Abstract
Background
Autism spectrum disorder (ASD) is a common neurodevelopmental diagnosis showing substantial phenotypic heterogeneity. A leading example can be found in verbal and nonverbal cognitive skills, which vary from elevated to impaired compared with neurotypical individuals. Moreover, deficits in verbal profiles often coexist with normal or superior performance in the nonverbal domain.
Methods
To study brain substrates underlying cognitive imbalance in ASD, we capitalized categorical and dimensional IQ profiling as well as multimodal neuroimaging.
Results
IQ analyses revealed a marked verbal to nonverbal IQ imbalance in ASD across 2 datasets (Dataset-1: 155 ASD, 151 controls; Dataset-2: 270 ASD, 490 controls). Neuroimaging analysis in Dataset-1 revealed a structure–function substrate of cognitive imbalance, characterized by atypical cortical thickening and altered functional integration of language networks alongside sensory and higher cognitive areas.
Conclusion
Although verbal and nonverbal intelligence have been considered as specifiers unrelated to autism diagnosis, our results indicate that intelligence disparities are accentuated in ASD and reflected by a consistent structure–function substrate affecting multiple brain networks. Our findings motivate the incorporation of cognitive imbalances in future autism research, which may help to parse the phenotypic heterogeneity and inform intervention-oriented subtyping in ASD.
Keywords: autism, verbal and nonverbal IQ, multimodal neuroimaging, neurosubtyping, cognitive imbalance
Introduction
A mounting literature emphasizes that one critical challenge to diagnostic and research advances in autism spectrum disorder (ASD) is phenotypic heterogeneity in cognitive and sensory processing (Lombardo et al. 2019; Hong et al. 2020). It ranges from deficits to normal, or even enhanced, abilities compared with typically developing individuals (Mottron et al. 2006), suggesting that the “disorder” term inherent to ASD may not equally qualify for all diagnosed individuals (Baron-Cohen 2017). Merging all individuals on the spectrum can, thus, potentially miss clinically important ASD subgroups, or blur common autism phenotypes (Lai et al. 2013; Lombardo et al. 2019; Mottron and Bzdok 2020). Ultimately, these challenges may hinder the ability to understand mechanisms of atypical function, and to develop therapies in autism.
ASD heterogeneity is particularly apparent when considering the variability in nonverbal and verbal competences (Munson et al. 2008). Prior studies show that verbal and nonverbal intelligence quotient (IQ) measures are highly heterogeneous, dramatically change during brain development, and are sometimes more discrepant among children with ASD compared with typically developing children (Joseph et al. 2002; Coolican et al. 2008; Ankenman et al. 2014; Lord et al. 2015; Nowell et al. 2015). In support of such increased discrepancy between verbal and nonverbal IQ, individuals with ASD who experience speech onset delay may perform adequately, or often even better than neurotypicals, on tasks that do not require verbal skills and target nonverbal perceptual reasoning (Dawson et al. 2007). Conversely, individuals with ASD who present with verbal abilities that are comparable with neurotypicals often show atypical sensory-perceptual processing (Bonnel et al. 2010; Samson et al. 2015). Although there has been debate about an overall diagnostic utility of cognitive imbalances in ASD (Lennen et al. 2010; Clements et al. 2020), it is commonly indicated that this atypical trait seems to affect at least a subgroup of ASD. Recognizing the importance, several clinical studies have assessed cognitive imbalances based on a ratio of verbal to nonverbal IQ (vnIQ), and reported significant IQ discrepancies in ASD at the group level (Caron et al. 2006; Ankenman et al. 2014; Nader et al. 2015).
Although neural substrates underlying cognitive imbalances in ASD remain to be investigated, it has been postulated that different functional systems may atypically compete during brain development in ASD, possibly due to altered synaptogenesis and neural plasticity (Mottron et al. 2014). Such a broad mechanism may affect the organization of multiple cortical areas, including sensory-motor, language, as well as higher cognitive regions (Silbereis et al. 2016). Neuroimaging, especially multimodal magnetic resonance imaging (MRI), allows for the study of typical and atypical brain organization and development in vivo (Betzel and Bassett 2017; Lerch et al. 2017; Gilmore et al. 2018; Larivière et al. 2019). Indeed, surface-based MRI analysis can examine morphological variations of cortical areas (Raznahan et al. 2010; Valk et al. 2015; Hong et al. 2018; Bedford et al. 2020), and resting-state functional MRI (rs-fMRI) taps into cortico–cortical connectivity (Di Martino et al. 2011, 2014; Müller et al. 2011; Craddock et al. 2012; Keown et al. 2013). At the level of brain structure, several studies have converged on the patterns of atypical cortical thickness increases in ASD relative to controls, in a spatial distribution largely affecting frontal and temporal lobe regions (Hardan et al. 2006; Valk et al. 2015; van Rooij et al. 2018; Bedford et al. 2020). Findings have, nevertheless, been somewhat inconsistent, with other work showing cortical thickness decreases (Wallace et al. 2010) or no significant findings (Haar et al. 2016). Inconclusive results have also been reported at the level of functional connectivity based on rs-fMRI analysis, revealing mosaic patterns of both under- and over-connectivity in ASD groups relative to neurotypicals (Müller et al. 2011; Di Martino et al. 2014). Recognizing this challenge, recent studies have performed data-driven decomposition on heterogeneous brain patterns in ASD. These approaches identified coherent patterns of brain phenotypes from subgroups of ASD, either based on categorical clustering approaches or by projecting brain imaging features onto entirely continuous dimensions representing a biological spectrum of this condition (Lombardo et al. 2019; Hong et al. 2020). Quantifying inherent variability within groups diagnosed with ASD, these findings highlight potential limitations of conventional case-control group comparisons in revealing heterogenous cerebral substrates associated to the condition.
Despite many prior neuroimaging studies having employed a variety of statistical tools to unveil atypical structure and connectivity in ASD, studies rarely took into consideration cognitive discrepancies as potential modulators of brain organization. Work in neurotypical individuals has suggested that discrepant IQ profiles may relate to variations in structural (Margolis et al. 2013) and functional brain network organization (Margolis et al. 2018). These relationships and their structural correlates, however, have not been assessed in ASD. To fill this gap, we assessed structural and functional network substrates of cognitive imbalances in ASD, capitalizing on multimodal neuroimaging and connectomics. Analysis of cognitive phenotypes included categorical and dimensional decompositions of verbal and nonverbal IQ profiles, together with an assessment of nvIQ ratios. Analyses were regionally unconstrained, operating at a cortex- and connectome-wide level. We, nevertheless, hypothesized in light of previous studies (Kleinhans et al. 2008; Eyler et al. 2012; Lindell and Hudry 2013) that the imbalance of verbal and nonverbal IQ dimensions in ASD would particularly manifest in the structure and functional connectivity of the language network. Although we expected those significant brain anomalies in ASD to be discovered at the group level, the dominant effects were primarily hypothesized to relate to ASD subtypes with more severe cognitive imbalances.
Materials and methods
Participants
Dataset-1
We studied a subsample of individuals with ASD and neurotypical individuals from the 2 waves of the Autism Brain Imaging Data Exchange initiative (ABIDE-I and -II; Di Martino et al. 2014, 2017). Inclusion criteria resembled our prior studies (Valk et al. 2015; Hong et al. 2019a; 2019b; Park et al. 2021). Specifically, we restricted our assessment to those sites that included both children and adults, with ≥10 individuals per diagnostic group (n = 406, 203/203 ASD/controls). Data availability and detailed quality control criteria were then used to select only the cases with verbal/performance IQ scores and acceptable MRI quality (see below). This screening resulted in 306 individuals (155 ASD [age mean ± SD = 17.9 ± 8.6, 150 males], 151 controls [age = 17.7 ± 7.3, 150 males]) from 4 different sites: (i) NYU Langone Medical Center (NYU, 65/70 ASD/controls); (ii) University of Utah, School of Medicine (USM, 52/40 ASD/controls); (iii) University of Pittsburgh, School of Medicine (PITT, 20/22 ASD/controls); and (iv) Trinity Centre for Health Sciences, Trinity College Dublin (TCD, 18/19 ASD/controls).
Dataset-2
After screening the cases for Dataset-1, the remaining samples from ABIDE-I and ABIDE-II (n = 1920) became candidates to replicate the IQ profiling (Dataset-2). From these cases, we excluded those without IQ or ADOS scores (as these measures are the main target of our analyses), which resulted in 760 cases (=270/490 [ASD/controls]). Notably, this dataset was overall younger compared with Dataset-1. Therefore, although these samples covered a similar age range than Dataset-1, the demographic difference should be considered when interpreting the results of replication analyses in the later section.
Sociodemographic details for Dataset-1 and Dataset-2 are presented in Table 1.
