Abstract
Objectives:
To measure baseline human papillomavirus (HPV) vaccination rates among tertiary and community-based Otolaryngology – Head and Neck Surgery (Oto-HNS) clinic patients and to determine risk factors for under-vaccination.
Methods:
Retrospective chart review of patients aged 9 to 26 years presenting to an Oto-HNS clinic from 2017 to 2019. Patients were considered complete for HPV vaccination if they received two doses of HPV vaccine with the first dose received before age 15 years or three doses of HPV vaccine otherwise.
Results:
8,532 unique patients met the criteria. At the index visit, 3,110 (36.5%) had completed the HPV series, 5,422 (63.5%) were due for one or more doses, with 4,981 (58.4%) eligible for vaccination at the time of their appointment. Of those dues, most (3,148/5,422 or 58%) were past due by age (≥13 years old). Of the 3,148 patients past due, 745 (23.7%) were partially vaccinated and 2,403 (76.3%) were vaccine naïve. Male sex and younger age were both independently associated with incomplete vaccination (p < 0.0001).
Conclusion:
This study demonstrates that the implementation of on-site HPV vaccination has the potential to increase the opportunities for vaccination among vaccine-eligible patients, especially among young males. Otolaryngologists have the potential to provide meaningful preventive services in the fight against HPV-mediated disease.
Keywords: feasibility studies, immunization programs, otolaryngology, papillomavirus vaccines, squamous cell carcinoma of head and neck
INTRODUCTION
Otolaryngology – head and neck surgery (Oto-HNS) represents a specialty particularly positioned to promote and increase human papillomavirus (HPV) vaccination rates. Like Obstetrics and Gynecology (OBGYN) providers, who frequently offer HPV vaccination in the clinic, Oto-HNS providers represent surgical subspecialists who frequently treat HPV-mediated disease and malignancy. Indeed, highlighting HPV’s links to head and neck cancer improves comfort in the discussion of the HPv vaccine.1 In addition, surgical providers may be able to make a strong recommendation for vaccination,2,3 supporting primary care providers in the effort to provide preventive healthcare. Moreover, Otolaryngologists are relatively likely to see children and adolescents in clinic, as tympanostomy tube placement and tonsillectomy represent two of the most common ambulatory pediatric surgeries.4 These patients are also often seen several times within a single year for work up, counseling, and follow up, creating the potential for vaccination completion with little impact on patient appointments. From recurrent respiratory papillomatosis (RRP) to HPV-mediated oropharynx cancer, the treatment of HPv-mediated disease is an essential component of every Oto-HNS practice, begging the question, can Otolaryngologists actively join the fight to prevent this infection?
The human papillomavirus (HPV) is the most common sexually transmitted infection (STI) in the United States and worldwide.5 The original 4-valent HPV vaccine, approved in 2006, was only recommended for females for the prevention of cervical cancer.5 This recommendation was extended to males aged 9–26 in 2009.6 Notably, in 2009, both the incidence and prevalence of HPV(+) oropharyngeal squamous cell carcinoma (OPSCC), which is 10-fold more common in males than females, surpassed cervical cancer in the U.S.7 The current vaccine that is utilized in the United States, Gardasil 9, is a 9-valent Food and Drug Administration (FDA)-approved vaccine for males and females aged 9–45 for the prevention of HPV infections that may lead to cervical, vaginal, vulvar, anal, penile, and oropharyngeal squamous cell carcinoma, as well as several forms of nonmalignant papillomatosis.5 Gardasil 9 was originally approved for females in 2014, and the FDA expanded its approval of the vaccine to include males aged 9–26 in December of 2015. In June 2020, the FDA approved Gardasil 9 for the prevention of OPSCC and other HPV-related head and neck cancers.8 The current schedule for vaccination requires two doses five months apart starting at age 9 among the immunocompetent, or three doses if the first dose is administered at age 15 or later9 or if the patient is immunocompromised.
The Centers for Disease Control and Prevention (CDC) estimates 92% of HPV-attributable cancers are preventable by HPV vaccination.10 However, adolescent HPV vaccination rates remain relatively low compared to other scheduled adolescent vaccinations in this age range such as the meningococcal and tetanus, diphtheria, and pertussis vaccines (Tdap).11 Notably, in 2019 only 54.2% of eligible adolescents had received their full vaccination schedule and were thus considered fully protected against HPV,12 far below the Healthy People 2030 HPV vaccination rate goal of 80%.13 Walling et al. demonstrated that increased vaccine availability reaches the greatest number of adolescents and is the most successful in improving vaccination rates.14 However, achieving complete vaccination in this patient population is difficult as few adolescents have medical encounters in primary care for acute care, chronic care, or preventive care.15 In addition, HPV vaccination is currently not routinely required for school immunizations.16
We hypothesized that a significant number of HPV-vaccine eligible patients are seen by Otolaryngologists both in a tertiary and community care setting with a visit frequency that would allow HPV vaccine series completion for a significant proportion of patients. We sought to quantify the annual number of patients aged 9–26 presenting to an Oto-HNS clinic at a Midwestern US tertiary health center or affiliated community Oto-HNS clinic, evaluate their HPV vaccination status to assess the potential impact of an HPV vaccination program in this setting and to determine risk factors for undervaccination against HPV. To our knowledge, no such programs currently exist.
