Abstract
Objective
Intervertebral disc degeneration (IDD) is the main cause of back pain, and its treatment is a serious socio‐economic burden. The safety and treatment of fecal microbiota transplantation (FMT) has been established. However, the relationship between FMT and IDD still unclear. We aimed to explore whether FMT plays a role in IDD to provide a reference for the treatment of IDD.
Methods
An experimental model of IDD was established using 2‐month‐old male Sprague–Dawley rats. FMT was performed by intragastric gavage of IDD rats with a fecal bacterial solution. Rat serum, feces, and vertebral disc tissue were collected after surgery for 2 months. The levels of TNF‐α, IL‐1β, IL‐6, matrix metalloproteinase (MMP)‐3, MMP‐13, Collagen II, and aggrecan in the serum or vertebral disc tissue were measured by an enzyme‐linked immunosorbent assay, immunohistochemistry, quantitative real‐time polymerase chain reaction, or western blotting. We also examined the pathology of the vertebral disc tissue using hematoxylin and eosin (HE) and safranin O‐fast green staining. Finally, we examined the gut microbiota in rat feces using 16 S rRNA gene sequencing.
Results
We found that the expression of TNF‐α, IL‐1β, IL‐6, MMP‐3, MMP‐13, NLRP3 and Caspase‐1 increased in the IDD group rats. In contrast, Collagen II and aggrecan levels were downregulated. Additionally, vertebral disc tissue was severely damaged in the IDD group, with disordered cell arrangement and uneven safranin coloration. FMT reversed the effects of IDD modeling on these factors and alleviated cartilage tissue damage. In addition, FMT increased the gut microbiota diversity and microbial abundance in rats treated with IDD.
Conclusion
Our findings suggest that FMT has a positive effect in maintaining cellular stability in the vertebral disc and alleviating histopathological damage. It affects the diversity and abundance of gut microbiota in rats with IDD. Therefore, FMT may serve as a promising target for amelioration of IDD.
Keywords: Autophagy, Fecal Microbiota Transplantation, Gut Microbiota, Inflammation, Intervertebral Disc Degeneration
Fecal microbiota transplantation (FMT) inhibited TNF‐α, IL‐1β, IL‐6, MMP‐3, MMP‐13, NLRP3, and Caspase‐1 levels, up‐regulated collagen II and aggrecan expression, restored gut microbiota homeostasis, thereby improving intervertebral disc degeneration in mice.
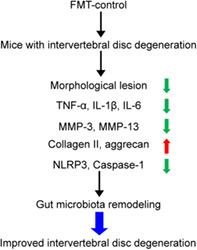
Introduction
Low back pain (LBP) is closely related to intervertebral disc degeneration (IDD). 1 , 2 The exact pathogenesis of IDD is believed to be the result of changes in the microenvironment within in vitro diagnostics caused by multiple factors. 3 , 4 The main strategy for all IDD treatments is to prevent cell loss due to programmed or regulated cell death. 5
The intervertebral disc is located between the vertebrae, 6 with gelatinous nucleus pulposus (NPs) as the central structure. NPs are surrounded by a fibrous plate annulus (AF) 7 and sandwiched between the upper and lower cartilage endplates (CEPs). 8 , 9 NPs play a role in maintaining homeostasis by secreting the extracellular matrix (ECM). A homeostatic imbalance between ECM anabolism and catabolism results in ECM reduction. 8 , 9 IDD is accompanied by changes in ECM homeostasis. Increased levels of secreted proinflammatory cytokines further promote ECM degradation and changes in diagnostic cell phenotypes. This results in vertebral disc protrusion and radicular pain. 10 In addition to changes in ECM homeostasis, IDD is associated with cellular inflammation and apoptosis. 11 , 12 , 13 , 14 Some studies have shown that cells die in the form of pyroptosis after inflammasome formation. 15 , 16 Pyroptosis releases large amounts of interleukin‐ (IL) 1β and IL‐18, leading to further inflammatory damage. 16
Gut microbiota also affects intervertebral disc homeostasis. 17 , 18 There were three potential ways of influence this: (1) the bacteria crossed the intestinal epithelial barrier into the intervertebral disc; (2) Bacteria regulate the mucosal and systemic immune system; (3) Bacteria regulate the metabolism of substances in the intestinal epithelium. 19 Clinical testing revealed nearly 60 overlapping bacterial strains between the intervertebral disc and the intestine. There are differences in biodiversity and abundance between healthy and diseased discs. Protective bacteria are abundant in normal discs, whereas putative pathogens are present in IDDs. 20 Therefore, regulation of the intestinal microbiota may affect the diversity and abundance of the microbiota in the intervertebral disc to regulate IDD.
Fecal microbiota transplantation (FMT) is associated with inflammation regulation and bacterial flora homeostasis in vivo. FMT is a therapeutic approach for colonizing the patient's gut with fecal suspension from a healthy individual. 21 Altering the composition of the host gut microbiota by fecal microbiota transplantation has been suggested as a potential novel strategy for regulating bone homeostasis. 22 Maternal FMT can restore the gut microbiota of infants born by cesarean section (CS) postpartum. 23 FMT has been shown to have favorable therapeutic effects in patients with severe antibiotic‐associated diarrhea (AAD) without infectious complications. 24 Researchers have provided limited evidence of a link between modic changes (a type of vertical disc degeneration) and gut microbiota by 16 S sequencing. 25 However, whether FMT has a therapeutic effect on IDD has not been further investigated. As the efficacy of FMT in related diseases has been established, we aimed to explore its potential role in IDD. The objectives of our study were as follows: (i) Clarify the effects of FMT on histopathological damage and intestinal flora in IDD rats; and (ii) Explore the regulation of FMT on ECM homeostasis, inflammation and pyrogenesis.
Methods
Animals Model
The experimental animals were Sprague–Dawley (SD) rats (2‐months‐old, male). The rats were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). Rats were randomly divided into three groups: control, IDD, and IDD + FMT. Rats were anesthetized with 2% pentobarbital (40 mg/kg). Grades 7–8 (Co7‐8) and 8–9 (Co8‐9) of the rat coccyx were punctured from the back with a 20‐gauge needle. 26 , 27 , 28 After the needle was completely inserted into the full thickness of the annulus fibrosus, it was rotated for 5 s and held for 30 s to ensure the effect of degenerative damage.
