Abstract
Recent guidelines have produced a consensus statement for perioperative care in hip and knee replacement. However, there is still a need for reanalysis of the evidence and recommendations. Therefore, we retrieved and reanalyzed the evidence of each recommended components of enhanced recovery after surgery (ERAS) based on the guidelines of total joint arthroplasty. For each one, we included for the highest levels of evidence and those systematic reviews and meta‐analyses were preferred. The full texts were analyzed and the evidence of all components were summarized. We found that most of the recommended components of ERAS are supported by evidence, however, the implementation details of each recommended components need to be further optimized. Therefore, implementation of a full ERAS program may maximize the benefits of our clinical practice but this combined effect still needs to be further determined.
Keywords: Enhanced recovery after surgery, Evidence‐based review, Perioperative care, Total hip arthroplasty, Total knee arthroplasty
Key Elements of Enhanced Recovery after TJA.
Introduction
Enhanced recovery after surgery (ERAS) aims to standardize perioperative management and improve clinical outcomes and has been most widely developed in orthopedic surgery in recent years. Implementation of the ERAS pathway enables faster and more efficient recovery in total joint arthroplasty (TJA). Studies 1 , 2 have shown that the adoption of ERAS significantly reduces the mortality rate, transfusion rate, incidence of complications, and length of stay (LOS) in patients with TJA without influencing the 30‐day readmission rate. Additionally, a recent guideline 3 described ERAS for total hip replacement and total knee replacement surgery.
The adoption of the ERAS pathway has spread informally, although there have been some notable coordinated initiatives. Currently, the guidelines vary by specialty but include at least 17 elements in TJA, 3 which were recommended by the ERAS Society; they are categorized into preoperative, intraoperative, and postoperative components. The interventions were listed in Table 1. However, the current and updated evidence and recommendations of the recommended components still needs to be further analyzed. 4 In addition, the evidence for modern practices and updated recommendations are barely reviewed in previous studies. 5 , 6 Thus, the aim of the present narrative review is to appraise each of the recommended components of ERAS in TJA, add the highest level of evidence of each recommendations, optimize their usage in clinical scenarios, and provide a basis for future clinical trials.
TABLE 1.
Recommended interventions for the perioperative care of hip and knee replacement studies by ERAS Society
| Number | Item | Evidence level |
|---|---|---|
| 1 | Preoperative information, education and counseling | Low |
| 2 | Preoperative optimization | Smoking: High; Alcohol: Low; Anemia: High |
| 3 | Preoperative fasting | Moderate |
| 4 | Standard anesthetic protocol | General anesthesia: moderate; neuraxial techniques: Moderate |
| 5 | Use of local anesthetics for infiltration analgesia and nerve blocks | LIA in knee replacement: High |
| 6 | Postoperative nausea and vomiting | Moderate |
| 7 | Prevention of perioperative blood loss | Tranexamic acid: High |
| 8 | Perioperative oral analgesia | Paracetamol: Moderate; NSAIDS: High |
| 9 | Maintaining normothermia | High |
| 10 | Antimicrobial prophylaxis | Moderate |
| 11 | Antithrombotic prophylaxis treatment | Moderate |
| 12 | Perioperative surgical factors | High |
| 13 | Perioperative fluid management | Fluid balance: Moderate |
| 14 | Postoperative nutritional care | Low |
| 15 | Early mobilization | Moderate |
| 16 | Criteria‐based discharge | Low |
| 17 | Continuous improvement and audit | Low |
Abbreviations: LIA: local infiltration analgesia; NSAIDs: non‐steroidal anti‐inflammatory drugs.
Methods
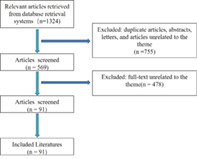
To obtain an overview of evidence‐based interventions utilized in ERAS programs for TJA, we searched the recommended components of ERAS combined with total hip or total knee arthroplasty in PubMed and Medline databases. For each component, a search was performed for the highest levels of evidence. The recommended interventions are listed in Table 1. We searched for English language studies published prior to September 2022. We included landmark studies, randomized controlled trials (RCTs), systematic review, and meta‐analysis that evaluated the influence of single or multiple items of the ERAS guidelines over meaningful clinical endpoints. Duplicate publications, case reports, editorials, and reviews were excluded. Two authors (CJ C and Y L) independently assessed the eligibility of articles for inclusion in the study. Inconsistencies were adjudicated by the senior author (PD K) until consensus was obtained. Then, the full texts were analyzed and the evidence of each of the components were summarized.
Results
Preadmission Phases
Preoperative Information, Education, and Expectation Counseling
Preoperative information enables patients to obtain the right information and support beforehand. The guidelines recommended preoperative patient education. 3 It has been found to not only reduce preoperative anxiety but also offer benefits in terms of pain, function, and adverse events. 7 Preoperative patient education or counseling has been widely used in recent ERAS protocols, including the implementation of specific joint and/or lung function exercises and setting a clear expectation for LOS. Furthermore, preoperative patient education and exercise have been shown to independently affect postoperative outcomes such as pain, function, and LOS. 8 Another two studies 9 , 10 found that changing patient expectations of LOS had a positive effect on LOS, which can result in discharging patients earlier from the hospital. Though the evidence level is still low, preoperative patient education should be performed in terms of perioperative exercise, rehabilitation, and expectations of LOS to reduce patient anxiety and facilitate discharge from the hospital.
Preoperative Phases
Preoperative Optimization
The guidelines recommended smoking cessation 4 weeks or more, alcohol cessation, and preoperative anemia correction. 3 Preoperative risk factors, such as smoking, alcohol consumption, and anemia, and low physical activity, remains prevalent in orthopedic surgery, which may lead to complications, including poor wound healing, myocardial infarction, cardiac arrest, pneumonia, urinary tract infection, sepsis, acute renal failure, and mortality. 11 , 12 , 13 Optimizing these risk factors may benefit a large proportion of TJA patients. As for evidence, one systematic review 14 found that the use of recombinant human erythropoietin and oral iron supplementation can reduce transfusion requirements and LOS and found that it is cost effective in THA. One RCT 15 on hip and knee replacement found a benefit of a smoking intervention program 6–8 weeks before surgery, which can reduce postoperative morbidities and the median length of stay. However, no studies have assessed alcohol cessation alone in TJA. A large retrospective study 16 found that alcohol misuse was associated with longer hospital stays and a higher possibility of medical‐ and surgery‐related complications in hip and knee replacement patients. Though the overall level of evidence is low, preoperative optimization may protect against postoperative complications even LOS.
