Abstract
Background
Understanding the pathological bases underlying the heterogeneity of motor decline in old age may lead to targeted treatments. We examined whether different brain pathologies are related to declining grip strength and gait function.
Methods
We examined postmortem brains of older adults who underwent annual motor testing. Postmortem exam measured 6 neurodegenerative and 5 cerebrovascular disease (CVD) pathologies. Grip strength was measured twice bilaterally using a hand-held dynamometer, and gait function was a composite measure based on time and steps taken to walk 8 ft and perform a 360° turn twice.
Results
In separate linear mixed-effects models including all autopsied adults (N = 1 217), neurodegenerative pathologies including tau tangles, TDP-43, and nigral neuronal loss were associated with declining grip strength, but not CVD pathologies. In contrast, although both CVD and neurodegenerative pathologies were associated with declining gait function, CVD pathologies accounted for 75% of the variance of declining rate of gait function explained by brain pathologies and neurodegenerative pathologies accounted for 25%. These findings were unchanged in adults (n = 970) without a history of stroke. Restricting analyses to only adults without dementia (n = 661), CVD pathologies continued to account for the majority of the variance of declining gait. However, we failed to detect in this subgroup the variance of declining grip strength explained by neurodegenerative or CVD pathologies.
Conclusion
Different pathologies accumulating in aging brains may contribute to the phenotypic heterogeneity of motor decline. Larger studies are needed in older adults without dementia to assess differences in the motor consequences of varied brain pathologies.
Keywords: Cerebrovascular disorders, Gait, Hand strength, Neurodegenerative diseases
Age-related loss of motor function is ubiquitous: No 75 year old can perform at the same level as they did when they were 25 years old (1). Although catastrophic events such as hip fracture, myocardial infarction, or stroke can cause the rapid onset of motor impairment, more commonly motor abilities such as walking speed (2) or grip strength decline insidiously over time in the absence of overt disability or clinical disease (3). There is marked heterogeneity of the severity of late-life motor impairment. For example, some older adults show minimal gait speed slowing, others manifest varying degrees of mobility disability and a minority may lose the ability to ambulate. In addition, a wide spectrum of motor deficits may be observed in older adults. For example, some show primarily reduced muscle strength and bulk (4) and others showed slowed gait speed (2) alone or together with reduced muscle strength in varied combinations and severity. Currently, only a small number of older adults manifest treatable causes of progressive motor decline for conditions such as hydrocephalus, Parkinson’s disease, or myelopathy. Filling knowledge gaps about the pathological bases underlying late-life motor impairment could have important clinical consequences. For example, currently, cerebrovascular disease (CVD) pathologies may be treatable, whereas treatments for neurodegenerative pathologies are lacking.
Our recent work has suggested that diverse neurodegenerative and CVD pathologies are related to late-life motor impairment (5–7). These studies have employed composite measures of motor function, which included both grip strength and gait function. Yet, these studies did not compare whether grip strength and gait function, which are commonly used to assess motor function in older adults (8,9), show differences in their associations with postmortem pathological indices. Prior brain MRI imaging studies of white matter hyperintensity (WMH), a marker of microvascular pathology, have reported an association with poor gait at baseline and a more rapid rate of declining gait function (10–12). However, conflicting results have been reported from the limited studies examining association of WMH with grip strength (13,14). In addition, grip and gait function have rarely been compared in the same individuals. Although multiple brain positron emission tomography studies have reported an association between imaging markers of amyloid-β (Aβ) and gait function (15,16), these studies have not examined grip strength or controlled for WMH. So, it is unknown whether Aβ and WMH are independently related to grip strength and gait function. Moreover, although brain MRI imaging is sensitive, it cannot resolve the presence of multiple comorbid neurodegenerative and CVD pathologies. Pathologies including transactive response DNA binding protein-43 (TDP-43) and microinfarcts and resilience markers can only be studied in postmortem brain tissues.
Concepts of chronic age-related neurological diseases are evolving. For example, we now recognize that the pathologies of Alzheimer’s disease (AD) and CVD are common in persons without clinically diagnosed dementia or stroke, and yet these subclinical pathologies cause mild clinical deficits. Moreover, these pathologies accumulate in aging brains years to decades before they manifest as age-related neurological diseases. This reconceptualization of aging phenotypes as continua was derived in part due to the unique study design of our cohort studies that included the full spectrum of older adults with no motor or cognitive deficits to those with mild or severe impairments in one or both domains that were followed until death.
Building on the success of this approach, the current study examined if indices of neurodegenerative and CVD brain pathologies are differentially associated with declining grip strength and gait function in older adults. To test this hypothesis, we used data from all adults in 2 cohort studies and examined associations of indices of varied brain pathologies with repeated measures of grip strength and gait function. Since the cohorts’ inception more than 2 decades ago, both studies have employed a structured autopsy protocol to collect diverse indices of neuropathologies from a fixed number of predefined brain sites. This approach was chosen to facilitate comparison of postmortem indices with diverse aging phenotypes from large numbers of older individuals. The sites chosen spanned diverse cortical, subcortical, and brainstem sites but does not comprehensively assess all known motor-related brain regions. Nonetheless, our decision to use these indices in the current study is supported by our prior publications (5–7) that have shown that the pathology indices collected in these studies are associated with longitudinal changes of composite measures of motor function.
Method
Participants
The data analyzed in this study were obtained from participants of one of the 2 ongoing community-based longitudinal clinical-pathological studies of aging: the Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP). ROS began enrollment in 1994 and enrolls nuns, priests, and brothers across the United States. MAP began in 1997 and enrolls adults from the greater Chicago metropolitan areas living in private homes, subsidized housings, and retirement facilities. Both studies employ harmonized protocols including motor function assessment and postmortem brain examination, and the data are collected by the same staff facilitating joint data analysis.
Eligible participants were 65 years or older and did not have known dementia at the time of study entry and consented to annual clinical testing, annual blood draws, and brain donation at the time of death. Additional details about both the studies are included in prior publications (17). A Rush University Medical Center Institutional Review Board approved each study. Data from both studies are available at www.radc.rush.edu, a National Institute of Aging sponsored data Sharing Hub.
The sample for these analyses (N = 1217) included 583 ROS and 634 MAP participants who had at least 2 motor assessments prior to death and had completed postmortem brain assessment at the time of these analyses and is illustrated in Supplementary Figures 1 and 2.
Assessment of Grip Strength and Gait Function
Grip strength
Annual motor testing assessed grip strength using a hand-held dynamometer (Lafayette Instruments, Lafayette, IN) scaled from 0 to 200 lb. Two trials were obtained from each hand and were averaged together to summarize grip strength (18).
