Abstract
Background
Sleep medications may contribute to dementia development or indicate sleep disturbances that are markers of or contributors to neurologic disease. The objective of this study was to examine the use of sleep medications and incident dementia in a community-based cohort of older adults. We hypothesize late-life sleep medication use is associated with a greater risk of dementia.
Methods
The Atherosclerosis Risk in Communities (ARIC) study is an ongoing community-based cohort study. ARIC participants taking barbiturates, benzodiazepines, antidepressants, non-benzodiazepine receptor agonists (Z-drugs), or other hypnotics in 2011–2013 were categorized as sleep medication users. Participants were followed through 2019 for incident dementia. Logistic regression propensity scores were used to match sleep medication users with nonusers (1:2). Cox proportional hazards regression models were used to estimate hazard ratios (HR) for time to dementia diagnosis with adjustment for demographics, lifestyle characteristics, and cardiovascular risk factors.
Results
One-quarter of the eligible ARIC participants used sleep medications. In the matched sample (N = 4 197; 69% female; mean age 75.3 + 5.0 years), 632 dementia cases were ascertained over a median follow-up of 6.5 years. In the fully adjusted model, sleep medication use compared to nonuse was associated with a 48% greater risk of dementia (HR: 1.48; 95% confidence interval (CI): 1.26–1.74).
Conclusion
To expand on these findings, studies with longer follow-up and earlier assessment of sleep medication use are needed. Furthermore investigation of the potential dose-response association of multiple sleep medications and the potential causal role of sleep medications in the development of dementia may be clinically meaningful.
Keywords: Cognition, Insomnia, Sleep disturbances
Background
The prevalence of dementia, estimated to reach 13.8 million by 2060, places a large burden on the aging population, their families, and the economy in the United States (US) (1,2). For adults aged 65 and older, the lifetime risk of Alzheimer’s dementia is over 20% for women and 10% for men (3). Poor sleep has been linked to dementia risk (4,5), although findings are inconsistent (6). To date, evidence suggests sleep disturbances are a risk factor for dementia, a symptom in the long natural history of dementia, or potentially both.
Approximately 50% of older adults report regularly experiencing sleep problems including poor sleep quality, insomnia (difficulty initiating and/or maintaining sleep), and frequently disrupted sleep (7–9). Patients with dementia commonly report sleep disturbances and insomnia (10,11). Many older adults seek treatment for their sleep problems, including pharmacological options. Sleep quality and quantity typically decline throughout the life-course (12), and concurrently sleep medication use increases in late-life (13). An estimated 9% of U.S. adults aged 65 and older are taking sedative/hypnotic drugs to treat poor sleep (14).
There are several classes of medications, with varying indications, used to treat sleep disorders and sleep disturbances (15). Medications approved by the U.S. Food and Drug Administration for the treatment of sleep disorders and sleep disturbances include GABAA receptor hypnotics that are either benzodiazepines (BZDs) or non-benzodiazepine sedatives (Z-drugs), orexin receptor antagonists, melatonin receptor agonists, and antidepressants. Of these, research has focused on the potential harmful effects of BZDs and hypnotics (not including antidepressants or off-label drugs), linking their use to a higher risk of cancer, incident stroke, and mortality (16,17).
Recently, sleep medication use has been examined in relation to dementia development. In a 2018 meta-analysis, BZDs users were at a 1.38 greater odds of developing dementia (1.07–1.77) compared to nonusers (18). However, this analysis was limited by variation across studies in exposure classification (ever use of BZDs vs nonusers), dementia classification (Diagnostic and Statistical Manual of Mental Disorders vs International Classification of Diseases), and the assessment of relevant confounders (18). Other cohort studies have found no evidence of an association between BZDs, Z-drugs, and incident dementia (19). The existing literature focuses largely on BZDs, and questions remain about the potential risks associated with sleep medication use overall and the possibility of confounding due to reverse causality given the long natural history of dementia (18,20–23). Better clarifying this association is important as sleep medication use may independently contribute to neurologic disease, or could be an indicator of sleep disturbances that are markers of and/or contributors to risk of neurologic degeneration.
With thoroughly-collected medication data and expert dementia adjudication, the Atherosclerosis Risk in Communities (ARIC) study cohort provides a unique opportunity to examine late-life sleep medication use and associations with incident dementia in a biracial community-based sample of older adults. In this study, we examine if late-life sleep medication use (assessed in 2011–2013) was associated with incident dementia over ~7 years of follow-up. Based on documented differences in prevalence of dementia and sleep medication use by sex and age, we examined if associations varied across participant subgroups. We hypothesized that late-life sleep medication use would be associated with greater dementia risk and that this association would not vary by participant sex and age.
Method
Study Design
Between 1987 and 1989, 15 792 adults (45–64 years) in 4 U.S. communities (Washington County, MD; Forsyth County, NC; Jackson, MS; and Minneapolis, MN) were enrolled in the ARIC study. Since baseline, up to 7 follow-up clinic visits have occurred. Participants are also contacted by phone (annually before 2012; semiannually since), with continuous surveillance for hospitalizations.
The initial analytic sample included 6 538 participants who attended ARIC Visit 5 (2011–2013). Participants were excluded if they were missing information on covariates (n = 327) or did not attend subsequent visits after Visit 5 (n = 373). Due to limited sample size, we excluded participants of race other than Black or White, and Black participants in Minnesota and Washington County cohorts (n = 42). Additionally, we excluded participants with baseline prevalent dementia (n = 357). From the initial Visit 5 sample, 5 439 participants were eligible for matching (see Statistical Analysis section). After matching, 4 197 (77.2%) participants were included in the final analytic sample (Supplementary Figure 1).
