Abstract
Previous research shows the beneficial effects of an intradialytic resistance training (IRT) on muscle function in haemodialysis patients. However, patients vary highly in their functional responses to IRT, may be due to effects of age and sex heterogeneities in adaptation. Therefore, the aim of this study was to investigate the degree to which the effects of IRT on the muscle function of haemodialysis patients vary by age and sex. We included 57 patients who completed a 12-week IRT (EXG) and 33 patients who received no IRT (CNG) during haemodialysis. Muscle function (MF) was assessed using dynamometry before and after a 12-week intervention and after a 12-week follow-up. After the 12-week intervention, we found a moderation effect of age in the relative (%) change (p = 0.011) and absolute (Δ) change (p = 0.027) of MF, and a moderation effect of sex in %MF (p = 0.001), but not in ΔMF (p = 0.069). Regarding patients’ age, the change of MF was only significantly different between EXG and CNG patients aged 60–70 years (%MF, EXG: + 34.6%, CNG: − 20.1%, p < 0.001; ΔMF, EXG: + 44.4 N, CNG: − 22.1 N, p < 0.001). Regarding patients’ sex, the change of MF was only significantly different between EXG and CNG female patients (%MF, EXG: + 23.9%, CNG: − 23.6%, p < 0.001). Age and sex did not significantly moderate changes in MF measures after 12 weeks of follow-up. We conclude that both age and sex of haemodialysis patients affect their functional response to IRT in the short term.
Trial Registration: Intradialytic Resistance Training in Haemodialysis Patients (IRTHEP)—#NCT03511924, 30/04/2018, https://clinicaltrials.gov/ct2/show/NCT03511924.
Subject terms: Renal replacement therapy, Rehabilitation
Introduction
Haemodialysis and kidney disease have been shown to negatively affect patients’ physical activity behaviour, physical functioning, musculoskeletal health, body composition and quality of life in haemodialysis patients (CKD-5D)1–3. The participation of kidney disease patients in regular physical activities is generally low, decreasing with kidney disease progression and reaching nadir in elderly CKD-5D patients1,2,4. Negative trends in patient’s behaviour are manifested in decreased muscle mass and function, bone mineral density, quality of bone structure, and resulted in declined mobility, health-related quality of life, and survival rates during therapy2–7.
Intradialytic resistance training (IRT) positively affected physical functions, mobility, nutritional status, body composition, quality of life, dialysis-related clinical outcomes and mortality in CKD-5D patients8–12. IRT improved muscle functions (MF) of lower extremities, positively affects survival in CKD-5D patients, and the change in MF was associated with the presence of diabetes mellitus and microribonucleic acid expression profiles as detailed in our previous studies13–16. Besides clinical efficiency in the prevention of physical function decline, large inter-individual differences in the physiological response to IRT have been reported among CKD5-D patients17–19. The heterogeneity of findings regarding the effectiveness of IRT (on muscle functions) in CKD-5D patients may be due to individual differences in the physiological adaptation to resistance training.
Strong evidence exists for the beneficial effects of resistance training on muscle mass and function and several authors have concluded that acute responses and chronic adaptation to resistance training in healthy subjects vary by age20–23 and by sex24–27. Therefore, age and sex differences in the effects of IRT may also exist among CKD-5D patients. In the general dialysis population, the volume and function of the skeletal muscle were lower in males than in females patients, and were negatively associated with age28–31. No differences were found between male and female CKD-5D patients in changes in body composition, muscle size, or muscular strength, after a 12-week resistance training intervention32. No differences were found in the change of physical functioning between the elderly and other age groups of patients after a walking exercise intervention33. In summary, the current evidence regarding age and sex heterogeneities in functional adaptation in CKD-5D patients is scarce and lacking34,35, and recommendations for intradialytic exercise are not specified by age and sex of patients36. Therefore, the aim of this study was to investigate whether age and sex moderated the effectiveness of IRT in CKD-5D patients.
Methods
Study design
We conducted a quasi-experimental, two-group, pre-post comparative study with an intervention of 12 weeks and a 12-weeks follow-up in 2018 at three dialysis centres to assess the effects of IRT on the lower extremity MF among CKD-5D patients. A comprehensive description of the objectives, design, methods and analysis of this study is provided elsewhere37. The Ethics Committee of Pavol Jozef Safarik University in Kosice reviewed and approved the study protocol (Approval no. 14N/2017). All methods, assessments and data acquisition were conducted in accordance with the Declaration of Helsinki of 1975 and with the Good Clinical Practice Principles of the International Council for Harmonization. The study was registered in ClinicalTrials.gov on 30/04/2018 (NCT03511924).
Subjects
For the purpose of this study we assessed the eligibility of patients treated at three dialysis centres (two dialysis centres located in Kosice, one dialysis centre located in Banska Bystrica). We selected three centres to meet the expected patient numbers in the study groups, and took specifically these three because they had identical patients' treatment regimens and a similar age and gender distribution. The inclusion criteria were as follows: age above 30 years, diagnosed with stage 5 chronic kidney disease, history of maintenance dialysis therapy for at least the last 3 months. Exclusion criteria were lower extremity amputation, severe dementia or retardation, presence of acute intercurrent disease and the probability of 1-year mortality higher than 25% according to the Charlson Comorbidity Index38. Informed consent was obtained from all individual participants included in the study.
Sample size calculation
For the purpose of this study, its statistical power was re-calculated with use of GPower 3.1® (Heinrich-Heine-University, Düsseldorf, Germany). We used a priori F test for an analysis of variance, with ten subgroups (two interventions by three age and two sex categories), a power of 80% and an effect size (Cohen’s f) of 0.40. We found that at least 64 patients totally are needed to detect differences in the change in MF by the intervention, age and sex.
Patient allocation
Patients attending dialysis therapy at both sites in Kosice were allocated to the experimental group (EXG, n = 57), while patients from the Banska Bystrica dialysis centre were allocated to the control group (CON, n = 33). After the allocation procedure, the investigatory team members and participating patients were informed about the group assignment structure16.
Intervention period—experimental condition
All EXG patients started the 12-week IRT programme according to clinical recommendations for exercise interventions in CKD-5D patients within a week after completion of the baseline assessments36. EXG subjects were asked to follow the prescribed IRT programme and not to make any significant lifestyle regimen and exercise behavioural changes during the time of the study, especially in the RT component. A detailed description of the methodology, periodization and progressivity of IRT applied in EXG patients is provided elsewhere37.
Intervention period—control condition
Patients allocated to the CNG received their standard nephrology care without any intervention increasing their physical activity during dialysis. These patients were requested to maintain their standard treatment regimen and to maintain their customary dietary and physical activity patterns, especially in the RT component. During the control period these patients received increased attention from the research team members but were physically inactive during haemodialysis sessions.
