Abstract
Objective
The objective of this study was to evaluate the utility of urine CD163 for detecting disease activity in childhood-onset SLE (cSLE) patients.
Methods
Sixty consecutive pediatric patients fulfilling four or more ACR criteria for SLE and 20 healthy controls were recruited for testing of urinary CD163 using ELISA. SLE disease activity was assessed using the SLEDAI-2K.
Results
Urine CD163 was significantly higher in patients with active LN than inactive SLE patients and healthy controls, with receiver operating characteristics area under the curve values ranging from 0.93 to 0.96. LN was ascertained by kidney biopsy. Levels of CD163 significantly correlated with the SLEDAI, renal SLEDAI, urinary protein excretion and C3 complement levels. Urine CD163 was also associated with high renal pathology activity index and chronicity index, correlating strongly with interstitial inflammation and interstitial fibrosis based on the examination of concurrent kidney biopsies.
Conclusion
Urine CD163 emerges as a promising marker for identifying cSLE patients with active kidney disease. Longitudinal studies are warranted to validate the clinical utility of urine CD163 in tracking kidney disease activity in children with lupus.
Keywords: childhood-onset SLE, CD163, biomarker, LN
Rheumatology key messages.
Soluble CD163 is increased in the urine of diseased patients with active LN.
In children with lupus, sCD163 urine levels correlate with LN clinical disease activity.
Urinary sCD163 levels correlate with both interstitial inflammation and fibrosis on biopsy.
Introduction
SLE is a chronic autoimmune disease characterized by multiorgan involvement and the production of diverse antinuclear antibodies. LN is a leading cause of morbidity and mortality in SLE and its presentation is influenced by many demographic variables, such as ethnicity, age and gender [1]. Childhood-onset SLE (cSLE) is characterized by onset prior to 18 years of age and LN manifests in up to 70% of cSLE patients. Because the diagnosis of LN positively correlates with an increased risk of kidney failure, patients affected by cSLE often face more aggressive or advanced disease [2–4]. In comparison to adult-onset SLE (aSLE), the clinical manifestation of cSLE is consistent with increased kidney disease damage, which demands longer and more advanced treatments [5]. Kidney biopsy remains the gold standard for accurate diagnosis of LN, but this process is highly invasive and rarely repeated in adolescents [6]. Adults have historically been at the focal point of research investigating the pathogenesis, course and long-term effects of LN, and there exists a paucity of information on the unique onset, complications and therapies associated with cSLE [7]. A greater understanding of the pathogenesis of SLE has led to many advances, such that positive outcomes have been obtained with few prescribed medications, but there remains the dire need for more clinical trials aimed at slowing the persistent damage accrual that occurs with cSLE [8, 9].
A previous study aimed to examine the prevalence and incidence rates of cSLE and LN among children in the USA enrolled in Medicaid between 2000 and 2004. The study identified 2929 cSLE cases, with 37% of these patients developing LN. Further, SLE and LN prevalence distinctly differed based upon demographic factors, including sex, race and residency [10]. Another study using electronic health record algorithms with cSLE data collected from 2009 to 2019 successfully identified 1508 SLE patients, with 537 positive for LN, indicating an LN prevalence of 36% in cSLE patients [11]. Notably, Hiraki et al. [10] and Wenderfer et al. [11] observed an overall prevalence of cSLE of 10 and 22, respectively, per 100 000 children, and these differences in prevalence could have resulted from referral patterns or differences in case definition. Collectively, these studies highlight the prevalence of cSLE and underscore the need for improving cSLE outcomes and practice in routine care.
Biomarker-led renal monitoring may give rise to improved routine clinical diagnosis and practice, and validation of improved biomarkers may render effective management of LN more readily available [12]. Current conventional serum biomarkers, such as anti-dsDNA and complement C3 and C4 levels, are inconsistent in their ability to predict renal flares and disease progression [13]. Additionally, current renal biomarker studies in children have been limited by the need to establish a normal age-specific profile for each biomarker and account for developmental factors [12]. For this reason, an ideal cSLE biomarker that correlates with renal activity, predicts renal flares and is sensitive to change would be significant in creating more reliable and easy-to-access testing [13].