MATERIAL AND METHODS
After obtaining IRB approval (IRB #: 21–006640), a retrospective chart review was performed for all patients from 9 to 26 years of age seen within the Department of Otorhinolaryngology – Head and Neck Surgery at a Mayo Clinic Midwest site from 2017 to 2019. Visits at the tertiary care center in Rochester, MN were categorized as academic (Acad-Oto) visits, while those performed at Mayo affiliated community clinics were characterized as community (Comm-Oto) visits. Patients who opted out of Minnesota Research Authorization or had unknown HPV vaccination status were excluded from the study.17,18 Patients aged 27 to 45 were not included in this analysis as HPV vaccine is not routinely recommended at this age, however, it is available as a shared decision between clinician and patient based on individual risk and benefit.19
Basic demographic information and clinical variables were collected to determine risk factors for under vaccination. These variables included: subspecialty or division of provider seen, HPV vaccination status, and date of HPV vaccinations. Vaccination status was calculated using the vaccine records from the electronic medical record (EMR) and dates of vaccinations. The EMR included vaccinations given on-site as well as those reported by the patient and identified within their vaccination records. These data were readily available in the medical record of most patients due to the existence of state-wide vaccination record repositories including the Wisconsin Immunization Registry, Minnesota Immunization Information Connection, and the Iowa Immunization Registry Information System, which are updated at the time of vaccination and automatically queried by the EMR.
Patients were considered fully vaccinated if they received two doses of the HPV vaccine with the first dose administered before age 15 years, with at least 5 months between doses. Alternatively, patients whose first dose was administered at 15 years or later were considered fully vaccinated if they received three doses of the HPV vaccine. Patients were classified into three groups based on vaccination status at their index appointment with an Otolaryngology provider during the time of interest: fully vaccinated (2–3 HPV vaccinations depending on series and age), partially vaccinated (1–2 HPV vaccinations depending on series and age), or vaccine naïve (no HPV vaccinations despite vaccine records available).
Logistic regression analysis was performed to evaluate relationships between vaccination status and patient demographics. Associations between vaccination status and sex, controlling for age and race/ethnicity, and between vaccination status and location of the appointment, controlling for age, sex, and race/ethnicity, were evaluated. Partially vaccinated and vaccine naïve patients were both coded as “not up-to-date” for the calculation of odds ratios and confidence intervals of full vaccination status. Statistical analyses were performed using SAS version 9.4 (SAS Institute; Cary, NC). All tests were two-sided and p-values <0.05 were considered statistically significant.
RESULTS
Patient Demographics
A total of 11,812 unique patients from age 9–26 years old presented to an outpatient Otolaryngology appointment at Acad-Oto site or an affiliated Midwest Comm-Oto site. A total of 8,532 patients (72.2%) met inclusion criteria after excluding 1,351 patients (11.4%) who declined Minnesota Research Authorization and 1,929 patients (16.3%) with unknown vaccination status. Patient demographics divided by age group and sex are summarized in Table I.
TABLE I.
Demographics of Patients Aged 9–26 Presenting to an Oto-HNS Department at a Midwest Tertiary Care Center or Affiliated Community Site From 2017 to 2019.