We collected 10 g of fresh feces from 2‐month‐old male rats. Fifty milliliters of sterile physiological saline was added and stirred at 37°C. The fecal solution was filtered through a double layer of sterile gauze. The filtered fecal residue was suspended in 100 mL of sterile physiological saline to obtain a fecal bacterial solution. Rats in the IDD + FMT group were intragastrically administered (5 mL/kg) by gavage once a day for 14 days (from the second day after the IDD operation). 29 , 30 The control and IDD groups were gavaged daily with the same volume of sterile physiological saline. Feces were collected 2 months after the operation, and the rats were sacrificed for subsequent analysis and detection.
Enzyme‐Linked Immunosorbent Assay
We determined the levels of TNF‐α, IL‐1β, IL‐6, matrix metalloproteinase (MMP)‐3, MMP‐13, Collagen II, and aggrecan in the serum and vertebral disc tissue. We used TNF‐α (RTA00, Bio‐Techne, Minneapolis, Minnesota, USA), IL‐1β (RLB00, Bio‐Techne, Minneapolis, Minnesota, USA), IL‐6 (437107, BioLegend, San Diego, CA, USA), MMP‐3 (SEA101Ra, Cloud‐Clone Corp, Houston, TX, USA), MMP‐13 (SEA099Ra, Cloud‐Clone Corp, Houston, TX, USA), Collagen II (ER0852, Wuhan Fine Biotech, Wuhan, China), and aggrecan (SEB908Ra, Cloud‐Clone Corp, Houston, TX, USA) Enzyme‐Linked Immunosorbent Assay (ELISA) kits to measure relative factors.
Immunohistochemical
Immunohistochemical (IHC) was used to measure the distribution of NLRP3, Caspase‐1, MMP‐3, and Collagen II in vertebral disc tissue. After dehydration, the sections were placed in 0.01 M citrate buffer (pH 6.0) and kept at a high temperature for 20 min. After the sections were cooled and washed, 1% periodic acid was used for 10 min to eliminate endogenous enzymes. Next, NLRP3 primary antibody (PA5‐79740, 1:400, Cell Signaling Technology, Danvers, MA, USA), Caspase‐1 primary antibody (22915‐1‐AP, 1:100, Proteintech, Chicago, IL, USA), MMP‐3 primary antibody (17873‐1‐AP, 1:100, Chicago, IL, Proteintech, USA), and Collagen II primary antibody (bs‐10589R, 1:200, Bioss, Beijing, China) were incubated. The secondary antibodies were then added for incubation. Subsequently, the sections were cultured with 3,3′‐diaminobenzidine (DAB), hematoxylin, and alcohol dehydration. Finally, the sections were blocked with a neutral resin and examined under a microscope (BA410T; Motic, Hongkong, China).
Hematoxylin and Eosin Staining
We performed a pathological examination of the vertebral disc tissue with hematoxylin and eosin (HE). Tissue sections were baked at 60°C, soaked in xylene, and dehydrated in alcohol. The sections were stained with hematoxylin for 5 min and stained with eosin for 5 min. After washing with distilled water, the sections were dehydrated in an alcohol gradient. Finally, the sections were blocked with neutral resin and examined under a microscope (BA410T; Motic, Hongkong, China).
Safranin O‐Fast Green (S‐O)
We also examined the pathology of the vertebral disc tissue with S‐O. Tissue sections were baked at 60°C, soaked in xylene, and dehydrated in alcohol. Sections were stained with fast green stain for 5 min and then stained with safranin O solution again for 30 s. Sections were dehydrated after washing with distilled water. Finally, the sections were blocked with neutral resin and examined under a microscope (BA410T; Motic, Hongkong, China).
16 S rRNA Gene Sequencing
First, we extracted fecal DNA using a genomic DNA extraction kit (dp328‐02, Tiangen, Beijing, China). Next, bacterial primers for the V3‐V4 region (341F:5’‐CCTACGGGNGGCWGCAG‐3′ and 805R:5’‐GACTACHVGGGTATCTAATCC‐3′) and the fusion enzyme (K1031, APExBIO, Houston, TX, USA) were used for PCR amplification. Raw data were obtained using an Illumina Novaseq6000 PE250. The Qiime 2 (2020.2) analysis process was used to call DADA2 for quality control in order to obtain useful clean data. Each amplicon sequence variant/OTU sequence was annotated using the silvA‐132‐99 database to obtain information on the corresponding species. The alpha diversity index of each sample was calculated using Qiime 2 software. The distance matrix was obtained based on the Bray–Curtis algorithm, which was used for beta diversity analysis and visualization.
Quantitative Real‐Time Polymerase Chain Reaction
We examined the expression of TNF‐α, IL‐1β, IL‐6, MMP‐3, MMP‐13, Collagen II, aggrecan, NLRP3, and Caspase‐1 in vertebral disc tissue. First, total RNA was extracted using TRIzol Reagent (15596–026, Invitrogen, Grand Island, NY, USA). Next, mRNA was reverse‐transcribed to cDNA using an mRNA reverse transcription kit (CW2569, Kangwei Century, Beijing, China). Primers for TNF‐α, IL‐1β, IL‐6, MMP‐3, MMP‐13, Collagen II, aggrecan, NLRP3, Caspase‐1, and β‐actin were provided by Sangon Biotech (Sangon Biotech, Shanghai, China) (Table 1). β‐actin was used as a reference. DNA amplification and detection were performed using a fluorescent quantitative PCR instrument (PIKOREAL96, Thermo Fisher, Waltham, MA, USA). 2−△△CT was used to assess relative mRNA levels.
TABLE 1.