CD163, a scavenger receptor, is exclusively expressed by macrophages and monocytes [14]. Macrophages polarize into subtypes with differing functions, including pro-inflammatory, anti-inflammatory and profibrotic functions. Macrophages can be classified into classically activated M1 and alternatively activated M2 macrophages, which are critical in inflammation and tissue repair, respectively. CD163 is prominent on the surface of M2c polarized macrophages in particular [15]. M2c macrophages release soluble CD163 (sCD163) into circulation following cleavage by metalloproteinases. One study measured the soluble ectodomain of MER (sMER), a member of the TAM-receptor tyrosine family, which plays a key role in the clearance of apoptotic cells. Increased plasma levels of sMER were found in patients with active lupus compared with healthy controls. Similarly, sMER and sCD163 are cleaved by the same metalloproteinase, and as researchers noted a significant correlation between lupus disease activity and sCD163, there is promising potential in further exploring both markers as they have been linked with M2c activation [16, 17]. Of relevance to this work, urinary CD163 has been reported to be a good biomarker in aSLE and has shown evidence of being associated with clinical disease activity, kidney pathology and long-term outcomes [22–25]. Given the outstanding performance of urinary CD163 as a disease biomarker in aSLE, here we investigate the clinical utility of CD163 in cSLE.
Materials and methods
Patients
Sixty consecutive pediatric patients (≤18 years of age) fulfilling four or more of the 1997 ACR classification criteria for SLE were enrolled into this cross-sectional study from the Texas Children’s Hospital (TCH) [18]. This study was approved by the institutional review board (IRB) at the Baylor College of Medicine (H-35050) and the University of Houston, Houston, TX, USA. All enrolled patients completed a written IRB-approved informed consent form based on good clinical practice and the Declaration of Helsinki. Demographic, clinical data and conventional measures of disease activity, including anti-dsDNA (ordinal variable, based on a Crithidia titer), complement 3 (C3) and C4 levels (continuous variable, in mg/dl), spot urinary protein:creatinine ratio (uPCR), serum creatinine levels and estimated glomerular filtration rate (estimated by the bedside Schwartz equation) were collected prospectively. Demographics and clinical characteristics are summarized in Table 1. As controls, healthy females of comparable ages were enrolled from the Gynecology and Adolescent Medicine Clinics at TCH.
Table 1.
Demographic and clinical information of the cSLE cohort
| Variable | Category | Healthy controls (n = 20) | Inactive SLE (n = 28) | Active nonrenal (n = 17) | Active renala (n = 15) |
|---|---|---|---|---|---|
| Age, years, mean (s.d.) | 15.4 (0.86) | 16.1 (1.02) | 14.7 (1.16) | 14.5 (1.44) | |
| Gender, n (%) | Female | 20 (100) | 23 (82) | 15 (88) | 13 (87) |
| Male | N/A | 5 (18) | 2 (12) | 2 (13) | |
| Ethnicity, n (%) | Asian | N/A | 2 (7) | 1 (6) | 1 (7) |
| Black | 3 (15) | 5 (18) | 7 (41) | 6 (40) | |
| Caucasian | 9 (45) | 4 (14) | N/A | N/A | |
| Hispanic | 8 (40) | 17 (60) | 9 (53) | 7 (47) | |
| Other | N/A | N/A | N/A | 1 (7) | |
| Clinical assessment, mean (95% CI) | SLEDAI | 0 | 2.61 (1.07) | 5.53 (1.60) | 18.1 (4.07) |
| rSLEDAI | 0 | 1.00 (0.87) | 0 | 9.33 (1.98) | |
| Laboratory measurement | uPCR (mg/mg), mean (95% CI) | N/A | 0.13 (0.05) | 0.10 (0.03) | 4.54 (3.26) |
| Pyuria (0, 1), % | N/A | 14% | 0% | 40% | |
| Hematuria (0, 1), % | N/A | 4.0% | 0% | 73% | |
| Casts (0, 1), % | N/A | 0% | 0% | 33% | |
| Serum creatinine, mean (95% CI) | N/A | 0.62 (0.05) | 0.54 (0.10) | 0.68 (0.10) | |
| Glomerular filtration rate, mean (95% CI) | N/A | 108 (8.44) | 114 (11.0) | 134 (64.7) | |
| dsDNA antibodies (titer), % positive (mean [95% CI]) | N/A | 39% (560 [488]) | 65% (612 [378]) | 67% (1304 [505]) | |
| C3, mean (95% CI) | N/A | 115 (8.52) | 83.0 (16.2) | 49.4 (19.2) |
As ascertained by a renal biopsy, the active LN patients had a mean activity score of 3.36 (s.d. 4.34) and a mean CI score of 0.91 (s.d. 1.38), with the following GN class distribution: class 1 (n = 1), class 2 (n = 1), class 3 (n = 3), class 4 (n = 2), class 5 (n = 3), mixed (n = 4).
