Abstract
Understanding the interplay between the microbiome and menopause holds promise for new interventions to alleviate menopausal symptoms and improve quality of life for women.
The world population is rapidly ageing. According to the 2020 United Nations Department of Economic and Social Affairs’ population report, the number of people aged 65 years and older will double by 2050, to reach 1.5 billion. As women tend to live longer than men, an older population will be predominately female. Research related to women’s health and ageing is therefore essential to maintain and improve quality of life, and to promote wellness, activity and resilience in older women.
Although menopause is a natural part of ageing, it is a much neglected aspect of women’s health. Over the past decade, a plethora of research has found that microbial communities residing in and on our body, including the vaginal microbiome, are integral to health maintenance, including healthy ageing. The vaginal microbiome is a dynamic ecosystem that varies substantially throughout a woman’s lifespan (Fig. 1). In most premenopausal women, the vaginal microbiome is dominated by Lactobacillus species1. These beneficial microorganisms acidify the vaginal microenvironment via lactic-acid fermentation and, consequently, protect a woman against invading pathogens and promote vaginal health. Notably, the vaginal microbiota structure is influenced by circulating sex hormones. High levels of oestrogen result in glycogen accumulation in the vaginal epithelium, which promotes Lactobacillus dominance. When a woman undergoes natural menopause (defined as the permanent cessation of menstruation due to loss of ovarian follicular function), circulating oestrogen levels drop dramatically, which has a profound effect on the vaginal microbiota. The existing paradigm presumes that declining levels of oestrogen and glycogen in the vaginal microenvironment during menopausal transition leads to decreased Lactobacillus colonization1, which consequently result in elevated vaginal pH. Clinical studies based on both microbial cultivation and 16S rRNA sequencing have demonstrated that, when compared with premenopausal women, the vaginal microbiota in postmenopausal women is characterized by a depletion of Lactobacillus species and the presence of anaerobes commonly associated with bacterial vaginosis (BV), such as Gardnerella, Prevotella, Anaerococcus, Peptoniphilus and Peptostreptococcus2,3. However, in contrast to BV, which is characterized by the bacterial overgrowth, postmenopausal women are colonized with reduced levels of these microorganisms2,4. The presence of enteric and skin pathobionts (Escherichia, Enterococcus, Streptococcus, Corynebacterium and Staphylococcus) in the vaginal microbiome of postmenopausal women has also been frequently reported.
