Abstract
Background
Northeast Brazil has the world's highest rate of Zika-related microcephaly. However, Zika case counts cannot accurately describe burden because mandatory reporting was only established when the epidemic was declining in the region.
Methods
To advance the study of the Zika epidemic, we identified hotspots of Zika in Pernambuco state, Northeast Brazil, using Aedes-borne diseases (dengue, chikungunya and Zika) and microcephaly data. We used Kulldorff's Poisson purely spatial scan statistic to detect low- and high-risk clusters for Aedes-borne diseases (2014–2017) and for microcephaly (2015–2017), separately. Municipalities were classified according to a proposed gradient of Zika burden during the epidemic, based on the combination of cluster status in each analysis and considering the strength of the evidence.
Results
We identified 26 Aedes-borne diseases clusters (11 high-risk) and 5 microcephaly clusters (3 high-risk) in Pernambuco. According to the proposed Zika burden gradient, our results indicate that the northeast of Pernambuco and the Sertão region were hit hardest by the Zika epidemic. The first is the most populous area of Pernambuco, while the second has one of the highest rates of social and economic inequality in Brazil.
Conclusion
We successfully identified possible hidden Zika hotspots using a simple methodology combining Aedes-borne diseases and microcephaly information.
Keywords: dengue, epidemiology, microcephaly, scan statistics, spatial analysis, Zika
Introduction
Accurately assessing a community's disease burden is a major goal in public health research and is critical to the study of epidemics and their consequences. When new viral diseases emerge, this task is challenging, as the reporting of cases may only be established after widespread transmission has occurred. Although the Zika virus (ZIKV) reached Brazil in 2014, mandatory reporting of cases did not begin until 2016, at least a full year into the epidemic.1,2 Given the difficulty of accurately assessing the Zika burden during the epidemic and the lack of data on Zika cases, identifying areas that were the hardest hit remains a challenge.
The first large outbreak of ZIKV, a flavivirus transmitted to humans mainly by Aedes aegypti mosquitoes, was on the island of Yap (Federated States of Micronesia) in 2007.3 According to genomics analyses, ZIKV was likely introduced to Brazil in the state of Pernambuco, possibly during 2013, and may have disseminated from there to other regions and even to other countries.4,5 Zika was considered a benign disease until October 2015, when an unusual increase in the number of neonates with microcephaly was detected in Pernambuco, Northeast Brazil. Microcephaly and other congenital malformations were later associated with ZIKV infection during pregnancy, and the congenital Zika syndrome was first described.1 From January 2015 to November 2016, 1950 infection-related microcephaly cases were confirmed in Brazil, of which 1487 (76.3%) were in the Northeast region.2 By the end of the Zika epidemic, nowhere else in the world had microcephaly rates as high as those observed in Northeast Brazil.
The task of identifying areas most affected by the Zika epidemic is hampered by some important factors. First, the mandatory reporting of Zika cases to Brazilian health authorities only began in 2016, a full year after the epidemic began, and well after the assumed peak of cases in most of the Northeast region.2,6 As a result, the number of reported Zika cases in this region and period of time is much lower than the reported cases of congenital microcephaly, despite well-established links between the two.7 In the absence of Zika data for 2014 and 2015, the number of microcephaly cases likely helps to identify areas that experienced Zika outbreaks. It is also important to note that microcephaly cases, given the severity of the condition, are much less likely to be under-reported than Zika cases. In a previous study, the incidence of Zika by municipality in Brazil was estimated directly from the rate of microcephaly cases.7 However, it is important to note that other factors may be acting to modify the risk of this congenital malformation when the infection occurs during pregnancy.8,9 As a consequence, a high incidence of Zika does not always translate to high microcephaly rates.
