Abstract
Cancer immunotherapy (CIT) has gained increasing attention and made promising progress in recent years, especially immune checkpoint inhibitors such as antibodies blocking programmed cell death 1/programmed cell death ligand 1 (PD-1/PD-L1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4). However, its therapeutic efficacy is only 10–30% in solid tumours and treatment sensitivity needs to be improved. The complex tissue environment in which cancers originate is known as the tumour microenvironment (TME) and the complicated and dynamic TME is correlated with the efficacy of immunotherapy. Ultrasound-targeted microbubble destruction (UTMD) is an emerging technology that integrates diagnosis and therapy, which has garnered much traction due to non-invasive, targeted drug delivery and gene transfection characteristics. UTMD has also been studied to remodel TME and improve the efficacy of CIT. In this review, we analyse the effects of UTMD on various components of TME, including CD8+ T cells, tumour-infiltrating myeloid cells, regulatory T cells, natural killer cells and tumour vasculature. Moreover, UTMD enhances the permeability of the blood-brain barrier to facilitate drug delivery, thus improving CIT efficacy in vivo animal experiments. Based on this, we highlight the potential of immunotherapy against various cancer species and the clinical application prospects of UTMD.
Subject terms: Cancer microenvironment, Tumour immunology, Cancer immunotherapy
Background
Cancer immunotherapy (CIT) is one of the standard therapies along with surgery, chemotherapy, radiotherapy and targeted therapy for cancer treatment, which produces antitumor effects by activating the body’s immune system [1]. Among oncology drug treatments, it is the fourth therapy after chemotherapy, targeted therapy, and hormone therapy. In recent years, immunotherapy, especially with immune checkpoints as therapeutic targets, has made an enormous difference for terminal cancer patients [2]. However, objective tumour response rates are still poor and treatment sensitivity needs to be further promoted [3]. The tumour microenvironment (TME) is the surroundings on which tumour cells rely, and the characteristics of TME significantly affect cancer progression and metastasis, correlating with the efficacy of CIT [4]. As tumour cells proliferate, the TME is dynamically changing, and the negative regulatory mechanisms of the immune system are used by TME to counteract the antitumor immune response and ultimately promote tumour immune escape [5]. However, one of the biggest challenges of current CIT is the narrow range of patients and the remarkable individual differences, which may be attributed to the heterogeneity of the TME [6, 7].
Traditionally, ultrasound is an imaging technique for diagnosis, but in the last few years, it has been developed for therapeutic purposes [8], one of which is ultrasound-targeted microbubble destruction (UTMD). UTMD has gained widespread popularity with regard to its non-invasive, targeted drug delivery and gene transfection characteristics [9]. Numerous studies on the application of UTMD to cancer immunotherapy have revealed the extraordinary possibility of remodelling TME and enhancing CIT efficacy [10–12], which has the potential to break down the heterogeneity of the TME and reach more patients.
In this review, we summarise the influences of UTMD on various components of TME to indicate its role in remodelling the TME. Meanwhile, the delivery role of this technology in vivo and its potentiation of CIT will also be discussed in this paper.
Ultrasound-targeted microbubble destruction
UTMD is an ultrasound therapy technique that uses low-frequency ultrasound to stimulate the cavitation of microbubbles in vivo, resulting in oscillation (alternating contraction and expansion) and rupture [13]. This process generates mechanical energy such as fluid flow, shear stress, shock wave and microjet, causing the cavitation effect or sonoporation [14, 15]. Unlike high-intensity focused ultrasound (HIFU), the low ultrasonic frequency of UTMD ranges from 20 kHz to 1 MHz, and its thermal effect is fragile [16]. The introduction of microbubbles (exogenous cavitation nuclei) can increase the density of cavitation nuclei, lower the cavitation threshold and improve the safety of ultrasound [17].
