P = 0.001). Luteal phase defects were also more common in women with compared to those without obesity, as defined by either integrated (76 vs 29%, P = 0.002) or maximum (71 vs 24%, P = 0.002) luteal progesterone.
LIMITATIONS, REASONS FOR CAUTION
This study was limited to an assessment of antral follicle dynamics and cannot inform on earlier stages of folliculogenesis. This study was observational and cannot address causation between obesity and altered antral follicle dynamics. Lastly, the data cannot be extrapolated to account for reduced fecundity and fertility in obesity.
WIDER IMPLICATIONS OF THE FINDINGS
The increasing global prevalence of obesity necessitates an understanding of the mechanisms that underlie obesity-related adverse reproductive health outcomes. Eumenorrheic women with obesity demonstrate altered ovarian antral follicle and endocrine dynamics compared to their counterparts without obesity. The degree to which abnormal granulosa cell assembly and/or activity underlie the suboptimal luteinization and subfertility requires further investigation.
STUDY FUNDING/COMPETING INTEREST(S)
Funding was provided by Cornell University, President’s Council of Cornell Women, United States Department of Agriculture (grant no. 8106), and National Institutes of Health (R01-HD0937848). B.Y.J. and H.V.B. were supported by doctoral training awards from the National Institutes of Health (T32-DK007158) and Canadian Institutes of Health Research (grant no. 146182), respectively.
TRIAL REGISTRATION NUMBER
Keywords: obesity, menstrual cycle, ovarian follicle, ultrasonography, luteal phase
Introduction
More than one-third of reproductive-aged women globally are living with obesity (Hruby and Hu, 2015; Hales et al., 2020; Vaamonde and Álvarez-Món, 2020). Obesity is associated with several adverse reproductive health outcomes, including increased likelihood of anovulation, longer time to pregnancy, reduced fertility, and increased risk of late menopause (Kyrou et al., 2018; Purcell and Moley 2011; Damodaran and Swaminathan, 2013; Shaw and Edelman, 2013; Dağ and Dilbaz, 2015; Goldsammler et al., 2018; Silvestris et al., 2018; Zhu et al., 2018). Despite these recognized impacts on reproductive health, a substantial percentage of women with obesity report regular menstrual cycles (Lasquety et al., 2012). Eumenorrheic women with obesity demonstrate reduced LH pulse amplitude (Jain et al., 2007), decreased FSH production during the follicular phase (De Pergola et al., 2006; Yeung et al., 2013), elevated estradiol (E2) concentrations (De Pergola et al., 2006; Yeung et al., 2013), and lower luteal progesterone (P4) concentrations (De Pergola et al., 2006; Jain et al., 2007; Yeung et al., 2013), consistent with endocrine hormone disruptions across the menstrual cycle. Precise control of endocrine hormone dynamics is critical for the maintenance of ovarian folliculogenesis and the timely release of a mature oocyte on a monthly basis. The nature of antral follicle dynamics in women with obesity, and how it aligns with their endocrine abnormalities, is unknown; yet both could underlie the adverse reproductive health outcomes that are common in this population.
Using transvaginal ultrasonography, antral follicle dynamics can be characterized by tracking the growth of uniquely identifiable follicles (i.e. Identity Method) (Pierson and Ginther, 1988; Knopf et al., 1989) or evaluating overall changes in follicle number and diameter (i.e. Non-Identity Method) (Baerwald et al., 2003; Rouleau et al., 2012; Vanden Brink et al., 2013) across the menstrual cycle. These approaches have been used to confirm wave-like patterns of antral follicle development in healthy, eumenorrheic women of reproductive age (Baerwald et al., 2003; 2004). Antral follicle dynamics are thought to be a primary factor determining menstrual cycle length (Baerwald et al., 2004) and may relate to fertility potential (Townson et al., 2002). However, the impact of obesity on follicle dynamics has not been prospectively evaluated and remains a significant knowledge gap given our substantial and growing rates of obesity. To that end, the objectives of this study were to contrast antral follicle growth and endocrine hormone dynamics between eumenorrheic women, with and without obesity, during an inter-ovulatory interval (IOI). We hypothesized that women with obesity would show differences in all key stages of follicle development including recruitment, selection, and ovulation, and that altered follicle dynamics would align with disruptions in endocrine hormone dynamics in both the follicular and luteal phases.
Materials and methods
Study participants
Female participants of reproductive age (18–38 years) were recruited from the general population between October 2009 and September 2021 to one of two studies. The first study was designed to contrast antral follicle dynamics in women with regular versus irregular menstrual cycles across a spectrum of adiposity. The second study was designed to evaluate the impact of weight loss on follicle dynamics in women with regular versus irregular menstrual cycles. Women were eligible to participate in the studies if they had consistent and optimal visualization of both ovaries on ultrasonography. Women were excluded if they: were using medications known or suspected to interfere with reproductive function in the 2 months prior; were pregnant or lactating in the 6 months prior; had a history of primary ovarian insufficiency; or had any confounding medical condition, including but not limited to untreated thyroid abnormalities or hyperprolactinemia. Both study protocols were approved by the Institutional Review Board at Cornell University and registered at ClinicalTrials.gov (Identifiers: NCT01927432, NCT01785719). Before procedures were performed, informed consent was obtained from all participants.
Participants who completed either study were retrospectively evaluated for inclusion in the current analysis (n = 112). Groups of interest were: (i) women with regular menstrual cycles and obesity, and (ii) women with regular menstrual cycles without obesity. Obesity was defined by a total percent body fat (PFT) ≥35% using whole-body dual x-ray absorptiometry (Valdez, 1991; Piqueras et al., 2021). Menstrual cycle regularity was defined by a self-reported menstrual cycle length of 21–35 days in the last year with menstrual cycle regularity confirmed post hoc using ultrasound monitoring of ovarian antral follicle development during an IOI (described below). All women included were normoandrogenic, as defined by a total testosterone (T) concentration ≤61.5 ng/dl based on a threshold derived in an internal reference cohort. Clinical measures of hyperandrogenism were not considered (hirsutism).
Ultrasonographic measurements
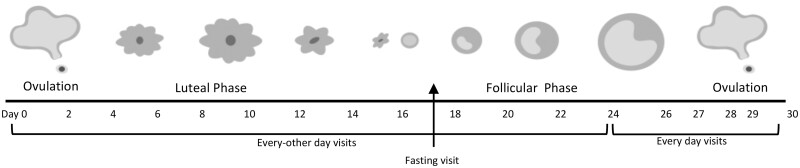
Serial transvaginal ultrasonography was used to evaluate antral follicle dynamics, as previously described (Baerwald et al., 2003; Rouleau et al., 2012; Vanden Brink et al., 2013; Jarrett et al., 2020). Briefly, participants visited the Human Metabolic Research Unit (Cornell University, Ithaca, NY, USA) for an ultrasound scan of the ovaries and blood draw approximately every-other-day for one IOI, with scans initiated on approximately cycle day 10 prior to ovulation (Fig. 1). An IOI was defined as the interval from one ovulation to the next ovulation, which represented the luteal phase following ovulation, menstruation, and the follicular phase preceding the subsequent ovulation. When a follicle ≥14–16 mm was detected, ultrasound scans transitioned to being performed daily until its fate, either regression or ovulation, was confirmed. Ovulation was defined as the sonographic detection of a corpus luteum during the IOI and was later confirmed with a rise in serum P4 of ≥1.5 ng/ml (Baerwald et al., 2005).