Table 1.
Demographic information.
| Dataset-I (306) | Dataset-II (760) | ||||
|---|---|---|---|---|---|
| ASD (155) |
Controls (151) | ASD (270) |
Controls (490) | ||
| Age (year) | 17.9 ± 8.6 (5.2–50.2) |
17.7 ± 7.3 (5.9–39.4) |
15.7 ± 7.1 (5.9–55.4) |
12.2 ± 4.1 (5.9–30.7) |
|
| Sex (male) | 150 | 150 | 240 | 362 | |
| Intelligence quotient (IQ) | Full | 105 ± 16 | 115 ± 13 | 108 ± 16 | 113 ± 13 |
| Verbal | 103 ± 17 | 114 ± 13 | 109 ± 17 | 114 ± 14 | |
| Nonverbal | 106 ± 17 | 113 ± 13 | 107 ± 16 | 110 ± 14 | |
Individuals with ASD underwent in-person interviews and had a diagnosis of Autistic, Asperger’s, or Pervasive Developmental Disorder Not-Otherwise-Specified established by expert clinical opinion aided by “gold standard” diagnostics: Autism Diagnostic Observation Schedule, ADOS and/or Autism Diagnostic Interview-Revised, ADI-R. These focus on 3 domains including reciprocal social interactions, communication and language, and restricted/repeated behaviors and interests. Full scale/nonverbal IQ/verbal IQ was measured via WASI, WAIS-III, and/or WISC-III. Controls had no history of mental disorders and were statistically matched for age to the ASD group at each site. There were no differences in age and sex between controls and ASD, and the datasets are based on studies approved by local IRBs, and data were fully anonymized.
MRI acquisition and processing
High-resolution T1-weighted images (T1w) and rs-fMRIs were available from all sites. Images were acquired on 3T scanners from Siemens (NYU, USM, and PITT) or Philips (IP and TCD). See Supplementary Materials for detailed scanning parameters. We utilized established multimodal image processing and co-registration routines to analyze MRI in the same reference frame, summarized below.
Structural MRI
T1w processing was based on FreeSurfer (v5.1;http://surfer.nmr.mgh.harvard.edu/; Fischl 2012). It included bias field correction, registration to stereotaxic space, intensity normalization, skull-stripping, and white matter segmentation. Triangular surface tessellation fitted a deformable mesh onto the white matter volume, providing gray–white and pial surfaces. We measured cortical thickness as the distance between gray–white and pial surfaces and registered individual surfaces to the fsaverage5 template, improving correspondence of measurements with respect to sulco-gyral patterns. Thickness data were smoothed using a surface-based kernel with a full-width-at-half-maximum (FWHM) of 20 mm, as in prior studies (Lerch and Evans 2005).
Resting-state functional MRI
For Dataset-1, the data were obtained from the Preprocessed Connectomes initiative (http://preprocessed-connectomes-project.org/abide/), while for Dataset-2, we performed equivalent preprocessing steps. Specifically, Processing was based on the Configurable Pipeline for the Analysis of Connectomes (CPAC; https://fcp-indi.github.io/), and included slice-time correction, head motion correction, skull-stripping, and intensity normalization. Statistical corrections removed effects of head motion, white matter, and cerebro-spinal fluid signals (Behzadi et al. 2007), as well as linear/quadratic trends. After band-pass filtering (0.01–0.1 Hz), we co-registered rs-fMRI and T1w data through combined linear and nonlinear transformations. Surface alignment was verified case-by-case and we interpolated voxel-wise rs-fMRI time-series along the mid-thickness surface model. We resampled rs-fMRI surface data to the Conte69 template (https://github.com/Washington-University/Pipelines) and applied 5-mm FWHM surface-based smoothing. To construct a functional connectivity matrix at individual, we used a recently proposed functional parcellation (https://github.com/ColeLab/ColeAnticevicNetPartition; Ji et al. 2019), which boundaries are constrained by those of a previously established atlas from the Human Connectome Project (Glasser et al. 2016). This parcellation (called “ColeAnticevicNetPartition”; 360 ROIs) includes a set of ROIs for the language network, which is a main target of our hypothesis. Based on this map, we averaged rs-fMRI time series at each parcel and computed an intrinsic functional connectivity matrix based on Pearson’s correlation of functional time series between every pair of parcels (360 × 360). Participants with faulty surface segmentations, imaging artifacts, or >0.3-mm averaged framewise displacement in the functional scans were excluded. Minor segmentation inaccuracies of all remaining cases were manually corrected by several raters (SLV and BCB), followed by pipeline reruns.
Analytical approaches
The goal of our analyses was a comprehensive assessment of imbalanced cognitive profiles in ASD, based on data-driven statistical learning and mapping of relevant multimodal brain imaging features. We performed 5 complementary analyses: a) group-level IQ profiling, b) IQ-based individual subtyping (categorical), c) identification of IQ-space dimensions to capture the variability of cognitive imbalance profiles (dimensional), d) direct association between IQ and brain imaging features, and e) Neurosynth-based functional decoding of anatomical areas related to cognitive imbalance.
Group-level IQ profiling
We first assessed differences in verbal and nonverbal IQ as well as their ratio (vnIQ; i.e. verbal IQ divided by nonverbal IQ) in ASD relative to controls, controlling for age and site effects. We also tested a replication dataset with a larger sample size (n = 760; see Participants for details) as well as another vnIQ metric, i.e. difference between verbal and nonverbal IQ rather than a ratio, to assess reproducibility. As biological heterogeneity is a hallmark of ASD, we furthermore tested whether the variance of those 3 IQ scores (i.e. verbal, nonverbal and ratio) is abnormally larger in ASD compared with the controls based on bootstrapping (with 10,000 iterations). Briefly, at each iteration we resampled the cases from each group (with replacement) and computed the group difference (i.e. ASD-controls) of IQ variance. The statistical model tested whether the mean of these variance differences between the groups is greater than zero (i.e. one-tailed z-test). Findings were corrected for multiple comparisons based on the false discovery rate at 5% (Benjamini and Hochberg 1995).
IQ-based categorical clustering
Following the group-level profiling, we carried out a categorical subtyping using IQ measures by applying agglomerative hierarchical clustering analysis (kernel: Ward’s linkage) on verbal and nonverbal IQ scores. To obtain an optimal solution k (=clustering number), we primarily relied on the Silhouette index (i.e. a measure of how similar an object is to its own cluster, compared with other clusters). The solution obtained by this index was further validated by another consensus-based algorithm called “NbClust” (Charrad et al. 2014), which provides the solution that has the highest agreement across 30 different established clustering-quality indices (e.g. Davies–Bouldin or Gap statistics). The clustering solution and the distribution of identified subtypes in the IQ space have been reproduced in Dataset-2. Identified subtypes were comprehensively profiled in terms of IQ scores and brain imaging features. For the latter, we compared the cortical thickness as well as whole-brain functional connectivity between each ASD subtype and neurotypical controls to identify distinct structure–function patterns across subtypes.
Dimensional IQ decomposition
We also applied principal component analysis (PCA) to verbal and nonverbal IQ scores. Given the number of input features (=2; verbal IQ and nonverbal IQ), we obtained 2 principal components. We mapped the component scores back to the individuals, and related these dimensional scores to cortical thickness and functional connectivity using group-interaction models (i.e. [ASD-control or control-ASD] × PC-scores).
Direct association between vnIQ and brain imaging features
The analyses in b) and c) targeted the underlying latent structures of IQ distribution, using fully data-driven techniques. Here, we assessed a direct association of vnIQ with brain imaging features based on linear models. To evaluate morphological substrates of IQ profiles, we computed the correlations between the vnIQ ratio and cortical thickness in both typically developing controls and ASD, and assessed group-by-vnIQ ratio interactions controlling for age and site effects. We repeated the same analysis for functional connectivity with vnIQ ratio.
Meta-analytic functional decoding
To identify potential functional associations of the brain areas discovered in the above analyses b–d), we performed a Neurosynth-based decoding of relevant cognitive functions. We first identified which brain areas showed significant effects of cortical thickness across the b–d) analyses (see Results for details). The areas of overlap on the cortical surface were then converted to the MRI volume using “mri_label2vol” in FreeSurfer, and fed into the Neurosynth, a meta-analytical framework identifying cognitive terms associated with the input cortical areas (Yarkoni et al. 2011).
Statistical test
The above brain imaging analyses a)–d) were conducted using SurfStat, a Matlab toolbox to perform surface-based statistical linear modeling (http://www.math.mcgill.ca/∼keith/surfstat; Worsley et al. 2009). We controlled for the effects of age, site, and framewise displacement (for functional connectivity measures) across all analyses. Surface-based anatomical findings were corrected for multiple comparisons using random field theory (Worsley et al. 1999).