Current researches conclude that the biophysical mechanism of UTMD is mainly the cavitation effect (or called sonoporation). The cavitation effect refers to the vibration of vapour or gas cavities under the action of acoustic waves. Then vapour or gas microbubbles expand, collapse and rupture when the acoustic pressure reaches a particular threshold [18]. The effect includes two forms. One is stable cavitation, where the microbubbles alternately expand and contract with changes in acoustic pressure, producing a microfluidic jet with low shear [19–21]. The other is inertial cavitation, where the microbubbles overextend and rupture to build a large shear [20, 21]. The cavitation effect induces the increase in the gap between vascular endothelial cells and the permeability of cell membrane [22, 23]. What’s more, microbubbles are an excellent delivery vehicle for targeted and specific delivery of bioactive molecules (such as drugs, nanoparticles, vaccines and therapeutic genes) to organs or tissues within ultrasound reach [24–26]. Microbubbles not only protect biologically active molecules from endogenous clearance during transport, but also release them in the focal area through inertial cavitation, enabling targeted therapies and reducing systemic toxic side effects [24, 27].
Tumour microenvironment and cancer immunotherapy
The TME is the complex environment where tumour cells or cancer stem cells grow. The comprehensive system consists of tumour cells, immunocytes, mesenchymal cells, tumour-associated endothelial cells (TAECs), extracellular matrix (ECM), cytokines, and other molecules that contribute to tumour growth and progression, such as immune checkpoint [28–30]. CIT is one of the most promising cancer treatment strategies, including bacterial immunotherapy, cytokine therapy, monoclonal antibody, immune checkpoint inhibitors (ICIs), cancer vaccines, adoptive cell therapy (ACT) and oncolytic virus therapy (OVT) [31–33]. These therapies can augment the recruitment of T cells in the TME, block the binding of immune checkpoints, enhance exposure to antigens or directly lyse tumour cells [33–36]. Although CIT has been successfully applied to many types of malignant tumours, only a small percentage of strictly selected advanced cancer patients benefit from these therapies [37].
The complicated and dynamic TME can influence the efficacy of immunotherapy. First, the TME contains immunosuppressive cells and a large number of cytokines such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), M2 type macrophages, IL-1, TGF-β, which retard the activation of effector cells especially T cells [38, 39]. Second, tumour cells reduce surface expression of HLA-I, making it difficult to be processed for presentation and not recognised by the immune system [40]. Third, tumour antigens are not adequately delivered to the lymphoid tissue due to the obstruction of the TME, predicting inefficient activation of T cells [41]. Moreover, the hypoxic and acidic microenvironment causes deactivation, senescence and depletion of infiltrating T cells [42].
In brief, the TME is the critical factor in influencing tumour immune response. The efficiency and sensitivity of CIT are closely correlated with the TME. An in-depth understanding of the interplay between the TME and immunotherapy is significant.
Effects of UTMD on intratumoral CD8+ T cells
CD8+ T cells play an essential role in tumour-mediated immune responses by recognising specific tumour antigens. Tumour cell necrosis induced by the perforin–granzyme pathway and apoptosis induced by the FAS pathway are two important antitumor mechanisms of CD8+ T cells [43]. Moreover, IFN-γ secreted by CD8+ T cells can cause tumour cell ferroptosis, an iron-dependent and non-apoptotic form of cell death [44]. However, CD8+ T cells are not sufficiently infiltrated and cannot be fully activated in most solid tumours [19]. There are many factors that impede CD8+ T-cell infiltration and activation during tumour progression, such as reduced tumour perfusion [45], blocked antigen presentation [40], high-potassium environment [46] and high expression of inhibitory receptors [47]. Appropriate ways to promote CD8+ T-cell infiltration and activation are significant.