Follow-up period
All patients enrolled in the study underwent a 12-week follow-up period after the completion of the experimental or control condition. During the follow-up, the patients were instructed not to participate in any structured physical activity during dialysis.
Measures—primary outcome
We used muscle function (MF) a primary outcome, measured as the maximal force produced by the patient during isometric contraction of hip extensor muscles. During the assessments, patients were in a supine position with arms safely and comfortably placed on the bed. The patient held the dominant leg in a straightened position, while the dynamometer was placed proximally to the ankle on the posterior surface of the lower leg. The patients were instructed to perform a maximal isometric contraction and hold it for 5 s. The tests were repeated within 30-s rest intervals, and the higher measured values of two consecutive tests were used for the analysis.
Before the assessments of MF took place, patients became familiar with test protocol and realized an exploratory set of the patient’s MF assessments with emphasis on the proper execution of muscle contractions. At the consecutive dialysis session, maximal isometric contraction force during the extension of the lower limb at the hip joint was assessed using a hand-held dynamometer (Universal digital force gauge HF 500, SAUTER GmbH, Balingen, Germany). The range of the dynamometer analyser was set from 0 to 500 N, with a recording interval of 0.1 N. These assessments of maximal isometric contraction force have excellent interrater reliability and accuracy39–41. The accuracy of the device used for assessments of muscle function in our study was verified with standard weights and the margin of error was below 5%. The absolute changes (ΔMF) of maximal isometric forces were calculated as post-intervention measure minus baseline measure and post-follow up measure minus baseline measure. The relative changes (%MF) of maximal isometric forces were calculated as the absolute value of post-intervention and post-follow up changes divided by baseline measure and multiplied by 100. All physical tests were administered by one member of the investigatory team (AZ).
Measures—background variables
We collected clinical measures (values registered from the last preceding serology and haematology tests) and body composition (patient’s body weight and height) from the medical database. We calculated the body mass index (BMI) for all patients as body weight in kilograms divided by the body height in metres squared (kg/m2). Patients’ age in decimals of years and sex (male/female) were collected from the medical database. For the analysis of differences between age groups, we categorised patients younger than 60.0 years in the middle-aged patients group (MA). Patients with an age between 60.0 and 70.0 were categorised in the younger-old group (YO), and patients older than 70.0 years were categorised in the older-old group (OO).
Background variables regarding the physical activity behaviour were assessed during an investigator-patient interview before and after exposure to the experimental and control conditions13. Regarding the individual physical activity we measured patient-reported frequency, duration and type of physical activities following the instructions of the Global Physical Activity Questionnaire42. A patient was considered to be physically inactive if he or she reported less than 3 × 30 min of moderate-intensity physical activity per week43.
Statistical analysis
First, we used the Kolmogorov–Smirnov test to assessed data normality, and the Levene’s test to analyse the homogeneity of variances in our database. Second, we assessed and quantified the flow and losses of subjects through the intervention and follow-up period of the study and briefly reported reasons for dropouts in each phase of the study according to the CONSORT statement recommendations44. Third, we assessed baseline primary outcome and background variables and compared them between the age and sex groups in EXG and CNG patients by one-way analysis of variance test. Data were presented as mean (M) ± standard deviation (SD). Fourth, we assessed whether effects of the experimental and control condition on the primary outcome (ΔMF and %MF) is moderated by patient’s sex or age, directly after the intervention and after the follow up period. We did so by adding these variables as moderator of group allocation in generalized linear model (GLM), and assessing the overall improvement of model fit based on that. We used the univariate GLM with the Bonferroni corrections for multiple comparisons to test main effects of allocation, age and sex (fixed factors), and moderation effects of allocation and age; and allocation and sex on the primary outcome (dependent value). Bonferroni post hoc tests were used to localize differences between the patients’ allocation and age or sex groups. Estimates of effects were presented as mean differences with 95% confidence interval (95% CI). We performed the analyses on an intention-to-treat basis, i.e., always including all 90 patients who had been enrolled in the study and had completed the baseline assessments regarding the primary outcomes. Statistical significance was defined as a p value below 0.05. Data analyses were carried out using the statistical software package IBM SPSS 22.0 (Version 22.0. Armonk, NY: IBM Corp.).
Results
Patient flow
We screened all 198 patients of three dialysis centres and regarding the inclusion and exclusion criteria, through their nephrologists. We identified 126 eligible patients and informed them about the possibility to participate in the study. In the end, 90 patients agreed to participate and signed a written informed consent prior to the study. Patients treated at two dialysis centres located in Kosice were allocated to the experimental group (EXG, n = 57). Patients treated at the dialysis centre in Banska Bystrica were allocated to the control group (CNG, n = 33).
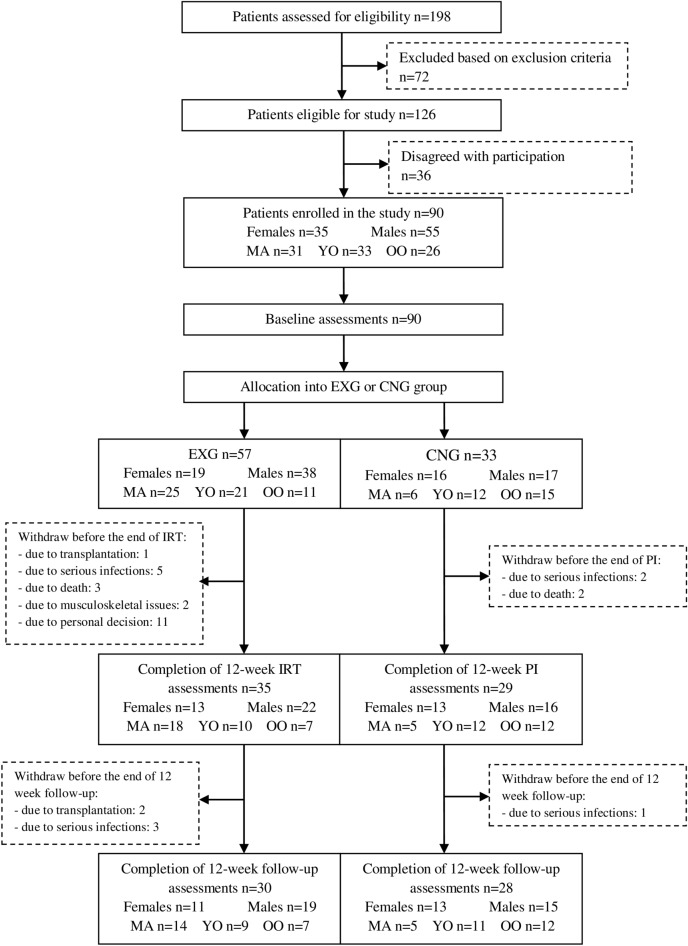
From the 57 patients initially included in the EXG, 22 patients discontinued participation in the study during the experimental condition. From the 33 patients who initially entered the CNG, four patients discontinued participation in the study during the control condition. During the 12-week follow-up, five patients in the EXG and one patient in the CNG dropped out due to mortality, transplantations, serious infections, personal decisions, and musculoskeletal issues. No adverse effects occurred during the application of exercise interventions or muscle strength assessments; see further the CONSORT flow diagram (Fig. 1)44. The resultant statistical power (1 − β error probability) of the study sample included in the data analysis was 0.93.
