Abstract
Background
Human papillomavirus (HPV) testing on self-samples represents a great opportunity to increase cervical cancer screening uptake among under-screened women.
Methods
A systematic review and meta-analysis on randomised controlled trials (RCTs) were performed to update the evidence on the efficacy of strategies for offering self-sampling kits for HPV testing compared to conventional invitations and to compare different self-sampling invitation scenarios. Four experimental invitational scenarios were considered. Women in the control group were invited for screening according to existing practice: collection of a cervical specimen by a healthcare professional. Random-effects models were used to pool proportions, relative participation rates and absolute participation differences.
Results
Thirty-three trials were included. In the intention-to-treat analysis, all self-sampling invitation scenarios were more effective in reaching under-screened women compared to controls. Pooled participation difference (PD) and 95% confidence interval (CI) for experimental vs. control was 13.2% (95% CI = 11.0–15.3%) for mail-to-all, 4.4% (95% CI = 1.2–7.6%) for opt-in, 39.1% (95% CI = 8.4–69.9%) for community mobilisation & outreach and 28.1% (23.5–32.7%) for offer at healthcare service. PD for the comparison opt-in vs. mail-to-all, assessed in nine trials, was −8.2% (95% CI = −10.8 to −5.7%).
Discussion
Overall, screening participation was higher among women invited for self-sampling compared to control, regardless of the invitation strategy used. Opt-in strategies were less effective than send-to-all strategies.
Subject terms: Cervical cancer, Population screening, Cancer prevention
Background
Despite being a preventable and treatable disease, cervical cancer (CC) remains the fourth most frequently diagnosed cancer and the fourth leading cause of cancer-related death in women globally [1, 2]. CC incidence and mortality vary widely worldwide. Cumulative rates of CC incidence and mortality in high-income countries (HICs) were 2–4 times lower than in low- and middle-income countries (LMICs) [1–3]. A range of social-cultural determinants of sexual health (e.g., education, economic status, customs, attitudes and beliefs) as well as the lack of high-quality screening programmes and the lack of widespread treatment for (pre-) cancerous lesions, which results from the absence of resources, infrastructures and qualified personnel, are some of the main reasons for the disparity observed LMICs vs. HICs [2–5]. Nevertheless, in many HICs, despite the higher availability and accessibility to cervical cancer screening (CCS) services, the overall screening coverage remains suboptimal.
Since it was discovered that persistent infection with oncogenic human papillomavirus (HPV) is the primary cause of cervical (pre-)cancer, novel primary and secondary prevention strategies have been continuously sought [6]. Substantial evidence supporting the use of high-risk HPV (hrHPV)-based tests for CCS has since then emerged, validating hrHPV testing as being more sensitive than cytology in primary screening [7, 8].
hrHPV testing is highly reproducible, less examiner-dependent and enhances the efficacy of screening while reducing the burden on healthcare systems by allowing longer screening intervals for HPV-negative women [7, 8]. Furthermore, women can be provided kits for self-collection of cervicovaginal specimens for HPV testing, allowing screening of comparable accuracy to that of clinician-collected samples when a validated PCR-based assay is used for testing [9–11].
The self-sampling modality for hrHPV testing represents a great opportunity to tackle another major challenge towards an optimal CC control—an adequate screening uptake. CC most commonly occurs in women who have been insufficiently or never-screened [12, 13]. Offering hrHPV self-samples to under-screened women has shown to be an effective strategy to lower the barriers to participation as it can be done at a location and time more convenient for the women, removes the anxiety most women feel about having a gynaecological exam and, at the same time, it limits the required healthcare resources [14]. The previous meta-analysis from randomised controlled trials found that mailing self-samples to the women’s home addresses is more effective in increasing screening participation response than when invitation/reminder letters to visit the primary care provider for the collection of a cervical specimen were sent. In the scenarios in which invitation was done through community campaigns and door-to-door visits, the relative participation was on average twice as high in the self-sampling versus control arms using invitation/reminder letters [10].
The primary objectives of the current review were to update the evidence on the efficacy of strategies offering self-sampling kits compared to invitations for conventional screening and in addition, to compare the efficacy between different self-sampling scenarios. The secondary objectives were to assess sample adequacy and test-positivity rate among screened women, and to explore follow-up adherence and detection rate of cervical (pre-)cancer in screen-test positive and invited women.
Methods
Research question and study selection
In this systematic review and meta-analysis, we aimed to answer the following research questions: Is CCS attendance higher when under-screened women are offered a self-sampling device for hrHPV testing (experimental group) compared to routine invitations/reminders to contact a healthcare provider (HCP) for collection of a cervical specimen (control group)? Also, how does attendance to screening vary when two different self-sampling invitation strategies are used?
The response rate in each intervention scenario, the relative response rate and response difference in the experimental versus control groups, were primary outcomes considered for this review. Another primary outcome was the response difference between two self-sampling invitation scenarios (opt-in vs. mail-to-all). Secondary outcomes concerned sample adequacy, test-positivity rate, follow-up adherence and detection of CIN2 + .
Studies were eligible for inclusion if the following criteria were met: the study population consisted of women who were irregularly screened, never-screened or did not respond to invitation/reminder letters offering screening done by a HCP; the study population included ≥400 women; the allocation to intervention arms (experimental or control group) was randomised, the participation rate was documented in both interventional groups; the experimental group consisted of women who were invited to provide a vaginal self-sample for hrHPV testing; and the control group was comprised of women who had the possibility to be screened according to the current clinical practice. In the absence of a control group, studies were also eligible for inclusion if screening participation between two self-sampling-based experimental groups was assessed.
Four different invitational scenarios were considered for the experimental group: (1) mail-to-all scenario, where an invitation to participate in the study accompanied by an hrHPV self-sampling kit was directly sent to the women at their home addresses; (2) opt-in scenario, where women received an invitation letter containing information on how a self-sampling kit could be requested or, alternatively, could be collected at their local clinic/pharmacy; (3) community mobilisation & outreach scenario, where women were offered a self-sampling kit either after attending a CCS awareness event or, at home/work upon a visit by a community healthcare worker (CHW) and (4) direct offer at a healthcare service, where women were offered a self-sampling kit at the end of an individual appointment when they visited a HCP for whatever reason.
For the women in the control group, the possibility to obtain screening according to current clinical practice either came after an invitation/reminder to visit an HCP or resulted from opportunistic screening upon woman’s request or on HCP recommendation, without organised invitation.
Literature search was conducted using a general search strategy translated to the syntax of two electronic databases, PubMed and Embase. The search strategy is identical to the one used in the previous review and is available in the supplementary materials [10]. No language restrictions were applied. All of the identified studies were retrieved until March 31, 2022. Two review authors (SC and BV) independently screened titles and abstracts. Full texts of the eligible studies were subsequently retrieved and re-assessed for inclusion. Studies fully meeting the eligibility criteria were added to those already included in the previous review. In addition, manual search was also performed on the reference lists of all the newly included studies and an assessment, identical to that described above, was carried out. Any disagreements were resolved by consensus or, when necessary, any unresolvable discordances were judged by MA. The protocol for this updated systematic review and meta-analysis can be found in the supplementary file.