Figure 1.
Ultrasound scanning and blood sampling schedule across an inter-ovulatory interval (IOI). Timeline shows a representative study visit schedule across a single IOI. Study visits were initiated in the late follicular phase with the goal of capturing a first ovulation. Scans were performed every other day until a dominant follicle emerged and reached 14–16 mm. Thereafter, scans were performed daily until ovulation was confirmed. Following ovulation, scans returned to an every-other-day frequency until emergence of the subsequent ovulatory dominant follicle at which time daily scans were repeated until the second ovulation was confirmed. Non-fasted blood samples were collected every other day throughout the IOI. One fasting blood sample was collected in the early follicular phase.
Scans were performed using a GE Voluson E8 Expert System or a GE Voluson E10 Expert System equipped with a 6–12 MHz 3D/4D transducer (GE Healthcare, Milwaukee, WI, USA). Ovaries were imaged from their inner to outer margins in the longitudinal plane using the automated volume modality. One of four sonographers conducted the ultrasound scans using a standardized protocol for three-dimensional image acquisition of the ovaries. Two-dimensional cineloops were archived and evaluated offline by three investigators [Sante DICOM Editor, Santesoft LTD, Athens, Greece]. For each scan during the IOI, follicle number and diameter were assessed for the left and right ovaries. In order to obtain reliable follicle counts, investigators used the grid system approach (Lujan et al., 2010). A reliability analysis based on 20 images that were randomized for evaluation confirmed high inter-rater agreement on follicle counts (single measures intraclass correlation coefficient (ICC) = 0.932), justifying the pooling of measures across raters. The diameter of each follicle was measured in the largest cross-sectional view and calculated as the average of its two orthogonal dimensions (i.e. length × width). If a large follicle (i.e. ≥10 mm) was detected, then orthogonal dimensions were repeated in a second plane and the four dimensions were averaged. Follicle diameter was rounded to the nearest whole number. Likewise, the largest cross-sectional view of the ovary in both the sagittal and transverse planes was used to calculate ovarian volume using the prolate ellipsoid formula. Ovarian volume was measured on a single scan for each participant during the follicular phase and presented as the average size of the left and right ovaries.
Growth and regression profiles of individual follicles that grew to 7 mm or greater were generated using the Identity Method (Baerwald et al., 2003; Rouleau et al., 2012; Vanden Brink et al., 2013; Jarrett et al., 2020). Briefly, all follicles ≥4 mm were sketched on paper to generate a map of antral follicles within each ovary. Maps were completed for each ovary at each visit of the IOI. Individual follicles were mapped for their location using anatomical landmarks and positions relative to other follicles within the ovary. Each follicle that grew to ≥7 mm was uniquely identified and changes in diameter were tracked over time from the day of first detection (i.e. at 4–5 mm) to last detection (i.e. at 4–5 mm or ovulation). Growth and regression rates of each uniquely identified follicle were then calculated. Sonographic presence was defined as the interval of time between the first and last days of sonographic detection of a follicle (Baerwald et al., 2009; Jarrett et al., 2020). The growth phase was defined as the interval of time from the day of first detection to the day of maximal follicle diameter (Baerwald et al., 2009; Jarrett et al., 2020). The regression phase was defined as the interval of time from the day of maximal diameter to the day of last detection (Baerwald et al., 2009; Jarrett et al., 2020). A follicle was considered to be in a static phase if it was observed within 1 mm of its maximal diameter for at least three consecutive days (or two consecutive visits) (Baerwald et al., 2009; Jarrett et al., 2020). The first and last days of a static phase coincided with the end of the growth phase and beginning of the regression phase, respectively.
A recruitment event was defined as the emergence of two or more follicles ≥4 mm within a 3-day (or two-visit) window, that further grew to ≥7 mm, in conjunction with an increase and subsequent decrease in the number of follicles ≥5 mm (adapted from Baerwald et al. (2003) and Baerwald et al. (2004)). Follicle waves were not characterized herein as described by Baerwald et al. due to our less frequent blood sampling protocol (Baerwald et al., 2003, 2004). Dominance was defined as the growth of a follicle to ≥10 mm that exceeded the next largest follicle by ≥2 mm (Baerwald et al., 2004). Selection was defined as the day when the future dominant follicle grew ≥1 mm larger than the subsequent follicles in the ovary and remained larger (Baerwald et al., 2003).
In the present analysis, no differences in the number of uniquely identified follicles between the left and right ovaries were detected (data not shown). Therefore, follicle number and diameter data from both ovaries were combined (Baerwald et al., 2003; Vanden Brink et al., 2013; Jarrett et al., 2020). The total number and proportion of follicles detected in different diameter categories were graphed for each participant over the IOI. Diameter categories of physiologic interest (i.e. antral follicle counts (AFCs)) included: ≥2, ≥5, 2–5, 6–9, and ≥10 mm. Growth profiles of uniquely identified follicles were also graphed for each participant.
Biochemical and other clinical measurements
Non-fasted blood samples were collected every other day during the IOI. Blood was collected into a clot-activated tube and allowed to sit at room temperature for 30–60 min. Serum was isolated by centrifugation and stored at–80°C until analysis. Chemiluminescence immunoassays (Immulite 2000, Siemens Medical Solutions Diagnostics, Deerfield, IL, USA) were performed to measure serum concentrations of FSH, LH, E2, and P4. Luteal phase defects (LPDs) were defined by decreased luteal phase length (<10 days) and/or biochemical measures of integrated luteal P4 < 80 ng/ml or peak P4 < 10 ng/ml, as per the American Society for Reproductive Medicine recommendations (Practice Committees of the American Society for Reproductive Medicine and the Society for Reproductive Endocrinology and Infertility, 2021). Inter- and intra-assay coefficients of variation (CV) were as follows: FSH (4.9%, 2.6%), LH (6.2%, 3.9%), E2 (9.7%, 8.6%), and P4 (11.8%, 7.2), respectively.
Fasted blood samples were also drawn on a single day of the IOI during the early follicular phase to assess androgens, anti-Müllerian hormone (AMH), and glucoregulatory status. Measurements were standardized such that no dominant follicles or active corpora lutea were present at the time of sampling. Serum sex hormone-binding globulin (SHBG) was measured by chemiluminescence immunoassay (inter-assay CV: 5.0%; intra-assay CV: 3.1%) and total T was measured by liquid chromatography–tandem mass spectrometry (Brigham Research Assay Core, Boston, MA, USA) [inter-assay CV: 6.4%]. Free androgen index (FAI) was calculated as: (total T (nmol/l)/SHBG (nmol/l)) × 100 (Vermeulen et al., 1999). Glucose was measured with a standard glucometer (Accu-Check Aviva, Roche Diabetes Care, Inc., Indianapolis, IN, USA) and insulin was measured by chemiluminescence immunoassay (inter-assay CV: 6.2%; intra-assay CV: 4.8%). The homeostatic model assessment for insulin resistance (HOMA-IR) was calculated as: (fasting glucose (nmol/l) × fasting insulin (mIU/ml)) ÷ 22.5 (Wallace et al., 2004). AMH was measured by enzyme-linked immunosorbent assay (Ansh Labs, Webster, TX, USA) [intra-assay CV: 2.9%].