Gait function
To gain more statistical power to identify brain pathologies associated with slope of gait changes over time, we used multiple indicators of gait rather than a simple indicator such as gait speed (19). In our prior studies examining multiple motor performances, we found that steps and time to complete an 8 feet walk and perform 360° turns loaded on one factor, which provided empiric support to summarize these measures as a composite measure (20). Participants were asked to walk 8 feet twice and then perform two 360° turns. Time (in seconds) and number of steps from both tasks were recorded. We first reciprocated the time and the steps measures, so that larger values indicated faster walking with fewer steps. The values of the 2 trials of walking and turning were averaged to obtain 4 measures: walking time, walking steps, turning time, and turning steps. These 4 measures were converted into z-scores, using the baseline mean and standard deviation of all study participants, and the 4 z-scores were averaged to summarize gait function.
Assessment of Postmortem Brain Pathologies
To allow for consistent analyses, the same structured autopsy was performed for all postmortem examinations as previously described (21). Following brain removal, one hemisphere was frozen for future studies. The other hemisphere was fixed with formaldehyde and cut into 1-cm slabs. Tissue sections were prepared from predetermined regions to collect indices of common neurodegenerative and CVD pathologies.
Neurodegenerative pathologies
Neuropathological diagnosis of AD
Sections from 5 cortical regions were stained by a modified Bielschowsky silver stain to visualize AD pathological hallmarks (diffuse plaques, neuritis plaques, and neurofibrillary tangles). A Board-certified neuropathologist, blinded to clinical data, adjudicated AD pathological diagnosis based on established criteria (22).
Aβ and tau assessment
Immunohistochemical analysis was used to calculate burden of Aβ and tau. Three antibodies specific for Aβ were used for immunostaining sections of 8 brain regions for plaques containing Aβ. Then, computer-assisted image analysis captured images, which were subsequently analyzed by automated algorithms for determination of the percent areas of the images occupied by Aβ. An antibody specific for phosphorylated tau was used for immunostaining sections of the same 8 regions for paired helical filament containing tau. Then, a computer-assisted microscope powered with StereoInvestigator software was employed for counting tau-labeled tangles in the sections. Percent area of Aβ and density of tau tangles were determined in each region and were averaged across regions to yield overall burden of Aβ and tau. Details of the procedure are described elsewhere (23).
Transactive response DNA binding protein-43
Immunohistochemical analysis was used to calculate burden of TDP-43. An antibody specific for phosphorylated TDP-43 was used for immunostaining sections of 6 brain regions looking for TDP-43 cytoplasmic inclusions in the glia or neurons. A recommended staging system was employed to quantify the presence of TDP-43 in the amygdala (Stage 1), hippocampus or entorhinal cortex (Stage 2), and neocortex (Stage 3). In this study, we used a bivariate variable indicating presence of advanced stages of TDP-43 (Stage 2 or 3), as was done in previous studies (24).
Parkinson’s disease pathologies
Sections of the midbrain containing substantia nigra, prepared from the level of third nerve exit, were stained by hematoxylin and eosin (H&E), and nigral neuronal loss was assessed by a semiquantitative scale (none, mild to severe). In this study, we used a bivariate variable indicating moderate or severe neuronal loss (24). Lewy body pathology was assessed immunohistochemically using an antibody against α-synuclein and was treated in this study as present versus absent.
Hippocampal sclerosis
Presence of hippocampal sclerosis was assessed by examining a unilateral coronal section of mid-hippocampus at the level of lateral geniculate body. Hippocampal sclerosis was defined as severe neuronal loss and gliosis in CA1 and/or subiculum (25).
CVD pathologies
Macroinfarcts
Fixed slabs from one hemisphere and pictures of fresh slabs from the other hemisphere were explored by naked eye looking for macroinfarcts. Detected lesions were confirmed microscopically too. We included only chronic, not acute or subacute, macroinfarcts as they are the lesions associated with motor decline over years prior to death, a method that was followed in our prior studies (26). We used a dichotomous variable for the current analyses indicating the presence or absence of macroinfarcts.
Microinfarcts
In contrast to macroinfarcts, microinfarcts are not visible to naked eyes. To detect microinfarcts, a minimum of 9 brain regions were examined. Similar to macroinfarcts, only chronic microinfarcts were included in the current analysis through a dichotomous variable indicating the presence or absence of them.
Atherosclerosis
Circle of Willis arteries were examined for the presence and severity of atherosclerosis. A semiquantitative scale (none, mild to severe) was adopted for the assessment of atherosclerosis based on the severity of atherosclerosis in each vessel and the number of vessels affected, as described previously (21). For the current analysis, we used a dichotomous variable indicating the presence or absence of moderate to severe atherosclerosis.
Arteriolosclerosis
Small arteries in the anterior basal ganglia were inspected for the presence of severity of arteriolosclerosis, which is hyaline thickening and narrowing of the lumen of vessels. A semiquantitative scale (none, mild to severe, complete occlusion) was used for scoring arteriolosclerosis (21). For the current analysis, we used a dichotomous variable indicating the presence or absence of moderate to severe arteriolosclerosis.
Cerebral amyloid angiopathy
Parenchymal and meningeal vessels in 4 brain regions were examined by immunostaining with an antibody specific for Aβ, and cerebral amyloid angiopathy (CAA) was scaled in each brain region using a semiquantitative scale (0–4). Brain regions’ CAA scales were averaged to make a CAA score, which was subsequently used to develop a semiquantitative scale (none, mild to severe) for the burden of CAA in the brain. For the current analysis, we used a dichotomous variable indicating the presence or absence of moderate to severe CAA.
Demographics and Other Clinical Covariates
Age was calculated from date of birth to date of last visit (age at the last visit) or death (age at death). Sex and years of formal education were obtained at the time of study enrollment.
Participants’ self-report was used to determine the presence of vascular risk factors and stroke. The total number of self-reported vascular risk factors (hypertension, diabetes mellitus, smoking) ranging from 0 to 3 and the presence or absence of stroke were examined in the analyses.
The presence of the ε4 allele of ApoE gene was based on sequencing rs429358 (codon 112) and rs7412 (codon 158) at exon 4 of the ApoE gene, as described previously (27).
Cognitive function was assessed by the annual administration of a battery of 17 neuropsychological tests that were reviewed by a neuropsychologist. Cognitive status at each visit was determined by a physician expert in the care of dementia, blinded to clinical data from prior cycles, who reviewed all clinical data including the neuropsychologist’s summary to determine a diagnosis of dementia based on established criteria (28).