Results
Dataset-1 was used for main phenotypic and neuroimaging analyses. Quality indices for structural and functional MRI data did not differ between ASD and controls (P > 0.43, t = 0.79 for cortical surface extraction, P > 0.11, t = 1.58 for head motion). Details on subject inclusion and quality control are provided in the Materials and Methods and Supplementary Fig. S1. Dataset-2 was used for the replication of IQ-related findings.
Group comparison of IQ profiles
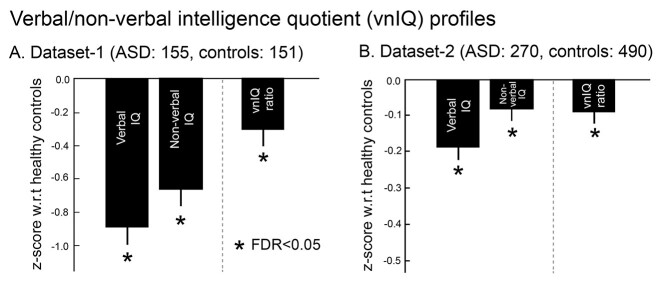
We first studied the verbal over nonverbal IQ (vnIQ) ratio to capture cognitive imbalance in a single score. In Dataset-1, ASD showed a reduced vnIQ ratio compared with controls (Cohen’s d = 0.27, P < 0.016). For further context, although both verbal and nonverbal IQ were reduced in ASD compared to controls, the decrease in verbal IQ was more severe (d = 0.72, P < 0.0001) than for nonverbal IQ (d = 0.45, P < 0.0001) in ASD. Moreover, the prevalence of such IQ discrepancy (i.e. verbal IQ < nonverbal IQ) was higher in ASD relative to neurotypicals (χ2 = 4.67, P < 0.03). Findings were similar in Dataset-2 (nvIQ reduction: d = 0.18, P < 0.015; verbal IQ reduction: d = 0.35, P < 0.0001; nonverbal IQ reduction: d = 0.16, P < 0.03; prevalence: χ2 = 8.83, P < 0.005) as well as when using the IQ difference as a metric (=verbal IQ − nonverbal IQ; reduction in ASD: d = 0.30, P < 0.007). Notably, the replication across datasets should be interpreted with a careful consideration of sample characteristics, as Dataset-2 had a different age range, sex composition, and sample size compared with the Dataset-1 (Fig. 1).
Fig. 1.
Verbal and nonverbal IQ profiles in ASD. A) Findings in Dataset-1. Z-scores of verbal IQ, nonverbal IQ, and the vnIQ ratio (verbal/nonverbal IQ) in ASD relative to neurotypical controls. Error bars present standard deviations. B) Replication in Dataset-2.
Notably, a bootstrapping analysis to test for IQ heterogeneity revealed that all 3 IQ metrics (i.e. verbal and nonverbal IQs, and their ratio) have significantly higher variances in ASD compared with the controls (P < 0.0001). This finding suggested that although at the group level there was clear evidence for both IQ reduction and imbalance in ASD, the individual patterns of IQ atypicality are highly variable, corroborating previous studies reporting heterogeneous cognitive profiles in ASD.
Categorical vs. dimensional approaches
Motivated by the previous analysis on IQ variance, we sought to explore the latent structure underlying the IQ distribution based on 2 complementary methods, i.e. categorical subtyping and dimensional decomposition. Subsequently, we assessed structural and functional substrates based on cortical thickness mapping and rs-fMRI connectomics.
Categorical subtyping of IQ profiles
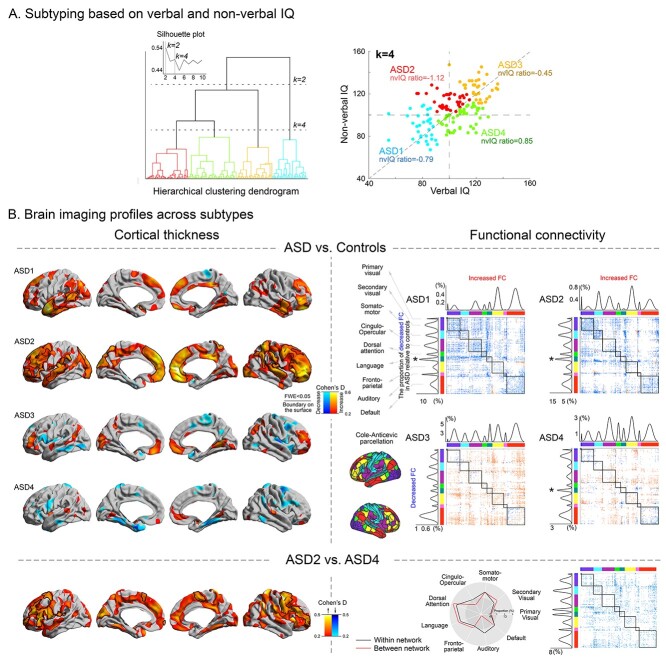
According to the Silhouette index (Fig. 2A, Supplementary Fig. S2), 2 or 4 clusters were considered as optimal. Although the 2-subtype solution split the ASD cohort along the general cognitive performance axis (i.e. low vs. high full-scale IQ), the 4-subtype solution was rather more sensitive to cognitive imbalances (reduced vs. increased vnIQ; Fig. 2A). Given its relevance to our hypothesis, we focused on this 4-subtype solution. Here, ASD1 had low full-scale IQ but slightly imbalanced verbal and nonverbal IQ (z-score of the vnIQ ratio relative to neurotypicals = −0.79), whereas ASD3 had average-high full-scale IQ without marked cognitive imbalance (z-score of the vnIQ ratio = −0.45). Compared with these 2 subtypes, ASD2 and ASD4 demonstrated dichotomized patterns of IQ imbalance (i.e. the vnIQ z-score in ASD2/ASD4 = −1.12/0.85) with relatively preserved overall cognitive performance (full-scale IQ [mean ± SD] = 106 ± 13/107 ± 14 for ASD2/ASD4). Please note that although the absolute z-score for the nvIQ ratio in each identified subtype was mostly below 1, effect size for the differences between each subtypes and TD averaged at 1.1 ± 0.8, suggesting noticeable differences.
Fig. 2.
Subtyping based on verbal and nonverbal IQ. A) Subtyping results shown at k = 2 and 4, the solutions resulting in the highest Silhouette index. B) A k = 4 solution provided subtypes reflecting cognitive imbalances, particularly when comparing ASD2 vs. ASD4. Cortical thickness and functional connectivity features were profiled across all 4 subtypes, by comparing these measures to neurotypical controls (effect sizes are presented as Cohen’s D). For the functional connectivity analysis, connections showing a significant between-group difference were counted within- and between-network separately and plotted left to the connectome for within-community comparisons and above the corresponding connectome for between-community analyses, for each subtype. The peak modulation among the functional network is marked by *. The language peak was observed in 3 out of the 4 identified subtypes. The bottom panels show targeted comparisons between ASD2 (high nonverbal compared with verbal IQ) and ASD4 (high verbal compared with nonverbal IQ).
See Supplementary Table S1 for more detailed clinico-demographic profiles of the identified subtypes. This subtype solution showed moderately similar patterns when using the clustering solution from nbClust as well as analyzing the independent Dataset-2 (Supplementary Fig. S3).
The IQ-derived ASD subtypes presented with differential cortical thickness alterations relative to neurotypical controls, in a spectrum that encompassed widespread thickness increases (ASD1 and ASD2) together with more mosaic patterns of increases and decreases in thickness (ASD3 and ASD4; Fig. 2B, left). Contrasting ASD2 and ASD4 indicated widespread increases in cortical thickness in the former group, which showed the lowest vnIQ ratio (=the most severely imbalanced cognitive profiles) among all other groups. It should be noted that these increases were not simply due to an effect from lower verbal IQ nor symptom severity, given that when comparing ASD1 (i.e. the group with the lowest verbal IQ and highest ADOS score) to ASD4, we did not observe marked changes in thickness both in cortex-wide and language networks. Moreover, when comparing the cortical thickness between the subtypes directly derived from verbal-IQ based clustering, those 2 subtypes showing a maximal verbal IQ difference did not reveal significant changes, speaking against a major role of verbal IQ on our neuroimaging finding (i.e. a main effect of cognitive imbalance on the brain substrate).