Data extraction and quality assessment
CCS participation data was extracted independently by SC and BV using a standardised electronic data entry form. Whenever available, data on sample adequacy, test-positivity rate, follow-up adherence among screen-positive women and detection of CIN2 + was also extracted for both experimental and control groups. Standardised tables were used to extract the following study characteristics: study design, type of target population, scenario of invitation, study size, and timeframe between invitation, participation and follow-up of screen-positive women.
The quality of the included RCTs was assessed using the Cochrane Collaboration’s tool for risk of bias in randomised trials [15]. Three parameters of the tool were particularly considered for assessment: selection, attrition and reporting bias. The two other parameters from the Cochrane tool, performance and detection bias, were not considered applicable given the nature of the interventions (participant-collected self-sample versus HCP-collected sample or VIA) not permitting blinding. The quality assessment was carried out independently by SC and BV. Any disagreements were resolved by consensus.
Statistical methods
The PRISMA 2020 guidelines for systematic reviews and meta-analysis were followed for transparent reporting [16].
Per-protocol (PP) and intention-to-treat (ITT) analyses were performed. In the PP analyses, only women who took an hrHPV self-sample in the experimental groups were counted as participants. In the ITT analyses, women who had been offered a self-sample but visited an HCP to have a sample taken instead, were also counted as participants. Where studies reported multiple time points of response assessment, the assessment at 12 months was used as an endpoint.
Addressing our primary objectives, the four different invitation scenarios for the experimental groups were considered and given the inherent differences between them, outcomes were pooled separately for each scenario.
To assess the contrast in participation between experimental and control groups, pooled proportions and 95% confidence intervals were computed by running a random-effects model using a statistical procedure for meta-analysis of binomial data, metaprop [17]. Statistical heterogeneity was assessed by using the I2 statistic, which describes the percentage of variation across studies due to inter-study heterogeneity rather than chance [18]. Relative participation rates (RP = proportionself-sampling/proportioncontrol) and absolute participation differences (PD = proportionself-sampling − proportioncontrol) were assessed by applying random-effects models for ratios or differences of proportions using metan [19, 20]. An identical analysis to that described above, was used to compare uptake between two experimental intervention scenarios (opt-in and mail-to-all) in studies reporting data for more than one self-sampling group. The opt-in scenario was considered as the index intervention and the mail-to-all as the comparator intervention.
As for our secondary objectives, firstly, we computed proportions for sample adequacy and test-positivity rate in the self-sampling group. A comparison of self-sampling vs. control was not possible because data for the two before mentioned parameters was not available for the control group. Secondly, we computed absolute and relative proportions, as well as proportion differences, to investigate the contrast between self-sampling and control groups in terms of follow-up adherence and detection rate of cervical (pre-)cancer in screen-positive women.
Statistical significance was defined as P < 0.05. StataSE 16 was used for all statistical analyses [21].
Results
Retrieval of studies
The PRISMA flowchart showing the selection of eligible trials, as well as, the study characteristics of included trials, can be found in the supplementary materials (Supplementary Fig. S1, Supplementary Tables S1 and S2, respectively).
To the 24 trials already included in the previous meta-analysis [10], 9 new studies were added, making up a total of 33 trial reports included in this review.
All the included trials, except one [22], comprised a control group involving standard of care for CCS and, one or more experimental arms offering a self-sampling device. In eight trials, besides the control group, data were reported for two experimental arms using different self-sampling invitation scenarios. The trial without a standard of care control group, only compared participation data for two self-sampling experimental arms, opt-in and mail-to-all [22].
The breakdown of the trials included in the experimental vs. control group analysis, according to invitation scenario, was as follows: 25 studies reported data for the ‘mail-to-all’ scenario [14, 23–46], 9 for the ‘opt-in’ [25, 35, 39–43, 46, 47], 5 for the ‘community mobilisation & outreach’ scenario [48–52] and one single report presented data for the ‘offer at the healthcare service’ [53]. Nine studies reported data for opt-in and mail-to-all scenarios and were included in the analysis exploring the contrast in participation between these two self-sampling experimental arms [22, 25, 35, 39–43, 46].
Quality of included studies
We judged the overall risk of bias in the included trials as moderate to high (Supplementary Table S3, Supplementary Material). Eight (24%) out of the 33 studies were given the low risk of bias score in all categories under assessment [25, 33–35, 40, 42, 43, 45]. In about half of the trials, details on the randomisation process [14, 27, 29, 31, 32, 36, 39, 44, 47–49, 51–53] and allocation concealment [23, 24, 26–32, 36, 39, 41, 47, 51] were not clearly documented. Attrition bias, referent to incomplete outcome data, was considered absent in all trials. Medium risk of bias for the category “reporting of timelines”, was attributed to nine trials since, the time evolved from invitation until responses were noted, was unclear [22, 26, 28, 31, 32, 36, 37, 47, 48, 50, 52, 53]. For the item “selective reporting”, ten studies were judged at medium risk of bias either because intention-to-treat results were not reported or exclusion of women was not explained [30–32, 36, 38, 41, 46, 48, 51, 52]. One study was judged at high-risk bias for the item “selective reporting” for both, not reporting intention-to-treat results and unexplained exclusion [26].
Cervical cancer screening uptake
The pooled absolute participation rate in the self-sampling and control arms as well as the relative participation and participation difference for self-sampling versus control arms are shown in Table 1 and in Supplementary Figs. S2–8.
Table 1.
Absolute proportion in self-sampling and control arm, relative participation and participation difference in the self-sampling versus control arm, by the scenario of invitation.
| Absolute participation | Relative participation | Participation difference | |||
|---|---|---|---|---|---|
| Self-sampling | Control | ||||
| Scenario of invitation | # | % (95% CI) | % (95% CI) | (95% CI) | % (95% CI) |
| Per-protocol | |||||
| Mail-to-all | 25/28† | 18.8 (15.7, 22.0) | 10.4 (7.8, 13.4) | 1.93 (1.51, 2.47) | 7.8 (4.7, 10.9) |
| Opt-in | 9/12† | 8.5 (5.6, 11.8) | 11.3 (8.2, 14.9) | 0.80 (0.58, 1.08) | −3.2 (−7.2, 0.9) |
| Community mobilisation & outreach | 5 | 92.5 (80.3, 99.1) | 52.7 (16.7, 87.1) | 1.92 (0.90, 4.10) | 38.5 (9.3, 67.7) |
| Offer at healthcare service | 1 | 42.0 (38.5, 45.6) | 21.6 (18.9, 24.6) | 1.95 (1.66, 2.28) | 20.4 (15.9, 25.0) |
| Intention-to-treat* | |||||
| Mail-to-all | 25/28† | 24.3 (21.5, 27.3) | 10.4 (7.8, 13.4) | 2.50 (2.08, 3.01) | 13.2 (11.0, 15.3) |
| Opt-in | 9/12† | 16.7 (10.5, 23.9) | 11.3 (8.2, 14.9) | 1.45 (1.16, 1.81) | 4.4 (1.2, 7.6) |
| Community mobilisation & outreach | 5 | 92.9 (82.3, 99.0) | 52.7 (16.7, 87.1) | 1.94 (0.89, 4.24) | 39.1 (8.4, 69.9) |
| Offer at healthcare service | 1 | 49.7 (46.1, 53.3) | 21.6 (18.9, 24.6) | 2.30 (1.98, 2.67) | 28.1 (23.5, 32.7) |
*Certain studies reported that some women, allocated to the self-sampling arm, had a Pap smear taken by a clinician. The sum of self-samples taken + Pap smears taken, were counted in the ITT analyses. In studies, where no such cases were reported, the number of events in the PP and ITT analyses were considered equal.
