Abstract
Background
Patients with inflammatory bowel disease (IBD) are at an increased risk of malnutrition. The goal of this study was to define the prevalence of malnutrition and micronutrient deficiencies in recently diagnosed IBD patients and to compare the performance of existing malnutrition screening tools in identifying IBD patients at increased risk for malnutrition.
Methods
This was a retrospective cohort study of adult patients with recently diagnosed IBD (≤18 months disease duration). A diagnosis of malnutrition was made utilizing the European Society for Clinical Nutrition and Metabolism malnutrition criteria. Serum micronutrient levels were included. The sensitivity of 5 malnutrition screening tools in identifying patients at moderate-high risk of malnutrition was determined based on the European Society for Clinical Nutrition and Metabolism malnutrition definition. Descriptive statistics summarized the data and univariate analyses tested associations.
Results
A total of 182 patients were included for analysis; 65 (36%) met criteria for malnutrition. A total of 135 (74%) patients had ≥1 micronutrient level checked and 105 (78%) had ≥1 deficiency. Patients with prior surgery (odds ratio [OR], 4.5; P = .004), active Crohn’s disease (OR, 2.8; P = .03), and diarrhea (OR, 2.1; P = .02) were more likely to be malnourished. The Malnutrition Universal Screening Tool and Saskatchewan IBD Nutrition Risk Tool had the highest sensitivity (100%) in predicting those at moderate-high risk of malnutrition at the time of screening.
Conclusions
Patients with recently diagnosed IBD have a high prevalence of malnutrition and micronutrient deficiencies. Both the Malnutrition Universal Screening Tool and Saskatchewan IBD Nutrition Risk Tool can be used to identify those at increased risk of malnutrition. Future studies and screening tool development are necessary to identify those at risk of developing malnutrition to facilitate timely referral for nutritional evaluation and prevent disease related complications.
Keywords: Malnutrition, Micronutrient Deficiencies, Inflammatory bowel disease, Screening
Key Messages.
- It is well known that malnutrition impacts many patients with inflammatory bowel disease and can have a profound impact on the clinical course.
- This study demonstrates that patients with recently diagnosed inflammatory bowel disease have a high prevalence of malnutrition as well as micronutrient deficiencies.
- The majority of patients with inflammatory bowel disease are not being screened for malnutrition routinely; this study highlights the importance of screening and identifies which tools have the highest sensitivity to screen patients in the ambulatory clinic setting.
Introduction
Inflammatory bowel disease (IBD) affects over 6.8 million people worldwide, and the incidence is steadily rising.1 While the pathogenesis of both Crohn’s disease (CD) and ulcerative colitis (UC) remains unclear, both diagnoses can lead to severe inflammation resulting in significant, often debilitating symptoms and decreased quality of life. Patients with IBD may also experience clinical malnutrition as defined by both the American and European Nutritional Societies.2,3 Malnutrition in IBD may result from many mechanisms including decreased oral intake, food trigger avoidance, medication effects, malabsorption, volume loss, altered anatomy due to prior surgery, and increased nutritional needs in the setting of active inflammation. In addition, although commonly overlooked, micronutrient deficiencies associated with restrictive diets, altered postsurgical anatomy, and untreated inflammation may occur in patients with IBD and is estimated to effect up to 50% of patients.4 Malnutrition has been associated with poor clinical outcomes in patients with IBD, including increased numbers of flares, impaired response to medical therapy, higher rates of surgical complications, reduced quality of life, and increased length of stay in those hospitalized for an IBD-related complication.5-7 The reported prevalence of malnutrition and micronutrient deficiencies among patients with IBD varies widely in the literature (from 20% to 80%), likely reflecting heterogeneity in studied populations and lack of standardization of measurement tools.5,8
The goal of this study was to define the prevalence of malnutrition and micronutrient deficiencies in a well-characterized population of recently diagnosed IBD patients and to compare the performance of existing malnutrition screening tools in this population to identify those who are currently malnourished as well as those at risk for the development of malnutrition in the future.
Methods
This was a single-center, retrospective cohort study of patients 18 years of age and older with a diagnosis of IBD within the preceding 18 months, as part of a large, prospective cohort of recently diagnosed patients with IBD who are enrolled in a registry at our center (comprehensive care for recently diagnosed patients with IBD [COMPASS-IBD] registry). This program includes sessions with an IBD physician as well as with an IBD focused nutritionist, pharmacist and social worker. Patient demographics and disease characteristics were collected at the initial (screening) IBD visit through structured questionnaires and medical record review. All patients were retrospectively evaluated for a diagnosis of malnutrition at the initial visit using the European Society for Clinical Nutrition and Metabolism (ESPEN) definition of malnutrition.