Assessment of SLE disease activity and flares
Subjects enrolled were either incident patients with biosamples collected prior to receiving immunosuppression, prevalent patients with new-onset lupus flare (prior to escalating immunosuppression) or prevalent patients in remission (on or off immunosuppression). At enrollment, the cSLE patients were categorized into three groups: active renal [with active nephritis, based on urine dipstick and microscopic urine sediment analysis performed by a trained pediatric nephrologist; renal SLEDAI (rSLEDAI) >0]), active nonrenal (with symptoms of active nonrenal organ involvement; rSLEDAI = 0) and inactive SLE (asymptomatic with no findings of organ activity, subclinical hematuria, hypocomplementemia and/or elevated autoantibodies allowed). After enrollment, SLE disease activity was formally assessed using the SLEDAI-2K, a validated tool in clinical practice and research [19]. Renal activity was assessed using renal domain scores of the SLEDAI (range 0–16; 0 = inactive LN). Disease damage was assessed using the SLICC/ACR Damage Index (SDI) (range 0–47; 0 = no SLE damage) [20].
Kidney histology
In the active renal group, the histopathologic features associated with active LN were assessed by performing a kidney biopsy, evaluated by a single pediatric nephropathologist (M.J.H.). The International Society of Nephrology/Renal Pathology Society criteria were used to assess specific histologic manifestations of active inflammation of LN as well as features of LN chronicity and degenerative damage [21]. Biopsy activity and chronicity indices (AI and CI, respectively) were used for biopsy assessment according to the standards of the National Institutes of Health (NIH). As ascertained by kidney biopsy, the active LN patients had a mean AI of 3.36 (s.d. 4.34) and a mean CI of 0.91 (s.d. 1.38), with the following GN classes: class 1 (n = 1), class 2 (n = 1), class 3 (n = 3), class 4 (n = 2), class 5 (n = 3) and mixed class (n = 4). Elevated AI and CI scores are considered as risk factors for poor LN outcomes over long-term follow-up [22, 23].
Biomarker assays
Urine samples were prepared, aliquoted and frozen at −80°C within 2 hours of sample collection, prior to batch processing. To avoid multiple freeze–thaw cycles, only one vial was retrieved each time for an assay. Urine levels of CD163 were measured using a human ELISA kit (RayBiotech, Peachtree Corners, GA, USA), following the manufacturer’s instructions. Briefly, diluted urine samples were added to anti-CD163 precoated 96-well microplates, followed by biotin-conjugated anti-CD163 detection antibody, streptavidin–horseradish peroxidase and substrate. Optical densities were measured at 450 nm using a microplate reader (ELX808, BioTek Instruments, Winooski, VT, USA) and sample concentrations were calculated using a standard curve. All measurements were assayed in duplicate. The levels of urine creatinine were assayed using the Creatinine Parameter Assay Kit (R&D Systems, Minneapolis, MN, USA). Urine CD163 values were normalized to urine creatinine before further analysis. Performers and readers of biomarker assays were blinded to clinical and reference information. Biomarker assays were performed with the assistance of Houston Omics Collaborative (https://hoc.bme.uh.edu/).
Statistical analysis
Data were analyzed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). The Mann–Whitney U test was used for comparisons between two groups and analysis of variance with subsequent posttest pairwise comparisons was used for comparison of multiple groups. Nonparametric Spearman correlation was performed for correlation analysis. Receiver operating characteristics (ROC) curves were used to compare the performance of urine CD163 vs other parameters and to determine the optimal cut-off values. A two-tailed P-value <0.05 was considered significant. To identify the CI for the area under the curve (AUC), the Delong method was used with a 95% confidence level. For sensitivity and specificity, CIs were calculated using the Clopper–Pearson method with a 95% confidence level.
Results
Urine CD163 was significantly elevated in cSLE patients
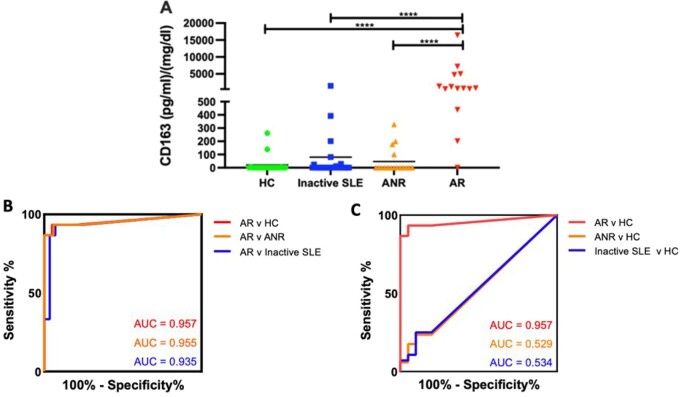
This cSLE cohort was comprised of 15 active renal, 17 active nonrenal and 28 inactive SLE patients, matched for demographics (Table 1). Urine CD163 levels were measured by ELISA in all SLE patients and healthy controls, as plotted in Fig. 1A. Notably, 80% of healthy control subjects had no detectable urine CD163. Creatinine normalized urine CD163 was significantly elevated in active LN compared with healthy controls [71 478.9 pg/mg (s.d. 265937.4) vs 20.4 (64.6), fold change (FC) = 3497.7, P < 0.0001]. Urine CD163 levels were also significantly higher in active LN patients compared with those with active nonrenal SLE and inactive SLE [79.4 pg/mg (s.d. 287.5), FC = 900.4, P < 0.0001]. Additionally, 75% of inactive SLE patients had a mean concentration of zero for urine CD163, further underscoring the elevation of CD163 levels, particularly in active LN patients.