Information on the distribution of other arboviruses endemic in Brazil, namely dengue and chikungunya, can also serve as indicators of Zika incidence during this period. A major factor linking the incidence of Zika, dengue and chikungunya is the fact that they share the same vector, the Aedes aegypti mosquito.10 By virtue of a shared carrier, a greater risk of one arbovirus in a given area may imply greater risk of another arbovirus. Furthermore, it can be difficult in a clinical setting to distinguish between symptoms of Zika and symptoms of dengue and chikungunya, and cases of one are sometimes mistakenly reported as cases of another.11 In fact, in the absence of a channel for reporting Zika cases in 2014 and 2015, the government in Pernambuco encouraged medical professionals to report Zika cases as dengue cases.12 Thus, the distinction between Zika, dengue and chikungunya during this period is blurred in official records for clinical as well as administrative reasons.
Therefore, we propose that an elevated burden of Zika during and immediately following the epidemic in Brazil (from 2014 to 2017) may be detected primarily by an increase in the incidence of microcephaly, as well as by increases in the incidence of dengue, chikungunya and Zika itself. Considering these factors together represents a way to identify which areas were hit hardest by the Zika epidemic and which areas were less affected. This study sought to identify these areas in the state of Pernambuco. Pernambuco is a poor and unequal state in Brazil, and one of the most severely affected states in the Zika epidemic, accounting for 16.8% of Brazil's reported cases of congenital Zika syndrome through the end of 2017.13
Methods
Study site
Pernambuco is located in Northeast Brazil, and has 185 municipalities divided into five regions: Agreste, Mata, Metropolitan of Recife, São Francisco and Sertão (Figure 1). The state is characterized by coastal and marshy terrain, with varying climate conditions ranging from humid tropical (predominant on the coast) and semiarid (predominant in the interior). The population of Pernambuco was estimated at 9674 793 in 2021, with 98.65 inhabitants per km2.14 The capital Recife had 1661 017 inhabitants and the highest population density (approximately 7590 inhabitants per km²) of the state, according to the estimates for 2021.15
Figure 1.
Regions and municipalities of Pernambuco state, Brazil.
Data
In Brazil, suspected cases of dengue, Zika or chikungunya identified at healthcare facilities are reported to the Notifiable Diseases Information System (Sistema de Informação de Agravos de Notificação [SINAN]) of the Brazilian Ministry of Health. We obtained data on reported cases of dengue (2014–2017), Zika (2016–2017) and chikungunya (2015–2017) confirmed by laboratory or clinical-epidemiological criteria from SINAN (ftp://ftp.datasus.gov.br/dissemin/publicos/SINAN/DADOS). We chose these years because Zika started causing outbreaks in the state in 2014. However, chikungunya and Zika cases data were only available after 2015 and 2016, respectively. The data that were not publicly available at the time of the analysis (Zika and chikungunya cases data) were requested from the Ministry of Health at https://esic.cgu.gov.br/, by using the Law of Access to Information. Cases were analyzed and aggregated by municipality of notification.
We obtained the anonymized individual records of live births from the Brazilian Ministry of Health's Live Births Information System (Sistema de Informações sobre Nascidos Vivos [SINASC]) from 2015 to 2017. The first Zika-related microcephaly cases were reported in 2015. The SINASC data are publicly available at ftp://ftp.datasus.gov.br/dissemin/publicos/SINASC/. The accumulated counts of live births by municipality for the study period are shown in Supplementary Figure 1. Microcephaly cases were identified as those with the code ‘Q02’ in any position of the variable corresponding to the classification of congenital anomalies according to the 10th Revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). Then the data were aggregated by municipality of residence of the mother. The complete data were also aggregated to obtain the number of live births by municipality.
Population projections by municipality estimated by Freire et al. were used in the analysis.16 We used the mean population by municipality from 2014 to 2017 (Supplementary Figure 2). Shapefiles were downloaded at the Brazilian Institute of Geography and Statistics Instituto Brasileiro de Geografia e Estatística website (https://www.ibge.gov.br/geociencias/organizacao-do-territorio/malhas-territoriais).
Statistical analysis
For the exploratory analysis, we calculated the incidence per 100 000 inhabitants for dengue, Zika and chikungunya, and the microcephaly incidence per 10 000 live births by municipality for all years combined. We excluded Fernando de Noronha municipality from the analysis as it is an island approximately 350 km away from the mainland.
We used the packages tidyverse (v. 1.3.0)17 and ggplot2 (v. 3.3.0)18 in R (R Core Team, Vienna, Austria, v. 3.6.3)19 to organize, analyze and visualize the data.
