Abstract
Oligonucleotide primers were designed and used to amplify, by PCR, partial 16S rRNA genes of members of the bacterial division Verrucomicrobia in DNA extracted from a pasture soil. By applying most-probable-number theory to the assay, verrucomicrobia appeared to contribute some 0.2% of the soil DNA. Amplified ribosomal DNA restriction analysis of 53 cloned PCR-amplified partial 16S rRNA gene fragments and comparative sequence analysis of 21 nonchimeric partial 16S rRNA genes showed that these primers amplified only 16S rRNA genes of members of the Verrucomicrobia in DNA extracted from the soil.
Liesack and Stackebrandt (20) recovered a group of cloned bacterial PCR-amplified 16S rRNA gene fragments from an Australian forest soil. At the time, these could not to be assigned to any known group of cultivated bacteria. Later, Ward-Rainey et al. (34) found that the aerobic, aquatic bacterium Verrucomicrobium spinosum was related, on the basis of 16S rRNA gene homology, to these uncultivated soil bacteria. Further pure culture isolates have been assigned to this group of bacteria, including four species of the aquatic aerobic genus Prosthecobacter (10, 11) and as yet unnamed strains of fermenting ultramicrobacteria from rice paddy soil (15). This group has been named Verrucomicrobia (11) and accorded division status (11, 12). Phylogenetically, these pure cultures represent only a small fraction of the diversity found within this group (12). On the basis of cloned 16S rRNA genes and reverse transcription-PCR products of rRNA, verrucomicrobia (members of Verrucomicrobia) have been detected in a wide range of habitats, including soils (4, 7, 17, 19, 20), and may represent an important part of the soil microflora (6, 19).
Previously, verrucomicrobia have been found serendipitously, whether by culturing or by detection of the 16S rRNA genes of uncultured representatives. It has not yet been possible to devise a means of selectively isolating members of this group, and the molecular detection of the group as a whole has been done in clone libraries of PCR-amplified 16S rRNA genes produced with oligonucleotide primers targeting a wide range of bacterial groups. We have used a PCR-based assay directed at the 16S rRNA genes only of members of Verrucomicrobia to estimate the numerical abundance of this group within the microbial community of a pasture soil and to survey the phylogenetic relationships of verrucomicrobia in this soil.
DNA extraction from soil.
Soil from depths of 0 to 5 cm was taken from a perennial ryegrass (Lolium perenne) and white clover (Trifolium repens) pasture of the Dairy Research Institute at Ellinbank, Victoria, Australia, in May 1997. The soil (27) is a krasnozem (red basaltic loam; Ferrosol [13]) with a pH (1:5 [wt/wt] soil:water) of 5.6. Plant and root material was removed with forceps, and the soil was sieved though a 1-mm sieve. A sample was dried to constant weight at 105°C and used to calculate the dry weight of soil used to extract DNA.
DNA was extracted from soil by using a bead-beating protocol described by McVeigh et al. (24) and then separated from the coextracted brown material (presumably containing humic acids) by gel electrophoresis in TAE-agarose consisting of 1% (wt/vol) low-melting-point agarose (Progen Industries, Darra, Queensland, Australia) in TAE buffer (40 mM Tris [pH 8.0 with acetic acid] plus 1 mM EDTA [pH 8.0 with NaOH]). The high-molecular-weight DNA fraction (>10,000 bp) was excised under UV illumination after staining with ethidium bromide. Two milliliters of TAE buffer was added per gram (wet weight) of gel, and the gel slices were melted at 65°C. After the mixture