Statistical Analysis
To examine bivariate associations of the last visit motor functions, we used Spearman correlation coefficient for quantitative covariates and t-test for dichotomous covariates. Then, we used linear mixed-effects models to examine the annual rate of change in grip strength and gait function prior to death and their associations with demographics and other covariates. In the core model, repeated measures of grip strength or gait function were the outcomes and the models’ fixed terms included intercept (the level of motor function prior to death), time (rate of change in motor function), age at death, sex, education, and their interaction with time. The core model also included 2 random effect terms: intercept and time. The random effect terms permitted models to include person-specific initial levels and rate of change in the motor function. The model also included 4 variance components: variances of the person-specific initial level and rate of change of motor function and their covariance, as well as a residual variance component. Next, in separate mixed-effects models, we examined whether vascular risk factors, stroke, dementia, or ApoE ε4 allele was associated with the rate of motor decline. Each model included the terms in the core model as well as terms for a different covariate and its interaction with time.
Then we employed a series of mixed-effect models to examine the associations of postmortem pathology indices with motor decline. There were 3 stages to our analyses. First, we examined the associations of each of the 11 individual brain pathologies with declining grip strength and then we repeated these models for declining gait function. Mixed-brain pathologies commonly accumulate in older adults and neurodegenerative and CVD pathologies are related to one another (25,29). So, in a second stage, we examined the 6 neurodegenerative pathologies together in one model and the 5 CVD pathologies in a separate model to identify which pathologies within each group of pathologies were independently associated with motor decline. In the third stage, we examined the associations of all 11 pathologies together in one model with declining grip strength and then repeated this model with declining gait function.
To contextualize our findings, we used the estimates of the variance of the person-specific rates of motor decline after adjustment for models’ terms to calculate the percentage of variance in the rates of grip strength and gait function decline explained by the neurodegenerative and CVD pathologies alone and together compared with demographic measures alone.
In a sensitivity analysis, we used functional mixed-effects (FME) models (30,31) that allow motor function changes to be nonlinear and also allow the associations of brain pathologies with grip strength and gait function decline to be nonlinear over time. To compare FME and linear mixed-effects models, we used Bayesian information criterion (BIC) where lower values indicate a better model fit. We used both graphical and numerical methods to verify models’ assumptions. The statistical significance was determined at the level of .05, and the analyses were done using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Clinical Characteristics of Study Participants at Last Visit and Postmortem Indices
Clinical characteristics
The clinical characteristics of the 1 217 older adults, who were followed for an average 7.4 years (SD = 3.8) before death, are summarized in Table 1. The average age at death was 90 years, and 2/3 were women. On average, the last clinical assessment was 10.4 (SD = 14.1) months before death. Average grip strength was 32.1 lbs (SD = 17.9, range = 0–112.0), and average gait function was 0.63 unit (SD = 0.32, range = 0–2.07). Older ages, female sex, and history of stroke were associated with lower levels of grip strength and gait function proximate to death (Supplementary Table 1).
Table 1.
Demographic and Clinical Characteristics of Participants at the Last Visit Before Death
| Mean (SD) or N (%) | |||||
|---|---|---|---|---|---|
| Covariates | No Stroke (n = 969) | History of Stroke (n = 247) | No Dementia (n = 556) | Dementia (n = 661) | All (n = 1 217) |
| Age at last visit (y), mean (SD) | 89.2 (6.5) | 89.7 (6.1) | 88.0 (6.5) | 90.9 (6.0) | 89.3 (6.5) |
| Age at death (y), mean (SD) | 90.1 (6.5) | 90.5 (6.0) | 89.0 (6.6) | 91.5 (5.9) | 90.2 (6.4) |
| Female, n (%) | 308 (32) | 66 (27) | 440 (67) | 402 (72) | 842 (69) |
| Years of education, mean (SD) | 16.3 (3.6) | 16.1 (3.6) | 16.3 (3.5) | 16.2 (3.6) | 16.3 (3.6) |
| Presence of ApoE ε4, n (%) | 240 (25) | 56 (23) | 116 (18) | 180 (32) | 296 (25) |
| Hypertension, n (%) | 658 (68) | 199 (81) | 470 (71) | 388 (70) | 858 (71) |
| Diabetes mellitus, n (%) | 211 (22) | 71 (29) | 150 (23) | 132 (24) | 282 (23) |
| Smoking history n (%) | 276 (28) | 79 (32) | 227 (34) | 128 (23) | 355 (29) |
| Number of vascular risk factors, median (IQR) | 1.0 (2.0) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (2.0) | 1.0 (2.0) |
| History of stroke, n (%) | 0 (0) | 247 (100) | 120 (18) | 127 (23) | 247 (20) |
| Dementia prior to death, n (%) | 429 (44) | 127 (51) | 0 (0) | 661 (100) | 556 (46) |
| Years of follow-up prior to death, mean (SD) | 7.4 (3.9) | 7.2 (3.6) | 7.5 (3.9) | 7.2 (3.8) | 7.4 (3.8) |
Notes: ApoE ε4 = ε4 allele of apolipoproteinE gene; IQR = interquartile range; SD = standard deviation.
Postmortem indices
The average postmortem interval was 9.4 (SD = 8.4) hours. AD was the most common brain pathology observed in approximately 2/3 of the all postmortem examinations (Supplementary Table 2). Yet, most examinations (81%, n = 986) showed evidence of multiple neurodegenerative and CVD pathologies compared with only 13% (n = 164) with a single pathology.
Declining Motor Function
In a linear mixed-effects model controlling for demographics, grip strength declined at an average rate of approximately 2 lb per year (estimate = −1.845, SE = 0.054, p < .001). To contextualize the average decline, we used the model-derived estimates (Supplementary Table 3, Model 1) to calculate change in the level of the grip strength in the last 10 years before death. In an average woman, who died at the age of 90 years old with 16 years of education, grip strength was reduced by 43% during the last decade of life.
In a separate linear mixed-effects model controlling for demographics, gait function declined at an average rate of approximately 0.04 unit per year (estimate = −0.043, SE = 0.001, p < .001). As described above, based on the model-derived estimates for declining gait function (Supplementary Table 3, Model 2), an average woman, died at the age of 90 years old with 16 years of education, manifested a 43% reduction in gait function during the last decade of her life.
Grip and gait function showed a similar percentage of decline during the last decade of life. So, we examined to what extent the person-specific rates of declining grip and gait function were correlated using the fixed and random effects of the above models to estimate person-specific rate of decline for both functions (Supplementary Figure 3). The annual rates of grip strength and gait function decline were weakly correlated (Spearman r = 0.27, p < .001), suggesting that they have less than 10% of shared variance.
Risk Factors and Declining Motor Function
Next, we examined to what extent known risk factors for specific-brain pathologies might be differentially associated with grip strength and gait function decline. As a proxy for risk factors associated with CVD pathologies, we examined self-reported history of vascular risk factors and stroke. As a proxy for degenerative brain pathologies, we examined ApoE ε4 allele because of its strong association with AD (27) and TDP-43 (32).
