Abstract
The dissimilatory Fe(III) reducer Geobacter metallireducens reduced Fe(III) bound in humic substances, but the concentrations of Fe(III) in a wide range of highly purified humic substances were too low to account for a significant portion of the electron-accepting capacities of the humic substances. Furthermore, once reduced, the iron in humic substances could not transfer electrons to Fe(III) oxide. These results suggest that other electron-accepting moieties in humic substances, such as quinones, are the important electron-accepting and shuttling agents under Fe(III)-reducing conditions.
Factors controlling the rate and extent of dissimilatory Fe(III) reduction are of interest because Fe(III) reduction has an impact on the geochemistry of a variety of sedimentary environments (4, 5). The presence of humic substances can significantly stimulate the oxidation of organic compounds coupled to Fe(III) reduction in aquifer sediments and in cultures (1, 6, 7, 12). When first noted in sediments, this phenomenon was presumed to be the result of humic substances solubilizing Fe(III) from insoluble Fe(III) oxides (12) because previous studies performed with the same sediments had demonstrated that solubilization of Fe(III) with synthetic chelators enhanced Fe(III) reduction (10–12). However, humic substances were found to solubilize too little Fe(III) to account for their stimulatory effect (6).
Subsequent studies suggested that humic substances stimulate Fe(III) reduction because humic substances can act as an electron shuttle between Fe(III)-reducing microorganisms and insoluble Fe(III) oxides (6, 7, 14). These studies also suggested that the important electron-shuttling groups in humic substances are quinone moieties. In this model, Fe(III)-reducing microorganisms transfer electrons to quinone moieties in humic substances, and the hydroquinone groups that are generated from quinone reduction can abiotically transfer electrons to Fe(III) oxides. This reoxidizes the humic substances into forms which can undergo another cycle of reduction and oxidation. Thus, in the presence of Fe(III) oxides, a small amount of soluble humic substances can become a major electron acceptor for organic matter oxidation because each humic substance molecule may be reused as an electron acceptor multiple times. This electron shuttling between Fe(III)-reducing microorganisms and Fe(III) oxides via humic substances accelerates the rate of Fe(III) reduction because (i) the Fe(III) reducers can access soluble humic substances more readily than they can establish direct contact with insoluble Fe(III) oxides, and (ii) microbially reduced humic substances can access insoluble Fe(III) oxides more readily than Fe(III)-reducing microorganisms can (7, 11).
Several lines of evidence support the concept that quinones are the important electron transfer moieties in electron shuttling between Fe(III)-reducing microorganisms and Fe(III) oxides. Studies in which semiquinones were quantified by electron spin resonance revealed that there was a direct correlation between the quinone contents of various humic substances and the electron-accepting capacities of the humic substances (14). It has also been demonstrated that microbial reduction of humic substances results in an increase in quinone radicals in direct proportion to the electron-accepting capacity of the humic substances, as would be expected if electrons were being transferred primarily to quinone moieties (14). Microorganisms that have the ability to transfer electrons to humic substances invariably have the ability to transfer electrons to extracellular quinones, whereas organisms that do not reduce extracellular quinones do not reduce humic substances (3, 6, 7). Low concentrations of extracellular quinones, such as the humic substance analog anthraquinone-2,6-disulfonate, can stimulate Fe(III) oxide reduction (6, 7) and enhanced anaerobic benzene oxidation in the petroleum-contaminated aquifer sediments in which addition of humic substances stimulated anaerobic benzene oxidation (1).
It has recently been suggested that iron bound in humic substances might also be involved in the humic substance redox processes associated with microbial Fe(III) reduction (2). This suggestion was based on the finding that commercially available Aldrich humic substances had an iron content that was comparable to the electron-accepting capacity of the humic substances estimated indirectly. However, it was not determined whether iron in the humic substances could in fact serve as an electron acceptor and/or an electron shuttle to Fe(III) oxide.
Fe(III) is a minor electron-accepting group in true humic substances.
