Abstract
Background
Past research has not investigated both lower-extremity power and upper-extremity strength in the same fall injury study, particularly nonfracture fall injuries.
Methods
In the Osteoporotic Fractures in Men Study (baseline: N = 5 994; age 73.7 ± 5.9 years; 10.2% non-White), fall injuries (yes/no) were assessed prospectively with questionnaires approximately every 3 years over 9 years. Maximum leg power (Watts) from Nottingham single leg press and maximum grip strength (kg) from handheld dynamometry were assessed at baseline and standardized to kg body weight. Physical performance included gait speed (6-m usual; narrow walk) and chair stands speed.
Results
Of men with ≥1/4 follow-ups (N = 5 178; age 73.4 ± 5.7 years), 40.4% (N = 2 090) had ≥1 fall injury. In fully adjusted repeated-measures logistic regressions, lower power/kg and grip strength/kg had higher fall injury risk (trend across quartiles: both p < .0001), with lower quartiles at significantly increased risk versus highest Q4 except for grip strength Q3 versus Q4. Fall injury risk was 19% higher per 1 standard deviation (SD) lower power/kg (95% confidence interval [CI]: 1.12–1.26) and 16% higher per SD lower grip strength/kg (95% CI: 1.10–1.23). In models including both leg power/kg and grip strength/kg, odds ratios (ORs) were similar and independent of each other and physical performance (leg power/kg OR per SD = 1.13, 95% CI: 1.06–1.20; grip strength/kg OR per SD = 1.11, 95% CI: 1.05–1.17).
Conclusions
Lower leg power/kg and grip strength/kg predicted future fall injury risk in older men independent of physical performance. Leg power potentially identifies fall injury risk better than grip strength at higher muscle function, though grip strength may be more suitable in clinical/practice settings.
Keywords: Falls, Muscle, Physical performance
Both muscle power and strength may be independently related to geriatric outcomes, such as falls that result in injuries, including nonfracture fall injury (NFFI) and fracture. Lower-extremity muscle power (force * velocity) is defined as the ability to exert force at maximum movement velocity (1). Grip strength has been suggested as a biomarker of aging (2) and low grip strength has been linked to mortality (3). Both muscle function measures have been related to chronic conditions, such as osteoarthritis, diabetes mellitus, and cardiovascular disease in aging populations (3,4). While both muscle power and strength are lower at older ages compared to younger ages, larger magnitudes of age-related declines have been described for power than for strength (5–10). Therefore, power rather than strength could potentially be an earlier indicator of age-related loss in muscle function and increased risk for falls and fall injuries.
Falls are the leading cause of both fatal and nonfatal injuries in older adults. In 2018, 10.2% of adults ≥65 years reported a fall injury in the past 12 months (11). Importantly, these fall-related injuries have been linked to mortality, morbidity, and disability (12,13). Recent findings have also noted a distinct increase in number and cost of fatal and nonfatal medically treated fall injuries (11,14). Therefore, establishing muscle function risk factors for fall injuries (both NFFI and fractures) may inform disability and mortality prevention in older adults.
Falls are common in older adults with 20%–35% self-reporting at least 1 fall annually (11,12,15–20). Lower leg power and strength (21,22) and grip strength (12,23–25) have been related to falls in studies of older adults. While a few studies have indicated that lower leg power (26), grip strength (12,24,25), and poor physical performance (27,28) were associated with fall injuries, these previous studies have typically focused on fracture outcomes and have not included NFFI. Additionally, previous studies have not evaluated whether both upper-extremity and lower-extremity muscle function are independently associated with fall injuries.
We previously found that higher leg power was significantly related to 18% lower fall risk over 4.5 years in community-dwelling men in the Osteoporotic Fractures in Men (MrOS) Study (29). However, injuries resulting from these falls, a clinically relevant indicator of fall severity, were not examined. In the current analysis we expanded on these earlier results and prospectively examined the relationship of baseline leg power and grip strength with incident fall injuries in a large population of older community-dwelling men. We hypothesized that lower leg power would be associated more strongly versus lower grip strength with higher fall injuries over 9 years, independent of physical performance.
Materials and Methods
Study Population
The MrOS Study (https://mrosonline.ucsf.edu) is a multicenter longitudinal cohort study designed to evaluate healthy aging with a particular focus on risk factors for osteoporosis and fractures. Baseline visits occurred between March 2000 and April 2002 at 6 U.S. sites (N = 5 994; aged 73.7 ± 5.9 years) (30,31). Initial eligibility criteria included age ≥65 years, the ability to walk without assistance or walking aid; provide self-reported data and informed consent; residence near a clinical site; and absence of bilateral hip replacement or any severe disease/condition that would result in imminent death. Of 5 994 men at baseline, N = 5 178 (86.4%) had fall injury data for ≥1 follow-up questionnaire, the Nottingham leg power measure, and grip strength. Nottingham leg power data were missing for N = 420 (7.0%) due to equipment failure and N = 89 (1.5%) who were unable/refused. Grip strength data were missing for N = 74 (1.2%) who were unable and N = 2 (0.02%) who refused and/or had missing data. Fall injury data on follow-up questionnaires were not available in N = 231 (3.9%) men.