Regarding functional connectivity, we targeted their differential profiles within and between different functional communities, which are defined by the parcellation map (i.e. ColeAnticevicNetPartition; Ji et al. 2019). All subtypes presented with a variable pattern of decreases and increases across multiple networks relative to controls. Notably, ASD1 and 2, which showed cortical thickening in the above analysis, presented with overall reductions of functional connectivity relative to controls (mean Cohen’s D = 0.34/0.33 for within-/between-community connectivity), whereas ASD3 and 4 showed mixed patterns of both increased (D = 0.31/0.30 for within-/between-community connectivity) and decreased (D = 0.31/0.29 for within-/between-community) functional connectivity. Most marked modulations were again observed in language networks (Fig. 2B, right). Directly contrasting ASD2 and ASD4 revealed hypoconnectivity in the former group (D = 0.34/0.33 for within-/between-community connectivity).
Dimensional IQ subtyping
Following the categorical clustering, we ran a PCA based on the verbal and nonverbal IQs. Instead of searching for discrete boundaries across the individuals, this analysis identified continuous dimensions that can explain the largest individual variabilities in terms of cognitive imbalance. Two components were identified, each explaining a distinct dimension underlying ASD IQ profile variance (PC1: 76%, PC2: 24%; Fig. 3A). PC1 reflected more closely the average of verbal and nonverbal IQ, whereas PC2 reflected more closely their imbalance.
Fig. 3.
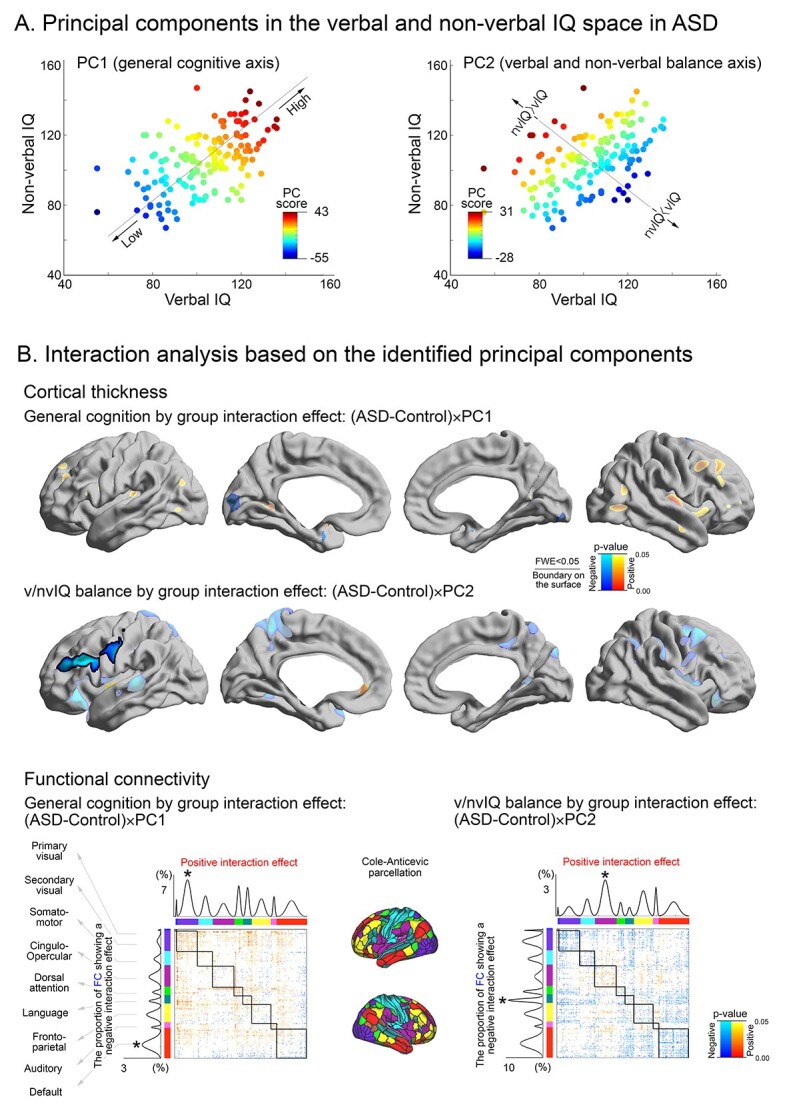
PCA for the distribution of verbal and nonverbal IQ in ASD. A) The direction and scores of the 2 principal components derived from verbal and nonverbal IQ profiles. PC1 reflected individual variability along a general cognitive axis, whereas PC2 reflected verbal to nonverbal IQ imbalance. B) Between-group interaction analysis of PC1 and PC2 on cortical thickness and functional connectivity. Upper panels show significant cortical thickness modulations were delineated by solid boundaries whereas uncorrected tendencies are shown in semi-transparent. Lower panels show the proportion of functional connections that undergo a significant between-group interaction for both PC1 and PC2. Findings were stratified according to functional communities as in the prior figures.
Notably, PC2 showed a clear spectrum (spanning from ASD2 to ASD4 from the above subtyping analysis), with one extreme characterized by high functioning nonverbal IQ, yet with a low verbal IQ as well as the other extreme showing enhanced verbal functioning but with reduced nonverbal IQ profiles. We observed similar IQ components in Dataset-2 (Supplementary Fig. S4).
Group-interaction analyses between PC scores and brain imaging features (Fig. 3B) revealed that (i) in PC1, the ASD group presented no significant effect but only a tendency of cortical thickening associated with higher general cognitive performance, whereas (ii) in PC2, increased thickness reflected a more marked vnIQ imbalance (more deficits in verbal compared with nonverbal IQ) mostly in the language areas in ASD compared with the neurotypicals. Functional connectivity also revealed similar group-dependent changes with respect to its relationship with each PC score. For the general cognitive dimension (PC1), both positive effects were most marked in the visual network (t = 1.96/1.97 for within- and between-community connectivity, respectively), whereas negative effects predominated in transmodal systems such as default mode network (t = 2.02/1.97 for within- and between-community connectivity). Notably, however, for PC2 reflecting verbal/nonverbal imbalance, the communities demonstrating significant interaction effects included the language and salience networks. In the language network, higher PC2 scores (=more severe vnIQ reduction) were related decreased connectivity in ASD relative to controls (t = 1.92/2.01 for within-/between-community connectivity). On the contrary, in the salience network, higher PC2 scores represented connectivity increases in ASD (t = 1.95/1.95 for within-/between-community connectivity).
Notably, both identified subtypes and PC scores were neither related to structural MRI quality indexed by Euler number (from FreeSurfer; Rosen et al. 2018) nor framewise displacement of fMRI signals (Supplementary Fig. S6).
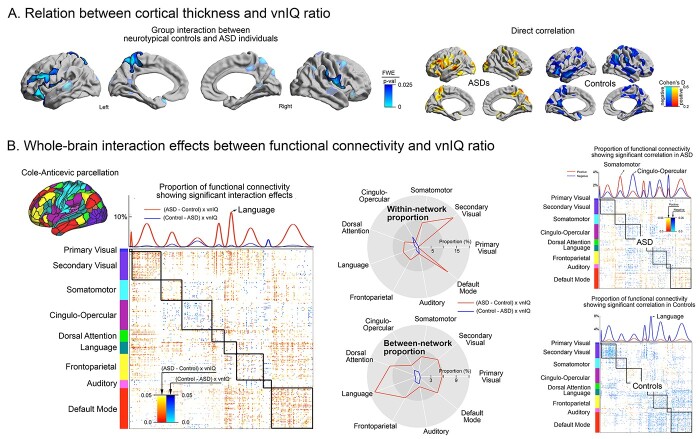
Direct association between vnIQ and brain imaging features
Finally, we directly associated vnIQ metrics (score and ratio) with brain imaging features. In this analysis, we assessed surface-wide interactions between the diagnostic groups (ASD, neurotypicals) and vnIQ metrics on cortical thickness and functional connectivity. Considering cortical thickness, there were only trends for interactions between group membership and verbal or non-verbal IQ scores. On the other hand, group by vnIQ ratio interactions were significant in multiple areas including the left insular, fronto-opercular, paracentral, posterior midline and right fronto-opercular cortices (Fig. 4A), suggesting that the ratio value is uniquely associated with cortical alterations in ASD. Notably, while increased cortical thickness in neurotypicals was associated with increased vnIQ, ASD showed an inverse pattern, with atypical thickening relating to lower scores (i.e. more severe cognitive imbalance). Repeating the above analysis for rs-fMRI connectivity confirmed significant interaction effects between vnIQ and diagnostic groups (ASD, controls) across multiple networks (Fig. 4B, left and middle). Post-hoc analyses highlighted alterations of within-community communication for the default mode (mean ± SD t = 2.12 ± 0.40) and sensory (t = 2.09 ± 0.34) networks, whereas particularly the language network (t = 2.07 ± 0.35) displayed interaction effects in connections to other networks. Although this functional interaction showed overall distinct patterns that are shifted towards a zero-centered distribution when performing a global mean signal regression (GSR), their relative patterns (i.e. those spanning within and across the subnetworks) appear to be preserved (r = 0.83 for effect size map correlation between with and without GSR), suggesting robustness (Supplementary Fig. S7).