| Age 9–12 | Age 13–17 | Age 18–26 | Grand Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female n = 1262 (14.8%) |
Male n = 1298 (15.2%) |
Combined n = 2560 (30.0%) |
Female n = 1154 (13.5%) |
Male n = 1109 (13.0%) |
Combined n = 2263 (26.5%) |
Female n = 2196 (25.7%) |
Male n = 1513 (17.7%) |
Combined n = 3709 (43.5%) |
8532 (100%) | |
| Appointment location | ||||||||||
| Tertiary care center | 500 (5.9) | 536 (6.3) | 1036 (12.1) | 438 (5.1) | 423 (5.0) | 861 (10.1) | 874 (10.2) | 584 (6.8) | 1458 (17.1) | 3355 (39.3) |
| Community Oto-HNS clinic | 762 (8.9) | 762 (8.9) | 1524 (17.9) | 716 (8.4) | 686 (8.0) | 1402 (16.4) | 1322 (15.5) | 929 (10.9) | 2251 (26.4) | 5177 (60.7) |
| Visit subspecialty | ||||||||||
| General Oto-HNS* | 551 (6.5) | 586 (6.9) | 1137 (13.3) | 621 (7.3) | 604 (7.1) | 1225 (14.4) | 1359 (15.9) | 893 (10.5) | 2252 (26.4) | 4614 (54.1) |
| Audiology | 440 (5.2) | 417 (4.9) | 857 (10.0) | 294 (3.4) | 268 (3.1) | 562 (6.6) | 496 (5.8) | 378 (4.4) | 874 (10.2) | 2293 (26.9) |
| Pediatric Oto-HNS | 211 (2.5) | 243 (2.8) | 454 (5.3) | 129 (1.5) | 135 (1.6) | 264 (3.1) | 23 (0.3) | 16 (0.2) | 39 (0.5) | 757 (8.9) |
| FPRS | 3 (0.0) | 5 (0.1) | 8 (0.1) | 13 (0.2) | 16 (0.2) | 29 (0.3) | 65 (0.8) | 50 (0.6) | 115 (1.3) | 152 (1.8) |
| Head & neck cancer | 5 (0.1) | 2 (0.0) | 7 (0.1) | 5 (0.1) | 11 (0.1) | 16 (0.2) | 83 (1) | 49 (0.6) | 132 (1.5) | 155 (1.8) |
| SLP | 11 (0.1) | 6 (0.1) | 17 (0.2) | 50 (0.6) | 19 (0.2) | 69 (0.8) | 33 (0.4) | 14 (0.2) | 47 (0.6) | 133 (1.6) |
| Rhinology | 1 (0.0) | 1 (0.0) | 2 (0.0) | 9 (0.1) | 10 (0.1) | 19 (0.2) | 56 (0.7) | 49 (0.6) | 105 (1.2) | 126 (1.5) |
| Neurotology | 17 (0.2) | 7 (0.1) | 24 (0.3) | 10 (0.1) | 21 (0.2) | 31 (0.4) | 26 (0.3) | 24 (0.3) | 50 (0.6) | 105 (1.2) |
| Laryngology | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (0.1) | 0 (0.0) | 5 (0.1) | 9 (0.1) | 5 (0.1) | 14 (0.2) | 19 (0.2) |
| Other** | 23 (0.3) | 31 (0.4) | 54 (0.6) | 18 (0.2) | 25 (0.3) | 43 (0.5) | 46 (0.5) | 35 (0.4) | 81 (0.9) | 178 (2.1) |
| Patient address | ||||||||||
| IA, MN, or WI | 1227 (14.4) | 1265 (14.8) | 2492 (29.2) | 1116 (13.1) | 1075 (12.6) | 2191 (25.7) | 2065 (24.2) | 1443 (16.9) | 3508 (41.1) | 8191 (96) |
| Other state | 32 (0.4) | 24 (0.3) | 56 (0.7) | 36 (0.4) | 26 (0.3) | 62 (0.7) | 112 (1.3) | 55 (0.6) | 167 (2) | 285 (3.3) |
| Non-US | 3 (0) | 9 (0.1) | 12 (0.1) | 2 (0) | 8 (0.1) | 10 (0.1) | 19 (0.2) | 15 (0.2) | 34 (0.4) | 56 (0.7) |
| Race/ethnicity | ||||||||||
| Nonwhite hispanic or latino | 67 (0.8) | 40 (0.5) | 107 (1.3) | 31 (0.4) | 36 (0.4) | 67 (0.8) | 37 (0.4) | 27 (0.3) | 64 (0.8) | 238 (2.8) |
| Nonwhite nonhispanic or latino | 101 (1.2) | 86 (1) | 187 (2.2) | 74 (0.9) | 77 (0.9) | 151 (1.8) | 121 (1.4) | 87 (1) | 208 (2.4) | 546 (6.4) |
| White hispanic or latino | 43 (0.5) | 42 (0.5) | 85 (1) | 29 (0.3) | 30 (0.4) | 59 (0.7) | 38 (0.4) | 27 (0.3) | 65 (0.8) | 209 (2.4) |
| White nonhispanic or latino | 996 (11.7) | 1068 (12.5) | 2064 (24.2) | 971 (11.4) | 922 (10.8) | 1893 (22.2) | 1904 (22.3) | 128315 | 3187 (37.4) | 7144 (83.7) |
| White unknown | 8 (0.1) | 6 (0.1) | 14 (0.2) | 9 (0.1) | 6 (0.1) | 15 (0.2) | 18 (0.2) | 22 (0.3) | 40 (0.5) | 69 (0.8) |
| Other race/ethnicity | 34 (0.4) | 41 (0.5) | 75 (0.9) | 31 (0.4) | 24 (0.3) | 55 (0.6) | 51 (0.6) | 41 (0.5) | 92 (1.1) | 222 (2.6) |
| Unknown race/ethnicity | 13 (0.2) | 15 (0.2) | 28 (0.3) | 9 (0.1) | 14 (0.2) | 23 (0.3) | 27 (0.3) | 26 (0.3) | 53 (0.6) | 104 (1.2) |
| Vaccination eligibility | ||||||||||
| Eligible at Oto-HNS appointment | 954 (11.2) | 1025 (12.0) | 1979 (23.2) | 484 (5.7) | 559 (6.6) | 1043 (12.2) | 847 (10.0) | 1112 (13.0) | 1959 (23.0) | 4981 (58.4) |
ENT = Ear, nose, and throat; FPRS = Facial plastic and reconstructive surgery; IA = Iowa; MN = Minnesota; SLP = Speech and language pathology; US = United States; WI = Wisconsin.