The primer sequence
| Primer ID | 5′‐3′ | Product length (bp) |
|---|---|---|
| β‐Actin | F: ACATCCGTAAAGACCTCTATGCC | 223 |
| R: TACTCCTGCTTGCTGATCCAC | ||
| TNF‐α | F: CCCCTCTATTTATAATTGCACCT | 167 |
| R: CTGGTAGTTTAGCTCCGTTT | ||
| IL‐1β | F: CAGCAGCATCTCGACAAGAG | 123 |
| R: AAAGAAGGTGCTTGGGTCCT | ||
| IL‐6 | F: TCACTATGAGGTCTACTCGG | 141 |
| R: CATATTGCCAGTTCTTCGTA | ||
| MMP‐3 | F: CCTCTGAGTCTTTTCATGGAGGG | 234 |
| R: TGTCTGTAGCCCAGGAGTGT | ||
| MMP‐13 | F: CAAAGACTATCCCCGCCTCA | 147 |
| R: ACTCTCACAATGCGATTACTCC | ||
| CollagenII | F: ACCCTCAACCCCAAAACAACACA | 100 |
| R: TCAGGTCAGCCATTCAGTGC | ||
| aggrecan | F: ACAGACACCCCTACCCTTGC | 193 |
| R: CCTCACATTGCTCCTGGTCGAT | ||
| NLRP3 | F: CACCTCTTCTCTGCCTACCTG | 181 |
| R: AGCTGTAAAATCTCTCGCAGT | ||
| Caspase‐1 | F: CTAGACTACAGATGCCAACCAC | 128 |
| R: GGCTTCTTATTGGCATGATTCCC |
Western Blot
We also measured the levels of TNF‐α, IL‐1β, IL‐6, MMP‐3, MMP‐13, Collagen II, aggrecan, NLRP3, and Caspase‐1 in vertebral disc tissue. First, the vertebral disc tissue was fully ground in RIPA Lysate (AWB0136; Abiowell, Changsha, China). Subsequently, the grinding fluid was centrifuged, and the supernatant was collected. The protein content was measured using a BCA protein kit (ab102536, Abcam, Cambridge, UK).
Based on the protein quantification results, the markers and denatured proteins were dotted into the wells. Electrophoresis was performed at a constant voltage of 75 V for 130 min. Electrophoresis was terminated when bromophenol blue was electrophoresed at the bottom of the gel. After the transfer, the membrane was removed and washed once with 1 × PBST. Subsequently, we disposed the membrane for 1.5 h under PBST (5% skimmed milk) powder. Next, we added primary and secondary antibodies. A chemiluminescence imaging system (ChemiScope6100, Clinx, Shanghai, China) was used to calculate the protein samples on the membrane. β‐actin was used as an internal reference. Antibody information is presented in Table 2.
TABLE 2.
The information of antibody
| Name | Article number | Source | Dilution rate | Molecular weight | Transfer film time | Company |
|---|---|---|---|---|---|---|
| TNF‐α | 60,291‐1‐Ig | Mouse | 1:5000 | 17–22 KDa | 40 min | Proteintech (Chicago, IL, USA) |
| IL‐1β | ab254360 | Rabbit | 1:1000 | 30 KDa | 50 min | Abcam (Cambridge, UK) |
| IL‐6 | 21,865‐1‐AP | Rabbit | 1:500 | 20 KDa | 40 min | Proteintech (Chicago, IL, USA) |
| MMP‐3 | 17,873‐1‐AP | Rabbit | 1:1000 | 45–47 KDa | 70 min | Proteintech (Chicago, IL, USA) |
| MMP‐13 | 18,165‐1‐AP | Rabbit | 1:1000 | 65–70 KDa | 90 min | Proteintech (Chicago, IL, USA) |
| CollagenII | ab188570 | Rabbit | 1:5000 | 141 KDa | 150 min | Abcam (Cambridge, UK) |
| aggrecan | ab36861 | Rabbit | 1 μg/mL | 110 KDa | 130 min | Abcam (Cambridge, UK) |
| NLRP3 | ab263899 | Rabbit | 1:1000 | 118 KDa | 150 min | Abcam (Cambridge, UK) |
| Caspase‐1 | ab179515 | Rabbit | 1:1000 | 45/10/12 KDa | 75 min | Abcam (Cambridge, UK) |
| β‐Actin | 66,009‐1‐Ig | Mouse | 1:5000 | 42 KDa | 60 min | Proteintech (Chicago, IL, USA) |
| HRP goat anti‐Rabbit IgG | SA00001‐2 | Rabbit | 1:6000 | / | 90 min | Proteintech (Chicago, IL, USA) |
| HRP goat anti‐mouse IgG | SA00001‐1 | Mouse | 1:5000 | / | 90 min | Proteintech (Chicago, IL, USA) |
Statistical Analysis
Graphpad Priam (version 9.0; GraphPad Software, La Jolla, CA, USA) was used for statistical analysis. Kolmogorov–Smirnov test was used to analyze whether the data conformed to a normal distribution. The normally distributed continuous variables were represented as the mean ± standard deviation (X̅ ± SD). One‐way analysis of variance (ANOVA) was used to compare the data differences between multiple groups. P‐Value <0.05 was considered statistically significant.
Results
Histopathological Analysis of Vertebral Disc after FMT
First, we analyzed the histopathological findings of the vertebral discs. The cells in the vertebral disc tissue of the control group were normal and regular, and no lesions were observed. In the IDD group, the number of cells decreased, and the arrangement was disordered. The IDD + FMT group showed a therapeutic effect, and tissue damage was effectively alleviated (Figure 1A). The S‐O results showed the same tissue damage trend as the HE results. No damage was observed in the control group, and safranine coloration was uniform. The vertebral disc tissue in the IDD group was severely damaged, and safranin coloration was uneven. The treatment effect was observed in the IDD + FMT group, and tissue damage was observed between the control and IDD groups (Figure 1B). Therefore, FMT alleviated histopathological damage caused by IDD.
Fig. 1.