As per the ESPEN criteria, patients were defined as malnourished if they had a body mass index (BMI) of <18.5 kg/m2 or if the patient reported unintentional weight loss of >10% in any time period or >5% over 3 months in addition to BMI <20 or <22 kg/m2 in those <70 years of age and in those ≥70 years of age, respectively.9 All patients were evaluated for risk of malnutrition utilizing 5 previously validated screening tools. Three of these tools were designed for the general population, including the Malnutrition Universal Screening Tool (MUST), the Short Nutritional Assessment Questionnaire (SNAQ), and the Nutritional Risk Index (NRI). Two additional screening tools were utilized, which were developed specifically for patients with IBD, including the Malnutrition Inflammatory Risk Tool (MIRT) and the Saskatchewan IBD Nutrition Risk Tool (SaskIBD-NR) (Supplemental Table 1). The risk of malnutrition was assessed retrospectively with data collected at time of cohort enrollment. The performance of each tool was determined by comparing the screening tool results to the established diagnostic criteria for malnutrition (ESPEN criteria). For the MUST, SNAQ and SaskIBD-NR, the moderate-risk and high-risk groups were combined and compared with the low-risk group based on previous literature utilizing these tools.10 NRI scores were combined to compare no risk and low risk with moderate risk and high risk. The MIRT was designed as a dichotomous variable (high risk vs not high risk) and therefore did not require modification. Patients who did not meet ESPEN criteria for malnutrition at the initial visit were then followed for 1 year (evaluated at 6 and 12 months) for the development of malnutrition.
In addition, serum micronutrient levels were assessed at the discretion of the treating physician and included when available. Vitamins and minerals evaluated included vitamin B6, vitamin B12, vitamin C, vitamin D, folate, ferritin, phosphorous, and zinc. Identified deficiencies in these micronutrients were recorded.
Descriptive statistics were used to summarize the data including Student t tests for continuous variables and Fisher exact tests for categorical (binary) variables. Sensitivity and specificity for each screening tool was calculated for malnutrition at baseline and their association with future development of malnutrition at 6 and 12 months. Univariable logistic regression was used to test associations between demographic and disease characteristics and the presence of malnutrition using SAS Statistical Software V.9.3 (SAS Institute, Cary, NC, USA).
Ethical Considerations
The study was conducted according to the ethical principles of the Declaration of Helsinki. This study was approved by the Institutional Review Board at the Icahn School of Medicine at Mount Sinai.
Results
A total of 207 patients were included in the COMPASS-IBD cohort of newly diagnosed IBD patients; of these, 182 had complete clinical data to retrospectively determine malnutrition risk and were therefore included in this study. The median time from diagnosis to initial visit was 2.5 (interquartile range [IQR], 1-6) months; 91 (50%) patients were male, 109 (59%) had CD, and the median age was 27 (IQR, 22-35) years. At baseline, 100 (55%) patients had a normal BMI (18.5-24.9 kg/m2), while 18 (10%) were underweight (BMI ≤18.5 kg/m2), 44 (24%) were overweight (BMI 25-29.9 kg/m2), and 20 (11%) were obese (BMI 30-39.9 kg/m2). Moreover, at baseline, 81 (45%) patients reported unintentional weight loss in the past 3 months, with a median weight loss of 15.5 (IQR, 10-24) pounds reported. Further cohort characteristics are provided in Table 1.
Table 1.
Demographic and disease characteristics of patients
| Characteristic | Overall Cohort (n = 182) | Malnourished at Screening Visit (n = 65) | Not Malnourished at Screening Visit (n = 117) |
|---|---|---|---|
| Women | 92 (51) | 31 (47.7) | 61 (52.1) |
| CD | 108 (59) | 45 (69.2) | 63 (53.8) |
| Age, y | 27 (22-35) | 25 (20-30) | 29 (23-37) |
| Disease location | |||
| Colon | 93 (51) | 40 (61.5) | 53 (45.3) |
| Small bowel | 89 (49) | 36 (55.4) | 53 (45.3) |
| Perianal | 10 (5) | 2 (3.1) | 8 (6.8) |
| Prior abdominal surgery | 10 (5) | 7 (10.7) | 3 (2.6) |
| Surgery type | |||
| ICR | 4 (2) | 3 (4.6) | 1 (0.9) |
| STC with EI | 1 (0.5) | 1 (1.5) | 0 (0) |
| STC with IPAA | 0 (0) | 0 (0) | 0 (0) |
| Small bowel resection | 5 (3) | 2 (3.1) | 3 (2.6) |
| Bariatric surgery | 1 (0.5) | 1 (1.5) | 0 (0) |
| Extraintestinal manifestations | 47 (26) | 17 (26.2) | 30 (25.6) |
| Time from diagnosis to baseline, mo | 2.5 (1-6) | 2 (1-6) | 3 (1-6.3) |
| Biologic naïve at baseline | 136 (75) | 43 (66) | 93 (79) |
| Weight at baseline visit, kg | 68.9 (58.5-78.3) | 59.4 (52.5-71.2) | 73 (62.1-81.6) |
| BMI at baseline visit, kg/m2 | 23.2 (20.9-26.0) | 20.6 (18.8-23.1) | 24.9 (22.5-27.0) |
| Disease activity at baseline visit | |||
| Partial Mayo score | 2 (0-4.3) | 3 (1-5.5) | 2 (0-4) |
| HBI | 2 (1-4) | 3 (1-5) | 2 (1-3.5) |
| Diarrhea reported at baseline visit | 63 (35) | 29 (44.6) | 34 (29.1) |
| Weight loss reported at baseline visit | 81 (45) | 48 (73.8) | 33 (28.2) |
Values are n (%) or median (interquartile range)
Abbreviations: BMI, body mass index; CD, Crohn’s disease; EI, end ileostomy; HBI, Harvey-Bradshaw Index; ICR, ileocolic resection; IPAA, ileal pouch–anal anastomosis; STC, subtotal colectomy.