was cooled to room temperature, 5 U of β-agarase (New England Biolabs/Genesearch, Arundel, Queensland, Australia) was added per g (wet weight) of gel, and the preparations were incubated at 37°C for 2 h. The DNA was then purified by sequential extractions with phenol (equilibrated to pH 8.0 [Sigma, Castle Hill, New South Wales, Australia]), phenol-chloroform-isoamyl alcohol (25:24:1 [vol/vol/vol]), and chloroform-isoamyl alcohol (24:1 [vol/vol]), followed by a sodium chloride (0.5 M) plus ethanol (66% [vol/vol]) precipitation (14). The DNA pellet was dissolved in TE buffer (10 mM Tris [pH 8.0 with HCl] plus 1 mM EDTA [pH 8.0 with NaOH]), and potassium acetate was added to a final concentration of 0.5 M. After a 90-min incubation on ice, the sample was centrifuged at 16,000 × g for 30 min, at 4°C. The supernatant was amended with 1/10 volume of 3 M sodium acetate (pH 5.2) and 2 volumes of 100% ethanol, and the mixture was incubated overnight at −20°C. The DNA was collected by centrifugation (16,000 × g for 15 min), washed in 70% (vol/vol) ethanol, air dried, and dissolved in 20 μl of TE buffer. Various amounts of soil DNA were electrophoresed in 1% (wt/vol) TAE-agarose gels containing 0.3 μg of ethidium bromide per ml. Known amounts of highly purified DNA from Escherichia coli (concentrations determined spectrophotometrically [26]) were electrophoresed in the same gel and used to generate a standard curve. The gel was photographed under UV illumination, the relative intensities of the bands were analyzed with the NIH Image program (National Institutes of Health, Bethesda, Md.), and the data were used to estimate the unknown DNA concentrations. When volume changes during the extraction procedure were compensated for, 9.85 μg of DNA was isolated per g of dry soil.
Primer design.
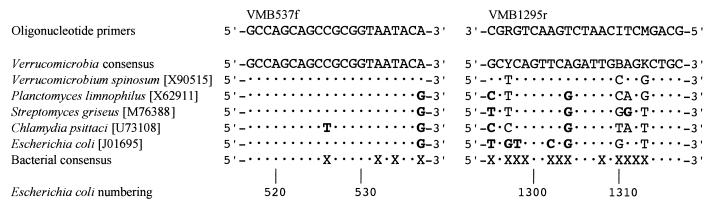
16S rRNA gene sequences of members of Verrucomicrobia, including those from pure cultures and genes recovered in clones libraries, were aligned. Regions of identity or high homology were selected as possible target sites for a PCR assay, and these regions were compared with the homologous regions of 16S rRNA genes from representatives of major lineages of bacterial descent. Two regions apparently unique to verrucomicrobia were identified, and the oligonucleotide primers VMB537f and VMB1295r, complementary to these regions, were designed (Fig. 1). The expected fragment size, determined from the published 16S rRNA gene sequence of V. spinosum (34), was 805 bp.
FIG. 1.
Alignments of oligonucleotide primers and target sequences. Mismatches are given in bold type. The nucleotide position numbering is taken from the work of Brosius et al. (5), the bacterial consensus sequence is from the review of Lane (18), and the GenBank accession numbers are given in square brackets. B, C or G or T; I, inosine; K, G or T; M, A or C; R, A or G; X, unspecified nucleotide; Y, C or T. Dots indicate identity with the Verrucomicrobia consensus. Mixed bases in the primers are equimolar.
The suitability of the primers was assessed by carrying out a search of GenBank (1, 3). Only members of Verrucomicrobia had gene sequences complementary to those of both primers. Since few cultured of members of the verrucomicrobia are available and since the soil may contain previously unknown organisms which may be detected by a set of specific primers, we decided to test the primers by carrying out a phylogenetic analysis of the products amplified from the soil being investigated. However, the current information on the phylogenetic diversity of the group may mean that the primers do not detect some as yet unknown members of Verrucomicrobia.