Includes chief resident clinic and nurse-only visits.
Includes Allergy, Medical Genetics, Physical and Occupational Therapy, and unknown.
Variation in Vaccination Rates by Age and Sex
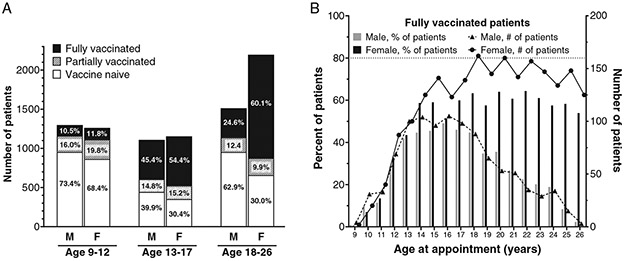
Of the 8,532 patients evaluated in this study, 4,219 (49.4%) were vaccine naïve. 4,313 (50.6%) had received at least one vaccination dose, and 3,110 (36.4%) were fully vaccinated. At the time of their appointment, 2,560 patients (30%) were 9–12 years old, 2,263 (26.5%) were 13–17, and 3,709 (43.5%) were 18–26. To determine whether vaccine status varied by age or sex, the patient population was first divided by sex and then further subdivided into three age groups: 9–12, 13–17, and 18–26 years. Sex was significantly associated with vaccination status (p < 0.0001) even after adjusting for patient age and race/ethnicity (Table II), with females 2.5 times more likely than males to be fully vaccinated (95% CI 2.2–2.7). Males were also more likely to be vaccine naïve compared to females (n = 2347/3920, 59.9% vs. n = 1872/4612, 40.6%, respectively), and to be due for at least one dose of vaccine (n = 2,907/3,920,74.2% vs. n = 2,515/4,612, 54.5%). This difference was particularly stark in later age ranges, with 62.9% (952/1,513) of males aged 18–26 vaccine naïve, compared to 30.0% (658/2,196) of females of the same age range (Fig. 1A). The gap between males and females who were fully vaccinated widened nearly every year beginning at age 18 (Fig. 1B).
TABLE II.
Odds Ratio Estimates for the Association Between Full Vaccination Status and Patient Demographics in Patients 9 to 26 Years of Age Presenting to Otolaryngology Clinic.
| Effect (n) | Patient count (%) | OR (95% CI) | p-value |
|---|---|---|---|
| Oto-HNS site | |||
| Oto-Comm | 5177 (61%) | 1.1 (1.0, 1.2) | 0.11 |
| Oto-Acad | 3355 (39%) | Ref | - |
| Race/ethnicity | |||
| Non-white hispanic | 238 (3%) | 1.3 (1.0, 1.7) | 0.00 |
| Non-white non-hispanic | 546 (6%) | 0.9 (0.7, 1.1) | 0.64 |
| White hispanic | 209 (2%) | 1.2 (0.9, 1.6) | 0.01 |
| Other | 222 (3%) | 0.6 (0.4, 0.9) | 0.04 |
| Unknown | 173 (2%) | 0.4 (0.3, 0.6) | <.0001 |
| White non-hispanic | 7144 (84%) | Ref | - |
| Sex | |||
| Female | 4612 (54%) | 2.5 (2.2, 2.7) | <.0001 |
| Male | 3920 (46%) | Ref | - |
| Age group | |||
| Age 9–12 | 2560 (30%) | 0.2 (0.1, 0.2) | <.0001 |
| Age 13–17 | 2263 (27%) | 1.3 (1.2, 1.4) | <.0001 |
| Age 18–26 | 3709 (43%) | Ref |
Fig. 1.
HPV vaccination status by age group and sex. (A) Patient vaccination status divided by sex and patient age group; values within each bar subset depict percentage of patients within the given age group and sex. (B) Rates of vaccination series completion by age and sex. Bar graph values depict percentage of fully vaccinated patients within given sex and age at time of visit, while line graph values depict the total number of fully vaccinated patients within specified age and sex. Dashed line at 80% represents the Healthy People 2030 vaccination goal
Variation in Vaccination Rates by Oto Clinic and Subspecialty
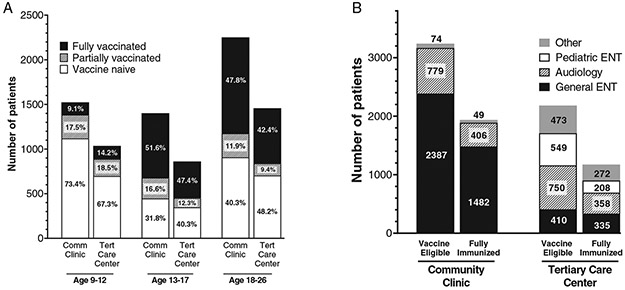
Visits within the department of Otolaryngology were then divided based on the subspecialty seen. The largest group, “General Oto-HNS,” included all visits to general Otolaryngology physicians, nurse practitioners, chief residents seeing a general clinic, and physician assistants, as well as nurse-only visits. “Other” included patients who presented to Allergy, Medical Genetics, Physical and Occupational therapy within Oto-HNS, and those with no documented visit type. When broken down by visit type, patients were most likely to be seen by general Oto-HNS providers (n = 4,614, 54.1%), followed by Audiology (n = 2,293, 26.9%) (Table I).
