Abstract
In accordance with Article 43 of Regulation (EC) No 396/2005, the European Commission requested EFSA to perform a risk assessment of the existing maximum residues levels (MRLs) for oxamyl considering the new toxicological reference values. Additionally, if needed to ensure adequate consumer protection, lower limits of quantification (LOQs) than those currently established in the legislation should be proposed. EFSA performed various consumer exposure calculation scenarios, considering the risk assessment values as available for the existing uses of oxamyl and the lowering of LOQs for several plant and animal commodities as suggested by the European Union Reference Laboratories for Pesticide Residues (EURLs). Based on the results of the consumer exposure assessment calculated considering the risk assessment values for crops with authorised oxamyl uses and the existing EU MRLs at the LOQ for remaining commodities (scenario 1), chronic consumer intake concerns were identified for 34 diets. Acute exposure concerns were identified for a wide range of crops, including crops with currently authorised oxamyl uses: bananas, potatoes, melons, cucumbers, carrots, watermelons, tomatoes, courgettes, parsnips, salsifies and aubergines/eggplants. Under exposure calculation scenario 3, which considered lowering of all MRLs to the lowest analytically achievable limits of quantification, EFSA concludes that chronic consumer exposure concerns can still not be excluded. Similarly, acute consumer exposure concerns were identified for 16 commodities, including crops with known authorised uses: potatoes, melons, watermelons and tomatoes, even though for these crops a lower LOQ as proposed by the EURLs were considered. Further refinements of the calculated exposure at the current stage were not possible by EFSA, but EFSA identified a list of commodities for which a lower LOQ than routinely achievable is expected to significantly reduce the consumer exposure and for which a risk management decision is required.
Keywords: oxamyl, consumer risk assessment, acute and chronic risk assessment, limit of quantification
Background
Oxamyl is a nematicide which was first assessed in 2005 for the inclusion in Annex I of the Council Directive 91/414/EEC1 (EFSA, 2005). Oxamyl is considered to be approved for use in the EU until 31 January 2023.2
On 1 October 2010, EFSA provided a reasoned opinion on the review of the existing maximum residue levels (MRLs) for the active substance oxamyl in compliance with Article 12(2) of Regulation (EC) No 396/20053 (MRL review) (EFSA, 2010). This risk assessment was performed using revision 2 of the EFSA Pesticide Residues Intake Model (PRIMo) and the calculated exposures were compared with the toxicological reference values (TRVs) for oxamyl valid at that time, i.e., acceptable daily intake (ADI) of 0.001 mg/kg body weight (bw) per day and acute reference dose (ARfD) of 0.001 mg/kg bw. The MRLs resulting from this review were implemented by Regulation (EU) No 61/2014.4 The MRLs for potatoes, carrots, parsnips, salsify, Brussels sprouts and sugar beet roots were implemented at the limit of quantification (LOQ) of 0.01 mg/kg. For oranges, mandarins, bananas, tomatoes and cucurbits with edible peel (cucumbers, courgettes, gherkins) tentative MRLs of 0.01 mg/kg (at the LOQ) and for aubergines/eggplants at 0.02 mg/kg were implemented. For the uses of oxamyl on melons, watermelons and sweet peppers/bell peppers, consumer intake concerns could not be excluded and therefore these uses were withdrawn and the MRL was set at the default LOQ of 0.01 mg/kg.
On 6 July 2018, the Codex Alimentarius Commission adopted new Codex maximum residue limits (CXLs) for oxamyl.5 EFSA provided scientific support by assessing the proposed CXLs (EFSA, 2018b). The CXLs that were found to be safe for European consumers, namely for melons and watermelons, were implemented by Regulation (EU) No 2019/552.6 For tomatoes, the MRL at the LOQ of 0.01 mg/kg was confirmed by the same Regulation; the use of oxamyl on tomatoes was also evaluated by the JMPR.
On 18 May 2022, in the framework of the procedure on the renewal of the approval of oxamyl under Regulation (EC) No 1107/2009,7 EFSA in the conclusions on the peer review (EFSA, 2022) proposed to lower by a factor of 10 the TRVs for oxamyl (i.e. ADI of 0.0001 mg/kg bw day and ARfD of 0.0001 mg/kg bw) and identified several areas of critical concern, inter alia, that the preliminary consumer dietary risk assessment indicates a large exceedance of the ARfD for all the representative uses. During the EU pesticides peer review, the screening assessment for all MRLs confirmed after the MRL review was also performed, considering the new lowered TRVs for oxamyl. The screening indicated that the LOQs are not sufficiently protective for European consumers, as the calculated theoretical maximum daily intake (TMDI) exceeded the new lowered TRVs (1,240% of the ADI (NL toddler) and a large exceedance of the ARfD for several commodities (top 3: 1,538% potatoes, 1,517% melons, 1,385% pears)).
The EU pesticides peer review also concluded that for the uses assessed in the MRL review, the Article 12 confirmatory data gaps are addressed for a metabolism study with a radioactive marker representative for the use of oxamyl by drip irrigation in fruits and fruiting vegetables and for a study demonstrating storage stability of oxamyl residues in commodities with high acid content. The Article 12 confirmatory data gap for four additional residues trials on oranges and four additional residues trials on mandarins compliant with southern outdoor GAPs for these crops is considered as obsolete as the use on citrus is no longer supported (EFSA, 2022). In addition, the consumer dietary risk assessment could not be finalised since the residue definition for risk assessment was set on provisional basis as oxamyl alone, pending the assessment of relevance of metabolites IN‐D2708, IN‐A2213, IN‐QKT34 and IN‐N0079 in various crops.
All oxamyl EU MRLs are currently set at the LOQs of 0.01* mg/kg, 0.02* mg/kg (herbs and edible flowers) and 0.05* mg/kg (teas, hops, spices, honey), with the exception of melons and watermelons (0.01 mg/kg), and aubergines/eggplants (0.02 mg/kg). The MRLs for melons and watermelons reflect Codex MRLs implemented in EU legislation while the MRL on aubergines/eggplants reflect a plant protection use authorised in the EU. No import tolerances exist.
The decision on the renewal of approval of oxamyl is expected in 2023. However, considering the significantly lowered TRVs and the acute consumer risks identified in the EFSA conclusions on the peer review resulting from the existing MRLs, the Commission requests EFSA to carry out a risk assessment of the existing MRLs in view of the consumer protection level they provide and to investigate whether the LOQs of 0.01* mg/kg, 0.02* mg/kg (herbs and edible flowers) and 0.05* mg/kg (teas, hops, spices, honey) are protective enough, and, if need be, to propose lower achievable LOQs that ensure adequate consumer protection.
Terms of reference (as provided by the requestor)
EFSA is requested, according to Article 43 of Regulation (EC) No 396/2005:
to perform a risk assessment of the existing MRLs considering the input values for risk assessment as derived during the MRL review and by the JMPR, the new toxicological reference values, the provisional residue definition for risk assessment (set as oxamyl only) and the newest version of PRIMo.
if need be, to propose lower LOQs than those currently established in the legislation that ensure adequate consumer protection.
Assessment
EFSA based the assessment on the following documents:
-
–
the conclusion on the peer review of the pesticide risk assessment of the active substance oxamyl (EFSA, 2022);
-
–
the reasoned opinion on the review of the existing MRLs for oxamyl according to Article 12 of Regulation (EC) No 396/2005 (EFSA, 2010);
-
–
the scientific support for preparing an EU position in the 50th Session of the Codex Committee on Pesticide Residues (CCPR) (EFSA, 2018b);
-
–
the Joint FAO/WHO Meeting on Pesticide residues (JMPR) Evaluation report (FAO, 2018);
-
–
the Report of the 50th Session of the Codex Committee on Pesticide Residues (FAO/WHO, 2018);
-
–
information provided upon request to European Commission by the European Union Reference Laboratories for Pesticide Residues (EURLs).
The deadline for delivering a statement according to Article 43 of Regulation (EC) No 396/2005 on the safety of the proposed MRLs for consumers was agreed to be 2 months from receipt of this mandate. EFSA accepted the mandate and included it in the EFSA Register of Questions with the reference number EFSA‐Q‐2022‐00833 and committed to provide the statement by 23 January 2023.
The additional information provided by the EURLs and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents and, thus, are made publicly available as background documents to this statement.8 Screenshots of the report sheets of the PRIMo are presented in Appendix C.
1. Toxicological reference values
The toxicological assessment of oxamyl was initially performed by the EU pesticides peer review for the inclusion of the active substance in Annex I of the Council Directive 91/414/EEC (EFSA, 2005). The ADI of 0.001 mg/kg bw per day and an acute reference dose (ARfD) of 0.001 mg/kg bw were derived (EFSA, 2005). These values were confirmed in the EU review report (European Commission, 2011) and implemented by Commission Directive 2006/16/EC9 on the inclusion of oxamyl as active substance in Annex I of Directive 91/414/EEC.
In the framework of the renewal of the approval of oxamyl, the toxicological reference values (TRVs) were derived from the same key study as selected by EFSA in 200510 (i.e. the rat acute oral neurotoxicity study, with a no observed adverse effect level (NOAEL) of 0.1 mg/kg bw based on neurotoxicity findings) (EFSA, 2022). Compared with the TRVs agreed for the first approval (European Commission, 2011), these newly agreed TRVs have been decreased by 10‐fold, by adding an extra‐factor of 10 to the standard uncertainty factor (UF) of 100. Consequently, the ADI of 0.0001 mg/kg bw per day and an ARfD of 0.0001 mg/kg bw were derived by EFSA in the framework of the EU pesticides peer review for renewal of the approval of oxamyl (EFSA, 2022). These values have not been implemented yet.