Participating center Institutional Review Boards approved study protocols and all participants provided written informed consent to participate in the study.
Sleep Medication Use
The primary exposure for this analysis was any sleep medication use at ARIC Visit 5. At each ARIC visit, participants were asked to bring all medications they had taken in the past 2 weeks. Medications were verified and recorded by ARIC staff. Based on the literature (15,16,18), the following medication classes were included in the composite sleep medication variable (Supplementary Table 1): Barbiturates, BZD derivatives (eg, Triazolam, Estazolam, Flurazepam, and Quazepam), antidepressants (eg, Doxepin and Trazodone; categorized as selective serotonin reuptake inhibitors [SSRIs] and non-SSRIs), other hypnotics, and non-benzodiazepine sedatives (Z-drugs). Participants taken any of these medications at baseline were categorized as sleep medication users.
Outcome Ascertainment
Participants were followed for incident dementia from their Visit 5 (2011–2013) exam through November 15, 2019. ARIC dementia classification has been detailed previously (24). Briefly, dementia was ascertained in 3 ways: (a) For participants attending in-person follow-up exams, dementia was adjudicated by an expert panel of neurologists and neuropsychologists based on cognitive decline determined from prior cognitive assessments, neuropsychological test battery at Visit 5, and informant interviews; (b) all participants were called semiannually, and the Six Item Screener (SIS) was administered (25), followed by an AD8 informant interview (26) when the SIS indicated potential cognitive impairment; and (c) additional dementia cases were identified from international classification of diseases (ICD-9) hospital discharge or death certificate diagnostic codes.
The panel’s determination of participant cognitive status at Visit 5 was categorized as normal, mild cognitive impairment (MCI), or dementia.
Covariates
Relevant covariates were obtained from ARIC Visit 5 questionnaires and physiologic assessments, except for demographic information obtained at Visit 1 and assumed static. Covariates in this analysis included: age, sex, race, study center, educational attainment, apolipoprotein E (APOE) ε4 genotype, smoking status, alcohol consumption, depressive symptoms, body mass index (BMI), systolic blood pressure, antihypertensive medication use, prevalent diabetes, and prevalent coronary heart disease (CHD). Standard with ARIC analyses, a 5-level race-study center variable (White participants from Minneapolis, MN; White participants from Washington County, MD; Black participants from Jackson, MS; Black participants from Forsyth County, NC; White participants from Forsyth County, NC) was created and used in analyses.
Interview-administered questionnaires were used to assess smoking status, alcohol consumption and presence of depressive symptoms. Participants were asked if they currently smoke cigarettes or drink alcohol and categorized as current, former, or never. The Center for Epidemiologic Studies Depression (CES-D) is a validated measure of frequency of depressive symptoms in the past week, with higher scores indicating greater depressive symptoms (27). The validated CES-D 11-item version was used in ARIC because it is believed to be better for older adults and correlates highly with the original full CES-D scale (28). The 11-item version responses are scored as 0 (<1 day in the past week), 1 (1–2 days in the past week), or 2 (3–7 days in the past week) for each question, with the scores summed for a total scale score ranging from 0 to 22 (28). Consistent with previous ARIC analyses (29), a cutoff score ≥9 was used to represent the presence of clinically significant depressive symptoms (30). For the full CES-D scale an analogous cutoff has been shown to have high sensitivity and specificity (as high as 100% and 88%, respectively) for detecting major depression among older adults (31). Educational attainment (Visit 1) was categorized as basic to high school graduate or some college or more.
Trained staff collected height, weight, blood pressure, and fasting blood measures. APOE ε4 genotyping was performed using the TaqMan assay (Applied Biosystems, Foster City, CA) (32). Participants with APOE ε4 (≥1 allele vs 0 alleles) were coded as 1. BMI was calculated as weight (kilogram) divided by height (meter) squared. Systolic blood pressure was measured 3 times, and the final 2 measurements were averaged. Participants taking hypertensive medications were coded as 1 for antihypertensive medication use (0: no antihypertensive medications). Prevalent diabetes was defined as fasting blood glucose concentration ≥126 mg/dL, nonfasting glucose concentration ≥200 mg/dL, self-reported physician-diagnosis, or pharmacological treatment for diabetes. Prevalent CHD was based on prior cardiovascular revascularization, presence of a previous myocardial infarction at Visit 1, or incident CHD prior to Visit 5. Events prior to Visit 1 were self-reported and incident events between Visit 1 and Visit 5 were adjudicated by ARIC physicians (33).
Propensity Score Matching
Logistic regression propensity score matching was used to match each sleep medication user with 2 medication nonusers by age, sex, race, and education level. Using the R MatchIT package, age, sex, race, and education were included in a regression model to calculate the probability of exposure (sleep medication use). This score was used to match sleep medication users with nonusers (1:2) using the nearest neighbor matching without replacement. After matching, covariate balance was assessed with a standardized mean difference <10% indicating acceptable balance.
Statistical Analysis
Follow-up time was defined as time from the participants’ Visit 5 in-clinic exam date until the occurrence of incident dementia, death, loss to follow-up, or November 15, 2019, whichever occurred first. We used the Kaplan Meier method to visualize estimated years free of incident dementia by late-life sleep medication use and conducted log-rank tests to examine differences between sleep medication users and nonusers.
Using the matched sample, Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) relating late-life sleep medication use with incident dementia. We performed a series of progressive adjustments. Model 1 was unadjusted. Model 2 adjusted for age, sex, race-study center, education, and APOE ε4. Model 3 (main model) further adjusted for smoking status, alcohol consumption, depressive symptoms, and BMI. Model 4 added adjustment for prevalent cardiovascular risk factors including systolic blood pressure, antihypertensive medication, prevalent diabetes, and prevalent CHD.