ApoE ε4 genotype was related to a faster grip strength decline (ApoE ε4 × Time: Estimate = −0.345, SE = 0.100, p < .001) but not vascular risk factors or stroke. History of stroke but not ApoE ε4 genotype was related to more rapid gait function decline (stroke × time: Estimate = −0.007, SE = 0.002, p = .003). The presence of dementia, a nonspecific proxy of brain atrophy caused by both neurodegenerative and CVD pathologies (33,34), was associated with both faster grip strength and gait function decline (Table 2; Supplementary Tables 4–7).
Table 2.
Association of Risk Factors and Brain Pathologies, Each in a Separate Model, With Grip Strength and Gait Function Decline Rate
| Estimates (SE), p-Values | ||
|---|---|---|
| Model’s Term | Grip Strength | Gait Function |
| Risk factors | ||
| Presence of ApoE ε4 × Time | −0.343 (0.100), <.001 | −0.001 (0.002), .676 |
| Number of vascular risk factors × Time | 0.026 (0.052), .619 | 0.001 (0.001), .477 |
| History of stroke × Time | 0.094 (0.109), .387 | −0.007 (0.002), .003 |
| Dementia × Time | −0.901 (0.084), <.001 | −0.012 (0.002), <.001 |
| Brain pathologies | ||
| Neurodegenerative | ||
| Amyloid × Time | −0.176 (0.039), <.001 | −0.001 (0.001), .140 |
| Tangles × Time | −0.195 (0.033), <.001 | −0.002 (0.001), .003 |
| TDP-43 × Time | −0.402 (0.093), <.001 | −0.004 (0.002), .065 |
| Hippocampal sclerosis × Time | −0.504 (0.138), <.001 | −0.003 (0.003), .405 |
| Lewy bodies × Time | −0.387 (0.097), <.001 | −0.005 (0.002), .019 |
| Nigral neuronal loss × Time | −0.539 (0.131), <.001 | −0.010 (0.003), <.001 |
| Cerebrovascular | ||
| Macroinfarcts × Time | −0.183 (0.092), .046 | −0.010 (0.002), <.001 |
| Microinfarcts × Time | −0.078 (0.093), .402 | −0.004 (0.002), .047 |
| Atherosclerosis × Time | −0.100 (0.097), .304 | −0.008 (0.002), <.001 |
| Arteriolosclerosis × Time | −0.237 (0.097), .014 | −0.006 (0.002), .001 |
| Cerebral amyloid angiopathy × Time | −0.237 (0.090), .009 | −0.003 (0.002), .107 |
Notes: ApoE ε4 = ε4 allele of apolipoproteinE gene; SE = standard error; TDP-43: transactive response DNA binding protein-43. Two series of 15 mixed-effects models examined the associations of each of the 4 risk factors, 6 neurodegenerative, and 5 cerebrovascular pathologies shown in the left column with either grip strength decline (middle column) or gait function decline (right column). Each model included 9 terms including time (the rate of change of either grip strength or gait function), cross-sectional terms for age at death, sex, education, and a single risk factor or pathology shown in the left column as well as the interaction of these 4 terms with time. Each cell in the middle and right columns shows the Estimate, SE, and p-value of the interaction between a specific risk factor or pathology with either the rate of grip strength decline or gait function decline.
Brain Pathologies and Declining Motor Function
There were 3 stages of our analyses to clarify the associations of neurodegenerative and CVD brain pathologies with declining grip strength and gait function.
Individual brain pathologies
In separate mixed-effects models, we examined the association of each individual pathology with declining grip strength and gait function (Table 2, Supplementary Tables 8–18). All 6 neurodegenerative pathologies and 3 of the 5 CVD pathologies were associated with faster grip strength decline. In contrast, 3 of the 6 neurodegenerative pathologies and 4 of the 5 CVD pathologies were associated with faster gait function decline.
Degenerative and CVD pathologies
Previous studies have shown that some brain pathologies are correlated (25,29,35), which underscores the importance of joint analyses to determine which indices are independently related to outcomes of interest. In addition, our initial findings described above suggest that the associations of risk factors for degenerative and CVD pathologies may differ between grip strength and gait function decline. Therefore, we examined the associations of degenerative pathologies and CVD pathologies in separate models with grip strength and gait function decline.
Among neurodegenerative pathologies, tangles, TDP-43, and nigral neuronal loss were independently related to declining grip (Table 3, model 1; Supplementary Table 19). Only tangles and nigral neuronal loss were independently related to declining gait function (Table 3, model 4; Supplementary Table 19). In the models with CVD pathologies, arteriolosclerosis and CAA pathologies were independently associated with declining grip strength (Table 3, model 2; Supplementary Table 20), and macroinfarcts and atherosclerosis were associated with declining gait function (Table 3, model 5; Supplementary Table 20).
Table 3.
Neurodegenerative Versus Cerebrovascular Pathologies and Declining Grip Strength and Gait Function
| Grip Strength Decline Estimates (SE), p-values |
Gait Function Decline Estimates (SE), p-values |
||||||
|---|---|---|---|---|---|---|---|
| Group of Pathologies | Neuropathology | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 |
| Neurodegenerative | Amyloid × Time | −0.082 (0.043), .057 | −0.079 (0.043), .068 | 0.000 (0.001), .939 | 0.000 (0.001), .810 | ||
| Tangles × Time | −0.132 (0.038), <.001 | −0.121 (0.039), .002 | −0.002 (0.001), .018 | −0.002 (0.001), .043 | |||
| TDP-43 × Time | −0.229 (0.099), .020 | −0.231 (0.010), .020 | 0.000 (0.001), .929 | −0.002 (0.002), .343 | |||
| Hippocampal sclerosis × Time | −0.245 (0.146), .093 | −0.222 (0.147), .130 | 0.000 (0.003), .931 | 0.001 (0.003), .864 | |||
| Lewy bodies × Time | −0.153 (0.105), .144 | −0.166 (0.105), .113 | −0.001 (0.002), .649 | −0.001 (0.002), .564 | |||
| Nigral neuronal loss × Time | −0.402 (0.140), .004 | −0.389 (0.140), .006 | −0.009 (0.003), .002 | −0.009 (0.003), .003 | |||
| Cerebrovascular disease | Macroinfarcts × Time | −0.145 (0.096), .129 | −0.153 (0.093), .101 | −0.008 (0.002), <.001 | −0.009 (0.002), <.001 | ||
| Microinfarcts × Time | −0.039 (0.096), .686 | −0.063 (0.093), .495 | −0.002 (0.002), .410 | −0.002 (0.002), .379 | |||
| Atherosclerosis × Time | −−0.027 (0.101), .793 | −0.011 (0.099), .912 | −0.005 (0.002), .011 | −0.005 (0.002), .019 | |||
| Arteriolosclerosis × Time | −0.209 (0.100), .038 | −0.141 (0.099), .156 | −0.004 (0.002), .059 | −0.003 (0.002), .121 | |||
| Cerebral amyloid angiopathy × Time | −0.240 (0.090), .008 | −0.075 (0.093), .419 | −0.003 (0.002), .076 | −0.002 (0.002), .290 | |||
| Model-derived variance component | |||||||
| Variance in the person-specific motor function decline rate | 1.095 (0.086), <.001 | 1.199 (0.092), <.001 | 1.095 (0.086), <.001 | 0.000410 (0.000040), <.001 | 0.000389 (0.000039), <.001 | 0.000381 (0.000039), <.001 | |
Notes: SE = standard error; TDP-43 = transactive response DNA binding protein-43. Each model shows a single mixed-effects model with the outcome of grip strength decline (Models 1–3) or gait function decline (Models 4–6). The terms for pathologies included in each model were different: either neurodegenerative (Model 1 or 4), cerebrovascular disease pathologies (Model 2 or 5), or both together (Model 3 or 6). Each model included a term for time (rate of change in motor function) with cross-sectional terms for age at death, sex, education and each of the pathologies listed in the left column as well as their interaction with time. Each cell in a column shows the Estimate, SE, and p-value for the interaction of the pathology with time to show whether the pathology metric was associated with either grip strength or gait function decline rate. Bolded cells were significant. The last 2 rows indicate variance of the person-specific grip strength (Models 1–3) and gait function (Models 4–6) decline rates derived from the corresponding models, which were used to determine the percentage of variance in a motor function decline rate explained by pathologies. For example, the variance of the person-specific rate of grip strength decline derived from the core model, including no pathology, is 1.215 and from the model 1 including the neurodegenerative pathologies is 1.095. The variance of the rate of grip strength decline explained by the neurodegenerative pathologies is calculated in the following way: (1.215–1.095)/1.215 = 0.099 or 9.9%.