Fig. 4.
Structure–function substrates of vnIQ imbalance in ASD. A) Cortical thickness analysis. Left. Interaction between diagnostic group (ASD, controls) and vnIQ scores. FWE-corrected clusters are shown in solid and with black outlines, uncorrected trends in semitransparent. Right. Correlation between cortical thickness and vnIQ in each group separately. B) Functional associations. Left. Using a functional parcellation that includes the language network (Ji et al. 2019), interaction analysis was performed at the level of parcel-to-parcel connections. Uncorrected P-values from this interaction analysis were sorted according to functional communities (primary visual, secondary visual, somatomotor, cingulo-opercular, dorsal attention, language, frontoparietal, auditory, and default mode). Parcel-wise significant interactions were summed within each network, and stratified into within- vs. between-community connections. Right. Direct correlation analysis between vnIQ and functional connectivity across different communities, carried out in ASD and controls separately. Positive/negative effects are indicated in blue/red.
We also explored connectome-wide correlations in each group separately (Fig. 4B, right). In ASD, the vnIQ ratio modulated both positively and negatively the functional connectivity of multiple networks, whereas associations in controls were mainly negative. Specifically, in ASD somatomotor, visual, and default mode systems showed more frequently positive associations, whereas cingulo-opercular, language, and auditory networks showed rather negative associations.
In both cortical thickness and functional connectivity findings, the effects were relatively consistent across the included sites and when repeating analyses within children or adults (that are split by the median age [16 years] to ensure equal group sample size; Supplementary Fig. S8 and S9).
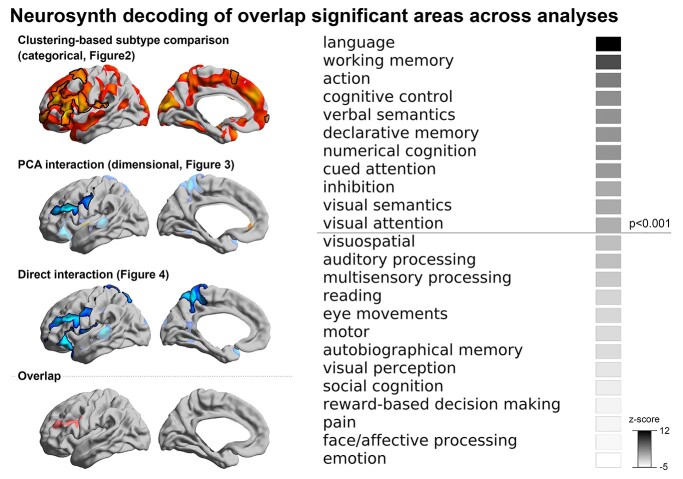
Meta-analytic functional decoding
For Neurosynth-based spatial decoding, we generated an overlap map of significant areas, intersecting the significant morphological findings from all 3 previous analyses (i.e. ASD2 vs. ASD4 subtype comparison, PC2 group-interaction and group-by-IQ interaction effects), and then fed it to the Neurosynth decoder. This analysis identified multiple terms, with “language” being the top-ranked one alongside with other language- and higher cognitive processes (e.g. “verbal semantics,” “working memory,” “cognitive control”; Fig. 5).
Fig. 5.
Functional decoding of the cortical area related to cognitive imbalance. The significant area commonly found across all previous 3 analyses was identified (i.e. left middle frontal area), and fed into the Neurosynth decoding framework. The significance of the decoding result was indicated at P < 0.001 (one tailed; z > 3.1).
Discussion
Inter-individual heterogeneity in biological, cognitive, and behavioral dimensions is increasingly recognized to hinder research and intervention in autism (Hong et al. 2018, 2020; Lombardo et al. 2019; Rødgaard et al. 2019). The current diagnostic classification of ASD is broad, and thus captures different severities and potential etiologies (Lai et al. 2013; Mottron and Bzdok 2020). Although this approach likely increases diagnostic sensitivity, it may reduce specificity, which motivates additional stratification to further calibrate diagnostics and guide intervention. Here, we targeted the brain correlates of autism heterogeneity using a “cognitive-first” perspective by studying verbal and nonverbal dimensions of intelligence in multisite datasets. In contrast to the current use of IQ measures as clinical specifiers without diagnostic indication, but in line with observations of discrepant cognitive profiles in autism (Joseph et al. 2002; Mottron et al. 2006; Coolican et al. 2008; Munson et al. 2008; Soulières et al. 2011; Ankenman et al. 2014; Nowell et al. 2015; Johnson et al. 2021), the current work provides evidence for a marked cognitive imbalance in ASD compared with neurotypicals. Indeed, capitalizing on 3 complementary data analytical strategies (i.e. IQ profile clustering, dimensional IQ profile decomposition, and linear associations to vnIQ ratio), we demonstrated converging findings showing imbalanced verbal to nonverbal intelligence in ASD. Moreover, utilizing brain imaging and connectomics, we could reveal brain structure–function substrates underlying such imbalances, which are characterized by atypical morphology and functional connectivity of language, sensory-motor, and higher cognitive systems. Phenotypic findings were replicated in an independent cohort. These findings motivate the incorporation of cognitive imbalances in autism research, which may help to stratify individuals prior to interventions.
Our analysis first quantified cognitive imbalances by calculating a simple ratio between verbal and nonverbal IQ profiles, as previously done in phenotypic assessments of individuals with typical and atypical brain development (Ankenman et al. 2014; Nader et al. 2015). Applying the same metric to 2 independent ABIDE sub-cohorts, we indeed found generalizable evidence of marked IQ imbalances in ASD, a result that remained the same when switching the metric from a ratio to a difference of the 2 IQ scores. After confirming their uneven cognitive profiles, we also sought to identify potential brain substrates underlying this heterogeneous phenotype based on multimodal neuroimaging. One of the chosen approaches, categorical clustering, is among the most frequently used methods in recent subtyping studies to identify homogeneous subgroups across various brain disorders such as autism, epilepsy, and depression (Bernhardt et al. 2015; Drysdale et al. 2017; Hong et al. 2018, 2020). In the current work, this approach also revealed clinically and biologically meaningful ASD subgroups, whose IQ profiles were particularly related to their cognitive imbalance. Specifically, a 4-subtype solution highlighted discrepant IQ profiles in ASD2 (with reduced verbal functioning) vs. ASD4 (with reduced nonverbal functioning). Directly comparing both subtypes revealed fronto-central cortical thickening in individuals with a lower IQ ratio, together with connectivity anomalies related to between-network connectivity in the same individuals. The results were further corroborated by a complementary PCA, which showed one of the primary IQ dimensions as the one sensitive to cognitive imbalances. Notably, this component reflected brain structure and function in a similar manner as the clustering findings, pointing to cortical thickening and functional connectivity modulations of frontal language networks by degrees of cognitive imbalance in ASD. A direct brain-IQ regression analysis provided similarly converging evidence, collectively suggesting robustness of findings across alternative analytical frameworks. Finally, Neurosynth-based decoding found that the spatial distribution of cognitive imbalance-related brain areas (in the cortical thickness analyses) was aligned to functional networks previously implicated in language processing. This finding follows the intention of the vnIQ ratio measure to be sensitive to language-related impairments in the presence of normal nonverbal functioning, a pattern frequently described in ASD (Dawson et al. 2007). In sum, our multi-method findings provide unique neural support for the clinical relevance of IQ discrepancy in ASD.
A recent analysis comparing the correlations between IQ measures and ADOS severity score using the same data source revealed that this symptom score, and more specifically the “communication” was mainly driven by verbal IQ rather than by vnIQ (Johnson et al. 2021). This result is predictable, considering the conceptual overlap between communication and language. Compared with this result, our findings suggest that while less directly associated to autism “severity,” vnIQ may be more informative in linking atypical cognitive functions of ASD to their brain substrates, compared with verbal IQ. The lack of association between vnIQ and symptom severity may be in part due to the fact that ADOS scores (as measured by standardized diagnostic instruments) are at risk of being affected by multiple associated/comorbid factors (Havdahl et al. 2016) and to group individuals at a too high hierarchical level in neurodevelopmental disorders (Frith 2021; Mottron 2021).