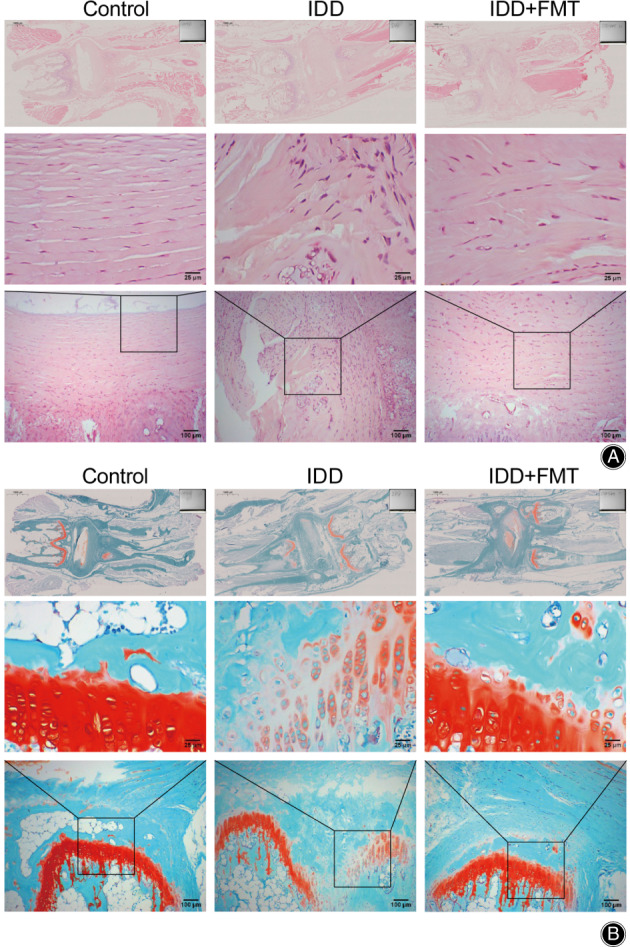
Histopathological analysis of vertebral disc after FMT. (A) A micrograph showing HE staining of rat intervertebral disc tissue. (Scale bar = 25/100/1000 μm). (B) A micrograph showing S‐O staining of rat intervertebral disc tissue. (Scale bar = 25/100/1000 μm). FMT, fecal microbiota transplantation.
FMT Changed the Levels of Inflammatory Factors in Rats
To investigate whether FMT affects inflammatory factors, we first assessed the expression of TNF‐α, IL‐1β, and IL‐6 in the rat serum and vertebral disc tissue. ELISA, quantitative real‐time polymerase chain reaction (qRT‐PCR), and western blotting showed that TNF‐α, IL‐1β, and IL‐6 were upregulated in the IDD group compared to the control group (Figure 2A‐C). FMT reversed the effects of IDD modeling on inflammatory factors and significantly downregulated their levels (Figure 2A‐C). Therefore, FMT led to the downregulation of inflammatory factors in IDD rats.
Fig. 2.
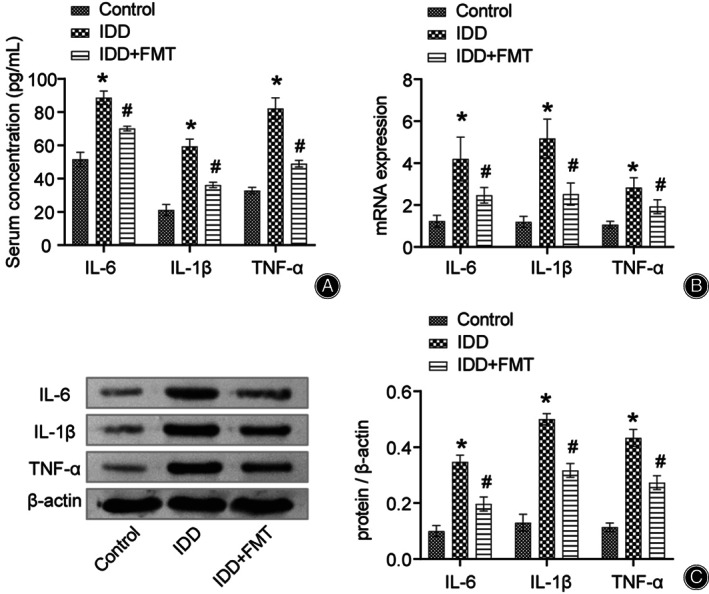
FMT alters inflammatory factor levels in rats. (A) Quantification of TNF‐α, IL‐1β, and IL‐6 in the rat serum. (B) Expression of TNF‐α, IL‐1β, and IL‐6 in rat intervertebral disc tissues. (C) Protein expression of TNF‐α, IL‐1β, and IL‐6 in the rat intervertesbral disc tissue. *P < 0.05 vs. control. #P < 0.05 vs. IDD. FMT, fecal microbiota transplantation; IDD, intervertebral disc degeneration.
FMT Altered Catabolic Enzyme (Matrix Metalloproteinase, MMP) and Extracellular Matrix (Collagen II and Aggrecan) Levels in the Vertebral Disc Tissue
Imbalance of the extracellular matrix in the vertebral disc tissue leads to problems such as abnormal stress in the vertebral disc and eventually causes IDD. Therefore, we investigated the effects of FMT on vertebral disc ECM homeostasis. We found that the levels of MMP‐3 and MMP‐13 in the IDD group were significantly upregulated by qRT‐PCR (Figure 3A). After FMT treatment, the levels of MMP‐3 and MMP‐13 decreased (Figure 3A). The changing trend of Collagen II and aggrecan was always opposite to that of MMP (Figure 3A). Additionally, we detected the above factors in the vertebral disc tissue by western blotting and ELISA (Figure 3B,C, respectively). The detection results were consistent with those of qRT‐PCR. Thus, FMT positively affects the maintenance of vertebral disc extracellular matrix homeostasis.
Fig. 3.