All brain pathologies together
In the third stage of these analyses, we examined the associations of all the brain pathologies together in a single model to identify which group and what individual pathologies within each group were most strongly associated with declining grip strength or gait function.
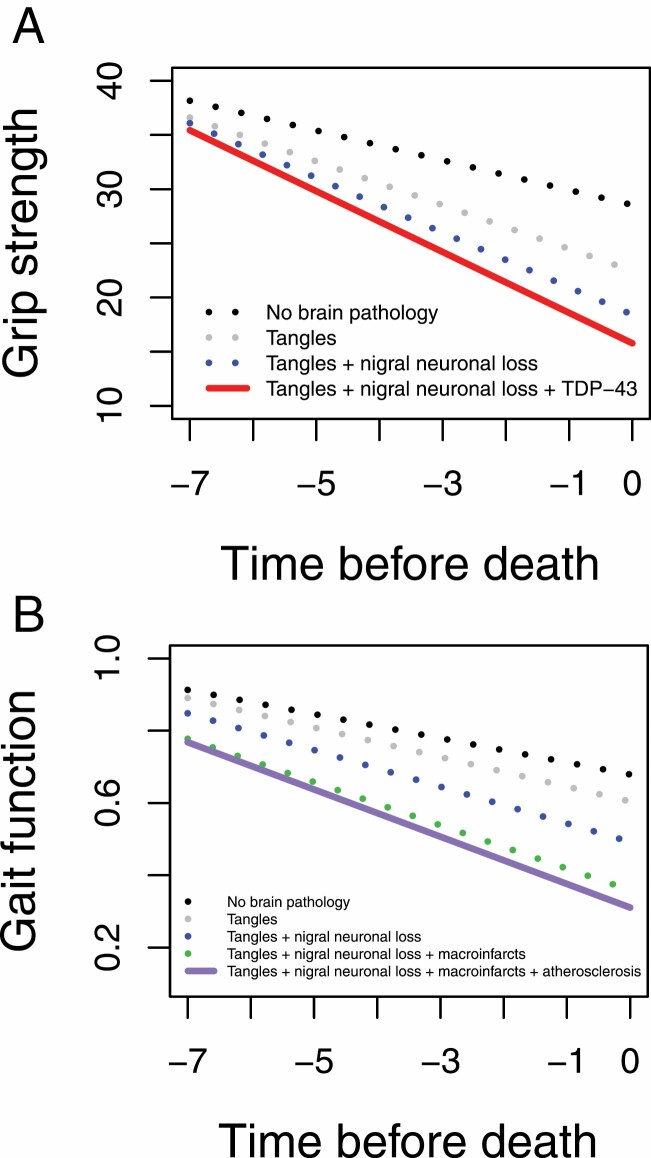
Neurodegenerative pathologies (tangles, TDP-43, and nigral neuronal loss) but none of the CVD pathologies were associated with declining grip strength (Table 3, model 3; Supplementary Table 21; Figure 1A). By contrast, both neurodegenerative (tangles and nigral neuronal loss) and CVD pathologies (macroinfarcts and atherosclerosis) were associated with declining gait function (Table 3, model 6; Supplementary Table 21; Figure 1B).
Figure 1.
Contributions of brain pathologies to grip strength and gait function decline. Model-derived trajectories of grip strength (A) and gait function (B) decline in an average 90-y-old women with 16 y of education and with increasing burden of brain pathologies. Neurodegenerative pathologies (tau tangles, nigral neuronal loss, and TDP-43 proteinopathy) were associated with a faster grip strength decline. By contrast, both neurodegenerative (tau tangles and nigral neuronal loss) and cerebrovascular disease (macroinfarcts and cerebral atherosclerosis) pathologies were associated with a faster gait function decline.
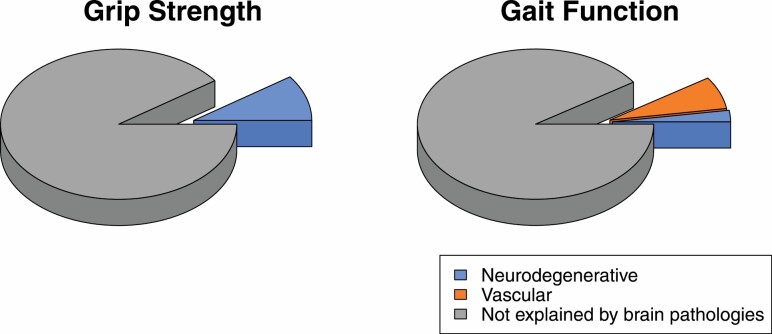
Calculating variance of person-specific rates of gait function decline indicated that neurodegenerative pathologies explained 20%–26% and CVD pathologies explained 74%–80%, depending on the order of pathologies’ entry, of the variance that brain pathologies explained in the rate of gait function decline (Table 3, Models 4–6; Figure 2). However, brain pathologies altogether explained only 10% and 9% of the variance in the rate of grip strength and gait function decline, respectively (Figure 2).
Figure 2.
Variance of grip strength and gait function decline rate explained by brain pathologies.