On the day of the fasting blood draw, anthropometry was performed. Participants were weighed on a standard digital scale and height measured using a stadiometer. Waist circumference was measured with a soft tape between the lowest rib and iliac crest. Dual x-ray absorptiometry (Discovery-A, Hologic, Inc., Bedford, MA, USA) was performed to estimate total adiposity as a measure of fat versus lean mass.
Statistical analysis
All analyses were performed using JMP Pro 14.0.1 (SAS Institute, Cary, NC, USA). Data were log-transformed if not normally distributed before analyses. Cross-sectional data were compared between groups using t-tests. Fisher’s exact tests were used to compare cross-sectional categorical data between groups. Follicular and endocrine data were centralized to the day of ovulation and evaluated by: (i) normalizing the data across the IOI and (ii) averaging the data across the luteal and follicular phases. Mixed-effect models evaluated longitudinal between-group differences in follicle number, follicle populations, growth parameters, and endocrine hormones (main fixed effect: obesity). Participant identifier was used as a random effect and day as a fixed effect across all models, with day being centralized to ovulation. The statistical significance threshold was set at P < 0.050.
Results
Participant characteristics
There were 42 women eligible for inclusion in the present analysis (with obesity: n = 21; without obesity: n = 21). Reproductive, anthropometric, and metabolic features are compared between groups in Table I. By design, women with obesity had a higher PFT (P < 0.0001), but similar menstrual cycle lengths (P = 0.582), compared to their counterparts without obesity. The groups did not differ in terms of age, total T, FAI, ovarian volume, or early follicular phase levels of LH, FSH, and LH:FSH (All P 0.050). However, AMH levels were significantly decreased in women with obesity (P = 0.007), and this decrease persisted when accounting for age (P = 0.01). As expected, women with obesity also had higher BMI, increased central adiposity, and impaired insulin sensitivity compared to those without obesity (Table I).
Table I.
Baseline characteristics of the study participants.
| Women without obesity | Women with obesity | |
|---|---|---|
| Participant (N) | 21 | 21 |
| Age (years) | 29 ± 6 | 29 ± 4 |
| Reproductive markers | ||
| Menstrual cycle length (days) | 29 ± 3 | 30 ± 2 |
| Luteal phase (days) | 16 ± 4 | 17 ± 4 |
| Follicular phase (days) | 13 ± 2 | 12 ± 2 |
| Total testosterone (ng/dl) | 21.9 ± 12.7 | 21.1 ± 11.3 |
| Free androgen index | 1.28 ± 0.74 | 1.93 ± 1.34 |
| LH:FSH | 0.73 ± 0.32 | 0.75 ± 0.47 |
| Anti-Müllerian hormone | 5.94 ± 2.49 | 4.40 ± 3.01* |
| Ovarian volume | 6.57 ± 2.11 | 6.53 ± 2.02 |
| Anthropometric markers | ||
| Percent total fat (%) | 27.5 ± 3.7 | 43.6 ± 4.9**** |
| BMI (kg/m2) | 22.9 ± 3.2 | 34.4 ± 5.1**** |
| Trunk fat percentage (%) | 23.8 ± 4.7 | 43.0 ± 6.1**** |
| Waist circumference (cm) | 79 ± 8 | 104 ± 18**** |
| Waist:hip ratio | 0.80 ± 0.05 | 0.85 ± 0.08 |
| Metabolic markers | ||
| Systolic blood pressure (mmHg) | 111 ± 10 | 115 ± 10 |
| Diastolic blood pressure (mmHg) | 68 ± 7 | 71 ± 9 |
| Fasting glucose (mg/dl) | 93.6 ± 12.2 | 92.1 ± 6.3 |
| Fasting insulin (mIU/l) | 4.29 ± 2.22 | 9.96 ± 5.60**** |
| HOMA-IR | 1.00 ± 0.56 | 2.27 ± 1.31** |
Data are presented as mean ± SD. Within rows, * denote significant differences between groups, adjusted values.
P < 0.05,
P < 0.01,
P < 0.0001. Reproductive, anthropometric, and metabolic endpoints were evaluated on a standardized day of the inter-ovulatory interval during the early follicular phase of the menstrual cycle.
HOMA-IR, homeostatic model assessment of insulin resistance.
Overall, all women demonstrated normal IOI (mean ± SD, 28 ± 6 days), follicular phase (16 ± 5 days), and luteal phase lengths (12 ± 3 days) (Bull et al., 2019), regardless of obesity status. Mean IOI, follicular phase, and luteal phase lengths did not differ between women with and without obesity (P0.100). Ovulation of a dominant follicle was observed at least twice in all women (i.e. at the beginning and end of the IOI). One participant without obesity ovulated two follicles (i.e. one from each ovary) at the end of their IOI.
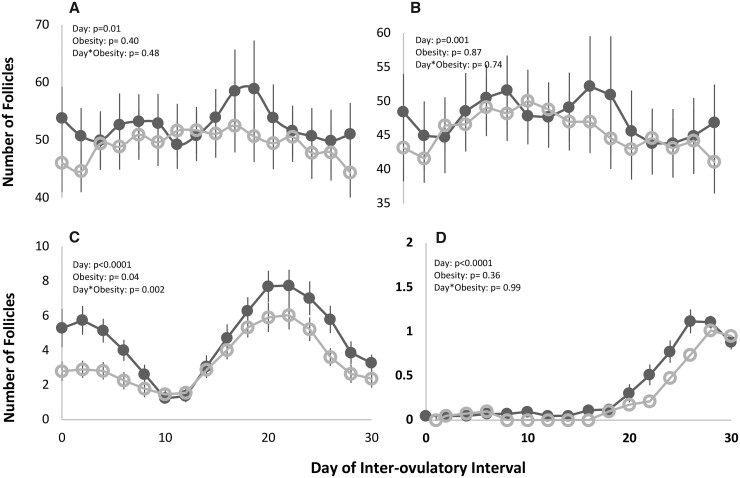
AFC across an IOI
Mean profiles of AFC 2 mm (Fig. 2A), AFC 2–5 mm (Fig. 2B), AFC 6–9 mm (Fig. 2C), and AFC ≥10 mm (Fig. 2D) are shown for both groups in Fig. 2. AFC 2 mm did not differ between groups on any given day of the IOI (POBESITY0.100). Likewise, there were no differences in AFC 2–5 mm or AFC ≥10 mm between groups across the IOI (POBESITY 0.100). By contrast, on any given day of the IOI, women with obesity displayed fewer 6–9 mm follicles than women without obesity (POBESITY = 0.040, PDAY*OBESITY = 0.002).
Figure 2.
Longitudinal profiles of total (A), 2–5 mm (B), 6–9 mm (C), and ≥10 mm (D) antral follicle counts across an inter-ovulatory interval (IOI) in non-obese (black ⬤) and obese women (gray ○). Day-to-day changes in total follicle counts per follicle size category were monitored using the Non-Identity Method. Mixed models showed a day effect for total, 2–5, 6–9, and ≥10 mm follicles, and an obesity effect for 6–9 mm follicles. Day-by-obesity effects were noted for 6–9 mm follicles.