Figure 1. Vaginal microbiota vary in women across the lifespan.
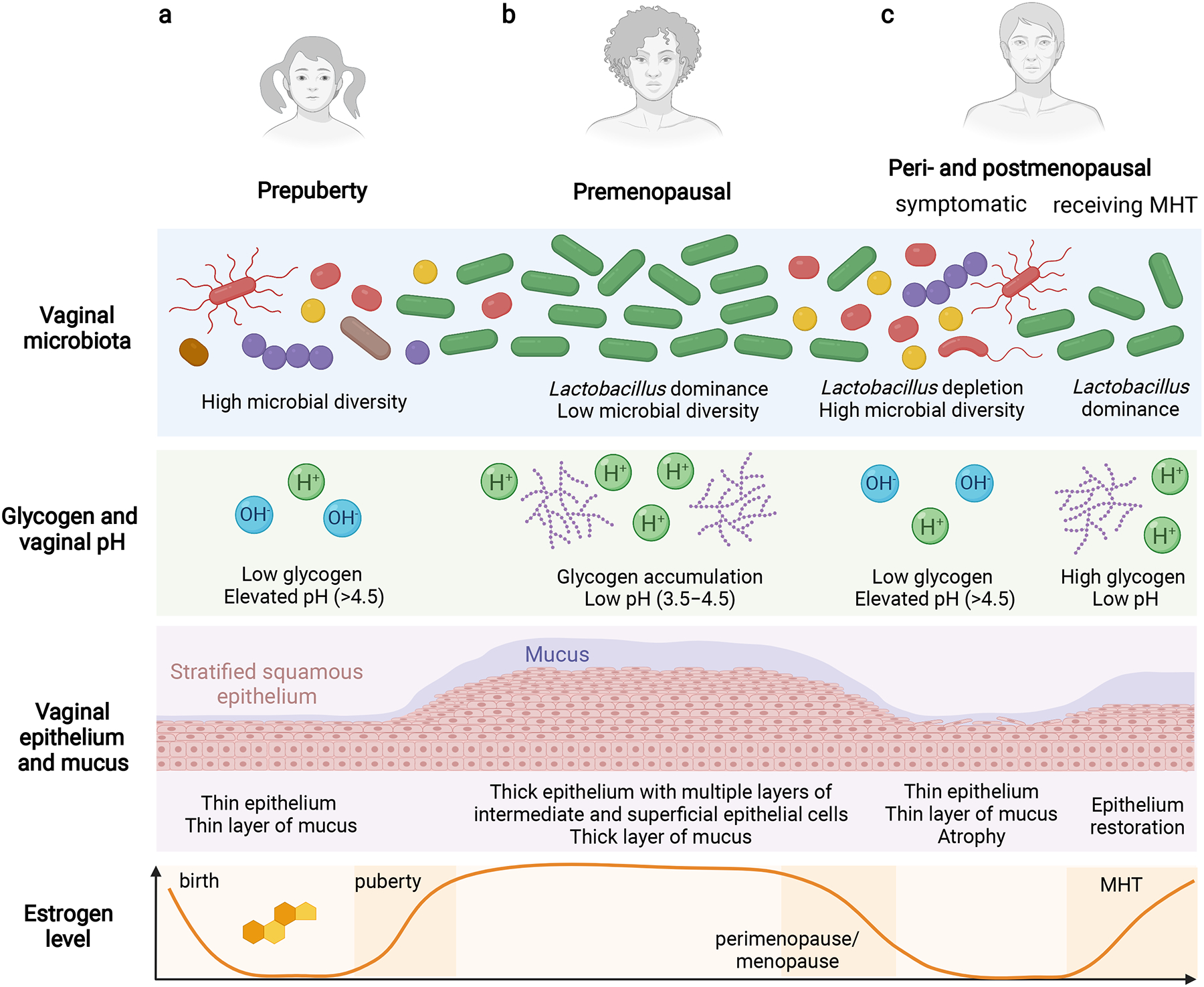
a, Before puberty, the vaginal microbiome has high microbial diversity and contains streptococci, enterococci and anaerobes. The vaginal microenvironment is also characterized by a neutral-to-alkaline pH (6–8). Due to low levels of oestrogen, vaginal stratified squamous epithelium is thin, with low deposition of glycogen and a thin layer of mucus. b, after puberty in healthy premenopausal women, the vaginal microbiome is dominated by one or few Lactobacillus species (L. crispatus, Lactobacillus iners, Lactobacillus jensenii or Lactobacillus gasseri), and has low microbial diversity. This contrasts with the gut microbiome, in which high microbial diversity is associated with better health. Intriguingly, sex hormones are inherently correlated with the composition of the vaginal microbiota. high levels of circulating oestrogen occurs when females are of reproductive age, stimulating glycogen accumulating in the vaginal stratified squamous epithelium. Vaginal Lactobacillus species depend on glycogen, which is released by exfoliated superficial epithelial cells, as an energy source. Fermentation of glycogen degradation products by lactobacilli yields lactic acid, which reduces vaginal pH to 3.5–4.5. The acidic vaginal microenvironment inhibits colonization by bacterial, viral, fungal and protozoan pathogens. c, During menopause, circulating oestrogen levels reduce from >120 pg ml–1 to <10 pg ml–1. Decreasing oestrogen levels in some peri- and postmenopausal women result in reduced glycogen accumulation in the vaginal epithelium, and consequently a decrease in Lactobacillus abundance. Vaginal epithelia that are less acidified are colonized by anaerobic bacteria that are associated with BV, or pathobionts, leading to higher microbial diversity. The hormonal changes also lead to thinning of the vaginal epithelium and atrophy. MHT, particularly low-dose vaginal oestrogens, can help to mitigate the adverse changes in the local microenvironment related to menopausal transition, including vaginal atrophy, dyspareunia, dysuria and other genitourinary symptoms, and restore a Lactobacillus-dominant vaginal microbiome. Overall, Lactobacillus colonization of the vaginal niche during menopause can contribute directly or indirectly to women’s wellness, including gynaecologic and sexual health.