Scan statistics
To detect low and high-risk clusters of Aedes-borne diseases and microcephaly we used Kulldorff's Poisson purely spatial scan statistic20 for all years combined for (i) Aedes-borne diseases (dengue + Zika + chikungunya) (2014–2017) and (ii) microcephaly cases (2015–2017).
Purely spatial scan statistics identify clusters through moving circles across space by comparing the observed number of cases with the expected number of cases inside the circle.20 The municipality was considered as part of the circle if its centroid was located within the circle. The clusters are ordered according to the log-likelihood ratio (LLR), where the cluster with the maximum LLR is the most likely cluster, that is, the cluster least likely to be due to chance.20 The LLR is calculated as follows:
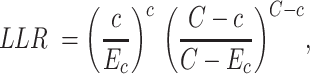 |
(1) |
where c is the number of cases inside the cluster, C is the total number of cases in the state and Ec is the expected number of cases inside the circle. Ec is calculated by:
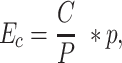 |
(2) |
where P is the population of the state and p is the population inside the cluster. A high-risk cluster is defined by an area with more cases than expected, while a low-risk cluster is an area with fewer cases than expected.
To assess statistical significance, we performed Monte Carlo simulations (n=999) for each analysis. Clusters were considered to be statistically significant if p<0.05. Clusters were restricted to not overlap geographically, to have at least five cases, to include a maximum of 50% of Pernambuco's population at risk and to have a maximum radius of 50 km. Because of the skewed population of Pernambuco, which is highly concentrated in the Metropolitan Region of Recife, we chose to use both the maximum population at risk and the maximum radius criteria to restrict the size of the clusters. For the maximum population at risk, 50% is the standard setting. We experimented with different sizes of maximum radius and chose 50 km, resulting in a reasonable combination of number and size of detected clusters.
SaTScan (v. 9.6) software (https://www.satscan.org/) was used within R (v. 3.6.3),19 along with the package rsatscan (v. 0.3.9200).21
Zika epidemic burden classification
To identify the municipalities that were most and least affected by the Zika epidemic, we propose a classification of Zika epidemic burden by combining the results from the two scan statistics analyses (Table 1). From each set of results (for microcephaly and for Aedes-borne diseases), we used whether the municipality was part of a cluster or not, and if so, of what type (i.e. low- or high-risk). First, we defined three major categories: (i) hotspots: municipalities that were part of any high-risk cluster; (ii) coldspots: municipalities that were part of any low-risk cluster and were not part of any high-risk cluster; and (iii) neutral: municipalities that were not part of any clusters.
Table 1.
Proposed criteria for classification of municipalities in terms of estimated Zika burden during the epidemic, based on cluster type of microcephaly and dengue, Zika and chikungunya (DZC)
| Zika burden classification | Cluster type | ||
|---|---|---|---|
| Major category | Gradient | Microcephaly | DZC |
| Hotspots | 5 | High-risk | High-risk |
| 4 | High-risk | - | |
| 3 | High-risk | Low-risk | |
| 2 | - | High-risk | |
| 1 | Low-risk | High-risk | |
| Neutral | 0 | - | - |
| Coldspots | −1 | - | Low-risk |
| −2 | Low-risk | - | |
| −3 | Low-risk | Low-risk | |
Then we further split the major categories into a proposed, diverging gradient, in which the extreme values represent the municipalities that were most and least affected by the Zika epidemic. This gradient was based on the different combinations of cluster types of microcephaly with the cluster types of Aedes-borne diseases. In our results, we had nine different combinations (Table 1, columns 3 and 4), hence, our proposed gradient has nine levels. Positive values were attributed for the combinations fitting the definition of hotspots, negative values for the coldspots and zero for the neutral category. To define the order of the combinations in the gradient we considered the strength of the evidence. In the extremes are the municipalities that were identified as part of clusters of the same type for both microcephaly and Aedes-borne diseases. For the intermediate levels, we considered microcephaly data to provide stronger evidence than Aedes-borne diseases because the latter are more likely to be under-reported. Therefore, we assumed that being part of a high-risk cluster for microcephaly represented a higher Zika burden than being part of a high-risk Aedes-borne disease cluster.