To determine the conditions for the assay, DNA extracted (14) from pure cultures of a number of organisms (at 1 ng of DNA per reaction) was used. The experiments reported here employed a 50-μl reaction mixture containing PCR buffer (10 mM Tris-HCl [pH 9.0 at 25°C], 50 mM KCl, and 0.1% [wt/vol] Triton X-100), DNA in a volume of 1 μl, 1 mM MgCl2, and 50 pmol of each primer, overlaid with 2 drops of mineral oil (Promega, Annandale, New South Wales, Australia). After an initial denaturation at 94°C for 5 min, 10 nmol of each deoxynucleoside triphosphate and 1.25 U of Taq DNA polymerase (Promega) were added to each reaction mixture followed by 42 cycles of 70°C for 90 s, 72°C for 120 s, 94°C for 60 s, and a final extension of 70°C for 90 s and then 72°C for 6 min. Thirty microliters of the reaction mixture was then electrophoresed on a 2% (wt/vol) TAE-agarose gel. PCR products of the expected size (approximately 800 bp) were obtained with DNA from V. spinosum DSM 4136 (obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany [DSMZ]) and from strain VeGlc2 (15). No products were obtained with DNA from E. coli W3110 and Streptomyces griseus Waksman 18-16 (both from the collection of the Department of Microbiology and Immunology, University of Melbourne), Chlamydia psittaci EBA (purified DNA obtained from Karin D. E. Everett, Department of Medical Microbiology, University of Georgia, Athens, Ga.), or Planctomyces limnophilus DSM 3776 (obtained from the DSMZ). S. griseus has homologous regions (potential target sites for the primers) of the 16S rRNA gene which have sequences similar to those of members of the Verrucomicrobia (Fig. 1), while the planctomycetes and chlamydiae have been postulated to be near phylogenetic relatives of Verrucomicrobia, although this is far from resolved (10, 11, 34).
MPN-PCR.
The oligonucleotide primers VMB537f and VMB1295r were used to amplify any 16S rRNA genes with complementary sequences present in various diluted samples of soil DNA. Amplicons could be detected in PCR assays receiving 10−8 g (five positive of five replicates), 10−9 g (four positive of five replicates), and 10−10 g (three positive of five replicates) of soil DNA but not in assays receiving 10−11 g of soil DNA (zero positive of five replicates). Similar assays were carried out with DNA extracted from a pure culture of V. spinosum. Amplicons could be detected in assays receiving 10−11 g (five positive of five replicates) and 10−12 g (four positive of five replicates) of V. spinosum DNA but not in those receiving only 10−13 g (zero positive of five replicates). The most-probable-number (MPN) results (2) for our assays are 2.7 × 109 (95% confidence interval of 1.3 × 109 to 6.8 × 109) targets detected per g of soil DNA and 1.3 × 1012 (95% confidence interval of 5 × 1011 to 3.9 × 1012) targets detected per g of DNA from V. spinosum. The genome size of V. spinosum is not known, but assuming a size similar to that of E. coli, 4.3 × 10−15 g (25), our assay had a lower detection limit of about 180 genomes. Applying this threshold, we estimate a population of about 5 × 106 verrucomicrobia per g of dry soil.
Our MPN data also suggest that verrucomicrobia contribute some 0.2% of the soil DNA (2.7 × 109 of 1.3 × 1012). The number of cells able to be stained with DAPI (4′,6-diamidino-2-phenylindole) in the same soil is about 1.5 × 109 cells per g of dry soil (9), so that verrucomicrobia may be present at a density of about 3 × 106 cells per g of dry soil. It should be stressed that our calculations are based on a number of as yet untested assumptions: (i) the amplification efficiency of 16S rRNA genes with our assay is similar for V. spinosum and other verrucomicrobia, (ii) the average genome size of the soil microflora is approximately equal to that of V. spinosum, and (iii) the average 16S rRNA gene copy number of the verrucomicrobia present is the same as that of V. spinosum. Previous investigations of verrucomicrobia in soils, one using a competitive quantitative PCR method directed at the cloned fragment EA25 (19) and another using a probe directed at reverse transcription-PCR-amplified RNA of the cloned fragment DA101 (6), have suggested that verrucomicrobia may be quantitatively important in soils. The number of EA25-like genes was estimated to be 2.17 × 108 copies per g of soil (19). Group-specific fluorescence-labeled oligonucleotide probes targeting the rRNA could be used to estimate the numbers of verrucomicrobia but could not simultaneously determine the phylogenetic diversity within the group.
16S rRNA gene sequence analysis.