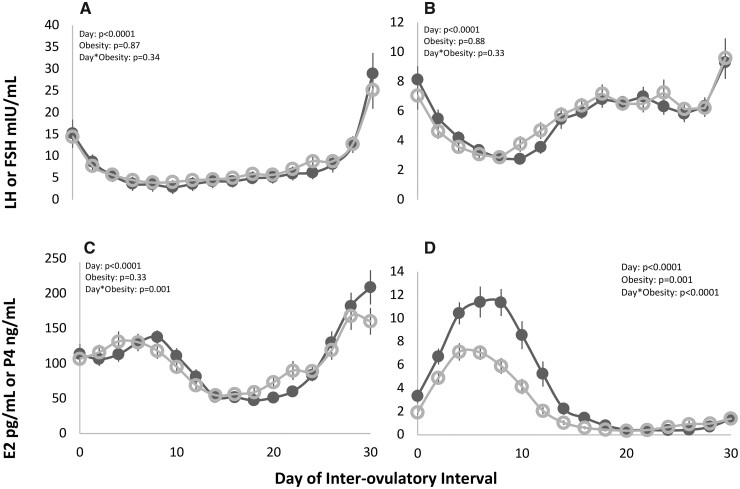
Endocrine hormones during an IOI
Mean profiles of endocrine hormones during an IOI are depicted for both groups in Fig. 3. There were no differences in LH or FSH on any given day between non-obese and obese groups (Fig. 3A and B, respectively; Both: POBESITY > 0.050). By contrast, changes in E2 concentrations differed across the IOI by obesity status (Fig. 3C;PDAY*OBESITY = 0.001) and P4 concentrations were lower on any day of the IOI in women with obesity (Fig. 3D;POBESITY = 0.001, PDAY*OBESITY < 0.001).
Figure 3.
Longitudinal profiles of luteinizing hormone (LH) (A), follicle-stimulating hormone (FSH) (B), estradiol (E2) (C), and progesterone (P4) (D) across an inter-ovulatory interval (IOI) in non-obese (black ⬤) and obese women (gray ○). Day-to-day changes in hormone concentrations were monitored by serial venipuncture. Mixed models showed a day effect for LH, FSH, E2, and P4. An obesity effect was noted for P4, and a day-by-obesity effect was noted for E2 and P4.
Follicle counts and endocrine hormones by menstrual cycle phase
Mean follicle populations and endocrine hormone concentrations are presented for the follicular and luteal phases in Table II. During the follicular phase, women with obesity displayed similar follicle counts and follicle size populations, as well as LH, FSH, and E2 concentrations compared to women without obesity (All: POBESITY0.050). By contrast, P4 concentrations were significantly lower in women with obesity during the follicular phase compared women without obesity (POBESITY = 0.027). In the luteal phase, there were no differences in AFC, AFC 2–5 mm, LH, FSH, or E2 levels (All: POBESITY > 0.05). The proportion of 2–5 mm follicles was significantly increased in women with obesity (POBESITY = 0.008). By contrast, AFC 6–9 mm and the proportion of 6–9 mm follicles were decreased in the luteal phases of women with obesity compared to women without obesity (Both: POBESITY < 0.050). Lastly, P4 concentrations were significantly lower across the luteal phase in the women with obesity (POBESITY = 0.001).
Table II.
Impact of day and obesity on average number of follicles per diameter category and hormone concentrations during the follicular and luteal phase.
| Women without obesity (n = 21) | Women with obesity (n = 21) | Day fixed effect | Obesity fixed effect | |
|---|---|---|---|---|
| Follicular phase | ||||
| AFC | 54 ± 28 | 50 ± 20 | P < 0.0001 | P = 0.671 |
| AFC 2–5 mm | 48 ± 30 | 46 ± 20 | P < 0.0001 | P = 0.854 |
| AFC 6–9 mm | 6 ± 4 | 4 ± 3 | P < 0.0001 | P = 0.056 |
| Proportion 2–5 mm (%) | 86.0 ± 9.6 | 89.5 ± 8.5 | P < 0.0001 | P = 0.162 |
| Proportion 6–9 mm (%) | 12.5 ± 9.4 | 9.0 ± 8.2 | P < 0.0001 | P = 0.139 |
| Mean LH (mIU/ml) | 9.57 ± 12.66 | 9.68 ± 11.69 | P < 0.0001 | P = 0.727 |
| Mean FSH (mIU/ml) | 6.76 ± 3.64 | 6.97 ± 3.50 | P = 0.158 | P = 0.845 |
| Mean E2 (pg/ml) | 100.39 ± 9.75 | 103.73 ± 88.45 | P < 0.0001 | P = 0.887 |
| Mean P4 (ng/ml) | 0.85 ± 1.41 | 0.41 ± 0.32 | P < 0.0001 | P = 0.027 |
| Luteal phase | ||||
| AFC | 59 ± 21 | 47 ± 19 | P = 0.974 | P = 0.559 |
| AFC 2–5 mm | 45 ± 22 | 45 ± 19 | P = 0.274 | P = 0.764 |
| AFC 6–9 mm | 4 ± 4 | 2 ± 2 | P < 0.0001 | P = 0.006 |
| Proportion 2–5 mm (%) | 90.9 ± 8.5 | 95.0 ± 5.0 | P < 0.0001 | P = 0.008 |
| Proportion 6–9 mm (%) | 8.9 ± 8.5 | 5.0 ± 5.0 | P < 0.0001 | P = 0.010 |
| Mean LH (mIU/ml) | 5.84 ± 6.44 | 6.27 ± 6.02 | P < 0.0001 | P = 0.770 |
| Mean FSH (mIU/ml) | 4.11 ± 2.61 | 3.93 ± 2.56 | P < 0.0001 | P = 0.637 |
| Mean E2 (pg/ml) | 115.59 ± 57.51 | 116.33 ± 5.00 | P < 0.0001 | P = 0.727 |
| Mean P4 (ng/ml) | 7.85 ± 5.74 | 4.95 ± 3.34 | P < 0.0001 | P = 0.001 |
Data are presented as mean ± SD. Groups were contrasted using generalized linear mixed models.
AFC, antral follicle count; E2, estradiol; P4, progesterone.
The prevalence rates of LPDs are presented according to three definitions. Based on biochemical measures, women with obesity had a greater incidence of LPDs compared to women without obesity. Namely, 16 women with obesity (76%) and 6 women without obesity (29%) displayed LPD by integrated luteal P4, while 15 women with obesity (71%) and 5 women without obesity (24%) had LPDs based on peak luteal P4 (Both: P < 0.010). The incidence of LPDs defined by luteal phase length did not differ between groups (P = 0.067).
Recruitment, selection, and ovulation
In women without obesity, two or three recruitment events were commonly observed during the IOI (Table III). In contrast, only one or two recruitment events were observed during the IOI in women with obesity (Table III). Ultimately, 91% of women with obesity and 95% of women without obesity exhibited at least one recruitment event across the IOI. There was a significant difference in the number of recruitment events between groups, with women with obesity displaying fewer events (P = 0.007) (Table III).
Table III.
Recruitment events in non-obese and obese women during natural cycles.
| Women without obesity (n = 21) | Women with obesity (n = 21) | |
|---|---|---|
| Recruitment | ||
| Number of recruitment events | 2 ± 1 | 1 ± 1*** |
| Distribution of events (N, %) | ||
| 0 | 1/21 (4.8) | 2/21 (9.5)** |
| 1 | 2/21 (9.5) | 13/21 (61.9)** |
| 2 | 9/21 (42.9) | 5/21 (23.8)** |
| 3 | 9/21 (42.9) | 1/21 (4.8)** |
Data are presented as mean ± SD or proportion (%). Within rows, *denote significant differences between groups, adjusted values.