Fall Injury
Fall injuries were assessed by questionnaires 4 times spaced approximately 3 years apart over 9 years, with questions asked on interim questionnaire 1 (Year 2, July 2002 to March 2004), at study visit 2 (Year 5, March 2005 to May 2006), at study visit 3 (Year 7, March 2007 to March 2009), and on interim questionnaire 2 (Year 9, March 2009 to February 2011). Any self-reported fall injury in the past 12 months was defined as an affirmative answer to the question: “During the past 12 months, have you fallen and landed on the floor or ground, or fallen and hit an object like a table or chair?” and if yes, subsequently indicating if ≥1 of the following injuries occurred: broken or fractured a bone, hit or injured head, had a sprain or a strain, had bruise or bleeding, and/or other injury (options were not mutually exclusive). These particular fractures were not necessarily linked to adjudication, though MrOS did centrally adjudicate fracture outcomes reported through other questionnaires that were administered every 4 months. However, this triannual assessment did not include NFFI.
Leg Power/kg
The Nottingham Power Rig was used to measure leg extension power (W) at baseline (21,22,32). Participants were instructed to push the pedal as hard and as fast as possible through a full range of motion, with exclusions for bilateral hip replacement in the past 6 months. The test was performed on each leg until power plateaued, or up to 5–10 trials to obtain peak power. Maximum peak power was used in analyses and normalized to body weight (W/kg body weight).
Grip Strength/kg
Jamar dynamometers (Sammons Preston Rolyan, Bolingbrook, IL) (33) were used to measure grip strength for 2 trials of each hand, with exclusions for recent hand pain/arthritis symptoms or hand surgery in the past 3 months. Maximum grip strength was normalized by body weight (kg/kg body weight).
Covariates
All descriptive and potential covariates were assessed at the initial clinic visit. Age, race, smoking status (current/past/never) and alcohol consumption (number of drinks per day) (34), and any hip/joint pain in the past year (yes/no) were obtained from self-administered questionnaires. Body mass index (BMI) was calculated from weight (balance beam or digital scales) and height (Harpenden stadiometers, Dyved, United Kingdom). Physical activity (PA) was measured by the Physical Activity Scale for the Elderly (PASE) (35). Systolic blood pressure in the right and left posterior tibial artery and the right brachial artery were measured twice after the participants were supine for at least 5 minutes. Pulses were detected by using a handheld 8 MHz Doppler (36). Ankle–brachial index (ABI) was calculated as the ratio of the average systolic pressure in the ankle to the average systolic pressure in the arm. ABI < 0.9 defined peripheral vascular disease and ABI > 1.3 defined arterial stiffening. Total fat and lean mass were assessed by dual energy X-ray absorptiometry (Hologic, Inc., Waltham, MA) (37). Global cognition was assessed using the Teng Modified Mini-Mental State (3MS) Examination (38) and executive function was measured using Trails B (39) completion time. Serum cystatin C concentrations were determined using a BN100 nephelometer (Dade Behring Inc., Deerfield, IL) using a particle-enhanced immunonepholometric assay (40). Cystatin C-based estimated glomerular filtration rate (eGFR) was computed using a Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation reexpressed for standardized cystatin C (41). Comorbidities included diabetes (self-report physician diagnosis, medication use, and/or baseline fasting glucose ≥126 mg/dL), hypertension (self-report physician diagnosis and/or medication use), and self-reported history of congestive heart failure, myocardial infarction, stroke, and Parkinson’s disease. Total number of medications was calculated from current prescription medications brought to the clinic visit (42). Physical performance measures included 6-m usual gait speed, narrow walk gait speed, and chair stands speed from the extended Short Physical Performance Battery (43). Usual gait speed was measured from the fastest time of 2 trials over 6 m. Narrow walk gait speed on a course of 6 m × 20 cm was used as an indirect measure of dynamic balance and participants were considered able to complete a trial if they had no more than 2 deviations from the lane. Men unable to complete either the usual gait speed test or narrow walk speed test were excluded. For men able to rise once from a standard chair without using arms, time to complete 5 repeated chair stands as fast as possible without using arms was recorded. Men who attempted but were unable to complete a single stand or 5 repeated stands were included in analyses with a value of 0 stands/second.