Patients were more likely to be seen in a Comm-Oto practice (5,177 (60.7%) vs. 3,355 (39.3%)). Within the Acad-Oto clinic, most eligible patients were seen by Audiology, while in Comm-Oto clinics, most were seen by general Otolaryngologists (Fig. 2). Visit location was included as a patient variable in the logistic regression analysis to determine whether this was associated with patient vaccination status. After controlling for age, sex, and race/ethnicity, there was no significant difference in odds of full vaccination between patients seen in a Comm-Oto practice and those seen in an Acad-Oto practice [OR 1.1 (95% CI 1.0–1.2)] (p = 0.11).
Fig. 2.
Patient vaccination status by visit type. (A) Vaccination status by age group, divided as either seen in community Oto-HNS clinic (Oto-Comm), or at a tertiary care center (Oto-Acad). Values within each bar subset depict percentage of patients within the given age group and visit type. (B) Vaccination status by subspecialty within Oto-HNS visit. Values in graph represent number of patients represented by each bar subset. Other contains general Oto-HNS, Audiology, Pediatric Oto-HNS, Rhinology, Neurotology, Laryngology, Speech Language Pathology (SLP), Head and Neck Cancer (H&N), Facial Plastic and Reconstructive Surgery (FPRS), Oto-HNS Physical and Occupational Therapy, Oto-HNS Allergy, Oto-HNS Medical Genetics, and Unknown
Evaluation of Patient Eligibility for Point-Of-Care vaccination (POCV)
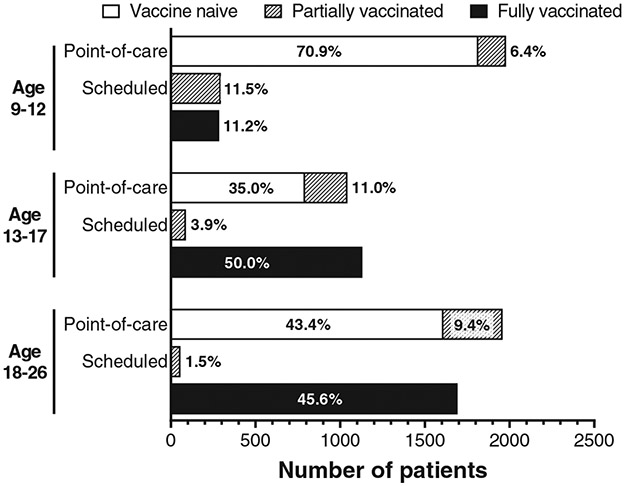
To further analyze the potential impact of Otolaryngology departments offering on-site, HPV point-of-care vaccination (POCV), patients were categorized as eligible or ineligible for vaccination at the time of their Oto-HNS visit. All vaccine-naïve patients were categorized as eligible for POCV, and all fully vaccinated patients were categorized as ineligible. By comparing patients’ vaccination dates and Otolaryngology appointment dates, partially vaccinated patients were categorized as “early” or “due.” These cutoffs were determined from the CDC HPV vaccination scheduling recommendations, which vary depending on the age at which a patient receives their first dose.20 Partially-vaccinated patients aged 9–14 were considered “early” if they received their first vaccine dose less than 5 months prior to their Otolaryngology appointment, while the rest were considered “due.” Partially vaccinated patients aged 15–26 were further divided into those who had received one dose by the time of their Otolaryngology appointment, and those who had received two doses. Of those who had received only one dose, patients were considered “early” if their dose had been administered within the past 28 days (4 weeks) of their Otolaryngology appointment. Patients who had received two doses were considered “early” if their second dose had been administered within 84 days (12 weeks) of their Otolaryngology appointment, or if their first dose had been administered less than 5 months prior.21 All other patients were considered “due.”