To determine whether humic substances contain microbially reducible Fe(III), studies were initially conducted with highly purified humic substances obtained from the International Humic Substances Society. Such humic substances are preferred over Aldrich humic substances for determining the properties of humic substances because the Aldrich humic substances may not adequately represent the chemical nature of true humic substances found in soils and sediments (13). As previously described (6), various purified humic substances (final concentration, 2 g/liter) were dissolved in 30 mM anaerobic bicarbonate buffer that contained 10 mM acetate under N2-CO2 (80:20). A washed suspension of Geobacter metallireducens cells was added to the humic substance solution, and the preparation was incubated at 30°C for 2 h. The G. metallireducens was then removed by anaerobically passing the preparation through a filter (pore diameter, 0.2 μm). An aliquot of the filtrate was acidified with HCl (final concentration, 0.5 N), an aliquot of the HCl extract was added to HEPES buffer containing the Fe(II) reagent ferrozine, and the absorbance at 562 nm (A562) was quantified in order to measure the Fe(II) content (9). In order to account for the absorbance due to the presence of humic substances in the filtrate, the A562 of a humic substance solution filtrate without ferrozine added was also determined. Furthermore, since adding the cell suspension added ca. 20 μM Fe(II), the amount of Fe(II) added with the cells was determined by adding the cell suspension to bicarbonate buffer without humic substances and then determining the Fe(II) content as described above. The amount of Fe(II) produced from microbial reduction of Fe(III) in the humic substances was calculated from the A562 of the various preparations as follows: (A562 from the ferrozine analysis of the microbially reduced humic substance filtrate) − (A562 attributed to humic substances) − [A562 due to Fe(II) added with the cell suspension].
The electron-accepting capacity of moieties in the humic substances other than Fe(III) was determined as previously described (6). Fe(III) citrate (final concentration, 10 mM) was added to a filtrate of microbially reduced humic substances, and after 15 min an aliquot was acid extracted and analyzed with ferrozine as described above. As in previous studies (6, 14), the amount of Fe(II) produced from the reduction of Fe(III) citrate was considered to represent the electron-accepting capacity of non-Fe(III) moieties in the humic substances and was calculated as follows: [A562 from the ferrozine analysis of the filtrates of microbially reduced humic substances amended with Fe(III) citrate] − [A562 from the ferrozine analysis of filtrates of microbially reduced humic substances not amended with Fe(III) citrate]. The latter absorbance value included the absorbance due to any Fe(II) produced as a result of microbial reduction of Fe(III) in humic substances, the absorbance due to Fe(II) introduced with the cell suspension, and the absorbance due to the humic substances.
Soil humic acids, which have been used most frequently for studies of the electron transfer capability of humic substances (6, 7), contained no detectable microbially reducible Fe(III), but as previously demonstrated (6, 14), these humic substances did have a significant electron-accepting capacity due to other moieties in them (Table 1). The non-Fe(III) electron-accepting capacity of these humic substances and the other humic substances evaluated was similar to the capacity observed previously (6), but in this study the results are presented on a per-gram basis. Peat humic acids also contained no detectable microbially reducible Fe(III) but could accept electrons from G. metallireducens. The other highly purified humic substances obtained from the International Humic Substances Society that were evaluated did contain small amounts of microbially reducible Fe(III) (Table 1). However, the electron-accepting capacity of the Fe(III) in these humic substances was ca. 10% or less of the electron-accepting capacity that was due to moieties other than Fe(III). These results demonstrate Fe(III) plays a very minor role as an electron-accepting group in a wide range of well-characterized humic substances.
TABLE 1.
Microbially reducible Fe(III) and non-Fe(III) electron-accepting capacities of diverse humic substances
| Humic substance sourcea | Microbially reducible Fe(III) concn (μmol/g) | Non-Fe(III) electron-accepting capacity (μmol/g) | % of total electron-accepting capacity due to Fe(III)b |
|---|---|---|---|
| Suwannee | 9.2 ± 1.0c | 106 ± 7.0 | 7.5 |
| Nymph | 22.0 ± 1.0 | 186 ± 8.4 | 10.4 |
| Soil | 0 | 342 ± 5.8 | 0 |
| Peat | 0 | 238 ± 6.8 | 0 |
| Leonardite | 18.2 ± 1.3 | 261 ± 5.8 | 6.5 |
| Summit | 16.7 ± 0.8 | 152 ± 2.3 | 10.1 |
| Aldrich | 115.8 ± 1.4 | 153 ± 3.9 | 43.5 |
All humic substances other than the Aldrich humic substances were obtained from the International Humic Substances Society.
{Microbially reducible Fe(III)/[microbially reducible Fe(III) + (non-Fe(III) electron-accepting capacity]} × 100.
Mean ± standard deviation based on triplicate analyses.