Fig. 1.
Discriminatory power of urine CD163 for active renal lupus
(A) Dot plot displaying creatinine normalized urine CD163 levels in SLE patients and healthy control groups. The y-axis shows the concentration of CD163 (pg/mg) and the x-axis shows the four groups under investigation (20 healthy controls, 28 inactive SLE, 17 active nonrenal and 15 active). Means are indicated. Only comparisons attaining statistical significance are indicated with P-values. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (B) Values in the plot indicate the AUC. Urine CD163 performed excellently in discriminating active renal from healthy controls. The 95% CIs for active renal vs healthy controls, active renal vs active nonrenal and active renal vs inactive SLE AUCs were 0.88 to 1.00, 0.87 to 1.00 and 0.84 to 1.00, respectively. (C) Values in the plot indicate the AUC of healthy control patients versus all other cohorts. The 95% CIs for active renal vs healthy control, active nonrenal vs healthy control and inactive SLE vs healthy control AUCs were 0.88 to 1.00, 0.34 to 0.72 and 0.37 to 0.70, respectively.
ROC analysis was performed comparing all patient groups to the active LN group (Fig. 1B), and fold change, AUC value, specificity and sensitivity with 95% CI for each comparison are displayed in Table 2. ROC analysis was also utilized to compare healthy control patients to all other groups (Fig. 1C). Urine CD163 significantly discriminated active renal patients from healthy controls [AUC 0.96 (95% CI 0.88, 1.00)], as shown in Fig. 1B and C. With a cut-off value of 170.1 pg/mg, urinary CD163 successfully differentiated active LN from healthy control subjects with 93% sensitivity and 95% specificity (Table 2). Urinary CD163 did not differentiate active nonrenal and inactive SLE patients from healthy controls (Fig. 1C), underscoring the need for disease activity to be within the kidneys in order to observe elevations in urine CD163. Urine CD163 distinguished active renal SLE from active nonrenal SLE with an AUC of 0.95 (95% CI 0.87, 1.00), 94% specificity and 93% sensitivity. In addition to this, urine CD163 distinguished active renal SLE from inactive SLE with an AUC of 0.93 (95% CI 0.84, 1.00), 93% specificity and 93% sensitivity (Fig. 1B, Table 2).
Table 2.
Performance characteristics of urine CD163 in cSLE
| Urine CD163 | Fold change | ROC AUC | Specificity | Sensitivity |
|---|---|---|---|---|
| Active renal vs healthy control | 3497.7 | 0.96 (0.88, 1.00) | 0.95 (0.75, 1.00) | 0.93 (0.68, 1.00) |
| Active renal vs active nonrenal | 1507.3 | 0.95 (0.87, 1.00) | 0.94 (0.71, 1.00) | 0.93 (0.68, 1.00) |
| Active renal vs inactive SLE | 900.4 | 0.93 (0.84, 1.00) | 0.93 (0.77, 0.99) | 0.93 (0.68, 1.00) |
Values are presented as diagnostic metrics with 95% CIs.
Urine CD163 was significantly correlated with clinical disease activity and laboratory measures of lupus activity
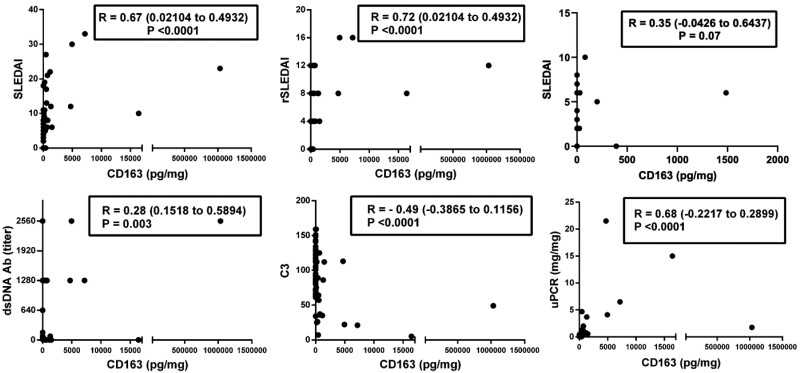
Spearman correlations were calculated between urine CD163, SLEDAI, rSLEDAI, uPCR, dsDNA antibodies and C3 using just the data from the patients, excluding the healthy controls. Urine CD163 levels exhibited remarkable correlation with SLEDAI, rSLEDAI and uPCR, with correlation coefficients ranging from 0.67 to 0.72 (P < 0.0001), as plotted in Fig. 2. For active nonrenal patients alone, urine CD163 exhibited a weak positive correlation with the SLEDAI (P = 0.07) (Fig. 2). Urinary CD163 also correlated positively with serum anti-dsDNA antibodies (P = 0.003) and negatively with C3 (P < 0.0001) (Fig. 2).