The proportional hazards assumption was confirmed by testing interactions between sleep medication use and the natural log of follow-up time, and by visual inspection of the survival function versus survival time.
In sensitivity analyses, we excluded (n = 35) participants with incident dementia within the first year (365 days) of the follow-up period. To explore possible effect modification of the association between sleep medication use and dementia by sex, age, education, depressive symptoms, or APOE ε4 we included cross-product interaction terms (eg, sleep medication use × sex) in Model 3. We present stratified results across categories and by median age (75 years). To explore if the association differed by baseline cognitive status, we stratified the sample by Visit 5 cognitive status (normal cognition or MCI). To explore the dose-response nature of the association, we categorized sleep medication users by number of medications used at baseline (0, 1, or ≥2). To test our matching, we repeated the propensity score matching procedure, matching on all covariates, and ran hazard regression models with this sample. Finally, to explore the association by sleep medication class, we ran models with the individual sleep medication classes (with sufficient sample sizes).
All analyses were performed using R version 3.5.1.
Results
From the initial sample, 5 439 participants were eligible for matching. After matching, 4 197 participants were included in the final analytic sample. Matched sample characteristics are presented in Table 1 by late-life sleep medication use and in Supplementary Table 2 compared to the prematched sample. Approximately 69% of the matched sample was female and on average 75.3 (standard deviation [SD]: 5.0) years old.
Table 1.
Matched Sample Participant Characteristics by Late-life Sleep Medication Use, ARIC Study 2011–2013 (N = 4 197)*
| Matched Nonusers (n = 2 798) | Sleep Medication Users (n = 1 399) | Standardized Mean Difference | |
|---|---|---|---|
| Demographics and genetics | |||
| Age, years | 75.3 ± 5.0 | 75.2 ± 5.0 | 0.006 |
| Age ≥75 years, n (%) | 1 403 (50.1) | 693 (49.5) | |
| Female sex, n (%) | 1 972 (70.5) | 990 (70.8) | 0.006 |
| White, n (%) | 2 325 (83.1) | 1 190 (85.1) | 0.05 |
| Education, n (%) | 0.002 | ||
| Basic to high school graduate | 1 579 (56.4) | 811 (58.0) | |
| Some college or more | 1 219 (43.6) | 588 (42.0) | |
| APOE ε4, n (%) | 734 (26.2) | 349 (24.9) | |
| Lifestyle + health status characteristics | |||
| Current smoker, n (%) | 160 (5.7) | 99 (7.1) | |
| Current alcohol consumption, n (%) | 1 432 (51.2) | 670 (47.9) | |
| High depressive symptoms (CES-D ≥ 9), n (%) | 131 (4.7) | 157 (11.2) | |
| BMI, kg/m2 | 28.6 ± 5.7 | 28.9 ± 5.9 | |
| Cardiovascular risk factors | |||
| Systolic blood pressure, mmHg | 130 ± 18.1 | 129 ± 18.0 | |
| Antihypertensive medication use, n (%) | 1 990 (71.1) | 1 084 (77.5) | |
| Prevalent diabetes, n (%) | 715 (25.6) | 415 (29.7) | |
| Prevalent CHD, n (%) | 370 (13.2) | 230 (16.4) | |
| Sleep medications | |||
| Barbiturates, n (%) | 17 (1.2) | ||
| Benzodiazepine derivatives, n (%) | 448 (32.0) | ||
| Non-SSRI antidepressants, n (%) | 367 (26.2) | ||
| SSRI antidepressants, n (%) | 505 (36.1) | ||
| Hypnotics, n (%) | 242 (17.3) | ||
| Z-drugs, n (%) | 149 (10.7) |
Note: ARIC = Atherosclerosis Risk in Communities; BMI = body mass index; CES-D = Center for Epidemiologic Study Depression Scale; CHD = coronary heart disease; SD = standard deviation; SSRI = selective serotonin reuptake inhibitor.
*Data presented as mean ± SD unless otherwise specified.
Relative to matched nonusers, sleep medication users were more likely to be current smokers, less likely to consume alcohol, and more likely to have high depressive symptoms. Sleep medication users were more likely to use antihypertensive medications and have prevalent diabetes and CHD.
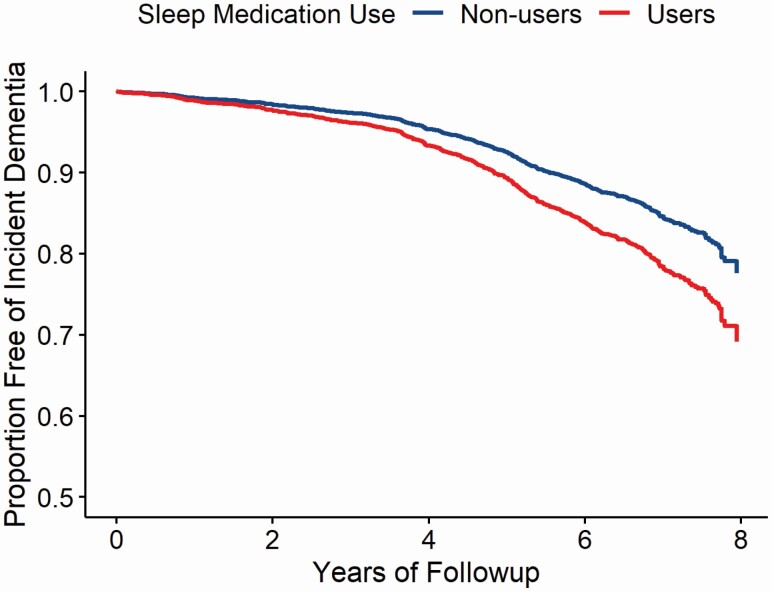
Over a median follow-up of 6.5 years (Interquartile range: 1.28) 632 incident dementia cases were identified. Figure 1 displays the estimated probability of years free of incident dementia by late-life sleep medication use. The risk of dementia significantly differed between sleep medication users versus nonusers (p < .001).