Even though Aldrich humic substances are not considered appropriate analogues of true humic substances in the environment (13), it was of interest to determine whether, as previously suggested (2), these humic substances contained microbially reducible Fe(III). The concentration of microbially reducible Fe(III) in the Aldrich humic substances was more than fourfold higher than the concentration of microbially reducible Fe(III) in any of the other humic substances evaluated, but even in these highly impure humic substances the electron-accepting capacity due to Fe(III) bound in the humic substances was less than the electron-accepting capacity due to other moieties in the humic substances (Table 1).
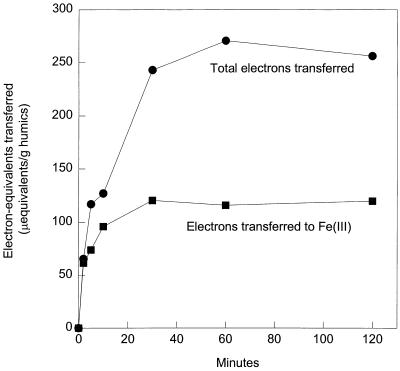
In order to determine if Fe(III) or other electron-accepting moieties in Aldrich humic substances were reduced preferentially, electron transfer was monitored over time (Fig. 1). Electrons were initially transferred primarily to Fe(III), and only after the Fe(III) was reduced were the other electron-accepting moieties significantly reduced (Fig. 1). This could have resulted from an initial transfer solely to Fe(III), but it is also likely that any quinone groups that initially accepted electrons reduced the Fe(III) in the humic substances, just as they reduced Fe(III) oxides. Only after the Fe(III) was completely reduced would the quinone moieties remain in a reduced state.
FIG. 1.
Electron equivalents transferred to Fe(III) and sum of electron equivalents transferred to Fe(III) and other electron-accepting groups in Aldrich humic acids by a cell suspension of G. metallireducens over time. The results are means based on duplicate incubations.
Lack of electron shuttling via iron.
Despite the fact that Fe(III) accounted for little, if any, of the electron-accepting capacity in highly pure humic substances, the finding that G. metallireducens initially reduced the Fe(III) in humic substances suggested that even small amounts of iron in humic substances might be important as an electron transfer agent in Fe(III)-reducing environments if, once reduced, the iron in humic substances could transfer electrons to Fe(III) oxide and thus reoxidize the Fe(II) in the humic substances back to Fe(III). Through such a recycling mechanism, even small amounts of iron could participate in significant electron transfer in sediments. Such regeneration mechanisms are necessary for any electron-accepting moiety in humic substances to play a significant role as an electron acceptor in sediments because, on a per-gram basis, the total electron-accepting capacity of humic substances is low compared to the electron-accepting capacities of other potential electron acceptors for anaerobic respiration. Therefore, the potential of iron in humic substances to function as an electron shuttle between Fe(III) reducers and Fe(III) oxides was evaluated.
The electron shuttling studies were conducted with Aldrich humic substances because they were the only humic substances available that contained high enough concentrations of Fe(III) to accurately monitor. Furthermore, it was data obtained with Aldrich humic substances which was the basis for the previous suggestion (2) that iron may be important in humic substances. As described above, the humic substances (final concentration, 2 g/liter) were dissolved in anaerobic bicarbonate buffer containing acetate. The concentration of Fe(II) was determined with ferrozine as described above, and the total iron concentration was determined after Fe(III) was reduced with hydroxylamine, as previously described (9). The difference between the total iron and Fe(II) concentrations represents the concentration of Fe(III) (9). The Aldrich humic substances were stored aerobically before they were added to the anaerobic buffer, and thus it is not surprising that most of the iron in the initial humic substance solution was recovered as Fe(III) (Table 2). As expected from the studies described above, reduction of the humic substances with G. metallireducens reduced all of the Fe(III) to Fe(II) (Table 2).
TABLE 2.
Potential for iron in humic substances to serve as an electron shuttle
| Prepn | Fe(II) concn (μmol/g) | Fe(III) concn (μmol/g) |
|---|---|---|
| Humic substances in solutiona | 28.8 ± 4.6b | 62.8 ± 2.9 |
| Microbially reduced humic substancesc | 117 ± 11.8 | 0 |
| Microbially reduced humic substances exposed to Fe(III) oxided | 224 ± 24.3 | 0 |
Values determined before G. metallireducens was introduced.
Mean ± standard deviation based on triplicate analyses.
Values determined after exposure to G. metallireducens.
Values determined after an anaerobic filtrate of the microbially reduced humic substances was exposed to Fe(III) oxide.