We highlight what is known about the microbiome in menopause and discuss the steps needed to improve our understanding of the interplay between the vaginal microbiota and female hormones during healthy ageing in women. Our commentary is focused on the vaginal and gut microbiota of cisgender peri- and postmenopausal females.
Microbiome-mediated symptoms
Symptoms of menopause include vasomotor symptoms (VMSs) (hot flushes and night sweats), sleep disturbances, mood changes and genitourinary symptoms (vulvovaginal atrophy (VVA), dyspareunia, dysuria and recurrent urinary tract infections (UTIs)). Although VMSs usually improve over time, the genitourinary syndrome of menopause (GSM) usually worsens without effective treatment. GSM affects approximately 50% of postmenopausal women and can significantly impair women’s health, sexual function and quality of life5. It has been postulated that the vaginal microbiome closely relates to vaginal health and GSM. A 2013 cross-sectional study (n = 87) showed that women with signs of mild or moderate VVA had greater odds to have highly diverse microbiota depleted of lactobacilli than microbiota dominated by Lactobacillus crispatus when compared to women with no VVA3. A small longitudinal study (n = 16) also found a positive correlation between vaginal dryness and VVA with Lactobacillus depletion and increased microbial diversity6. However, two clinical studies (n = 88 and n = 120) published in 2017 and 2021 found no association between severity of vulvovaginal symptoms and vaginal microbiota composition7,8. Therefore, further observational studies and randomized controlled trials are needed to determine whether Lactobacillus species can directly alleviate postmenopausal GSM, vaginal discomfort and severity of symptoms.
It is acknowledged that the vaginal microbiota interact with other microbial communities at both proximal and distal body sites (for example, the urinary and gastrointestinal tract) (Fig. 2). These bidirectional interactions, referred to as microbiome axes, occur during and after menopause and probably relate to specific menopause symptoms. Previous studies based on whole-genome sequencing and expanded quantitative urine culture techniques have shown an interconnectivity of microbiota within the urogenital tract (the vagina–bladder axis). Vaginal Lactobacillus species have been also hypothesized to play a protective role within the urinary tract. Dysuria and recurrent UTIs are common symptoms in postmenopausal women. A 2021 study identified anaerobic bacteria (for example, Prevotella and Porphyromonas) to be associated with recurrent UTIs treated with antibiotics9. Overall, restoration of vaginal lactobacilli might help alleviate some urinary symptoms and protect against UTIs. Intriguingly, a study of postmenopausal women (n = 62) with overactive bladder symptom also revealed that increased levels of Lactobacillus in the bladder associate with modest improvement in urgency and incontinence symptoms10. From the opposite direction, the urinary tract might serve as a reservoir of vaginal Lactobacillus species and aid with the re-colonization of the reproductive tract after dysbiosis occurs.
Figure 2. Microbiome axes in healthy ageing and menopause.