Sensitivity Analyses
We examined whether our findings were driven by participants having stroke or dementia by including terms for stroke, dementia, and their interaction with time in all of our models. Controlling for stroke and dementia did not change our findings that neurodegenerative pathologies, not CVD ones, explained grip strength decline and CVD pathologies explained most (90%) of the variance in the rate of gait function decline explained by brain pathologies (Supplementary Table 22).
In further sensitivity analyses, our findings were unchanged when we excluded participants with a history of stroke (n = 247). In adults without a history of stroke, CVD pathologies accounted for the majority of the variance of gait decline and neurodegenerative pathologies were the only pathologies related to declining grip strength (Supplementary Table 23).
Next, we excluded about 50% of the analytic cohort who had clinical dementia at the time of death (n = 556). CVD pathologies still accounted for the majority of the variance of declining gait; however, neither CVD nor neurodegenerative pathologies explained variance of grip strength decline rate, possibly due to smaller sample size as well as truncated variance of grip strength after excluding adults with dementia (Supplementary Table 24).
Our analytic cohort derived from 2 independent studies (MAP and ROS). Adding a term for study (MAP vs ROS) and its interaction with time did not change our findings of preferential association of neurodegenerative pathologies with grip strength decline and CVD pathologies with gait function decline (Supplementary Table 25).
Motor decline may show linear and nonlinear changes during the last decade of life. In further analyses, we used FME models that allow both person-specific trajectories of motor decline and the associations of predictors to be nonlinear over time. In models controlled for age at death, sex, and education, FME models (Supplementary Figure 4) had better model fit statistics than linear models in examining both grip strength (BIC: FME = 60036.1, Linear = 60506.4) and gait function (BIC: FME = −2305.4, Linear = −1803.8) longitudinal assessments. Then, we used FME models to examine the associations of tangles and macroinfarcts (the pathologies with the strongest associations with declining rates of grip strength and gait function, Supplementary Tables 26 and 27) with nonlinear decline. FME models showed similar results to the linear models; neurodegenerative pathologies (tangles) were more strongly associated with grip strength and CVD pathologies (macroinfarcts) with gait function decline (Supplementary Figures 5 and 6). However, the FME models are complex and unable in providing quantitative parameters for the variance of person-specific decline rates explained by the models’ terms, which was a major focus of this study.
Discussion
The current study found a dissociation between the brain pathologies associated with declining grip strength and gait function. Neurodegenerative brain pathologies and not CVD pathologies were associated with declining grip strength. In contrast, CVD rather than neurodegenerative pathologies accounted for most of the variance of brain pathologies with declining gait function. These findings add evidence to the idea that the accumulation of different types of pathologies, that is, neurodegenerative or CVD pathologies, in aging brains may account in part for the phenotypic heterogeneity of motor decline in older adults. These may have important clinical consequences because profiling more diverse motor performances may detect motor signatures that may lead to targeted interventions for specific pathologies to prevent specific motor deficits.
The pathological basis for the heterogeneity of late-life motor impairment is unclear. Although previous studies have reported that the burden of accumulating neurodegenerative and CVD brain pathologies contribute to late-life motor impairment, these studies employed composite measures that summarized multiple motor performances. Thus, it is unknown if the associations of brain pathologies are different for commonly tested performances such as grip strength and gait function. Brain imaging has advanced our understanding of the complex biology underlying late-life motor decline. However, most imaging studies have focused on gait function and have not compared grip strength with gait function (10–16). Moreover, current brain imaging studies cannot identify and control for the range of comorbid mixed neurodegenerative or CVD pathologies commonly observed in postmortem examination of aging brains. The current study leveraged novel postmortem data in well-characterized older adults to fill the knowledge gap in prior studies.
The current study extends prior studies (5–7) by showing that mixed-brain pathologies are associated with both declining grip strength and gait function in the same older adults. In addition, by examining indices of several neurodegenerative and CVD pathologies together in the same models, this study found that the burden of accumulating neurodegenerative and CVD pathologies is different for declining grip strength and gait function. Neurodegenerative brain pathologies but not CVD pathologies contribute to declining grip strength and CVD pathologies account for the majority of variance of declining gait function. These findings suggest that adults with poor gait function may benefit from more aggressive treatment to reduce vascular risk factors and diseases. Moreover, adding indices of gait function to conventional vascular risk scores like the Framingham risk score may improve the specificity of this risk score for identifying adults at risk for developing CVD pathologies (36). Similarly, screening adults for poor grip strength may improve the homogeneity of older adults recruited to clinical trials of treatments for neurodegenerative pathologies like AD. Recent work suggests that combinations of sensor metrics obtained during instrumented motor testing may yield more granular motor signatures that may help stratify older adults for targeted treatments of specific-brain pathologies (37).
Advances in our understanding of age-related disorders like AD and Parkinson’s disease have led to their reconceptualization as continua that develop over many years (38). During their earliest asymptomatic stages, neurodegenerative and CVD brain pathologies accumulate in adults with normal motor and cognitive function. As these pathologies accumulate, mild clinical deficits manifest and are followed in later stages with more severe findings, that is, dementia or stroke. These latter stages are associated with a higher burden of varied brain pathologies. Analyzing postmortem pathologies in large numbers of older adults that encompassed the full clinical spectrum of older adults, that is, with and without dementia, was crucial for providing data that led to this paradigm shift in conceptualizing AD and related disorders. Thus, our primary analysis included all adults with and without dementia. In a secondary analysis, we repeated analyses after excluding almost 50% of the analytic sample with dementia prior to death. As we observed in our primary analyses, CVD pathologies accounted for the majority of the variance of declining gait function. However, we failed to detect in this subgroup variance of declining grip strength explained by neurodegenerative or CVD pathologies. We suspect that the latter finding was due to decreased power and truncated variance as the adults with dementia on average have a higher burden of mixed-brain pathologies compared to those with no dementia (Supplementary Table 1). Further larger clinical autopsy studies in older adults without dementia will be needed to test this hypothesis.
The mechanisms underlying the different associations of neurodegenerative and CVD brain pathologies with grip strength and gait function decline are not clear. Many brain regions such as primary sensorimotor, premotor, and supplementary motor cortices are crucial for both grip and gait functions. Other brain regions may underlie specific performances such as inferior parietal cortex for grip strength and parahippocampal cortex during gait (39,40). Regional differences in the accumulation of pathologies may be due to selective vulnerability of the underlying tissue to varied pathologies. For example, selective vulnerability of neurons in brain networks have been reported for neurodegenerative pathologies like AD (41,42). Brain infarcts are most frequently seen in the basal ganglia (43), an important region for control of gait (44). In contrast, AD pathology accumulates in the basal ganglia in the later stages of AD (45). As brain imaging techniques for specific pathologies evolve, they may be used to map the topography of neural networks underlying specific motor performances based on their preferential associations with different pathologies.
