Graphical Abstract:
Introduction
Persons with chronic kidney disease (CKD) are at twofold increased risk for cardiovascular events (CVE: incident myocardial infarction [MI], stroke, or cardiovascular death) compared to non-CKD individuals following coronary angiography1. Multiple clinical factors, including diabetes mellitus, heart failure, high blood pressure, and kidney disease, have been proposed to increase the risk of CVE among CKD patients following coronary intervention2. Excess activation of counter-regulatory pathways such as renin-angiotensin-aldosterone and natriuretic peptide system, inflammation, endothelial dysfunction, and vascular calcification have been attributed to the elevated CV risk among patients with CKD3. Biomarker measurement is a parsimonious approach to gauge these underlying pathophysiologic processes. Hence, a biomarker panel may guide healthcare professionals in stratifying patients at increased risk of CVE. In this study, using a panel of biomarkers developed via targeted proteomics, we hypothesized that a distinct set of biomarkers could stratify the risk of developing CVE following coronary angiography.
Methods
To explore this question, we utilized the resources of the Catheter Sampled Blood Archive in Cardiovascular Diseases (CASABLANCA) cohort study (NCT00842868)4. 1251 patients were enrolled in CASABLANCA. Specific to this sub-study, we excluded those who underwent peripheral angiography (n=152), leaving 1,099 patients in this study. A subset of the 172 patients were selected as external validation. A cohort of 927 remaining patients was split with a 70:30 ratio into separate derivation and internal validation sets, resulting in a derivation set of 649 patients and an internal validation set of 278 patients. The derivation set was used to identify the prognostic panel and develop the prognostic model, and the internal validation set was initially used with the validation of this model. The validation cohort for this study consists of the combined internal and external validation sets (n = 278 + 172 = 450). Four patients had a missing estimated glomerular filtration rate (eGFR) value were removed, resulting in a final validation cohort of 446 participants (Figure 1). We applied a previously-developed biomarker panel to predict incident cardiovascular events (HART CVE, Prevencio, Kirkland WA). The detail of artificial intelligence-leveraged algorithm was described elsewhere(2). Briefly, 15 ml of blood was obtained immediately before angiography and after the completion of angiographic procedure(s) through a centrally placed vascular access sheath. The samples were processed and were stored in a −80 C freezer until analysis. After a single freezethaw cycle, 200 ml of plasma was analyzed for a panel of 109 biomarkers on a Luminex 100/200 xMAP technology platform. The 109 biomarkers were not individually chosen but were acquired in the form of a commercially available kit, known as the Myriad RBM MAP. This incorporates biomarkers that reflect a wide variety of pathways associated with plaque rupture/erosion and includes acute phase reactants, inflammatory markers, and biomarkers of atherosclerosis. Least absolute shrinkage and selection operator (Lasso)was implemented to select candidate final biomarker. Monte Carlo cross-validation with 1,000 iterations followed with 80/20 training/test split. In cases when a variable was not selected in all 1,000 Monte Carlo iterations or its coefficients had a p-value >0.05, the variable was removed, and the analysis repeated. The ultimate result of this process was our final panel of 4 proteins (N-terminal pro-B type natriuretic peptide, kidney injury molecule-1, osteopontin, and tissue inhibitor of matrix metalloproteinase-1). The prognostic model was linearly transformed into a scaled score of 0 to 10. Patients in the higher risk group had a score greater than or equal to the optimal cutoff for the score, which was determined to be 5.526 using the optimal Youden’s index (with the model’s output rescaled to the range of 0 to 10). Patients in the lower-risk group had a score <5.526.
Figure 1.