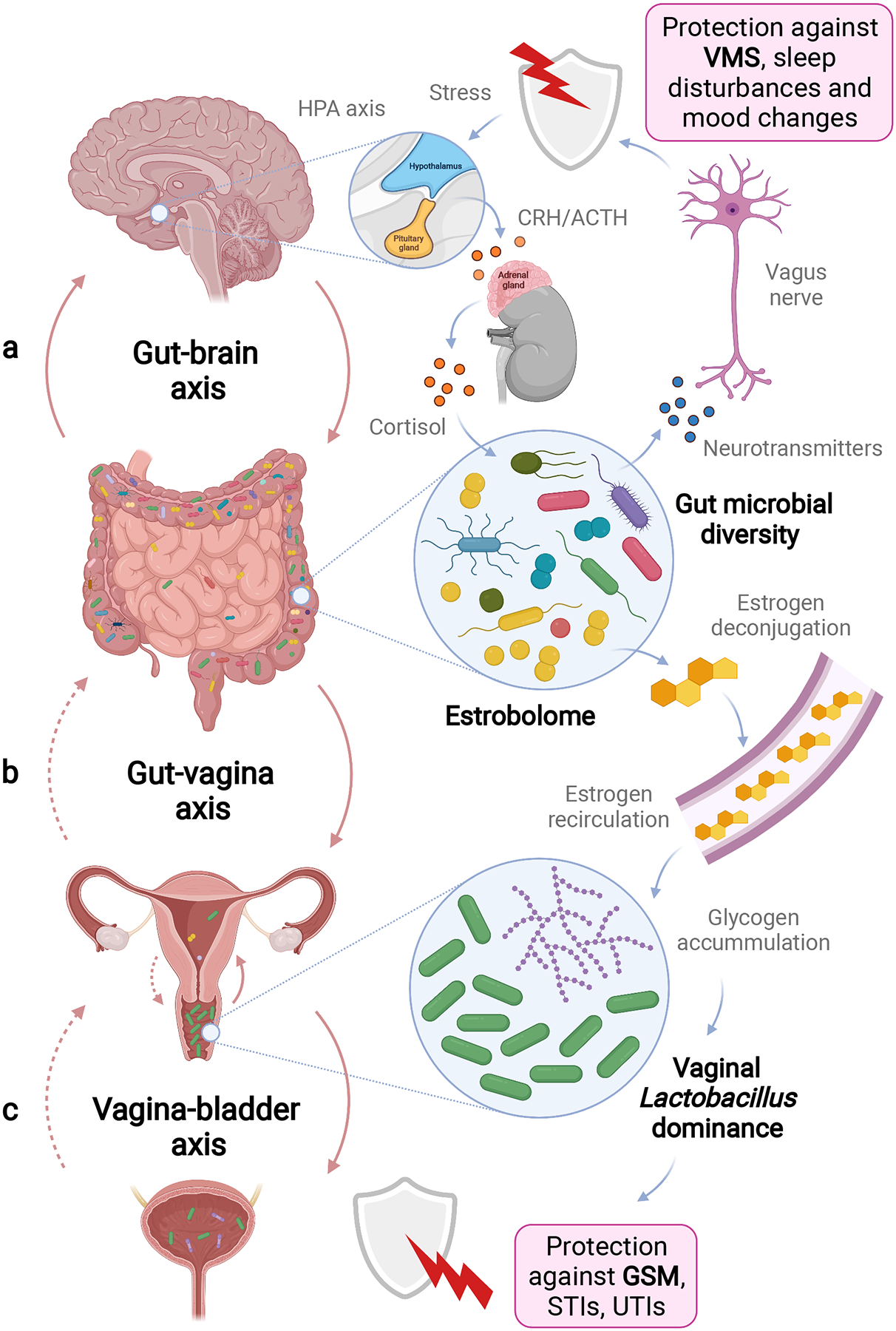
a–c, The microbiome axes play a critical role in maintaining health and homeostasis throughout the body. In ageing women in particular, interactions between the gut and brain (a), gut and vagina (b) and vagina and bladder (c) might function in mediation and alleviation of menopausal symptoms, such as VMSs and GSM. Microbiome axes exhibit bidirectional interactions that can affect distal and local sites and impact both health, symptoms and quality of life in menopausal women. Putative interactions that need more scientific evidence are indicated by dotted arrows. In a, the gut microbiota communicate with the enteric nervous system and brain (gut–brain axis). The gut bacteria produce metabolites acting as neurotransmitters. These compounds can affect local neuronal cells, as well as the vagus nerve that signals directly to the brain. Thus, changes in gut microbiota composition in ageing women can potentially impact VMSs, sleep and mental health. reciprocally, the nervous system can also influence gut microbiota composition. For example, stress activates the hypothalamic–pituitary–adrenal (HPA) axis response that involves production of corticotropin receptor hormone (CRH) in the brain and release of adrenocorticotrophic hormone (ACTH), triggering the synthesis of cortisol in adrenal glands. circulating cortisol can affect the gut barrier integrity via neuroimmune signalling, and consequently alter the gut microbiota. Vaginal microbiota interact with other microbial communities at other mucosal sites, including the gastrointestinal and urinary tracts. In b, the gut microbiota influence the vaginal microbial community via an oestrobolome-mediated mechanism (vagina–gut axis). Inactive conjugated oestrogens can be metabolized by enteric microorganisms. This metabolic process results in reabsorption of deconjugated active oestrogens to circulation. afterwards, high levels of circulating oestrogen facilitate Lactobacillus colonization in the vagina by stimulating glycogen production. at the same time, the gastrointestinal tract can be a reservoir for pathobionts replacing vaginal lactobacilli when a woman undergoes menopause. In c, health-associated Lactobacillus species can be also detected in the bladder (vagina–bladder axis), suggesting translocation of vaginal bacteria in the female urogenital tract. Lactobacillus residing in the urethra and bladder may have a protective role against UTIs. Overall, colonization of the vagina with Lactobacillus-dominant microbiota protects against urogenital infections, and probably contributes to a symptom-free menopause. STIs, sexually transmitted infections.
The gut microbiota can also impact microbial communities in the vagina via the oestrobolome — the collection of enteric microorganisms (and their genes) capable of metabolizing oestrogens. Indeed, some enteric bacteria can produce enzymes, such as β-glucuronidases11, and reactivate hepatically conjugated oestrogens, which consequently decrease their excretion and stimulate their reabsorption to circulation. The free oestrogen can be transported to distal sites, such as the reproductive tract, and promote Lactobacillus dominance