In the current study, brain pathologies accounted for only about 10% of late-life motor decline (Figure 2). However, this highlights another important knowledge gap about the pathological bases for late-life motor impairment (46). Motor performance is a complex volitional behavior whose networks originate in the brain, but control networks for both grip and gait extend outside the brain to influence motoneurons in the spinal cord that via peripheral nerves regulate muscle firing and movement in the periphery. Nonetheless, nearly all studies to date have focused on brain pathologies and late-life motor impairment while animal studies have identified gait centers in the brainstem (such as midbrain locomotor region and pontine and medullary reticular formation) and their circuits with spinal cord structures crucial for motor function, that is, central pattern generator (44). Although brain imaging can be obtained in living adults, CNS regions outside the brain cannot be interrogated with the same resolution in living adults (39) and have not been examined together with peripheral nerve and muscle tissues crucial for all motor functions. Recent work suggests that both neurodegenerative and CVD pathologies commonly observed in the brain may show different patterns of accumulation in brainstem and spinal cord structures (47,48). This emphasizes the necessity of further studies to interrogate pathologies in all portions of the distributed motor pathways outside the brain to determine the predominant pathologies contributing to declining grip strength and gait function in older adults.
The study has several strengths. The data come from 2 longitudinal community-based studies, collected by the same staff following uniform protocols, with more than 90% follow up rate and more than 80% autopsy rate, minimizing risks of biases. The participants were followed annually for an average of 7 years providing reliable estimate of motor function changes over time. A structured pathological examination was performed by staff blinded to clinical data providing indices of diverse neurodegenerative and CVD brain pathologies. However, the study has also limitations. The study participants were composed mainly of highly educated Whites, which necessitates replication of the study in other populations. The study did not include MRI data to examine whether the motor functions’ declines are associated with MRI vascular lesions, including WMHs and enlarged perivascular spaces, beyond the associations of the pathologies. Moreover, the full extent of the associations of degenerative and CVD pathologies underlying motor decline cannot be inferred from studies limited to brain. Selection of brain regions for pathological assessment did not focus on motor-related regions underlying grip and gait function but mostly focused on cognitive-related brain regions. So, future studies examining brain pathologies in motor regions such as primary motor cortex may account for more variance of motor performances. The pathological assessment for all postmortem indices except for macroinfarcts was limited to one hemisphere. As left and right hemispheres’ regions are not equally involved in some motor performances (49,50), future pathological studies that examine both hemispheres are needed.
Supplementary Material
Acknowledgments
We are sincerely grateful to participants of the Religious Orders Study and the Rush Memory and Aging project. Also, we appreciate Traci Colvin, MPH, Tracey Nowakowski, MA, and Karen Skish, MS for study coordination, Shengying Chen, MS, for statistical analyses, and other staff of the Rush Alzheimer’s Disease Center. Sponsor did not have any role in data acquisition and analyses, interpretation of data, or writing the manuscript.
Contributor Information
Shahram Oveisgharan, Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, Illinois, USA; Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois, USA.
Lei Yu, Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, Illinois, USA; Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois, USA.
Tianhao Wang, Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, Illinois, USA; Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois, USA.
Julie A Schneider, Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, Illinois, USA; Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois, USA; Department of Pathology, Rush University Medical Center, Chicago, Illinois, USA.
David A Bennett, Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, Illinois, USA; Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois, USA.
Aron S Buchman, Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, Illinois, USA; Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois, USA.
Funding
This work was supported by National Institute of Health grants, R01AG043379, R01AG047679, R01AG056352, R01AG017917, R01NS078009, P30AG10161, and R01AG15819.
Conflict of Interest
None declared.
Author Contributions
S.O.: design and conceptualized study; interpretation of the data; drafted the manuscript for intellectual content. L.Y.: analysis and interpretation of data; revising the manuscript for intellectual content. T.W.: analysis and interpretation of data; revising the manuscript for intellectual content. J.A.S.: acquisition of data; revising the manuscript for intellectual content. D.A.B.: acquisition of data; interpretation of the data; revising the manuscript for intellectual content. A.S.B.: design and conceptualized study; interpretation of the data; acquisition of data; revising the manuscript for intellectual content.
References
- 1. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882–887. doi: 10.15585/mmwr.mm6732a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility—giving mobility clinical visibility: a mobility working group recommendation. JAMA. 2014;311(20):2061–2062. doi: 10.1001/jama.2014.3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Picavet HS, van den Bos GA. The contribution of six chronic conditions to the total burden of mobility disability in the Dutch population. Am J Public Health. 1997;87(10):1680–1682. doi: 10.2105/ajph.87.10.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–2646. doi: 10.1016/s0140-6736(19)31138-9 [DOI] [PubMed] [Google Scholar]
- 5. Buchman AS, Yu L, Wilson RS, et al. Progressive parkinsonism in older adults is related to the burden of mixed brain pathologies. Neurology 2019;92(16):e1821–e1830. doi: 10.1212/WNL.0000000000007315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchman AS, Wang T, Yu L, Leurgans SE, Schneider JA, Bennett DA. Brain pathologies are associated with both the rate and variability of declining motor function in older adults. Acta Neuropathol (Berl). 2020;140(4):587–589. doi: 10.1007/s00401-020-02212-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol Biol Sci Med Sci. 2014;69(12):1536–1544. doi: 10.1093/gerona/glu117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carson RG. Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging. 2018;71:189–222. doi: 10.1016/j.neurobiolaging.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 9. Peel NM, Kuys SS, Klein K. gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol Biol Sci Med Sci. 2013;68(1):39–46. doi: 10.1093/gerona/gls174 [DOI] [PubMed] [Google Scholar]