The data underlying this article and the script of the analysis are available at https://github.com/laispfreitas/PE_satscan.22
Results
In the state of Pernambuco there were 123 934 dengue, 167 Zika and 32 983 chikungunya cases from 2014 to 2017, and 800 microcephaly cases from 2015 to 2017 (Supplementary Table 1). Of the 167 Zika reported cases, 96 were in Recife. In 2016, 25 municipalities reported at least one case of Zika.
The cumulative incidence of dengue, Zika and chikungunya during 2014–2017 peaked at 11 742.9 cases per 100 000 inhabitants, with the highest incidence identified in the Agreste region (Figure 2A). In this period, four municipalities did not report dengue, Zika, chikungunya or microcephaly cases. Higher microcephaly incidence rates were observed in the Sertão region, peaking at 98.4 cases per 10 000 live births (Figure 2B). Fifty-one out of 184 municipalities did not report any cases of microcephaly.
Figure 2.
Cumulative incidence of dengue, Zika and chikungunya (DZC) per 100 000 inhabitants, 2014–2017 (A) and of microcephaly per 10 000 live births, 2015–2017 (B), Pernambuco state, Brazil.
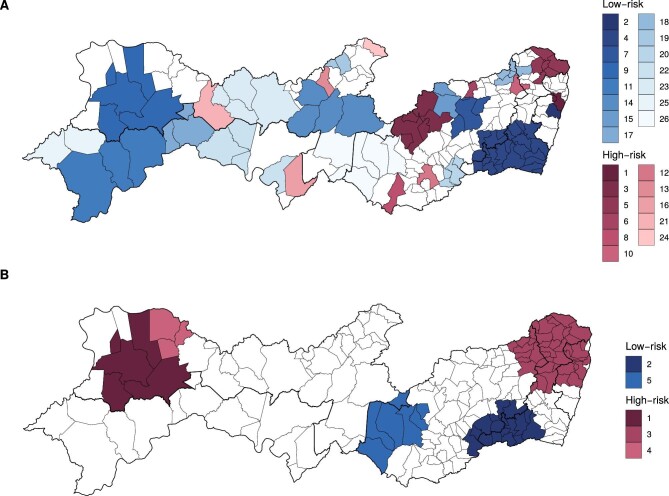
There were 26 clusters of dengue, Zika and chikungunya detected in 2014–2017 using the purely spatial scan statistics, with 11 high-risk and 15 low-risk clusters (Supplementary Table 2). The most likely high-risk cluster and the most likely low-risk cluster were both detected in the Metropolitan Region of Recife (Figure 3A). For microcephaly, five clusters were detected in 2015–2017, with three high-risk and two low-risk clusters (Supplementary Table 3). The most likely low-risk cluster was detected in the Agreste and Mata regions, and the most likely high-risk cluster in the northwest of Sertão region (Figure 3B).
Figure 3.
Low- and high-risk clusters of dengue, Zika and chikungunya cases, 2014–2017 (A) and of microcephaly, 2015–2017 (B), in Pernambuco state, Brazil. Clusters are ordered by likelihood ratio.
Combining the results of both scan statistics analyses (i.e. Aedes-borne diseases and microcephaly), of the 50 municipalities constituting high-risk microcephaly clusters, 10 were also high-risk for dengue, Zika and chikungunya (Table 2). Of the 24 municipalities constituting low-risk microcephaly clusters, 19 were also low-risk for dengue, Zika and chikungunya. The names of the municipalities in each category from Table 2 are available in Supplementary Table 4.
Table 2.