Our results collectively suggest that cognitive imbalance may represent an important source of phenotypic heterogeneity in autism, and that its non-consideration might have contributed to inconclusive findings across previous case-control studies in ASD. Indeed, although some prior MRI findings suggested altered thickening in frontal and temporal cortices in ASD relative to neurotypicals (Hardan et al. 2006; Valk et al. 2015; van Rooij et al. 2018; Bedford et al. 2020), other studies have shown cortical thinning or only subtle effects (Wallace et al. 2010), limiting consistency (Duerden et al. 2012). Similar to the structural imaging literature, rs-fMRI studies often followed a case-control design without incorporation of IQ profiles. Specifically, some reports emphasized reductions in both short- and long-range cortico-cortical connections (Khan et al. 2013; Tomasi and Volkow 2019; Hong et al. 2019b), whereas others suggested atypical organization of local and subcortical–cortical connectivity (Mizuno et al. 2006; Keown et al. 2013; Nair et al. 2013; Cerliani et al. 2015; Park et al. 2021). Although such divergences may be partially attributed to methodological choices and motion-related confounds (Müller et al. 2011; Nair et al. 2013), inherent heterogeneity across individuals with ASD may also contribute (Bernhardt et al. 2017; Dickie et al. 2018; Hong et al. 2018; Lombardo et al. 2019; Nunes et al. 2019; Rødgaard et al. 2019; Benkarim et al. 2020). A recent model that consolidated behavioral, neuroimaging, and genetic findings suggests that genetic mutations may trigger brain reorganization in individuals with a low plasticity threshold, particularly in association cortices with a high number of synapses (Mottron et al. 2014). These reorganizations, and their role in the cortical hierarchies may in turn lead to alterations in perceptual pathways, and the cascading effect may account for (i) atypical visual and auditory perceptual processing (Wang et al. 2007; O’Connor 2011), ranging from enhancements to impairments (Mottron et al. 2013), and (ii) difficulties in more integrative, social cognitive functions (Leekam 2016). It may thus be tempting to speculate that findings showing differential modulations of brain structure and function by cognitive imbalance in ASD may relate to developmental processes similar to cross-modal compensation (Mottron et al. 2014). However, as neurobiological substrates identified through cortical thickness and functional connectivity analysis remain somewhat unclear in the context of ASD, it remains to be investigated how far our findings reflect ASD-related effects on brain network plasticity. Previous post-mortem work suggested intracortical cellular and laminar anomalies (Bauman and Kemper 1985; Hutsler et al. 2007), together with ectopic neurons in the white matter in ASD individuals (Bauman and Kemper 2008; Avino and Hutsler 2010). These findings are complemented by work showing alterations in dendritic spine densities on cortical projection neurons in ASD, showing increased spine densities in supragranular layers in frontal regions and in infragranular layers in temporal cortices (Hutsler and Zhang 2010). These authors discussed the possibility that increased spine densities may result from deficient postnatal culling of connections and may thus have downstream effects on the interplay of excitation and inhibition in local microcircuits, which may be in line with recent connectome modeling studies (Trakoshis et al. 2020; Park et al. 2021). Other reports have shown focal “patches” of disorganized cortical layers in ASD (Stoner et al. 2014) and emphasized altered columnar arrangement, again with prominent findings in temporal and frontal cortices (Casanova et al. 2002, 2006).
Despite such biologically supportive findings on the structure–function substrates of cognitive imbalance in autism, our study has several limitations. First, as other samples in ASD neuroimaging, the ABIDE sample is known to be predominantly male. However, to be maximally inclusive, Dataset-I and Dataset-II included females as well, despite the imbalanced sex composition. Second, the ABIDE datasets have only limited phenotypic characterization available, which precluded additional investigation into factors and confounders that might relate to IQ imbalance in ASD. For instance, information on medication, co-occurring conditions, and indices of adaptive functioning and overall wellbeing were not systematically available. Finally, despite our efforts in image quality control and confound correction, we cannot rule out that MRI data quality and head motion effects did not contribute to the observed findings.
In future work, it will be important to extend the data-driven and multimodal neuroimaging approaches presented here to more deeply characterized individuals and to assess generalizability to samples with a higher proportion of females. Such work may also benefit from the inclusion of task-based functional imaging paradigms to study neural dynamics, for instance during verbal vs. nonverbal reasoning (Kobayashi et al. 2007). Moreover, multimodal explorations into the complex neural substrates of autism may naturally benefit from ongoing advances in MRI acquisition, processing, and modeling, which may allow for gains in sensitivity and specificity to map structural as well as functional changes associated with ASD.
We close by highlighting that our study does not imply that researchers need to control for IQ measures as a variable of no interest when comparing individuals with ASD to neurotypical controls. As our results emphasize, verbal and nonverbal aspects of intelligence and their disparities are instead an important dimension of the diverse autism phenotype, which encompasses a high prevalence of impaired as well as enhanced abilities compared with neurotypicals (Mottron et al. 2014). Although etiological factors that contribute to these imbalances remain to be studied further, our findings showing a robust structure–function substrate of imbalances point to atypical large-scale brain reorganization centered around language-related networks, potentially downstream to perturbations of cross-network plasticity.
Funding
This work was supported by funding from the Brain & Behavior Research Foundation (NARSAD Young Investigator grant; #28436) and the Institute for Basic Science (IBS-R15-D1) in Korea for SJH. SL acknowledges funding from Fonds de la Recherche du Québec—Santé (FRQ-S) and the Canadian Institutes of Health Research (CIHR). RVdW received support from the Richard and Ann Sievers Award. BP is supported by a Molson Engineering Fellowship and the National Research Foundation of Korea (NRF-2020R1A6A3A03037088), the National Research Foundation of Korea (NRF-2021R1F1A1052303), Institute for Information and Communications Technology Planning and Evaluation (IITP) funded by the Korea Government (MSIT) (2020-0-01389, Artificial Intelligence Convergence Research Center, Inha University; 2021-0-02068, Artificial Intelligence Innovation Hub), and Institute for Basic Science (IBS-R015-D1). AdM acknowledges funding from NIMH (R21MH107045-01, R01MH105506, R01MH115363). IS acknowledges funding from FRQ-S and CIHR AM acknowledges research funding from NIEHS (R01 ES030950, R01 ES027424, K23ES026239), NIH (1UG3OD023290), and the NVLD Project. BCB acknowledges research funding from the SickKids Foundation (NI17-039), the National Sciences and Engineering Research Council of Canada (NSERC; Discovery-1304413), CIHR (FDN-154298), Azrieli Center for Autism Research (ACAR), an MNI-Cambridge collaboration grant, BrainCanada (Azrieli Future Leaders), FRQS, and the Canada Research Chairs Tier 2 program. Conflict of interest statement: The authors have no conflict of interest to disclose.
Supplementary Material
Affiliations 1 and 2 contributed equally to this work.
Contributor Information
Seok-Jun Hong, Multimodal Imaging and Connectome Analysis Laboratory, McConnell Brain Imaging Centre, Department of Neurology and Neurosurgery and Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, Quebec H3A2B4, Canada; Center for Neuroscience Imaging Research, Institute for Basic Science, Sungkyunkwan University, Seobu-ro 2066, Jangan-gu, Suwon 16419, South Korea; Department of Biomedical Engineering, Sungkyunkwan University, Seobu-ro 2066, Jangan-gu, Suwon 16419, South Korea; Center for the Developing Brain, Child Mind Institute, 101 East 56th Street, New York, NY 10022, United States.
Laurent Mottron, Centre de Recherche du CIUSSSNIM and Department of Psychiatry and Addictology, Université de Montréal, 7070 boulevard Perras, Montréal, Quebec H1E 1A4, Canada.
Bo-yong Park, Multimodal Imaging and Connectome Analysis Laboratory, McConnell Brain Imaging Centre, Department of Neurology and Neurosurgery and Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, Quebec H3A2B4, Canada; Center for Neuroscience Imaging Research, Institute for Basic Science, Sungkyunkwan University, Seobu-ro 2066, Jangan-gu, Suwon 16419, South Korea; Department of Data Science, Inha Univerisity, Incheon 22212, South Korea.
Oualid Benkarim, Multimodal Imaging and Connectome Analysis Laboratory, McConnell Brain Imaging Centre, Department of Neurology and Neurosurgery and Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, Quebec H3A2B4, Canada.
Sofie L Valk, Multimodal Imaging and Connectome Analysis Laboratory, McConnell Brain Imaging Centre, Department of Neurology and Neurosurgery and Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, Quebec H3A2B4, Canada; Otto Hahn group Cognitive neurogenetics, Max Planck Institute for Human Cognitive and Brain Sciences, Stephanstraβe 1A. Leipzig D-04103, Germany; Institute of Neuroscience and Medicine, Research Centre Wilhelm-Johnen-Strasse, Jülich 52425, Germany; Institute of Systems Neuroscience, Heinrich Heine University, Moorenstr. 5, Düsseldorf 40225, Germany.