Soils are systems containing a high diversity of microbial species (29) from many different lineages (7, 17, 21). rRNA genes from previously undiscovered organisms are sometimes recovered in clone libraries generated with oligonucleotide primers targeting specific groups, because the primers have (sufficient) homology to the genes of nontarget bacterial groups to allow the amplification of those genes (20, 24). To determine if the amplicons detected in the PCR assay originated from 16S rRNA genes from verrucomicrobia, further reactions were carried out with 10−10 g of soil DNA per reaction mixture. Products of five amplification reactions were pooled and purified by gel electrophoresis followed by phenol extraction (14). The PCR product was resuspended in 50 μl of TE buffer, and a second PCR was performed, with 1 μl of the purified PCR product, under the same conditions as the primary PCR. PCR products were separated on a 1% (wt/vol) TAE-agarose gel, excised, purified with the Geneclean kit (BIO 101, Vista, Calif.), and eluted in TE buffer. Following the standard A-tailing procedure as outlined in the manufacturer’s instructions, the purified PCR products were ligated into the cloning vector pGEM-T Easy (Promega) and transformed into JM109 high-efficiency competent E. coli cells (Promega). Clones containing an insert of the expected size were identified by colony PCR with primers GEM189r (5′-AGCGGATAACAATTTCACACAGG-3′) and GEM2987f (5′-CCCAGTCACGACGTTGTAAAACG-3′), targeting regions flanking the cloning site. White colonies were picked from the plate and denatured for 10 min at 94°C in PCR buffer, 1 mM MgCl2 and 100 pmol of each primer before the addition of 20 nmol of each deoxynucleoside triphosphate and 2.5 U of Taq DNA polymerase (Promega) to each 100-μl reaction mixture, followed by 40 cycles of 56°C for 60 s, 72°C for 60 s, and 94°C for 60 s. Aliquots of the PCR-amplified inserts of 53 clones were subjected to restriction pattern analyses (amplified ribosomal DNA restriction analysis [ARDRA] [32]) by separate digestion with the restriction endonucleases RsaI and Sau3AI. Nine unique restriction patterns were observed (Table 1). Approximately half of the inserts of each restriction pattern group, totalling 23 of the cloned partial 16S rRNA genes, were sequenced.
TABLE 1.
Grouping of cloned amplified partial 16S rRNA genes
| Restriction group | No. of cloned fragments | Cloned fragment(s) sequenced | Subdivision of Verrucomicrobiaa |
|---|---|---|---|
| 1 | 5 | EV101, EV118 | 2 |
| 2 | 1 | EV102 | 3 |
| 3 | 3 | EV103, EV116 | 3 |
| 4 | 2 | EV104, EV134 | 3 |
| 5 | 23 | EV105, EV107,b EV109, EV111, EV151, EV154, EV155 | 3 |
| 6 | 11 | EV106, EV108, EV152, EV153 | 3 |
| 7 | 6 | EV110, EV117, EV139 | 3 |
| 8 | 1 | EV112b | Noneb |
| 9 | 1 | EV133 | 3 |
Designations are those of Hugenholtz et al. (12).
Possibly of chimeric origin.
The PCR products were purified with QIAquick PCR purification kits (Qiagen, Clifton Hill, Victoria, Australia) and eluted in distilled water. Sequencing was performed as previously described (14), with the primers VMB537f and VMB1295r. The lengths of the sequenced fragments varied from 720 to 767 bp, thus representing approximately 50% of the 16S rRNA gene. None of the sequences were identical. The most similar sequences differed from each other in 2 of 761 common nucleotide positions (between the pair EV105 and EV154 and also the pair EV110 and EV154). BLAST analysis (1) and a reconstruction of the phylogeny (see below) revealed that all of the 23 inserts sequenced originated from organisms closely related to members of Verrucomicrobia as defined Hedlund et al. (11) and Hugenholtz et al. (12). Two cloned fragments (EV107 and EV112) were identified as being of possible chimeric origin by the program Chimera Check, version 2.7 (23), and were excluded from further analyses. In both of these cases, the 5′ regions of the chimeric cloned fragments (representing 35 and 48% of the sequence information, respectively) exhibited high similarity to members of subdivision 2 of Verrucomicrobia, while the 3′ regions showed high similarity to members of subdivision 3.
rRNA secondary structure predictions.