If the iron in the humic substances could shuttle the electrons received from G. metallireducens to Fe(III) oxide, then exposure of microbially reduced humic substances to Fe(III) oxide should have oxidized the Fe(II) in the humic substances to Fe(III). To evaluate this, an anaerobic slurry of poorly crystalline Fe(III) oxide (final concentration, 10 mmol per liter), prepared as previously described (8), was added to a filtrate of microbially reduced humic substances. After 1 h, the Fe(III) oxide was removed by filtration, and the Fe(II) and Fe(III) concentrations in the humic substance solution were determined. No Fe(III) was detected in the humic substance solution after exposure to Fe(III) oxide, indicating that there was no electron transfer between the Fe(II) in the humic substances and the Fe(III) oxide (Table 2). As expected from the study described above (Table 1), there was an increase in the soluble Fe(II) content after exposure of the microbially reduced humic substances to Fe(III) oxide which could be attributed to other reduced moieties, such as hydroquinones, that transferred electrons to the Fe(III) oxide.
Conclusions.
Our results demonstrate that the electron-accepting capacities of a wide range of highly purified humic substances were much higher than the electron-accepting capacities of the microbially reducible Fe(III), which showed that Fe(III) in humic substances can at best account for a small amount of the initial electron transfer to humic substances in environments in which Fe(III) reduction is the terminal electron-accepting process. Furthermore, unlike quinone moieties, the iron in humic substances cannot function as an electron shuttle between Fe(III)-reducing microorganisms and Fe(III) oxides. The ability to cycle between oxidized and reduced forms is essential for moieties in humic substances to be quantitatively significant as electron acceptors in Fe(III)-reducing environments. Thus, the role of iron bound in humic substances as an electron transfer agent in Fe(III)-reducing sediments is likely to be minimal.
Acknowledgments
This research was supported by grant N0014-96-1-0382 from the Office of Naval Research and grant DE FG02-97ER62475 from the Department of Energy.
REFERENCES
- 1.Anderson R T, Lovley D R. Naphthalene and benzene degradation under Fe(III)-reducing conditions in petroleum-contaminated aquifers. Bioremed J. 1999;3:121–135. [Google Scholar]
- 2.Benz M, Schink B, Brune A. Humic acid reduction by Propionibacterium freudenreichii and other fermenting bacteria. Appl Environ Microbiol. 1998;64:4507–4512. doi: 10.1128/aem.64.11.4507-4512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coates J D, Ellis D J, Roden E, Gaw K, Blunt-Harris E L, Lovley D R. Recovery of humic-reducing bacteria from a diversity of environments. Appl Environ Microbiol. 1998;64:1504–1509. doi: 10.1128/aem.64.4.1504-1509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovley D R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovley D R. Microbial reduction of iron, manganese, and other metals. Adv Agron. 1995;54:175–231. [Google Scholar]
- 6.Lovley D R, Coates J D, Blunt-Harris E L, Phillips E J P, Woodward J C. Humic substances as electron acceptors for microbial respiration. Nature. 1996;382:445–448. [Google Scholar]
- 7.Lovley D R, Fraga J L, Blunt-Harris E L, Hayes L A, Phillips E J P, Coates J D. Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim Hydrobiol. 1998;26:152–157. [Google Scholar]
- 8.Lovley D R, Phillips E J P. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986;51:683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovley D R, Phillips E J P. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol. 1987;53:1536–1540. doi: 10.1128/aem.53.7.1536-1540.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovley D R, Woodward J C. Mechanisms for chelator stimulation of microbial Fe(III)-oxide reduction. Chem Geol. 1996;132:19–24. [Google Scholar]
- 11.Lovley D R, Woodward J C, Chapelle F H. Stimulated anoxic biodegradation of aromatic hydrocarbons using Fe(III) ligands. Nature. 1994;370:128–131. doi: 10.1038/370128a0. [DOI] [PubMed] [Google Scholar]
- 12.Lovley D R, Woodward J C, Chapelle F H. Rapid anaerobic benzene oxidation with a variety of chelated Fe(III) forms. Appl Environ Microbiol. 1996;62:288–291. doi: 10.1128/aem.62.1.288-291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malcolm R L, MacCarthy P. Limitations in the use of commercial humic acids in water and soil research. Environ Sci Technol. 1986;20:904–911. doi: 10.1021/es00151a009. [DOI] [PubMed] [Google Scholar]
- 14.Scott D T, McKnight D M, Blunt-Harris E L, Kolesar S E, Lovley D R. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ Sci Technol. 1998;32:2984–2989. [Google Scholar]