Preoperative Fasting
The guidelines recommended intake of clear fluids until 2 hours before the induction of anesthesia, and a 6‐hour fast for solid food. 3 Preoperative fasting is conducted for reducing the risk of aspiration at anesthesia, however, prolonged fasting may induce catabolism and a surgical stress response, and lead to insulin resistance, hyperglycemia, and muscle breakdown. 17 Shortened fasting time can increase patients' postoperative comfort, improve insulin resistance, and reduce stress responses. 18 According to the updated guidelines for many operations, 19 fasting has changed from overnight to allowing carbohydrate drinks 2 hours before surgery. However, few studies have assessed carbohydrate loading or fasting alone in TJA. Though the direct evidence for fasting in TJA is lacking, preoperative fasting should be specifically arranged according to the guidelines and each patient's surgery time.
Perioperative Oral Analgesia
Perioperative oral analgesia is one of the cornerstones of exemplar ERAS. The guidelines recommended routine use of paracetamol, Non‐steroidal anti‐inflammatory drugs (NSAIDs), and oxycodone, however, gabapentinoids are not currently recommended. 3 One RCT 20 on TKA found that preoperative oral rofecoxib can reduce opioid consumption, pain, vomiting, and sleep disturbance, with improved knee range of motion. Another RCT 21 found that preoperative celecoxib exhibits better analgesia efficacy than postoperative administration in TKA. Although ERAS programs emphasize minimizing the use of opioids postoperatively to minimize side effects, opioids are still effective in reducing acute and chronic moderate‐to‐severe pain after surgery. One RCT 22 found that morphine/oxycodone offers an attractive alternative to oxycodone/ acetaminophen for the management of moderate‐to‐severe postoperative pain. Though currently not recommended, perioperative pregabalin can result in improved pain and decreased opioid consumption for 1 week after hospital discharge for THA surgery. 23 Thus, though low overall level of evidence is existing, the benefit of perioperative oral analgesia is consistent.
During Surgery Phases
Standard Anesthetic Protocol
The guidelines recommended for routine use of epidural analgesia and moderately recommended use of general anesthesia and neuraxial techniques. 3 A standardized anesthetic protocol is a core component of TJA within the ERAS pathway and the techniques vary. Spinal anesthesia is widely preferred. A recent meta‐analysis 24 found that spinal anesthesia was associated with a significantly reduced occurrence of nausea and length of hospital stay compared with general anesthesia. And a network meta‐analysis 25 compared the efficiency of various interventions for postoperative pain management in THA and found that spinal anesthesia is the best intervention to reduce pain in the first 24 hours; lumbar plexus block is a better choice to reduce pain 12 to 48 hours postoperatively, and neuraxial techniques have become commonplace in TJA. 3 A meta‐analysis 26 and systematic review 27 have found neuraxial anesthesia have reduced length of stay and surgical site infections compared with general anesthesia, although some of the results are conflicting. Thus, the benefits of epidural analgesia are consistent, however, the effects of neuraxial anesthesia still need to be further determined.
Use of Local Anesthetics for Infiltration Analgesia and Nerve Blocks
The guidelines recommended use of local infiltration analgesia (LIA) for knee replacement but not for hip replacement, while nerve blocks are not recommended as an essential ERAS component. 3 LIA has an advantage over nerve blocks without motor blockade, which enables earlier ambulation. 3 A meta‐analysis 28 published in 2018 demonstrated that local infiltration analgesia can significantly reduce early perioperative pain and total narcotic consumption compared with placebo; however, the pain‐relieving effect was short in duration. Furthermore, a meta‐analysis 29 published in 2020 found that local infiltration provided superior analgesia and morphine‐sparing effects than spinal (intrathecal) analgesia within the first 72 hours. A systematic review 30 found that regional blocks in THA can reduce postoperative pain, morphine consumption, and nausea and vomiting without influencing length of stay or rehabilitation. Thus, use of LIA for pain control is beneficial, while whether use of nerve blocks should consider its effects on motor blockade.
Prevention of Perioperative Blood Loss
The guidelines recommended use of tranexamic acid (TXA) to reduce perioperative blood loss and the requirement for postoperative allogenic blood transfusion. 3 TKA and THA have been associated with pronounced blood loss, and the resultant anemia can lead to severe complications, such as higher rates of postoperative infection, slower physical recovery, increased length of hospital stay, and increased morbidity and mortality. 31 In addition, postoperative hidden blood loss after TKA is one of the factors related to lower limb swelling. 32 Nowadays, TXA is increasingly used in total hip and knee arthroplasties, and it has been found that TXA is safe and effective for reducing the need for blood transfusions without increasing associated complications, irrespective of patient high‐risk status at baseline. 33 Moreover, several recent meta‐analyses 34 , 35 , 36 , 37 , 38 have demonstrated that topical, intravenous, and oral TXA all have similar positive effects in reducing total blood loss and the need for blood transfusions without increasing the risk of postoperative complications, such as thromboembolic events, both in TKA and THA. The evidence level of use of TXA in TJA is high, and it should be therefore used.
Maintaining Normothermia
The guidelines recommended to maintain normal body temperature peri‐ and postoperatively through pre‐warming and the active warming of patients intraoperatively.3 Perioperative hypothermia is a well‐known risk factor for postoperative complications, especially in the elderly, as it might cause coagulation and platelet function abnormalities, increased cardiac morbidity, surgical site infection, and pressure ulcer incidence levels. Research suggests that maintaining normothermia helps reduce blood loss and shortens the discharge time. 39 , 40 Among active perioperative hypothermia prevention strategies, forced‐air warming and fluid warming were the most commonly mentioned. Active warming prevents perioperative hypothermia, of which a forced‐air warming system has been shown to be an effective way to reduce perioperative hypothermia. 41 , 42 Although some scholars 43 have concerns regarding the infection risk associated with the forced air‐warming system, a recent RCT 44 found that forced air warming results in a similar number of surgical site infections compared with resistive fabric warming; both are similarly effective at maintaining normothermia. Moreover, an RCT 45 and meta‐analysis 46 found that the air‐free warming system and reflective blanket were as effective as forced air warming devices in maintaining normothermia. No meta‐analysis has investigated fluid warming in TJA, although one RCT 47 found that warming infusion could reduce the incidence of perioperative hypothermia and improve outcomes (such as shorter time to spontaneous breathing, eye opening, consciousness recovery, and extubation, and a decreased incidence of shivering and postoperative cognitive dysfunction) in the elderly during bilateral hip replacement. Though evidence level is comparatively low, maintaining normothermia via varied methods during the surgery is beneficial for TJA patients.