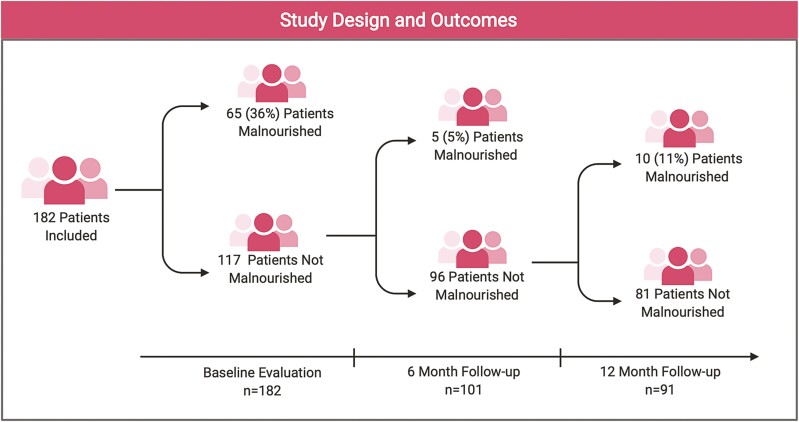
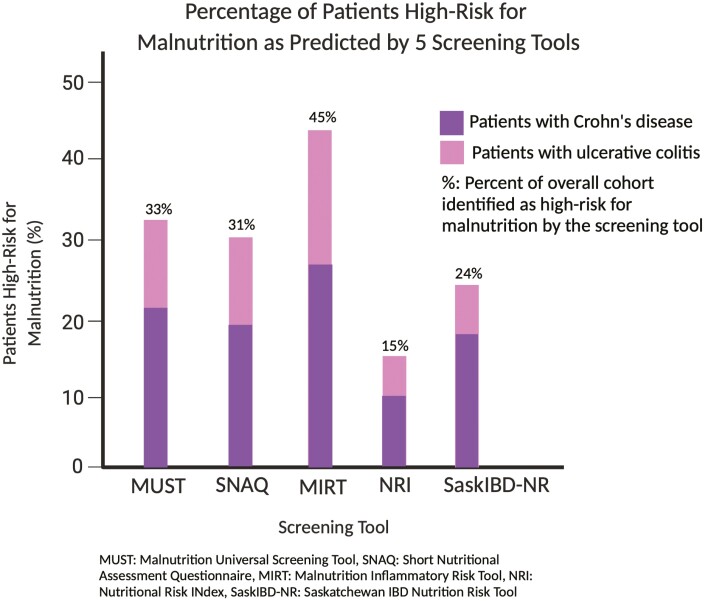
A flow diagram of the cohort is displayed in Figure 1 with the proportion of patients who met criteria for malnutrition (ESPEN) at the different time points in the study. Based on the ESPEN criteria, 65 (36%) patients were malnourished at baseline. Of those malnourished at baseline, 45 (69%) had a diagnosis of CD and 20 (31%) had UC. Univariate analysis demonstrated that patients with a prior IBD-related surgery (odds ratio [OR], 4.5; 95% confidence interval [CI], 1.1-18.2; P = .04), those who reported diarrhea (OR, 2.1; 95% CI, 1.1-4.0; P = .02), and those with active CD (Harvey-Bradshaw Index ≥ 5) (OR, 2.8; 95% CI, 1.1-7.4; P = .03) were more likely to be malnourished. Comparing the 5 previously validated malnutrition screening tools, 60 (33%) patients were identified as high risk for malnutrition by the MUST, 55 (31%) were high risk by the SNAQ, and 27 (15%) were high risk by the NRI. In addition, the MIRT and SaskIBD-NR identified 81 (45%) and 44 (24%) patients as high risk for malnutrition, respectively (Figure 2). The performance of the malnutrition screening tools for identifying malnourished patients is summarized in Table 2. Evaluating those who were moderate to high risk, the highest sensitivity was seen with the MUST and SaskIBD-NR (both had a sensitivity of 100%). Comparatively, the NRI had the lowest sensitivity (76.6%) (Table 2). The sensitivity of predicting the development of malnutrition at 6- and 12-month follow-up was significantly lower for all of the tools evaluated in this study; once again, the SaskIBD-NR and MUST had the highest sensitivity at predicting malnutrition at 6 months (60%), and the SaskIBD-NR had the highest sensitivity at 12-month follow-up (50%).