Figure 1.
The CONSORT flow diagram of patients summarising patients’ eligibility assessment, enrolment and allocation into the experimental (EXG) and control group (CNG) of the study and distribution of patients regarding age and sex subgroups (EC experimental condition, CC control condition, MA middle-aged, YO younger-old, OO older-old group).
Characteristics of the study participants
Patients’ baseline characteristics enrolled in the EXG and the CNG arm by age and sex are presented in Tables 1 and 2, respectively. The baseline assessments of the physical activity behaviour showed that 83 patients (92%) did not participate in customary physical activities.
Table 1.
Baseline patient characteristics, by arm and age.
| Variable | Experimental condition EXG (n = 57) | Control condition CNG (n = 33) | ||||||
|---|---|---|---|---|---|---|---|---|
| MA (n = 25) | YO (n = 21) | OO (n = 11) | p value | MA (n = 6) | YO (n = 12) | OO (n = 15) | p value | |
| Age in years (M, SD) | 47.6 (10.2) | 65.5 (3.3) | 76.2 (3.7) | < 0.001* | 51.9 (5.1) | 65.2 (3.1) | 75.6 (4.5) | < 0.001* |
| Body weight in kg (M, SD) | 77.9 (13.0) | 81.8 (20.3) | 68.7 (15.5) | 0.111 | 63.3 (8.1) | 75.5 (12.4) | 68.0 (17.2) | 0.202 |
| Body mass index in kg/m2 (M, SD) | 26.6 (5.2) | 28.1 (6.7) | 25.2 (4.7) | 0.384 | 22.8 (3.3) | 26.2 (4.2) | 24.2 (5.5) | 0.326 |
| Dialysis adequacy in Kt/V (M, SD) | 1.5 (0.3) | 1.6 (0.4) | 1.6 (0.4) | 0.859 | 2.0 (0.3) | 1.9 (0.4) | 2.1 (0.3) | 0.499 |
| Over-hydration index in % (M, SD) | 11.8 (9.6) | 12.2 (5.6) | 12.0 (3.5) | 0.983 | 9.9 (7.2) | 14.3 (5.0) | 11.3 (7.8) | 0.360 |
| C-reactive protein in mg/l (M, SD) | 10.3 (14.7) | 12.6 (13.8) | 11.7 (5.2) | 0.837 | 2.9 (3.5) | 8.4 (9.2) | 12.3 (14.3) | 0.236 |
| iPTH in pg/ml (M, SD) | 495.4 (436.9.0) | 362.9 (281.3) | 196.2 (113.7) | 0.057 | 186.2 (114.3) | 467.5 (560.4) | 370.7 (338.9) | 0.407 |
| Haemoglobin in g/l (M, SD) | 112.0 (14.2) | 112.0 (11.3) | 112.2 (14.7) | 0.999 | 114.8 (5.9) | 111.8 (17.0) | 113.2 (13.9) | 0.913 |
| Albumin in g/l (M, SD) | 39.4 (2.6) | 39.1 (3.2) | 39.6 (2.0) | 0.882 | 37.3 (1.0) | 35.8 (5.7) | 37.6 (4.4) | 0.567 |
| Ferritin in ng/ml (M, SD) | 558.8 (451.7) | 609.3 (466.7) | 649.6 (749.3) | 0.881 | 878.5 (355.1) | 841.4 (358.1) | 834.9 (352.8) | 0.967 |
| Phosphates in mml/l (M, SD) | 1.8 (0.5) | 1.6 (0.3) | 1.9 (0.5) | 0.078 | 1.4 (0.3) | 1.5 (0.6) | 1.4 (0.4) | 0.710 |
| Calcium in mmol/l (M, SD) | 2.2 (0.2) | 2.2 (0.2) | 2.0 (0.3) | 0.128 | 2.3 (0.1) | 2.2 (0.1) | 2.4 (0.1) | 0.367 |
| Potassium in mEq/l (M, SD) | 5.1 (0.7) | 5.0 (0.6) | 5.5 (1.0) | 0.167 | 5.5 (0.6) | 5.1 (0.8) | 5.1 (1.0) | 0.583 |
| Sodium in mEq/l (M, SD) | 138.2 (3.8) | 138.0 (2.4) | 138.0 (2.9) | 0.949 | 138.7 (2.7) | 137.8 (2.2) | 138.6 (2.9) | 0.664 |
| Hip extension in N (M, SD) | 178.6 (55.0) | 171.3 (66.3) | 113.7 (53.4) | 0.011# | 160.3 (57.0) | 147.8 (52.6) | 133.8 (36.5) | 0.476 |
iPTH intact parathyroid hormone, N Newton, EXG experimental group, CNG control group, MA middle-aged, YO younger-old, OO older-old group. Data are presented as mean (M) ± standard deviation (SD), p values determined by analysis of variance tests. #Differences between groups significant at p < 0.05. *Differences between groups significant at p < 0.001.
Table 2.
Baseline patient characteristics, by arm and sex.