Antimicrobial Prophylaxis and Skin Preparation
The guidelines recommended to receive systemic antimicrobial prophylaxis for TJA patients. 3 Infection after hip and knee replacement is an infrequent yet serious complication, of which antimicrobial prophylaxis plays an important role in reducing the rate of infections. Currently, no universally defined guidelines have been released for antibiotic/antiseptic prophylaxis in TJA. In current orthopedic surgery, an antimicrobial prophylaxis regimen is routinely used to fight against the most likely contaminating microorganisms. A recent meta‐analysis 48 found no differences in infection risk for orthopedic procedures where implants are utilized between single and multiple doses of prophylactic antibiotics, which is in line with the recent guidelines issued by the Centers for Disease Control and Prevention (CDC). Similarly, another meta‐analysis 49 confirmed the benefit of surgical antibiotic prophylaxis utilization in total joint arthroplasty and found that the added benefit of postoperative surgical antibiotic prophylaxis or continuation beyond 24 hours was not evident. Regarding the efficiency of different antimicrobial prophylaxis regimens, one prospective study 50 evaluated different strategies in patients undergoing TJA and regimens including cefazolin, cefuroxime, or vancomycin, alone or combined with gentamicin. They found that the evaluated regimens showed bactericidal activity and achieved plasma levels above the minimum inhibitory concentrations in almost all of the intraoperative isolates, of which cefazolin and gentamicin‐containing regimens had higher serum bactericidal titers. In addition, another two systematic reviews 51 , 52 revealed a significant protective effect of intrawound antibiotic powder, such as vancomycin, for the prevention of surgical site infection with low‐quality evidence. However, in TKA, antibiotic‐loaded bone cement was not recommended because one systematic review 53 found that antibiotic‐loaded bone cement rendered limited improvement in terms of the rate of deep or superficial surgical site infection compared with plain bone cement. Furthermore, skin preparation prior to surgery is also important to prevent surgical site infections. One RCT found that patients who received alcohol and povidone‐iodine before draping have a significantly reduce rate of surgical site infection compared with patients who received only a single alcohol and povidone‐iodine before draping. Another two meta‐analyses 54 , 55 supported preoperative bathing with chlorhexidine kin preparation in TJA to reduce the risk of infection, the incidence of revision surgery, or the length of stay. The antimicrobial prophylaxis and skin preparation to prevent infection is beneficial, however, these recommendations can be impaired due to the overall low quality of evidence. In order to reduce the risks of infection after TJA, patients should receive systemic antimicrobial prophylaxis on the base of proper skin preparation.
Perioperative Surgical Factors
Surgical Approach and Technique
Different surgical approaches may influence surgical outcomes, complications, and recovery, of which minimally invasive surgery may have putative benefits, such as reductions in stress and pain. 3 However, the preferred surgical approach was not highlighted or recommended in the guidelines due to the inconclusive evidence. 3 As for TKA, a recent meta‐analysis 56 found that the efficacy of different surgical approaches, including midvastus, subvastus, mini‐parapatellar, quadriceps‐sparring (QS) and parapatellar, was similar in TKA, and no differences were found in functional outcomes over short or medium terms, but subvastus seemed to have increased ROM at 6 months post‐surgery. For THA, an RCT 57 found that factors such as family education, patient preconditioning, preemptive analgesia, and accelerated preoperative and postoperative rehabilitation, rather than the size of the incision, play a dominant role in influencing the outcome of THA. However, a meta‐analysis 58 still found that short‐term recovery favors limited incision over standard incision, and the former had better LOS, VAS pain at discharge, blood loss, and the Harris hip score at 3 months postoperatively. In terms of the efficiency of the direct anterior approach (DAA) in THA, the results are conflicting. One observational study 59 favored DAA as a valuable addition to enhanced recovery pathways to reduce patient LOS; however, another study 60 found no superior benefits of DAA compared with the posterior approach when the ERAS pathway was used and DAA had a significantly higher rate of periprosthetic femoral fractures. Thus, considering the low level of evidence of current studies, there is no conclusive evidence regarding which surgical approach should be preferred.
Drainage
The guidelines recommended not routinely use of surgical drains for hip and knee replacement. 3
The use of suction drains after orthopedic surgery seems to be a logical and effective way to reduce the size of postoperative wound hematomas. Studies 61 have shown comparable outcomes in TJA patients with or without surgical drains, suggesting that these drains are not necessary. A 2018 meta‐analysis 62 concluded that the use of closed suction drains after TKA is probably not superior to no drains for most outcome measures, and drainage patients had an increased risk of homologous transfusion and a longer time to regain straight‐leg raising, although the need for dressing change was decreased. Another meta‐analysis 63 shared similar viewpoints that no clear benefit or drawback was found in the use of closed drainage after TKA. The same proposition can also be found in THA. A meta‐analysis 64 concluded that the routine use of closed suction drainage systems post primary hip arthroplasty is not supported because closed suction drainage is associated with increased total blood loss and blood transfusion requirements but similar surgical site infection and hematoma formation compared with nondrainage. In terms of urinary drainage, one RCT found that catheterization might not be necessary in nondrainage TKA using combined spinal epidural anesthesia because postoperative urinary retention incidence, clinical outcomes and knee scores and functions were similar. Furthermore, a systematic review 65 found that prolonged wound drainage and urinary catheterization were the identified risk factors associated with surgical site infection. Though the overall level of evidence is low, the routinely used drainages should be reduced.
Perioperative Fluid Management
The guidelines recommended judiciously use of intravenous fluids, which may discourage in favor of early oral intake. 3 Maintaining fluid balance, a prominent component of ERAS pathways, is important in patients undergoing TJA. Restricted and balanced fluid management protocols have been advocated within ERAS, but meaningful comparison is challenging in TJA due to limited intraoperative blood and fluid loss. 3 One RCT 66 for TKA within an ERAS pathway found that compared with restrictive fluid management, a liberal fluid regimen (median 4250 mL, range 3150–5200 mL) may lead to significant hypercoagulability and a reduction in vomiting without influencing postoperative hypoxemia, exercise capacity or hospital stay. However, patients who receive high volumes of intraoperative fluid (>2 L) combined with a history of prior urinary retention are identified as risk factors for postoperative urinary retention for THA under spinal anesthesia. 67 Goal‐directed fluid therapy (GDT), guided by assessment of fluid responsiveness, aims to optimize systemic oxygenation, protect organs particularly at risk of perioperative hypoperfusion, and reduce postoperative gastrointestinal complications. 68 One RCT 69 in THA found that GDT can decrease postoperative complications. However, the quality of evidence of GDT’ benefits in TJA is still low and its role in TJA need to be further confirmed. Thus, based on current literatures, high volumes of intraoperative fluid in TJA is improper, and balanced fluid management or Goal‐directed fluid therapy may be encouraged, however, their effects still need to be further determined.