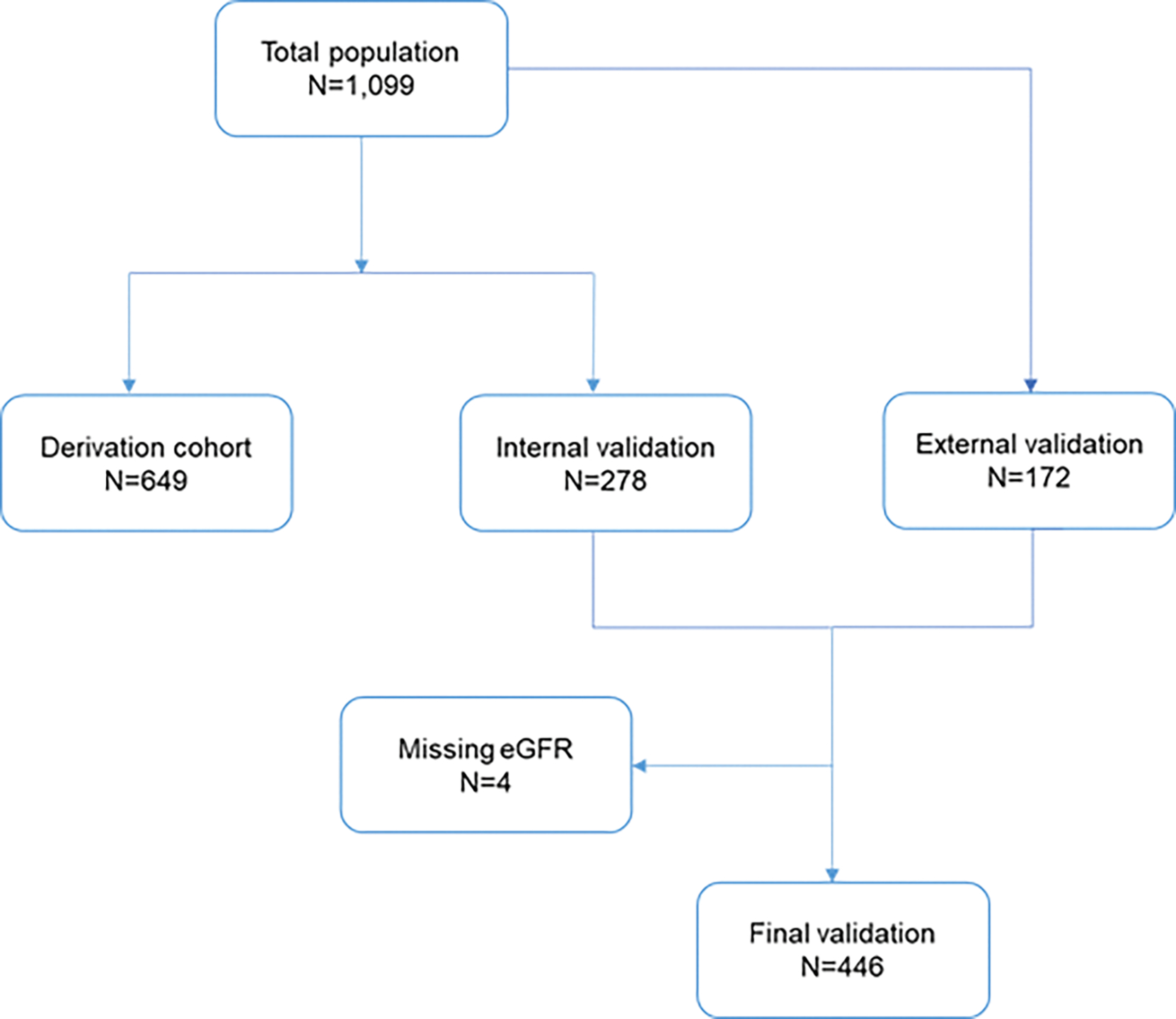
CONSORT flow diagram
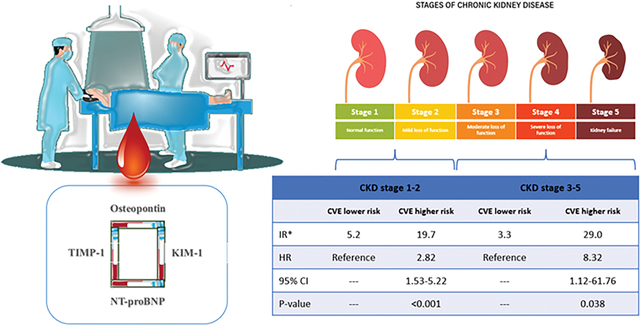
We defined CKD stage 1–2 as eGFR less than 120 mL/min/1.72m2 and more than 60 mL/min/1.72m2 and CKD stage 3–5 as eGFR less than 60 mL/min/1.72m2. Patients were categorized into 4 groups: 1) CKD stage 1–2/CVE Lower-risk, 2) CKD stage 1–2/CVE Higher-risk, 3) CKD stage 3–5/CVE Lower-risk, 4) CKD stage 3–5/CVE Higher-risk, and were followed for 2 years for incident cardiovascular events. Cox proportional hazard regression was used to assess the association of four categories with incident CVE. Harrell C statistic was reported as a measure of the discrimination ability of the risk model.