#Number of studies.
†Giorgi et al. [25] and Giorgi et al. [35] had two control groups (one in which a Pap smear was taken by a clinician and another in which a sample for hrHPV testing was taken by a clinician). Kellen et al. [41] also had two control arms (one with recall letters and another without recall letters.
Self-sampling arm
In the mail-to-all scenario PP participation varied from 6.4 to 34.0%, with a pooled average of 18.8% (95% CI 15.7%, 22.0%). In opt-in scenario, the PP participation varied between trials from 1.5 to 17.5%, with a pooled average of 8.5% (95% CI 5.6%, 11.8%). Participation in the community mobilisation & outreach scenario, varied from 79.8 to 99.2%, with a pooled participation of 92.5% (95% CI 80.3%, 99.1%). In the offer at healthcare service scenario, which included one single study, the participation was of 42.0% (95% CI 38.5%, 45.6%) (Table 1 and Supplementary Fig. S2).
In the mail-to-all and opt-in scenarios, the ITT results were higher than the PP results. The pooled ITT participation was 24.3% (95% CI 21.5%, 27.3%) in the mail-to-all and 16.7% (95% CI 10.5%, 23.9%) in the opt-in scenario, which were 5.5% and 8.2% higher, respectively, than PP participation. For the community mobilisation & outreach, ITT and PP results were quasi-equal (PP: 92.5% (95% CI 80.3%, 99.1%) vs. ITT: 92.9% (95% CI 82.3%, 99.0%)). In the offer at healthcare service scenario, the ITT participation was 49.7% (95% CI 46.1%, 53.3%), which was 7.7% higher than in PP analysis (Table 1 and Supplementary Fig. S3).
Control arm
In the control group the participation in the mail-to-all scenario was 10.4% (95% CI 7.8%, 13.4%) and in the opt-in it was 11.3% (95% CI 8.2%, 14.9%). The average participation in the community mobilisation & outreach invitation scenario was 52.7% (95% CI 16.7%, 87.1%) and in the single study assessing participation with offer at healthcare service scenario, the screening participation in the control arm was 21.6% (95% CI 18.9%, 24.6%) (Table 1 and Supplementary Fig. S4).
Participation in self-sampling versus control arms
Among women randomised to self-sampling in a mail-to-all scenario, participation was significantly higher than in the control groups: RP = 1.93 (95% CI 1.51, 2.47) in PP and 2.50 (95% CI 2.08, 3.01) in ITT analyses. In the opt-in scenario, the average participation was lower (but not significantly) than in the control group in PP analysis (RP = 0.80; 95% CI 0.58, 1.08), whereas in ITT analysis the pooled participation was significantly higher than in the control group: RP = 1.45 (95% CI 1.16, 1.81). In the community mobilisation & outreach scenario, the participation was higher compared to the control group, the differences were not significant: RP = 1.92 (95% CI 0.90, 4.10) and 1.94 (95% CI 0.89, 4.24), in PP and ITT analyses respectively. In the offer at the healthcare service, the relative participation in the self-sampling arm was on average about twice as high when compared to control in both PP and ITT analyses, 1.95 (95% CI 1.66, 2.28) and 2.30 (95% CI 1.98, 2.67), respectively (Table 1, PP: Supplementary Fig. S5 and ITT: Supplementary Fig. S6).
No evidence of publication bias was found. Harbord’s test for funnel plot asymmetry in the relative participation for mail-to-all or opt-in scenarios was not significant (Supplementary Table S5).
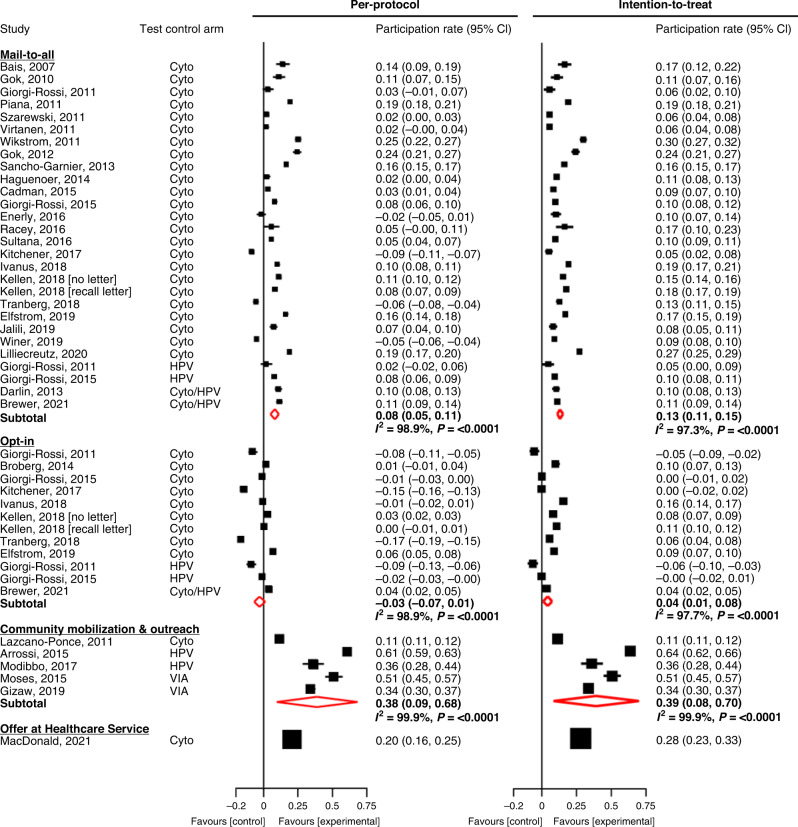
The difference in participation (PD) between self-sampling and control arms for the mail-to-all scenario was 7.8% (95% CI 4.7%, 10.9%) in PP analysis. In the mail-to-all scenario, in four of the 25 trials [36, 39, 42, 44] the PD was negative, and this difference was significant in three of them when the PP analysis was considered. However, in the ITT analysis the PD was positive in all cases with a pooled value of 13.2% (95% CI 11.0%, 15.3%). In the opt-in scenario, PP participation difference between experimental and control groups tended to be negative in most trials with a pooled PD of −3.5% (95% CI −7.2%, 0.9%). However, in ITT the pooled PD was significantly higher than zero (4.4%; 95% CI 1.2%, 7.6%). For community mobilisation & outreach, the PD in all trials was significantly higher than zero in PP and ITT analyses: pooled PD = 38.5% (95% CI 9.3%, 67.7%) and 39.1% (95% CI 8.4%, 69.9%), respectively. Offer at the healthcare service yielded a PD between the self-sampling and control arms of 20.4% (95% CI 15.9%, 25.0%) and 28.1% (95% CI 23.5%, 32.7%) in PP and ITT analyses, respectively (Table 1 and Fig. 1).