Figure 1.
Retrospective cohort study design and the development of malnutrition over 6 and 12 months. Figure created with BioRender.com.
Figure 2.
Percentage of patients identified as high-risk for malnutrition by the 5 screening tools utilized in this study. Figure created with BioRender.com. MIRT, Malnutrition Inflammatory Risk Tool; MUST, Malnutrition Universal Screening Tool; NRI, Nutritional Risk Index; SaskIBD-NR, Saskatchewan Inflammatory Bowel Disease, Nutrition Risk Tool; SNAQ, Short Nutrition Assessment Questionnaire.
Table 2.
Comparison of performance of malnutrition screening tools at baseline as well as at 6- and 12-month follow-up
| Screening Tool | Months From Baseline/Screening Visit | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| MUST | 0 | 100.0 | 66.7 |
| 6 | 60.0 | 69.8 | |
| 12 | 40.0 | 69.1 | |
| SNAQ | 0 | 93.6 | 79.5 |
| 6 | 40.0 | 80.2 | |
| 12 | 40.0 | 82.7 | |
| MIRT | 0 | 95.4 | 83.3 |
| 6 | 20.0 | 84.2 | |
| 12 | 10.0 | 82.5 | |
| NRI | 0 | 76.7 | 71.7 |
| 6 | 60.0 | 73.1 | |
| 12 | 10.0 | 70.9 | |
| SaskIBD-NR | 0 | 100.0 | 63.3 |
| 6 | 60.0 | 66.7 | |
| 12 | 50.0 | 69.1 |
Abbreviations: MIRT, Malnutrition Inflammatory Risk Tool; MUST, Malnutrition Universal Screening Tool; NRI, Nutritional Risk Index; SaskIBD-NR, Saskatchewan Inflammatory Bowel Disease, Nutrition Risk Tool; SNAQ, Short Nutrition Assessment Questionnaire.
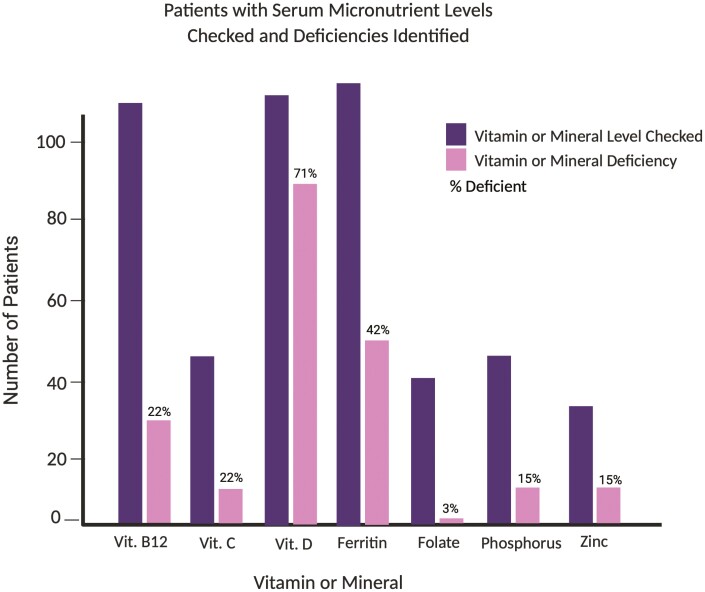
The prevalence of micronutrient deficiencies at baseline was determined among those patients with serum micronutrient levels checked at the discretion of their treating physician; 135 (74%) patients had at least 1 micronutrient level checked, and of these patients, 105 (78%) had at least 1 deficiency (Figure 3) More specifically, 71% of 116 with vitamin D 25-OH levels were deficient, 42% of the 119 tested had low ferritin, 22% of those 115 tested were deficient in vitamin B12, and 22% of 46 measured had low vitamin C levels. In addition, 15% (n = 5 of 34) of zinc and 15% (n = 7 of 46) of phosphorus levels checked were deficient. Only 1 case of folate deficiency was identified among 40 patients who were tested. Of the 65 patients who met criteria for malnutrition at the time of screening, 10 (15%) did not have serum micronutrient levels checked.