| Variable | Experimental condition EXG (n = 57) | Control condition CNG (n = 33) | ||||
|---|---|---|---|---|---|---|
| Females (n = 19) | Males (n = 38) | p value | Females (n = 16) | Males (n = 17) | p value | |
| Age in years (M, SD) | 64.6 (14.2) | 57.3 (12.6) | 0.055 | 69.2 (10.1) | 65.9 (9.4) | 0.332 |
| Body weight in kg (M, SD) | 75.2 (19.6) | 78.8 (15.5) | 0.449 | 64.7 (14.1) | 74.7 (13.8) | 0.046# |
| Body mass index in kg/m2 (M, SD) | 28.5 (7.3) | 26.1 (4.6) | 0.122 | 24.6 (5.2) | 24.8 (4.5) | 0.932 |
| Dialysis adequacy in Kt/V (M, SD) | 1.8 (0.3) | 1.5 (0.3) | 0.001† | 2.2 (0.3) | 1.8 (0.3) | 0.002† |
| Over-hydration index in % (M, SD) | 10.4 (4.5) | 12.7 (8.3) | 0.260 | 12.3 (7.4) | 12.0 (6.4) | 0.923 |
| C-reactive protein in mg/l (M, SD) | 8.1 (4.6) | 13.1 (15.3) | 0.175 | 9.1 (13.2) | 9.3 (10.1) | 0.959 |
| iPTH in pg/ml (M, SD) | 480.7.8 (424.3) | 342.9 (308.4) | 0.167 | 491.8 (554.6) | 259.9 (155.8) | 0.108 |
| Haemoglobin in g/l (M, SD) | 109.8 (12.8) | 113.2 (13.3) | 0.361 | 108.1 (10.4) | 117.6 (15.3) | 0.048# |
| Albumin in g/l (M, SD) | 38.8 (3.1) | 3739.6 (2.5) | 0.317 | 36.2 (4.9) | 37.5 (4.1) | 0.403 |
| Ferritin in ng/ml (M, SD) | 695.2 (544.8) | 627.6 (452.0) | 0.304 | 932.4 (248.1) | 763.1 (405.6) | 0.161 |
| Phosphates in mml/l (M, SD) | 1.7 (0.4) | 1.8 (0.5) | 0.679 | 1.5 (0.5) | 1.5 (0.4) | 0.905 |
| Calcium in mmol/l (M, SD) | 2.1 (0.2) | 2.2 (0.3) | 0.387 | 2.4 (0.1) | 2.3 (0.1) | 0.003† |
| Potassium in mEq/l (M, SD) | 5.4 (0.8) | 5.0 (0.7) | 0.056 | 5.1 (1.0) | 5.3 (0.6) | 0.489 |
| Sodium in mEq/l (M, SD) | 138.6 (3.5) | 137.8 (2.9) | 0.354 | 138.6 (2.0) | 138.1 (3.1) | 0.589 |
| Hip extension in N (M, SD) | 113.1 (52.2) | 188.5 (52.5) | < 0.001* | 122.0 (32.6) | 164.1 (48.7) | 0.007† |
iPTH intact parathyroid hormone, N Newton, EXG experimental group, CNG control group. Data are presented as mean (M) ± standard deviation (SD), p values determined by analysis of variance tests. #Differences between groups significant at p < 0.05. †Differences between groups significant at p < 0.01. *Differences between groups significant at p < 0.001.
Differences in effects of 12-week intervention period on the primary outcome by age and sex
After 12-week intervention, we found a significant effect of the intervention on %MF (η2 = 0.199, p = 0.001,) and ΔMF (η2 = 0.137, p = 0.004). Effects on both measures of MF were significantly greater in the EXG compared to the CNG group (%MF: difference 25.2%, 95% CI = 11.8 to 38.6%, p = 0.001; ΔMF: difference 28.9 N, 95% CI = 9.7–48.1 N, p = 0.004).
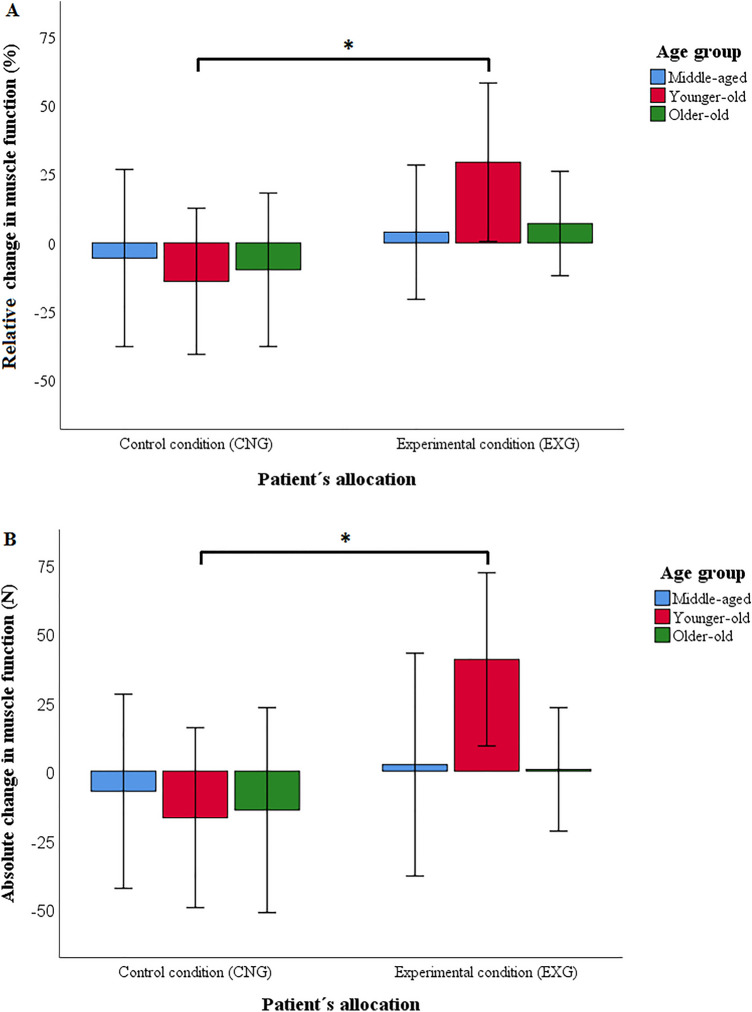
We found a significant effect of age on the effect of the intervention on of %MF (η2 = 0.145, p = 0.011) and on ΔMF (η2 = 0.119, p = 0.027). Both measures of MF change differed significantly between EXG and CNG in YO patients (%MF: difference = 54.7%, 95% CI = + 32.2 to + 77.1%, p < 0.001; ΔMF: difference = 66.5 N, 95% CI = + 34.3 to + 98.7 N, p < 0.001; see Fig. 2A,B). However, they did not differ in MA (%MF: difference = 10.2%, 95% CI = − 14.5 to + 35.0%, p = 0.441; ΔMF: difference = 9.9 N, 95% CI = − 25.5 to + 45.4 N, p = 0.577; see Fig. 2A,B) and neither in OO patients (%MF: difference = 10.8%, 95% CI = − 12.8 to + 34.4%, p = 0.364; ΔMF: difference = 10.2 N, 95% CI = − 23.6 to + 44.0 N, p = 0.548; see Fig. 2A,B).
Figure 2.
Relative (A) and absolute (B) changes in muscle function by patients’ allocation and age after the 12-week intervention (CNG control condition, EXG experimental condition). Data were presented as mean ± standard deviation. *Differences between groups significant at p < 0.001. p values calculated for intention-to-treat analysis (n = 90).