Casey Paquola, Multimodal Imaging and Connectome Analysis Laboratory, McConnell Brain Imaging Centre, Department of Neurology and Neurosurgery and Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, Quebec H3A2B4, Canada; Institute of Neuroscience and Medicine, Research Centre Wilhelm-Johnen-Strasse, Jülich 52425, Germany.
Sara Larivière, Multimodal Imaging and Connectome Analysis Laboratory, McConnell Brain Imaging Centre, Department of Neurology and Neurosurgery and Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, Quebec H3A2B4, Canada.
Reinder Vos de Wael, Multimodal Imaging and Connectome Analysis Laboratory, McConnell Brain Imaging Centre, Department of Neurology and Neurosurgery and Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, Quebec H3A2B4, Canada.
Janie Degré-Pelletier, Multimodal Imaging and Connectome Analysis Laboratory, McConnell Brain Imaging Centre, Department of Neurology and Neurosurgery and Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, Quebec H3A2B4, Canada; Department of Psychology, Université du Québec à Montréal, 100 rue Sherbrooke Ouest, Montréal, Québec H2X 3P2, Canada.
Isabelle Soulieres, Department of Psychology, Université du Québec à Montréal, 100 rue Sherbrooke Ouest, Montréal, Québec H2X 3P2, Canada.
Bruce Ramphal, Department of Psychiatry, The New York State Psychiatric Institute and the College of Physicians Surgeons, Columbia University, 1051 Riverside Drive, New York, NY 10032, United States.
Amy Margolis, Department of Psychiatry, The New York State Psychiatric Institute and the College of Physicians Surgeons, Columbia University, 1051 Riverside Drive, New York, NY 10032, United States.
Michael Milham, Center for the Developing Brain, Child Mind Institute, 101 East 56th Street, New York, NY 10022, United States; Center for Biomedical Imaging and Neuromodulation, Nathan Kline Institute, 140 Old Orangeburg Road, Orangeburg, NY 10962, United States.
Adriana Di Martino, Autism Center, Child Mind Institute, 101 East 56th Street, New York, NY 10022, United States.
Boris C Bernhardt, Multimodal Imaging and Connectome Analysis Laboratory, McConnell Brain Imaging Centre, Department of Neurology and Neurosurgery and Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, Quebec H3A2B4, Canada.
References
- Ankenman K, Elgin J, Sullivan K, Vincent L, Bernier R. Nonverbal and verbal cognitive discrepancy profiles in autism spectrum disorders: influence of age and gender. Am J Intellect Dev Disabil. 2014:119:84–99. [DOI] [PubMed] [Google Scholar]
- Avino TA, Hutsler JJ. Abnormal cell patterning at the cortical gray-white matter boundary in autism spectrum disorders. Brain Res. 2010:1360:138–146. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Editorial perspective: neurodiversity - a revolutionary concept for autism and psychiatry. J Child Psychol Psychiatry. 2017:58:744–747. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985:35:866–874. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. The neuropathology of the autism spectrum disorders: what have we learned? In: Autism: neural basis and treatment possibilities. Novartis Foundation symposium. Chichester, UK: John Wiley & Sons, Ltd.; 2008. pp. 112–128. [PubMed] [Google Scholar]
- Bedford SA, MTM P, Devenyi GA, Tullo S, Germann J, Patel R, Anagnostou E, Baron-Cohen S, Bullmore ET, Chura LR, et al. Large-scale analyses of the relationship between sex, age and intelligence quotient heterogeneity and cortical morphometry in autism spectrum disorder. Mol Psychiatry. 2020:25:614–628. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007:37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Method. 1995:57:289–300. [Google Scholar]
- Benkarim O, Paquola C, Park B-Y, Hong S-J, Royer J, de Wael RV, Lariviere S, Valk S, Bzdok D, Mottron L, et al. Functional idiosyncrasy has a shared topography with group-level connectivity alterations in autism. 2020: bioRxiv. https://www.nature.com/articles/s42003-021-02572-6.
- Bernhardt BC, Hong S-J, Bernasconi A, Bernasconi N. Magnetic resonance imaging pattern learning in temporal lobe epilepsy: classification and prognostics. Ann Neurol. 2015:77:436–446. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Di Martino A, Valk SL, Wallace GL. Neuroimaging-based phenotyping of the autism spectrum. Curr Top Behav Neurosci. 2017:30:341–355. [DOI] [PubMed] [Google Scholar]
- Betzel RF, Bassett DS. Multi-scale brain networks. NeuroImage. 2017:160:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnel A, McAdams S, Smith B, Berthiaume C, Bertone A, Ciocca V, Burack JA, Mottron L. Enhanced pure-tone pitch discrimination among persons with autism but not Asperger syndrome. Neuropsychologia. 2010:48:2465–2475. [DOI] [PubMed] [Google Scholar]
- Caron M-J, Mottron L, Berthiaume C, Dawson M. Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain. 2006:129:1789–1802. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002:58:428–432. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IAJ, Switala AE, van Engeland H, Heinsen H, Steinbusch HWM, Hof PR, Trippe J, Stone J, Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006:112:287–303. [DOI] [PubMed] [Google Scholar]
- Cerliani L, Mennes M, Thomas RM, Di Martino A, Thioux M, Keysers C. Increased functional connectivity between subcortical and cortical resting-state networks in autism spectrum disorder. JAMA Psychiat. 2015:72:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrad M, Ghazzali N, Boiteau V, Niknafs A. NbClust: AnRPackage for determining the relevant number of clusters in a data set. J Stat Softw. 2014:61. [Google Scholar]
- Clements CC, Sparding T, Schultz RT, Yerys BE, Watkins MW. DAS-II cognitive profiles are not diagnostically meaningful for autism: a ROC analysis. Autism Res. 2020:13:2143–2154. [DOI] [PubMed] [Google Scholar]
- Coolican J, Bryson SE, Zwaigenbaum L. Brief report: data on the Stanford-Binet Intelligence Scales (5th ed.) in children with autism spectrum disorder. J Autism Dev Disord. 2008:38:190–197. [DOI] [PubMed] [Google Scholar]
- Craddock RC, James GA, Holtzheimer PE 3rd, Hu XP, Mayberg HS. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp. 2012:33:1914–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M, Soulières I, Gernsbacher MA, Mottron L. The level and nature of autistic intelligence. Psychol Sci. 2007:18:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo X-N, Mennes M, Mairena MA, Lord C, Castellanos FX, Milham MP. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 2011:69:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Yan C-G, Li Q, Denio E, Castellanos FX, Alaerts K, Anderson JS, Assaf M, Bookheimer SY, Dapretto M, et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 2014:19:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, O’Connor D, Chen B, Alaerts K, Anderson JS, Assaf M, Balsters JH, Baxter L, Beggiato A, Bernaerts S, et al. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci Data. 2017:4:170010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie EW, Ameis SH, Shahab S, Calarco N, Smith DE, Miranda D, Viviano JD, Voineskos AN. Personalized intrinsic network topography mapping and functional connectivity deficits in autism spectrum disorder. Biol Psychiatry. 2018:84:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017:23:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden EG, Mak-Fan KM, Taylor MJ, Roberts SW. Regional differences in grey and white matter in children and adults with autism spectrum disorders: an activation likelihood estimate (ALE) meta-analysis. Autism Res. 2012:5:49–66. [DOI] [PubMed] [Google Scholar]
- Eyler LT, Pierce K, Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 2012:135:949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. Free surfer. NeuroImage. 2012:62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U. When diagnosis hampers research. Autism Res. 2021:14(10):2235–2236. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018:19:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016:536:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar S, Berman S, Behrmann M, Dinstein I. Anatomical abnormalities in autism? Cereb Cortex. 2016:26:1440–1452. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Muddasani S, Vemulapalli M, Keshavan MS, Minshew NJ. An MRI study of increased cortical thickness in autism. Am J Psychiatry. 2006:163:1290–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havdahl KA, Hus Bal V, Huerta M, Pickles A, Øyen A-S, Stoltenberg C, Lord C, Bishop SL. Multidimensional influences on autism symptom measures: implications for use in etiological research. J Am Acad Child Adolesc Psychiatry. 2016:55:1054–1063.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S-J, Valk SL, Di Martino A, Milham MP, Bernhardt BC. Multidimensional neuroanatomical subtyping of autism Spectrum disorder. Cereb Cortex. 2018:28:3578–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S-J, Hyung B, Paquola C, Bernhardt BC. The superficial White matter in autism and its role in connectivity anomalies and symptom severity. Cereb Cortex. 2019a:29:4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S-J, Vos de Wael R, Bethlehem RAI, Lariviere S, Paquola C, Valk SL, Milham MP, Di Martino A, Margulies DS, Smallwood J, et al. Atypical functional connectome hierarchy in autism. Nat Commun. 2019b:10:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Vogelstein JT, Gozzi A, Bernhardt BC. Towards neurosubtypes in autism. Biologicals. 2020;88(1):111–128. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010:1309:83–94. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, Love T, Zhang H. Histological and magnetic resonance imaging assessment of cortical layering and thickness in autism spectrum disorders. Biol Psychiatry. 2007:61:449–457. [DOI] [PubMed] [Google Scholar]