After Surgery Phases
Antithrombotic Prophylaxis Treatment
The guidelines recommended to mobilize as soon as possible post‐surgery and receive anti‐.
thrombotic prophylaxis treatment. 3 Patients undergoing orthopedic surgery for bone are at increased venous thromboembolism (VTE) risk, which is a significant contributor to postoperative morbidity and mortality. The main risk factors for venous thromboembolism after TJA include a history of VTE, varicose veins, and congestive cardiac failure, followed by female sex, age (≥80), hypertension, (active) cancer, obesity (BMI ≥30), and (black) race. 70 A 2012 guideline recommended by the American College of Chest Physicians suggested a minimum of 10 to 14 days of antithrombotic prophylaxis for patients undergoing TJA. 71 Strategies for thromboprophylaxis include pharmacologic and mechanical methods. Pharmacologic methods for thromboprophylaxis include vitamin K antagonists (e.g., warfarin), low‐molecular‐weight heparin (LMWH), factor Xa inhibitors (e.g., rivaroxaban, darexaban, and apixaban), or direct thrombin inhibitors (e.g., ximelagatran and dabigatran etexilate). 72 Two meta‐analyses 72 , 73 found that low‐molecular‐weight heparin is associated with a higher rate of venous thromboembolism (VTE) than factor Xa inhibitors. Some of them also found that compared with LMWH, direct thrombin inhibitors have a higher risk of major bleeding in TJA patients and a lower rate of VTE in THA patients but a similar effectiveness in deep vein thrombosis (DVT) and pulmonary embolism prophylaxis in TJA patients. Accordingly, one meta‐analysis 74 found that new anticoagulants, such as rivaroxaban, dabigatran, or apixaban, were generally associated with a higher bleeding tendency. Another meta‐analysis 75 found that the addition of intermittent mechanical leg compression augments the efficacy of anticoagulation in preventing DVT in TJA patients. Though the level of evidence is not high, TJA patients should receive combined pharmacological and mechanical prophylaxis to reduce the risk of venous thromboembolism.
Postoperative Nausea and Vomiting
The guidelines support use of multimodal postoperative nausea and vomiting (PONV) prophylaxis for patients undergoing hip and knee replacement. 3 PONV is a common complication after arthroplasty that can complicate discharge and increase patient suffering and dissatisfaction. The risk factors 76 , 77 include bilateral TJA, motion sickness, a history of migraines, lower body mass index (BMI), female sex, nonsmoking, and use of postoperative opioid use (ropivacaine and hydromorphone, patient‐controlled epidural analgesia). The methods of postoperative nausea and vomiting prophylaxis vary, including the use of dexamethasone. PONV can be reduced or minimized by administering multimodal antiemetic prophylaxis. Ondansetron, palonosetron, ramosetron, and 5‐hydroxytryptamine receptor 3 (5‐HT3) antagonists are commonly used for preventing PONV. 78 RCTs 77 , 79 have demonstrated that preoperative or intraoperative ramosetron was more effective and had a longer‐lasting effect for preventing PONV after TKA than ondansetron under spinal anesthesia. Glucocorticoids, especially dexamethasone, are also safe, efficacious, and inexpensive for such prophylaxis in patients undergoing TJA, including diabetic patients. 80 Most studies recommend glucocorticoid administration prior to or during surgery. Two meta‐analyses 81 , 82 found that preoperative or perioperative intravenous glucocorticoids can not only alleviate pain intensity within 48 hours but also decrease the occurrence of PONV after TJA. However, a recent RCT 83 found that the pain and nausea controlling effect of preoperative and postoperative glucocorticoids only lasts for 24 hours; additional administration of glucocorticoids at 1 day postoperatively was needed to provide prolonged benefits. Though the level of evidence is low, PONV prophylaxis via multiple ways should be encouraged.
Postoperative Nutritional Care and Intervention
The guidelines recommended an early return to normal diet for TJA patients. 3 Traditionally, early oral feeding or feeding within the first 6 h postoperatively and postoperative carbohydrate supplementation were mostly used. Although vomiting is a risk factor for early postoperative oral intake, it is proved to be safe to have an early feeding. An RCT 84 demonstrated tolerable outcomes with early postoperative feeding, which found that early feeding (at 4 h postoperatively) and late feeding (⩾8 h postoperatively) showed no difference in nausea, return of bowel function, and length of hospital stay without increasing postoperative morbidity.
Malnutrition is an important risk factor for in‐hospital death, postoperative complications, total mortality, and reoperation rates in THA; thus, delicate nutritional care must be implemented for these patients to mitigate the increased risks. 85 , 86 A retrospective cohort study 87 demonstrated the benefits of postoperative nutritional supplementation and found that postoperative nutritional supplementation is a protective factor for surgical site infection, periprosthetic joint infection, and 30‐day readmission in geriatric patients with hypoalbuminemia undergoing THA. A retrospective study 88 also investigated the direct association of postoperative nutritional intervention with accelerated discharge in TJA. They found that compared with malnourished patients, malnourished patients with nutritional intervention (a high‐protein, anti‐inflammatory diet) had a shorter hospital LOS and lower primary hospitalization charges and 90‐day total charges. Though the overall level of evidence is still low, return to normal food intake as soon as possible is recommended for TJA patients, and proper postoperative nutritional intervention is also beneficial.
Early Mobilization
The guidelines recommended that patients should mobilize as early as possible. 3 Postoperative mobilization may be restricted by pain or medical paraphernalia, however, the benefits of early mobilization are obvious. A large observational study 89 conducted in 19 Australian hospitals found that early mobilization was associated with improved health outcomes and reduced rates of VTE. And a systematic review 90 concluded that early mobilization in TJA can result in a reduced length of stay of approximately 1.8 days without an increase in negative outcomes, which can be achieved within 24 hours of operation. Another RCT 91 had a similar finding in THA: compared with mobilization on the day after surgery, mobilization on the day of surgery significantly increases the probability of discharge and decreases the time to readiness for discharge. The level of evidence is consistent and strong, and patients should mobilize as early as possible to facilitate an earlier discharge.
Criteria‐Based Discharge and Continuous Improvement and Audit
The guidelines recommended to use some objective discharge criteria to facilitate patient discharge directly to their home as well as routinely audit the process measures, clinical outcomes, cost effectiveness, patient satisfaction/experience, and changes to the pathway. 3 However, a high level of evidence for this strategy in TJA is still lacking.