While some of the sequences recovered from the pasture soil were phylogenetically separated from each other, others were shown to be very closely related through a reconstruction of their phylogeny. However, none of the sequences had 100% identity. Unless obvious mismatches in pairing regions of the resultant predicted rRNA molecule can be identified, it is difficult to determine whether differences between sequences are real or due to artifacts which arise in multiple rounds of amplification in the PCR (33). The predicted secondary structures of helices 28, 29, and 30 of the resultant rRNA molecules were analyzed (helix numbering according to Van de Peer et al. [30, 31]) with the software package Mfold, version 3.0 (M. Zuker, Institute of Biomedical Computing, Washington University, St. Louis, Mo.). Six unique sequence types were found in the region covering helices 28, 29, and 30. All of the differences between sequence types either were in nonpairing regions or resulted in permissible pairings.
Verrucomicrobia in the pasture soil.
The 21 nonchimeric partial 16S rRNA gene sequences were aligned against homologous sequences of selected reference organisms and members (Fig. 1) of Verrucomicrobia (11, 12), obtained from GenBank (3), by using the alignment program Pileup implemented in the Australian National Genomic Information Service system (22). This alignment was then manually checked and corrected, and regions of uncertain alignment were eliminated, with the software Se-Al, version 1.d1 (A. Rambaut, Department of Zoology, University of Oxford). Further analyses were restricted to the unambiguously aligned regions totalling 596 positions. Evolutionary analyses were carried out as described elsewhere (14), according to the Jukes and Cantor (16) nucleotide substitution model. A phylogenetic dendrogram was constructed by the method of Fitch and Margoliash (8).
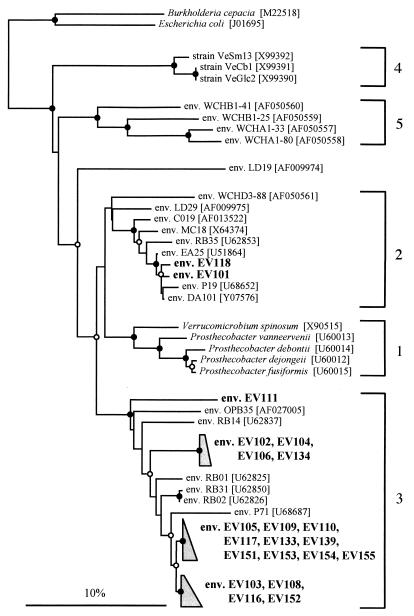
Nineteen of the cloned fragments fell within subdivision 3 of Verrucomicrobia, as defined by Hugenholtz et al. (12). These formed three clusters within subdivision 3, each of very similar sequence types, while one sequence branched more deeply (Fig. 2). Two cloned fragments apparently originated from organisms which would be classified in subdivision 2 according to Hugenholtz et al. (12) (Fig. 2). No cultivated representatives of these two subdivisions are known. The closest cultured relatives of the verrucomicrobia detected in this study had evolutionary distances (16) of between 8.9 and 13.8% to the new sequence types. In a group such as the Proteobacteria, such evolutionary distances encompass vastly different metabolic groups (28).
FIG. 2.
Phylogenetic dendrogram showing the relationships between cloned 16S rRNA gene fragments from the Ellinbank pasture soil (env. EV101 to env. EV155) and members of Verrucomicrobia. The 16S rRNA gene sequence from E. coli was used to root the dendrogram. Sequences prefixed with “env.” are cloned fragments from environmental samples. The GenBank accession numbers are given in square brackets. Nodes recovered in 90% or more of the 1,000 bootstrap dendrograms are indicated by filled circles, those recovered in 70 to 89% are indicated by open circles, and those recovered in less than 70% have no symbol. Bar, 10 inferred nucleotide substitutions per 100 positions.