Results
Of 446 patients with CKD included in this study, mean (standard deviation) age of study participants was 66.5 (11.2) years, and 29.2% (n=130) were female; 84.8% (n=378) were at stage 1–2 CKD and 15.2% (n=68) were at stage 3–5 CKD (Table 1).
Table1.
Baseline characteristics of study population
| variable | All | CKDs 1–2 | CKDs 3–5 | p-value |
|---|---|---|---|---|
|
| ||||
| Age, year | 66.5 (11.2) | 65.6 (10.9) | 71.5 (11.6) | 0.001 |
| Male | 316 / 446 (70.9%) | 277 / 378 (73.3%) | 39 / 68 (57.4%) | 0.01 |
| Caucasian | 411 / 446 (92.2%) | 353 / 378 (93.4%) | 58 / 68 (85.3%) | 0.05 |
| Morbidities | ||||
| Hypertension | 319 / 446 (71.5%) | 259 / 378 (68.5%) | 60 / 68 (88.2%) | 0.001 |
| Diabetes Mellitus | 113 / 446 (25.3%) | 83 / 378 (22%) | 30 / 68 (44.1%) | 0.001 |
| Dyslipidemia | 292 / 445 (65.6%) | 253 / 378 (66.9%) | 39 / 67 (58.2%) | 0.17 |
| Heart failure | 79 / 446 (17.7%) | 56 / 378 (14.8%) | 23 / 68 (33.8%) | 0.001 |
| Coronary artery disease | 217 / 446 (48.7%) | 173 / 378 (45.8%) | 44 / 68 (64.7%) | 0.005 |
| Smoker | 58 / 441 (13.2%) | 52 / 373 (13.9%) | 6 / 68 (8.8%) | 0.33 |
| Atrial fibrillation/flutter | 83 / 446 (18.6%) | 71 / 378 (18.8%) | 12 / 68 (17.6%) | 1 |
| CVA/TIA | 43 / 446 (9.6%) | 36 / 378 (9.5%) | 7 / 68 (10.3%) | 0.82 |
| Prior angioplasty | 40 / 446 (9%) | 33 / 378 (8.7%) | 7 / 68 (10.3%) | 0.65 |
| Prior stent | 120 / 446 (26.9%) | 101 / 378 (26.7%) | 19 / 68 (27.9%) | 0.88 |
| Prior CABG | 84 / 446 (18.8%) | 68 / 378 (18%) | 16 / 68 (23.5%) | 0.31 |
| Laboratory tests | ||||
| Sodium, mg/dl | 138.932 (3.232) | 139.012 (3.117) | 138.508 (3.78) | 0.33 |
| BUN, mg/dl | 18 (15, 24) | 17 (14, 21) | 34 (26, 42.75) | 0.001 |
| Blood glucose, mg/dl | 102 (91, 118) | 101 (92, 116) | 107 (90, 128) | 0.18 |
| Creatinine, mg/dl | 1.04 (0.87, 1.28) | 1 (0.84, 1.17) | 1.78 (1.4, 3.78) | 0.001 |
| eGFR, ml/min/1.73 m2 | 97.2 (73.9, 110.4) | 102.2 (84.8, 111.8) | 38.6 (18.3, 46.9) | 0.001 |
| NT proBNP, pg/mL | 1550 (574, 4423) | 1280 (509, 3030) | 7890 (3290, 15975) | 0.001 |
| KIM-1, ng/mL | 0.04 (0.02, 0.07) | 0.03 (0.02, 0.06) | 0.08 (0.05, 0.18) | 0.001 |
| Osteopontin, ng/mL | 28 (20, 42.75) | 26 (19, 37) | 65.5 (37.75, 108) | 0.001 |
| TIMP-1, ng/mL | 72 (58, 92.75) | 68 (57, 85) | 109.5 (86.5, 127) | 0.001 |