Fig. 2.
Urine CD163 levels and its correlation with clinical and laboratory indices in cSLE
The number of patients included in the inactive SLE, active nonrenal and active renal groups were 28, 17 and 15, respectively. The y-axes show the concentration of creatinine normalized urine CD163 (pg/mg) and the x-axes display various clinical and laboratory metrics: SLEDAI, rSLEDAI, SLEDAI of the active nonrenal cohort only, uPCR, dsDNA antibody (titer) and C3. Plots indicate the Spearman correlation coefficients along with the 95% CIs. The 95% CIs for the following metrics were SLEDAI (0.02104, 0.4932), rSLEDAI (0.02104, 0.4932), SLEDAI of active nonrenal only (−0.0426, 0.6437), uPCR (0.1518, 0.5894), dsDNA antibody (titer) (−0.3865, 0.1156) and C3 (−0.2217, 0.2899).
Urine CD163 was predictive of concurrent kidney pathology changes
Pathological classification of patients with LN was obtained via kidney biopsy, performed concurrently with urine collection. Among the active LN cohort, the GN class distribution included class 1 (n = 1), class 2 (n = 1), class 3 (n = 3), class 4 (n = 2), class 5 (n = 3), class 3 + 5 (n = 1) and class 4 + 5 (n = 2). As ascertained by a kidney biopsy, the active LN patients had a mean AI of 3.36 (s.d. 4.34) and a mean CI of 0.91 (s.d. 1.38). Although patients with proliferative LN exhibited higher levels of urinary CD163, numbers were too small to ascertain statistical significance. As summarized in Table 3, active LN patients with elevated AI and CI scores exhibited significantly increased urine CD163 levels (P = 0.012). Urine CD163 significantly correlated with interstitial inflammation and interstitial fibrosis (Spearmen correlation coefficient 0.77; P < 0.05 for both) on concurrent renal biopsies. Kidney pathology glomerular cellular infiltrates were also associated with urine CD163, although it barely missed significance (P = 0.07).
Table 3.
Association of urine CD163 with AI and CI in cSLE with active LN
| Variable | N | Urine CD163 mean | Fold change | P-value |
|---|---|---|---|---|
| Nonproliferative LN | 5 | 1572 | 84.5 | NS |
| Proliferative | 8 | 132 951 | ||
| Low AI (0–6) | 8 | 1216 | 7.8 | 0.012 |
| High AI (≥7) | 3 | 9511 | ||
| Low CI (0–2) | 8 | 1216 | 7.8 | 0.012 |
| High CI (≥3) | 3 | 9511 |
Only cSLE patients with active LN are included in this analysis.
Pathology information is based on concurrent renal biopsies.
Discussion
Urinary CD163 has been reported as a marker of kidney disease in adults with SLE. Many studies in adult SLE patients have reported increased urinary CD163 levels in active LN patients compared with individuals without LN, inactive SLE patients and healthy controls [24–28]. One study performed separate analyses of urinary CD163 levels based on ethnicity, including Black, White and Asian American cohorts. Researchers concluded that for all ethnicities, urine CD163 levels were higher in active LN patients compared with active nonrenal, inactive SLE and healthy control subjects. Among LN patients of all three groups, the Caucasian cohort had lower levels of urine CD163 compared with African American and Asian patients, suggesting that ethnicity may notably factor into disease severity and biomarker levels. That study also showed a strong correlation between CD163, SLEDAI, rSLEDAI and Physician’s Global Assessment [24]. Serum and urine CD163 have been at the center of many aSLE studies, with one revealing that the accumulation of CD163-positive macrophages was increased in proliferative LN patients. In further evaluating the accuracy of urine CD163 compared with monocyte chemoattractant protein-1 (MCP-1), CD163 was hypothesized to be more specific than MCP-1 at accurately reflecting kidney damage associated with macrophage infiltration, given that MCP-1 was elevated in various autoimmune diseases, while CD163 remained highly correlated with M2 macrophages [25].