ARDRA clearly distinguished the amplified fragments originating from members of subdivision 2 (restriction group 1) from those originating from members of subdivision 3 (restriction groups 2 to 9) of Verrucomicrobia (Table 1), as well as some differentiation within subdivision 3. This suggests that any of the 53 cloned fragments analyzed by ARDRA and not belonging to these divisions or not belonging to Verrucomicrobia would have been identified by a different ARDRA pattern. The oligonucleotide primers VMB537f and VMB1295r therefore appear to facilitate the specific amplification of partial 16S rRNA genes of verrucomicrobia in the presence of 16S rRNA genes from the diverse soil microbial community.
Acknowledgments
This work was supported by an Australian Research Council grant and a University of Melbourne Special Initiatives Grant.
We thank Cameron Gourley (Dairy Research Institute, Ellinbank), for his cooperation in obtaining soil samples, and Karin D. E. Everett (University of Georgia), for supplying DNA.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Beliaeff B, Mary J Y. The “most probable number” estimate and its confidence limits. Water Res. 1993;27:799–805. [Google Scholar]
- 3.Benson D A, Boguski M S, Lipman D J, Ostell J, Ouellette B F F, Rapp B A, Wheeler D L. GenBank. Nucleic Acids Res. 1999;27:12–17. doi: 10.1093/nar/27.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borneman J, Triplett E W. Molecular microbial diversity in soils from eastern Amazonia: evidence for unusual microorganisms and microbial population shifts associated with deforestation. Appl Environ Microbiol. 1997;63:2647–2653. doi: 10.1128/aem.63.7.2647-2653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosius J, Palmer M L, Kennedy P J, Noller H F. Complete nucleotide sequence of the 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felske A, Akkermans A D L. Prominent occurrence of ribosomes from an uncultured bacterium of the Verrucomicrobiales cluster in grassland soils. Lett Appl Microbiol. 1998;26:219–223. doi: 10.1046/j.1472-765x.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- 7.Felske A, Wolterink A, Van Lis R, Akkermans A D L. Phylogeny of the main bacterial 16S rRNA sequences in Drentse A grassland soils (The Netherlands) Appl Environ Microbiol. 1998;64:871–879. doi: 10.1128/aem.64.3.871-879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitch W M, Margoliash E. Construction of phylogenetic trees. Science. 1967;155:279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- 9.Grinton, B., and P. H. Janssen. Unpublished data.
- 10.Hedlund B P, Gosink J J, Staley J T. Phylogeny of Prosthecobacter, the fusiform caulobacters: members of a recently discovered division of the Bacteria. Int J Syst Bacteriol. 1996;46:960–966. doi: 10.1099/00207713-46-4-960. [DOI] [PubMed] [Google Scholar]
- 11.Hedlund B P, Gosink J J, Staley J T. Verrucomicrobia div. nov., a new division of the Bacteria containing three new species of Prosthecobacter. Antonie Leeuwenhoek. 1997;72:29–38. doi: 10.1023/a:1000348616863. [DOI] [PubMed] [Google Scholar]
- 12.Hugenholtz P, Goebel B M, Pace N R. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol. 1998;180:4765–4774. doi: 10.1128/jb.180.18.4765-4774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isbell R F. Australian soil classification. Collingwood, Victoria, Australia: CSIRO Publishing; 1996. [Google Scholar]
- 14.Janssen P H, O’Farrell K A. Succinispira mobilis gen. nov., sp. nov., a succinate-decarboxylating anaerobic bacterium. Int J Syst Bacteriol. 1999;49:1009–1013. doi: 10.1099/00207713-49-3-1009. [DOI] [PubMed] [Google Scholar]
- 15.Janssen P H, Schuhmann A, Mörschel E, Rainey F A. Novel anaerobic ultramicrobacteria belonging to the Verrucomicrobiales lineage of bacterial descent isolated by dilution culture from anoxic rice paddy soil. Appl Environ Microbiol. 1997;63:1382–1388. doi: 10.1128/aem.63.4.1382-1388.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jukes T H, Cantor C R. Evolution of protein molecules. In: Munro H N, editor. Mammalian protein metabolism. New York, N.Y: Academic Press; 1969. pp. 21–132. [Google Scholar]