Conclusions and Limitations
After the introduction of the ERAS concept, an increasing number of studies have been conducted and have demonstrated the superiority of the ERAS pathway over traditional treatment. ERAS programs have been demonstrated to be safe, efficacious, acceptable, and widely applicable in the perioperative period and share a number of similarities across a range of specialties. By dispensing with dogma and accelerating care, orthopedic surgeons have made significant gains in hospital stay and patient outcome. Consequently, providing evidence‐based and similar ERAS protocols can safely benefit TJA patients, and it is reasonable to hypothesize that the gains should be greater.
Our results indicate the recommended components of ERAS have a bound base of evidence and implementation of a full ERAS program may maximize the benefits of our clinical practice.
Based on the recent guidelines in TJA, we review the evidence for the components of ERAS to provide recommendations. However, our study has a number of limitations. First, although a comparatively comprehensive literature search was conducted, we may omit some relevant studies, which may render potential publication bias. Due to the difficulty of performing high‐quality RCTs, the effectiveness of individual components within established programs is difficult to determine. Furthermore, not all of the identified studies were systematic reviews or meta‐analyses.
Therefore, the conclusion of this study should be interpreted with caution. These recommendations had not been discussed by the experts in related field and most of the recommendations had a low level of evidence. Our results should be regarded as a supplement of viewpoints to the guidelines. Furthermore, these recommendations still need to be further determined with higher level of evidence.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81974333); 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University(ZYJC18040).
Author Contributions
CJ C and YL participated in the drafting, writing, and revising of the manuscript. X Z and C LYL participated in the data selection and analysis. PD K participated in the conception and design of the study and contributed to analysis and interpretation of the data and they approved the final version of the manuscript to be submitted, and agreed to be accountable for all aspects of the work.
References
- 1. Zhu S, Qian W, Jiang C, Ye C, Chen X. Enhanced recovery after surgery for hip and knee arthroplasty: a systematic review and meta‐analysis. Postgrad Med J. 2017;93(1106):736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deng QF, Gu HY, Peng WY, Zhang Q, Huang ZD, Zhang C, et al. Impact of enhanced recovery after surgery on postoperative recovery after joint arthroplasty: results from a systematic review and meta‐analysis. Postgrad Med J. 2018;94(1118):678–93. [DOI] [PubMed] [Google Scholar]
- 3. Wainwright TW, Gill M, McDonald DA, Middleton RG, Reed M, Sahota O, et al. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: enhanced recovery after surgery (ERAS(®)) society recommendations. Acta Orthop. 2020;91(1):3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kehlet H, Memtsoudis SG. ERAS guidelines for hip and knee replacement ‐ need for reanalysis of evidence and recommendations? Acta Orthop. 2020;91(3):243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morrell AT, Layon DR, Scott MJ, Kates SL, Golladay GJ, Patel NK. Enhanced recovery after primary Total hip and knee arthroplasty: a systematic review. J Bone Joint Surg Am. 2021;103(20):1938–47. [DOI] [PubMed] [Google Scholar]
- 6. Rutherford RW, Jennings JM, Dennis DA. Enhancing recovery after Total knee arthroplasty. Orthop Clin North Am. 2017;48(4):391–400. [DOI] [PubMed] [Google Scholar]
- 7. McDonald S, Page MJ, Beringer K, Wasiak J, Sprowson A. Preoperative education for hip or knee replacement. Cochrane Database Syst Rev. 2014;2014(5):Cd003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moyer R, Ikert K, Long K, Marsh J. The value of preoperative exercise and education for patients undergoing Total hip and knee arthroplasty: a systematic review and meta‐analysis. JBJS Rev. 2017;5(12):e2. [DOI] [PubMed] [Google Scholar]
- 9. Tanzer D, Smith K, Tanzer M. Changing patient expectations decreases length of stay in an enhanced recovery program for THA. Clin Orthop Relat Res. 2018;476(2):372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Padilla JA, Feng JE, Anoushiravani AA, Hozack WJ, Schwarzkopf R, Macaulay WB. Modifying patient expectations can enhance Total hip arthroplasty postoperative satisfaction. J Arthroplasty. 2019;34(7 s):S209–s14. [DOI] [PubMed] [Google Scholar]
- 11. Debbi EM, Rajaee SS, Spitzer AI, Paiement GD. Smoking and Total hip arthroplasty: increased inpatient complications, costs, and length of stay. J Arthroplasty. 2019;34(8):1736–9. [DOI] [PubMed] [Google Scholar]
- 12. Akhavan S, Nguyen LC, Chan V, Saleh J, Bozic KJ. Impact of smoking cessation counseling prior to Total joint arthroplasty. Orthopedics. 2017;40(2):e323–e8. [DOI] [PubMed] [Google Scholar]
- 13. Agrawal S, Ingrande J, Said ET, Gabriel RA. The Association of Preoperative Smoking with Postoperative Outcomes in patients undergoing Total hip arthroplasty. J Arthroplasty. 2021;36(3):1029–34. [DOI] [PubMed] [Google Scholar]
- 14. Alexander DP, Frew N. Preoperative optimisation of anaemia for primary total hip arthroplasty: a systematic review. Hip Int. 2017;27(6):515–22. [DOI] [PubMed] [Google Scholar]
- 15. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet (London, England). 2002;359(9301):114–7. [DOI] [PubMed] [Google Scholar]
- 16. Best MJ, Buller LT, Gosthe RG, Klika AK, Barsoum WK. Alcohol misuse is an independent risk factor for poorer postoperative outcomes following primary Total hip and Total knee arthroplasty. J Arthroplasty. 2015;30(8):1293–8. [DOI] [PubMed] [Google Scholar]
- 17. Findlay JM, Gillies RS, Millo J, Sgromo B, Marshall RE, Maynard ND. Enhanced recovery for esophagectomy: a systematic review and evidence‐based guidelines. Ann Surg. 2014;259(3):413–31. [DOI] [PubMed] [Google Scholar]