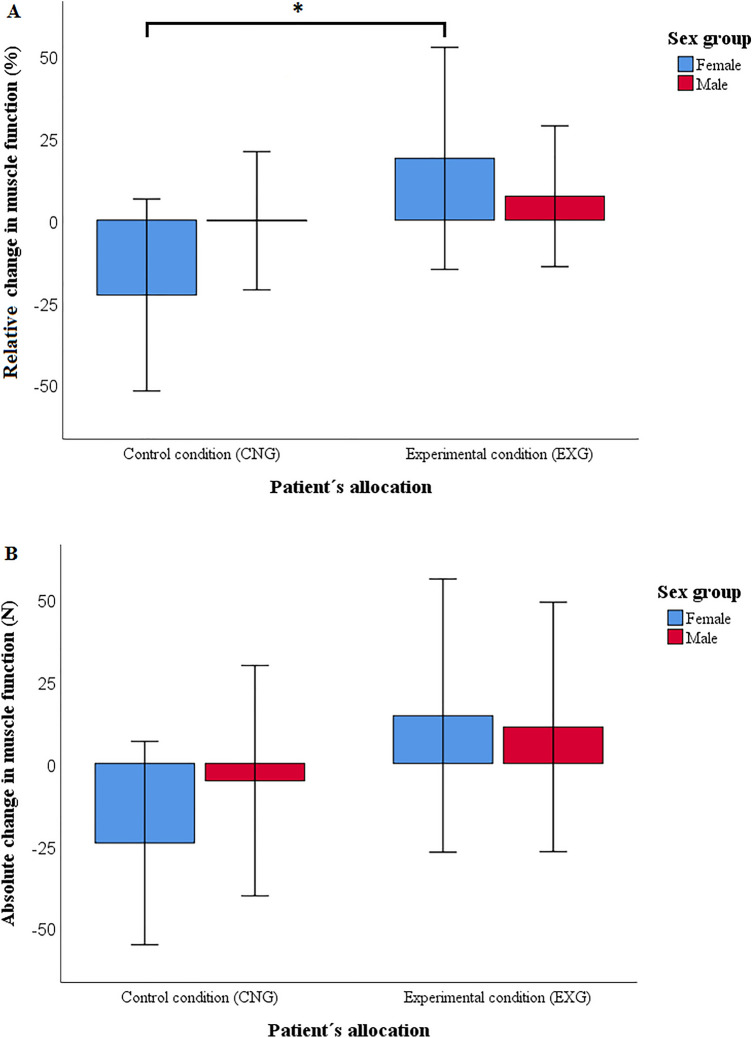
We found a significant effect of sex on the effect of the intervention in the change of %MF (η2 = 0.165, p = 0.001), however not for change of ΔMF (η2 = 0.057, p = 0.069). The %MF change differed significantly between EXG and CNG female patients (%MF: difference = 47.5%, 95% CI = 27.7 to 67.3%, p < 0.001), but not between EXG and CNG male patients (%MF: difference = 3.0%, 95% CI = − 14.9 to + 20.8%, p = 0.741, see Fig. 3A,B). Differences in the changes of %MF and ΔMF in the CNG and EXG patients, by age and sex, are presented in Tables 3 and 4, respectively.
Figure 3.
Relative (A) and absolute (B) changes in muscle function by patients’ allocation and sex after the 12-week intervention (CNG control condition, EXG experimental condition). Data were presented as mean ± standard deviation. *Differences between groups significant at p < 0.001. p values calculated for intention-to-treat analysis (n = 90).
Table 3.
Comparison of differences in relative change of muscle function and 95% confidence intervals (CI) from baseline to first post-measurement, between the experimental and control group, regarding patient’s age and sex.
| Group | Middle aged | Younger old | Older old | Female | Male |
|---|---|---|---|---|---|
| EXG | + 7.4 (5.7) | + 34.6 (8.2) | + 3.2 (9.4) | + 23.9 (7.0) | + 6.2 (5.7) |
| CNG | − 2.8 (10.9) | − 20.1 (7.7) | − 7.6 (7.1) | − 23.6 (7.1) | + 3.3 (6.9) |
| Mean difference | 10.2 (12.3) | 54.7* (11.2) | 10.8 (11.8) | 47.5* (9.9) | 3.0 (8.9) |
| 95% CI | − 14.5 to + 35.0 | + 32.2 to + 77.1 | − 12.8 to + 34.4 | + 27.7 to + 67.3 | − 14.9 to + 20.8 |
Data are presented as means (relative changes in muscle function) and standard deviations. EXG, experimental group; CNG, control group. p-value calculated for intention-to-treat analysis (n = 90, nEXG = 57, nCNG = 33). Difference between groups significant at p < 0.001 is marked by *.
Table 4.
Comparison of differences in absolute change of muscle function and 95% confidence intervals (CI) from baseline to first post-measurement, between the experimental and control group, regarding patient’s age and sex.
| Group | Middle aged | Younger old | Older old | Female | Male |
|---|---|---|---|---|---|
| EXG | + 4.9 (8.2) | + 44.4 (11.7) | − 2.1 (13.5) | + 22.0 (10.0) | + 9.4 (8.2) |
| CNG | − 5.1 (15.7) | − 22.1 (11.0) | − 12.3 (10.2) | − 24.5 (10.1) | − 1.8 (9.8) |
| Mean difference | 9.9 (17.7) | 66.5* (16.1) | 10.2 (16.9) | 46.5 (14.2) | 11.2 (12.8) |
| 95% CI | − 25.6 to + 45.4 | + 34.3 to + 98.7 | − 23.6 to + 44.0 | + 18.1 to + 75.0 | − 14.4 to + 36.9 |
Data are presented as means (absolute changes in muscle function) and standard deviations. EXG, experimental group; CNG, control group. p-value calculated for intention-to-treat analysis (n = 90, nEXG = 57, nCNG = 33). Difference between groups significant at p < 0.001 is marked by *.
Differences in effects of 12-week follow-up on the primary outcome by age and sex
We did not find a significant effect of age (%MF: η2 = 0.105, p = 0.053; ΔMF: η2 = 0.075, p = 0.128), and neither of sex (%MF: η2 = 0.056, p = 0.082; ΔMF: η2 = 0.043, p = 0.127), on the effect of 12-week follow-up in the changes of %MF and ΔMF.
Discussion
IRT positively affects MF in CKD-5D patients. We found that the beneficial effects of IRT on MF manifested only in YO and female CKD-5D patients.