Similarly, in an aSLE eastern Chinese cohort, serum CD163 levels were found to be significantly elevated in patients with LN compared with healthy controls, and in addition to CD163 being positively correlated with AI and CI, the renal survival rate of individuals with high CD163 was reported to be lower [26]. A separate study demonstrated that increasing levels of urinary CD163 in a cross-sectional and longitudinal cohort did in fact reflect clinical severity and histological activity of the disease. In that study, urine CD163 levels of active LN patients were highest compared with those without LN and individuals with other glomerular diseases. Mejia-Vilet et al. [27] observed that urinary CD163 varied by LN class and significantly correlated with the severity of the renal flare. Additionally, urine CD163 decreased in patients who had complete or partial clinical response over the course of treatment, suggesting that CD163 may function in guiding future induction therapies in SLE patients. Lastly, Gupta et al. [28] observed higher levels of urinary CD163 in an active LN cohort compared with healthy controls as well as RA patients, and these levels decreased with treatment. Based on the above findings, CD163 has been shown to be superior at predicting a renal response in adult cohorts. When compared with current conventional biomarkers, recent data have shown a good correlation with disease activity and predictive outcomes in aSLE, suggesting that similar outcomes may be plausible in cSLE.
Here we investigate if urine CD163 has comparable diagnostic potential in cSLE. In this study, urine CD163 was significantly higher in cSLE patients with active LN when compared with inactive SLE participants and healthy controls. Similar to previous studies reported in aSLE patients, CD163 had a strong correlation with SLEDAI, rSLEDAI, uPCR and C3 (P < 0.0001 for all). CD163 performed excellently in discriminating active renal LN patients from inactive SLE and healthy controls with high specificity. Moreover, urine CD163 was also significantly elevated in cSLE patients with increased renal pathology AI and CI scores, correlating strongly with interstitial inflammation and interstitial fibrosis. Hence this urine marker demonstrates excellent potential for future longitudinal studies in cSLE, as its ability to detect renal disease is on par with previous literature in aSLE cohorts.
Conventional clinical biomarkers for SLE include anti-dsDNA and complement proteins, including C1q, C1r, C2, C3 and C4, but these lack sufficient sensitivity and/or specificity [13, 29]. Previous studies have reported promising single serum and urine biomarkers in cSLE [10]. For example, neutrophil gelatinase–associated lipocalin (NGAL) has been found to be elevated in conjunction with acute kidney injury and various inflammatory conditions [13, 30]. Brunner et al. [30] recognized NGAL as a promising biomarker in cSLE following elevated urine NGAL levels in biopsy-proven SLE patients compared with healthy controls and patients with JIA. Furthermore, there was a strong correlation between urinary NGAL and renal disease activity and damage. In addition, Watson et al. [31] investigated urine MCP-1 as a candidate biomarker in cSLE. MCP-1 shows promise in predicting renal histology with a good AUC value (0.81), high specificity and high sensitivity (0.70 and 0.71, respectively). Compared with these competing urinary biomarkers, urine CD163 demonstrates superior diagnostic metrics in distinguishing cSLE patients with active LN.
While kidney biopsy remains the gold standard for detecting LN activity, CD163 may contribute to early LN diagnosis, as detection of urine CD163 could pave the way for less invasive methods of diagnosis as well as potentially allow for more accurate differentiation between disease states [15, 32]. In regard to cSLE, a simple urine test could potentially be decisive in whether or not to institute more aggressive immunosuppressive induction therapy. Urine is easy to obtain noninvasively, and a urine CD163 test can be performed repeatedly with significantly reduced costs and risks compared with a kidney biopsy. Ultimately, urine tests lend themselves to point-of-care testing and even home monitoring of disease status.
CD163 belongs to the cysteine-rich scavenger receptor superfamily and is subject to cleavage, resulting in various isoforms. CD163 staining is primarily found on resident tissue macrophages, such as spleen cells and elsewhere in the immune system, Kupffer cells located in the liver and interstitial and alveolar macrophages in the lungs. CD163 is also a marker of anti-inflammatory macrophages [14, 33, 34]. Macrophages are categorized into M1 and M2 macrophages, and M2 macrophages can be further divided into M2a, M2b and M2c subtypes that participate in renal repair, immune response and anti-inflammatory and profibrotic actions. Using immunohistochemical analysis of renal tissue, Olmes et al. [35] reported that M2c-like macrophages amass in large quantities in LN. CD163-positive cells were primarily found in cellular crescents and proliferative glomerular lesions, and they accumulated in areas of acute tubular injury, indicating that CD163 may play a pathogenic role in this disease [36]. Hence, one might postulate that elevated urinary CD163 in LN might be reflective of elevated CD163-positive M2 macrophages within the inflamed kidneys, although such a direct relationship remains to be established. In our present study, CD163 levels significantly correlated (P < 0.05) with interstitial inflammation and interstitial fibrosis. Interestingly, cSLE patients exhibiting the highest sCD163 levels (1 032 658, 16 421, 7161, 4712 and 4951 pg/mg) generally showed scoring >0 for glomerular infiltrates, cellular crescents, interstitial inflammation and interstitial fibrosis. This analysis follows scoring of kidney biopsies as either 0, 1, 2 or 3 based on 0%, 1–25%, 25–50% or >50% glomeruli, tubule–interstitium or interstitium involvement.