- 18. Xu D, Zhu X, Xu Y, Zhang L. Shortened preoperative fasting for prevention of complications associated with laparoscopic cholecystectomy: a meta‐analysis. J Int Med Res. 2017;45(1):22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292–8. [DOI] [PubMed] [Google Scholar]
- 20. Buvanendran A, Kroin JS, Tuman KJ, Lubenow TR, Elmofty D, Moric M, et al. Effects of perioperative administration of a selective cyclooxygenase 2 inhibitor on pain management and recovery of function after knee replacement: a randomized controlled trial. JAMA. 2003;290(18):2411–8. [DOI] [PubMed] [Google Scholar]
- 21. Liu J, Wang F. Preoperative celecoxib analgesia is more efficient and equally tolerated compared to postoperative celecoxib analgesia in knee osteoarthritis patients undergoing total knee arthroplasty: a randomized, controlled study. Medicine. 2018;97(51):e13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richards P, Gimbel JS, Minkowitz HS, Kelen R, Stern W. Comparison of the efficacy and safety of dual‐opioid treatment with morphine plus oxycodone versus oxycodone/acetaminophen for moderate to severe acute pain after total knee arthroplasty. Clin Ther. 2013;35(4):498–511. [DOI] [PubMed] [Google Scholar]
- 23. Clarke H, Pagé GM, McCartney CJ, Huang A, Stratford P, Andrion J, et al. Pregabalin reduces postoperative opioid consumption and pain for 1 week after hospital discharge, but does not affect function at 6 weeks or 3 months after total hip arthroplasty. Br J Anaesth. 2015;115(6):903–11. [DOI] [PubMed] [Google Scholar]
- 24. Pu X, Sun JM. General anesthesia vs spinal anesthesia for patients undergoing total‐hip arthroplasty: a meta‐analysis. Medicine. 2019;98(16):e14925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu P, Wu Y, Liang Z, Deng Y, Meng Q. Comparing the efficacy of pain managements after total hip arthroplasty: a network meta‐analysis. J Cell Biochem. 2019;120(3):4342–54. [DOI] [PubMed] [Google Scholar]
- 26. Zorrilla‐Vaca A, Grant MC, Mathur V, Li J, Wu CL. The impact of neuraxial versus general anesthesia on the incidence of postoperative surgical site infections following knee or hip arthroplasty: a meta‐analysis. Reg Anesth Pain Med. 2016;41(5):555–63. [DOI] [PubMed] [Google Scholar]
- 27. Johnson RL, Kopp SL, Burkle CM, Duncan CM, Jacob AK, Erwin PJ, et al. Neuraxial vs general anaesthesia for total hip and total knee arthroplasty: a systematic review of comparative‐effectiveness research. Br J Anaesth. 2016;116(2):163–76. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Z, Shen B. Effectiveness and weakness of local infiltration analgesia in total knee arthroplasty: a systematic review. J Int Med Res. 2018;46(12):4874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cai AL, Liu SJ, Wu B, Liu G. Intrathecal versus local infiltration analgesia for pain control in total joint arthroplasty. J Orthop Surg Res. 2020;15(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anaesthesia improve outcome after total hip arthroplasty? A systematic review. Br J Anaesth. 2009;103(3):335–45. [DOI] [PubMed] [Google Scholar]
- 31. Levine BR, Haughom B, Strong B, Hellman M, Frank RM. Blood management strategies for total knee arthroplasty. J Am Acad Orthop Surg. 2014;22(6):361–71. [DOI] [PubMed] [Google Scholar]
- 32. Gao FQ, Li ZJ, Zhang K, Huang D, Liu ZJ. Risk factors for lower limb swelling after primary total knee arthroplasty. Chin Med J (Engl). 2011;124(23):3896–9. [PubMed] [Google Scholar]
- 33. Poeran J, Chan JJ, Zubizarreta N, Mazumdar M, Galatz LM, Moucha CS. Safety of tranexamic acid in hip and knee arthroplasty in high‐risk patients. Anesthesiology. 2021;135:57–68. [DOI] [PubMed] [Google Scholar]
- 34. Taeuber I, Weibel S, Herrmann E, Neef V, Schlesinger T, Kranke P, et al. Association of Intravenous Tranexamic Acid with Thromboembolic Events and Mortality: a systematic review, meta‐analysis, and meta‐regression. JAMA Surg. 2021;156:e210884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li H, Bai L, Li Y, Fang Z. Oral tranexamic acid reduces blood loss in total‐knee arthroplasty: a meta‐analysis. Medicine. 2018;97(45):e12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao‐Yu C, Yan G, Wei C, Yuejv L, Ying‐Ze Z. Reduced blood loss after intra‐articular tranexamic acid injection during total knee arthroplasty: a meta‐analysis of the literature. Knee Surg Sports Traumatol Arthroscopy. 2014;22(12):3181–90. [DOI] [PubMed] [Google Scholar]
- 37. Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Shores P, Mullen K, et al. The efficacy of tranexamic acid in Total hip arthroplasty: a network meta‐analysis. J Arthroplasty. 2018;33(10):3083–9.e4. [DOI] [PubMed] [Google Scholar]
- 38. Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Shores P, Mullen K, et al. The efficacy of tranexamic acid in Total knee arthroplasty: a network meta‐analysis. J Arthroplasty. 2018;33(10):3090–8.e1. [DOI] [PubMed] [Google Scholar]
- 39. Yi J, Liang H, Song R, Xia H, Huang Y. Maintaining intraoperative normothermia reduces blood loss in patients undergoing major operations: a pilot randomized controlled clinical trial. BMC Anesthesiol. 2018;18(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Casati A, Fanelli G, Ricci A, Musto P, Cedrati V, Altimari G, et al. Shortening the discharging time after total hip replacement under combined spinal/epidural anesthesia by actively warming the patient during surgery. Minerva Anestesiol. 1999;65(7–8):507–14. [PubMed] [Google Scholar]
- 41. de Brito PV, Clark AM, Galvão CM. A systematic review on the effectiveness of prewarming to prevent perioperative hypothermia. J Clin Nurs. 2013;22(7–8):906–18. [DOI] [PubMed] [Google Scholar]
- 42. Xu H, Xu G, Ren C, Liu L, Wei L. Effect of forced‐air warming system in prevention of postoperative hypothermia in elderly patients: a prospective controlled trial. Medicine. 2019;98(22):e15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McGovern PD, Albrecht M, Belani KG, Nachtsheim C, Partington PF, Carluke I, et al. Forced‐air warming and ultra‐clean ventilation do not mix: an investigation of theatre ventilation, patient warming and joint replacement infection in orthopaedics. J Bone Joint Surg. 2011;93(11):1537–44. [DOI] [PubMed] [Google Scholar]