We found that the intervention had effects on %MF and ΔMF in YO patients, but did not find such differences in effects on MF measures for MA and OO patients. A possible explanation for this age-dependency of effects may be that the prescribed IRT and its progressivity were really suited for YO patients but did not suit for MA and OO patients. In the OO patients, the relatively frequent cardiovascular and metabolic comorbidities may have lowered their functional adaptability to IRT45,46. This partially aligns with some previous reports on CKD-5D patients that found beneficial effects of exercise on MF in patients aged above 60 and 65 years33,47,48 and with reports of no improvements in lower extremity muscle strength after intradialytic exercise in patients aged above 70 and above 80 years49,50. However, other studies reported no age-differences in functional adaptation after intradialytic- and home-based exercise in CKD-5D patients19,44. A first explanation of these discrepancies might regard the different shares of age groups in the various studies. The studies that reported no age differences included experimental subjects with a mean age 68 ± 13 years, and 72 (69–79) years; and control subjects in mean age of 68 ± 11 years, and 76 (69–78) years, respectively19,44. In our study, we included experimental subjects with a mean age of 60 ± 13 years and control subjects with a mean age of 68 ± 10 years. This lower average age of our patients might be a source of the contrasting conclusions. A second explanation regards the type of physical activity intervention as assessed. The studies that reported no age-heterogeneity used a combination of aerobic and resistance exercise as the intervention. In contrast, we applied resistance training as the intervention which may be more effective in CKD-5D patients.
We found that the intervention had effects in female patients, but not in male patients. This is in contrast with the findings of previous studies on changes in MF among patients with chronic kidney disease, which reported no sex-related differences in MF change51 and beneficial effects in male dialysis patients but not in female52. A reason could be that we included patients diagnosed with stage 5 CKD on maintenance haemodialysis therapy, whereas the previous study included stage 4 and 5 CKD patients who were on pre-dialysis therapy51,52. The higher severity of the disease and application of maintenance haemodialysis therapy in patients enrolled in our study might have contributed to different conclusions regarding the role of patients’ sex in functional adaptation. Alternatively, this may simply be a chance finding, given that one study reports no sex differences, a second one effectiveness in males and ours effectiveness in females. Evidently, this requires further study.
This study has several important strengths. First, we carried out procedures during the haemodialysis therapy, which enabled us to obtain more realistic data in a reproducible design. Second, the allocation of patients into the EXG and CNG groups according to the geographical location of care-providing dialysis centres minimised the likelihood of contamination of the CNG subjects by the intervention throughout the study.
Our study also has some limitations, however. First, we used a quasi-experimental design with the allocation of patients into arms based on the dialysis centre location, which may have led to differing samples per arm. However, to control baseline imbalances between subgroups in body weight, dialysis adequacy, haemoglobin, calcium, we analysed differences in primary outcome measures using models adjusted for these patients’ characteristics. This hardly affected our findings. Second, assessors of the outcome measures were not blinded, which may have caused information bias. However, the assessments were highly standardised, limiting the potential effect of this. Third, we used hand-held dynamometry for MF measures assessments. Compared to isokinetic dynamometry, handheld dynamometry is a less reliable diagnostic instrument, which may have produced differences in MF assessments between male and female healthy subjects, potentially leading to bias53. However, another study reported a high reliability and validity for the assessment of MF by handheld dynamometry in female and male CKD-5D patients54. Fourth, we did not assess the muscle quantity in CKD-5D patients, and therefore we were not able to report associations between observed age- and sex-related heterogeneity in the change of MF measures and muscle tissue structure indicators. Fifth, the proportion of YO female patients was lower in EXG (15%) compared to CNG (23%), and the proportion of YO patients was lower in females (19%) compared to males (41%). However, we found that all reported differences between subgroups were not statistically significant.
Patients’ age and sex play an important role in the response of MF to IRT. We found that the IRT is much more effective in YO patients and in female patients, implying special attention is needed for organisation of exercise interventions for MA and OO patients and male patients. This implies that age and gender should be considered regarding individual intensity, duration and frequency prescriptions for RT. Regarding other implications of our results for clinical practice, our study provided interesting evidence about assessment of muscle function among CKD-5D patients. The associations between patients’ physical functions, mortality and importance of physical performance assessments in CKD-5D patients are well described55–57. However, assessment methods of physical function applied in clinical practice differs in applicability and accuracy58. We found different effect sizes for age and sex between the measures of %MF and ΔMF. Both measures of MF change were moderated by allocation and age, however only %MF was moderated by allocation and sex. It may be assumed that both calculation methods of MF changes are feasible for CKD-5D patients; however assessment of patients’ %MF may provide more sex-specific information on functional adaptation after physical interventions.
Future research might focus on the effectiveness of exercise prescriptions tailored to the CKD-5D patients’ characteristics2,19,36. Furthermore, the functional assessments for age and sex heterogeneity analyses in CKD-5D patients might be realized by isokinetic dynamometry and after the application of other types of exercise and nutritional interventions46.
Acknowledgements
We appreciate the co-operation of the representatives and staff of the dialysis centres involved in the implementation of this study. Special acknowledgements go to Peter Mizla MD and Peter Javorsky MD for their organisational support during the study planning and implementation.
Author contributions
A.Z., J.R., P.K., A.M.G., J.P.D. and S.A.R. conceptualised and designed the work. A.Z., J.R., P.K., A.M.G., J.P.D. and S.A.R. formally analysed research data. A.Z. and J.R. acquired funding for the work. A.Z., J.R., P.K., A.M.G., J.P.D. and S.A.R. formulated and supervised the research methodology. A.Z., J.R. and P.K. realised project administration. A.Z., J.R. and P.K. realised investigation and research data acquisition. A.Z., J.R., P.K., A.M.G., J.P.D. and S.A.R. validated, analysed and interpreted research data. A.Z., J.R., P.K., A.M.G., J.P.D. and S.A.R. created and validated visual resources used in the manuscript (Tables 1, 2, 3, 4; Figs. 1, 2, 3). A.Z., J.R., P.K., A.M.G., J.P.vD. and S.A.R. wrote (drafted, edited and reviewed) the manuscript. All authors reviewed the manuscript, approved the submitted version and agreed to be personally accountable for the author’s contributions and to ensure that questions related to the accuracy or integrity of any part of the work.
Funding
This work was supported by the Slovak Research and Development Agency under Contract no. APVV-16-0490 and was supported by the Internal Research Grant System of Pavol Jozef Safarik University under Contract no. VVGS-2019-1069. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available in the ZENODO repository, at: 10.5281/zenodo.7019159; reference number: 7019159.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Aurel Zelko, Jaroslav Rosenberger and Peter Kolarcik.