Statistical Analyses
Descriptive statistics included 2-sided t tests and Chi-square tests of proportions (or Fishers Exact, if appropriate) to compare characteristics at the baseline study visit (2000–2002) in men with any fall injury versus without fall injury. Generalized estimating equations (GEEs) with binomial distributions were used to model the outcome of fall injury during the approximate 9-year follow-up period, with the responses to the fall injury questions over time as repeated assessments. Baseline leg power/kg and grip strength/kg were the primary predictors and were entered both in separate models and additionally together in the same model after assessing correlation between leg power/kg and grip strength/kg. All GEE models were built using an unstructured correlation as this model had the highest Bayesian information criterion. Models were built with both continuous predictors and as quartiles for each measure. Leg power quartiles (W/kg) were defined as: Q1: ≤2.05; Q2: 2.06–2.53; Q3: 2.54–2.99; Q4: >2.99. Grip strength quartiles (kg/kg body weight) were defined as: Q1: ≤0.44; Q2: 0.45–0.51; Q3: 0.52–0.59; Q4: >059. Q4 represented men with the highest leg power or grip strength relative to their body weight and was considered the reference group for analyses. Cochran–Mantel–Haenszel tests of trends across quartiles were performed. Baseline covariates in Table 1 (except weight, BMI, total body fat mass, and total lean mass because leg power and grip strength were normalized to kg body weight) were entered using forward stepwise GEE modeling in the following order, and only retained if significant at α ≤ 0.10: age; race, site, and height; smoking status and alcohol consumption; PA; ABI; hip/joint pain; hypertension; diabetes; congestive heart failure, myocardial infarction, stroke, and Parkinson’s disease; Teng 3MS score; Trails B; total number of medications; self-reported ≥1 fall in past year; self-reported ≥2 falls in past year; cystatin C; eGFR. Age, race, site, height, hypertension, stroke, and total number of medications were retained in all final models since significant at α ≤ 0.10 in any model. As a sensitivity analysis to determine if physical performance attenuated the relationship between muscle function and fall injury, physical performance measures that were weakly correlated (weak: r ≤ 0.40; moderate 0.40 < r < 0.60; strong r ≥ 0.60) with leg power/kg or grip strength/kg were subsequently added to final model; 6-m usual gait speed, narrow walk speed, and chair stand speed were all entered separately.
Table 1.
Descriptive Characteristics of Men by Fall Injury Status
| % or Mean ± Standard Deviation | Total (N = 5 178) | Any Fall Injury (N = 2 090) | No Fall Injury (N = 3 088) | p Value (any fall injury vs no fall injury) |
|---|---|---|---|---|
| Demographics | ||||
| Age at baseline, years | 73.4 ± 5.7 | 74.0 ± 5.8 | 72.9 ± 5.6 | <.0001 |
| White race, % | 89.8 | 91.9 | 88.4 | <.0001 |
| Anthropometry | ||||
| Height, cm | 174.4 ± 6.8 | 174.3 ± 6.9 | 174.3 ± 6.7 | .53 |
| Weight, kg | 83.2 ± 13.0 | 83.5 ± 13.2 | 83.0 ± 12.9 | .17 |
| Body mass index, kg/m2 | 27.3 ± 3.7 | 27.4 ± 3.8 | 27.3 ± 3.7 | .24 |
| Total body fat mass, kg | 21.7 ± 7.0 | 22.0 ± 7.2 | 21.5 ± 6.9 | .006 |
| Total lean mass, kg | 57.0 ± 7.1 | 56.9 ± 7.1 | 57.0 ± 7.2 | .67 |
| Lifestyle characteristics | ||||
| Current smoker, % | 5.3 | 4.2 | 5.9 | .02 |
| >1 alcohol drink/week, % | 53.3 | 55.7 | 51.7 | .004 |
| Physical activity, PASE score | 148.8 ± 67.8 | 146.7 ± 66.7 | 150.3 ± 68.6 | .06 |
| Clinical measures | ||||
| Ankle–brachial index, % | .14 | |||
| ≤0.9 (low) | 5.5 | 6.1 | 5.0 | |
| 1.0–1.4 (normal) | 90.7 | 89.8 | 91.4 | |
| ≥1.4 (high) | 3.8 | 4.1 | 3.7 | |
| Hip/joint pain, % | 23.0 | 26.3 | 21.2 | <.0001 |
| Teng 3MS score | 93.5 ± 5.6 | 93.7 ± 5.1 | 93.4 ± 5.9 | .05 |
| Trails B, seconds to complete | 130.8 ± 56.3 | 129.8 ± 54.4 | 131.4 ± 57.5 | .31 |
| Cystatin C, mg/L | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.3 | .50 |
| GFR, CKD-EPI cystatin C equation | 73.7 ± 17.5 | 72.7 ± 17.0 | 74.5 ± 17.9 | .07 |
| History of falls | ||||
| Self-reported ≥1 fall in past year, % | 20.6 | 29.3 | 14.9 | <.0001 |
| Self-reported ≥2 falls in past year, % | 8.6 | 13.9 | 5.0 | .0001 |
| Comorbidities | ||||
| Hypertension, % | 42.2 | 44.3 | 41.0 | .02 |
| Diabetes, % | 14.6 | 15.3 | 14.2 | .23 |
| Congestive heart failure, % | 4.5 | 5.0 | 4.2 | .24 |
| Myocardial infarction, % | 12.9 | 14.8 | 11.6 | .001 |
| Stroke, % | 5.1 | 6.1 | 4.4 | .006 |
| Parkinson’s disease, % | 0.7 | 0.9 | 0.6 | .23 |
| Medications | ||||
| Total # of medications | 4.0 ± 3.5 | 4.5 ± 3.9 | 3.7 ± 3.3 | <.0001 |
| Physical performance | ||||
| 6-m usual gait speed, m/s | 1.26 ± 0.23 | 1.25 ± 0.23 | 1.27 ± 0.22 | .02 |
| Narrow walk speed, m/s | 1.16 ± 0.27 | 1.14 ± 0.27 | 1.17 ± 0.26 | .002 |
| Chair stands speed, #/second | 0.48 ± 0.14 | 0.47 ± 0.15 | 0.49 ± 0.14 | <.0001 |
| Muscle function | ||||
| Nottingham leg power, W/kg body weight | 2.53 ± 0.70 | 2.47 ± 0.68 | 2.59 ± 0.70 | <.0001 |
| Grip strength, kg/kg body weight | 0.51 ± 0.11 | 0.50 ± 0.11 | 0.52 ± 0.11 | <.0001 |
Notes: 3MS = Modified Mini-Mental State Examination; eGFR = estimated glomerular filtration rate; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; PASE = Physical Activity Scale for the Elderly.