Figure 1.
Estimated probability of years free of incident dementia by late-life sleep medication use in the Atherosclerosis Risk in Communities (ARIC) study. Adjusted for age, sex, race-center, education, APOE ε4, smoking status, alcohol consumption, depressive symptoms, and BMI. BMI = body mass index.
Table 2 presents the Cox proportional hazard regression analysis results. In the unadjusted model, late-life sleep medication use was associated with a 53% higher risk of dementia (HR: 1.53; 95% CI: 1.31, 1.79) compared to nonuse. After adjustment for demographics and genetics (Model 2), sleep medication users were at a 59% greater risk of dementia (HR: 1.59; 95% CI: 1.36, 1.86) than nonusers. After further adjustment for lifestyle characteristics and depressive symptoms (Model 3), this association persisted (HR: 1.50; 95% CI: 1.27, 1.76). The association between sleep medication use and incident dementia further persisted after additional adjustment for prevalent cardiovascular risk factors (Model 4), including systolic blood pressure, antihypertensive medication use, prevalent diabetes, and prevalent CHD (HR: 1.48; 95% CI: 1.26, 1.74). When individuals with incident dementia within 1 year of baseline were excluded (n = 35), results remained mostly unchanged (eg, Model 3 HR: 1.45; 95% CI: 1.23, 1.71; Supplementary Table 3).
Table 2.
Late-life Sleep Medication Use and Incident Dementia: ARIC Study (N = 4 197)*
| Matched Nonusers (n = 2 798) | Sleep Medication Users (n = 1 399) | |
|---|---|---|
| Incident dementia, n | 367 | 265 |
| Cumulative incidence | 13.1% | 18.9% |
| Incidence rate, per 100 person-years | 2.11 | 3.17 |
| Model† | Hazard ratio (95% confidence intervals) | |
| Model 1 | (reference) | 1.53 (1.31, 1.79) |
| Model 2 | (reference) | 1.59 (1.36, 1.86) |
| Model 3 | (reference) | 1.50 (1.27, 1.76) |
| Model 4 | (reference) | 1.48 (1.26, 1.74) |
Note: Bold text represents statistically significant results. ARIC = Atherosclerosis Risk in Communities; BMI = body mass index; CHD = coronary heart disease.
*Matched sample 2:1.
†Model adjustments―Model 1: crude unadjusted; Model 2: adjusted for age, sex, race-center, education, and APOE ε4; Model 3: Model 2 + adjustment for smoking status, alcohol consumption, depressive symptoms, and BMI; Model 4: Model 3 + adjustment for systolic blood pressure, antihypertensive medications, prevalent diabetes, and prevalent CHD.
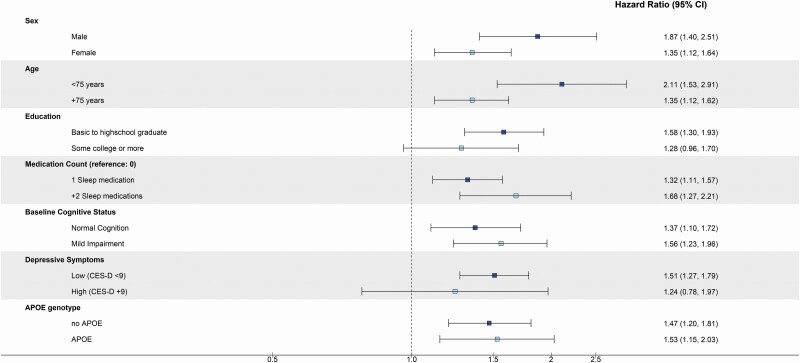
When sleep medication users were stratified by number of medications used, there was some evidence of dose-response, whereby the risk of dementia was even greater for participants taking 2 or more medications as compared to nonusers (Figure 2; HRsleep medication use vs nonuse: 1.68; 95% CI: 1.27, 2.21) and directly compared to those taking 1 medication, (data not shown: HR+2 sleep medications vs 1 sleep medication: 1.24; 95% CI: 0.93, 1.65) though this difference was not statistically significant.
Figure 2.
Adjusted hazard ratios (95% confidence intervals) of incident dementia among late-life sleep medication users, stratified by subgroups, ARIC study (N = 4 197). Sex (n): male (1 235), female (2 962); Age (n): <75 years (2 101), +75 years (2 096); Education: basic to high school (2 390), some college (1 807); Medication count (n): 1 sleep medication (1 107), +2 sleep medications (292); Baseline cognitive status (n): normal cognitive status (3 317), mild cognitive impairment (877); Depressive symptoms (n): CES-D < 9 (3 909), CES-D ≥ 9 (288); APOE ε4 (n): no APOE ε4 (2 940), APOE ε4 (1 083); Interaction p values: sex: p = .16, age: p = .20; Education (basic): p = .09, education (some college): p = .76; Baseline cognitive status: p = .56; Depressive symptoms p = .74; APOE ε4 p = .84. Models adjusted for the following except for the variable stratified on (besides variable stratified on): age, sex, race-center, education, APOE ε4, smoking status, alcohol consumption, depressive symptoms, and BMI. ARIC = Atherosclerosis Risk in Communities; BMI = body mass index; CES-D = Center for Epidemiologic Studies Depression.