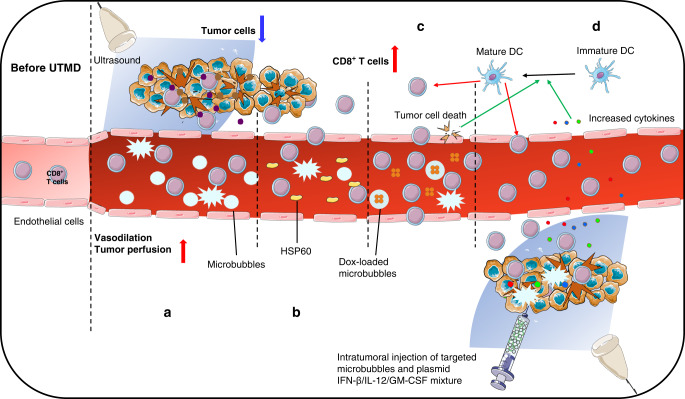
One recent study showed that the UTMD treatment increased the percentage of infiltrating CD8+ T cells by 24.28%, and the absolute number was higher with a low mechanical index (MI = 0.4). Furthermore, the combination therapy of UTMD with anti-programmed cell death ligand 1 (anti-PD-L1) boosted the secretion of IFN-γ and granzyme B, indicating the activation and function of infiltrating CD8+ T cells. Vasodilation and enhanced tumour perfusion contributed to this phenomenon because the suppressive tumour immune response was mitigated by improving tumour hypoxia [48] (Fig. 1a). In addition, mechanical stress by UTMD promotes heat-shock protein (HSP60) overexpression, thereby increasing the infiltration of CD8+ T cells into the tumour [49] (Fig. 1b). The underlying mechanism may be that HSP binding to scavenger receptor SREC-I or LOX-1 (HSP-specific receptors expressed on dendritic cells) activates dendritic cells and mediates the cross-presentation of antigenic peptides from tumour cells, thereby triggering the T-cell-mediated adaptive immune responses [50]. Other studies also found similar results by combining microbubbles with plasmids encoding cytokines, such as pIFN-β and pIL-12 [51, 52] (Fig. 1d). What’s more, UTMD was reported to enhance Dox-mediated immunogenic cell death (ICD) to activate more dendritic cells (DCs), eliciting an increment in the percentage of CD8+ T cells [53] (Fig. 1c). Similarly, UTMD with GM-CSF plasmids was shown to conduct recruitment of CD8+ T cells by upregulating DCs maturation [54] (Fig. 1d). Inertial cavitation mediated by UTMD exposes intracellular nucleic acids, pathogen-associated molecular patterns (PAMP), or damage-associated molecular patterns (DAMP) [55]. Cytosolic double-stranded DNA (dsDNA) and dsRNA are detected by cyclic GMP-AMP synthase (cGAS) and retinoic acid inducible gene I (RIG-I), which are pattern-recognition receptors (PRRs), resulting in the activation of stimulator of interferon genes (STING) [56]. The ubiquitinated STING elicits the expression of pro-inflammatory factors through IRF3 and NFκB-dependent pathways. Meanwhile, activated STING in antigen-presenting cells stimulates the T-cell-mediated adaptive immune responses and exerts anti-tumour effects [57]. In consequence, UTMD enhances CD8+ T-cell infiltration and T-cell-mediated adaptive immune responses by dilating blood vessels, promoting HSP expression, boosting tumour antigen exposure, and facilitating cytokine secretion.
Fig. 1. UTMD increases the infiltration of CD8+ T cells.
a Vasodilation and enhanced tumour perfusion. b HSP60 attracts infiltration of CD8+ T cells. c and d Dox-mediated tumour cell death and increased cytokines promote the maturation of DCs to cause CD8+ T-cell infiltration.
Effects of UTMD on tumour-infiltrating myeloid cells
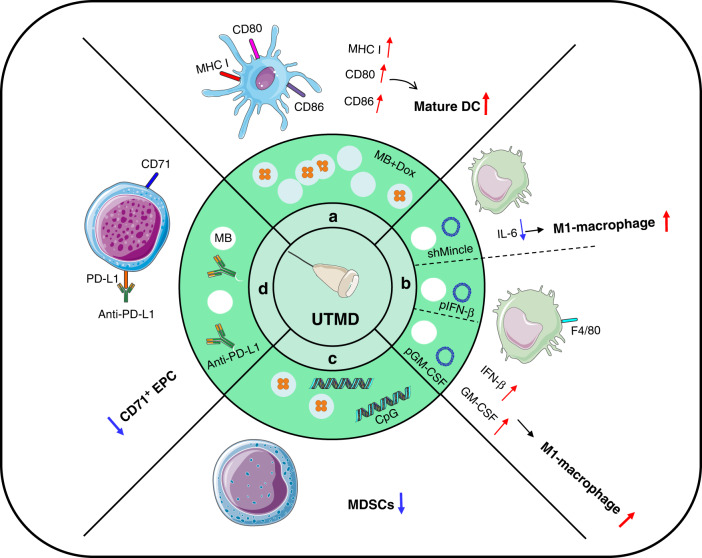
Tumour-infiltrating myeloid cells (TIMs) are a non-negligible regulatory component in tumour progression, including mast cells, DCs, monocytes, and macrophages [58, 59]. DCs are a specialised class of antigen-presenting cells (APCs) derived from the hematopoietic bone marrow [60]. Tumour-infiltrating DCs process and present tumour antigens and gradually differentiate into mature DCs that express co-stimulatory molecules CD80 or CD86 to interact with T cells to resist tumours [61]. UTMD was explored to heighten antigen processing via MHC class I to increase CD86 expression in the meninges, indicating the maturity of DCs. UTMD alone or combined with Dox mediated the release of DAMPs (such as HMGB-1, HSP, ATP) from tumour cells [53, 62]. These molecules bind to pattern-recognition receptors (such as Toll-like receptors, and purinergic receptors) to stimulate DCs maturation [63]. Upon DCs maturation, an increased expression of TAP1 and TAP2 was detected, which promotes the loading of MHC-I [62]. In addition to the enriched CD86, other studies confirmed the maturation and activation of DCs because of the enhanced CD80 expression and INF-γ (active marker) secretion by UTMD [64] (Fig. 2a).