Fig. 1. Difference in participation rate between the self-sampling and the control arms of randomised trials.
Cyto cytology, HPV human papillomavirus, VIA visual inspection with acetic acid. In intention-to-treat analysis both, hrHPV tests on self-samples and Pap smears were accounted for. Participation rates displayed as fractions.
Participation in self-sampling trials comparing opt-in versus mail-to-all scenarios
In all the nine trials comparing two self-sampling scenarios, the absolute participation rates were always significantly higher in the mail-to-all than in the opt-in scenario. The pooled participation in the opt-in arm was 8.4% (95% CI 5.8%, 11.3%) in PP and 15.6% (95% CI 9.5%, 22.9%) in ITT analyses. In the mail-to-all arms, the pooled participation was 18.3% (95% CI 14.8, 22.1%) in PP and 24.4% (95% CI 19.4%, 29.8%) in ITT analyses (Table 2, PP: Supplementary Figs. S9 and S10, ITT: Supplementary Figs. S11 and S12).
Table 2.
Absolute proportion in self-sampling arm, and relative proportion and difference in the opt-in vs. mail-to-all self-sampling invitation scenarios.
| Absolute participation | Relative participation | Participation difference | |||
|---|---|---|---|---|---|
| Opt-in | Mail-to-all | ||||
| Analysis | # | % (95% CI) | % (95% CI) | (95% CI) | % (95% CI) |
| Per-protocol | 9 | 8.4 (5.8, 11.3) | 18.3 (14.8, 22.1) | 0.46 (0.40, 0.53) | −9.7 (−11.5, −8.0) |
| Intention-to-treat* | 9 | 15.6 (9.5, 22.9) | 24.4 (19.4, 29.8) | 0.61 (0.50, 0.74) | −8.2 (−10.8, −5.7) |
*Certain studies reported that some women, allocated to the self-sampling arm, had a Pap smear taken. The sum of self-samples taken + Pap smears taken, were counted in the ITT analyses. In studies, where no such cases were reported, the number of events in the PP and ITT analyses were considered as equal.
#Number of studies.
Women invited to participate in CCS through the opt-in scenario were significantly less likely to participate compared to those who were directly mailed the self-sampling kit in both PP and ITT analyses, RP = 0.46 (95% CI 0.40, 0.53) and 0.61 (95% CI 0.50, 0.74), respectively (Table 2, PP: Supplementary Fig. S13 and ITT: Supplementary Fig. S14).
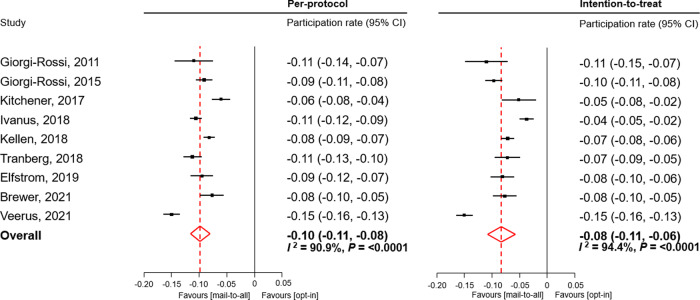
The PD for opt-in vs. mail-to-all was −9.7% (95% CI −11.5, −8.0) and −8.2% (95% CI −10.8, −5.7) in PP and ITT analyses, respectively (Table 2 and Fig. 2).
Fig. 2. Difference in participation rate between opt-in and mail-to-all experimental groups of randomised trials.
In intention-to-treat analysis both, hrHPV tests on self-samples and Pap smears were accounted for. Participation rates displayed as fractions.
Sample adequacy, test-positivity rate, follow-up adherence and detection of CIN2+
The results for sample adequacy, test-positivity rate, follow-up adherence and detection of CIN2 + , are presented in Table 3.
Table 3.
Absolute proportion in self-sampling arm, and relative proportion and difference in proportions between the self-sampling arm versus control arm (cytology) regarding rate of inadequate specimens, compliance of screen-positive women to follow-up and detection of cervical precancer (CIN2 + ).
| Absolute proportion self-sampling arm | Relative proportion | Proportion Difference | |||
|---|---|---|---|---|---|
| Parameter | # | (95% CI) | # | (95% CI) | (95% CI) |
| Inadequate sample | 20 | 1.1% (0.4, 2.1%) | – | – | – |
| Test positivity* | 29 | 11.1% (10.0, 12.2%) | – | – | – |
| Compliance to follow-up | 24 | 79.0% (67.9, 88.3%) | 11 | 0.93 (0.84, 1.04) | −6.7% (−17.4, 4.0%) |
| CIN2 + /1000 invited | 21 | 2.7‰ (1.7, 3.2‰) | 17 | 2.43 (1.65, 3.59) | 1.8‰ (0.6, 3.1‰) |
| CIN2 + /1000 screened | 21 | 11.4‰ (8.5, 14.7‰) | 17 | 1.24 (0.77, 1.99) | 3.3‰ (−0.4, 7.1‰) |
*Test positivity of hrHPV test in the self-sampling arm (per-protocol).
#Number of studies.
For 20 of the included trials, data on the adequacy of self-samples was available [23–28, 30–38, 44, 46, 50–52]. The proportion of inadequate self-samples was generally low (overall = 1.1%; 95% CI 0.4%, 2.1%) except in one study [52] where this proportion was extremely high, 16.8% (95% CI 14.6%, 19.2%) (Supplementary Fig. S17). The exclusion of this study from the meta-analysis results for inadequate self-samples led to further decrease on the overall rate to 0.6% (95% CI 0.4%, 0.9%).
Data on hrHPV test positivity were reported in 29 trials. The test-positivity rate in the self-sampling arm ranged from 5.7% and 29.4% with a pooled proportion of 11.1% (95% CI 10.0%, 12.2%) (Supplementary Fig. S18). The follow-up adherence for women with a positive test result on a self-sample, among the 24 included studies, ranged between 41.0% and 100.0%, with pooled proportion of 79.0% (95% CI 67.9%, 88.3%) (Supplementary Fig. S19). The detection rate of CIN2 + was 2.7‰ (95% CI 1.7‰, 4.1‰) and 11.4‰ (95% CI 8.5‰, 14.7‰) among invited and screened women, respectively (Supplementary Figs. S20 and S21).
Discussion
CC has for long been known as a disease of inequities and its elimination, as a global public health problem, is one of the most pressing priorities for the World Health Organization (WHO) [4]. Secondary prevention, by identifying and treating women with (pre-)cancerous lesions, plays an essential role in reducing CC incidence and mortality. However, the success of CCS services is highly dependent on the motivation of women to attend a health centre. In response to this barrier, offering self-sampling kits for HPV testing has previously shown to be a highly effective method for reaching under-screened women [10, 54]. The worldwide use of HPV self-sampling for CCS remains relatively low and as of February 2021, only 17 countries of the 139 for which official screening recommendations were identified, reported having already introduced the self-sampling modality as a screening option in their programmes. In eight of these countries, self-sampling was reported as a strategy aimed only at under-screened women (Argentina, Australia, Denmark, Ecuador, Finland, France, Myanmar and Sweden). As for the other nine countries, such as the case of The Netherlands and Malaysia, HPV self-sampling has been made available for all women as a primary screening approach [55]. Nevertheless, since July 2022, both in Sweden and Australia, all women eligible for CCS have been given a choice between self-sampling or clinician sampling irrespective of their screening status [56, 57].