The metabolites IN‐D2708, IN‐A2213, IN‐QKT34 (IN‐A2213 glucoside conjugate) and IN‐N0079 which were present in tomatoes and potatoes (representative uses assessed by the EU peer review on the renewal of the approval) are major rat metabolites, and therefore, the toxicological reference values of the parent compound are applicable to these compounds (EFSA, 2022).
As requested by the present mandate, the consumer exposure assessment for oxamyl will be performed considering the TRVs derived in the renewal of the approval process of oxamyl.
2. Residue definitions
The enforcement residue definition established for plant and animal commodities in Regulation (EC) No 396/2005 comprises the parent compound oxamyl alone. The same enforcement residue definition for plant commodities has been agreed by the EU pesticides peer review on the renewal of the approval of oxamyl (EFSA, 2022). The enforcement residue definition for commodities of animal origin could not be concluded by the EU pesticides peer review on the renewal of the approval pending the submission of residue trials on crops that could be fed to livestock to estimate the livestock dietary burden (EFSA, 2022).
The residue definition for the risk assessment was derived as parent oxamyl by the EU pesticides peer review for the inclusion of the active substance in Annex I of the Council Directive 91/414/EEC (EFSA, 2005). The EU pesticides peer review on the renewal of the approval on the basis of metabolism studies with fruit crops, root crops, leafy crops and pulses/oilseeds concluded that the residue definition for risk assessment in primary and rotational crops can be set as ‘oxamyl’ on provisional basis, pending the submission of residue trials on tomatoes and potatoes (representative uses considered for the renewal of the approval of oxamyl) analysing residues of metabolites IN‐D2708, IN‐A2213, IN‐QKT34 (IN‐A2213 glucoside conjugate) and IN‐N0079. In processed commodities, parent oxamyl is unstable and degrades to metabolite IN‐A2213. In animal commodities the risk assessment residue definition has not been concluded since, pending the residue data on potatoes and rotational crops, it is not known if the livestock dietary burden triggers the setting of residue definitions for products of animal origin (EFSA, 2022).
The data gaps set by the EU pesticides peer review on the renewal of the approval (EFSA, 2022) have not been addressed so far and the assessment of the impact of these data gaps on the outcome of the consumer exposure is not within the remit of the present assessment.
Noting the terms of reference of the mandate, the residue definition for risk assessment and enforcement in plant and animal commodities applicable for the present consumer risk assessment is ‘oxamyl’ alone, as set by the EU peer review for the approval of oxamyl (EFSA, 2005) and confirmed by the MRL review (EFSA, 2010).
3. Analytical methods for enforcement
The availability of the analytical enforcement methods for the determination of residues of oxamyl according to the existing enforcement residue definition (i.e., oxamyl alone), was investigated both in the MRL review and in the EU pesticides peer review on the renewal of the approval of the active substance (EFSA, 2010, 2022).
The MRL review concluded that suitable analytical methods are available for enforcement of parent oxamyl in commodities with high water content, high acid content and dry commodities at the validated LOQ of 0.01 mg/kg (EFSA, 2010). The availability of analytical enforcement method for the determination of residues in commodities of animal origin was not further investigated due to insignificant livestock exposure to oxamyl residues.
The EU pesticides peer review on the renewal of the approval concluded that oxamyl residues can be monitored in food and feed of plant origin by the quick, easy, cheap, effective and safe (QuEChERS) method using high‐performance liquid chromatography with tandem mass spectrometry (HPLC–MS/MS) with a LOQ of 0.01 mg/kg in the four major plant matrices and dried tobacco leaf. The lack of studies on matrix effects and the verification of the extraction efficiency were identified as data gaps (EFSA, 2022). In food of animal origin oxamyl residues can be determined either by a multi‐residue QuEChERS method or by a single residue method using HPLC–MS/MS determination with a validated LOQ of 0.01 mg/kg in all animal matrices. Also, for these methods matrix effects were not examined and the extraction efficiency has not been verified (EFSA, 2022).
Noting potential consumer intake concerns related to oxamyl residues at the LOQ, the European Commission requested the EURLs to investigate whether a lower LOQs could be achieved in plant and animal matrices. The EURLs provided information that a lower LOQs could be achieved for the following crops/commodities:
-
–
0.002 mg/kg in oranges and tomatoes.
-
–
0.001 mg/kg in commodities of high water and high acid content11: citrus fruits (except oranges), pome fruits, stone fruits, berries and small fruits, miscellaneous fruit (except table olives, avocados), root and tuber vegetables, bulb vegetables, fruiting vegetables (except tomatoes), brassica vegetables, leaf vegetables, fresh herbs and edible flowers, legume vegetables, stem vegetables, fungi, sugar plants.
-
–
0.005 mg/kg in avocados, cereals, meat of mammals12 and bird's eggs.
-
–
0.001 mg/kg in cow's milk.
The information provided by the EURLs will be further considered in the consumer exposure assessment.
4. Consumer risk assessment
As a basis for this risk assessment and in accordance with the internationally agreed methodology for pesticide residues (FAO, 2016), EFSA performed a chronic and acute consumer risk assessment for the existing oxamyl MRLs as established in the Regulation (EU) 2019/552, considering the newest version of EFSA PRIMo rev. 3.1 (EFSA, 2018a, 2019).
The existing EU MRLs are set on the basis of the known authorised uses of oxamyl in the EU for bananas, potatoes, carrots, parsnips, salsifies, aubergines/eggplants, cucumbers, gherkins, courgettes, Brussels sprouts and sugar beet (roots), assessed during the MRL review (EFSA, 2010). For melons and watermelons, the existing EU MRL is set on the basis of CXL. The uses on tomatoes were assessed by the MRL review in 2010 (resulting in tentative MRL of 0.01* mg/kg as implemented by Regulation (EU) No 61/2014), the EU pesticides peer review in 2022 (provisional MRL proposal of 0.01* mg/kg) and by the JMPR in 2018 (CXL of 0.01* mg/kg confirmed by Regulation (EU) 2019/552) resulting in the same MRL proposal at the LOQ of 0.01 mg/kg. For the remaining commodities of plant origin, the existing EU MRLs for oxamyl are set at the LOQs of 0.01, 0.02 and 0.05 mg/kg (depending on the matrices). For commodities of animal origin, the existing EU MRLs are set at the LOQ of 0.01 mg/kg.
4.1. Scenario with available risk assessment values (scenario 1)
4.1.1. Input values
The consumer exposure was calculated for the existing oxamyl EU MRLs as established in the Regulation (EU) 2019/552. For those crops on which known oxamyl uses exist, the risk assessment values, namely the median residue values (STMR) for the chronic exposure and the highest residue values (HR) for the acute exposure, as derived by the MRL review (EFSA, 2010) or by the JMPR (FAO, 2018) were used to refine the exposure assessments. For melons and watermelons, the input values for residues in pulp were available. For the remaining commodities of plant and animal origin the existing EU MRLs (set at LOQ) were used as input values.
All input values considered in the risk assessment scenario 1 are reported in Appendix A.1.
4.1.2. Results
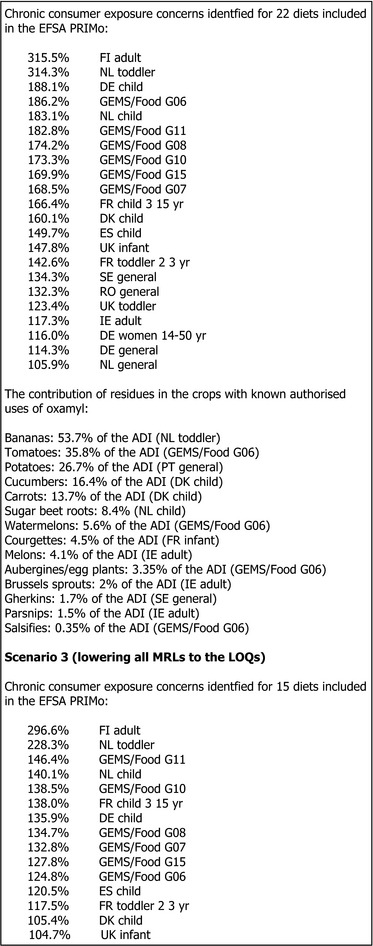
The chronic consumer exposure exceeded the ADI for a total of 34 diets, with the highest exposure being 1,219% of the ADI as calculated for NL toddler diet. The main contributing commodities (in % of the ADI) were cattle milk (from 30% in UK adult diet to 597% in NL toddler diet), coffee beans (278%, FI adult diet), apples (125%, DE child diet), sugar beet roots (84.4% NL child diet), wheat (72% GEMS/Food G06), maize/corn (70%, NL toddler diet), rye (55% rye, DK child diet) and was individually below 50% of the ADI for other commodities.
The contribution of residues (in % of the ADI) in the crops with known oxamyl authorised uses was the highest for sugar beet root (84.4% NL child diet), bananas (53.7% NL toddler diet), tomatoes (35.8% GEMS/Food G06 diet), potatoes (26.7% PT general diet) and was below 20% of the ADI for other crops (for details see Appendix B).