Number and percentage of municipalities by cluster type for dengue, Zika and chikungunya (DZC) cases (2014–2017) and for microcephaly (2015–2017), Pernambuco state, Brazil
| Microcephaly cluster type, n (%) | |||||
|---|---|---|---|---|---|
| High | No cluster | Low | Total, n (%) | ||
| DZC cluster type | High | 10 (5.4) | 12 (6.5) | 1 (0.5) | 23 (12.5) |
| No cluster | 29 (15.8) | 54 (29.3) | 4 (2.1) | 87 (47.3) | |
| Low | 11 (6.0) | 44 (23.9) | 19 (10.3) | 74 (40.2) | |
| Total | 50 (27.2) | 110 (59.8) | 24 (13.0) | 184 (100.0) | |
In Figure 4 we show the proposed Zika burden classification after combining the results from the scan statistics analyses to identify the municipalities most and least affected by the epidemic. The municipalities identified as probable Zika hotspots are depicted in warm colors. Sixty-three out of 184 municipalities were identified as probable hotspots for Zika. The results indicate that the municipalities in the northeast of Pernambuco state and in the Sertão region were hardest hit by the Zika epidemic (Figure 4).
Figure 4.
Proposed Zika burden classification by municipality, 2014–2017, Pernambuco state, Brazil.
Discussion
Mandatory reporting of confirmed Zika cases came late in the epidemic in Brazil, hindering reliable identification of areas most affected by the disease. To address this issue, we identified spatial clusters of Zika, dengue and chikungunya—three arboviruses that share the same vector, the Ae. aegypti mosquito—and of microcephaly cases in neonates to identify hidden Zika hotspots in the state of Pernambuco, one of the most affected by the epidemic.
Our results indicate that the northeast of Pernambuco—including parts of the Metropolitan Region of Recife, Mata and Agreste—and the more western part of the Sertão region were hit hardest by the Zika epidemic. These two regions are on opposite sides of the state. The first has the state's highest population density and urbanization rate. Because other arboviruses are more frequent in urban areas, these areas might see a magnified risk of Zika. A study in Recife has described an association between precarious living conditions and higher microcephaly incidence.23 The urban poor in Brazil often live in households and areas that lack solid infrastructure, such as proper plumbing systems and waste disposal, leading to poor environmental hygiene associated with mosquito breeding. The Sertão region has one of the highest rates of social and economic inequality in Brazil and is also characterized by precarious healthcare access. The distribution of health services in the Sertão region is well below the state average, while child and maternal mortality are higher than the state average.24,25
Fifty municipalities constituted high-risk microcephaly clusters, with only 10 of these also constituting high-risk clusters for Aedes-borne diseases. Because most cases of microcephaly in the region were caused by Zika,26 the pattern of high risk of microcephaly combined with a low risk of dengue, Zika and chikungunya suggests that there was under-reporting of Aedes-borne diseases in these municipalities. The under-reporting of acute infectious diseases is usually higher in poorer areas, as a consequence of lower access to healthcare services. Therefore, using only Aedes-borne data would bias the identification of Zika high-risk areas. By combining the analyses using such data with analyses using microcephaly data, we successfully identified potential hidden Zika hotspots. Of note, the identification of Sertão region as a Zika hotspot was only possible because the scan statistics results for dengue, Zika and chikungunya were combined with the results for microcephaly.
Of 23 municipalities constituting high-risk clusters for Aedes-borne diseases, 10 were also high risk for microcephaly. It is possible that in locations that were high-risk for Aedes-borne diseases but not for microcephaly, dengue and/or chikungunya was or were more prevalent than Zika. However, these results reinforce the hypothesis of a higher Zika incidence not necessarily translating into higher microcephaly incidence.8,9 Microcephaly rates as high as those observed in Northeast Brazil were unprecedented and not observed anywhere else in the world where Zika has knowingly caused large epidemics. It seems that underlying factors may be acting to modify the risk of developing microcephaly given the infection during pregnancy.23,27 Even today, the reason why some regions of Northeast Brazil presented such high Zika-related microcephaly rates remains an open question.
The coldspots need to be interpreted with caution. It is possible that low-risk areas were identified as such due to under-reporting or because, in fact, the Zika burden was low in these locations. If the latter is true, the population of municipalities classified as Zika coldspots may be at risk of future outbreaks. Seroprevalence studies can explore this issue. The healthcare and epidemiological surveillance coverage in Pernambuco, especially of reference services, decreases the further one moves to the interior of the state (from the coast to the Sertão region), with the exception of the municipality of Petrolina (São Francisco region), a regional development hub. These ‘assistance gaps’ directly impact the coverage and quality of information systems, including SINASC and SINAN, responsible for capturing and reporting live births and compulsory notification diseases, respectively.