P < 0.01,
P < 0.001.
In women with obesity, 6.5% of all 2–5 mm antral follicles grew to ≥7 mm, compared to 9.4% in women without obesity. As a result, women with obesity displayed fewer selectable follicles (6–9 mm) compared to women without obesity (P < 0.001). Of those follicles that progressed from the selectable pool to dominance, there were no differences in the maximum diameter of anovulatory follicles at selection (P = 0.323). However, ovulatory follicles were selected at significantly smaller diameters in women with obesity compared to those without obesity (P < 0.010) (Table IV), although the day of selection did not differ between groups (P = 0.810). Overall, women with obesity displayed fewer dominant follicles (P = 0.041) which manifested as fewer anovulatory follicles (P = 0.040) (Table IV). However, a similar relative proportion of selectable follicles achieved dominance [30 of 121 (25%) vs 40 of 197 (20%) follicles] in the group with compared to those without obesity, respectively (P = 0.348). Of the anovulatory dominant follicles, maximal diameters did not differ between groups (P = 0.763). By design, all women experienced ovulatory dominant follicles. There was no difference in maximal diameters achieved by the ovulatory follicles between groups (P = 0.628) (Table IV).
Table IV.
Follicle kinetics of dominant follicles in non-obese and obese women.
| Women without obesity (n = 21) | Women with obesity (n = 21) | |
|---|---|---|
| Characteristics of anovulatory dominant follicles | ||
| Total number over the IOI (N) | 18 | 9* |
| Prevalence (% of participants) | 12/21 (57.1) | 6/21 (28.6)** |
| Prevalence in the follicular phase (N participants, %) | 10/21 (47.6) | 4/21 (19.0)* |
| Prevalence in the luteal phase (N participants, %) | 5/21 (23.8) | 2/21 (9.5) |
| Diameter at selection (mm) | 7.4 ± 1.0 | 6.6 ± 1.1 |
| Sonographic presence (days) | 16.31 ± 3.90 | 15.83 ± 2.32 |
| Growth phase (days) | 7.38 ± 2.36 | 7.50 ± 2.88 |
| Growth rate (mm/day) | 0.94 ± 0.24 | 0.96 ± 0.35 |
| Static phase (days) | 1.31 ± 0.75 | 1.50 ± 1.22 |
| Regression phase (days) | 7.62 ± 3.59 | 6.83 ± 2.64 |
| Regression rate (mm/day) | 0.75 ± 0.26 | 0.82 ± 0.15 |
| Maximum diameter (mm) | 10.7 ± 1.0 | 10.5 ± 0.8 |
| Characteristics of ovulatory dominant follicles | ||
| Total number over the IOI (N) | 22 | 21 |
| Prevalence (% of participants) | 21/21 (100) | 21/21 (100) |
| Emergence to ovulation | ||
| Day of emergence (day) | 13.8 ± 4.1 | 14.5 ± 3.9 |
| Growth phase (days) | 15.4 ± 3.1 | 14.8 ± 2.7 |
| Growth rate (mm/day) | 1.03 ± 0.22 | 1.05 ± 0.23 |
| Selection to ovulation | ||
| Diameter at selection (mm) | 9.5 ± 1.9 | 7.5 ± 1.7** |
| Day of selection (day) | 21.0 ± 3.9 | 20.0 ± 3.0 |
| Growth phase (days) | 8.9 ± 1.9 | 9.0 ± 2.4 |
| Growth rate (mm/day) | 1.22 ± 0.27 | 1.26 ± 0.24 |
| Maximum diameter of ovulatory dominant follicles (mm) | 19.8 ± 2.9 | 19.5 ± 2.8 |
Data are presented as mean ± SD or proportion (%). Within rows, * denote significant differences between groups, adjusted values.
P < 0.05,
P < 0.01.
IOI, inter-ovulatory interval.
Follicle kinetics
Complete growth and regression profiles were available for 121 uniquely identifiable follicles in the group with obesity and 197 follicles in the group without obesity. Of those uniquely identified follicles, 30 follicles progressed to dominance in women with obesity, compared to 40 follicles in women without obesity. The kinetics of anovulatory dominant follicles did not differ between groups. The length of the growth, static and regression phases, as well as the growth and regression rates of the anovulatory dominant follicles, were all similar between groups (All: P > 0.050). Full growth profiles of ovulatory follicles were available for all women (Table IV). Across the groups, ovulatory follicles emerged on similar days of the menstrual cycle and displayed similar growth phases and growth rates from emergence to ovulation and selection to ovulation (All: P > 0.050) (Table IV).
Discussion
To our knowledge, this study provides the most comprehensive evaluation of ovarian antral follicle and endocrine dynamics in women with obesity and regular menstrual cycles conducted to date. Our findings are consistent with evidence of suppressed antral follicle development in women with obesity. Namely, fewer selectable-sized (6–9 mm) follicles were detected in women with obesity despite similar numbers of follicles in the recruitable size pool compared to their non-obese counterparts. Recruitment events occurred less often during IOIs in women with obesity and fewer dominant follicles emerged per participant. Further, ovulatory follicles were selected at smaller diameters in those with obesity. The timing and growth kinetics of ovulatory follicles did not differ between women with and without obesity, although P4 production was substantially lower in those with obesity post-ovulation. Together, this new knowledge suggests that despite regular, ovulatory menstrual cycles, women with obesity display differences in antral follicle development alongside alterations in endocrine hormone production, compared to their non-obese counterparts, which may underlie the suboptimal reproductive health outcomes common in this population.
Previous research in primarily non-obese women with regular cycles has shown that follicular recruitment occurs in two or three waves throughout an IOI (Baerwald et al., 2003). The number of follicular waves is posited to reflect fertility potential in bovine models wherein animals with more follicular waves exhibit higher fertilization and pregnancy rates than those with fewer follicular waves (Ahmad et al., 1997; Townson et al., 2002). In our cohorts, women with obesity commonly exhibited one recruitment event whereas women without obesity displayed primarily two or three recruitment events. Therefore, these data suggest that a decreased number of recruitment events may underlie the decreased fertility and fecundity that is commonly observed in women with obesity (Yilmaz et al., 2009; Broughton and Moley, 2017; Silvestris et al., 2018). However, because fertility was not an endpoint in the present study, we are unable to validate this hypothesis.
By definition, follicle waves include concomitant rises and falls in FSH, reflecting the gonadotropin-dependence of antral follicles of the recruited cohort (Ginther et al., 2000, 2001). Our definition on a recruitment event was strictly morphologic due to our relatively infrequent blood sampling methods. However, the definition of recruitment events used herein was similar to previous reports of follicle waves (Baerwald et al., 2003), which were later corroborated to align with fluctuations in FSH (Baerwald et al., 2004). That said, FSH concentrations did not differ between cohorts across the IOI, although FSH tended to be lower in those with obesity during the luteal phase. Reduced FSH production has been reported in women with obesity, regular cycles, and presumptive fertility in the follicular phase (De Pergola et al., 2006) and across the menstrual cycle (Yeung et al., 2013). However, others have shown no obesity-related differences in FSH across the menstrual cycle, when using serial daily sampling of urinary FSH metabolites (Jain et al., 2007). It is difficult to explain the discrepancies between studies. The degree of adiposity does not appear to be a factor as our study participants had a similar BMI to those enrolled in the studies reporting lower FSH levels (De Pergola et al., 2006; Yeung et al., 2013). Likewise, our groups were comparable in age and had similar exclusion criteria. Whether there are relevant metabolically related mechanisms, not captured by BMI or PFT, which underlie suppression of FSH should be pursued in future research.