References
- 1.Wilkinson TJ, et al. Prevalence and correlates of physical activity across kidney disease stages: An observational multicentre study. Nephrol. Dial. Transplant. 2021;36:641–649. doi: 10.1093/ndt/gfz235. [DOI] [PubMed] [Google Scholar]
- 2.Zelle DM, Klaassen G, van Adrichem E, Bakker SJ, Corpeleijn E, Navis G. Physical inactivity: A risk factor and target for intervention in renal care. Nat. Rev. Nephrol. 2017;13:152–168. doi: 10.1038/nrneph.2016.187. [DOI] [PubMed] [Google Scholar]
- 3.Battaglia Y, et al. Ultrasonography of quadriceps femoris muscle and subcutaneous fat tissue and body composition by BIVA in chronic dialysis patients. Nutrients. 2020;12:1388. doi: 10.3390/nu12051388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JC, Young Do J, Kang SH. Comparisons of physical activity and understanding of the importance of exercise according to dialysis modality in maintenance dialysis patients. Sci. Rep. 2021;11:21487. doi: 10.1038/s41598-021-00924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonard MB, et al. A multi-imaging modality study of bone density, bone structure and the muscle-bone unit in end-stage renal disease. Bone. 2019;127:271–279. doi: 10.1016/j.bone.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura M, et al. The impact of muscle mass loss and deteriorating physical function on prognosis in patients receiving hemodialysis. Sci. Rep. 2021;11:22290. doi: 10.1038/s41598-021-01581-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McIntyre CW, Selby NM, Sigrist M, Pearce LE, Mercer TH, Naish PF. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol. Dial. Transplant. 2006;21:2210–2216. doi: 10.1093/ndt/gfl064. [DOI] [PubMed] [Google Scholar]
- 8.Rosa CSC, et al. Effect of continuous progressive resistance training during hemodialysis on body composition, physical function and quality of life in end-stage renal disease patients: A randomized controlled trial. Clin. Rehabil. 2018;32:899–908. doi: 10.1177/0269215518760696. [DOI] [PubMed] [Google Scholar]
- 9.Rhee SY, et al. Intradialytic exercise improves physical function and reduces intradialytic hypotension and depression in hemodialysis patients. Korean J. Intern. Med. 2019;34:588–598. doi: 10.3904/kjim.2017.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes LCC, et al. Intradialytic resistance training improves functional capacity and lean mass gain in individuals on hemodialysis: A randomized pilot trial. Arch. Phys. Med. Rehabil. 2019;100:2151–2158. doi: 10.1016/j.apmr.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Corrêa HL, et al. Resistance training improves sleep quality, redox balance and inflammatory profile in maintenance hemodialysis patients: A randomized controlled trial. Sci. Rep. 2020;10:11708. doi: 10.1038/s41598-020-68602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Exel AL, et al. Effectiveness of a resistance exercise program for lower limbs in chronic renal patients on hemodialysis: A randomized controlled trial. Hemodial. Int. 2021 doi: 10.1111/hdi.12918. [DOI] [PubMed] [Google Scholar]
- 13.Spakova I, et al. MicroRNA molecules as predictive biomarkers of adaptive responses to strength training and physical inactivity in haemodialysis patients. Sci. Rep. 2020;10:15597. doi: 10.1038/s41598-020-72542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudacek M, Zelko A, Rosenberger J. Intradialytic resistance training has positive effect on survival in hemodialysis patients independently of their nutritional status measured by body composition monitoring. Nephrol. Dial. Transplant. 2021;36(Supp 1):gfab102.0015. doi: 10.1093/ndt/gfab102.0015. [DOI] [Google Scholar]
- 15.Kissova V, Zelko A, Rosenberger J, Geckova AM. The role of diabetes mellitus in the effectiveness of intradialytic exercise intervention on patients' muscle function. Endocrinol. Diabetes Nutr. (Engl Ed) 2022;69:112–121. doi: 10.1016/j.endien.2022.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Zelko A, et al. The effects of an intradialytic resistance training on lower extremity muscle functions. Disabil. Rehabil. 2022;44:275–281. doi: 10.1080/09638288.2020.1766581. [DOI] [PubMed] [Google Scholar]
- 17.Baker LA, et al. Clinical practice guideline exercise and lifestyle in chronic kidney disease. BMC Nephrol. 2022;23:75. doi: 10.1186/s12882-021-02618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilund KR, Viana JL, Perez LM. A critical review of exercise training in hemodialysis patients: Personalized activity prescriptions are needed. Exerc. Sport Sci. Rev. 2020;48:28–39. doi: 10.1249/JES.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valenzuela PL, et al. Intradialytic exercise: One size doesn't fit all. Front. Physiol. 2018;9:844. doi: 10.3389/fphys.2018.00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemmer JT, et al. Age and gender responses to strength training and detraining. Med. Sci. Sports Exerc. 2000;32:1505–1512. doi: 10.1097/00005768-200008000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Psatha M, et al. Age-related changes in the effects of strength training on lower leg muscles in healthy individuals measured using MRI. BMJ Open Sport Exerc. Med. 2017;3:e000249. doi: 10.1136/bmjsem-2017-000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negaresh R, Ranjbar R, Habibi A, Mokhtarzade M, Fokin A, Gharibvand MM. The effect of resistance training on quadriceps muscle volume and some growth factors in elderly and young men. Adv. Gerontol. 2017;30:880–887. [PubMed] [Google Scholar]
- 23.Phillips BE, Williams JP, Greenhaff PL, Smith K, Atherton PJ. Physiological adaptations to resistance exercise as a function of age. JCI Insight. 2017;2:e95581. doi: 10.1172/jci.insight.95581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ansdell P, Thomas K, Hicks KM, Hunter SK, Howatson G, Goodall S. Physiological sex differences affect the integrative response to exercise: Acute and chronic implications. Exp. Physiol. 2020;105:2007–2021. doi: 10.1113/EP088548. [DOI] [PubMed] [Google Scholar]
- 25.Jones MD, Wewege MA, Hackett DA, Keogh J, Hagstrom AD. Sex differences in adaptations in muscle strength and size following resistance training in older adults: A systematic review and meta-analysis. Sports Med. 2021;51:503–517. doi: 10.1007/s40279-020-01388-4. [DOI] [PubMed] [Google Scholar]
- 26.Melnyk JA, Rogers MA, Hurley BF. Effects of strength training and detraining on regional muscle in young and older men and women. Eur. J. Appl. Physiol. 2009;105:929–938. doi: 10.1007/s00421-008-0979-0. [DOI] [PubMed] [Google Scholar]
- 27.Da Boit M, et al. Sex differences in the response to resistance exercise training in older people. Physiol. Rep. 2016;4:e12834. doi: 10.14814/phy2.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Constantin-Teodosiu D, et al. Gender and age differences in plasma carnitine, muscle strength, and exercise tolerance in haemodialysis patients. Nephrol. Dial. Transplant. 2002;17:1808–1813. doi: 10.1093/ndt/17.10.1808. [DOI] [PubMed] [Google Scholar]