The contribution of MRLs set at the LOQ was 1,068% in the NL toddler diet.
When only crops with known authorised uses are considered (disregarding MRLs at the LOQ for remaining commodities of plant and animal origin), the chronic consumer intake concerns are identified for two diets with the maximum calculated exposure of 151% of the ADI for NL toddler diet and 134% of the ADI for NL child diet. When only crops with MRLs above the LOQ of 0.01 mg/kg are considered (i.e., watermelons, melons and aubergines/eggplants) the chronic exposure amounts to 11% of the ADI (GEMS/Food G08 diet).
Acute consumer intake concerns could not be excluded for 82 commodities (the crops with known authorised uses are reported in bold):
-
–
with acute exposure above 1,000% of the ARfD: pears, oranges, cattle milk, apples, pineapples.
-
–
with acute exposure between 500% and 1,000% of the ARfD: bananas (971%), peaches, mangoes, grapefruits, potatoes (769%), melons (758%), table grapes, cucumbers (656%), carrots 634%), kiwi fruits, watermelons (611%), sweet peppers/bell peppers, mandarins, leeks, tomatoes (581%), cauliflowers, beetroots, celeriacs/turnip rooted, granate apples/pomegranates, kohlrabies, swedes/rutabagas, avocados.
-
–
with acute exposure between 100% and 500% of the ARfD: kaki/Japanese persimmons, courgettes (465%), head cabbages, kales, sweet corn, papayas, plums, broccoli, escaroles/broad‐leaved, witloofs/Belgian endives, carobs/Sain John's bread, carambolas, lettuces, celeries, rhubarbs, parsnips (361%), turnips, apricots, lemons, Chinese cabbages‐pe‐tsai, yams, salsifies (310%), pumpkins, aubergines/eggplants (250%), quinces, radishes, goat milk, onions, spinaches, prickly pears/cactus fruits, guavas, limes, asparagus, beans, honey and other apiculture products, globe artichokes, poultry muscle/meat, cultivated fungi, strawberries, Florence fennels, cocoa beans, spring onions/green onions, chards/beet leaves, cherimoyas, wheat, coconuts, medlar, rice, chicken eggs, cherries (sweet), swine muscle/meat, litchis/lychees, figs, beans (with pods), blackberries.
From the crops on which the authorised uses of oxamyl exist in Europe, no acute intake concerns were identified only for Brussels sprouts (84% of the ARfD) and gherkins (28% of the ARfD). For sugar beet root the acute exposure is not calculated as no consumption data are available.
The detailed results of the calculations are presented in Appendix B.
4.2. Scenario with lower limits of analytical quantification, except crops with known authorised uses (scenario 2)
4.2.1. Input values
Under this scenario, further attempts were made to refine the exposure calculated under scenario 1. For those crops/commodities for which the EURLs confirmed that lower LOQs could be achieved (see Section 3), the lower LOQ values were used in the exposure calculation. For the crops with known existing authorised uses, the input values were the same as in scenario 1, except for sugar beet root where the input value was a lower LOQ of 0.001 mg/kg considering the unlikely concentration of residues in sugar.
All input values considered in the risk assessment scenario 2 are reported in Appendix A.1.
4.2.2. Results
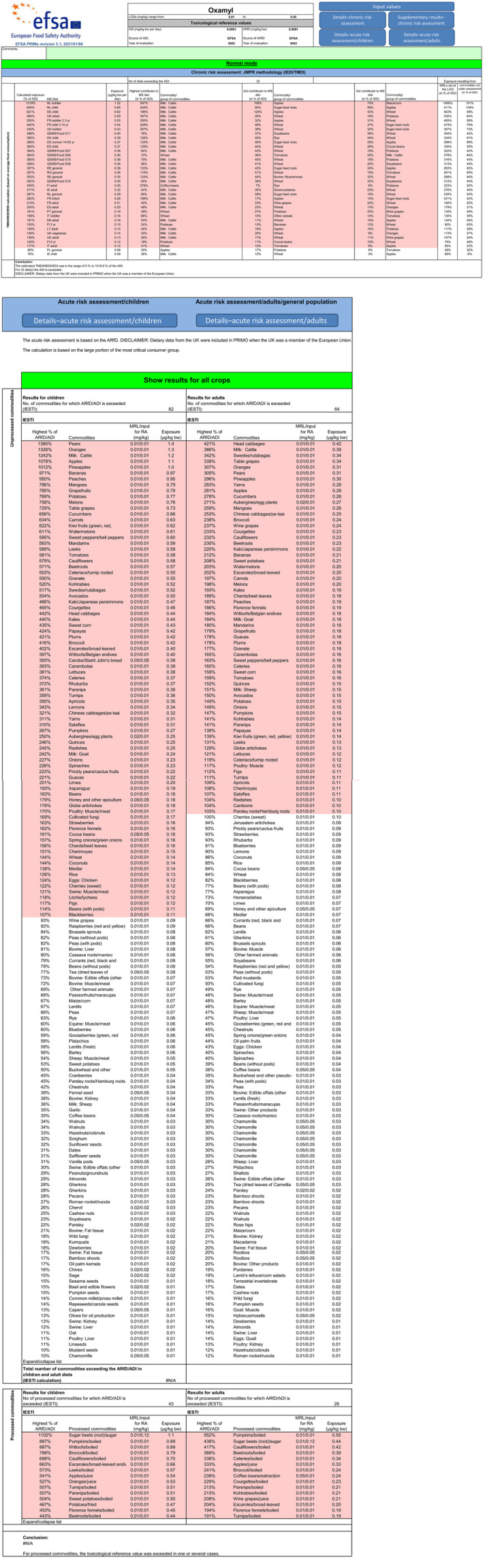
The chronic exposure calculated under scenario 2 indicated intake concerns for 22 diets, with the highest exposure of 315% of the ADI calculated for Finnish adult diet. The main contributing commodities (in % of the ADI) were coffee beans (278%, FI adult diet), cattle milk (60%, NL toddler diet), bananas (54%, NL toddler diet) and was individually below 50% of the ADI for other commodities.
The contribution of residues (in % of the ADI) in the crops on which there exist known oxamyl uses was the same as calculated in the scenario 1, except for sugar beet root where the exposure was reduced to 8.4% of the ADI (NL child diet).
The contribution of MRLs set at the LOQ accounted for 294% of the ADI in the Finnish adult diet.
When only crops with known authorised uses are considered (disregarding MRLs at the LOQ for remaining commodities of plant and animal origin), the chronic consumer intake concerns are identified for NL toddler diet with a maximum calculated exposure of 104% of the ADI.
Acute consumer intake concerns could not be excluded for 23 commodities (the crops with known authorised uses are reported in bold):
-
–
with acute exposure between 500% and 1,000% of the ARfD: bananas (971%), potatoes (769%), melons (758%), cucumbers (656%), carrots (634%), watermelons (611%), tomatoes (581%).
-
–
with acute exposure between 100% and 500% of the ARfD: courgettes (465%), carobs/Saint John's bread, parsnips (361%), salsifies (310%), oranges, avocados, aubergines/eggplants (250%), goat milk, beans, honey and other apiculture, cocoa beans, coconuts, pears, cattle milk, apples, pineapples.
From the crops with known authorised uses of oxamyl, no intake concerns are identified for Brussels sprouts and gherkins. For sugar beet root the acute exposure is not calculated as no consumption data are available.
The detailed results of the calculations are presented in Appendix B.
4.3. Scenario with lowering of all oxamyl MRLs to the existing LOQ or lower LOQ (scenario 3)
4.3.1. Input values
Under this scenario the lowering of all EU MRLs of oxamyl to the lowest analytically achievable LOQs and the impact on the consumer exposure was investigated. According to the information provided by the EURLs, for several commodities/commodity groups a lower LOQ could be potentially achieved, thus for these commodities the input values were as reported by the EURLs (see Section 3). For the commodities of plant and animal origin for which the EURLs did not provide any indication of lower analytically achievable LOQs, the input values were the MRLs at the LOQs (of 0.01 mg/kg, 0.02 mg/kg or 0.05 mg/kg, depending on the matrix) as established by Regulation (EU) 2019/552.
All input values considered in the risk assessment scenario 3 are reported in Appendix A.1.