in the vagina (via the gut–vagina axis). Thus, it is imperative to study interactions between the gut and vaginal microbiota in peri- and postmenopausal women, as a reduction in the diversity of the gut microbiota and oestrobolome can contribute to a decrease in circulating oestrogen levels, as well as genitourinary symptoms of menopause. Notably, these interactions and the functional activity of the oestrobolome might also contribute to other hypoestrogenic conditions (including cardiovascular disease and osteoporosis) among postmenopausal women. In addition, the impact of oestrobolome activity on hyperestrogenic conditions such as oestrogen-mediated malignancies (for example, breast, ovarian and endometrial cancers) is another controversial topic that might be elucidated in future studies. Overall, further investigations are required to better understand microbiome-related risk factors for these hormone- and menopause-related conditions. Finally, reciprocal impact of the vaginal microbiome on the gut microbiome structure is mostly unknown; nevertheless, the link between obesity, gut dysbiosis and vaginal microbiota composition suggests bidirectional mucosal axes12.
Intriguingly, gut bacteria can also communicate with the brain (via the gut– brain axis). Some metabolites produced by the gut microbiota — for example, short-chain fatty acids — have neuroactive properties and can affect the enteric nervous system, as well as the brain via the vagus nerve. Thus, potential imbalances in the gut microbiota composition during and after menopause might impact the mental health of ageing women. Moreover, enteric microorganisms via the oestrobolome activity might also contribute to the alleviation of VMSs such as hot flushes in perimenopausal women, when reactivated and reabsorbed oestrogen is transported to the brain. Reciprocally, neuropsychiatric conditions, such as stress or anxiety, can influence gut epithelial integrity and gut microbiota composition via the cascade of endocrine and neuroimmune signalling, involving the release of cortisol. Yet, our knowledge on sex and age differences in the gut microbiota is rudimentary. More mechanistic studies on the microbiome and hormone axes are needed to elucidate the role of the microbiome in menopause and women’s ageing.
Microbiome-targeting therapies
Multiple strategies are currently available to provide relief of menopause symptoms. Over-the-counter vaginal lubricants or moisturizers are first-line therapies for women with mild or moderate genitourinary symptoms. Although lubricants are frequently recommended, only a few randomized placebo-controlled studies have assessed the safety and efficacy of these products. The long-lasting effect of lubricants and other vaginally applied feminine products on the vaginal microbiome is also generally unknown. Limited in vitro studies indicate that some lubricants can damage vaginal epithelial cells (due to hyperosmolarity) and/or inhibit the growth and adherence of vaginal Lactobacillus species (due to presence of excipients and antimicrobial compounds in formulations)13. Nevertheless, the clinical importance of these findings still require confirmation in clinical studies.