While interaction terms were not statistically significant, the association between sleep medication use and incident dementia appeared to differ somewhat by sex, age, depressive symptoms, and education in stratified analyses (Figure 2). The sleep medication use and dementia association appeared to be stronger among males (HRsleep medication use vs nonuse: 1.87; 95% CI: 1.40, 2.51) compared to females (HRsleep medication use vs nonuse: 1.35; 95% CI: 1.12, 1.64) and among younger participants (HRsleep medication use vs nonuse: 2.11; 95% CI: 1.53, 2.91) as compared to older participants (HRsleep medication use vs nonuse: 1.35; 95% CI: 1.12, 1.62). Additionally, the association was more pronounced for participants with a basic to high school education (HRsleep medication use vs nonuse: 1.58; 95% CI: 1.30, 1.93) as compared to participants with some college education (HRsleep medication use vs nonuse: 1.28; 95% CI: 0.96, 1.70). The association did not differ markedly by baseline cognitive status (normal cognition: HRsleep medication use vs nonuse: 1.37; 95% CI: 1.10, 1.72; vs MCI: HRsleep medication use vs nonuse: 1.56; 95% CI: 1.23, 1.96).
Importantly, the association appeared to be stronger among participants with low depressive symptoms (HRsleep medication use vs nonuse: 1.51; 95% CI: 1.27, 1.79) as compared to participants categorized as having higher depressive symptoms (HRsleep medication use vs nonuse: 1.24; 95% CI: 0.78, 1.97) and the association only differed minimally by APOE ε4 status (no APOE: HRsleep medication use vs nonuse: 1.47; 95% CI: 1.20, 1.81; vs APOE ε4: HRsleep medication use vs nonuse: 1.53; 95% CI: 1.15, 2.03).
In models exploring sleep medication class, BZDs, non-SSRI, and SSRI antidepressants were associated with incident dementia in the crude models, but only the association for non-SSRI and SSRI antidepressants remained after adjustment (model 3: HRnon-SSRI antidepressant users vs no sleep mediation use: 1.45; 95% CI: 1.13, 1.86; HRSSRI antidepressant users vs no sleep mediation use: 1.85; 95% CI: 1.51, 2.26; Table 3). When sleep medication users were matched with nonusers on all covariates, results remained consistent (Supplementary Table 4).
Table 3.
Late-life Sleep Medication Use and Incident Dementia, by Sleep Medication Type (N = 4 197)*
| Matched Nonusers (n = 2 798) | Sleep Medication Users (n = 1 399) | |
|---|---|---|
| Benzodiazepine derivatives (BZDs) | ||
| n (%) | 0 (0) | 448 (32.0) |
| Hazard ratio (95% confidence intervals) | ||
| Crude model | (reference) | 1.34 (1.06, 1.68) |
| Adjusted model† | (reference) | 1.17 (0.93, 1.48) |
| Non-SSRI antidepressants | ||
| n (%) | 0 (0) | 367 (26.2) |
| Hazard ratio (95% confidence intervals) | ||
| Crude model | (reference) | 1.39 (1.09, 1.78) |
| Adjusted model† | (reference) | 1.45 (1.13, 1.86) |
| SSRI antidepressants | ||
| n (%) | 0 (0) | 505 (36.1) |
| Hazard ratio (95% confidence intervals) | ||
| Crude model | (reference) | 1.83 (1.50, 2.24) |
| Adjusted model† | (reference) | 1.85 (1.51, 2.26) |
| Hypnotics | ||
| n (%) | 0 (0) | 242 (17.3) |
| Hazard ratio (95% confidence intervals) | ||
| Crude model | (reference) | 1.08 (0.78, 1.50) |
| Adjusted model† | (reference) | 1.06 (0.77, 1.47) |
| Z-drugs | ||
| n (%) | 0 (0) | 149 (10.7) |
| Hazard ratio (95% confidence intervals) | ||
| Crude model | (reference) | 0.94 (0.61, 1.46) |
| Adjusted model† | (reference) | 1.18 (0.76, 1.83) |
Note: Bold text represents statistically significant results. BMI = body mass index; SSRI = selective serotonin reuptake inhibitor.
*Matched sample 2:1.
†Adjusted model: adjusted for age, sex, race-center, education, APOE ε4, smoking status, alcohol consumption, depressive symptoms, and BMI.
Discussion
Dementia and sleep medication use are both highly prevalent among U.S. older adults (14,34). Previous studies have focused on specific classes of sleep medications, including BZDs and Z-drugs, and risk of dementia (18,20–23). However, older adults are using more than just BZDs and Z-drugs to treat their sleep disorders or sleep disturbances, and many adults are using more than 1 type of medication for different indications (13,15). For this study, we examined sleep medication use more broadly in a community-based cohort of older adults. In our eligible sample, 27.5% of participants were taking any medication that may be prescribed to treat disordered or disturbed sleep. In our matched sample, late-life sleep medication users were consistently at a higher risk for dementia than nonusers. If sleep medications are associated with an even moderate increase in dementia risk, more awareness to the increasing prevalence of sleep medication use among U.S. older adults is warranted.