The present meta-analysis, despite the observed inter-study heterogeneity, confirmed in ITT analyses that hrHPV self-sampling methods are more effective in reaching women than the traditional invitations, regardless of the invitation strategy that was used. However, the PP participation in the opt-in scenario was overall not significantly more effective than with conventional invitations. The highest participation was observed when strategies involved a face-to-face invitation. Our updated meta-analysis evaluated also the participation in trials with different self-sampling strategies. The mail-to-all strategy was undoubtedly more effective in generating uptake than the opt-in.
The comparison of CCS uptake according to the different invitation strategies demonstrated the highest absolute gain in reaching under-screened populations when self-sampling kits were offered through community mobilisation & outreach. Over 90% of the women in the experimental group participated in screening by taking a self-sample in accordance to the strategy they were allocated to at randomisation. The face-to-face recruitment potentially led to an increase in the women’s confidence to perform the self-collection, whether because these women had to take part in a pre-screening explanatory event or because they were verbally instructed on how to use the self-sampling kit at the moment the kit was received. In addition, the high participation rate with self-sampling in the studies included in this scenario could be explained by the fact that women who agreed to provide a sample after attending the CCS awareness event had to take the sample on location and hand-it over immediately to the CHW [52] or received verbal instructions on how to return a sample taken at home [51]. Participating women who received the visit of the CHW at home/work were asked to provide the sample immediately upon invitation in two of the studies [48, 50] and in another [49], the CHW collected the used kits either later on the same day or the day after the invitation visit. The immediate requirement or short-time interval given to women for providing the sample in this invitation scenario potentially made it harder for the participants to fail or forget to return the kit for laboratory testing.
The offer at a healthcare service, also seems to be a promising strategy to improve screening participation rates since similarly to the community mobilisation & outreach scenario, it benefits from the face-to-face invitation, also giving women the chance to clarify any doubts that might exist with regards to the instructions for use or the reliability of the hrHPV self-sampling test [58, 59]. Moreover, a trustworthy relationship between the women and their HCP seems to be linked to their willingness to accept the offer of screening services [59]. Similar conclusions were found in a small RCT carried out at a GP practice in Belgium, which compared the response between the direct offer of a self-sampling kit by a GP versus a GP recommendation to have a Pap smear taken. The participation rate in the self-sampling arm (78%) was significantly higher than that in the control arm (51%, P = 0.009) [60]. Because this study comprised a small study population (n < 400), it was not considered eligible for the present meta-analysis. Our results for the offer at healthcare service scenario, in ITT analysis, showed that about half of women randomised to the experimental arm and 22% of those randomised to control, accepted the screening services. However, only one study from New Zealand investigating this invitation strategy met the inclusion criteria and therefore the results obtained for these can not be generalised to the context of other countries [53].
Directly sending a self-sampling kit to the women’s homes has also demonstrated to be an effective strategy for reaching under-screened women compared to the standard invitations. From an environmental, logistical and economic point of view, however, this strategy might not be the most attractive or realistic to implement at a global scale. In addition to that, considerable heterogeneity was observed on the effect size of the studies included in this scenario. Nevertheless, mail-to-all invitations, on a country-by-country situation have shown to be successful in improving screening engagement. All of the studies investigating this scenario in our meta-analysis were carried out in high-income countries most likely benefiting from effective and fast postal mail services, an aspect which is essential for the success of this invitation method.
When women were invited to perform self-sampling screening but given the additional responsibility of either collecting the kit elsewhere or making a request to receive one (opt-in scenario), engagement was still noticed but at a lower scale when compared to the other scenarios. In PP analysis, participation in the opt-in scenario was not significantly higher than in the control group. However, it is relevant to notice that in ITT analysis the overall participation rate after opt-in invitation was double to that observed when only screening with self-sampling was considered. The first two years of the new HPV-based screening programme in the Netherlands (2017–2018) revealed that only 7% of all screened women used a self-sampling kit. However, since then, self-sampling was more actively promoted and the manner of requesting a kit became more easy, resulting in a substantial increase on the proportion of screened women using self-sampling (16% in 2020) [61]. While this invitation strategy might not be the key to overcome the low screening rates among under-screened women, these results show that the provision of screening options to choose from can work as an improvement to the standard reminder. A pilot opt-in implementation study carried out in Denmark among non-attenders found that offering different methods for women to request the self-sampling kits resulted in different response rates to the invitation. Among the women who ordered the kit, 61% did it by regular mail, 37% via the study webpage, 1% via phone call and 1% made the request by email [62]. Most recently, the large follow-up study of that pilot, came to revalidate the importance of offering different methods to order the self-sampling kit. During the four years in between studies, the women’s preferences towards the method used in response to invitation changed, with the majority (63%) of the participants resorting to the study website to place a request. Overall, the amount of requests done by letter suffered a drastic drop to about half of the percentage noted in the pilot study, 29.1%, while the phone and email options saw a modest increase to 5.6% and 2.3%, respectively [63]. Empowering women by giving them the control over their healthcare choices, in this case by allowing a certain level of autonomy to choose a method of ordering a self-sampling kit or the option to pick a screening strategy of their liking and convenience, can potentially improve their engagement level.
Our meta-analysis clearly showed that overall, an invitation to collect or order a kit for HPV self-sampling is less effective in generating screening participation than when these kits are directly sent to the women’s home address, both when participation was computed according to the PP and ITT analyses. However, when looking into the results at study level, in some cases the gain in participation for the mail-to-all scenario compared to opt-in was very small [39, 40]. Thus, in context of specific real-life screening settings inviting women to order a self-sampling kit might result in similar screening uptake to that observed when kits are directly sent in the mail, while being superior in terms of cost-effectiveness. Furthermore, there are also reported instances for which mail-to-all strategy revealed to be ineffective in prompting screening uptake to a level that justifies the implementation of such strategy in the detriment of the standard screening [27]. The implementation of one strategy or the other, should be preceded by local pilot projects, to evaluate the feasibility, effectiveness and sustainability in the context of a specific country or region. This suggestion is further substantiated by the findings of Tranberg et al. [64]. In this study, a randomised controlled effectiveness trial that was enriched by data on socioeconomic register-based variables, it was found that mailing self-sampling kits directly to women resulted in a higher participation rate than an opt-in invitation across all socioeconomic groups however, significant contrasts in the participation difference for mail-to-all vs. opt-in were observed across ethnic groups. Western immigrants seemed to benefit particularly more from the mail-to-all strategy than non-Western immigrants and native Danish women [64].