CVA: cerebrovascular accident, TIA: transient ischemic attack, CABG: coronary artery bypass graft, BUN: blood urea nitrogen, eGFR: estimated glomerular filtration rate, NTproBNP: N terminal pro B type natriuretic peptides, KIM-1: kidney injury molecule-1, TIMP-1: tissue inhibitor of matrix metalloproteinase-1
During the 2-year follow-up, 74 CVE were ascertained. 51 (13.5%) events occurred in stage 1–2 CKD and 23 (33.8%) events occurred in stage 3–5 CKD. The C-statistic for predicting 2-years cardiovascular events in all 446 patients was 0.77 (0.72, 0.82). The model was well-calibrated (Hosmer-Lemeshow test p-value > 0.40). Considering patients at CVE lower-risk within each CKD staging group as a reference, the hazard ratio (95 % confidence interval) of cardiovascular events was 2.82 (1.53, 5.22) for CKD stage 1–2/CVE higher-risk, and 8.32 (1.12, 61.76) for CKD stage 3–5/CVE higher-risk (Table 2).
Table 2.
Hazard ratios of cardiovascular events
| Category | Incidence rate | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|---|
|
| ||||
| Stage CKD 1–2 | ||||
| CVE lower-risk | 5.2 | Referent | Referent | Referent |
| CVE higher-risk | 19.7 | 2.82 | 1.53, 5.22 | <0.001 |
| Stage CKD 3–5 | ||||
| CVE lower-risk | 3.3 | Referent | Referent | Referent |
| CVE higher-risk | 29.0 | 8.32 | 1.12, 61.76 | 0.038 |
Incidence rates are presented as cases per 100 person years.
Abbreviations: CKD: chronic kidney disease, CVE: cardiovascular event, CI: confidence interval
Discussion
The results of this study indicate the HART CVE panel identified patients at high risk of CVE among the patient population at different stages of CKD. This is important given the sharp increase in risk of CVE that occurs with a decline in GFR, particularly among patients with GFR below 60 mL/min/1.72m2. Biomarker measurement is a parsimonious method to stratify risk for CVE in those with CKD, although a decline in kidney function may interfere with the discrimination of prognostic biomarkers. Thus, whenever possible, prognostic panels should be evaluated in the context of decline in kidney function in order to better understand how this situation affects performance. It is noteworthy that among those with CKD Stage 3–5, a “lower risk” HART CVE score was associated with similar event rates as lower-risk individuals with Stage 1–2 CKD and a more than 8-fold lower risk for events compared to study participants with corresponding kidney function and “higher risk” HART CVE score.