A total of 1,203 patients were partially vaccinated. Of these, 748 patients received their first vaccine prior to age 15, while 455 received their first vaccine at age 15 or later. Additionally, 398 (53.2%) of partially vaccinated, two-dose patients were due for their second and final vaccine dose at the time of their Oto-HNS appointment, while 364 of 455 (80%) of partially vaccinated, 3-dose patients were due for either their second (n = 183) or third dose (n = 181) at the time of their appointment. Consequently, nearly two-thirds of the 1,203 partially vaccinated patients (762/1203, 63.3%), were considered due, and 579 patients would have been eligible for their final vaccine dose at the time of their visit. Including vaccine-naïve patients, a total of 4981/8532 (58.4%) patients were considered due at the time of their appointment and would have been eligible for a POCV during their visit (Fig. 3).
Fig. 3.
Patients eligible for point-of-care vaccination by age group. After dividing patients by age group, partially vaccinated patients were categorized either as eligible or ineligible for point-or-care vaccination, based off the date of their most recent vaccine dose and vaccine scheduling parameters. Unvaccinated and eligible partially vaccinated patients were categorized as eligible for a point-of-care (“Point-of-care” in graph) vaccine dose. Patients were categorized as eligible for next vaccine dose scheduling (“Scheduled” in graph) if they had received their vaccine dose too recently to receive an additional dose at the time of their visit. Values in graph bars represent the percent of patients represented by the bar subset within a given age group
Variation in Vaccination Rates by Race and Ethnicity
84% of patients seen in both Acad-Oto and Comm-Oto clinics in this study were White Non-Hispanic or Latino (n = 7,144, 83.7%), limiting the ability to compare vaccination status by race and ethnicity. Race/ethnicity was associated with vaccination status, after controlling for age and sex. Interestingly, non-White Hispanic/Latino and White Hispanic/Latino patients were both slightly more likely to be fully vaccinated compared to White-non-Hispanic/Latino patients (OR 1.35, 95% CI 1.0–1.7, p = 0.00, and OR 1.2, 95% CI 0.9–1.6, p = 0.01, respectively), (Table II). Patients who selected “other” or chose not to disclose their race and/or ethnicity were both less likely to be fully vaccinated compared to the reference group (OR 0.6, 95% CI 0.4–0.9, p = 0.04, and OR 0.4, 95% CI0.3–0.6, p < 0.0001, respectively).
DISCUSSION
This study of Otolaryngology patients demonstrates that the current vaccination rates of Midwest Otolaryngology academic and community clinics largely mirror those of the general population, with high rates of vaccine-naïve or under-vaccinated patients who could benefit from a point-of-care, on-site HPV vaccination program. In fact, vaccination rates among the Otolaryngology patients evaluated in this study were lower than both Minnesota state and national vaccination rates, according to the 2018 National Immunization Survey (NIS) and Minnesota Immunization Information Connection (MIIC) (13–17 years).22 In addition, a Midwest HPV POCV program within Otolaryngology practices has the potential to catch patients who are both due and late for their vaccination, without significantly impacting patient appointment schedules. Finally, this program has the potential to provide a significant benefit to male patients, especially over the age of 18.
Vaccination rates within Otolaryngology clinics were lower than expected when compared to NIS and MIIC rates. For example, among Otolaryngology patients aged 13–17, 65% (1470/2263) had received at least one dose of the HPV vaccine, compared to 68% of adolescents nationwide and 76% in Minnesota.22 Only 50.0% (1131/2263) of Oto-HNS adolescents had completed their HPV vaccination series, which is comparable to 51% nationally, but below state rates of 58% in Minnesota.23 The low rate of HPV vaccination among Otolaryngology patients highlights the potential to target missed opportunities (MO) at Otolaryngology appointments. Missed clinical opportunities to strongly recommend and administer the HPV vaccine are considered one of the most important reasons for HPV low series completion.24 MOs have been defined as an event where an eligible patient is due for a vaccine dose at an appointment, but that dose was not administered.25,26 MO analysis in OBGYN clinics showed 67.1% of vaccine eligible women had at least one MO.25 This is largely in line with the cohort studied here, where 65.7% of patients had at least one MO. Moreover, it is notable that thousands of MO were identified within a relatively small specialty at a single institution, highlighting the sheer scale of HPV under vaccination more broadly across all medical encounters.