4.3.2. Results
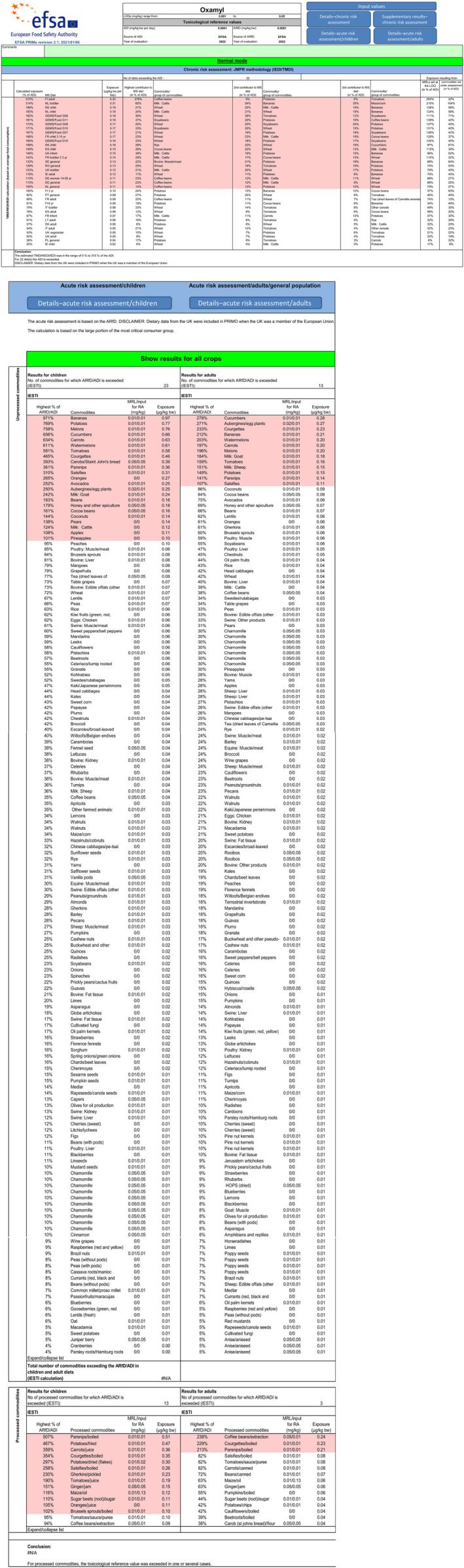
The chronic exposure calculated under scenario 3 indicated intake concerns for 15 diets, with the highest exposure of 297% of the ADI calculated for Finnish adult diet.
The highest contributing commodities (>10% of the ADI) were coffee beans (278%, FI adult diet), cattle milk (60%, NL toddler diet and 39% UK infant diet), soyabeans (37%, GEMS/Food G11 diet), wheat (36%, GEMS/Food G06 diet), maize/corn (35%, NL toddler diet), rye (27.5%, DK child diet), cocoa beans (26%, ES child diet), bovine muscle/meat (22%, SE general population diet), apples (12%, DE child diet) and swine muscle/meat (11%, DK child diet).
All calculated exposure is attributed to residues at the LOQ.
Acute consumer intake concerns (in % of the ARfD) could not be excluded for 16 commodities (the crops with known authorised uses are reported in bold): carobs (393%), oranges (265%), avocados (252%), goat milk (242%), beans (183%), honey (179%), cocoa beans (161%), potatoes (154%), melons (152%), sheep milk (151%), coconuts (144%), pears (138%), cattle milk (124%), watermelons (1225), tomatoes (116%), apples (108%) and pineapples (101%).
The detailed results of the calculations are presented in Appendix B.
Based on scenario 3, EFSA identified a list of commodities for which a lower LOQ is expected to significantly reduce the consumer exposure.
5. Uncertainties related to exposure calculations
The different consumer exposure calculations are affected by the following uncertainties:
-
–
the conclusions of the EU pesticides peer review on the renewal of the approval have not been taken into consideration in this assessment (scenarios 1, 2 and 3);
-
–
no information is available on national authorizations of oxamyl in EU on commodities other than those assessed by the MRL review in 2010 (scenarios 1 and 2);
-
–
the LOQ of 0.001 mg/kg applied for commodities of plant origin with high water and high acid content is provisional and further validation data would be needed to confirm this LOQ (scenarios 2 and 3);
-
–
none of the LOQs proposed by the EURLs have been validated according to the requirements for the post‐registration methods as currently applicable by EU Guidance document SANTE/2020/12830 (scenarios 2 and 3).
6. Conclusions and recommendations
Based on the results of the consumer exposure assessment calculated considering the risk assessment values for crops with authorised oxamyl uses and the existing EU MRLs at the LOQ for remaining commodities as reported in the Regulation (EU) 2019/552, chronic and acute consumer exposure concerns cannot be excluded.
When all oxamyl MRLs are lowered to the routinely achievable LOQ or to a lower LOQ as reported by the European Reference Laboratories, the chronic consumer exposure is lower, but intake concerns remain. Acute intake concerns can also not be excluded with those lower LOQs for a range of commodities, including several crops with known authorised uses of oxamyl: potatoes, melons, watermelons and tomatoes. Furthermore, the exposure assessment is affected by uncertainties related to insufficient validation data package for these lower LOQs in several plant and animal matrices.
Thus, it was not possible for EFSA at the current stage to identify a safe consumer exposure scenario. However, EFSA identified a list of commodities for which a lower LOQ than routinely achievable is expected to significantly reduce the consumer exposure and for which risk management decision is required.
The recommendations of EFSA are compiled in the table below (Table 1).
Table 1.
Summary table
| Code (a) | Commodity (b) | Existing MRL (mg/kg)/ Source | Exisiting CXL | Outcome of the risk assessment | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
|
Enforcement residue definition (EU): Oxamyl Enforcement residue definition (JMPR): Oxamyl | |||||
| 0110010 | Grapefruits |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. |
| 0110020 | Oranges |
0.01* (ft 1) (EFSA, 2010) |
Not established | Further consideration needed |
Chronic and acute (265% ARfD) consumer intake concerns cannot be excluded. An LOQ of 0.002 mg/kg is sufficiently validated, but a lower LOQ would be necessary to ensure sufficient consumer protection, considering the acute intake concern. According to the EU pesticides peer review, the use on citrus fruits is no more authorised in EU (EFSA, 2022). Contribution of residues to the chronic exposure 8% ADI. |
| 0110030 | Lemons |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
| 0110040 | Limes | ||||
| 0110050 | Mandarins |
0.01* (ft 1) (EFSA, 2010) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. According to the EU pesticides peer review, the use on citrus fruits is no more authorised in EU. Contribution of residues to the chronic exposure <1% ADI. |
| 0120010 | Almonds |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.01* | Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
| 0120020 | Brazil nuts | ||||
| 0120030 | Cashew nuts | ||||
| 0120040 | Chestnuts | ||||
| 0120050 | Coconuts | Further consideration needed |
Chronic and acute (144%) exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 5% ADI. An LOQ lower than 0.01 mg/kg would be necessary to ensure sufficient consumer protection, considering the acute intake concern. |
||
| 0120060 | Hazelnuts/cobnuts | 0.01* |
Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
||
| 0120070 | Macadamia | ||||
| 0120080 | Pecans | ||||
| 0120090 | Pine nut kernels | ||||
| 0120100 | Pistachios | ||||
| 0120110 | Walnuts | ||||
| 0130010 | Apples |
0.01* (Reg. 2019/552) |
Not considered (d) | Further consideration needed |
Chronic and acute (108% ARfD) exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 12% ADI. An LOQ lower than 0.001 mg/kg would be necessary to ensure sufficient consumer protection, considering the acute intake concern. |
| 0130020 | Pears | Further consideration needed |
Chronic and acute (138% ARfD) exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 4% ADI. An LOQ lower than 0.001 mg/kg would be necessary to ensure sufficient consumer protection, considering the acute intake concern. |
||
| 0130030 | Quinces | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
||
| 0130040 | Medlars | ||||
| 0130050 | Loquats/Japanese medlars | ||||
| 0140010 | Apricots |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
| 0140020 | Cherries (sweet) | ||||
| 0140030 | Peaches | ||||
| 0140040 | Plums | ||||
| 0151010 | Table grapes |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 1.5% ADI. |
| 0151020 | Wine grapes |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 2.5% ADI. |
|||
| 0152000 | Strawberries | 0.01* (Reg. 2019/552) | Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
| 0153000 | Cane fruits | ||||
| 0153010 | Blackberries | ||||
| 0153020 | Dewberries | ||||
| 0153030 | Raspberries (red and yellow) | ||||
| 0153990 | Other cane fruits | ||||
| 0154000 | Other small fruits & berries | ||||
| 0154010 | Blueberries | ||||
| 0154020 | Cranberries | ||||
| 0154030 | Currants (red, black and white) | ||||
| 0154040 | Gooseberries (green, red and yellow) | ||||
| 0154050 | Rose hips | ||||
| 0154060 | Mulberries (black and white) | ||||
| 0154070 | Azarole/Mediterranean medlar | ||||
| 0154080 | Elderberries | ||||
| 0161010 | Dates |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
| 0161020 | Figs | ||||
| 0161030 | Table olives | 0.01* |
Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. |
||
| 0161040 | Kumquats |
0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
||
| 0161050 | Carambolas | ||||
| 0161060 | Kaki/Japanese persimmons | ||||
| 0161070 | Jambuls/jambolans | ||||
| 0162010 | Kiwi fruits (green, red, yellow) | ||||
| 0162020 | Litchis/lychees | ||||
| 0162030 | Passionfruits/maracujas | ||||
| 0162040 | Prickly pears/cactus fruits | ||||
| 0162050 | Star apples/cainitos | ||||
| 0162060 | American persimmon/Virginia kaki | ||||
| 0163010 | Avocados |
0.01* (Reg. 2019/552) |
Not considered (d) | Further consideration needed |
Chronic and acute (252% ARfD) consumer intake concerns cannot be excluded. An LOQ of 0.005 mg/kg is sufficiently validated, but a lower LOQ would be necessary to ensure sufficient consumer protection, considering the acute intake concern. Contribution of residues to the chronic exposure <1% ADI |
| 0163020 | Bananas |
0.01* (ft 2) (EFSA, 2010) |
Not established | 0.001* |
Lower LOQ provisional pending further validation. Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 5.4% ADI. A narrow safety margin regarding acute exposure is noted (97% ARfD). According to the EU pesticides peer review, the Article 12 confirmatory data gap has been addressed (EFSA, 2022). |
| 0163030 | Mangoes |
0.01* (Reg. 2019/552) |
Not considered (d) |
0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
| 0163040 | Papayas | ||||
| 0163050 | Granate apples/pomegranates | ||||
| 0163060 | Cherimoyas | ||||
| 0163070 | Guavas | ||||
| 0163080 | Pineapples | Further consideration needed |
Chronic and acute (101% ARfD) exposure concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI An LOQ lower than 0.001 mg/kg would be necessary to ensure sufficient consumer protection, considering the acute intake concern. |