Results
At the initial visit, 20.6% reported a fall and 8.6% reported recurrent falls in the year prior to baseline (Table 1). Over the 9-year prospective follow-up period, 40% (N = 2 090) self-reported any fall injury (N = 1 003 reported an NFFI only, N = 314 reported a fracture only, and N = 773 reported both). NFFI were 3 times more common than fractures (48% with NFFI only vs 15% with fractures only), and typically occurred earlier than fractures for those with both an NFFI and fracture (95% [N = 732/773] had an NFFI first; 5% [N = 41/773] had a fracture first). Men with any fall injury had lower leg power/kg (2.47 ± 0.68 W/kg vs 2.59 ± 0.70 W/kg) and grip strength/kg (0.50 ± 0.11 kg/kg vs 0.52 ± 0.11 kg/kg) compared to those without a fall injury (Table 1; p < .001 for both). Those with fall injuries were older, more likely to be White, had higher total body fat mass, were less likely to smoke and more likely to use alcohol, and more likely to have hip/joint pain, higher cognitive function, history of any and recurrent falls, hypertension, myocardial infarction, stroke, higher total number of medications, and poorer physical performance. An equal number of men with any fall injury were in each leg power and grip strength quartile (Table 2).
Table 2.
Among Men With Fall Injury (N = 2 090), Number and Percent in Each Leg Power and Grip Strength Quartile
| N = 2 090 | Leg Power N (%) |
Grip Strength N (%) |
|---|---|---|
| Q1 | 581 (27.8) | 585 (28.0) |
| Q2 | 546 (26.1) | 550 (26.3) |
| Q3 | 526 (25.2) | 496 (23.7) |
| Q4 | 437 (20.9) | 459 (22.0) |
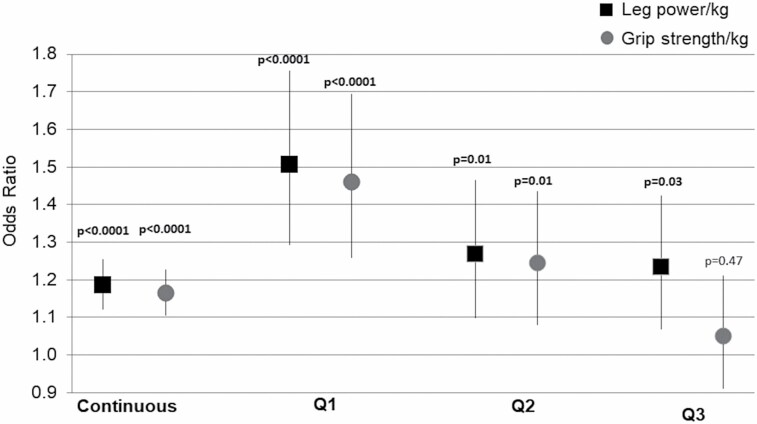
A 1 standard deviation (SD) lower power/kg was associated with a 19% (odds ratio [OR] = 1.19; 95% confidence interval [CI]: 1.12–1.26) increased fall injury risk, adjusting for age, race, site, height, hypertension, stroke, and total number of medications (Figure 1). Men in lower power/kg quartiles had a significantly higher fall injury risk compared to men in the highest quartile (Q1 OR = 1.51, 95% CI: 1.29–1.76; Q2 OR = 1.27, 95% CI: 1.10–1.46; Q3 OR = 1.23, 95% CI: 1.10–1.42) with significant trend across quartiles (p < .001; Figure 1). Results were similar for grip strength when assessed continuously (OR = 1.16, 95% CI: 1.10–1.23, p < .0001). Similar to the pattern of results for leg power quartiles, men in grip strength quartiles Q1 and Q2 had an increased fall injury risk versus Q4 (Q1 OR = 1.46, 95% CI: 1.26–1.69; Q2 OR = 1.25, 95% CI: 1.08–1.44; both p < .0001), but risk for Q3 versus Q4 was not significant (OR = 1.05, 95% CI: 0.91–1.21; p = .50). However, the trend across grip strength quartiles was statistically significant (p < .001; Figure 1). Because leg power/kg and grip strength/kg were only moderately correlated (r = 0.44; p < .0001), these were entered into the fall injury model simultaneously to determine independent contributions. In models with both leg power/kg and grip strength/kg, fall injury risk estimates per 1 SD decrease in each measure were similar in magnitude and independent of each other (leg power/kg OR = 1.13, 95% CI: 1.06–1.20; grip strength/kg OR = 1.11, 95% CI: 1.05–1.17).
Figure 1.
Odds ratio (95% confidence intervals) for leg power and grip strength associated with fall injury*. Notes: All models adjusted for age, race, site, height, hypertension, stroke, and total number of medications; *p values for Q1, Q2, and Q3 are from models with comparison to Q4.