Late-life Sleep Medication Use and Dementia
In this study, we aimed to examine the use of any medications commonly prescribed to treat insomnia, disordered sleep, or sleep disturbances. Among over 4 000 matched sleep medication users and nonusers we found a consistent association of late-life sleep medication use with incident dementia over 6.5 years of follow-up. Our results are consistent with results reported in a longitudinal study among a nationally representative sample of older adults by Robbins et al. in which routine self-reported sleep medication use was associated with incident dementia (HR: 1.30; 95% CI: 1.10, 1.53) (35). Our results build on this evidence, with a broader operationalization of sleep medication use in a cohort of older adults with rigorous dementia ascertainment.
The observed associations are consistent in both direction and magnitude with a number of meta-analyses and systematic reviews that found an association between BZD and Z-drug use and incident dementia (18,22,23,36). Given the potential harmful effects of BZDs, several major medical and psychiatric organizations, including the American Geriatrics Society (37), advised against the prescription of BZDs, hypnotics, and Z-drugs for treatment of sleep disorders in older adults (38). Despite these recommendations, the prescription of BZDs continues, as does the prescription of other pharmacological sleep aids (37). In a 2020 review of BZD risks by Ettcheto et al., the authors suggest that as an alternate to BZDs, other sleep medications, such as melatonin agonists and SSRI antidepressants should be considered (23). When we explored medication class in sensitivity analyses, however, the association between sleep medication use and dementia appeared to be driven in part by the use of non-SSRI and SSRI antidepressants. While these results can be interpreted to suggest the association may be driven by the use of antidepressants for depression, the results of our analysis stratified by high depressive symptoms revealed the association appeared somewhat stronger among participants with low depressive symptoms. These findings on antidepressants are hypothesis-generating and warrant further investigation in studies with information on medication indication.
The association observed between late-life sleep medication use and dementia was particularly stronger in males, younger participants (<75 years), those with less years of education, those with low depressive symptoms, and those taking more than 1 class of sleep medication. This last result is consistent with findings by Tseng et al. in a cohort of older adults in the Taiwan National Health Insurance Research Database (20). Tseng et al. found participants taking two or more classes of BZDs or hypnotics were at a threefold greater odds (Odds Ratios: 2.82–3.28) for dementia compared to those using only 1 class of drug (20). Taken together with these results, our exploratory findings suggest more attention may be needed to the prescription and unintentional chronic use of multiple medications, with potentially varying indications, as these individuals may be at elevated dementia risk.
Controversy on Causality
The association between BZDs and other sedative/hypnotics and incident dementia has been controversial. This controversy is centered on the limited causal evidence linking the pharmacological agents to dementia development and the likelihood that sleep disorders, anxiety, and depression may all be symptoms co-occurring in the prolonged early stages of dementia, and therefore not causally associated with dementia (21,22). To address reverse causality, Penninkilampi et al. conducted a meta-analysis of observational studies accounting for study lag time, and among studies with a lag time ≥ 5 years reported a pooled HR of 1.30 for BZD use and risk of dementia (22). The authors concluded their meta-analysis provides convincing evidence that BZD use is associated with an increased risk of dementia, and the association is not due to protopathic bias (22). Given the prolonged period of pathophysiological changes prior to dementia onset (39), a lag time of 5 years, particularly in older adults, may not be adequate to rule out reverse causality. In restricted analyses, excluding participants with dementia within 1 year of baseline, our results remained unchanged. While our results do not provide evidence of a causal association, our findings suggest older adults taking sleep medications may need closer monitoring for incident cognitive impairment.
Sleep Medication Use and Participant Characteristics
In our sample, sleep medication users were more likely to have a number of comorbid conditions (eg, high depressive symptoms, diabetes mellitus, hypertension, and CHD). The higher prevalence of sleep medication use among individuals with poorer health suggests that sleep medication use may be an indicator of individuals struggling with sleep disturbances and a sequela of co-occurring chronic conditions, all of which may individually or collectively contribute to dementia risk. Of these conditions, sleep medication users were more likely to have high depressive symptom scores than nonusers. Depression is a possible risk factor for dementia and has bidirectional associations with sleep disturbances (40), therefore it is a likely confounder of the sleep medication and dementia association. Furthermore, antidepressants (eg, Doxepin, Trazodone, and Amitriptyline) are commonly prescribed to treat depressive symptoms and insomnia or poor sleep simultaneously. In our analyses, we attempted to disentangle to the role of depression as a potential confounder. In the preliminary analysis, the association between sleep medication and dementia persisted even after adjustment for high depressive symptoms. Furthermore, in stratified analyses, the association appeared to be stronger among those with low depressive symptoms compared to those with high depressive symptoms. We are not able to rule out residual confounding; however, our results suggest the association of sleep medication use and dementia is independent of depression. More research is needed to disentangle the relationship between depression, sleep medication use, and dementia in older adults.
Strengths and Limitations
This study has many strengths. For ARIC, participants brought medication bottles to study visits and trained staff recorded information directly from the medication bottles. Additionally, the study included expert adjudication of dementia cases by neurologists and neuropsychologists. We used propensity score matching and lag time analysis methods to address confounding and reverse causality. However, the study is not without limitations. While medication data were thoroughly collected, we are not able to discern medication indications (sleep disturbances vs another indication) and therefore indication bias may be possible. Additionally, we are not able to assess medication adherence over time. Furthermore, not all participants with sleep disturbances are taking sleep medications and are not captured in this analysis, nor are those with sleep disorders who do not receive pharmacological treatments (obstructive sleep apnea). We do not have a measure of sleep quality and are unable to adjust for sleep disturbances in our analyses. Finally, given the observational nature of this study, causal inference is limited.