Women with obesity in our study had a decreased pool of selectable-sized follicles (6–9 mm) across the IOI, despite similar numbers of recruitable-sized (2–5 mm) follicles. Growth from the recruitable cohort to the selectable stage is FSH-dependent (Macklon and Fauser, 2001), and AMH is thought to exert a negative paracrine effect, inhibiting their transition to the growing phase at the antral stages (Weenen et al., 2004; Themmen, 2005; Nilsson et al., 2011; Chen et al., 2020). While FSH concentrations were comparable in women with and without obesity, AMH levels were significantly depressed in women with obesity. AMH is known to regulate the transition of primordial follicles into the growing follicle pool, and at later stages becomes a brake in follicle development with the transition to dominance (Weenen et al., 2004). Therefore, lack of AMH may underly the decreased pool of 6–9 mm follicles. That said, our study cannot address causation and we cannot rule out the possibility that lower AMH in obesity simply reflects the smaller 6–9 mm pool, as antral follicles sized 5–8 mm have been shown to produce the most AMH (Jeppesen et al., 2013).
Follicles were selected at smaller diameters in women with obesity (7.5 mm). In previous reports of antral follicle dynamics in healthy women of reproductive age, selection typically occurred in the range of 9.2 to 10.4 mm (Baerwald et al., 2003, 2004; Vanden Brink et al., 2013; Bashir et al., 2018), with one report of selection occurring closer to 12.0 mm (Jarrett et al., 2020). Selection was defined by functional evidence of a future dominant follicle that grew ≥1 mm larger than the subsequent subordinate follicles. This preferential growth is associated with the acquisition of LH receptors and transition to LH-dependent growth (Zeleznik, 2004). A smaller size at selection may reflect earlier responsiveness to LH which has been shown in other anovulatory conditions associated with obesity, such as polycystic ovary syndrome (Hillier, 1994; Willis et al., 1998). Increased insulin signaling in granulosa and theca cells has been posited as a potential mechanism promoting premature acquisition of LH receptors in antral follicles (Poretsky et al., 1999; Wang et al., 2017). The women with obesity in our study had higher levels of fasting insulin compared to their non-obese counterparts as well as evidence of insulin resistance based on HOMA-IR. As such, it is possible that insulin concentrations in obesity may have been sufficient to alter the timing of granulosa cell LH receptor acquisition. A smaller diameter at selection could also relate to the decreased AMH levels detected in the women with obesity in our study. AMH inhibits the induction of LH receptor expression on granulosa cells by FSH (Di Clemente et al., 1994). Therefore, AMH levels in obesity may be insufficient to negatively modulate the FSH-dependent induction of LH receptor acquisition, leading to their earlier expression. It is important to note that selection occurred at the same time point in women with and without obesity, although the diameter of the follicle at selection differed. This finding could be attributed to the lack of differences in LH and FSH between groups given that the follicles transitioned from FSH-dependence to LH-dependence at the expected time. The lack of a detectable change in gonadotropin production suggests that metabolic factors may converge directly on the ovary to modulate follicular transitions in the context of obesity.
By design, all women in our study exhibited at least one dominant ovulatory follicle. However, anovulatory dominant follicles are capable of emerging at multiple time points during an IOI (Baerwald et al., 2004). To that point, we found that 85% of participants with obesity exhibited ovulatory dominant follicles that emerged within a recruitment event and 78% of anovulatory follicles emerged within a recruitment event. Similarly, 90% of non-obese participants displayed emergence of an ovulatory dominant follicle that was associated with a recruitment event and 78% of anovulatory follicles emerged within a recruitment event. Together, these observations are consistent with the maintenance coordinated follicular growth throughout the IOI in the context of obesity and regular menstrual cycles. That said, we detected evidence of overall suppression of 6–9 mm follicles and subsequently proportionally fewer anovulatory dominant follicles in the participants with obesity. It is important to note that once a follicle reached dominance, as defined by a diameter ≥10 mm, whether ovulatory or anovulatory, we noted no differences in the growth kinetics or maximum diameters achieved by the dominant follicles of the obese versus non-obese groups. There were also no differences in LH, FSH, or E2 levels between the groups once dominance was achieved (data not shown). Therefore, our data are consistent with suppression, and not disruption, of morphologic or endocrinologic dominant follicle development in eumenorrheic women with obesity.
Our data support the well-described findings of reductions in luteal P4 in eumenorrheic women with obesity (Jain et al., 2007; Carlson et al., 2012; Yeung et al., 2013) and provide a physiological basis underlying the need for P4 supplementation in patients with obesity undergoing IVF (Whynott et al., 2021). We found higher rates of LPDs in participants with obesity based on integrated and peak luteal P4 levels. Causes of LPDs in women with obesity are uncertain but may be a result of abnormally functioning granulosa cells of the pre-ovulatory follicle and/or reduced number of luteinized granulosa cells in the corpus luteum (Terranova, 2017). Evidence in obese, non-human primates has shown impaired granulosa cell function in the peri-ovulatory follicle (Bishop et al., 2019), as well as reduced luteal P4 production that was associated with decreased vascularization of the corpus luteum (Bishop et al., 2018, 2021). Our study cannot attest to any changes in the vascularity of corpora lutea. However, our findings of premature follicle selection in women with obesity could conceivably result in insufficient FSH stimulation of the ovulatory follicle (Terranova, 2017), leading to abnormal luteinization after ovulation (Wilks et al., 1976; Rice et al., 1998).
This study was strengthened by its use of a well-characterized cohort of women recruited consecutively from the general population. In using PFT measured by dual-energy x-ray absorptiometry to define obesity, we used a more direct marker of excess adiposity than BMI to accurately determine any impact of obesity on follicle development (Rothman, 2008; De Lorenzo et al., 2016). That said, 18 of the 21 participants with obesity in our study had a BMI ≥30 kg/m2, and 20 of the 21 participants without obesity had a BMI <30 kg/m2. As such, there is sufficient overlap across definitions for obesity to allow for our findings to be generalizable to standard clinical practices. Further, we were careful to exclude participants with androgen excess in order to eliminate factors that could confound antral follicle dynamics in the context of obesity (Jarrett et al., 2020). We relied on a biochemical, and not clinical, measure of androgen excess as hirsutism scores have not been shown to consistently reflect current androgen levels (Ewing and Rouse, 1978; Legro et al., 2010). However, we acknowledge that we did not assess other androgens (i.e., androstenedione and dehydroepiandrosterone) and therefore, more subtle forms of biochemical hyperandrogenism may have been present. Other limitations include the homogeneity of our study population wherein 81% of participants identified as Caucasian and 90% identified as not Hispanic or Latino. This limits the generalizability of our findings to other races and ethnicities, as both obesity rates (Petersen et al., 2019) and ovarian reserve (Ho et al., 2012) are known to differ by race. We appreciate that additional research should be performed in larger, more diverse populations before large-scale conclusions can be made about antral follicle and endocrine dynamics in obesity.