Figure 3.
Number of patients with serum micronutrient levels checked and deficiencies identified. Figure created with BioRender.com. Vit., vitamin.
Discussion
In this large, single-center cohort study, we observed that malnutrition was highly prevalent (>30%) in newly diagnosed IBD patients and that both the MUST and SaskIBD-NR have a high sensitivity for detecting a moderate-to-high risk of malnutrition at the time of screening. Patients with prior IBD-related surgery, diarrhea, and clinically active CD (Harvey-Bradshaw Index ≥ 5) were more likely to be malnourished at the time of initial screening. Although the MUST and SaskIBD-NR performed well at identifying those at moderate-high risk for malnutrition at the time of screening, all 5 of the tools included in this study were of lower utility in identifying patients who would develop malnutrition at 6 and 12 months after screening. Roughly three-quarters of patients in this study had 1 or more serum micronutrient levels checked at the initial visit and of these, 78% had at least 1 deficiency. While many of these included deficiencies commonly associated with IBD (such as vitamin D, iron, and vitamin B12), deficiencies in less frequently checked vitamins and minerals such as vitamin C and zinc were also identified.
The prevalence of malnutrition in patients with newly diagnosed IBD has not been previously defined. In 2019, Bachand et al9 reported a prevalence of malnutrition of 15% among patients with long-standing quiescent CD. While this study certainly highlighted a high prevalence of malnutrition among patients in clinical remission, the highest-risk patients (those with active disease) were excluded. A subsequent study identified malnutrition in 42% of patients with IBD undergoing surgery.11 While numerous similar studies have reported a prevalence of malnutrition in a subset of IBD patients, the majority excluded patients with UC and many only include those requiring hospitalization or surgery. In contrast, our study uniquely evaluated malnutrition in patients with CD and UC at diagnosis and included patients with both active and quiescent disease who were seen in an ambulatory clinic.
Given the high prevalence of malnutrition in patients with newly diagnosed IBD identified in this study and the poor outcomes associated with malnutrition in patients with IBD, the ability to screen for malnutrition is crucial. In our study, the MUST and SaskIBD-NR performed the best at identifying malnutrition. Of note, the components of the MUST overlap significantly with the ESPEN diagnostic criteria for malnutrition. Therefore, it is not surprising that the MUST performed so well in predicting malnutrition at the time of the screening. However, the SaskIBD-NR questionnaire components do not overlap with the ESPEN diagnostic criteria, yet this tool still performed extremely well in predicting malnutrition at the time of screening. To date, there is no single, universally accepted malnutrition screening tool to accurately and noninvasively invasively identify those at risk for malnutrition in the clinic. While the ESPEN guidelines recommend screening for malnutrition in all patients with newly diagnosed IBD and at routine follow-up, the guidelines do not delineate which tool should be used in this high-risk population.4 In a systematic review evaluating nutritional screening and assessment in patients with IBD, Li et al12 stated that although malnutrition and sarcopenia are highly prevalent among IBD patients, they were unable to identify a single, best nutritional screening or diagnostic tool based on the current available literature.12 In contrast, Bachand et al9 compared 5 different screening tools and concluded that the SNAQ was best at identifying malnutrition in patients with CD. More recently, a nutritional care pathway was published from the IBD Qorus and utilized a modified MUST score to screen a cohort of IBD patients.13 This was one of the first studies to propose a formal algorithm to screen patients with IBD; however, the modified MUST screening tool that was used was not previously validated in the IBD population.13 These studies certainly illustrate the significant heterogeneity in the literature on malnutrition screening tools in IBD and highlight the need for a single, universally utilized tool in this high-risk patient population.