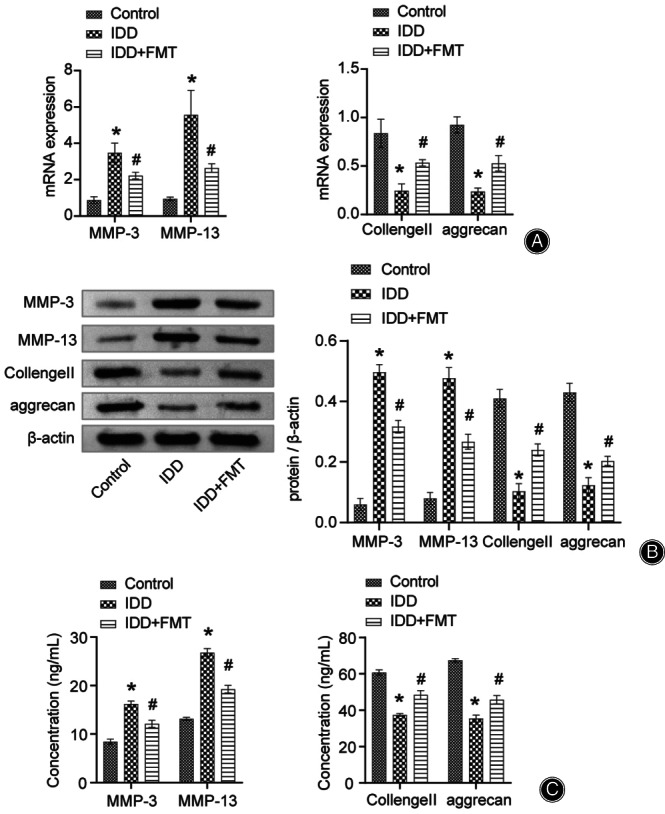
FMT altered catabolic enzymes and extracellular matrix levels in vertebral disc tissue. (A) qRT‐PCR data representing the expression of MMP‐3, MMP‐13, Collagen II, and aggrecan in rat intervertebral disc tissue. (B) Western blot data representing the protein expression of MMP‐3, MMP‐13, Collagen II, and aggrecan in rat intervertebral disc tissue. (C) ELISA data representing the quantification of MMP‐3, MMP‐13, Collagen II, and aggrecan in rat serum. *P < 0.05 vs. control. #P < 0.05 vs. IDD. FMT, fecal microbiota transplantation; IDD, intervertebral disc degeneration; qRT‐PCR, quantitative real‐time polymerase chain reaction; MMP, matrix metalloproteinase.
FMT Changed the Level of Pyroptosis Factor in Vertebral Disc Tissue
Next, the influence of FMT on vertebral disc tissue pyroptosis factor was examined. We assessed changes in pyroptotic factors (NLRP3 and Caspase‐1) in vertebral disc tissues using qRT‐PCR, western blotting, and IHC. The changes in the levels of pyroptosis factors in the three assays were consistent (Figure 4A‐D). IDD modeling experiments upregulated the expression of NLRP3 and Caspase‐1. However, FMT alleviated the modeling damage and significantly downregulated the levels of these factors. Therefore, FMT also has a positive effect on the alleviation of pyroptosis in IDD.
Fig. 4.
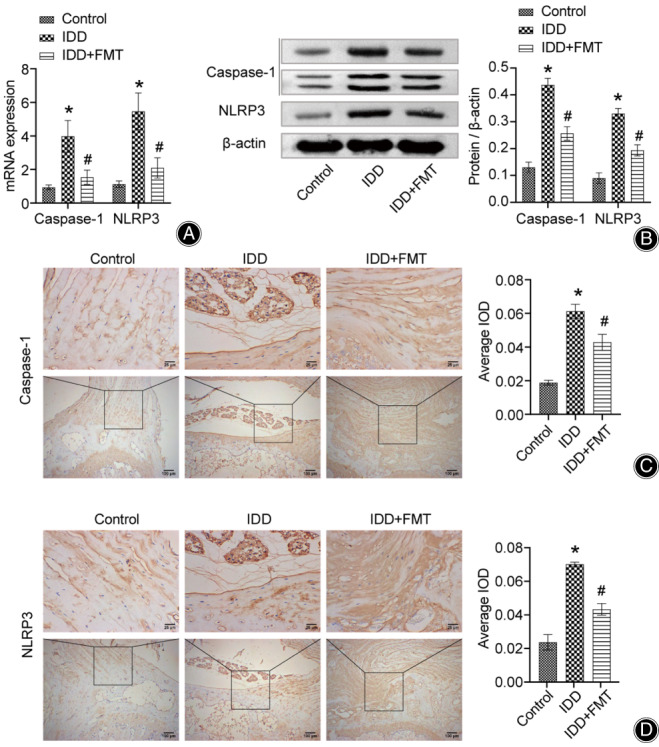
FMT changed the level of pyroptosis in vertebral disc tissue. (A) Expression of Caspase‐1 and NLRP3 in rat intervertebral disc tissues. (B) Protein expression of Caspase‐1 and NLRP3 in rat intervertebral disc tissue. (C) Micrograph showing IHC staining for Caspase‐1 in rat intervertebral disc tissue. (Scale bar = 25/100 μm). (D) Micrograph showing IHC staining of NLRP3 in rat intervertebral disc tissues. (Scale bar = 25/100 μm). *P < 0.05 vs. control. #P < 0.05 vs. IDD. FMT, fecal microbiota transplantation; IDD, intervertebral disc degeneration; IHC, immunohistochemical.
Changes in Gut Microbiota after FMT
Finally, we performed 16 S rRNA gene sequencing of rat feces. First, we used a Venn diagram to visualize the common amplicon sequence variants (ASVs) in both groups (Figure 2A). We found 742 ASVs specific to the control, 539 ASVs specific to the IDD, 501 ASVs specific to the IDD + FMT, 868 ASVs common to the controls and IDD, and 1005 ASVs common to the IDD and IDD + FMT (Figure 5A). The histogram shows that the genus level microbes mainly included Muribaculaceae, Lactobacillus, Clostridia_UCG‐014, Prevotellaceae Ga6A1 group, Lachnospiraceae, Ruminococcus, Lachnospiraceae NK4A136 group, UCG‐005, Clostridium sensu stricto 1, Bacilli, Treponema, Monoglobus, Romboutsia, RF39, Prevotella, Oscillospiraceae, Turicibacter, [Eubacterium] coprostanoligenes group, Ruminococcaceae, and Roseburia (Figure 5B).
Fig. 5.