||
| 0163090 | Breadfruits | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI |
||
| 0163100 | Durians | ||||
| 0163110 | Soursops/guanabanas | ||||
| 0211000 | Potatoes |
0.01* (EFSA, 2010) |
0.01* |
Further consideration needed |
Chronic and acute (154% ARfD) exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 5.3% ADI. An LOQ lower than 0.001 mg/kg would be necessary to ensure sufficient consumer protection, considering the acute intake concern. |
| 0212010 | Cassava roots/manioc |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. |
| 0212020 | Sweet potatoes |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 3.5% ADI. |
|||
| 0212030 | Yams |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
|||
| 0212040 | Arrowroots | ||||
| 0213010 | Beetroots |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. |
| 0213020 | Carrots |
0.01* (EFSA, 2010) |
0.01* (FAO, 2018)(c) |
0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 1.4% ADI. |
| 0213030 | Celeriacs/turnip rooted celeries |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
| 0213040 | Horse radishes | ||||
| 0213050 | Jerusalem artichokes | ||||
| 0213060 | Parsnips |
0.01* (EFSA, 2010) |
0.01* (FAO, 2018)(c) |
||
| 0213070 | Parsley roots/Hamburg roots parsley |
0.01* (Reg. 2019/552) |
Not considered (d) | ||
| 0213080 | Radishes | ||||
| 0213090 | Salsifies |
0.01* (EFSA, 2010) |
Not established | ||
| 0213100 | Swedes/rutabagas |
0.01* (Reg. 2019/552) |
Not considered (d) | ||
| 0213110 | Turnips | ||||
| 0220010 | Garlic |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
| 0220020 | Onions | ||||
| 0220030 | Shallots | ||||
| 0220040 | Spring onions/green onions and Welsh onions | ||||
| 0231010 | Tomatoes |
0.01* (FAO, 2018) |
0.01* (FAO, 2018)(c) |
Further consideration needed |
Chronic and acute (116% ARfD) consumer intake concerns cannot be excluded. An LOQ of 0.002 mg/kg is sufficiently validated, but a lower LOQ would be necessary to ensure sufficient consumer protection, considering the acute intake concern. Contribution of residues to the chronic exposure 7% ADI. |
| 0231020 | Sweet peppers/bell peppers |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
| 0231030 | Aubergines/egg plants |
0.02 (ft 2) (EFSA, 2010) |
0.01* (FAO, 2018)(c) |
||
| 0231040 | Okra/lady's fingers |
0.01* (Reg. 2019/552) |
Not considered (d) | ||
| 0232010 | Cucumbers |
0.01* (ft 2) (EFSA, 2010) |
0.02 (FAO, 2018)(c) |
0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 1.6% ADI. According to the EU pesticides peer review, the Article 12 confirmatory data gap has been addressed (EFSA, 2022). |
| 0232020 | Gherkins |
0.02 (FAO, 2018)(c) |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. According to the EU pesticides peer review, the Article 12 confirmatory data gap has been addressed (EFSA, 2022). |
||
| 0232030 | Courgettes |
0.04 (FAO, 2018)(c) |
|||
| 0233010 | Melons |
0.01 (FAO, 2018) |
0.01 (FAO, 2018)(c) |
Further consideration needed |
Chronic and acute (152% ARfD) consumer intake concerns cannot be excluded. An LOQ lower than 0.001 mg/kg would be necessary to ensure sufficient consumer protection considering the acute intake concern. Contribution of residues to the chronic exposure <1% ADI. |
| 0233020 | Pumpkins |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. |
| 0233030 | Watermelons |
0.01 (FAO, 2018) |
0.01 (FAO, 2018)(c) |
Further consideration needed |
Chronic and acute (122% ARfD) consumer intake concerns cannot be excluded. An LOQ lower than 0.001 mg/kg would be necessary to ensure sufficient consumer protection considering the acute intake concern. Contribution of residues to the chronic exposure 1% ADI. |
| 0234000 | Sweet corn |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. |
| 0241010 | Broccoli | ||||
| 0241020 | Cauliflowers | ||||
| 0242010 | Brussels sprouts |
0.01* (EFSA, 2010) |
0.01* (FAO, 2018)(c) |
0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. |
| 0242020 | Head cabbages |
0.01* (Reg. 2019/552) |
Not considered (d) |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 1.4% ADI. |
|
| 0243010 | Chinese cabbages/pe‐tsai |
0.01* (Reg. 2019/552) |
Not considered (d) |
0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
| 0243020 | Kales | ||||
| 0244000 | Kohlrabies | ||||
| 0251010 | Lamb's lettuce/corn salads |
0.01* (Reg. 2019/552) |
Not considered (d) |
0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
| 0251020 | Lettuces | ||||
| 0251030 | Escaroles/broad‐leaved endives | ||||
| 0251040 | Cress and other sprouts and shoots | ||||
| 0251050 | Land cress | ||||
| 0251060 | Roman rocket/rucola | ||||
| 0251070 | Red mustards | ||||
| 0251080 | Baby leaf crops (including brassica species) | ||||
| 0252010 | Spinaches | ||||
| 0252020 | Purslanes | ||||
| 0252030 | Chards/beet leaves | ||||
| 0252990 | Other spinach and similar | ||||
| 0253000 | Grape leaves and similar species | ||||
| 0254000 | Watercress | ||||
| 0255000 | Witloofs/Belgian endives | ||||
| 0256010 | Chervil | ||||
| 0256020 | Chives | ||||
| 0256030 | Celery leaves | ||||
| 0256040 | Parsley | ||||
| 0256050 | Sage | ||||
| 0256060 | Rosemary | ||||
| 0256070 | Thyme | ||||
| 0256080 | Basil and edible flowers | ||||
| 0256090 | Laurel/bay leaves | ||||
| 0256100 | Tarragon | ||||
| 0260010 | Beans (with pods) | ||||
| 0260020 | Beans (without pods) | ||||
| 0260030 | Peas (with pods) | ||||
| 0260040 | Peas (without pods) | ||||
| 0260050 | Lentils (fresh) | ||||
| 0270010 | Asparagus | ||||
| 0270020 | Cardoons | ||||
| 0270030 | Celeries | ||||
| 0270040 | Florence fennels | ||||
| 0270050 | Globe artichokes | ||||
| 0270060 | Leeks | ||||
| 0270070 | Rhubarbs | ||||
| 0270080 | Bamboo shoots | ||||
| 0270090 | Palm hearts | ||||
| 0280000 | Fungi | ||||
| 0280010 | Cultivated fungi | ||||
| 0280020 | Wild fungi | ||||
| 0280990 | Mosses and lichens | ||||
| 0290000 | Algae and prokaryotes organisms | ||||
| 0300010 | Beans |
0.01* (Reg. 2019/552) |
Not considered (d) | Further consideration needed |
Chronic and acute (183% ARfD) consumer intake concerns cannot be excluded. An LOQ lower than 0.01 mg/kg would be necessary to ensure sufficient consumer protection, considering the acute intake concern. Contribution of residues to the chronic exposure 7.7% ADI. |
| 0300020 | Lentils | 0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure individually <3% ADI. |
||
| 0300030 | Peas | ||||
| 0300040 | Lupins/lupini beans | ||||
| 0401010 | Linseeds |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 1.5% ADI. |
| 0401020 | Peanuts/groundnuts | 0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 2.7% ADI. |
||
| 0401030 | Poppy seeds | 0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. |
||
| 0401040 | Sesame seeds | 0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. |
||
| 0401050 | Sunflower seeds | 0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 6.7% ADI. |
||
| 0401060 | Rapeseeds/canola seeds | 0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 9.5% ADI. |
||
| 0401070 | Soyabeans | Further consideration needed |
Chronic consumer intake concerns cannot be excluded. Considering the high contribution of residues in soybean (37% ADI) to the total exposure, a lowering of the existing LOQ would be required to ensure consumer protection |
||
| 0401080 | Mustard seeds |
0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. |
||
| 0401090 | Cotton seeds | 0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 3% ADI. |
||
| 0401100 | Pumpkin seeds | 0.01* | Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. | ||
| 0401110 | Safflower seeds | ||||
| 0401120 | Borage seeds | ||||
| 0401130 | Gold of pleasure seeds | ||||
| 0401140 | Hemp seeds | ||||
| 0401150 | Castor beans | ||||
| 0402010 | Olives for oil production |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 8% ADI. |
| 0402020 | Oil palm kernels |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 6% ADI. |
|||
| 0402030 | Oil palm fruits |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 7.8% ADI. |
|||
| 0402040 | Kapok |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 2.9% ADI. |
|||
| 0500010 | Barley |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.005* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 4.4% ADI. An LOQ of 0.005 mg/kg is sufficiently validated. |
| 0500020 | Buckwheat and other pseudo‐cereals | Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 1.4% ADI. An LOQ of 0.005 mg/kg is sufficiently validated | |||
| 0500030 | Maize/corn |
Further consideration needed |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 35% ADI. An LOQ of 0.005 mg/kg is sufficiently validated, but a lower LOQ would be necessary to ensure sufficient consumer protection, considering the high contribution of residues in maize/corn to the total exposure. |
||
| 0500040 | Common millet/proso millet | 0.005* | Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. An LOQ of 0.005 mg/kg is sufficiently validated | ||
| 0500050 | Oat | 0.005* | Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 2.9% ADI. An LOQ of 0.005 mg/kg is sufficiently validated. | ||