Prior studies have demonstrated the prevalence and clinical importance of micronutrient deficiencies in patients with IBD, highlighting the importance of screening for malnutrition and micronutrient deficiencies early in the disease course to prevent disease-related complications.4 The most common micronutrient deficiencies in patients with IBD include iron, vitamin B12, vitamin D, folic acid, selenium, zinc, and vitamin B6, and this is estimated to affect more than 50% of the IBD population.14 Vitamin and mineral deficiencies not only are highly prevalent in patients with IBD, but also have been demonstrated to lead to clinically significant comorbid conditions, such as anemia, osteoporosis, thrombophilia, poor wound healing, worsening of chronic inflammation, and carcinogenesis.15 Although there are no formal guidelines for the assessment of micronutrients in patients with IBD, many studies have suggested that those with active disease and those in clinical remission should be routinely assessed for vitamin and mineral deficiencies.15 Moreover, prior clinical and basic science studies have highlighted the significance of more commonly overlooked vitamin and mineral deficiencies, such as the development of clinical scurvy in ambulatory IBD patients from vitamin C deficiency and worsening colitis in a mouse model in the setting of zinc deficiency.16,17 Screening for micronutrient deficiencies at the time of diagnosis allows for early detection and prevention of malnutrition and IBD-related complications including osteoporosis, poor wound healing, and symptoms that may confound the clinical picture including anemia and diarrhea from iron and zinc deficiencies, respectively. Moreover, some of these complications associated with micronutrient deficiencies such as posterolateral cord syndrome from vitamin B12 deficiency or myelopathy from copper deficiency are irreversible, and therefore it is crucial to identify and treat these deficiencies early in the disease course.18,19
Strengths of this study include the size of the cohort as well as the prospective nature of the larger cohort with routine collection of data necessary to determine malnutrition risk at screening and at follow-up. While previous studies on malnutrition in IBD specifically studied patients in remission or included only those with CD, this study included patients with both active and quiescent disease as well as all IBD types. This study highlights the prevalence of malnutrition in those with both CD and UC as well as in those with quiescent disease and those with active inflammation, emphasizing the importance of screening for malnutrition in all patients with IBD both at the time of diagnosis and routinely thereafter.
Malnutrition is commonly defined by either the ESPEN diagnostic criteria, which take into account BMI and unintentional weight loss, or the criteria from the American Society for Parenteral and Enteral Nutrition (ASPEN). The ASPEN diagnostic criteria include an estimate of energy intake and a nutrition focused physical exam evaluating for muscle wasting and edema as well as a history of unintentional weight loss. The ASPEN criteria are thought to be more subjective, given the variability in the nutrition focused physical exam (findings may vary by examiner). Given the retrospective nature of this study, the ASPEN criteria were not utilized to define malnutrition. However, the ESPEN criteria place a large emphasis on BMI; it does not take into account the impact of acute inflammation or reduced oral intake and does not include a measure of sarcopenia. More recently, the Global Leadership Initiative on Malnutrition proposed novel malnutrition diagnostic criteria, which include phenotypic criteria of unintentional weight loss, low BMI, and reduced muscle mass as well as etiologic criteria of poor oral intake and inflammation or acute illness. Of note, these criteria have been neither validated in ambulatory IBD patients nor adopted into routine use. The Global Leadership Initiative on Malnutrition criteria certainly include a more comprehensive definition of malnutrition including an evaluation of sarcopenia and, once validated in the outpatient IBD population, will likely provide improved diagnostic criteria for this high-risk patient population.
This study has several potential limitations. First, the study was limited to young adults with IBD. While malnutrition can have a profound impact on children with IBD due to their increased nutritional needs, the screening tools studied were not designed for use in the pediatric setting.20 Similarly, there were very few older patients (>65 years of age) included in the study. The significant impact of malnutrition on clinical outcomes is perhaps best described in the geriatric literature, and having a young study population likely resulted in an underestimation of the malnutrition risk. Moreover, the predominance of young patients in this study limits the generalizability of the results to patients across all age groups.21,22 Additionally, malnutrition is a broad term that includes both undernutrition and overnutrition. However, the malnutrition screening tools utilized in this study only identify those at risk of undernutrition and do not take into account sarcopenic obesity. Furthermore, although we serve a large local population of patients with IBD, we are a tertiary care center and, therefore, a referral bias may be present, reducing the generalizability of the findings in this study. In addition, for patients that were not previously seen in our medical system, we had to rely on reported weight loss or gain in the prior 3 to 6 months, which may have introduced recall bias. Of note, the prevalence of malnutrition at 6 and 12 months was low and therefore this limits the evaluation of the sensitivity of the screening tools in predicting malnutrition. Finally, given the retrospective nature of this study, we were reliant on the data in the electronic medical records. Ideally, future, prospective studies would confirm the findings in this study.
Conclusions
In summary, we observed that patients with newly diagnosed IBD have a high prevalence of malnutrition and micronutrient deficiencies and that both the MUST and SaskIBD-NR can be used to noninvasively identify those at risk of malnutrition with high sensitivity at the time of screening. None of the tools evaluated had a high sensitivity for predicting the development of malnutrition at 6 or 12 months. Given that over one-third of patients met ESPEN criteria for malnutrition at their initial visit to our center, perhaps malnutrition may predate other symptoms of IBD, and therefore, a new diagnosis of malnutrition or micronutrient deficiencies in young patients with atypical symptoms should prompt further evaluation. Future studies and screening tool development are necessary to identify patients who are at risk of developing malnutrition to facilitate timely referral for nutritional evaluation and ultimately prevent clinically significant malnutrition and disease-related complications.