The proportion of self-samples with an inadequate result was very low, supporting the usability of this screening method and an argument which could be used to reassure women who doubt their competencies for performing a sample themselves. The timely treatment of 90% of the detected precancerous lesions is one of the requisites of the WHO global strategy to accelerate the elimination of CC [4]. Therefore, the engagement of women with positive test results in follow-up procedures is essential to achieve that goal. In our meta-analysis, overall compliance with follow-up was high but varied considerably across studies (average 79% range 41–100%). It is important, however, to remember that these studies were carried out under controlled study conditions which may be difficult to replicate in real-world screening programmes. Particularly among minority groups and socially disadvantaged women, while self-collection has been deemed as particularly suitable to increase screening attendance, barriers to follow-up (i.e., anxiety, lack of knowledge or appropriate guidance, lack of time) identical to those identified within cytology-based programmes remain a reality in context of self-collection screening [65]. In-person, clinician-led follow-up appointments or reminders, continue to be a necessary to assure appropriate follow-up. Additional research is needed to identify alternative strategies for improving the level of compliance.
Study limitations
While this study provides an updated overview of the impact of offering a self-sampling device as an alternative to the current clinical practice for CCS, some limitations should be taken into account. The observed between-study heterogeneity affected our overall confidence in the conclusions that were reached. As a consequence, no universal conclusion is possible with regards to one invitation strategy being distinctly superior to other. Thus, local engagement of the community and pilot studies should precede implementation. Moreover, the generalisability of our results is somehow limited to the context of the countries where the studies included within each scenario were carried out, given that the interventions included in the community mobilisation & outreach scenario were only investigated in low- and middle-income countries opposite to the mail-to-all and opt-in scenarios that were only explored in high-income context. Other limitation to this study, inherent to most meta-analyses using aggregated data, is the limited data on influential factors. Meta-regression and sub-group analysis were not possible since for most studies, data on covariates was not made available or when provided, different categorisations were used across studies.
Conclusion
HPV self-sampling as an alternative to traditional invitations for screening by a HCP has the potential to increase participation among under- and never-screened women. Face-to-face offers of self-sampling kits for hrHPV testing, either in context of community mobilisation & outreach or offered at the healthcare service, seems to be the most effective approach to increase screening in under-screened populations. The mail-to-all strategy has also led to an improvement of participation rates in high-income countries. The opt-in invitations resulted in the lowest gain in screening uptake. While our pooled results support the use of self-sampling strategies to accelerate CC elimination, large inter-study heterogeneity was noted between and within invitation scenarios. Pilot implementation studies should be set up to identify and tailor the best strategy to introduce HPV self-sampling to the population and evaluate its viability and appropriateness in the specific local context before general roll-out.
Supplementary information
Acknowledgements
Horizon 2020 Framework Programme for Research and Innovation of the European Commission and the US National Cancer Institute/NIH/DHHS.
Author contributions
MA designed the study concept and protocol. MA, SC and BV contributed to the data extraction, and SC conducted the statistical analysis. SC and BV co-wrote the drafts of the manuscript. MA and PEC provided critical revisions for the manuscript, and all co-authors provided final approval to publish. All authors had full access to the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
MA was supported by the Horizon 2020 Framework Programme for Research and Innovation of the European Commission, through the RISCC Network (Grant No. 847845); Sciensano the employer of MA and SC received funding in the framework VALHUDES, a researcher-induced protocol for evaluation of HPV tests on self-samples (see Arbyn et al. [66]). MA and SC did not receive any financial or material benefit from this project. BV was supported by the Horizon 2020 Framework Programme for Research and Innovation of the European Commission, through the ELEVATE project (Grant No. 825747). PEC was supported by the intramural research program (ZIACP101237-01) of the US National Cancer Institute/NIH/DHHS.
Data availability
The datasets generated during and/or analysed during this study are available from the corresponding author on reasonable request.
Competing interests
PEC has received HPV tests and assays for research at a reduced or no cost from Roche, Becton Dickinson, Cepheid and Arbor Vita Corporation.
Ethics approval and consent to participate
This study used publicly available data extracted from published studies. Ethical approval by an Institutional Review Board was not required.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Stefanie Costa, Bo Verberckmoes.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-02094-w.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catarino R, Petignat P, Dongui G, Vassilakos P. Cervical cancer screening in developing countries at a crossroad: emerging technologies and policy choices. World J Clin Oncol. 2015;6:281. doi: 10.5306/wjco.v6.i6.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Global strategy to accelerate the elimination of cervical cancer as a public health problem. Geneva, CH: World Health Organization; 2020.
- 5.Scarinci IC, Garcia FAR, Kobetz E, Partridge EE, Brandt HM, Bell MC, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010;116:2531–42. doi: 10.1002/cncr.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Human papillomaviruses. Vol. 90. Lyon, FR: International Agency for Research on Cancer; 2007.
- 7.Arbyn M, Ronco G, Anttila A, Meijer CJLM, Poljak M, Ogilvie G, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30:F88–99. doi: 10.1016/j.vaccine.2012.06.095. [DOI] [PubMed] [Google Scholar]
- 8.Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJF, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–32. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 9.Arbyn M, Verdoodt F, Snijders PJF, Verhoef VMJ, Suonio E, Dillner L, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15:172–83. doi: 10.1016/S1470-2045(13)70570-9. [DOI] [PubMed] [Google Scholar]
- 10.Arbyn M, Smith SB, Temin S, Sultana F, Castle P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823. doi: 10.1136/bmj.k4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbyn M, Castle PE. Offering self-sampling kits for HPV Testing to reach women who do not attend in the regular cervical cancer screening program. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2015;24:769–72. doi: 10.1158/1055-9965.EPI-14-1417. [DOI] [PubMed] [Google Scholar]
- 12.Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007;45:93–106. doi: 10.1016/j.ypmed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Andrae B, Kemetli L, Sparén P, Silfverdal L, Strander B, Ryd W, et al. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst. 2008;100:622–9. doi: 10.1093/jnci/djn099. [DOI] [PubMed] [Google Scholar]
- 14.Jalili F, O’Conaill C, Templeton K, Lotocki R, Fischer G, Manning L, et al. Assessing the impact of mailing self-sampling kits for human papillomavirus testing to unscreened non-responder women in Manitoba. Curr Oncol. 2019;26:167–72. doi: 10.3747/co.26.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris R, Bradburn M, Deeks DJJ, Harbord HR, Altman ADG, Sterne J. metan: fixed- and random-effects meta-analysis. Stata J. 2008;8:3–28. doi: 10.1177/1536867X0800800102. [DOI] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.StataCorp. Stata: Release 16. Statistical software. College Station, Texas, USA: College Station, Texas, USA: Stata Corp LLC; 2019.