| 0500060 | Rice | 0.005* | Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 7.8% ADI. An LOQ of 0.005 mg/kg is sufficiently validated. | ||
| 0500070 | Rye |
Further consideration needed |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 27.5% ADI. An LOQ of 0.005 mg/kg is sufficiently validated, but a lower LOQ would be necessary to ensure sufficient consumer protection, considering the high contribution of residues in rye to the total exposure. |
||
| 0500080 | Sorghum | 0.005* | Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. An LOQ of 0.005 mg/kg is sufficiently validated. | ||
| 0500090 | Wheat | Further consideration needed |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 36% ADI. An LOQ of 0.005 mg/kg is sufficiently validated, but a lower LOQ would be necessary to ensure sufficient consumer protection, considering the high contribution of residues in wheat to the total exposure. |
||
| 0610000 | Tea (dried leaves of Camellia sinensis) |
0.05* (Reg. 2019/552) |
Not considered (d) | 0.05* | Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 7% ADI. |
| 0620000 | Coffee beans |
0.05* (Reg. 2019/552) |
Not considered (d) | Further consideration needed |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 278.5% ADI. An LOQ lower than 0.05 mg/kg would be necessary to ensure sufficient consumer protection, considering the high contribution of residues in coffee beans to the chronic exposure. |
| 0631010 | Chamomille |
0.05* (Reg. 2019/552) |
Not considered (d) | 0.05* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
| 0631020 | Hibiscus/roselle | ||||
| 0631030 | Rose | ||||
| 0631040 | Jasmine | ||||
| 0631050 | Lime/linden | ||||
| 0632000 | Herbal infusions (dried leaves) | ||||
| 0632010 | Strawberry leaves | ||||
| 0632020 | Rooibos | ||||
| 0632030 | Mate/maté | ||||
| 0633010 | Valerian root | ||||
| 0633020 | Ginseng root | ||||
| 0640000 | Cocoa beans | 0.05* (Reg. 2019/552) | Not considered (d) | Further consideration needed |
Chronic and acute (161% ARfD) consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 26% ADI. An LOQ lower than 0.05 mg/kg would be necessary to ensure sufficient consumer protection, considering the acute intake concern and the high contribution of residues in cocoa beans to the chronic exposure. |
| 0650000 | Carobs/Saint John's bread |
0.05* (Reg. 2019/552) |
Not considered (d) | Further consideration needed |
Chronic and acute (393% ARfD) consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. An LOQ lower than 0.05 mg/kg would be necessary to ensure sufficient consumer protection, considering acute exposure concerns. |
| 0700000 | HOPS (dried) |
0.05* (Reg. 2019/552) |
Not considered (d) | 0.05* | Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
| 0800000 | SPICES |
0.05* (Reg. 2019/552) |
Not considered (d) | 0.05* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI, except for vanilla pods (1%) and capers (2.9%). |
| 0900010 | Sugar beet roots |
0.01* (EFSA, 2010) |
0.01* (FAO, 2018) (c) |
0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 8.4% ADI. |
| 0900020 | Sugar canes |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 1.9% ADI. |
| 0900030 | Chicory roots |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.001* |
Lower LOQ provisional pending further validation. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. |
| 1011010 | Swine: Muscle/meat |
0.01* (Reg. 2019/552) |
Not considered (d) | Further consideration needed |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 11% ADI. An LOQ of 0.005 mg/kg is sufficiently validated, but a lower LOQ would be necessary to ensure sufficient consumer protection, considering the high contribution of residues in swine muscle/meat to the total exposure. |
| 1011020 | Swine: Fat tissue | 0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure individually below 3% ADI. |
||
| 1011030 | Swine: Liver | ||||
| 1011040 | Swine: Kidney | ||||
| 1011050 | Swine: Edible offals (other than liver and kidney) | ||||
| 1012010 | Bovine: Muscle/meat |
0.01* (Reg. 2019/552) |
Not considered (d) | Further consideration needed |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure 22% ADI. An LOQ of 0.005 mg/kg is sufficiently validated, but a lower LOQ would be necessary to ensure sufficient consumer protection, considering the high contribution of residues in bovine muscle/meat to the total exposure. |
| 1012020 | Bovine: Fat tissue | 0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure individually <2% ADI. |
||
| 1012030 | Bovine: Liver | ||||
| 1012040 | Bovine: Kidney | ||||
| 1012050 | Bovine: Edible offals (other than liver and kidney) | ||||
| 1013010 | Sheep: Muscle/meat |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.005* | Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure individually <3% ADI. |
| 1013020 | Sheep: Fat tissue |
0.01* |
|||
| 1013030 | Sheep: Liver | ||||
| 1013040 | Sheep: Kidney | ||||
| 1013050 | Sheep: Edible offals (other than liver and kidney) | ||||
| 1014010 | Goat: Muscle/meat |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.005* |
Lower LOQ sufficiently validated. Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. |
| 1014020 | Goat: Fat tissue | 0.01* | Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI (for several matrices no consumption data available). | ||
| 1014030 | Goat: Liver | ||||
| 1014040 | Goat: Kidney | ||||
| 1014050 | Goat: Edible offals (other than liver and kidney) | ||||
| 1015010 | Equine: Muscle/meat | 0.005* |
Lower LOQ sufficiently validated. Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure <1% ADI. |
||
| 1015020 | Equine: Fat tissue | 0.01* | Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI (for several matrices no consumption data available). | ||
| 1015030 | Equine: Liver | ||||
| 1015040 | Equine: Kidney | ||||
| 1015050 | Equine: Edible offals (other than liver and kidney) | ||||
| 1016010 | Poultry: Muscle/meat |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.005* |
Lower LOQ sufficiently validated. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 7% ADI. |
| 1016020 | Poultry: Fat tissue | 0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI. |
||
| 1016030 | Poultry: Liver | ||||
| 1016040 | Poultry: Kidney | ||||
| 1016050 | Poultry: Edible offals (other than liver and kidney) | ||||
| 1017010 | Other farmed animals: Muscle/meat |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.005* |
Lower LOQ sufficiently validated. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 1.3% ADI. |
| 1017020 | Other farmed animals: Fat tissue | 0.01* |
Chronic consumer intake concerns cannot be excluded. Contribution of residues to the chronic exposure individually <1% ADI (for several matrices no consumption data available). |
||
| 1017030 | Other farmed animals: Liver | ||||
| 1017040 | Other farmed animals: Kidney | ||||
| 1017050 | Other farmed animals: Edible offals (other than liver and kidney) | ||||
| 1020010 | Milk: Cattle |
0.01* (Reg. 2019/552) 0.01 |
Not considered (d) | Further consideration needed |
Chronic and acute (124%) exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 59.7% ADI An LOQ of 0.001 mg/kg is sufficiently validated, but a lower LOQ would be necessary to ensure sufficient consumer protection, considering the acute intake concern and the high contribution of residues in cattle milk to the chronic exposure. |
| 1020020 | Milk: Sheep | Further consideration needed |
Chronic and acute (151%) exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 2.4% ADI. A lowering of the existing LOQ of 0.01 mg/kg would be necessary to ensure sufficient consumer protection, considering the acute intake concern. |
||
| 1020030 | Milk: Goat | Further consideration needed |
Chronic and acute (242%) exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 3.6% ADI. A lowering of the existing LOQ of 0.01 mg/kg would be necessary to ensure sufficient consumer protection, considering the acute intake concern. |
||
| 1020040 | Milk: Horse |
0.01* |
Chronic exposure concerns cannot be excluded. No consumption data available to estimate contribution of residues to the total chronic exposure. |
||
| 1030010 | Eggs: Chicken |
0.01* (Reg. 2019/552) |
Not considered (d) | 0.005* |
Lower LOQ sufficiently validated. Chronic exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 6.7% ADI. |
| 1030020 | Eggs: Duck |
Lower LOQ sufficiently validated. Chronic exposure concerns cannot be excluded. No consumption data available to estimate contribution of residues to the total chronic exposure. |
|||
| 1030030 | Eggs: Goose | ||||
| 1030040 | Eggs: Quail | ||||
| 1040000 | Honey and other apiculture products |
0.05* (Reg. 2019/552) |
Not considered (d) | Further consideration needed |
Chronic and acute (179%) exposure concerns cannot be excluded. Contribution of residues to the chronic exposure 5% ADI. A lowering of the existing LOQ of 0.05 mg/kg would be necessary to ensure sufficient consumer protection, considering the acute intake concern. |
MRL: maximum residue level; CXL: codex maximum residue limit; JMPR: Joint FAO/WHO Meeting on Pesticide Residues; LOQ: limit of quantification; ADI: acceptable daily intake; ARfD: acute reference dose.