Supplementary Material
Contributor Information
Stephanie L Gold, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Loren G Rabinowitz, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
Laura Manning, Susan and Leonard Feinstein Inflammatory Bowel Disease Clinical Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Laurie Keefer, Susan and Leonard Feinstein Inflammatory Bowel Disease Clinical Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
William Rivera-Carrero, Susan and Leonard Feinstein Inflammatory Bowel Disease Clinical Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Stephanie Stanley,, University of New England College of Osteopathic Medicine, Biddeford, ME, USA.
Alexis Sherman, Susan and Leonard Feinstein Inflammatory Bowel Disease Clinical Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Ana Castillo, Susan and Leonard Feinstein Inflammatory Bowel Disease Clinical Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Stacy Tse, Susan and Leonard Feinstein Inflammatory Bowel Disease Clinical Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Amanda Hyne,, Susan and Leonard Feinstein Inflammatory Bowel Disease Clinical Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Kristina Matos,, Susan and Leonard Feinstein Inflammatory Bowel Disease Clinical Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Benjamin Cohen, Department of Gastroenterology, Hepatology and Nutrition, Cleveland Clinic, Cleveland, OH, USA.
Ari Grinspan, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Jean-Frederic Colombel, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Bruce E Sands, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Susan and Leonard Feinstein Inflammatory Bowel Disease Clinical Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Marla C Dubinsky, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Department of Pediatrics, Susan and Leonard Feinstein Inflammatory Bowel Disease Clinical Center, Icahn School of Medicine Mount Sinai, New York, NY, USA.
Ryan C Ungaro, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Funding
R.C.U. is supported by a National Institutes of Health K23 Career Development Award (K23 KD111995-01A1).
Conflicts of Interest
M.C.D. has served as a consultant for AbbVie, Arena, BMS, Boehringer Ingelheim, Eli Lilly, Janssen, Pfizer, Prometheus Labs, Takeda, and UCB. B.E.S. has received research grants from Takeda, Pfizer, Theravance Biopharma R&D, Janssen; has received consulting fees from 4D Pharma, Abivax, AbbVie, Alimentiv, Allergan, Amgen, Arena Pharmaceuticals, AstraZeneca, Bacainn Therapeutics, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Calibr, Capella Bioscience, Celgene, Celltrion Healthcare, ClostraBio, Enthera, F. Hoffmann-La Roche, Ferring, Galapagos, Gilead, Glaxo SmithKline, GossamerBio, Immunic, Index Pharmaceuticals, Innovation Pharmaceuticals, Ironwood Pharmaceuticals, Janssen, Kaleido, Kallyope, Lilly, MiroBio, Morphic Therapeutic, Oppilan Pharma, OSE Immunotherapeutics, Otsuka, Palatin Technologies, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Redhill Biopharma, Rheos Medicines, Salix Pharmaceuticals, Seres Therapeutics, Shire, Sienna Biopharmaceuticals, Sun Pharma, Surrozen, Takeda, Target PharmaSolutions, Teva Branded Pharmaceutical Products R&D, Thelium, Theravance Biopharma R&D, TLL Pharma, USWM Enterprises, Ventyx Biosciences, Viela Bio, Vivante Health, Vivelix Pharmaceuticals; and owns stock for Vivante Health and Ventyx Biosciences. J.-F.C. has received research grants from AbbVie, Janssen Pharmaceuticals, and Takeda; has received payment for lectures from AbbVie, Amgen, Allergan, Ferring Pharmaceuticals, Shire, and Takeda; has received consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene Corporation, Eli Lilly, Ferring Pharmaceuticals, Galmed Research, GlaxoSmithKline, Geneva, Iterative Scopes, Janssen Pharmaceuticals, Kaleido Biosciences, Landos, Otsuka, Pfizer, Prometheus, Sanofi, Takeda, and TiGenix; and holds stock options in Intestinal Biotech Development. R.C.U. has served as an advisory board member or consultant for AbbVie, Bristol-Myers Squibb, Janssen, Pfizer, and Takeda; and received research support from AbbVie, Boehringer Ingelheim, Eli Lilly, and Pfizer. L.K. has served as consultant for Lilly, Takeda, AbbVie, and Reckitt Health; and is co-founder of and owns equity in Trellus Health. S.L.G., L.G.R., L.M., W.R.-C., S.S., A.S., A.C., S.T., A.H., K.M., and A.G. have no conflicts of interest and nothing to disclose. B.C. has served on the advisory boards and as a consultant for AbbVie, Celgene-Bristol-Myers Squibb, Pfizer, Sublimity Therapeutics, Takeda, TARGET RWE, CME Companies: Cornerstones, and Vindico; has served as a speaker for AbbVie; and has received educational grant support from Pfizer.