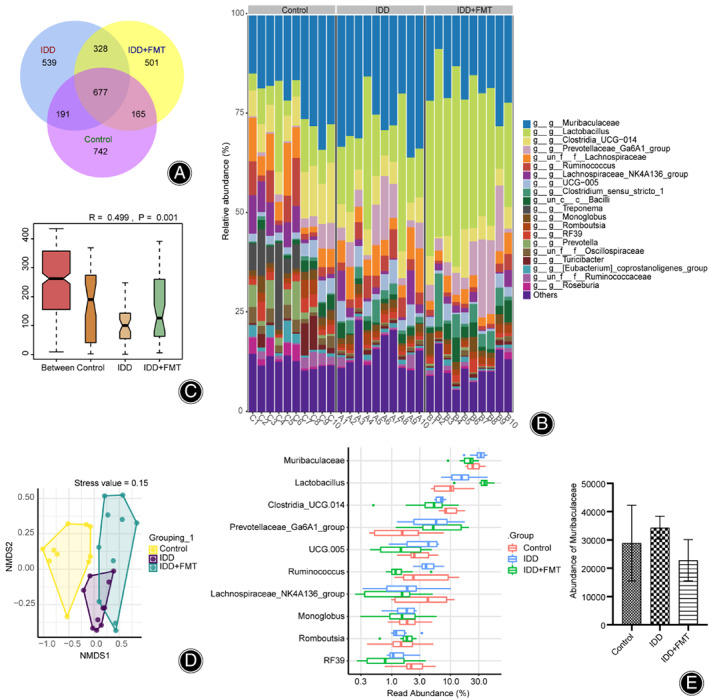
Changes in gut microbiota after FMT. (A) Venn diagram. The number on the petal represents the number of ASVs unique to the sample. (B) Histogram of the relative abundance of top 20 ASVs at the genus level. (C) Anosim analyses. This was used to analyze differences between groups. (D) NMDS index shows the differences between groups. E. Read abundance of top 10 dominant flora at the genus level. FMT, fecal microbiota transplantation; ASV, amplicon sequence variant; NMDS, non‐metric multidimensional analysis.
IDD modeling experiments upregulated the relative abundance of Muribaculaceae. FMT down‐regulated this relative abundance. Next, we explored differences in community structure among the groups. The obtained value (R) = 0.263, and P = 0.001 indicated that there was a significant difference between the groups (Figure 5C). Non‐metric multidimensional analysis (NMDS) revealed differences among the control, IDD, and IDD + FMT groups (Figure 5D). Figure 5E shows the top 10 dominant flora with relative abundances at the genus level. IDD increased the abundance of Lactobacillus and decreased that of ClostridiaUCG‐014. FMT further increased the abundance of Lactobacillus and decreased that of Clostridia_UCG‐014. Thus, FMT increased the gut microbiota diversity and microbial abundance in rats treated with IDD.
Discussion
IDD is a common musculoskeletal disorder that causes heavy socioeconomic burden. 31 , 32 Unfortunately, current diagnostic techniques and treatments do not reduce related pain. 33 , 34 Therefore, we attempted to treat the IDD rat model using FMT, and explore FMT's potential role in IDD. In this work, we studied the effects of FMT on histopathological damage, gut microbiota, inflammation, ECM homeostasis, and pyroptosis. We found that the expression of inflammatory factors (TNF‐α, IL‐1β, and IL‐6), catabolic enzyme (MMP‐3 and MMP‐13), and pyroptotic factors (NLRP3 and Caspase‐1) increased in the IDD group rats. In contrast, extracellular matrix (Collagen II and aggrecan) levels were downregulated. Additionally, vertebral disc tissue was severely damaged in the IDD group, with disordered cell arrangement and uneven safranin coloration. FMT reversed the effects of IDD modeling on these factors and alleviated cartilage tissue damage. Moreover, we also found that FMT increased the gut microbiota diversity and microbial abundance in rats with IDD.
FMT Positively Affects the Maintenance of Vertebral Disc ECM Homeostasis
A key factor in IDD degeneration is how NPs sense and respond to the disease‐causing signals. For example, acid‐sensing ion channel (ASIC) 1/3 induces pyroptosis and NLRP3 inflammasome activation by upregulating ROS/NF‐κB signaling. 35 We found that the levels of catabolic enzymes (matrix metalloproteinases [MMPs] ‐3 and MMP‐13) were upregulated and the levels of ECM (Collagen II and aggrecan) were downregulated in the vertebral disc tissue of IDD rats. FMT treatment reversed this trend. MMPs play an important role in mediating ECM disruption in cells. 36 They degrade various ECM proteins. 37 M2a macrophages promote ECM metabolic imbalance in NP cells, with enhanced transcription of Collagen II and aggrecan and decreased expression of MMP‐3 and MMP‐9. 38 Three interacting events cause IDD degeneration: loss of NPs, ECM dehydration, and decreased cellular function. 11 , 12 , 13 , 14 The loss of localized cells was attributed to a reduction in matrix components, such as Collagen II, with a concomitant loss of water from hyaluronic acid aggregated into proteoglycans. The reduction in these components is associated with the replacement of the matrix by other fibers and proteoglycans. 39 , 40 Therefore, FMT positively affects the maintenance of vertebral disc ECM homeostasis.
FMT Alleviated Inflammation and Pyroptosis in IDD Rats
In the IDD group, the expression of inflammatory factors (TNF‐α, IL‐1β, and IL‐6) and pyroptosis factors (NLRP3 and Caspase‐1) increased, and FMT reversed these changes. Previous studies have shown that the expression of TNF‐α, IL‐1β, and IL‐6 decreases when inflammation is decreased. 41 Ethanol treatment of hepatocytes results in TXNIP overexpression, activation of the NLRP3 inflammasome, and Caspase‐1‐mediated pyroptosis. 42 Pyroptosis is an inflammatory form of regulatory cell death (RCD). 43 NLRP3‐mediated pyroptosis has been implicated in disc degeneration. 44 NLRP3 inflammasome is thought to contribute to the induction of pyroptosis via TXNIP. 45 The major destructive effect of pyroptosis depends on the release of inflammatory factors IL‐1β and IL‐18. 46 When pattern recognition receptors recognize damage‐associated molecular patterns, the inflammasome activates Caspase‐1 followed by the activation of IL‐1β, IL‐18, and gasdermin‐D (GSDMD). 47 Thus, FMT alleviated inflammation and pyroptosis in IDD rats.
Changes in Gut Microbiota after FMT
Although fecal microbiota supernatants contain pro‐inflammatory metabolites, they can be removed by repeated washing. 48 The synergistic action of phages and host bacteria after FMT restored the recipient gut microbiota. 49 FMT prevented the decline in endogenous insulin production in patients newly diagnosed with T1D. Several microbiota are associated with preserved residual β‐cell function. 50 We found that IDD increased the abundance of Muribaculaceae and Lactobacillus, and decreased the abundance of Clostridia_UCG‐014. FMT further increased the abundance of Lactobacillus and decreased that of Clostridia_UCG‐014. These studies demonstrate that IDD may have a potential therapeutic effect through the auxiliary metabolism of gut microbes.