- 10. Rosario BL, Rosso AL, Aizenstein HJ, et al. Cerebral white matter and slow gait: contribution of hyperintensities and normal-appearing parenchyma. J Gerontol A Biol Sci Med Sci. 2016;71(7):968–973. doi: 10.1093/gerona/glv224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosso AL, Studenski SA, Longstreth WT, Brach JS, Boudreau RM, Rosano C. Contributors to poor mobility in older adults: integrating white matter hyperintensities and conditions affecting other systems. J Gerontol A Biol Sci Med Sci. 2017;72(9):1246–1251. doi: 10.1093/gerona/glw224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sullivan KJ, Ranadive R, Su D, et al. Imaging-based indices of neuropathology and gait speed decline in older adults: the Atherosclerosis Risk in Communities study. Brain Imaging Behav. 2021;15(5):2387–2396. doi: 10.1007/s11682-020-00435-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sachdev PS, Wen W, Christensen H, Jorm AF. White matter hyperintensities are related to physical disability and poor motor function. J Neurol Neurosurg Psychiatry. 2005;76(3):362–367. doi: 10.1136/jnnp.2004.042945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aribisala BS, Gow AJ, Bastin ME, et al. Associations between level and change in physical function and brain volumes. PLoS One. 2013;8(11):e80386. doi: 10.1371/journal.pone.0080386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wennberg AMV, Lesnick TG, Schwarz CG, et al. Longitudinal association between brain amyloid-beta and gait in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2018;73(9):1244–1250. doi: 10.1093/gerona/glx240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tian Q, Resnick SM, Bilgel M, Wong DF, Ferrucci L, Studenski SA. β-Amyloid burden predicts lower extremity performance decline in cognitively unimpaired older adults. J Gerontol A Biol Sci Med Sci. 2017;72(5):716–723. doi: 10.1093/gerona/glw183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(suppl 1):S161–S189. doi: 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology. 2007;29(1-2):66–73. doi: 10.1159/000109498 [DOI] [PubMed] [Google Scholar]
- 19. von Oertzen T, Hertzog C, Lindenberger U, Ghisletta P. The effect of multiple indicators on the power to detect inter-individual differences in change. Br J Math Stat Psychol. 2010;63(Pt 3):627–646. doi: 10.1348/000711010X486633 [DOI] [PubMed] [Google Scholar]
- 20. Boyle PA, Wilson RS, Buchman AS, et al. Lower extremity motor function and disability in mild cognitive impairment. Exp Aging Res. 2007;33(3):355–371. doi: 10.1080/03610730701319210 [DOI] [PubMed] [Google Scholar]
- 21. Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol. 2018;136(6):887–900. doi: 10.1007/s00401-018-1920-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hyman BT, Trojanowski JQ. Editorial on consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(10):1095–1097. doi: 10.1097/00005072-199710000-00002 [DOI] [PubMed] [Google Scholar]
- 23. Oveisgharan S, Wilson RS, Yu L, Schneider JA, Bennett DA. Association of early-life cognitive enrichment with Alzheimer disease pathological changes and cognitive decline. JAMA Neurol. 2020;77(10):1217–1224. doi: 10.1001/jamaneurol.2020.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oveisgharan S, Dawe RJ, Leurgans SE, et al. Total daily physical activity, brain pathologies, and parkinsonism in older adults. Ehlers DK, ed. PLoS One. 2020;15(4):e0232404. doi: 10.1371/journal.pone.0232404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nag S, Yu L, Capuano AW, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease: HS and TDP-43 in Aging and AD. Ann Neurol. 2015;77(6):942–952. doi: 10.1002/ana.24388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buchman AS, Yu L, Oveisgharan S, Farfel JM, Schneider JA, Bennett DA. Person-specific contributions of brain pathologies to progressive parkinsonism in older adults. J Gerontol Biol Sci Med Sci. 2020;76:615–621. doi: 10.1093/gerona/glaa176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oveisgharan S, Buchman AS, Yu L, et al. APOE ε2ε4 genotype, incident AD and MCI, cognitive decline, and AD pathology in older adults. Neurology. 2018;90(24):e2127–e2134. doi: 10.1212/WNL.0000000000005677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Capuano AW, Wilson RS, Schneider JA, Leurgans SE, Bennett DA. Global odds model with proportional odds and trend odds applied to gross and microscopic brain infarcts. Biostat Epidemiol. 2018:1–16. doi: 10.1080/24709360.2018.1500089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilson RS, Wang T, Yu L, Bennett DA, Boyle PA. Normative cognitive decline in old age. Ann Neurol. 2020;87(6):816–829. doi: 10.1002/ana.25711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo W. Functional mixed effects models. Biometrics. 2002;58(1):121–128. doi: 10.1111/j.0006-341x.2002.00121.x [DOI] [PubMed] [Google Scholar]
- 32. Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503–1527. doi: 10.1093/brain/awz099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu L, Boyle PA, Dawe RJ, Bennett DA, Arfanakis K, Schneider JA. Contribution of TDP and hippocampal sclerosis to hippocampal volume loss in older-old persons. Neurology. 2020;94(2):e142–e152. doi: 10.1212/WNL.0000000000008679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crystal H, Schneider J, Bennett D, Leurgans S, Levine S. Associations of cerebrovascular and Alzheimer’s disease pathology with brain atrophy. Curr Alzheimer Res. 2014;11(4):309–316. doi: 10.2174/1567205011666140302194358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. 2016;139(11):2983–2993. doi: 10.1093/brain/aww224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oveisgharan S, Yu L, Capuano A, et al. Late-life vascular risk score in association with postmortem cerebrovascular disease brain pathologies. Stroke. 2021;52(6):2060–2067. doi: 10.1161/STROKEAHA.120.030226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buchman AS, Dawe RJ, Leurgans SE, et al. Different combinations of mobility metrics derived from a wearable sensor are associated with distinct health outcomes in older adults. J Gerontol A Biol Sci Med Sci. 2020;75(6):1176–1183. doi: 10.1093/gerona/glz160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jack CR, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. la Fougère C, Zwergal A, Rominger A, et al. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. NeuroImage 2010;50(4):1589–1598. doi: 10.1016/j.neuroimage.2009.12.060 [DOI] [PubMed] [Google Scholar]
- 40. Ward NS, Frackowiak RSJ. Age-related changes in the neural correlates of motor performance. Brain J Neurol. 2003;126(Pt 4):873–888. doi: 10.1093/brain/awg071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leng K, Li E, Eser R, et al. Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease. Nat Neurosci. 2021;24(2):276–287. doi: 10.1038/s41593-020-00764-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Das RR, Seshadri S, Beiser AS, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham Offspring Study. Stroke. 2008;39(11):2929–2935. doi: 10.1161/STROKEAHA.108.516575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Takakusaki K. Functional neuroanatomy for posture and gait control. J Mov Disord. 2017;10(1):1–17. doi: 10.14802/jmd.16062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jelistratova I, Teipel SJ, Grothe MJ. Longitudinal validity of PET-based staging of regional amyloid deposition. Hum Brain Mapp. 2020;41(15):4219–4231. doi: 10.1002/hbm.25121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68(11):1379–1386. doi: 10.1093/gerona/glt089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buchman AS, Leurgans SE, Nag S, et al. Spinal arteriolosclerosis is common in older adults and associated with parkinsonism. Stroke. 2017;48(10):2792–2798. doi: 10.1161/STROKEAHA.117.017643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buchman AS, Nag S, Leurgans SE, et al. Spinal Lewy body pathology in older adults without an antemortem diagnosis of Parkinson’s disease. Brain Pathol. 2018;28(4):560–568. doi: 10.1111/bpa.12560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62(9):1048–1055. doi: 10.1093/gerona/62.9.1048 [DOI] [PubMed] [Google Scholar]
- 50. White O, Davare M, Andres M, Olivier E. The role of left supplementary motor area in grip force scaling. PLoS One. 2013;8(12):e83812. doi: 10.1371/journal.pone.0083812 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.