The two high-risk microcephaly clusters were identified in the northeast and in the Sertão region. A recent study estimated the spatiotemporal distribution of microcephaly in Pernambuco using a conditional autoregressive model and found high microcephaly incidences also in the middle portion of the state.28 In addition to having used a different methodology, the authors used data from a different source, the Public Health Event Registry (Registro de Evento de Saúde Pública [RESP]) system, explaining the differences in the results. The case definition for notification in the RESP system is different from that of SINASC, and has undergone several changes over time. The RESP system was implemented in November 2015 for the notification of cases of microcephaly and any other congenital anomalies.
Despite its important contributions, this study has some limitations. Due to the awareness surrounding the microcephaly epidemic, it is possible that microcephaly reporting was over-reported and that a proportion of cases were misdiagnosed. To counterbalance this, we used information from the Live Births System, SINASC, instead of the RESP system, as it is more robust and less prone to bias caused by disease awareness. SINASC data are collected only by authorized staff from medical facilities, at the moment of the birth of the child. Any person with an internet connection was able to report a case to RESP at any moment, which could lead to cases being reported more than once and other data quality issues. However, SINASC only captures the microcephaly cases diagnosed at birth, while some cases may be diagnosed later in life. As already mentioned, there is under-reporting and misclassification of Aedes-borne diseases cases. To address the latter, we combined the three diseases—dengue, Zika and chikungunya—in the analysis. Different levels of under-reporting across the municipalities were expected, both for Aedes-borne diseases (SINAN data) and microcephaly cases (SINASC data), and could bias our results. Finally, we did not consider covariates that might help identify spatial hotspots.
This analysis provides a much-needed classification of Zika burden in the state most affected by the Zika epidemic. In doing so, our results have the potential to be used by policymakers to target interventions in the identified hotspots to reduce the social, economic and societal impacts of the Zika epidemic.1 This study also provides a foundation for addressing the potential double jeopardy of two successive novel infectious disease outbreaks. Brazil is the country most affected by the Zika epidemic and has been an epicenter of the COVID-19 pandemic, with more than 22 million confirmed cases and more than 618 thousand deaths by 25 December 2021.29 The Brazilian population is therefore experiencing two successive outbreaks with reproductive health consequences. There is now evidence that pregnant women have higher chances of developing the severe form of COVID-19.30 Such double jeopardy will directly impact how Brazilian women experience reproductive health and childbearing in future cohorts.
Our study provides a foundation for research investigating the social and environmental factors associated with Zika. Merging data on socioeconomic characteristics, health surveillance infrastructure and environmental conditions at the municipality level to our classification scheme represents an important next step in addressing this question. Our study also provides an important basis for analyses that identify high-risk areas for illnesses that are historically under-reported, a common issue, particularly in low- and middle-income countries. The applied methodology has the potential to be adapted to instances in which a novel disease emerges and where under-reporting is also expected. As an example, excess deaths, influenza-like illness and hospitalizations due to severe acute respiratory illness, could be used to identify and classify high-risk areas for COVID-19.
Conclusions
The identification of areas most affected by the Zika epidemic has important research and policy implications. By combining Zika with other arboviruses and microcephaly, our approach offers a broader and potentially more reliable classification scheme for identifying Zika hotspots: information that can be used to inform public health research and policy. Importantly, our analysis identifies areas that might be particularly vulnerable to under-reporting, as suggested by the clusters that had a high microcephaly risk but a low Aedes-borne diseases risk.
Supplementary Material
Acknowledgements
We would like to thank the current and former members of the Decode Zika project: Ana Paula Portella, Irene Rossetto, Karlos Ramos, Kristine Hopkins, Luiz Gustavo Fernandes Sereno and Ryan Lloyd.
Contributor Information
Laís Picinini Freitas, Population Research Center, University of Texas at Austin, Austin, Texas 78712-1699, USA.