Limitations and Strengths of the Study
The advantage of this study were: (i) FMT maintained cellular stability, alleviated histopathological damage; and (ii) FMT influenced the gut microbiota in IDD rats. FMT may serve as a promising target for ameliorating IDD and for palliative therapy.
However, several limitations of this study must be considered. Owing to the limitations of experimental funds and conditions, we did not conduct experiments to explore the influence of specific components of fecal bacteria on IDD. In addition, the maintenance time of FMT treatment effect on IDD needs to be further explored.
Conclusion
These findings suggest that FMT has a positive effect on maintaining cellular stability and alleviating histopathological damage. FMT decreased level of inflammatory factors, catabolic enzyme, and pyroptotic factors, and increased extracellular matrix levels. Besides, FMT alleviated the injury of intervertebral disc tissue in IDD rats. In addition, we found that FMT affected the gut microbiota diversity and microbial abundance in IDD rats. Therefore, FMT may serve as a promising target for ameliorating IDD and palliative therapy.
Ethics Statement
All animal experiments were approved by the Animal Ethics Committee of Xiangya Third Hospital of Central South University (No.2020‐S292).
Funding
This study was supported by the Hunan Health Commission's 2019 Medical Service and Support Capability Improvement Subsidy Fund (No. 2019‐57) and General Items of the Hunan Provincial Health Commission (No. 202204074627).
Author Contributions
Ziwei Han and Li Wan conceived the design; Bo Yao, Youquan Cai, and Weiguo Wang carried out the experiment; Jia Deng, Lei Zhao, Ziwei Han, and Li Wan carried out the data visualization. Bo Yao wrote the first draft, and Ziwei Han and Li Wan directed the revisions. All authors have checked the final manuscript.
Contributor Information
Ziwei Han, Email: studiobabyfish@163.com.
Li Wan, Email: 718685@wwhospital.com.
References
- 1. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990‐2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;386(9995):743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Battié MC, Videman T, Parent E. Lumbar disc degeneration: epidemiology and genetic influences. Spine (Phila Pa 1976). 2004;29(23):2679–90. [DOI] [PubMed] [Google Scholar]
- 4. Nakamichi R, Ito Y, Inui M, Onizuka N, Kayama T, Kataoka K, et al. Mohawk promotes the maintenance and regeneration of the outer annulus fibrosus of intervertebral discs. Nat Commun. 2016;7:12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohnishi T, Iwasaki N, Sudo H. Causes of and molecular targets for the treatment of intervertebral disc degeneration: a review. Cell. 2022;11(3):394–410. 10.3390/cells11030394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shapiro IM, Vresilovic EJ, Risbud MV. Is the spinal motion segment a diarthrodial polyaxial joint: what a nice nucleus like you doing in a joint like this? Bone. 2012;50(3):771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–4. [DOI] [PubMed] [Google Scholar]
- 8. Freemont AJ, Watkins A, Le Maitre C, et al. Current understanding of cellular and molecular events in intervertebral disc degeneration: implications for therapy. J Pathol. 2002;196(4):374–9. [DOI] [PubMed] [Google Scholar]
- 9. Cherif H, Bisson DG, Mannarino M, Rabau O, Ouellet JA, Haglund L. Senotherapeutic drugs for human intervertebral disc degeneration and low back pain. Elife. 2020;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lyu FJ, Cui H, Pan H, MC Cheung K, Cao X, Iatridis JC, et al. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Res. 2021;9(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ji ML, Jiang H, Zhang XJ, Shi PL, Li C, Wu H, et al. Preclinical development of a microRNA‐based therapy for intervertebral disc degeneration. Nat Commun. 2018;9(1):5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang B, He Y, Li S, Wei X, Liu J, Shan Z, et al. The RCAN1.4‐calcineurin/NFAT signaling pathway is essential for hypoxic adaption of intervertebral discs. Exp Mol Med. 2020;52(5):865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Che H, Li J, Li Y, Ma C, Liu H, Qin J, et al. p16 deficiency attenuates intervertebral disc degeneration by adjusting oxidative stress and nucleus pulposus cell cycle. Elife. 2020;9:e52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li ZJ, Choi DK, Sohn KC, Seo MS, Lee HE, Lee Y, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134(11):2747–56. [DOI] [PubMed] [Google Scholar]
- 16. Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Munawar N, Ahsan K, Muhammad K, Ahmad A, Anwar MA, Shah I, et al. Hidden role of gut microbiome dysbiosis in schizophrenia: antipsychotics or Psychobiotics as therapeutics? Int J Mol Sci. 2021;22(14):7671–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zaiss MM, Joyce Wu HJ, Mauro D, Schett G, Ciccia F. The gut‐joint axis in rheumatoid arthritis. Nat Rev Rheumatol. 2021;17(4):224–37. [DOI] [PubMed] [Google Scholar]
- 19. Li W, Lai K, Chopra N, Zheng Z, das A, Diwan AD. Gut‐disc axis: a cause of intervertebral disc degeneration and low back pain? Eur Spine J. 2022;31(4):917–25. [DOI] [PubMed] [Google Scholar]
- 20. Rajasekaran S, Soundararajan DCR, Tangavel C, Muthurajan R, Sri Vijay Anand KS, Matchado MS, et al. Human intervertebral discs harbour a unique microbiome and dysbiosis determines health and disease. Eur Spine J. 2020;29(7):1621–40. [DOI] [PubMed] [Google Scholar]
- 21. Wang JW, Kuo CH, Kuo FC, Wang YK, Hsu WH, Yu FJ, et al. Fecal microbiota transplantation: review and update. J Formosan Med Assoc. 2019;118(Suppl 1):S23–s31. [DOI] [PubMed] [Google Scholar]
- 22. Li C, Pi G, Li F. The role of intestinal Flora in the regulation of bone homeostasis. Front Cell Infect Microbiol. 2021;11:579323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Korpela K, Helve O, Kolho KL, Saisto T, Skogberg K, Dikareva E, et al. Maternal fecal microbiota transplantation in cesarean‐born infants rapidly restores Normal gut microbial development: a proof‐of‐concept study. Cell. 2020;183(2):324–334.e325. [DOI] [PubMed] [Google Scholar]