The percentage of patients with at least one MO among males was even higher, at 76.1%. This is particularly relevant because HPV-mediated OPSCC is ten times more common in males than females. Several factors have likely contributed to this discrepancy. Importantly, the relationship between HPV infection and oropharyngeal cancer is a relatively new discovery; indeed, the HPV vaccine did not receive FDA approval for the prevention of head and neck cancers until 2020.8 Consequently, this new knowledge has not yet fully permeated the medical community, resulting in understandably low awareness of the role of HPV vaccination in head and neck cancer prevention among both patients and providers.27 Another probable reason for this sex-mediated vaccine discrepancy could be the continuity of care that female patients have with OBGYN providers, who are frequently equipped to administer the HPV vaccine. Finally, targeted marketing campaigns associating the HPV vaccine with protection from cervical cancer, which strongly associates the risk of malignancy with the sexual transmission in females and typically does not address the risk to males, may also have played a role in low vaccination rates among males.28
While primary care providers represent a critical first line in the distribution of HPV vaccinations to eligible patients, surgical subspecialties, especially those who treat HPV-mediated diseases, may be particularly impactful in supporting primary care vaccination efforts. In addition to helping increase HPV vaccination rates, Otolaryngologist can provide patient education on HPV-related diseases of the head and neck including oropharyngeal cancer, RRP, and inverted papilloma, among others. The implementation of vaccination clinics by OBGYN providers has improved female HPV vaccination rates. A clinical interventions bundle, including offering no-cost HPV vaccination, initiated at an OBGYN clinic resulted in statistically significant increases in patients both initiating and completing the HPV vaccination series, with an increase of 29.7% and 20.3% respectively.25 A 2021 study by Torabi et al demonstrated that only 30.6% of patients understood the relationship between HPV infection and OPSCC, and that learning about this relationship may increase vaccine uptake.27 This study also revealed 36% of patients selected an Otolaryngologist as their ideal way to be educated on HPV’s role in OPSCC.27 Otolaryngology practices, including those with an imbedded Audiology practice who see a significant number of eligible patients, are in a unique position to speak to HPV related diseases, see a large volume of both adolescent male and female patients who are due for HPV POCV, may be able to give a strong recommendation for vaccination, and facilitate its administration to decreased MO and increase community vaccination rates.
One consideration for Oto-HNS providers is whether adding vaccination capabilities would place a significant burden on the clinic to make vaccination fiscally or logistically unviable. Further studies are needed to assess the financial impact, training of staff, and logistics of storing and ordering vaccines. Government-sponsored programs such as the Vaccines for Children (VFC) program provide the vaccine free of charge to providers for those patients 18 years and younger and permit them to bill up to $21.22 per dose to the patient’s insurance. Of the 5,422 patients requiring at least one vaccine dose, only 10.6% (n = 579) would have been eligible for their final dose at the time of their visit. The remaining patients would need to schedule at least one follow-up visit to be considered fully vaccinated, either with Otolaryngology or their primary care provider. Notably, 43% (3679/8532) of patients were seen by Oto-HNS at least twice over the course of a year, suggesting that many patients requiring more than one dose could potentially incorporate their vaccine doses in their follow-up appointments.
This study has several limitations. First, the retrospective nature of the study precludes further understanding of what, if any, reasoning underlies why a patient has not completed their HPV vaccination series. Second, over a quarter of the patients seen (27.7%) were excluded based on our exclusion criteria of incomplete vaccination records and opting out of Minnesota Research Authorization. In addition, this study was limited to a relatively homogeneous population of mostly White, non-Hispanic/Latino patients living in the Midwestern United States, and therefore may not be generalizable to more diverse populations. Finally, this study does not investigate the willingness of patients to receive an HPV vaccination in an Oto-HNS clinic, or Otolaryngology provider’s comfort with or willingness to give a strong recommendation for the vaccine and administer it within their clinic. Despite these limitations, the results included in this study reveal that an HPV vaccination clinic offered by Otolaryngology departments would provide thousands of opportunities to improve HPV vaccination and decrease MO within our communities. Future research efforts should focus on the implementation of an HPV vaccination clinic in the Otolaryngology department and evaluate its effectiveness in eliminating MO. While it is important to investigate all opportunities to improve patient vaccination rates, the most effective way to increase overall vaccination rates may be to implement an on-site vaccination program integrated into clinic operations that allow patients to be vaccinated without further scheduling, referrals, or appointments.
CONCLUSION
This study demonstrates that Oto-HNS clinics see a significant number of patients who would benefit from point-of-care HPV vaccination (58.4%), and that many of these opportunities occur when patients visit general Oto-HNS, audiology, or pediatric Oto-HNS. Moreover, males are particularly likely to benefit from point-of-care HPV vaccination as Otolaryngologists are uniquely prepared to discuss oropharyngeal cancer with their patients. These results support the development of a pilot vaccination program within Oto-HNS providers.
ACKNOWLEDGEMENTS
Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Footnotes
2022 Triological Combined Sections Meeting, Coronado, CA, USA on January 20–22 2022.
Internal departmental funding was utilized without commercial sponsorship or support.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Level of Evidence: 3
Contributor Information
Victoria J. Palacios, Reno School of Medicine, University of Nevada, Reno, Nevada, USA.
Dante J. Merlino, Department of Otorhinolaryngology-Head and Neck Surgery, Mayo Clinic, Rochester, Minnesota, USA.
Stephanie S. Anderson, Kern Center for the Science of Healthcare Delivery, Mayo Clinic, Rochester, Minnesota, USA.
Sarah R. Yeakel, Department of Otorhinolaryngology-Head and Neck Surgery, Mayo Clinic, Rochester, Minnesota, USA.
Garret W. Choby G, Department of Otorhinolaryngology-Head and Neck Surgery, Mayo Clinic, Rochester, Minnesota, USA.