- 17.Kuske C R, Barns S M, Busch J D. Diverse uncultured bacterial groups from soils of the arid southwestern United States that are present in many geographic regions. Appl Environ Microbiol. 1997;63:3614–3621. doi: 10.1128/aem.63.9.3614-3621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane D J. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester, United Kingdom: John Wiley & Sons; 1991. pp. 115–175. [Google Scholar]
- 19.Lee S Y, Bollinger J, Bezdicek D, Ogram A. Estimation of the abundance of an uncultured soil bacterial strain by a competitive quantitative PCR method. Appl Environ Microbiol. 1996;62:3787–3793. doi: 10.1128/aem.62.10.3787-3793.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liesack W, Stackebrandt E. Occurrence of novel groups of the domain Bacteria as revealed by analysis of genetic material isolated from an Australian terrestrial environment. J Bacteriol. 1992;174:5072–5078. doi: 10.1128/jb.174.15.5072-5078.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liesack W, Janssen P H, Rainey F A, Ward-Rainey N L, Stackebrandt E. Microbial diversity in soil: the need for a combined approach using molecular and cultivation techniques. In: van Elsas J D, Trevors J T, Wellington E M H, editors. Modern soil microbiology. New York, N.Y: Marcel Dekker; 1997. pp. 375–439. [Google Scholar]
- 22.Littlejohn T G, Bucholtz C A, Campbell R M M, Gaëta B A, Huynh C, Kim S H. Computing for biotechnology—WebANGIS. Australas Biotechnol. 1996;6:211–217. [Google Scholar]
- 23.Maidak B L, Cole J R, Parker C T, Jr, Garrity G M, Larsen N, Li B, Lilburn T G, McCaughey M J, Olsen G J, Overbeek R, Pramanik S, Schmidt T M, Tiedje J M, Woese C R. A new version of the RDP (Ribosomal Database Project) Nucleic Acids Res. 1999;27:171–173. doi: 10.1093/nar/27.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McVeigh H P, Munro J, Embley T M. Molecular evidence for the presence of novel actinomycete lineages in a temperate forest soil. J Ind Microbiol. 1996;17:197–204. [Google Scholar]
- 25.Moat A G, Foster J W. Microbial physiology. 3rd ed. New York, N.Y: Wiley-Liss; 1995. [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Sargeant I J, Skene J K M. Some properties of the krasnozems of southern Victoria, Australia. Aust J Soil Res. 1970;8:281–295. [Google Scholar]
- 28.Stackebrandt E. Unifying phylogeny and phenotypic diversity. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. Berlin, Germany: Springer-Verlag; 1992. pp. 19–47. [Google Scholar]
- 29.Torsvik V, Goksøyr J, Daae F L. High diversity in DNA of soil bacteria. Appl Environ Microbiol. 1990;56:782–787. doi: 10.1128/aem.56.3.782-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van de Peer Y, Nicolaï S, De Rijk P, De Wachter R. Database on the structure of small ribosomal subunit RNA. Nucleic Acids Res. 1996;24:86–91. doi: 10.1093/nar/24.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van de Peer Y, Chapelle S, De Wachter R. A quantitative map of nucleotide substitution rates in bacteria rRNA. Nucleic Acids Res. 1996;24:3381–3391. doi: 10.1093/nar/24.17.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaneechoutte M, Rossau R, De Vos P, Gillis M, Janssens D, Paepe N, De Rouck A, Fiers T, Claeys G, Kersters K. Rapid identification of bacteria of the Comamonadaceae with amplified ribosomal DNA-restriction analysis (ARDRA) FEMS Microbiol Lett. 1992;93:227–234. doi: 10.1111/j.1574-6968.1992.tb05102.x. [DOI] [PubMed] [Google Scholar]
- 33.Ward D M, Ferris M J, Nold S C, Bateson M M. A natural view of microbial diversity within hot spring cyanobacterial mat communities. Microbiol Mol Biol Rev. 1998;62:1353–1370. doi: 10.1128/mmbr.62.4.1353-1370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward-Rainey N, Rainey F A, Schlesner H, Stackebrandt E. Assignment of hitherto unidentified 16S rDNA species to a main line of descent within the domain Bacteria. Microbiology (Reading) 1995;141:3247–3250. [Google Scholar]