Indicates that the MRL is set at the limit of quantification.
Commodity code number, as listed in Annex I of Regulation (EC) No 396/2005.
Crops on which authorised uses were reported by the MRL review (EFSA, 2010) or an MRL was implemented on the basis of the CXL, are reported in bold.
Based on EU GAP.
Not considered relevant for the present assessment.
(ft 1): The European Food Safety Authority identified some information on storage stability, crop metabolism and residue trials as unavailable. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 25 January 2016, or, if that information is not submitted by that date, the lack of it.
(ft 2): The European Food Safety Authority identified some information on crop metabolism as unavailable. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 25 January 2016, or, if that information is not submitted by that date, the lack of it.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- bw
body weight
- CAC
Codex Alimentarius Commission
- CCPR
Codex Committee on Pesticide Residues
- CXL
codex maximum residue limit
- EMS
evaluating Member State
- EURLs
European Union Reference Laboratories for Pesticide Residues (former CRLs)
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- LOAEL
lowest observed adverse effect level
- LOQ
limit of quantification
- MRL
maximum residue level
- NEDI
national estimated daily intake
- NESTI
national estimated short‐term intake
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- PAFF
Standing Committee on Plants, Animals, Food and Feed
- PRIMo
(EFSA) Pesticide Residues Intake Model
- RA
risk assessment
- RD
residue definition
- SANCO
Directorate‐General for Health and Consumers
- SCPAFF
Standing Committee on Plants, Animals, Food and Feed (formerly: Standing Committee on the Food Chain and Animal Health; SCFCAH)
- STMR
supervised trials median residue
- WHO
World Health Organization
Appendix A – Input values for the exposure calculations
A.1 Input values consumer risk assessment – Scenario 1
| Commodity | Existing/ Proposed MRL (mg/kg) | Source | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |||
| Risk assessment residue definition: oxamyl | ||||||
| Bananas | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Potatoes | 0.01* | EFSA (2010) | 0.005 | STMR (EFSA, 2010) | 0.005 | HR (EFSA, 2010) |
| Carrots | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Parsnips | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Salsifies | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Tomatoes | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Aubergines/ eggplants | 0.02 | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Cucumbers, gherkins, courgettes | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Melons, watermelones | 0.01 | FAO (2018) | 0.005 | STMR (pulp) (FAO, 2018) | 0.005 | HR (pulp) (FAO, 2018) |
| Brussels sprouts | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Sugar beet root | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Other commodities of plant and animal origin |
0.01* or 0.02* or 0.05* |
EU MRL (Regulation (EU) 2019/552) |
0.01 or 0.02 or 0.05 |
EU MRL (Regulation (EU) 2019/552) |
0.01 or 0.02 or 0.05 |
EU MRL (Regulation (EU) 2019/552) |
MRL: maximum residue level; STMR: supervised trials median residue in raw agricultural commodity; HR: highest residue in raw agricultural commodity
Indicates that the M.RL is set at the limit of quantification.
A.2 Input values consumer risk assessment – Scenario 2
| Commodity | Existing/ Proposed MRL (mg/kg) | Source | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |||
| Risk assessment residue definition: oxamyl | ||||||
| Bananas | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Potatoes | 0.01* | EFSA (2010) | 0.005 | STMR (EFSA, 2010) | 0.005 | HR (EFSA, 2010) |
| Carrots | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Parsnips | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Salsifies | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Tomatoes | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Aubergines/ eggplants | 0.02 | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Cucumbers, gherkins, courgettes | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Melons, watermelones | 0.01 | FAO (2018) | 0.005 | STMR (pulp) (FAO, 2018) | 0.005 | HR (pulp) (FAO, 2018) |
| Brussels sprouts | 0.01* | EFSA (2010) | 0.01 | STMR (EFSA, 2010) | 0.01 | HR (EFSA, 2010) |
| Citrus fruits (except oranges), pome fruits, stone fruits, berries and small fruits, miscellaneous fruit (except table olives and avocados | 0.01* | EU MRL (Regulation (EU) 2019/552) | 0.001 | Lowest analytical validation level (EURLs) | 0.001 | Lowest analytical validation level (EURLs) |
| Oranges | 0.01* | LOQ | 0.002 | Lowest analytical validation level (EURLs) | 0.002 | Lowest analytical validation level (EURLs) |
| Avocados | 0.01* | LOQ |
0.005 |
Lowest analytical validation level (EURLs) | 0.005 | Lowest analytical validation level (EURLs) |
| Root and tuber vegetables (except potatoes, carrots, parsnips, salsifies) | 0.01* | EU MRL (Regulation (EU) 2019/552) | 0.001 | Lowest analytical validation level (EURLs) | 0.001 | Lowest analytical validation level (EURLs) |
| Bulb vegetables | 0.01* | EU MRL (Regulation (EU) 2019/552) | 0.001 | Lowest analytical validation level (EURLs) | 0.001 | Lowest analytical validation level (EURLs) |
| Fruiting vegetables (except tomatoes, aubergines/eggplants, cucumbers, gherkins, courgettes, melons and watermelons) | 0.01* | EU MRL (Regulation (EU) 2019/552) | 0.001 | Lowest analytical validation level (EURLs) | 0.001 | Lowest analytical validation level (EURLs) |
|
Brassica vegetables (except Brussesl sprouts) Leaf vegetables, herbs and edible flowers Legume vegetables Stem vegetables, Fungi |
0.01* | EU MRL (Regulation (EU) 2019/552) | 0.001 | Lowest analytical validation level (EURLs) | 0.001 | Lowest analytical validation level (EURLs) |
| Cereals | 0.01* | LOQ | 0.005 | Lowest analytical validation level (EURLs) | 0.005 | Lowest analytical validation level (EURLs) |
| Sugar plants | 0.01* | EU MRL (Regulation (EU) 2019/552) | 0.001(a) | Lowest analytical validation level (EURLs) | 0.001(a) | Lowest analytical validation level (EURLs) |
| Meat of swine, bovine, sheep, goat, equine, poultry, other farmed terrestrial animals | 0.01* | LOQ | 0.005 | Lowest analytical validation level (EURLs) | 0.005 | Lowest analytical validation level (EURLs) |
| Bird's Eggs | 0.01* | LOQ | 0.005 | Lowest analytical validation level (EURLs) | 0.005 | Lowest analytical validation level (EURLs) |
| Cattle milk | 0.01* | LOQ | 0.001 | Lowest analytical validation level (EURLs) | 0.001 | Lowest analytical validation level (EURLs) |
| Tree nuts, table olives; Pulses; Oilseeds; Oilfruits | 0.01* | EU MRL (Regulation (EU) 2019/552) | 0.01 | LOQ (Regulation (EU) 2019/552) | 0.01 | LOQ (Regulation (EU) 2019/552) |
| Tea, coffee, herbal infusions; Hops; Spices | 0.05* | EU MRL (Regulation (EU) 2019/552) | 0.05 | LOQ (Regulation (EU) 2019/552) | 0.05 | LOQ (Regulation (EU) 2019/552) |
| Fat, liver, kidney, edible offal of swine, bovine, sheep, goat, equine, poultry, other farmed terrestrial animals | 0.01* | EU MRL (Regulation (EU) 2019/552) | 0.01 | LOQ (Regulation (EU) 2019/552) | 0.01 | LOQ (Regulation (EU) 2019/552) |
| Milk of sheep, goat, horse | 0.01* | EU MRL (Regulation (EU) 2019/552) | 0.01 | LOQ (Regulation (EU) 2019/552) | 0.01 | LOQ (Regulation (EU) 2019/552) |
| Honey and other apiculture products | 0.05* | EU MRL (Regulation (EU) 2019/552) | 0.05 | LOQ (Regulation (EU) 2019/552) | 0.05 | LOQ (Regulation (EU) 2019/552) |
MRL: maximum residue level; STMR: supervised trials median residue in raw agricultural commodity; HR: highest residue in raw agricultural commodity; EURLs: European Union Reference Laboratories for Pesticide Residues; LOQ: limit of quantification.
Indicates that the MRL is set at the limit of quantification.
No concentration of residues is expected in sugar and therefore in sugar beet root the input value is the lowest valdiation level of 0.001 mg/kg as reported by EURLs for high water content matrices.