There are limitations to this study. The cross-sectional design does not allow for evaluating changes in disease activity over time. Future studies will aim to monitor CD163 as a measurable biomarker for evaluating kidney disease progression in a longitudinal cohort. Prospective studies in larger cSLE cohorts would help validate the feasibility of using urine CD163 across a more demographically diverse patient population and reaffirm the role of CD163 in differentiating between diseased states within the spectrum of disease progression. It will also be important to assess if urine CD163 can be used to predict response to induction therapy in cSLE and long-term disease outcome.
In conclusion, urinary CD163 emerges as a useful biomarker in children with LN given that the biomarker demonstrated high accuracy, sensitivity and specificity for active LN. Validating its clinical utility in larger cohorts would shed light on the specific roles this biomarker could play in disease management. Finally, mechanistic studies are also warranted to better understand the pathogenic role of CD163-bearing macrophages in LN.
Acknowledgements
H.I., K.V. and J.C. performed the experiments and data analyses. M.J.H. performed all pathology analyses. S.W. and C.M. designed the studies and reviewed all data. H.I., S.W. and C.M. wrote the manuscript. All authors reviewed the manuscript and concurred with the findings. This study was approved by IRB at the Baylor College of Medicine (H-35050) and the University of Houston, Houston, TX, USA. All enrolled patients completed an IRB-approved informed consent form based on good clinical practice and the Declaration of Helsinki.
Funding: This work is supported by NIH grant R01 AR074096.
Disclosure statement: The authors have declared no conflicts of interest.
Contributor Information
Haleigh Inthavong, Department of Biomedical Engineering, University of Houston.
Kamala Vanarsa, Department of Biomedical Engineering, University of Houston.
Jessica Castillo, Department of Biomedical Engineering, University of Houston.
M John Hicks, Department of Pathology, Texas Children’s Hospital; Department of Immunology and Pathology, Baylor College of Medicine.
Chandra Mohan, Department of Biomedical Engineering, University of Houston.
Scott E Wenderfer, Renal Section, Texas Children’s Hospital; Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA.
Data availability statement
All data reported in this work are freely available to interested readers by contacting the corresponding authors.
References
- 1. Mohan C, Putterman C.. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat Rev Nephrol 2015;11:329–41. [DOI] [PubMed] [Google Scholar]
- 2. Davidson A. What is damaging the kidney? Nat Rev Rheumatol 2016;12:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soliman S, Mohan C.. Lupus nephritis biomarkers. Clin Immunol 2017;185:10–20. [DOI] [PubMed] [Google Scholar]
- 4. Abulaban KM, Brunner HI.. Biomarkers for childhood-onset systemic lupus erythematosus. Curr Rheumatology Rep 2015;17:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tucker LB, Uribe AG, Fernandez M. et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus 2008;17:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mok CC, Mohan C.. Urinary biomarkers in lupus nephritis: are we there yet? Arthritis Rheum 2021;73:194–6. [DOI] [PubMed] [Google Scholar]
- 7. Stichweh D, Arce E, Pascual V.. Update on pediatric systemic lupus erythematosus. Curr Opin Rheumatol 2004;16:577–87. [DOI] [PubMed] [Google Scholar]
- 8. Rodriguez-Smith J, Brunner HI.. Update on the treatment and outcome of systemic lupus erythematosus in children. Curr Opin Rheumatol 2019;31:464–70. [DOI] [PubMed] [Google Scholar]
- 9. Brunner HI, Gladman DD, Ibañez D, Urowitz MD, Silverman ED.. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheumotol 2008;58:556–62. [DOI] [PubMed] [Google Scholar]
- 10. Hiraki LT, Feldman CH, Liu J. et al. Prevalence, incidence, and demographics of systemic lupus erythematosus and lupus nephritis from 2000 to 2004 among children in the US Medicaid beneficiary population. Arthritis Rheum 2012;64:2669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wenderfer SE, Chang JC, Goodwin Davies A. et al. Using a multi-institutional pediatric learning health system to identify systemic lupus erythematosus and lupus nephritis: development and validation of computable phenotypes. Clin J Am Soc Nephrol 2022;17:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith EMD, Jorgensen AL, Midgley A. et al. International validation of a urinary biomarker panel for identification of active lupus nephritis in children. Pediatr Nephrol 2017;32:283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mok CC. Biomarkers for lupus nephritis: a critical appraisal. J Biomed Biotechnol 2010;2010:638413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou D, Wang Y, Chen LU, Zhang W, Luan J.. Soluble CD163: a novel biomarker with diagnostic and therapeutic implications in autoimmune diseases. J Rheumatol 2016;43:830. [DOI] [PubMed] [Google Scholar]