Rachel Lowe, Department of Earth Sciences, Barcelona Supercomputing Center (BSC), Barcelona 08034, Spain; Catalan Institution for Research and Advanced Studies (ICREA), Barcelona, Spain; Centre on Climate Change & Planetary Health and Centre for Mathematical Modelling of Infectious Diseases, London School of Hygiene & Tropical Medicine, London WC1E 7HT, UK.
Andrew E Koepp, Population Research Center, University of Texas at Austin, Austin, Texas 78712-1699, USA; Department of Human Development and Family Sciences, University of Texas at Austin, Austin, Texas 78712, USA.
Sandra Valongueiro Alves, Post-graduation Program of Public Health, Centro de Ciências Médicas, Universidade Federal de Pernambuco, Recife, Pernambuco 50670-901, Brazil.
Molly Dondero, Department of Sociology, American University, Washington, D.C. 20016-8072, USA.
Letícia J Marteleto, Population Research Center, University of Texas at Austin, Austin, Texas 78712-1699, USA; Department of Sociology, University of Texas at Austin, Austin, Texas 78712, USA.
Author's contributions
LPF, RL and LJM conceived the study; LPF carried out data collection, organization and analysis; LPF, RL and LJM interpreted the data and results. LPF and AEK drafted the manuscript; RL, SVA, MD and LJM revised the draft and contributed to the writing. All authors read and approved the final version of the manuscript.
Funding
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (https://www.nichd.nih.gov/): grant R01HD091257 awarded to LJM (PI: LJM, co-investigator: MD), and grants P2CH042849 and T32HD007081 awarded to the Population Research Center at the University of Texas at Austin. RL was supported by a Royal Society Dorothy Hodgkin Fellowship (https://royalsociety.org/grants-schemes-awards/grants/dorothy-hodgkin-fellowship/). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests
None declared.
Ethical approval
This study was conducted under Institutional Review Board approval #2018–01-0055 from the University of Texas at Austin and the Brazilian National Commission for Research Ethics (Comissão Nacional de Ética em Pesquisa [CONEP]), study approval CAAE: 34032920.1.0000.5149.
Data availability
The data underlying this article and the script of the analysis are available at https://github.com/laispfreitas/PE_satscan.
References
- 1. Lowe R, Barcellos C, Brasil Pet al. The Zika virus epidemic in Brazil: from discovery to future implications. Int J Environ Res Public Health. 2018;15(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Oliveira WK, de França GVA, Carmo EHet al. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet North Am Ed. 2017;390(10097):861–70. [DOI] [PubMed] [Google Scholar]
- 3. Kindhauser MK, Allen T, Frank Vet al. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ. 2016;94(9):675–686C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costa LC, Veiga RV, Oliveira JFet al. New insights on the Zika virus arrival in the americas and spatiotemporal reconstruction of the epidemic dynamics in Brazil. Viruses. 2020;13(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faria NR, Quick J, Claro IMet al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature. 2017;546(7658):406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Magalhaes T, Braga C, Cordeiro MTet al. Zika virus displacement by a chikungunya outbreak in Recife, Brazil. PLoS NeglTrop Dis. 2017;11(11):e0006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brady OJ, Osgood-Zimmerman A, Kassebaum NJet al. The association between Zika virus infection and microcephaly in Brazil 2015–2017: an observational analysis of over 4 million births. PLoS Med. 2019;16(3):e1002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodrigues LC, Paixao ES.. Risk of Zika-related microcephaly: stable or variable? Lancet North Am Ed. 2017;390(10097):824–6. [DOI] [PubMed] [Google Scholar]
- 9. Costa F, Ko AI.. Zika virus and microcephaly: Where do we go from here? Lancet Infect Dis. 2018;18(3):236–7. [DOI] [PubMed] [Google Scholar]