- 22.Veerus P, Hallik R, Jänes J, Jõers K, Paapsi K, Laidra K, et al. Human papillomavirus self-sampling for long-term non-attenders in cervical cancer screening: a randomised feasibility study in Estonia. J Med Screen. 2022;29:53–60. doi: 10.1177/09691413211052499. [DOI] [PubMed] [Google Scholar]
- 23.Bais AG, van Kemenade FJ, Berkhof J, Verheijen RHM, Snijders PJF, Voorhorst F, et al. Human papillomavirus testing on self-sampled cervicovaginal brushes: an effective alternative to protect nonresponders in cervical screening programs. Int J Cancer. 2007;120:1505–10. doi: 10.1002/ijc.22484. [DOI] [PubMed] [Google Scholar]
- 24.Gök M, Heideman DAM, van Kemenade FJ, Berkhof J, Rozendaal L, Spruyt JWM, et al. HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study. BMJ. 2010;340:c1040. doi: 10.1136/bmj.c1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giorgi Rossi P, Marsili LM, Camilloni L, Iossa A, Lattanzi A, Sani C, et al. The effect of self-sampled HPV testing on participation to cervical cancer screening in Italy: a randomised controlled trial (ISRCTN96071600) Br J Cancer. 2011;104:248–54. doi: 10.1038/sj.bjc.6606040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piana L, Leandri FX, Le RL, Heid P, Tamalet C, Sancho-Garnier H. L’auto-prélèvement vaginal à domicile pour recherche de papilloma virus à haut risque. Une solution de remplacement pour les femmes ne participant pas au dépistage cytologique des cancers du col de l’utérus. Campagne expérimentale du département des Bouches-duRhône. Bull Cancer. 2011;98:723–31. doi: 10.1684/bdc.2011.1388. [DOI] [PubMed] [Google Scholar]
- 27.Szarewski A, Cadman L, Mesher D, Austin J, Ashdown-Barr L, Edwards R, et al. HPV self-sampling as an alternative strategy in non-attenders for cervical screening—a randomised controlled trial. Br J Cancer. 2011;104:915–20. doi: 10.1038/bjc.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virtanen A, Nieminen P, Luostarinen T, Anttila A. Self-sample HPV tests as an intervention for nonattendees of cervical cancer screening in Finland: a randomized trial. Cancer Epidemiol Biomark Prev. 2011;20:1960–9. doi: 10.1158/1055-9965.EPI-11-0307. [DOI] [PubMed] [Google Scholar]
- 29.Wikström I, Lindell M, Sanner K, Wilander E. Self-sampling and HPV testing or ordinary Pap-smear in women not regularly attending screening: a randomised study. Br J Cancer. 2011;105:337–9. doi: 10.1038/bjc.2011.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gök M, van Kemenade FJ, Heideman DAM, Berkhof J, Rozendaal L, Spruyt JWM, et al. Experience with high-risk human papillomavirus testing on vaginal brush-based self-samples of non-attendees of the cervical screening program. Int J Cancer. 2012;130:1128–35. doi: 10.1002/ijc.26128. [DOI] [PubMed] [Google Scholar]
- 31.Darlin L, Borgfeldt C, Forslund O, Hénic E, Hortlund M, Dillner J, et al. Comparison of use of vaginal HPV self-sampling and offering flexible appointments as strategies to reach long-term non-attending women in organized cervical screening. J Clin Virol. 2013;58:155–60. doi: 10.1016/j.jcv.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Sancho-Garnier H, Tamalet C, Halfon P, Leandri FX, Retraite LL, Djoufelkit K, et al. HPV self-sampling or the Pap-smear: a randomized study among cervical screening nonattenders from lower socioeconomic groups in France: HPV self-sampling or the Pap-smear for screening among nonattenders women? Int J Cancer. 2013;133:2681–7. doi: 10.1002/ijc.28283. [DOI] [PubMed] [Google Scholar]
- 33.Haguenoer K, Sengchanh S, Gaudy-Graffin C, Boyard J, Fontenay R, Marret H, et al. Vaginal self-sampling is a cost-effective way to increase participation in a cervical cancer screening programme: a randomised trial. Br J Cancer. 2014;111:2187–96. doi: 10.1038/bjc.2014.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cadman L, Wilkes S, Mansour D, Austin J, Ashdown-Barr L, Edwards R, et al. A randomized controlled trial in non-responders from Newcastle upon Tyne invited to return a self-sample for human papillomavirus testing versus repeat invitation for cervical screening. J Med Screen. 2015;22:28–37. doi: 10.1177/0969141314558785. [DOI] [PubMed] [Google Scholar]
- 35.Giorgi Rossi P, Fortunato C, Barbarino P, Boveri S, Caroli S, Del Mistro A, et al. Self-sampling to increase participation in cervical cancer screening: an RCT comparing home mailing, distribution in pharmacies, and recall letter. Br J Cancer. 2015;112:667–75. doi: 10.1038/bjc.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enerly E, Bonde J, Schee K, Pedersen H, Lönnberg S, Nygård M. Self-sampling for human papillomavirus testing among non-attenders increases attendance to the Norwegian cervical cancer screening programme. PLoS ONE. 2016;11:e0151978. doi: 10.1371/journal.pone.0151978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Racey CS, Gesink DC, Burchell AN, Trivers S, Wong T, Rebbapragada A. Randomized intervention of self-collected sampling for human papillomavirus testing in under-screened rural women: uptake of screening and acceptability. J Women’s Health. 2016;25:489–97. doi: 10.1089/jwh.2015.5348. [DOI] [PubMed] [Google Scholar]
- 38.Sultana F, English DR, Simpson JA, Drennan KT, Mullins R, Brotherton JML, et al. Home-based HPV self-sampling improves participation by never-screened and under-screened women: results from a large randomized trial (iPap) in Australia: home-based HPV self-sampling in never- and under-screened women. Int J Cancer. 2016;139:281–90. doi: 10.1002/ijc.30031. [DOI] [PubMed] [Google Scholar]
- 39.Kitchener H, Gittins M, Cruickshank M, Moseley C, Fletcher S, Albrow R, et al. A cluster randomized trial of strategies to increase uptake amongst young women invited for their first cervical screen: the STRATEGIC trial. J Med Screen. 2018;25:88–98. doi: 10.1177/0969141317696518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivanus U, Jerman T, Fokter AR, Takac I, Prevodnik VK, Marcec M, et al. Randomised trial of HPV self-sampling among non-attenders in the Slovenian cervical screening programme ZORA: comparing three different screening approaches. Radio Oncol. 2018;52:399–412. doi: 10.2478/raon-2018-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kellen E, Benoy I, Vanden Broeck D, Martens P, Bogers JP, Haelens A, et al. A randomized, controlled trial of two strategies of offering the home-based HPV self-sampling test to non-participants in the Flemish cervical cancer screening program: home-based HPV self-sampling test. Int J Cancer. 2018;143:861–8. doi: 10.1002/ijc.31391. [DOI] [PubMed] [Google Scholar]
- 42.Tranberg M, Bech BH, Blaakær J, Jensen JS, Svanholm H, Andersen B. Preventing cervical cancer using HPV self-sampling: direct mailing of test-kits increases screening participation more than timely opt-in procedures—a randomized controlled trial. BMC Cancer. 2018;18:273. doi: 10.1186/s12885-018-4165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elfström KM, Sundström K, Andersson S, Bzhalava Z, Carlsten Thor A, Gzoul Z, et al. Increasing participation in cervical screening by targeting long-term nonattenders: randomized health services study. Int J Cancer. 2019;145:3033–9. doi: 10.1002/ijc.32374. [DOI] [PubMed] [Google Scholar]