In summary, our data are consistent with suppressed follicle dynamics in obesity and their alignment with reductions in AMH and luteal phase dysfunction. Given the rise of obesity globally, an understanding of antral follicle development in the context of obesity is critical for improving women’s reproductive health. Further research should elaborate on mechanisms driving earlier selection and insufficient luteinization post-ovulation in obesity. This knowledge may be used to inform improvements in contraception and infertility treatments, both of which are known to be suboptimal in women with obesity (Dağ and Dilbaz, 2015; Silvestris et al., 2018).
Acknowledgements
The authors would like to thank the research participants, whose contributions were invaluable to the completion of this project. They would also like to thank Anna Sear RDMS, Rene Hellwitz-Black RDMS, Erica Bender CNM NP-Ob/Gyn, Tara Bailey CPT, and Bailey Drewes MS, for their assistance in facilitating data collection.
Contributor Information
Alexis L Oldfield, Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA.
Heidi Vanden Brink, Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA.
Faith E Carter, Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA.
Brittany Y Jarrett, Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA.
Marla E Lujan, Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
Authors’ roles
Participation in study design: A.L.O., H.V.B., M.E.L., and B.Y.J. Data collection: H.V.B., M.E.L., A.L.O., and B.Y.J. Image analysis: A.L.O., F.E.C., and B.Y.J. Data analysis and interpretation: A.L.O., H.V.B., and M.E.L. Manuscript drafting and critical discussion: A.L.O., H.V.B., F.E.C., B.Y.J., and M.E.L.
Funding
Funding was provided by Cornell University, President's Council of Cornell Women, United States Department of Agriculture (grant no. 8106), and National Institutes of Health (R01-HD0937848). B.Y.J. and H.V.B. were supported by doctoral training awards from the National Institutes of Health (T32-DK007158) and Canadian Institutes of Health Research (grant no. 146182), respectively.
Conflict of interest
The authors report no conflict of interest.
References
- Ahmad N, Townsend EC, Dailey RA, Inskeep EK.. Relationships of hormonal patterns and fertility to occurrence of two or three waves of ovarian follicles, before and after breeding, in beef cows and heifers. Anim Reprod Sci 1997;49:13–28. [DOI] [PubMed] [Google Scholar]
- Baerwald AR, Adams GP, Pierson RA.. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril 2003;80:116–122. [DOI] [PubMed] [Google Scholar]
- Baerwald AR, Adams GP, Pierson RA.. Characterization of ovarian follicular wave dynamics in women. Biol Reprod 2004;69:1023–1031. [DOI] [PubMed] [Google Scholar]
- Baerwald AR, Adams GP, Pierson RA.. Form and function of the corpus luteum during the human menstrual cycle. Ultrasound Obstet Gynecol 2005;25:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerwald AR, Walker RA, Pierson RA.. Growth rates of ovarian follicles during natural menstrual cycles, oral contraception cycles, and ovarian stimulation cycles. Fertil Steril 2009;91:440–449. [DOI] [PubMed] [Google Scholar]
- Bashir ST, Baerwald AR, Gastal MO, Pierson RA, Gastal EL.. Follicle growth and endocrine dynamics in women with spontaneous luteinized unruptured follicles versus ovulation. Hum Reprod 2018;33:1130–1140. [DOI] [PubMed] [Google Scholar]
- Bishop CV, Mishler EC, Takahashi DL, Reiter TE, Bond KR, True CA, Slayden OD, Stouffer RL.. Chronic hyperandrogenemia in the presence and absence of a western-style diet impairs ovarian and uterine structure/function in young adult rhesus monkeys. Hum Reprod 2018;33:128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CV, Reiter TE, Erikson DW, Hanna CB, Daughtry BL, Chavez SL, Hennebold JD, Stouffer RL.. Chronically elevated androgen and/or consumption of a western-style diet impairs oocyte quality and granulosa cell function in the nonhuman primate periovulatory follicle. J Assist Reprod Genet 2019;36:1497–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CV, Takahashi D, Mishler E, Slayden OD, Roberts CT, Hennebold J, True C.. Individual and combined effects of 5-year exposure to hyperandrogenemia and western-style diet on metabolism and reproduction in female rhesus macaques. Hum Reprod 2021;36:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton DE, Moley KH.. Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril 2017;107:840–847. [DOI] [PubMed] [Google Scholar]
- Bull JR, Rowland SP, Scherwitzl EB, Scherwitzl R, Gemzell Danielsson K, Harper J.. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit Med 2019;2:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MJ, Thiel KW, Yang S, Leslie KK.. Catch it before it kills: progesterone, obesity, and the prevention of endometrial cancer. Discov Med 2012;14:215–222. [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yang W, Shi X, Zhang C, Song G, Huang D.. The factors and pathways regulating the activation of mammalian primordial follicles in vivo. Front Cell Dev Biol 2020;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dağ ZÖ, Dilbaz B.. Impact of obesity on infertility in women. J Turk Ger Gynecol Assoc 2015;16:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damodaran S, Swaminathan K.. Obesity and contraception. Obesity 2013;57:69–89. [Google Scholar]
- De Lorenzo A, Soldati L, Sarlo F, Calvani M, Di Lorenzo N, Di Renzo L.. New obesity classification criteria as a tool for bariatric surgery indication. World J Gastroenterol 2016;22:681–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pergola G, Maldera S, Tartagni M, Pannacciulli N, Loverro G, Giorgino R.. Inhibitory effect of obesity on gonadotropin, estradiol, and inhibin B levels in fertile women. Obesity (Silver Spring) 2006;14:1954–1960. [DOI] [PubMed] [Google Scholar]
- Di Clemente N, Goxe B, Remy JJ, Cate R.. Inhibitory effect of AMH upon the expression of aromatase and LH receptors by cultured granulosa cells of rat and porcine immature ovaries. 1994;2:553–558. [Google Scholar]
- Ewing JA, Rouse BA.. Hirsutism, race and testosterone levels: comparison of East Asians and Euroamericans. Hum Biol 1978;50:209–215. [PubMed] [Google Scholar]
- Ginther OJ, Beg MA, Bergfelt DR, Donadeu FX, Kot K.. Follicle selection in monovular species. 2001;647:638–647. [DOI] [PubMed] [Google Scholar]
- Ginther OJ, Bergfelt DR, Kulick LJ, Kot K.. Selection of the dominant follicle in cattle: role of estradiol. 2000; 389:383–389. [DOI] [PubMed] [Google Scholar]
- Goldsammler M, Merhi Z, Buyuk E.. Role of hormonal and inflammatory alterations in obesity-related reproductive dysfunction at the level of the hypothalamic-pituitary-ovarian axis. Reprod Biol Endocrinol 2018;16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM, Carroll MD, Fryar CD, Ogden CL.. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief 2020;360:1–8. [PubMed] [Google Scholar]
- Hillier SG. Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod 1994;9:188–191. [DOI] [PubMed] [Google Scholar]
- Ho MV, Martin KC, Lee J-A.. 基因的改变NIH Public Access. Bone 2012;23:1–7. [Google Scholar]
- Hruby A, Hu FB.. The epidemiology of obesity: a big picture. Pharmacoeconomics 2015;33:673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, Gibbs K, Polotsky HN, Feng S, Isaac B. et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab 2007;92:2468–2473. [DOI] [PubMed] [Google Scholar]
- Jarrett BY, Vanden Brink H, Oldfield AL, Lujan ME.. Ultrasound characterization of disordered antral follicle development in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2020;105:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, Raine-Fenning N, Campbell BK, Yding Andersen C.. Which follicles make the most anti-Müllerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod 2013;19:519–527. [DOI] [PubMed] [Google Scholar]
- Knopf L, Kastelic JP, Schallenberger E, Ginther OJ.. Ovarian follicular dynamics in heifers: test of two-wave hypothesis by ultrasonically monitoring individual follicles. Domest Anim Endocrinol 1989;6:111–119. [DOI] [PubMed] [Google Scholar]
- Kyrou I, Randeva HS, Tsigos C, Kaltsas G, Weickert MO. Clinical Problems Caused by Obesity. In: Feingold KR et al. (eds). Endotext. MDText.com, Inc.,2018.