Conclusion
Late-life sleep disturbances may be a risk factor for dementia, or they may also be a symptom in the natural history of dementia. Untangling the complex association between sleep medication use and incident dementia requires further investigation. However, given our consistent findings of an association between late-life sleep medication use and incident dementia, our study suggests greater awareness should be paid to the increasing prevalence of sleep medication use among older adults and the possible harmful consequences.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the Atherosclerosis Risk in Communities (ARIC) study for their important contributions.
Contributor Information
Kelsie M Full, Division of Epidemiology and Community Health, University of Minnesota, Minneapolis, Minnesota, USA.
Snigdha Pusalavidyasagar, Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, University of Minnesota, Minneapolis, Minnesota, USA.
Priya Palta, Division of General Medicine, Columbia University Irving Medical Center, New York, New York, USA.
Kevin J Sullivan, Department of Medicine, University of Mississippi Medical Center, Jackson, Mississippi, USA.
Jung-Im Shin, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Rebecca F Gottesman, Stroke Branch, National Institute of Neurological Disorders and Stroke Intramural Research Program, Bethesda, Maryland, USA.
Adam P Spira, Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine, Baltimore, Maryland, USA; Department of Psychiatry and Behavioral Sciences, Johns Hopkins Center on Aging and Health, Baltimore, Maryland, USA; Department of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Matthew P Pase, Turner Institute for Brain and Mental Health, Monash University, Clayton, Victoria, Australia.
Pamela L Lutsey, Division of Epidemiology and Community Health, University of Minnesota, Minneapolis, Minnesota, USA.
Funding
This work and the Atherosclerosis Risk in Communities Study was supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I). Neurocognitive data collection was supported by the National Heart, Lung, and Blood Institute, National Institute of Aging, National Institute of Neurological Disorders and Stroke, and The National Institute on Deafness and Other Communication Disorders (grant numbers 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917, and HL70825). K.M.F. was supported by an National Heart, Lung, and Blood Institute training grant (grant number T32 HL007779). P.P. is supported by the National Institutes of Health. A.P.S. is supported in part by the National Institute on Aging (grant numbers 1R01AG050507, 1RF1AG050745). R.F.G. was previously supported by the National Institute of Aging (grant numbers K24 AG052573, RF1AG050745). J.-I.S. is supported by the National Institute of Diabetes and Digestive and Kidney Disease (grant number 1K01DK121825). M.P.P. is supported by a National Heart Foundation of Australia Future Leader Fellowship (GTN102052) with sleep and dementia research funding from the National Health and Medical Research Council of Australia (GTN2009264; GTN1158384), National Institute on Aging (R01 AG062531-01A1), and Alzheimer’s Association (2018-AARG-591358). P.L.L. is supported in part by the National Institute on Aging (grant number R01AG062531) and the National Heart, Lung, and Blood Institute (grant number K24 HL159246).
Conflict of Interest
S.P. is a voluntary committee member for the American Board of Sleep Medicine and board member for Minnesota Sleep Society; K.S. has received NIH support for travel and conference attendance; J.-I.S. received support from a Merck grant; R.F.G. received NIH support for lectures at University of Alabama and University of Michigan; A.P.S. has received honoraria for serving as a consultant to Merck, from Springer Nature Switzerland AG, for guest editing special issues of Current Sleep Medicine Reports, and for presenting lectures at University of British Columbia, University of Pennsylvania, University of Toronto, and West Virginia University; M.P.P. serves as a member of the ADDF and Rebecca L. Cooper Medical Research Foundation boards; Additionally, this article was partially prepared while R.F.G. was employed at the Johns Hopkins University School of Medicine. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States Government.
Disclaimer
This article was partially prepared while R.F.G. was employed at the Johns Hopkins University School of Medicine. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States Government.
References
- 1. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. lzheimer’s Association A. 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021;17:327–406. doi: 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 3. Chêne G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimer’s Dement. 2015;11(3):310–320. doi: 10.1016/j.jalz.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen J-C, Espeland MA, Brunner RL, et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimer’s Dement. 2016;12(1):21–33. doi: 10.1016/J.JALZ.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luojus MK, Lehto SM, Tolmunen T, Brem A-K, Lönnroos E, Kauhanen J. Self-reported sleep disturbance and incidence of dementia in ageing men. J Epidemiol Community Health. 2017;71(4):329–335. doi: 10.1136/jech-2016-207764 [DOI] [PubMed] [Google Scholar]
- 6. Lutsey PL, Misialek JR, Mosley TH, et al. Sleep characteristics and risk of dementia and Alzheimer’s disease: the Atherosclerosis Risk in Communities Study. Alzheimer’s Dement. 2018;14(2):157–166. doi: 10.1016/J.JALZ.2017.06.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. LeBlanc M, Mérette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32(8):1027–1037. doi: 10.1093/sleep/32.8.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. doi: 10.1093/sleep/18.6.425 [DOI] [PubMed] [Google Scholar]
- 9. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186 [DOI] [PubMed] [Google Scholar]