Age-adjusted Pearson correlations showed that physical performance measures were weakly correlated with leg power/kg (6-m usual gait speed: r = 0.29; narrow walk speed: r = 0.25; chair stands speed: r = 0.35; all p < .0001) and grip strength/kg (6-m usual gait speed: r = 0.21; narrow walk speed: r = 0.21; chair stands speed: r = 0.26; all p < .0001). When added to fall injury models separately, physical performance covariates did not attenuate risk estimates and were not statistically significant in models including leg power/kg or grip strength/kg (leg power/kg models with 6-m usual gait speed: std β = 0.11, p = .36; narrow walk speed: std β = 0.16, p = .13; chair stands speed: std β = 0.33, p = .10; grip strength/kg models with 6-m usual gait speed: std β = .16, p = .17; narrow walk speed: std β = 0.17, p = .12; chair stands speed: std β = 0.13, p = .11).
Discussion
Both lower leg power/kg and lower grip strength/kg were individually and independently associated with increased fall injury risk in men. To our knowledge, this is the first study to prospectively and simultaneously examine the relationships of lower-extremity leg power and upper-extremity grip strength with prospective fall injury risk over nearly a decade. Associations of these measures with fall injury across muscle function quartiles were similar for leg power and grip strength when comparing groups with lower muscle function (Q1 and Q2) to highest muscle function (Q4). However, relationships for leg power may be more able to distinguish fall injury risk among those with higher function (Q3 vs Q4). This suggests that leg power potentially identifies fall injury risk similarly as grip strength at lower muscle function though better than grip strength among men with higher muscle function. In a past MrOS Study, values of jump power had wide ranges in the oldest adults compared to lower-extremity strength (jump force measure), and in those with functional limitations, suggesting that power may be able to differentiate within subsets of not only highest but also poorest functioning individuals (5). The absolute differences for leg power and grip strength among men with a fall injury versus without a fall injury were small in magnitude, though leg power was 4.6% lower and grip strength was 3.8% lower in those with a fall injury, which is beyond what is attributable to the age effect (5,10). Therefore, establishing the clinical relevance of these differences and examining the potential for inclusion in fall risk assessment tools for early detection should be evaluated in future studies. While leg power and grip strength were similarly related to fall injury risk, grip strength may be more easily measured in clinical and practice settings. Additionally, these relationships were independent of physical performance measures of gait speed or chair stands speed, which may indicate that both strength and power may be stronger predictors of fall injury risk than physical performance in older men.
While fall injuries are common in older adult populations, the relationships between various muscle function measures and fall injuries have not been extensively studied. In particular, lower-extremity muscle function contributes to functional tasks such as walking and maintaining balance, and therefore higher muscle function may prevent falls and fall injuries. However, the few past studies of muscle function and fall injury have only included upper-extremity grip strength (26) or indirect, task-based measures of leg muscle function using the chair stands test of physical performance (26,27). In older men and women (age = 78.1 ± 5.4 years; 29% with a fall injury), individuals in the slowest chair stand time quartile had a significantly higher fall injury risk over 4.3 years compared to those in the fastest quartile, and sex was not significant in models (27). Our results of leg power quartiles were not attenuated when adjusting for chair stand speed and we found higher estimates of fall injury risk at each quartile of leg power. In another previous study of our MrOS cohort, Nottingham leg power and grip strength were not related to hip fracture in older men over 5.3 years of follow-up (26). However, this study had follow-up approximately 2 times shorter than our study and only included hip fracture outcomes leading to a much lower fall injury rate (1.3%) than observed in the current study. In the past MrOS Study and most other fall injury studies in older adults, NFFI were not included as an outcome, which likely led to fall injury estimates (range: 1.3%–24%) (11,12,26,44) that were lower than the observed 40% in the current study. Estimating risk for more comprehensive fall injury outcomes may have implications for understanding how muscle function and different types of fall injuries may influence disability and mortality outcomes in older adults.
Muscle function decline likely precedes physical function impairments and disability in older adults (45). Lower leg power and grip strength were associated with increased fall injury risk independent of physical performance (gait speed and chair stands) in the current study. However, this is in contrast to previous studies examining the relationships of physical performance with falls (19,29), fall injuries (27), or incident radiographic vertebral fracture (28), in which physical performance was related to these outcomes in cross-sectional studies or longitudinal studies with much shorter follow-up than our study. Because our study assessed initial muscle power, strength, and physical performance and subsequent fall injuries over 9 years, we may have detected the early declines in muscle power and strength that occurred prior to any later physical performance decline. However, we are unable to establish temporality between muscle function and physical performance in this study. Muscle power and strength may be considered stronger predictors than physical performance measures for a wider range of fall injury outcomes (eg, NFFI and fractures) because muscle function decline likely occurs prior to substantial, clinically significant, and detectable decline in physical performance.
Strengths
The prospective design over 9 years allowed an assessment of fall injury risk in a large sample of men aged 70 years and older who are at high risk for falls and the associated injuries. Collection of leg power and grip strength allowed comparison of both upper-extremity and lower-extremity muscle function with fall injury risk. The prospective questionnaires utilized were not limited to fractures as information on other NFFI such as sprains, strains, bruises, and contusions were also collected. MrOS also collected data on many covariates to adjust for the independent associations of muscle power and strength with fall injury.