- 44. Kümin M, Deery J, Turney S, Price C, Vinayakam P, Smith A, et al. Reducing implant infection in Orthopaedics (RIIiO): results of a pilot study comparing the influence of forced air and resistive fabric warming technologies on postoperative infections following orthopaedic implant surgery. J Hosp Infect. 2019;103(4):412–9. [DOI] [PubMed] [Google Scholar]
- 45. Tjoakarfa C, David V, Ko A, Hau R. Reflective blankets are as effective as forced air warmers in maintaining patient normothermia during hip and knee arthroplasty surgery. J Arthroplasty. 2017;32(2):624–7. [DOI] [PubMed] [Google Scholar]
- 46. Liu S, Pan Y, Zhao Q, Feng W, Han H, Pan Z, et al. The effectiveness of air‐free warming systems on perioperative hypothermia in total hip and knee arthroplasty: a systematic review and meta‐analysis. Medicine. 2019;98(19):e15630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ma H, Lai B, Dong S, Li X, Cui Y, Sun Q, et al. Warming infusion improves perioperative outcomes of elderly patients who underwent bilateral hip replacement. Medicine. 2017;96(13):e6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ryan SP, Kildow BJ, Tan TL, Parvizi J, Bolognesi MP, Seyler TM. Is there a difference in infection risk between single and multiple doses of prophylactic antibiotics? A meta‐analysis. Clin Orthop Relat Res. 2019;477(7):1577–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Siddiqi A, Forte SA, Docter S, Bryant D, Sheth NP, Chen AF. Perioperative antibiotic prophylaxis in Total joint arthroplasty: a systematic review and meta‐analysis. J Bone Joint Surg Am. 2019;101(9):828–42. [DOI] [PubMed] [Google Scholar]
- 50. Rivera A, Sánchez A, Luque S, Mur I, Puig L, Crusi X, et al. Intraoperative bacterial contamination and activity of different antimicrobial prophylaxis regimens in primary knee and hip replacement. Antibiotics. 2020;10(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fernicola SD, Elsenbeck MJ, Grimm PD, Pisano AJ, Wagner SC. Intrasite antibiotic powder for the prevention of surgical site infection in extremity surgery: a systematic review. J Am Acad Orthop Surg. 2020;28(1):37–43. [DOI] [PubMed] [Google Scholar]
- 52. Heckmann ND, Mayfield CK, Culvern CN, Oakes DA, Lieberman JR, Della Valle CJ. Systematic review and meta‐analysis of intrawound vancomycin in Total hip and Total knee arthroplasty: a call for a prospective randomized trial. J Arthroplasty. 2019;34(8):1815–22. [DOI] [PubMed] [Google Scholar]
- 53. Schiavone Panni A, Corona K, Giulianelli M, Mazzitelli G, Del Regno C, Vasso M. Antibiotic‐loaded bone cement reduces risk of infections in primary total knee arthroplasty? A systematic review. Knee Surg Sports Traumatol Arthroscopy. 2016;24(10):3168–74. [DOI] [PubMed] [Google Scholar]
- 54. Wang Z, Zheng J, Zhao Y, Xiang Y, Chen X, Zhao F, et al. Preoperative bathing with chlorhexidine reduces the incidence of surgical site infections after total knee arthroplasty: a meta‐analysis. Medicine. 2017;96(47):e8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cai Y, Xu K, Hou W, Yang Z, Xu P. Preoperative chlorhexidine reduces the incidence of surgical site infections in total knee and hip arthroplasty: a systematic review and meta‐analysis. Int J Surg. 2017;39:221–8. [DOI] [PubMed] [Google Scholar]
- 56. Bouché PA, Corsia S, Nizard R, Resche‐Rigon M. Comparative efficacy of the different surgical approaches in Total knee arthroplasty: a systematic‐review and network meta‐analysis. J Arthroplasty. 2021;36(3):1187–94.e1. [DOI] [PubMed] [Google Scholar]
- 57. Pour AE, Parvizi J, Sharkey PF, Hozack WJ, Rothman RH. Minimally invasive hip arthroplasty: what role does patient preconditioning play? J Bone Joint Surg Am. 2007;89(9):1920–7. [DOI] [PubMed] [Google Scholar]
- 58. Moskal JT, Capps SG. Is limited incision better than standard total hip arthroplasty? A meta‐analysis. Clin Orthop Relat Res. 2013;471(4):1283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Free MD, Owen DH, Agius PA, Pascoe EM, Harvie P. Direct anterior approach Total hip arthroplasty: an adjunct to an enhanced recovery pathway: outcomes and learning curve effects in surgeons transitioning from other surgical approaches. J Arthroplasty. 2018;33(11):3490–5. [DOI] [PubMed] [Google Scholar]
- 60. Malek IA, Royce G, Bhatti SU, Whittaker JP, Phillips SP, Wilson IR, et al. A comparison between the direct anterior and posterior approaches for total hip arthroplasty: the role of an 'Enhanced Recovery' pathway. Bone Joint J. 2016;98‐b(6):754–60. [DOI] [PubMed] [Google Scholar]
- 61. Kosins AM, Scholz T, Cetinkaya M, Evans GRD. Evidence‐based value of subcutaneous surgical wound drainage: the largest systematic review and meta‐analysis. Plast Reconstr Surg. 2013;132(2):443–50. [DOI] [PubMed] [Google Scholar]
- 62. Zhang Q, Liu L, Sun W, Gao F, Zhang Q, Cheng L, et al. Are closed suction drains necessary for primary total knee arthroplasty?: a systematic review and meta‐analysis. Medicine. 2018;97(30):e11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Si HB, Yang TM, Zeng Y, Shen B. No clear benefit or drawback to the use of closed drainage after primary total knee arthroplasty: a systematic review and meta‐analysis. BMC Musculoskelet Disord. 2016;17:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kelly EG, Cashman JP, Imran FH, Conroy R, O'Byrne J. Systematic review and meta‐analysis of closed suction drainage versus non‐drainage in primary hip arthroplasty. Surg Technol Int. 2014;24:295–301. [PubMed] [Google Scholar]
- 65. Noailles T, Brulefert K, Chalopin A, Longis PM, Gouin F. What are the risk factors for post‐operative infection after hip hemiarthroplasty? Systematic review of literature. Int Orthop. 2016;40(9):1843–8. [DOI] [PubMed] [Google Scholar]
- 66. Holte K, Kristensen BB, Valentiner L, Foss NB, Husted H, Kehlet H. Liberal versus restrictive fluid management in knee arthroplasty: a randomized, double‐blind study. Anesth Analg. 2007;105(2):465–74. [DOI] [PubMed] [Google Scholar]
- 67. Lawrie CM, Ong AC, Hernandez VH, Rosas S, Post ZD, Orozco FR. Incidence and risk factors for postoperative urinary retention in Total hip arthroplasty performed under spinal anesthesia. J Arthroplasty. 2017;32(12):3748–51. [DOI] [PubMed] [Google Scholar]
- 68. Giglio MT, Marucci M, Testini M, Brienza N. Goal‐directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta‐analysis of randomized controlled trials. Br J Anaesth. 2009;103(5):637–46. [DOI] [PubMed] [Google Scholar]