Women with severe and persistent symptoms may require prescription therapies. Compelling evidence from multiple clinical trials indicates that menopausal hormone therapy (MHT) is highly effective for controlling menopausal symptoms; although, balancing the potential benefits and risks is required before initiating the treatment. Locally administrated low-dose oestrogens (including vaginally applied oestradiol, oestrone and conjugated oestrogens) are preferred over systemic therapy, owing to effectiveness and safety concerns5. Indeed, observational studies did not associate use of vaginal oestrogen with an increased risk of cancer or cardiovascular disease; although, clinical trial data related to long-term usage of vaginal oestrogens are still lacking. It is also important to note that common adverse effects in postmenopausal women that are receiving vaginal oestrogen therapy include vulvovaginal candidiasis (which relates to promotion of Candida growth, germination and adherence to epithelial cells by oestrogen and glycogen).
Regarding the vaginal microbiome, most clinical trials that have investigated low-dose vaginal oestrogens reported a reduction in vaginal pH, which strongly suggests that these treatments impact the vaginal microbiota and probably restore Lactobacillus dominance. Limited sequencing studies confirmed differences in vaginal microbiota composition between women receiving and not receiving MHT. A 2019 study (n = 45) showed that women receiving MHT more often had vaginal microbiomes dominated by Lactobacillus, an increased number of bacteria and lower vaginal pH, in addition to improved genitourinary symptoms, relative to women not receiving MHT4. A 2021 secondary analysis (n = 120) of samples collected in the randomized clinical trial (n = 302) also demonstrated that more women treated with the vaginal oestrogen had Lactobacillus-dominant microbiota and lower microbial diversity at 12 weeks than women treated with the moisturizer or dual placebo8. Nevertheless, additional clinical studies that include larger cohorts of women are warranted to determine how the vaginal microbiota relate to genitourinary symptoms and whether the microbial communities impact treatment responses.
Other available and approved therapies for postmenopausal women suffering from dyspareunia or VVA due to menopause include vaginal dehydroepiandrosterone (DHEA) inserts and oral ospemifene. Treatment with DHEA, which is an intermediate in the biosynthesis of androgens and oestrogens, demonstrated an improvement of GSM symptoms and a decrease in vaginal pH (indicative of Lactobacillus dominance)5. Similarly, treatment with ospemifene, an oestrogen agonist/antagonist, showed a decrease in vaginal pH and reduction in recurrent UTIs, suggesting reestablishment of Lactobacillus dominance. More comprehensive investigations are needed in the future to assess the efficacy of these treatments to restore the vaginal microbiome and homeostasis.
Hyaluronic acid, phytoestrogens (plant-based xenoestrogens) and testosterone have also been investigated but not approved for the treatment of GSM. However, available data are not adequate to provide evidence of efficacy and safety of these strategies, as well as their impact on lactobacilli and the composition of vaginal microbiota. Finally, in the past 10 years, the use of vulvovaginal energy-based devices (such as fractional CO2 lasers and radiofrequency devices) has substantially increased and been marketed as a therapy for vaginal ‘rejuvenation’. Yet, there is not enough scientific data demonstrating efficacy and safety of lasers and other energy-based devices for GSM or other urogynecologic conditions.