Limitations
Our fall injury outcomes were based on self-report and fractures were not necessarily linked to adjudication, as the fracture outcomes centrally adjudicated by MrOS were from separate questionnaires administered every 4 months that did not assess fall injuries. Fall injuries were likely underreported because these self-reported injuries were in the past 12 months and collection was approximately every 3 years. This recall or reporting bias may have led to underestimation of fall injuries. Our analysis included baseline muscle function and physical performance measures and did not update assessment values over time and could not assess temporality. Muscle power was measured with a seated power test that may exclude older adults unable to get into the seated position, with difficulty sitting or pushing in the required positions with fixed hip and knee angles or those with certain joint conditions, such as arthritis. We were not able to include all types of physical performance assessments; therefore, future studies could evaluate attenuation with additional measures. The community-dwelling, largely White population of men healthy enough to attend a clinic visit limits generalizability. By excluding those who are extremely frail or physically impaired and could not attend a visit, we likely observed more moderate fall injury risk estimates.
Conclusion
Leg power and grip strength were related to increased fall injury risk, including both NFFI and fracture, in separate models. Although leg power potentially identifies fall injury risk similarly as grip strength at lower muscle function though better than grip strength among men with higher muscle function, grip strength may be more suitable for measurement in clinical and practice settings. Importantly, physical performance did not explain the significant association of muscle power and strength with fall injuries assessed over 9 years. Poorer muscle function, both power and strength, may predict higher risk of future fall injury in older men.
Acknowledgments
Results were presented to The Gerontological Society of America 69th Annual Scientific Meeting, November 18, 2016, New Orleans, LA. The Osteoporotic Fractures in Men (MrOS) Research Group: Administrative Center (Oregon Health & Sciences University): E. Orwoll (principal investigator), J. Lapidus (coinvestigator), C. Nielson (coinvestigator), L. Marshall (coinvestigator), C. Pedersen (project director), M. Abrahamson, Y. Wang, J. Wiedrick, N. Fino, E. Hooker, J. Nava; Coordinating Center (California Pacific Medical Center Research Institute and University of California, San Francisco): S. R. Cummings (principal investigator), D. C. Bauer (coinvestigator), D. M. Black (coinvestigator), P. M. Cawthon (coinvestigator), K. L. Stone (coinvestigator), R. Collins (project director), B. Black, T. Blackwell, A. Burghardt, L. Concepcion, S. Ewing, S. L. Harrison, L. Y. Lui, S. Majumdar, C. Navy, N. Parimi, S. Patel, K. Peters, A. Schafer, C. Schambach, A. Schwartz, A. Yu; University of Alabama, Birmingham: J. Shikany (principal investigator), C. Lewis (coinvestigator), M. Kilgore (coinvestigator), P. Johnson (project director), M. Young (study coordinator), N. Webb, S. Felder, C. Collier, K. Hardy; University of Minnesota: K. Ensrud (principal investigator), H. Fink (coinvestigator), S. Diem (coinvestigator), J. Schousboe (coinvestigator), B. Taylor (coinvestigator), L. Langsetmo (coinvestigator), S. Potter (project director), N. Nelson (clinic coordinator), P. Van Coevering (program director), K. Jacobson, A. Kats, S. Luthi, K. Moen, E. Penland-Miller, T. Vo; Stanford University: M. Stefanick (principal investigator), A. Hoffman (coinvestigator), N. Ellsworth, K. Kent; University of Pittsburgh: J. Cauley (principal investigator), J. Zmuda (coinvestigator), E. Strotmeyer (coinvestigator), D. Cusick (project director), C. Newman, A. Flaugh, S. Happe; University of California, San Diego: D. Kado (principal investigator), E. Barrett-Connor (coinvestigator), L. Claravall (project director), M. L. Carrion-Petersen, P. Miller, M. Stephens, J. Smith.
Contributor Information
Mary E Winger, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Paolo Caserotti, Department of Sports Science and Clinical Biomechanics, University of Southern Denmark, Odense, Denmark.
Jane A Cauley, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Robert M Boudreau, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Sara R Piva, Department of Physical Therapy and Clinical and Translational Science Institute, School of Health and Rehabilitation Sciences, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Peggy M Cawthon, Research Institute, California Pacific Medical Center, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA.
Eric S Orwoll, Oregon Health and Science University, Portland, Oregon, USA.
Kristine E Ensrud, Department of Medicine and Division of Epidemiology and Community Health, University of Minnesota Center for Care Delivery and Outcomes Research, Minneapolis VA Health Care System, Minneapolis, Minnesota, USA.
Deborah M Kado, Geriatrics Section, Stanford University School of Medicine, Palo Alto, California, USA; Geriatrics Research Education and Clinical Center, Veterans Health Administration, Palo Alto, California, USA.