- Ji JL, Spronk M, Kulkarni K, Repovš G, Anticevic A, Cole MW. Mapping the human brain’s cortical-subcortical functional network organization. NeuroImage. 2019:185:35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CN, Ramphal B, Koe E, Raudales A, Goldsmith J, Margolis AE. Cognitive correlates of autism spectrum disorder symptoms. Autism Res. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. J Child Psychol Psychiatry. 2002:43:807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keown CL, Shih P, Nair A, Peterson N, Mulvey ME, Müller R-A. Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Rep. 2013:5:567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Gramfort A, Shetty NR, Kitzbichler MG, Ganesan S, Moran JM, Lee SM, Gabrieli JDE, Tager-Flusberg HB, Joseph RM, et al. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proc Natl Acad Sci U S A. 2013:110:3107–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Müller R-A, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008:1221:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi C, Glover GH, Temple E. Children’s and adults’ neural bases of verbal and nonverbal “theory of mind.”. Neuropsychologia. 2007:45:1522–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Chakrabarti B, Baron-Cohen S. Subgrouping the autism “spectrum”: reflections on DSM-5. PLoS Biol. 2013:11:e1001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larivière S, Vos de Wael R, Paquola C, Hong S-J, Mišić B, Bernasconi N, Bernasconi A, Bonilha L, Bernhardt BC. Microstructure-informed connectomics: enriching large-scale descriptions of healthy and diseased brains. Brain Connect. 2019:9:113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam S. Social cognitive impairment and autism: what are we trying to explain? Philos Trans R Soc Lond Ser B Biol Sci. 2016:371:20150082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennen DT, Lamb GD, Dunagan BJ, Hall TA. Verbal prowess equals higher IQ: implications for evaluating autism. Res Autism Spectr Disord. 2010:4:95–101. [Google Scholar]
- Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. NeuroImage. 2005:24:163–173. [DOI] [PubMed] [Google Scholar]
- Lerch JP, van der Kouwe AJW, Raznahan A, Paus T, Johansen-Berg H, Miller KL, Smith SM, Fischl B, Sotiropoulos SN. Studying neuroanatomy using MRI. Nat Neurosci. 2017:20:314–326. [DOI] [PubMed] [Google Scholar]
- Lindell AK, Hudry K. Atypicalities in cortical structure, handedness, and functional lateralization for language in autism spectrum disorders. Neuropsychol Rev. 2013:23:257–270. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Lai M-C, Baron-Cohen S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol Psychiatry. 2019:24:1435–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Bishop S, Anderson D. Developmental trajectories as autism phenotypes. Am J Med Genet C Semin Med Genet. 2015:169:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis A, Bansal R, Hao X, Algermissen M, Erickson C, Klahr KW, Naglieri JA, Peterson BS. Using IQ discrepancy scores to examine the neural correlates of specific cognitive abilities. J Neurosci. 2013:33:14135–14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis AE, Davis KS, Pao LS, Lewis A, Yang X, Tau G, Zhao G, Wang Z, Marsh R. Verbal-spatial IQ discrepancies impact brain activation associated with the resolution of cognitive conflict in children and adolescents. Dev Sci. 2018:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller R-A. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006:1104:160–174. [DOI] [PubMed] [Google Scholar]
- Mottron L. A radical change in our autism research strategy is needed: back to prototypes. Autism Res. 2021:14(10):2213–2220. [DOI] [PubMed] [Google Scholar]
- Mottron L, Bzdok D. Autism spectrum heterogeneity: fact or artifact? Mol Psychiatry. 2020:25:3178–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006:36:27–43. [DOI] [PubMed] [Google Scholar]
- Mottron L, Bouvet L, Bonnel A, Samson F, Burack JA, Dawson M, Heaton P. Veridical mapping in the development of exceptional autistic abilities. Neurosci Biobehav Rev. 2013:37:209–228. [DOI] [PubMed] [Google Scholar]
- Mottron L, Belleville S, Rouleau GA, Collignon O. Linking neocortical, cognitive, and genetic variability in autism with alterations of brain plasticity: the Trigger-Threshold-Target model. Neurosci Biobehav Rev. 2014:47:735–752. [DOI] [PubMed] [Google Scholar]
- Müller R-A, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011:21:2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J, Dawson G, Sterling L, Beauchaine T, Zhou A, Elizabeth K, Lord C, Rogers S, Sigman M, Estes A, et al. Evidence for latent classes of IQ in young children with autism spectrum disorder. Am J Ment Retard. 2008:113:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader A-M, Jelenic P, Soulières I. Discrepancy between WISC-III and WISC-IV cognitive profile in autism spectrum: what does it reveal about autistic cognition? PLoS One. 2015:10:e0144645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Müller R-A. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013:136:1942–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell KP, Brewton CM, Allain E, Mire SS. The influence of demographic factors on the identification of autism spectrum disorder: a review and call for research. Rev J Autism Dev Disord. 2015:2:300–309. [Google Scholar]
- Nunes AS, Peatfield N, Vakorin V, Doesburg SM. Idiosyncratic organization of cortical networks in autism spectrum disorder. NeuroImage. 2019:190:182–190. [DOI] [PubMed] [Google Scholar]
- O’Connor K. Auditory Processing in Autism Spectrum Disorder: A Review of the Literature: Neuroscience & Biobehavioral Reviews; 2011:36(2):836–854. [DOI] [PubMed]
- Park B-Y, Hong S-J, Valk SL, Paquola C, Benkarim O, Bethlehem RAI, Di Martino A, Milham MP, Gozzi A, Yeo BTT, et al. Differences in subcortico-cortical interactions identified from connectome and microcircuit models in autism. Nat Commun. 2021:12:2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, Bolton PF, Paus T, Murphy DGM. Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb Cortex. 2010:20:1332–1340. [DOI] [PubMed] [Google Scholar]
- Rødgaard E-M, Jensen K, Vergnes J-N, Soulières I, Mottron L. Temporal changes in effect sizes of studies comparing individuals with and without autism: a meta-analysis. JAMA. Psychiatry. 2019:76(11):1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AFG, Roalf DR, Ruparel K, Blake J, Seelaus K, Villa LP, Ciric R, Cook PA, Davatzikos C, Elliott MA, et al. Quantitative assessment of structural image quality. NeuroImage. 2018:169:407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F, Zeffiro TA, Doyon J, Benali H, Mottron L. Speech acquisition predicts regions of enhanced cortical response to auditory stimulation in autism spectrum individuals. J Psychiatr Res. 2015:68:285–292. [DOI] [PubMed] [Google Scholar]
- Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N. The cellular and molecular landscapes of the developing human central nervous system. Neuron. 2016:89:248–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulières I, Dawson M, Gernsbacher MA, Mottron L. The level and nature of autistic intelligence II: what about Asperger syndrome? PLoS One. 2011:6:e25372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, Wynshaw-Boris A, Colamarino SA, Lein ES, Courchesne E. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014:370:1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Reduced local and increased long-range functional connectivity of the thalamus in autism spectrum disorder. Cereb Cortex. 2019:29:573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakoshis S, Martínez-Cañada P, Rocchi F, Canella C, You W, Chakrabarti B, Ruigrok AN, Bullmore ET, Suckling J, Markicevic M, et al. Intrinsic excitation-inhibition imbalance affects medial prefrontal cortex differently in autistic men versus women. elife. 2020:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk SL, Di Martino A, Milham MP, Bernhardt BC. Multicenter mapping of structural network alterations in autism. Hum Brain Mapp. 2015:36:2364–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Busatto GF, Calderoni S, Daly E, Deruelle C, Di Martino A, et al. Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results from the ENIGMA ASD working group. Am J Psychiatry. 2018:175:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 2010:133:3745–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Mottron L, Peng D, Berthiaume C, Dawson M. Local bias and local-to-global interference without global deficit: a robust finding in autism under various conditions of attention, exposure time, and visual angle. Cogn Neuropsychol. 2007:24:550–574. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Andermann M, Koulis T, MacDonald D, Evans AC. Detecting changes in nonisotropic images. Hum Brain Mapp. 1999:8:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Taylor JE, Carbonell F, Chung MK, Duerden E, Bernhardt B, Lyttelton O, Boucher M, Evans AC. SurfStat: a Matlab toolbox for the statistical analysis of univariate and multivariate surface and volumetric data using linear mixed effects models and random field theory. NeuroImage. 2009:47:S102. [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011:8:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.