- 69. Cecconi M, Fasano N, Langiano N, Divella M, Costa MG, Rhodes A, et al. Goal‐directed haemodynamic therapy during elective total hip arthroplasty under regional anaesthesia. Crit Care. 2011;15(3):R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang J, Chen Z, Zheng J, Breusch SJ, Tian J. Risk factors for venous thromboembolism after total hip and total knee arthroplasty: a meta‐analysis. Arch Orthop Trauma Surg. 2015;135(6):759–72. [DOI] [PubMed] [Google Scholar]
- 71. Falck‐Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S–325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sun G, Wu J, Wang Q, Liang Q, Jia J, Cheng K, et al. Factor Xa inhibitors and direct thrombin inhibitors versus low‐molecular‐weight heparin for thromboprophylaxis after Total hip or Total knee arthroplasty: a systematic review and meta‐analysis. J Arthroplasty. 2019;34(4):789–800.e6. [DOI] [PubMed] [Google Scholar]
- 73. Lu X, Lin J. Low molecular weight heparin versus other anti‐thrombotic agents for prevention of venous thromboembolic events after total hip or total knee replacement surgery: a systematic review and meta‐analysis. BMC Musculoskelet Disord. 2018;19(1):322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gómez‐Outes A, Terleira‐Fernández AI, Suárez‐Gea ML, Vargas‐Castrillón E. Dabigatran, rivaroxaban, or apixaban versus enoxaparin for thromboprophylaxis after total hip or knee replacement: systematic review, meta‐analysis, and indirect treatment comparisons. BMJ. 2012;344:e3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kakkos SK, Warwick D, Nicolaides AN, Stansby GP, Tsolakis IA. Combined (mechanical and pharmacological) modalities for the prevention of venous thromboembolism in joint replacement surgery. J Bone Joint Surg. 2012;94(6):729–34. [DOI] [PubMed] [Google Scholar]
- 76. Wang Y, Yang Q, Lin J, Qian W, Jin J, Gao P, et al. Risk factors of postoperative nausea and vomiting after total hip arthroplasty or total knee arthroplasty: a retrospective study. Ann Transl Med. 2020;8(17):1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hahm TS, Ko JS, Choi SJ, Gwak MS. Comparison of the prophylactic anti‐emetic efficacy of ramosetron and ondansetron in patients at high‐risk for postoperative nausea and vomiting after total knee replacement. Anaesthesia. 2010;65(5):500–4. [DOI] [PubMed] [Google Scholar]
- 78. Ryu JH, Jeon YT, Min B, Hwang JY, Sohn HM. Effects of palonosetron for prophylaxis of postoperative nausea and vomiting in high‐risk patients undergoing total knee arthroplasty: a prospective, randomized, double‐blind, placebo‐controlled study. PLoS One. 2018;13(5):e0196388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pinsornsak P, Teeyaphudit M, Ruetiwarangkoon C, Chaiwuttisak A. Comparison of Ramosetron with ondansetron for prevention of intrathecal morphine‐induced nausea and vomiting after primary Total knee arthroplasty: a randomized control trial. J Arthroplasty. 2017;32(3):1040–3. [DOI] [PubMed] [Google Scholar]
- 80. Godshaw BM, Mehl AE, Shaffer JG, Meyer MS, Thomas LC, Chimento GF. The effects of peri‐operative dexamethasone on patients undergoing Total hip or knee arthroplasty: is it safe for diabetics? J Arthroplasty. 2019;34(4):645–9. [DOI] [PubMed] [Google Scholar]
- 81. Yang Q, Zhang Z, Xin W, Li A. Preoperative intravenous glucocorticoids can decrease acute pain and postoperative nausea and vomiting after total hip arthroplasty: a PRISMA‐compliant meta‐analysis. Medicine. 2017;96(47):e8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen P, Li X, Sang L, Huang J. Perioperative intravenous glucocorticoids can decrease postoperative nausea and vomiting and pain in total joint arthroplasty: a meta‐analysis and trial sequence analysis. Medicine. 2017;96(13):e6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kim JK, Ro DH, Lee HJ, Park JY, Han HS, Lee MC. Efficacy of systemic steroid use given one day after Total knee arthroplasty for pain and nausea: a randomized controlled study. J Arthroplasty. 2020;35(1):69–75. [DOI] [PubMed] [Google Scholar]
- 84. Kim JW, Park YG, Kim JH, Jang EC, Ha YC. The optimal time of postoperative feeding after Total hip arthroplasty: a prospective, randomized controlled trial. Clin Nurs Res. 2020;29(1):31–6. [DOI] [PubMed] [Google Scholar]
- 85. Newman JM, Sodhi N, Khlopas A, Piuzzi NS, Yakubek GA, Sultan AA, et al. Malnutrition increases the 30‐day complication and re‐operation rates in hip fracture patients treated with total hip arthroplasty. Hip Int. 2020;30(5):635–40. [DOI] [PubMed] [Google Scholar]
- 86. Li S, Zhang J, Zheng H, Wang X, Liu Z, Sun T. Prognostic role of serum albumin, Total lymphocyte count, and mini nutritional assessment on outcomes after geriatric hip fracture surgery: a meta‐analysis and systematic review. J Arthroplasty. 2019;34(6):1287–96. [DOI] [PubMed] [Google Scholar]
- 87. He Y, Xiao J, Shi Z, He J, Li T. Supplementation of enteral nutritional powder decreases surgical site infection, prosthetic joint infection, and readmission after hip arthroplasty in geriatric femoral neck fracture with hypoalbuminemia. J Orthop Surg Res. 2019;14(1):292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schroer WC, LeMarr AR, Mills K, Childress AL, Morton DJ, Reedy ME. 2019 Chitranjan S. Ranawat Award: elective joint arthroplasty outcomes improve in malnourished patients with nutritional intervention: a prospective population analysis demonstrates a modifiable risk factor. Bone Joint J. 2019;101‐B(7 Suppl C):17–21. [DOI] [PubMed] [Google Scholar]
- 89. Chua MJ, Hart AJ, Mittal R, Harris IA, Xuan W, Naylor JM. Early mobilisation after total hip or knee arthroplasty: a multicentre prospective observational study. PLoS One. 2017;12(6):e0179820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Guerra ML, Singh PJ, Taylor NF. Early mobilization of patients who have had a hip or knee joint replacement reduces length of stay in hospital: a systematic review. Clin Rehabil. 2015;29(9):844–54. [DOI] [PubMed] [Google Scholar]
- 91. Okamoto T, Ridley RJ, Edmondston SJ, Visser M, Headford J, Yates PJ. Day‐of‐surgery mobilization reduces the length of stay after elective hip arthroplasty. J Arthroplasty. 2016;31(10):2227–30. [DOI] [PubMed] [Google Scholar]