Probiotics and live biotherapeutic products (LBPs) are exciting strategies that have been pursued for vaginal microbiome modulation. However, to date, a very limited number of studies have examined the impact of probiotics on the vaginal microbiota in postmenopausal women. A 2013 randomized clinical trial (n = 87) of Gynoflor® vaginal tablets (consisting of low-dose oestrogen and Lactobacillus acidophilus KS400) showed improvement of genitourinary symptoms and an increase in lactobacilli when compared to a placebo14. Subsequent studies also evaluated this combination therapy for treatment of vaginal atrophy in breast cancer patients on aromatase inhibitors. Other LBPs, such as Lactin-V (a vaginal suppository with L. crispatus CTV-05), have been tested in randomized clinical trials for the treatment of BV, as well as recurrent UTIs15. However, in these clinical trials, only premenopausal women were recruited. Based on promising data in reproductive-age women, use of vaginal probiotics as an adjuvant therapy might be a valuable strategy for restoring vaginal microbiota in menopausal women to protect them against recurrent UTIs and other genitourinary sequelae.
Intriguing future strategies to restore the vaginal microbiome in menopausal women may include ‘precision probiotics’ (that is, Lactobacillus strains tailored to the individual woman), Lactobacillus-derived metabolites with antimicrobial or homeostasis-promoting properties, bacteriophages targeting anaerobes and enteric pathobionts, and some combination therapies. Vaginal microbiota transplantation (VMT) from healthy donors is a provocative treatment option, which is under investigation for recurrent BV and modulation of the vaginal microbiome, and may be adapted for microbiome restoration in menopausal women in the future. In addition, for women undergoing surgical menopause or women receiving anti-oestrogen therapies (for example, aromatase inhibitors for breast cancer), autologous VMT may be a viable treatment strategy. Collectively, women need more options that are backed by evidence-based medicine and robust research.
Outlook
Our understanding of the clinical relevance of the microbiome in menopause and women’s healthy ageing is growing but we need to better understand the impact of specific microorganisms and polymicrobial communities on genitourinary symptoms and other adverse outcomes, such as cardiovascular disease and osteoporosis after menopause. In addition, further insights into the role(s) of the vaginal and gut microbiomes in symptoms associated with hypoestrogenism due to surgery (for example, oophorectomy) and cancer therapies (for example, chemotherapy, radiation and aromatase inhibitors) might help to reduce adverse events and toxicities and improve women’s quality of life. Notably, most clinical studies on the microbiome and menopause have been conducted on predominantly Caucasian populations. Future investigations need to include women of different racial, ethnic and socioeconomic backgrounds as geography, race, ethnicity and lifestyle have been attributed to substantial differences in the human microbiota composition. Finally, future studies might benefit from the design of personalized approaches for restoration of vaginal microbiota during or after menopause.
Acknowledgements
We are thankful to the members of the Herbst-Kralovetz laboratory and clinical collaborators for discussions related to this topic. We apologize to authors whose work was not included herein due to space limitations. M.M.H.-K. is funded by the Flinn Foundation (no. 2244), Banner Foundation Women Inspiring Science and Health (WISH), and the National Institutes of Health National Cancer Institute (U54CA143924). We would also like to thank all of the women who have participated in our past and ongoing studies that will aid in enhancing our understanding of the microbiome and menopause.
Footnotes
Competing interests
M.M.H.-K. is a paid consultant for Freya Biosciences. None of this work related to, was shared with, or was licensed to this company or any other commercial entity. P.Ł. declares no competing interests.
References
- 1.Muhleisen AL & Herbst-Kralovetz MM Maturitas 91, 42–50 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Hillier SL & Lau RJ Clin. Infect. Dis 25, S123–S126 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Brotman RM et al. Menopause 21, 450–458 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gliniewicz K et al. Front. Microbiol 10, 193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The North American Menopause Society Menopause 27, 976–992 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Hummelen R et al. PLoS ONE 6, e26602 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell CM et al. Menopause 24, 1160–1166 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell CM et al. Am. J. Obstet. Gynecol 225, 159 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaughan MH et al. J. Urol 206, 1222–1231 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas-White K et al. Am. J. Obstet. Gynecol 223, 727 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ervin SM et al. J. Biol. Chem 294, 18586–18599 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Si J et al. Cell Host Microbe 21, 97–105 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Łaniewski P et al. Sex. Transm. Dis 48, 63–70 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaisamrarn U et al. Climacteric 16, 347–355 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen CR et al. N. Engl. J. Med 382, 1906–1915 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]