- 10. Kraemer MUG, Reiner RC, Brady OJet al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nature Microbiology. 2019;4(5):854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paixão ES, Teixeira MG, Costa M da CNet al. Symptomatic dengue during pregnancy and congenital neurologic malformations. Emerg Infect Dis. 2018;24(9):1748–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Brito CAA, de Brito CCM, Oliveira ACet al. Zika in Pernambuco: rewriting the first outbreak. Rev Soc Bras Med Trop. 2016;49(5):553–8. [DOI] [PubMed] [Google Scholar]
- 13. Ministério da Saúde do Brasil . Monitoramento integrado de alterações no crescimento e desenvolvimento relacionadas à infecção pelo vírus Zika e outras etiologias infecciosas, até a Semana Epidemiológica 52 de 2017. Boletim Epidemiológico. 2018;49(6). Available from: https://www.saude.gov.br/images/pdf/2018/fevereiro/20/2018-003-Final.pdf [accessed 3 August 2020]. [Google Scholar]
- 14. IBGE . Panorama - IBGE - Cidades - Pernambuco. Instituto Brasileiro de Geografia e Estatística. Available from: https://cidades.ibge.gov.br/brasil/pe/panorama [accessed 3 August 2020]. [Google Scholar]
- 15. IBGE . Panorama - IBGE - Cidades - Recife. Instituto Brasileiro de Geografia e Estatística. Available from: https://cidades.ibge.gov.br/brasil/pe/recife/panorama [accessed 3 August 2020]. [Google Scholar]
- 16. Freire FHM de A, Gonzaga MR, Gomes MMF.. Projeções populacionais por sexo e idade para pequenas áreas no Brasil. Revista Latinoamericana de Población. 2019;14(26):124–49. [Google Scholar]
- 17. Wickham H, Averick M, Bryan Jet al. Welcome to the Tidyverse. J Open Source Software. 2019;4(43):1686. [Google Scholar]
- 18. Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; 2016. Available from: https://ggplot2.tidyverse.org/ [accessed 18 April 2020]. [Google Scholar]
- 19. The R Foundation for Statistical Computing . R. The R Foundation; 2020. Available from: https://www.r-project.org/ [accessed 18 April 2020]. [Google Scholar]
- 20. Kulldorff M. A spatial scan statistic. Commun Stat Theory Methods. 1997;26(6):1481–96. [Google Scholar]
- 21. Kleinman K. rsatscan: tools, classes, and methods for interfacing with satscan stand-alone software. 2015. Available from: https://CRAN.R-project.org/package=rsatscan [accessed 19 February 2022].
- 22. Freitas LP. laispfreitas/PE_satscan [Internet]. Zenodo; 2022. Available from: https://zenodo.org/record/4610624 [accessed 19 February 2022]. [Google Scholar]
- 23. de Souza W V, de Albuquerque M de FPM, Vazquez Eet al. Microcephaly epidemic related to the Zika virus and living conditions in Recife, Northeast Brazil. BMC Public Health. 2018;18:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alves SV. Maternal mortality in Pernambuco, Brazil: What has changed in ten years? Reprod Health Matters. 2007;15(30):134–44. [DOI] [PubMed] [Google Scholar]
- 25. Murakami GF, Guimarães MJB, Sarinho SW.. Desigualdades sociodemográficas e causas de morte em menores de cinco anos no Estado de Pernambuco. Rev Bras Saude Mater Infant. 2011;11(2):139–52. [Google Scholar]
- 26. de Araújo TVB, Ximenes RA de A, Miranda-Filho D de Bet al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case-control study. Lancet Infect Dis. 2018;18(3):328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carvalho MS, Freitas LP, Cruz OGet al. Association of past dengue fever epidemics with the risk of Zika microcephaly at the population level in Brazil. Sci Rep. 2020;10:1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alexander N D E, Souza WV, Rodrigues LCet al. Spatiotemporal analysis of the population risk of congenital microcephaly in Pernambuco State, Brazil. Int J Environ Res Public Health. 2020;17(3):700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. WHO . WHO Coronavirus Disease (COVID-19) Dashboard; 2021. Available from: https://covid19.who.int/table [accessed 30 August 2021].
- 30. CDC . CDC updates, expands list of people at risk of severe COVID-19 illness. Centers for Disease Control and Prevention;2020. Available from: https://www.cdc.gov/media/releases/2020/p0625-update-expands-covid-19.html [accessed 8 March 2021]. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article and the script of the analysis are available at https://github.com/laispfreitas/PE_satscan.