Four biomarkers included in the panel reflect a different aspect of underlying pathophysiologies enhanced among patients with CKD. KIM-1, a novel marker of acute kidney injury, is a transmembrane glycoprotein and highly upregulated following ischemia-reperfusion injury in the kidneys5. Many studies have shown adverse outcomes associated with acute kidney injury during coronary angiography6, 7. Osteopontin is synthesized in various tissues and has multiple roles in several biological processes, including inflammatory response, bone resorption, vascular calcification, and extracellular matrix remodeling8. Increased expression of osteopontin was reported following myocardial infarction. Studies have linked elevated osteopontin level with increased cardiac fibrosis as osteopontin regulates myofibroblast differentiation and production of collage 1 production9. TIMP-1 is involved in the degradation of extracellular matrix. Increased expression of TIMP-1 was observed following acute pressure overload in myocardium and prolonged activation is linked to cardiac fibrosis and hypertrophy10. In a cohort of patients undergoing coronary angiography, TIMP-1 was an independent predictor of subsequent mortality and ischemic events11. Lastly, NT-proBNP is widely used in clinical practice as cardiac stress biomarker, and predictive ability of NT-proBNP for adverse outcomes among patients with coronary artery disease is well-established12. Given the specific role of each biomarker in the pathophysiology of cardiovascular disease, the developed panel may be used as a unique tool for prediction of CVE.
Conclusion
With the rapid rise in CKD, better tools to recognize risk and intervene proactively are needed. Biomarker panels such as HART CVE may be useful to individualize CVE risk assessment and foster aggressive primary and secondary cardiovascular prevention. Future clinical trials need to assess the efficacy of the HART CVE panel-guided approach in lowering the incidence of CVE among patients with CKD.
Highlights.
Patients with chronic kidney disease are at increased risk of cardiovascular events following coronary catheterization. Preceding coronary catheterization is an ideal time to measure biomarkers and estimate risk for future cardiovascular events.
Using a machine learning algorithm and targeted proteomics, we demonstrated that measuring four biomarkers (kidney injury molecule-1, N-terminal pro B-type natriuretic peptide, osteopontin, and tissue inhibitor of metalloproteinase-1) can individualize cardiovascular events risk assessment among patients with chronic kidney disease
Funding:
Dr. Mohebi is supported by the Barry Fellowship. Dr. McCarthy is supported by the NHLBI T32 postdoctoral training grant (5T32HL094301–12). Dr. Januzzi is supported by the Hutter Family Professorship.
Footnotes
Disclosures: Dr. van Kimmenade has received research grants from Novartis. Mr. Magaret, Dr. Barnes, and Ms. Rhyne are employees of Prevencio, Inc. Dr. Gaggin has received research grant support from Roche Diagnostics, Jana Care, Ortho Clinical, Novartis, Pfizer, Alnylam, Akcea (IONIS), Eidos; consulting income from Amgen, Eko, Merck, Pfizer; Stock ownership for Eko; Research payments for clinical endpoint committees from Baim Institute for Clinical Research for Abbott, Siemens and Beckman Coulter and from ACI Clinical for Abbott Laboratories. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife and Novartis; has received consulting income from Abbott Diagnostics, Boehringer-Ingelheim, Janssen, Novartis, Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda and Vifor.