Joshua P. Wiedermann, Department of Otorhinolaryngology-Head and Neck Surgery, Mayo Clinic, Rochester, Minnesota, USA.
Eric J. Moore, Department of Otorhinolaryngology-Head and Neck Surgery, Mayo Clinic, Rochester, Minnesota, USA.
Thomas J. O’Byrne, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, Minnesota, USA.
Robert M. Jacobson, Department of Pediatric and Adolescent Medicine, Mayo Clinic, Rochester, Minnesota, USA..
Kathryn M. Van Abel, Department of Otorhinolaryngology-Head and Neck Surgery, Mayo Clinic, Rochester, Minnesota, USA.
REFERENCES
- 1.Berger MH, Haidar YM, Bitner B, Trent M, Tjoa T. Practice patterns and knowledge among California pediatricians regarding human papillomavirus and its relation to head and neck cancer. Am J Otolaryngol. 2019;40: 525–529. [DOI] [PubMed] [Google Scholar]
- 2.Oh NL, Biddell CB, Rhodes BE, Brewer NT. Provider communication and HPV vaccine uptake: a meta-analysis and systematic review. Prev Med. 2021;148:106554. [DOI] [PubMed] [Google Scholar]
- 3.Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics. 2017;139. doi: 10.1542/peds.2016-1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharyya N Ambulatory pediatric otolaryngologic procedures in the United States: characteristics and perioperative safety. Laryngoscope. 2010;120:821–825. [DOI] [PubMed] [Google Scholar]
- 5.Gerald Julian OMSII, David Go OMSII, Shubrook DFJH. Preventing cancer with two injections, a clinical review of the HPV vaccination. Osteopathic Family Phys. 2019;11:24–29. [Google Scholar]
- 6.FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the advisory committee on immunization practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:630–632. [PubMed] [Google Scholar]
- 7.Van Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Benard VB. Trends in human papillomavirus-associated cancers - United States, 1999–2015. MMWR Morb Mortal Wkly Rep. 2018;67:918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration. Supplement accelerated approval. Available at: https://www.fda.gov/media/138949/download. Accessed December 1, 2021.
- 9.Administering HPV Vaccine. Available at: https://www.cdc.gov/vaccines/vpd/hpv/hcp/administration.html. 2021.
- 10.Senkomago V, Henley SJ, Thomas CC, Mix JM, Markowitz LE, Saraiya M. Human papillomavirus-attributable cancers - United States, 2012–2016. MMWR Morb Mortal Wkly Rep. 2019;68:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dang JHT, Stewart SL, Blumberg DA, Rodriguez HP, Chen MS. Patient and clinician factors associated with uptake of the human papillomavirus (HPV) vaccine among adolescent patients of a primary care network. Vaccine. 2021;39:3528–3535. [DOI] [PubMed] [Google Scholar]
- 12.Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services – Office of Disease Prevention and Health Promotion. Increase the proportion of adolescents who get recommended doses of the HPV vaccine — IID-08. Available at: https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-adolescents-who-get-recommended-doses-hpv-vaccine-iid-08.
- 14.Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138. doi: 10.1542/peds.2015-3863 [DOI] [PubMed] [Google Scholar]
- 15.Nordin JD, Solberg LI, Parker ED. Adolescent primary care visit patterns. Ann Fam Med. 2010;8:511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.State Information: HPV Vaccine Mandates for Elementary and Secondary Schools. Available at: https://www.immunize.org/laws/hpv.asp.
- 17.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester epidemiology project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester epidemiology project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405–1408. [DOI] [PubMed] [Google Scholar]
- 21.Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–304. [PMC free article] [PubMed] [Google Scholar]
- 22.National Immunization Survey and the Minnesota Immunization Information Connection. Adolescent Immunization Coverage in Minnesota. Available at: https://www.health.state.mn.us/people/immunize/stats/adol/coverdata.html. 2021.
- 23.Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities:provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine. 2011;29:8634–8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deshmukh U, Oliveira CR, Griggs S, et al. Impact of a clinical interventions bundle on uptake of HPV vaccine at an OB/GYN clinic. Vaccine. 2018;36:3599–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira CR, Rock RM, Shapiro ED, et al. Missed opportunities for HPV immunization among young adult women. Am J Obstet Gynecol. 2018;218:326.e321–326.e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torabi SJ, Su-Velez BM, Kasle DA, Yarbrough WG, St John M, Judson BL. Assessing human papillomavirus awareness and the role of oropharyngeal squamous cell carcinoma education on improving intention to vaccinate. Laryngoscope. 2021;132:528–537. [DOI] [PubMed] [Google Scholar]
- 28.Rim SH, Polonec L, Stewart SL, Gelb CA. A national initiative for women and healthcare providers: CDC’s inside knowledge: get the facts about gynecologic cancer campaign. J Womens Health (Larchmt). 2011;20:1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]