- 44.Winer RL, Lin J, Tiro JA, Miglioretti DL, Beatty T, Gao H, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi: 10.1001/jamanetworkopen.2019.14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lilliecreutz C, Karlsson H, Spetz, Holm AC. Participation in interventions and recommended follow-up for non-attendees in cervical cancer screening -taking the women’s own preferred test method into account—a Swedish randomised controlled trial. PLoS ONE. 2020;15:e0235202. doi: 10.1371/journal.pone.0235202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brewer N, Bartholomew K, Grant J, Maxwell A, McPherson G, Wihongi H, et al. Acceptability of human papillomavirus (HPV) self-sampling among never- and under-screened Indigenous and other minority women: a randomised three-arm community trial in Aotearoa New Zealand. Lancet Reg Health West Pac. 2021;16:100265. doi: 10.1016/j.lanwpc.2021.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broberg G, Gyrd-Hansen D, Miao Jonasson J, Ryd ML, Holtenman M, Milsom I, et al. Increasing participation in cervical cancer screening: offering a HPV self-test to long-term non-attendees as part of RACOMIP, a Swedish randomized controlled trial. Int J Cancer. 2014;134:2223–30. doi: 10.1002/ijc.28545. [DOI] [PubMed] [Google Scholar]
- 48.Lazcano-Ponce E, Lorincz AT, Cruz-Valdez A, Salmerón J, Uribe P, Velasco-Mondragón E, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011;378:1868–73. doi: 10.1016/S0140-6736(11)61522-5. [DOI] [PubMed] [Google Scholar]
- 49.Arrossi S, Thouyaret L, Herrero R, Campanera A, Magdaleno A, Cuberli M, et al. Effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): a population-based cluster-randomised trial. Lancet Glob Health. 2015;3:e85–94. doi: 10.1016/S2214-109X(14)70354-7. [DOI] [PubMed] [Google Scholar]
- 50.Moses E, Pedersen HN, Mitchell SM, Sekikubo M, Mwesigwa D, Singer J, et al. Uptake of community-based, self-collected HPV testing vs. visual inspection with acetic acid for cervical cancer screening in Kampala, Uganda: preliminary results of a randomised controlled trial. Trop Med Int Health. 2015;20:1355–67. doi: 10.1111/tmi.12549. [DOI] [PubMed] [Google Scholar]
- 51.Modibbo F, Iregbu KC, Okuma J, Leeman A, Kasius A, de Koning M, et al. Randomized trial evaluating self-sampling for HPV DNA based tests for cervical cancer screening in Nigeria. Infect Agent Cancer. 2017;12:11. doi: 10.1186/s13027-017-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gizaw M, Teka B, Ruddies F, Abebe T, Kaufmann AM, Worku A, et al. Uptake of cervical cancer screening in Ethiopia by self-sampling HPV DNA compared to visual inspection with acetic acid: a cluster randomized trial. Cancer Prev Res Philos Pa. 2019;12:609–16. doi: 10.1158/1940-6207.CAPR-19-0156. [DOI] [PubMed] [Google Scholar]
- 53.MacDonald EJ, Geller S, Sibanda N, Stevenson K, Denmead L, Adcock A, et al. Reaching under-screened/never-screened indigenous peoples with human papilloma virus self-testing: a community-based cluster randomised controlled trial. Aust N. Z J Obstet Gynaecol. 2021;61:135–41. doi: 10.1111/ajo.13285. [DOI] [PubMed] [Google Scholar]
- 54.Verdoodt F, Jentschke M, Hillemanns P, Racey CS, Snijders PJF, Arbyn M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: a systematic review and meta-analysis of randomised trials. Eur J Cancer. 2015;51:2375–85. doi: 10.1016/j.ejca.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Serrano B, Ibáñez R, Robles C, Peremiquel-Trillas P, de Sanjosé S, Bruni L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev Med. 2022;154:106900. doi: 10.1016/j.ypmed.2021.106900. [DOI] [PubMed] [Google Scholar]
- 56.Elfström KM, Dillner J. Cervical cancer screening improvements with self-sampling during the COVID-19 pandemic. medRxiv. [Preprint] 2022. Available from: https://www.medrxiv.org/content/10.1101/2022.07.19.22277806v1.full. [DOI] [PMC free article] [PubMed]
- 57.Australian Government - Department of Health and Aged Care. Self collection to increase choice within the National Cervical Screening Program [Internet]. 2021 [cited 2022 Sep 28]. https://www.health.gov.au/news/self-collection-to-increase-choice-within-the-national-cervical-screening-program
- 58.Nishimura H, Yeh PT, Oguntade H, Kennedy CE, Narasimhan M. HPV self-sampling for cervical cancer screening: a systematic review of values and preferences. BMJ Glob Health. 2021;6:e003743. doi: 10.1136/bmjgh-2020-003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLachlan E, Anderson S, Hawkes D, Saville M, Arabena K. Completing the cervical screening pathway: factors that facilitate the increase of self-collection uptake among under-screened and never-screened women, an Australian pilot study. Curr Oncol. 2018;25:17–26. doi: 10.3747/co.25.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peeters E, Cornet K, Devroey D, Arbyn M. Efficacy of strategies to increase participation in cervical cancer screening: GPs offering self-sampling kits for HPV testing versus recommendations to have a pap smear taken—a randomised controlled trial. Papillomavirus Res. 2020;9:100194. doi: 10.1016/j.pvr.2020.100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aitken CA, Inturrisi F, Kaljouw S, Nieboer D, Siebers AG, Melchers WJG, et al. Sociodemographic characteristics and screening outcomes of women preferring self-sampling in the Dutch cervical cancer screening programme: a population-based study. Cancer Epidemiol Biomarkers Prev. 2022. 10.1158/1055-9965.EPI-22-0712. [DOI] [PMC free article] [PubMed]
- 62.Lam JUH, Rebolj M, Møller Ejegod D, Pedersen H, Rygaard C, Lynge E, et al. Human papillomavirus self-sampling for screening nonattenders: opt-in pilot implementation with electronic communication platforms. Int J Cancer. 2017;140:2212–9. doi: 10.1002/ijc.30647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ejegod DM, Pedersen H, Pedersen BT, Serizawa R, Bonde J. Operational experiences from the general implementation of HPV self-sampling to Danish screening non-attenders. Prev Med. 2022;160:107096. doi: 10.1016/j.ypmed.2022.107096. [DOI] [PubMed] [Google Scholar]
- 64.Tranberg M, Bech BH, Blaakær J, Jensen JS, Svanholm H, Andersen B. HPV self-sampling in cervical cancer screening: the effect of different invitation strategies in various socioeconomic groups—a randomized controlled trial. Clin Epidemiol. 2018;10:1027–36. doi: 10.2147/CLEP.S164826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paolino M, Gago J, Le Pera A, Cinto O, Thouyaret L, Arrossi S. Adherence to triage among women with HPV-positive self-collection: a study in a middle-low income population in Argentina. ecancermedicalscience. 2020;14:1138. [DOI] [PMC free article] [PubMed]
- 66.Arbyn M, Peeters E, Benoy I, Vanden Broeck D, Bogers J, De Sutter P, et al. VALHUDES: A protocol for validation of human papillomavirus assays and collection devices for HPV testing on self-samples and urine samples. J Clin Virol. 2018;107:52–56. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during this study are available from the corresponding author on reasonable request.