A.3 Input values consumer risk assessment – Scenario 3
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: oxamyl | ||||
| Citrus fruits (except oranges), pome fruits, stone fruits, berries and small fruits, miscellaneous fruit (except table olives and avocados | 0.001 | Lowest analytical validation level (EURLs) | 0.001 | Lowest analytical validation level (EURLs) |
| Oranges | 0.002 | Lowest analytical validation level (EURLs) | 0.002 | Lowest analytical validation level (EURLs) |
| Avocados | 0.005 | Lowest analytical validation level (EURLs) | 0.005 | Lowest analytical validation level (EURLs) |
|
Root and tuber vegetables, Bulb vegetables, Fruiting vegetables (except tomatoes), Brassica vegetables, Leaf vegetables, Herbs and edible flowers Legume vegetables Stem vegetables, Fungi, Sugar plants |
0.001 | Lowest analytical validation level (EURLs) | 0.001 | Lowest analytical validation level (EURLs) |
| Tomatoes | 0.002 | Lowest analytical validation level (EURLs) | 0.002 | Lowest analytical validation level (EURLs) |
| Cereals | 0.005 | Lowest analytical validation level (EURLs) | 0.005 | Lowest analytical validation level (EURLs) |
| Meat of swine, bovine, sheep, goat, equine, poultry, other farmed terrestrial animals | 0.005 | Lowest analytical validation level (EURLs) | 0.005 | Lowest analytical validation level (EURLs) |
| Bird's Eggs | 0.005 | Lowest analytical validation level (EURLs) | 0.005 | Lowest analytical validation level (EURLs) |
| Cattle milk | 0.001 | Lowest analytical validation level (EURLs) | 0.001 | Lowest analytical validation level (EURLs) |
| Tree nuts, table olives; Pulses; Oilseeds; Oilfruits | 0.01 | LOQ (Regulation (EU) 2019/552) | 0.01 | LOQ (Regulation (EU) 2019/552) |
| Tea, coffee, herbal infusions; Hops; Spices | 0.05 | LOQ (Regulation (EU) 2019/552) | 0.05 | LOQ (Regulation (EU) 2019/552) |
| Fat, liver, kidney, edible offal of swine, bovine, sheep, goat, equine, poultry, other farmed terrestrial animals | 0.01 | LOQ (Regulation (EU) 2019/552) | 0.01 | LOQ (Regulation (EU) 2019/552) |
| Milk of sheep, goat, horse | 0.01 | LOQ (Regulation (EU) 2019/552) | 0.01 | LOQ (Regulation (EU) 2019/552) |
| Honey and other apiculture products | 0.05 | LOQ (Regulation (EU) 2019/552) | 0.05 | LOQ (Regulation (EU) 2019/552) |
EURLs: European Union Reference Laboratories for Pesticide Residues; LOQ: limit of quantification.
Appendix B – Consumer risk assessment





Appendix C – Pesticide Residue Intake Model (PRIMo)
PRIMo (scenario 1)
PRIMo (scenario 2)
PRIMo (scenario 3)
Appendix D – Used compound codes
| Code/trivial name (a) | IUPAC name/SMILES notation/InChiKey (b) | Structural formula (c) |
|---|---|---|
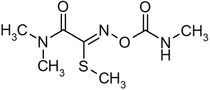
| Oxamyl |
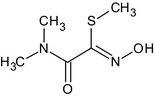
methyl (EZ)‐2‐(dimethylamino)‐N‐[(methylcarbamoyl)oxy]‐2‐oxothioacetimidate KZAUOCCYDRDERY‐UHFFFAOYSA‐N O=C(C(=N\OC(=O)NC)/SC)N(C)C |
|
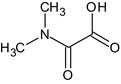
| IN‐D2708 |
(dimethylamino)(oxo)acetic acid CN(C)C(=O)C(=O)O YKFGLGXRUVEMNF‐UHFFFAOYSA‐N |
|
| IN‐A2213 |
methyl (1Z)‐2‐(dimethylamino)‐N‐hydroxy‐2‐oxoethanimidothioate CN(C)C(=O)C(=N\O)\SC KIDWGGCIROEJJW‐XQRVVYSFSA‐N |
|
|
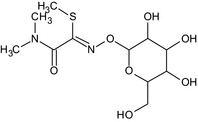
IN‐QKT34 (IN‐A2213 glucoside) |
1‐O‐{(Z)‐[2‐(dimethylamino)‐1‐(methylsulfanyl)‐2‐oxoethylidene]amino}hexopyranose CN(C)C(=O)C(=N\OC1OC(CO)C(O)C(O)C1O)\SC BVJZJNMSARVECQ‐XFXZXTDPSA‐N |
|
| IN‐N0079 |
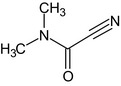
[(cyanocarbonyl)azanediyl]dimethane CN(C)C(=O)C#N DNRRZLQWEDPRRM‐UHFFFAOYSA‐N |
|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; InChiKey: International Chemical Identifier Key.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2021.1.3 ACD/Labs 2021.1.3 (File Version N15E41, Build 123,232, 7 July 2021).
ACD/ChemSketch 2021.1.3 ACD/Labs 2021.1.3 (File Version C25H41, Build 123,835, 28 August 2021).
Suggested citation: EFSA (European Food Safety Authority) , 2023. Statement on the risk assessment of maximum residue levels (MRLs) for oxamyl in view of consumer protection. EFSA Journal 2023;21(3):7823, 53 pp. 10.2903/j.efsa.2023.7823
Requestor: European Commission
Question number: EFSA‐Q‐2022‐00833
Declarations of interest: If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Approved: 20 January 2023
Notes
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32.
Commission Implementing Regulation (EU) 2021/2068 of 25 November 2021 amending Implementing Regulation (EU) No 540/2011 as regards the extension of the approval periods of the active substances benfluralin, dimoxystrobin, fluazinam, flutolanil, mecoprop‐P, mepiquat, metiram, oxamyl and pyraclostrobin. OJ L 421, 26.11.2021, p. 25–27.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Commission Regulation (EU) No 61/2014 of 24 January 2014 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for cyromazine, fenpropidin, formetanate, oxamyl and tebuconazole in or on certain products. OJ L 22, 25.1.2014, p. 1–32.
Joint FAO/WHO food standards programme Codex Alimentarius Commission. Appendix II. Forty‐first Session. Rome, Italy, 2–6 July 2018.
Commission Regulation (EU) No 2019/552 of 4 April 2019 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for azoxystrobin, bicyclopyrone, chlormequat, cyprodinil, difenoconazole, fenpropimorph, fenpyroximate, fluopyram, fosetyl, isoprothiolane, isopyrazam, oxamyl, prothioconazole, spinetoram, trifloxystrobin and triflumezopyrim in or on certain products. OJ L 96, 5.4.2019, p. 6–49.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Background documents to this reasoned opinion are published on OpenEFSA and are available at the following link: https://open.efsa.europa.eu/study-inventory/EFSA-Q-2022-00833
Commission Directive 2005/16/EC of 7 February 2006 amending Council Directive 91/414/EEC to include oxamyl as active substance. OJ L 36, 8.2.2006, p.37‐39.
The same study and NOAEL were also selected by EFSA in 2005 for setting of the oxamyl TRVs at 0.001 mg/kg bw day (EFSA, 2005).
The EURLs assume that lower levels of 0.001 mg/kg can be successfully validated in matrices with high water and high acid content, however, experiments are pending.
For other commodities of animal origin the EURLs confirm that there are no experimental data to support lower LOQs.
References
- EFSA (European Food Safety Authority) , 2005. Conclusion regarding the peer review of the pesticide risk assessment of the active substance oxamyl. EFSA Scientific Report 2005;3(3):26r, 78 pp. 10.2903/j.efsa.2005.26r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2010. Reasoned Opinion on the review of the existing maximum residue levels (MRLs) for oxamyl according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2010;8(10):1830, 34 pp. 10.2903/j.efsa.2010.1830 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2018. Scientific support for preparing an EU position in the 50th Session of the Codex Committee on Pesticide Residues (CCPR). EFSA Journal 2018;16(7):5306, 229 pp. 10.2903/j.efsa.2018.5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou M, Brancato A, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J, Theobald A, Verani A, 2019. Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1 (update of EFSA PRIMo revision 3). EFSA supporting publication 2019:EN‐1605, 15 pp. 10.2903/sp.efsa.2019.EN-1605 [DOI]
- EFSA (European Food Safety Authority) , Alvarez F, Arena M, Auteri D, Binaglia M, Federica Castoldi A, Chiusolo A, Colagiorgi A, Colas M, Crivellente F, De Lentdecker C, Egsmose M, Fait G, Ferilli F, Gouliarmou V, Herrero Nogareda L, Ippolito A, Istace F, Jarrah S, Kardassi D, Kienzler A, Lanzoni A, Lava R, Leuschner R, Linguadoca A, Lythgo C, Magrans O, Mangas I, Miron I, Molnar T, Padovani L, Parra Morte JM, Serafimova R, Sharp R, Szentes C, Terron A, Theobald A, Tiramani M and Villamar‐Bouza L, 2022. Conclusion on the peer review of the pesticide risk assessment of the active substance oxamyl. EFSA Journal 2022;20(5):7296, 36 pp. 10.2903/j.efsa.2022.7296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2011. Review report for the active substance for the active substance oxamyl, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 15 July 2005 in view of the inclusion of oxamyl in Annex I of Directive 91/414/EEC. SANCO/10212/05 final rev 1, 17 June 2011.
- FAO (Food and Agriculture Organization of the United Nations) , 2016. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 3rd Edition. FAO Plant Production and Protection Paper 225, 298 pp.
- FAO (Food and Agriculture Organization of the United Nations) , 2018. Oxamyl. In Pesticide residues in food ‐2017. Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group Evaluations, Part I, Residues. FAO Plant Production and Protection Paper 233. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations)/WHO (World Health Organisation) , 2018. Report of the 50th Session of the Codex Committee on Pesticide Residues, REP18/PR.