References:
- 1.Wada H; Shinozaki T; Suzuki M; Sakagami S; Ajiro Y; Funada J; Matsuda M; Shimizu M; Takenaka T; Morita Y; Yonezawa K; Matsubara H; Ono Y; Nakamura T; Fujimoto K; Ninomiya A; Kato T; Unoki T; Takagi D; Wada K; Wada M; Iguchi M; Yamakage H; Kusakabe T; Yasoda A; Shimatsu A; Kotani K; Satoh-Asahara N; Abe M; Akao M; Hasegawa K; Investigators E-JS, Impact of Chronic Kidney Disease on the Associations of Cardiovascular Biomarkers With Adverse Outcomes in Patients With Suspected or Known Coronary Artery Disease: The EXCEED-J Study. J Am Heart Assoc 2022, 11 (3), e023464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohebi R; Karimi Galougahi K; Garcia JJ; Horst J; Ben-Yehuda O; Radhakrishnan J; Chertow GM; Jeremias A; Cohen DJ; Cohen DJ; Maehara A; Mintz GS; Chen S; Redfors B; Leon MB; Stuckey TD; Rinaldi MJ; Weisz G; Witzenbichler B; Kirtane AJ; Mehran R; Dangas GD; Stone GW; Ali ZA, Long-Term Clinical Impact of Contrast-Associated Acute Kidney Injury Following PCI: An ADAPT-DES Substudy. JACC Cardiovasc Interv 2022, 15 (7), 753–766. [DOI] [PubMed] [Google Scholar]
- 3.Valdivielso JM; Rodriguez-Puyol D; Pascual J; Barrios C; Bermudez-Lopez M; Sanchez-Nino MD; Perez-Fernandez M; Ortiz A, Atherosclerosis in Chronic Kidney Disease: More, Less, or Just Different? Arterioscler Thromb Vasc Biol 2019, 39 (10), 1938–1966. [DOI] [PubMed] [Google Scholar]
- 4.Gaggin HK; Liu Y; Lyass A; van Kimmenade RR; Motiwala SR; Kelly NP; Mallick A; Gandhi PU; Ibrahim NE; Simon ML; Bhardwaj A; Belcher AM; Harisiades JE; Massaro JM; D’Agostino RB Sr.; Januzzi JL Jr., Incident Type 2 Myocardial Infarction in a Cohort of Patients Undergoing Coronary or Peripheral Arterial Angiography. Circulation 2017, 135 (2), 116–127. [DOI] [PubMed] [Google Scholar]
- 5.Geng J; Qiu Y; Qin Z; Su B, The value of kidney injury molecule 1 in predicting acute kidney injury in adult patients: a systematic review and Bayesian meta-analysis. J Transl Med 2021, 19 (1), 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lupu L; Abukatash H; Banai A; Rozenfeld KL; Lewit D; Merdler I; Loewenstein I; Bornstein G; Banai S; Shacham Y, Relation of Baseline Neutrophil Gelatinase-Associated Lipocalin (NGAL) Levels and Contrast-Induced Nephropathy following Percutaneous Coronary Intervention among Chronic Kidney Disease Patients. J Clin Med 2021, 10 (22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibrahim NE; McCarthy CP; Shrestha S; Lyass A; Li Y; Gaggin HK; Simon ML; Massaro JM; D’Agostino RB Sr.; Garasic JM; van Kimmenade RR; Januzzi JL Jr., Blood kidney injury molecule-1 predicts short and longer term kidney outcomes in patients undergoing diagnostic coronary and/or peripheral angiography-Results from the Catheter Sampled Blood Archive in Cardiovascular Diseases (CASABLANCA) study. Am Heart J 2019, 209, 36–46. [DOI] [PubMed] [Google Scholar]
- 8.Lok ZSY; Lyle AN, Osteopontin in Vascular Disease. Arterioscler Thromb Vasc Biol 2019, 39 (4), 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh M; Foster CR; Dalal S; Singh K, Osteopontin: role in extracellular matrix deposition and myocardial remodeling post-MI. J Mol Cell Cardiol 2010, 48 (3), 538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heymans S; Schroen B; Vermeersch P; Milting H; Gao F; Kassner A; Gillijns H; Herijgers P; Flameng W; Carmeliet P; Van de Werf F; Pinto YM; Janssens S, Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation 2005, 112 (8), 1136–44. [DOI] [PubMed] [Google Scholar]
- 11.Cavusoglu E; Ruwende C; Chopra V; Yanamadala S; Eng C; Clark LT; Pinsky DJ; Marmur JD, Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am Heart J 2006, 151 (5), 1101 e1–8. [DOI] [PubMed] [Google Scholar]
- 12.James SK; Lindahl B; Siegbahn A; Stridsberg M; Venge P; Armstrong P; Barnathan ES; Califf R; Topol EJ; Simoons ML; Wallentin L, N-terminal pro-brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: a Global Utilization of Strategies To Open occluded arteries (GUSTO)-IV substudy. Circulation 2003, 108 (3), 275–81. [DOI] [PubMed] [Google Scholar]