Elsa S Strotmeyer, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Osteoporotic Fractures in Men (MrOS) Research Group:
E Orwoll, J Lapidus, C Nielson, L Marshall, C Pedersen, M Abrahamson, Y Wang, J Wiedrick, N Fino, E Hooker, J Nava, S R Cummings, D C Bauer, D M Black, P M Cawthon, K L Stone, R Collins, B Black, T Blackwell, A Burghardt, L Concepcion, S Ewing, S L Harrison, L Y Lui, S Majumdar, C Navy, N Parimi, S Patel, K Peters, A Schafer, C Schambach, A Schwartz, A Yu, J Shikany, C Lewis, M Kilgore, P Johnson, M Young, N Webb, S Felder, C Collier, K Hardy, K Ensrud, H Fink, S Diem, J Schousboe, B Taylor, L Langsetmo, S Potter, N Nelson, P Van Coevering, K Jacobson, A Kats, S Luthi, K Moen, E Penland-Miller, T Vo, M Stefanick, A Hoffman, N Ellsworth, K Kent, J Cauley, J Zmuda, E Strotmeyer, D Cusick, C Newman, A Flaugh, S Happe, D Kado, E Barrett-Connor, L Claravall, M L Carrion-Petersen, P Miller, M Stephens, and J Smith
Funding
The Osteoporotic Fractures in Men Study is supported by National Institutes of Health (NIH) funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Center for Advancing Translational Sciences, and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, R01 AG066671, and UL1 TR000128. Additional support was provided by the Department of Epidemiology, University of Pittsburgh, NIH National Institute on Aging (T32-AG000181; A. B. Newman).
Conflict of Interest
None declared.
References
- 1. Bean JF, Kiely DK, Herman S, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50(3):461–467. doi: 10.1046/j.1532-5415.2002.50111.x [DOI] [PubMed] [Google Scholar]
- 2. Sayer AA, Kirkwood TB. Grip strength and mortality: a biomarker of ageing? Lancet (London, England). 2015;386(9990):226–227. doi: 10.1016/S0140-6736(14)62349-7 [DOI] [PubMed] [Google Scholar]
- 3. Bohannon RW. Grip strength: an indispensable biomarker for older adults. Clin Interv Aging. 2019;14:1681–1691. doi: 10.2147/CIA.S194543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strollo SE, Caserotti P, Ward RE, Glynn NW, Goodpaster BH, Strotmeyer ES. A review of the relationship between leg power and selected chronic disease in older adults. J Nutr Health Aging. 2015;19(2):240–248. doi: 10.1007/s12603-014-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strotmeyer ES, Winger ME, Cauley JA, et al. Normative values of muscle power using force plate jump tests in men aged 77–101 years: the Osteoporotic Fractures in Men (MrOS) Study. J Nutr Health Aging. 2018;22(10):1167–1175. doi: 10.1007/s12603-018-1081-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dietzel R, Gast U, Heine T, Felsenberg D, Armbrecht G. Cross-sectional assessment of neuromuscular function using mechanography in women and men aged 20–85 years. J Musculoskelet Neuronal Interact. 2013;13(3):312–319. [PubMed] [Google Scholar]
- 7. Stephenson ML, Smith DT, Heinbaugh EM, et al. Total and lower extremity lean mass percentage positively correlates with jump performance. J Strength Cond Res. 2015;29(8):2167–2175. doi: 10.1519/JSC.0000000000000851 [DOI] [PubMed] [Google Scholar]
- 8. Zengin A, Pye SR, Cook MJ, et al. Associations of muscle force, power, cross-sectional muscle area and bone geometry in older UK men. J Cachexia Sarcopenia Muscle. 2017;8(4):598–606. doi: 10.1002/jcsm.12198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dionyssiotis Y, Galanos A, Michas G, Trovas G, Lyritis GP. Assessment of musculoskeletal system in women with jumping mechanography. Int J Womens Health. 2010;1:113–118. doi: 10.2147/ijwh.s5889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caserotti P, Aagaard P, Larsen JB, Puggaard L. Explosive heavy-resistance training in old and very old adults: changes in rapid muscle force, strength and power. Scand J Med Sci Sports. 2008;18(6):773–782. doi: 10.1111/j.1600-0838.2007.00732.x [DOI] [PubMed] [Google Scholar]
- 11. Moreland B, Kakara R, Henry A. Trends in nonfatal falls and fall-related injuries among adults aged ≥65 years—United States, 2012–2018. MMWR Morb Mortal Wkly Rep. 2020;69(27):875–881. doi: 10.15585/mmwr.mm6927a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–1707. doi: 10.1056/NEJM198812293192604 [DOI] [PubMed] [Google Scholar]
- 13. Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989;261(18):2663–2668. doi: 10.1001/jama.1989.03420180087036 [DOI] [PubMed] [Google Scholar]
- 14. Florence CS, Bergen G, Atherly A, Burns E, Stevens J, Drake C. Medical costs of fatal and nonfatal falls in older adults. J Am Geriatr Soc. 2018;66(4):693–698. doi: 10.1111/jgs.15304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348(1):42–49. doi: 10.1056/NEJMcp020719 [DOI] [PubMed] [Google Scholar]
- 16. Karlsson MK, Ribom EL, Nilsson JA, et al. International and ethnic variability of falls in older men. Scand J Public Health. 2014;42(2):194–200. doi: 10.1177/1403494813510789 [DOI] [PubMed] [Google Scholar]