- 15. Moller HJ. Soluble CD163. Scand J Clin Lab Invest 2012;72:1–13. [DOI] [PubMed] [Google Scholar]
- 16. Nakayama W, Jinnin M, Makino K. et al. CD163 expression is increased in the involved skin and sera of patients with systemic lupus erythematosus. Eur J Dermatol 2012;22:512–7. [DOI] [PubMed] [Google Scholar]
- 17. Zizzo G, Guerrieri J, Dittman LM, Merrill JT, Cohen PL.. Circulating levels of soluble MER in lupus reflect M2c activation of monocytes/macrophages, autoantibody specificities and disease activity. Arthritis Res Ther 2013;15:R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classificiation of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 19. Gladman DD, Ibanez D, Urowitz MB.. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 20. Gladman D, Ginzler E, Goldsmith C. et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 21. Bajema IM, Wilhelmus S, Alpers CE. et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 2018;93:789–96. [DOI] [PubMed] [Google Scholar]
- 22. Austin HA, Muenz LR, Joyce KM, Antonovych TT, Balow JE.. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int 1984;25:689–95. [DOI] [PubMed] [Google Scholar]
- 23. Hiramatsu N, Kuroiwa T, Ikeuchi H. et al. Revised classification of lupus nephritis is valuable in predicting renal outcome with an indication of the proportion of glomeruli affected by chronic lesions. Rheumatology 2008;47:702–7. [DOI] [PubMed] [Google Scholar]
- 24. Zhang T, Li H, Vanarsa K. et al. Association of urine CD163 with proliferative lupus nephritis, fibrinoid necrosis, and cellular crescents and intrarenal M2 macrophages. Front Immunol 2020;11:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Endo N, Tsuboi N, Furuhashi K. et al. Urinary soluble CD163 level reflects glomerular inflammation in human lupus nephritis. Nephrol Dial Transplant 2016;31:2023–33. [DOI] [PubMed] [Google Scholar]
- 26. Yang G, Guo N, Yin J, Wu J.. Elevated soluble CD163 predicts renal function deterioration in lupus nephritis: a cohort study in eastern China. J Int Med Res 2021;49:3000605211049963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mejia-Vilet JM, Zhang XL, Cruz C. et al. Urinary soluble CD163: a novel noninvasive biomarker of activity for lupus nephritis. J Am Soc Nephrol 2020;31:1335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta R, Yadav A, Aggarwal A.. Urinary soluble CD163 is a good biomarker for renal disease activity in lupus nephritis. Clin Rheumatol 2021;40:941–8. [DOI] [PubMed] [Google Scholar]
- 29. Arriens C, Wren JD, Munroe ME, Mohan C.. Systemic lupus erythematosus biomarkers: the challenging quest. Rheumatology (Oxford) 2017;56:i32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brunner HI, Mueller M, Rutherford C. et al. Urinary neutrophil gelatinase–associated lipocalinas a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum 2006;54:2577–84. [DOI] [PubMed] [Google Scholar]
- 31. Watson L, Tullus K, Pilkington C. et al. Urine biomarkers for monitoring juvenile lupus nephritis: a prospective longitudinal study. Pediatric Nephrol 2014;29:397–405. [DOI] [PubMed] [Google Scholar]
- 32. Schwartz N, Michaelson JS, Putterman C.. Lipocalin-2, TWEAK, and other cytokines as urinary biomarkers for lupus nephritis. Ann N Y Acad Sci 2007;1109:265–74. [DOI] [PubMed] [Google Scholar]
- 33. Bleesing J, Prada A, Siegel DM. et al. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor α-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum 2007;56:965–71. [DOI] [PubMed] [Google Scholar]
- 34. Gorp HV, Delputte PL, Nauwynck HJ.. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol 2010;47:1650–60. [DOI] [PubMed] [Google Scholar]
- 35. Olmes G, Buttner-Herold M, Ferrazzi F. et al. CD163+ M2c like macrophages predominate in renal biopsies from patients with lupus nephritis. Arthritis Res Ther 2016;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li J, Liu CH, Xu DL, Gao B.. Significance of CD163-positive macrophages in proliferative glomerulonephritis. Am J Med Sci 2015;350:387–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data reported in this work are freely available to interested readers by contacting the corresponding authors.