- 24. Dai M, Liu Y, Chen W, Buch H, Shan Y, Chang L, et al. Rescue fecal microbiota transplantation for antibiotic‐associated diarrhea in critically ill patients. Crit Care. 2019;23(1):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Freidin MB, Wells P, Stalteri M, Williams FMK. Pilot analysis of association between gut microbiome and modic changes. Orthop Proc. 2019;101‐B(SUPP_10):1–1. [Google Scholar]
- 26. Liao Z, Luo R, Li G, Song Y, Zhan S, Zhao K, et al. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics. 2019;9(14):4084–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu X, Liu Y, Guo X, Zhou W, Wang L, Shi J, et al. Prolactin inhibits the progression of intervertebral disc degeneration through inactivation of the NF‐κB pathway in rats. Cell Death Dis. 2018;9(2):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liao Z, Li S, Liu R, Feng X, Shi Y, Wang K, et al. Autophagic degradation of Gasdermin D protects against nucleus pulposus cell Pyroptosis and retards intervertebral disc degeneration In vivo. Oxid Med Cell Longev. 2021;2021:5584447–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang D, Shao S, Zhang Y, Zhao D, Wang M. Insight into polysaccharides from Panax ginseng C. A. Meyer in improving intestinal inflammation: modulating intestinal microbiota and autophagy. Front Immunol. 2021;12:683911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xi S, Wang Y, Wu C, Peng W, Zhu Y, Hu W. Intestinal epithelial cell exosome launches IL‐1β‐mediated neuron injury in sepsis‐associated encephalopathy. Front Cell Infect Microbiol. 2021;11:783049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cieza A, Causey K, Kamenov K, Hanson SW, Chatterji S, Vos T. Global estimates of the need for rehabilitation based on the global burden of disease study 2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2021;396(10267):2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990‐2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Binch ALA, Fitzgerald JC, Growney EA, Barry F. Cell‐based strategies for IVD repair: clinical progress and translational obstacles. Nat Rev Rheumatol. 2021;17(3):158–75. [DOI] [PubMed] [Google Scholar]
- 34. Foster NE, Anema JR, Cherkin D, Chou R, Cohen SP, Gross DP, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018;391(10137):2368–83. [DOI] [PubMed] [Google Scholar]
- 35. Zhao K, An R, Xiang Q, Li G, Wang K, Song Y, et al. Acid‐sensing ion channels regulate nucleus pulposus cell inflammation and pyroptosis via the NLRP3 inflammasome in intervertebral disc degeneration. Cell Prolif. 2021;54(1):e12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cirillo N, Prime SS. A scoping review of the role of metalloproteinases in the pathogenesis of autoimmune pemphigus and pemphigoid. Biomolecules. 2021;11(10):1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amar S, Minond D, Fields GB. Clinical implications of compounds designed to inhibit ECM‐modifying metalloproteinases. Proteomics. 2017;17(23‐24). [DOI] [PubMed] [Google Scholar]
- 38. Li L, Wei K, Ding Y, Ahati P, Xu H, Fang H, et al. M2a macrophage‐secreted CHI3L1 promotes extracellular matrix metabolic imbalances via activation of IL‐13Rα2/MAPK pathway in rat intervertebral disc degeneration. Front Immunol. 2021;12:666361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yee A, Lam MP, Tam V, et al. Fibrotic‐like changes in degenerate human intervertebral discs revealed by quantitative proteomic analysis. Osteoarthr Cartil. 2016;24(3):503–13. [DOI] [PubMed] [Google Scholar]
- 40. Erwin WM, DeSouza L, Funabashi M, Kawchuk G, Karim MZ, Kim S, et al. The biological basis of degenerative disc disease: proteomic and biomechanical analysis of the canine intervertebral disc. Arthritis Res Ther. 2015;17(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Zeng Y. Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4 /MyD88/NF‐κB signal pathway. Drug Dev Res. 2019;80(3):353–9. [DOI] [PubMed] [Google Scholar]
- 42. Heo MJ, Kim TH, You JS, Blaya D, Sancho‐Bru P, Kim SG. Alcohol dysregulates miR‐148a in hepatocytes through FoxO1, facilitating pyroptosis via TXNIP overexpression. Gut. 2019;68(4):708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang Y, et al. Pyroptosis: a new frontier in cancer. Biomed Pharmacother. 2020;121:109595. [DOI] [PubMed] [Google Scholar]
- 44. Zhang J, Zhang J, Zhang Y, Liu W, Ni W, Huang X, et al. Mesenchymal stem cells‐derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. J Cell Mol Med. 2020;24(20):11742–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. He D, Zhou M, Bai Z, Wen Y, Shen J, Hu Z. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell pyroptosis via NLRP3‐dependent pathway. Biochem Biophys Res Commun. 2020;526(3):772–9. [DOI] [PubMed] [Google Scholar]
- 46. Tang G, Han X, Lin Z, Qian H, Chen B, Zhou C, et al. Propionibacterium acnes accelerates intervertebral disc degeneration by inducing Pyroptosis of nucleus pulposus cells via the ROS‐NLRP3 pathway. Oxid Med Cell Longev. 2021;2021:4657014–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kamali A, Ziadlou R, Lang G, Pfannkuche J, Cui S, Li Z, et al. Small molecule‐based treatment approaches for intervertebral disc degeneration: current options and future directions. Theranostics. 2021;11(1):27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang T, Lu G, Zhao Z, Liu Y, Shen Q, Li P, et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell. 2020;11(4):251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fujimoto K, Kimura Y, Allegretti JR, Yamamoto M, Zhang YZ, Katayama K, et al. Functional restoration of bacteriomes and Viromes by fecal microbiota transplantation. Gastroenterology. 2021;160(6):2089–2102.e2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Groot P, Nikolic T, Pellegrini S, Sordi V, Imangaliyev S, Rampanelli E, et al. Faecal microbiota transplantation halts progression of human new‐onset type 1 diabetes in a randomised controlled trial. Gut. 2021;70(1):92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]