- 17. Rosengren B, Ribom EL, Nilsson JA, et al. There is in elderly men a group difference between fallers and non-fallers in physical performance tests. Age Ageing. 2011;40(6):744–749. doi: 10.1093/ageing/afr108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faulkner KA, Chan BK, Cauley JA, et al. Histories including number of falls may improve risk prediction for certain non-vertebral fractures in older men. Inj Prev. 2009;15(5):307–311. doi: 10.1136/ip.2009.021915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Rekeneire N, Visser M, Peila R, et al. Is a fall just a fall: correlates of falling in healthy older persons. The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51(6):841–846. doi: 10.1046/j.1365-2389.2003.51267.x [DOI] [PubMed] [Google Scholar]
- 20. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged >/=65 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(37):993–998. doi: 10.15585/mmwr.mm6537a2 [DOI] [PubMed] [Google Scholar]
- 21. Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol. 1990;60(5):385–390. doi: 10.1007/BF00713504 [DOI] [PubMed] [Google Scholar]
- 22. Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond). 1992;82(3):321–327. doi: 10.1042/cs0820321 [DOI] [PubMed] [Google Scholar]
- 23. Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing. 1997;26(4):315–318. doi: 10.1093/ageing/26.4.315 [DOI] [PubMed] [Google Scholar]
- 24. Cawthon PM, Manini T, Patel SM, et al. Putative cut-points in sarcopenia components and incident adverse health outcomes: an SDOC analysis. J Am Geriatr Soc. 2020;68(7):1429–1437. doi: 10.1111/jgs.16517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cawthon PM, Travison TG, Manini TM, et al. Establishing the link between lean mass and grip strength cut points with mobility disability and other health outcomes: proceedings of the Sarcopenia Definition and Outcomes Consortium conference. J Gerontol A Biol Sci Med Sci. 2020;75(7):1317–1323. doi: 10.1093/gerona/glz081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cawthon PM, Fullman RL, Marshall L, et al. Physical performance and risk of hip fractures in older men. J Bone Miner Res. 2008;23(7):1037–1044. doi: 10.1359/JBMR.080227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ward RE, Leveille SG, Beauchamp MK, et al. Functional performance as a predictor of injurious falls in older adults. J Am Geriatr Soc. 2015;63(2):315–320. doi: 10.1111/jgs.13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cawthon PM, Blackwell TL, Marshall LM, et al. Physical performance and radiographic and clinical vertebral fractures in older men. J Bone Miner Res. 2014;29(9):2101–2108. doi: 10.1002/jbmr.2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan BK, Marshall LM, Winters KM, Faulkner KA, Schwartz AV, Orwoll ES. Incident fall risk and physical activity and physical performance among older men: the Osteoporotic Fractures in Men Study. Am J Epidemiol. 2007;165(6):696–703. doi: 10.1093/aje/kwk050 [DOI] [PubMed] [Google Scholar]
- 30. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the Osteoporotic Fractures in Men (MrOS) Study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 31. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the Osteoporotic Fractures in Men Study (MrOS). Contemp Clin Trials. 2005;26(5):557–568. doi: 10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 32. Blackwell T, Cawthon PM, Marshall LM, Brand R. Consistency of leg extension power assessments in older men: the Osteoporotic Fractures in Men (MrOS) Study. Am J Phys Med Rehabil. 2009;88(11):934–940. doi: 10.1097/PHM.0b013e3181bbddfb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harkonen R, Harju R, Alaranta H. Accuracy of the Jamar dynamometer. J Hand Ther. 1993;6(4):259–262. doi: 10.1016/s0894-1130(12)80326-7 [DOI] [PubMed] [Google Scholar]
- 34. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252(14):1905–1907. doi: 10.1001/jama.252.14.1905 [DOI] [PubMed] [Google Scholar]
- 35. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4 [DOI] [PubMed] [Google Scholar]
- 36. Cauley JA, Kassem AM, Lane NE, Thorson S. Prevalent peripheral arterial disease and inflammatory burden. BMC Geriatr. 2016;16(1):213. doi: 10.1186/s12877-016-0389-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA. 2002;288(15):1889–1897. doi: 10.1001/jama.288.15.1889 [DOI] [PubMed] [Google Scholar]
- 38. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 39. Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. doi: 10.2466/pms.8.7.271-276 [DOI] [Google Scholar]
- 40. Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N latex cystatin C assay on the Dade Behring nephelometer II system. Scand J Clin Lab Invest. 1999;59(1):1–8. doi: 10.1080/00365519950185940 [DOI] [PubMed] [Google Scholar]
- 41. Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58(4):682–684. doi: 10.1053/j.ajkd.2011.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. doi: 10.1007/BF01719664 [DOI] [PubMed] [Google Scholar]
- 43. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 44. Ward RE, Boudreau RM, Caserotti P, et al. Sensory and motor peripheral nerve function and longitudinal changes in quadriceps strength. J Gerontol A Biol Sci Med Sci. 2015;70(4):464–470. doi: 10.1093/gerona/glu183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nagi S. Some conceptual issues in disability and rehabilitation. In: Sussman MB, ed. Sociology and Rehabilitation. Washington, DC: American Sociological Association; 1965;100–113. [Google Scholar]