- Lasquety MG, Rodriguez D, Fehring RJ.. The influence of BMI levels on phases of the menstrual cycle and presumed ovulation. Linacre Q 2012;79:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Schlaff WD, Diamond MP, Coutifaris C, Casson PR, Brzyski RG, Christman GM, Trussell JC, Krawetz SA, Snyder PJ. et al. ; Reproductive Medicine Network. Total testosterone assays in women with polycystic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab 2010;95:5305–5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan ME, Brooks ED, Kepley AL, Chizen DR, Pierson RA, Peppin AK.. Grid analysis improves reliability in follicle counts made by ultrasonography in women with polycystic ovary syndrome. Ultrasound Med Biol 2010;36:712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklon NS, Fauser BCJM.. Follicle-stimulating hormone and advanced follicle development in the human. Arch Med Res 2001;32:595–600. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Schindler R, Savenkova MI, Skinner MK.. Inhibitory actions of anti-Müllerian hormone (AMH) on ovarian primordial follicle assembly. PLoS One 2011;6:e20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R, Pan L, Blanck HM.. Racial and ethnic disparities in adult obesity in the United States: CDC’s tracking to inform state and local action. Prev Chronic Dis 2019;16:E46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson RA, Ginther OJ.. Ultrasonic imaging of the ovaries and uterus in cattle. Theriogenology 1988;29:21–37. [Google Scholar]
- Piqueras P, Ballester A, Durá-Gil JV, Martinez-Hervas S, Redón J, Real JT.. Anthropometric indicators as a tool for diagnosis of obesity and other health risk factors: a literature review. Front Psychol 2021;12. 10.3389/fpsyg.2021.631179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC.. The insulin-related ovarian regulatory system in health and disease. Endocr Rev 1999;20:535–582. [DOI] [PubMed] [Google Scholar]
- Practice Committees of the American Society for Reproductive Medicine and the Society for Reproductive Endocrinology and Infertility. Diagnosis and treatment of luteal phase deficiency: a committee opinion. Fertil Steril 2021;115:1416–1423. [DOI] [PubMed] [Google Scholar]
- Purcell SH, Moley KH.. The impact of obesity on egg quality. J Assist Reprod Genet 2011;28:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice VM, Limback SD, Roby KF, Terranova PF.. Differential responses of granulosa cells from small and large follicles to follicle stimulating hormone (FSH) during the menstrual cycle and acyclicity: effects of tumour necrosis factor-α. Hum Reprod 1998;13:1285–1291. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes 2008;32:S56–S59. [DOI] [PubMed] [Google Scholar]
- Rouleau D, Case A, Gamelin A, Lim H, Baerwald A.. A practical method for ultrasonographically monitoring the day-to-day growth of individual ovarian follicles in women undergoing assisted reproduction. Ultrasound Med Biol 2012;38:1004–1010. [DOI] [PubMed] [Google Scholar]
- Shaw KA, Edelman AB.. Obesity and oral contraceptives: a clinician’s guide. Best Pract Res Clin Endocrinol Metab 2013;27:55–65. [DOI] [PubMed] [Google Scholar]
- Silvestris E, de Pergola G, Rosania R, Loverro G.. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol 2018;16:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova P. Luteal Phase Defects. Reference Module in Biomedical Sciences. Elsevier, 2017. 10.1016/B978-0-12-801238-3.05125-4. [DOI]
- Themmen APN. Anti-Müllerian hormone: its role in follicular growth initiation and survival and as an ovarian reserve marker. J Natl Cancer Inst Monogr 2005;34:18–21. [DOI] [PubMed] [Google Scholar]
- Townson DH, Tsang PCW, Butler WR, Frajblat M, Griel LC, Johnson CJ, Milvae RA, Niksic GM, Pate JL.. Relationship of fertility to ovarian follicular waves before breeding in dairy cows. J Anim Sci 2002;80:1053–1058. [DOI] [PubMed] [Google Scholar]
- Vaamonde JG, Álvarez-Món MA.. Obesity and overweight. Medicine (Spain) 2020;13:767–776. [Google Scholar]
- Valdez R. A simple model-based index of abdominal adiposity. J Clin Epidemiol 1991;44:955–956. [DOI] [PubMed] [Google Scholar]
- Vanden Brink H, Chizen D, Hale G, Baerwald A.. Age-related changes in major ovarian follicular wave dynamics during the human menstrual cycle. Menopause 2013;20:1243–1254. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM.. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672. [DOI] [PubMed] [Google Scholar]
- Wallace TM, Levy JC, Matthews DR.. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang S, Zhang Z, Lin Q, Liu Y, Xiao Y, Xiao K, Wang Z.. Defective insulin signaling and the protective effects of dimethyldiguanide during follicular development in the ovaries of polycystic ovary syndrome. Mol Med Rep 2017;16:8164–8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weenen C, Laven JSE, von Bergh ARM, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BCJM, Themmen APN.. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 2004;10:77–83. [DOI] [PubMed] [Google Scholar]
- Whynott RM, Summers KM, Jakubiak M, Van Voorhis BJ, Mejia RB.. The effect of weight and body mass index on serum progesterone values and live birth rate in cryopreserved in vitro fertilization cycles. F S Rep 2021;2:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks JW, Hodgen GD, Ross GT.. Luteal phase defects in the rhesus monkey: the significance of serum FSH: LH ratios. J Clin Endocrinol Metab 1976;43:1261–1267. [DOI] [PubMed] [Google Scholar]
- Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S.. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab 1998;83:3984–3991. [DOI] [PubMed] [Google Scholar]
- Yeung EH, Zhang C, Albert PS, Mumford SL, Ye A, Perkins NJ, Wactawski-Wende J, Schisterman EF.. Adiposity and sex hormones across the menstrual cycle: the BioCycle Study. Int J Obes (Lond) 2013;37:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz N, Kilic S, Kanat-Pektas M, Gulerman C, Mollamahmutoglu L.. The relationship between obesity and fecundity. J Womens Health (Larchmt) 2009;18:633–636. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ. The physiology of follicle selection. Reprod Biol Endocrinol 2004;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Chung HF, Pandeya N, Dobson AJ, Kuh D, Crawford SL, Gold EB, Avis NE, Giles GG, Bruinsma F. et al. Body mass index and age at natural menopause: an international pooled analysis of 11 prospective studies. Eur J Epidemiol 2018;33:699–710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.