References
- 1. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. White JV, Guenter P, Jensen G, et al. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr. 2012;36(3):275–283. [DOI] [PubMed] [Google Scholar]
- 3. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - an ESPEN Consensus Statement. Clin Nutr. 2015;34(3):335–340. [DOI] [PubMed] [Google Scholar]
- 4. Forbes A, Escher J, Hebuterne X, et al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36(2):321–347. [DOI] [PubMed] [Google Scholar]
- 5. Balestrieri P, Ribolsi M, Guarino MPL, Emerenziani S, Altomare A, Cicala M.. Nutritional aspects in inflammatory bowel diseases. Nutrients 2020;12(2):372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jansen I, Prager M, Valentini L, Buning C.. Inflammation-driven malnutrition: a new screening tool predicts outcome in Crohn’s disease. Br J Nutr. 2016;116(6):1061–1067. [DOI] [PubMed] [Google Scholar]
- 7. Takaoka A, Sasaki M, Nakanishi N, et al. Nutritional screening and clinical outcome in hospitalized patients with Crohn’s disease. Ann Nutr Metab. 2017;71(3-4):266–272. [DOI] [PubMed] [Google Scholar]
- 8. Bertani L, Ribaldone DG, Bellini M, Mumolo MG, Costa F.. Inflammatory bowel diseases: is there a role for nutritional suggestions? Nutrients 2021;13(4):1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bachand MP, Ruel J, Plamondon S.. Comparison between different tools for screening and assessment of nutritional status of patients with Crohn’s disease in an ambulatory setting. J Clin Nutr Diet 2019;4(3):1. [Google Scholar]
- 10. Stratton RJ, King CL, Stroud MA, Jackson AA, Elia M.. “Malnutrition Universal Screening Tool” predicts mortality and length of hospital stay in acutely ill elderly. Br J Nutr. 2006;95(2):325–330. [DOI] [PubMed] [Google Scholar]
- 11. Fiorindi C, Luceri C, Dragoni G, et al. GLIM criteria for malnutrition in surgical IBD patients: a pilot study. Nutrients 2020;12(8):2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li S, Ney M, Eslamparast T, et al. Systematic review of nutrition screening and assessment in inflammatory bowel disease. World J Gastroenterol. 2019;25(28):3823–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hwang C, Issokson K, Giguere-Rich C, et al. Development and pilot testing of the inflammatory bowel disease nutrition care pathway. Clin Gastroenterol Hepatol. 2020;18(12):2645–2649.e4. [DOI] [PubMed] [Google Scholar]
- 14. Weisshof R, Chermesh I.. Micronutrient deficiencies in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2015;18(6):576–581. [DOI] [PubMed] [Google Scholar]
- 15. Hwang C, Ross V, Mahadevan U.. Micronutrient deficiencies in inflammatory bowel disease: from A to zinc. Inflamm Bowel Dis. 2012;18(10):1961–1981. [DOI] [PubMed] [Google Scholar]
- 16. Dunleavy KA, Ungaro RC, Manning L, Gold S, Novak J, Colombel JF.. Vitamin C deficiency in inflammatory bowel disease: the forgotten micronutrient. Crohns Colitis 360 2021;3(1):otab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higashimura Y, Takagi T, Naito Y, et al. Zinc deficiency activates the IL-23/Th17 axis to aggravate experimental colitis in mice. J Crohns Colitis 2020;14(6):856–866. [DOI] [PubMed] [Google Scholar]
- 18. Pandey S, Holla VV, Rizvi I, Qavi A, Shukla R.. Can vitamin B12 deficiency manifest with acute posterolateral or posterior cord syndrome? Spinal Cord Ser Cases 2016;2:16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaiser SR, Winston GP.. Copper deficiency myelopathy. J Neurol. 2010;257(6):869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moeeni V, Day AS.. Impact of inflammatory bowel disease upon growth in children and adolescents. ISRN Pediatr 2011;2011:365712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Faye AS, Colombel JF.. Age is just a number-frailty associates with outcomes of patients with inflammatory bowel disease. Gastroenterology 2020;158(8):2041–2043. [DOI] [PubMed] [Google Scholar]
- 22. Faye AS, Wen T, Soroush A, et al. Increasing prevalence of frailty and its association with readmission and mortality among hospitalized patients with IBD. Dig Dis Sci. 2021;66(12):4178–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.