- 10. Rothman SM, Mattson MP. Sleep disturbances in Alzheimer’s and Parkinson’s diseases. Neuromolecular Med. 2012;14(3):194–204. doi: 10.1007/s12017-012-8181-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med. 2005;6(4):347–352. doi: 10.1016/j.sleep.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 12. Crowley K. Sleep and sleep disorders in older adults. Neuropsychol Rev. 2011;21(1):41–53. doi: 10.1007/s11065-010-9154-6 [DOI] [PubMed] [Google Scholar]
- 13. Chong Y, Fryar CDH, Gu Q.. Prescription Sleep Aid Use Among Adults: United States, 2005–2010. NCHS data brief, no 127. Hyattsville, MD: National Center for Health Statistics. 2013. [Google Scholar]
- 14. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136–142. doi: 10.1001/jamapsychiatry.2014.1763 [DOI] [PubMed] [Google Scholar]
- 15. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340–2372. doi: 10.1016/j.clinthera.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 16. Sivertsen B, Madsen IEH, Salo P, Tell GS, Øverland S. Use of sleep medications and mortality: the Hordaland Health Study. Drugs―Real World Outcomes. 2015;2(2):123–128. doi: 10.1007/s40801-015-0023-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sivertsen B, Salo P, Pentti J, Kivimäki M, Vahtera J. Use of sleep medications and risk of cancer: a matched case–control study. Sleep Med. 2015;16(12):1552–1555. doi: 10.1016/j.sleep.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 18. Lucchetta RC, da Mata BPM, Mastroianni PdC. Association between development of dementia and use of benzodiazepines: a systematic review and meta-analysis. Pharmacother J Hum Pharmacol Drug Ther. 2018;38(10):1010–1020. doi: 10.1002/phar.2170 [DOI] [PubMed] [Google Scholar]
- 19. Osler M, Jørgensen MB. Associations of benzodiazepines, Z-drugs, and other anxiolytics with subsequent dementia in patients with affective disorders: a nationwide cohort and nested case-control study. Am J Psychiatry. 2020;177(6):497–505. doi: 10.1176/appi.ajp.2019.19030315 [DOI] [PubMed] [Google Scholar]
- 20. Tseng L-Y, Huang S-T, Peng L-N, Chen L-K, Hsiao F-Y. Benzodiazepines, z-hypnotics, and risk of dementia: special considerations of half-lives and concomitant use. Neurotherapeutics. 2019;17:1–9. doi: 10.1007/s13311-019-00801-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takada M, Fujimoto M, Hosomi K. Association between benzodiazepine use and dementia: data mining of different medical databases. Int J Med Sci. 2016;13(11):825–834. doi: 10.7150/ijms.16185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Penninkilampi R, Eslick GD. A systematic review and meta-analysis of the risk of dementia associated with benzodiazepine use, after controlling for protopathic bias. CNS Drugs. 2018;32(6):485–497. doi: 10.1007/s40263-018-0535-3 [DOI] [PubMed] [Google Scholar]
- 23. Ettcheto M, Olloquequi J, Sánchez-López E, et al. Benzodiazepines and related drugs as a risk factor in Alzheimer’s disease dementia. Front Aging Neurosci. 2020;11(January):344. doi: 10.3389/fnagi.2019.00344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the atherosclerosis risk in communities neurocognitive study (ARIC-NCS). Alzheimer’s Dement (Amsterdam, Netherlands). 2016;2:1–11. doi: 10.1016/j.dadm.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40(9):771–781. doi: 10.1097/00005650-200209000-00007 [DOI] [PubMed] [Google Scholar]
- 26. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. doi: 10.1212/01.wnl.0000172958.95282.2a [DOI] [PubMed] [Google Scholar]
- 27. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 28. Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health. 1993;5(2):179–193. doi: 10.1177/089826439300500202 [DOI] [PubMed] [Google Scholar]
- 29. Sonsin-Diaz N, Gottesman RF, Fracica E, et al. Chronic systemic inflammation is associated with symptoms of late-life depression: the ARIC study. Am J Geriatr Psychiatry. 2020;28(1):87–98. doi: 10.1016/j.jagp.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takeshita J, Masaki K, Ahmed I, et al. Are depressive symptoms a risk factor for mortality in elderly Japanese American men?: the Honolulu-Asia aging study. Am J Psychiatry. 2002;159(7):1127–1132. doi: 10.1176/appi.ajp.159.7.1127 [DOI] [PubMed] [Google Scholar]
- 31. Beekman ATF, Deeg DJH, Van Limbeek J, et al. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in the Netherlands. Psychol Med. 1997;27(1):231–235. doi: 10.1017/S0033291796003510 [DOI] [PubMed] [Google Scholar]
- 32. Volcik KA, Barkley RA, Hutchinson RG, et al. Apolipoprotein E polymorphisms predict low density lipoprotein cholesterol levels and carotid artery wall thickness but not incident coronary heart disease in 12,491 ARIC study participants. Am J Epidemiol. 2006;164(4):342–348. doi: 10.1093/aje/kwj202 [DOI] [PubMed] [Google Scholar]
- 33. White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0 [DOI] [PubMed] [Google Scholar]
- 34. Patterson C. World Alzheimer Report 2018: The state of the art of dementia research: New frontiers. London, UK: Alzheimer’s Disease International (ADI). 2018. doi: 10.1111/j.0033-0124.1950.24_14.x [DOI] [Google Scholar]
- 35. Robbins R, DiClemente RJ, Troxel AB, et al. Sleep medication use and incident dementia in a nationally representative sample of older adults in the US. Sleep Med. 2021;79:183–189. doi: 10.1016/j.sleep.2020.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhong G, Wang Y, Zhang Y, Zhao Y. Association between benzodiazepine use and dementia: a meta-analysis. Aleman A, ed. PLoS One. 2015;10(5):e0127836. doi: 10.1371/journal.pone.0127836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–2246. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 38. Markota M, Rummans TA, Bostwick JM, Lapid MI. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632–1639. doi: 10.1016/j.mayocp.2016.07.024 [DOI] [PubMed] [Google Scholar]
- 39. Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bao YP, Han Y, Ma J, et al. Cooccurrence and bidirectional prediction of sleep disturbances and depression in older adults: meta-analysis and systematic review. Neurosci Biobehav Rev. 2017;75:257–273. doi: 10.1016/j.neubiorev.2017.01.032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.