- 29.Isoyama N, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2014;9:1720–1728. doi: 10.2215/CJN.10261013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, et al. Gender-specific associations of skeletal muscle mass and arterial stiffness among peritoneal dialysis patients. Sci. Rep. 2018;8:1351. doi: 10.1038/s41598-018-19710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uchiyama K, et al. Exercise capacity and association with Quality of Life in peritoneal dialysis patients. Perit. Dial. Int. 2019;39:66–73. doi: 10.3747/pdi.2018.00075. [DOI] [PubMed] [Google Scholar]
- 32.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolonedecanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J. Am. Soc. Nephrol. 2006;17:2307–2314. doi: 10.1681/ASN.2006010034. [DOI] [PubMed] [Google Scholar]
- 33.Baggetta R, et al. Effect of a home based, low intensity, physical exercise program in older adults dialysis patients: A secondary analysis of the EXCITE trial. BMC Geriatr. 2018;18:248. doi: 10.1186/s12877-018-0938-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souweine JS, et al. Dynapaenia and sarcopaenia in chronic haemodialysis patients: Do muscle weakness and atrophy similarly influence poor outcome? Nephrol. Dial. Transplant. 2021;36:1908–1918. doi: 10.1093/ndt/gfaa353. [DOI] [PubMed] [Google Scholar]
- 35.Cha RH, Lee GS, Yoo JY, Rhee OB, Jeon YD. Hand grip and leg muscle strength in hemodialysis patients and its determinants. J. Korean Med. Sci. 2021;36:e76. doi: 10.3346/jkms.2021.36.e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smart NA, et al. Exercise and Sports Science Australia (ESSA) position statement on exercise and chronic kidney disease. J. Sci. Med. Sport. 2013;16:406–411. doi: 10.1016/j.jsams.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Zelko A, et al. The effects of intradialytic resistance training on muscle strength, psychological well-being, clinical outcomes and circulatory micro-ribonucleic acid profiles in haemodialysis patients: Protocol for a quasi-experimental study. Medicine (Baltimore) 2019;98:e15570. doi: 10.1097/MD.0000000000015570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 39.Mentiplay BF, et al. Assessment of lower limb muscle strength and power using hand-held and fixed dynamometry: A reliability and validity study. PLoS One. 2015;10:e0140822. doi: 10.1371/journal.pone.0140822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chamorro C, Armijo-Olivo S, De la Fuente C, Fuentes J, Javier Chirosa L. Absolute reliability and concurrent validity of hand held dynamometry and isokinetic dynamometry in the hip, knee and ankle joint: Systematic review and meta-analysis. Open Med. (Wars) 2017;12:359–375. doi: 10.1515/med-2017-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grootswagers P, Vaes A, Hangelbroek R, Tieland M, van Loon L, de Groot L. Relative validity and reliability of isometric lower extremity strength assessment in older adults by using a handheld dynamometer. Sports Health. 2022 doi: 10.1177/19417381211063847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong T, Bull F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ) J. Public Health. 2006;14:66–70. doi: 10.1007/s10389-006-0024-x. [DOI] [Google Scholar]
- 43.Thivel D, Tremblay A, Genin PM, Panahi S, Rivière D, Duclos M. Physical activity, inactivity, and sedentary behaviors: Definitions and implications in occupational health. Front. Public Health. 2018;6:288. doi: 10.3389/fpubh.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulz KF, Altman DG, Moher D, CONSORT Group CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:332. doi: 10.1136/bmj.c332. [DOI] [PubMed] [Google Scholar]
- 45.Endo Y, Nourmahnad A, Sinha I. Optimizing skeletal muscle anabolic response to resistance training in aging. Front. Physiol. 2020;11:874. doi: 10.3389/fphys.2020.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garibotto G, et al. How to overcome anabolic resistance in dialysis-treated patients? Front. Nutr. 2021;8:701386. doi: 10.3389/fnut.2021.701386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cha RH, Lee GS. Steady exercise improves hand grip and leg muscle strength in hemodialysis patients. J. Exerc. Rehabil. 2021;17:435–443. doi: 10.12965/jer.2142616.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuzawa R, et al. Exercise training in elderly people undergoing hemodialysis: A systematic review and meta-analysis. Kidney Int. Rep. 2017;2:1096–1110. doi: 10.1016/j.ekir.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esteve Simo V, et al. Benefits of a low intensity exercise programme during haemodialysis sessions in elderly patients. Nefrologia. 2015;35:385–394. doi: 10.1016/j.nefro.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Yabe H, Kono K, Yamaguchi T, Ishikawa Y, Yamaguchi Y, Azekura H. Effects of intradialytic exercise for advanced-age patients undergoing hemodialysis: A randomized controlled trial. PLoS One. 2021;16:e0257918. doi: 10.1371/journal.pone.0257918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uchiyama K, et al. Home-based aerobic exercise and resistance training for severe chronic kidney disease: A randomized controlled trial. J. Cachexia Sarcopenia Muscle. 2021;12:1789–1802. doi: 10.1002/jcsm.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hiraki K, et al. Effects of home-based exercise on pre-dialysis chronic kidney disease patients: A randomized pilot and feasibility trial. BMC Nephrol. 2017;18:198. doi: 10.1186/s12882-017-0613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katoh M, et al. Isometric knee muscle strength measurement using a belt-stabilized hand-held dynamometer and an isokinetic dynamometer with and without trunk fixation: Investigation of agreement of measurement values and factors influencing measurement. J. Phys. Ther. Sci. 2019;31:878–883. doi: 10.1589/jpts.31.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez-Dominguez B, Lopez-Brull A, Plaza-Carrasco M, Casaña-Granell J, Garcia-Testal A, Benitez-Martinez J. Test-retest reliability, validity, and minimal detectable change of the measurement of lower limb muscular strength with handheld dynamometry in patients undergoing hemodialysis. Int. J. Nephrol. 2022;2022:5330608. doi: 10.1155/2022/5330608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Notomi S, Sawase K, Harada T, Funakoshi S, Mukae H, Nishino T. The impact of muscle mass loss and deteriorating physical function on prognosis in patients receiving hemodialysis. Sci. Rep. 2021;11:22290. doi: 10.1038/s41598-021-01581-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanden Wyngaert K, Van Biesen W, Eloot S, Van Craenenbroeck AH, Calders P, Holvoet E. The importance of physical performance in the assessment of patients on haemodialysis: A survival analysis. PLoS One. 2022;17:e0268115. doi: 10.1371/journal.pone.0268115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshikoshi S, et al. Associations between dynapenia, cardiovascular hospitalizations, and all-cause mortality among patients on haemodialysis. J. Cachexia Sarcopenia Muscle. 2022;13:2417–2425. doi: 10.1002/jcsm.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carrero JJ, Johansen KL, Lindholm B, Stenvinkel P, Cuppari L, Avesani CM. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016;90:53–66. doi: 10.1016/j.kint.2016.02.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in the ZENODO repository, at: 10.5281/zenodo.7019159; reference number: 7019159.