Fig. 2. Effects of UTMD on tumour-infiltrating myeloid cells.
a US + MB + Dox treatment promotes the maturation of DCs. b US + MB + shMincle/pIFN-β/GM-CSF treatment increases M1-macrophage infiltration. c US + MB + Dox+CpG treatment reduces the frequency and absolute amount of MDSCs. d US + MB + anti-PD-L1 treatment decreases the frequency of CD71+ erythroid progenitor cells.
Tumour-associated macrophages (TAMs) and MDSCs play an immunosuppressive role in the TME to promote malignant tumour progression [65, 66]. TAMs are generally divided into M1 and M2 phenotypes and are interchangeable between the two, with the former being antitumor and the latter pro-tumour [67]. Xue et al. bound shMincle to microbubbles and then applied UTMD to deliver the complexes to the tumour region. Ultimately, they found that UTMD blocked the Mincle/Syk/NF-κB axis by targeting Mincle and, more excitingly, promoted the conversion of the M2 phenotype to the M1 phenotype [68] (Fig. 2b). Complementarily, an increase in the number of F4/80+ macrophages infiltrates was also observed by combining plasmids with microbubbles [51, 54] (Fig. 2b). Macrophages have been reported to stimulate the expression of pro-inflammatory factors via the TLR4-MyD88 pathway [69]. Accordingly, we speculate that DAMPs generated by the cavitation effect are recognised by TLRs and then activate the downstream signalling molecule interferon regulatory factor (IRF)-5 via the TLR4-MyD88 pathway [70]. IRF-5 phosphorylates and enters the nucleus to perform transcriptional functions, thus promoting macrophage polarisation to the M1 phenotype [71]. Moreover, Kheirolomoom established a bilateral metastatic breast cancer model in which one side was treated with UTMD combined immune adjuvant CpG and the other was left untreated. CpG as an agonist of TLR9 has an essential supporting role in reversing the suppressive TME [72]. Within the both sides, there was a substantial drop in the frequency and absolute amount of MDSCs after performing the treatment procedure [73] (Fig. 2c). Similarly, one recent study elucidated that UTMD decreased the frequency of CD71+ erythroid progenitor cells, which suppress the immune response [74] (Fig. 2d). In conclusion, UTMD facilitates the expression of co-stimulatory molecules, triggers the maturation of DCs, promotes the polarisation to pro-inflammatory macrophages and curbs MDSCs to regulate the immune-suppressive TME.
Effects of UTMD on intratumoral regulatory T cells
Tregs are a group of immunosuppressive cells that maintain immune homoeostasis in the body. Tregs inhibit antitumor immune responses and promote tumour progression [75]. The chemokine receptor CCR4 expressed by Tregs selectively binds to the chemokine ligand CCL22, which is highly expressed in most tumour tissues [76, 77]. The immune response is downregulated mainly in two ways. First, Tregs directly inhibit effector cells through releasing perforin, granzyme or expressing inhibitory receptors programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) or secreting inhibitory cytokines such as TGF-β, IL-10 and IL-35 [78, 79]. Second, Tregs indirectly suppress T-cell activation by interacting with APCs. CTLA-4 expressed on Tregs conjugates with the co-stimulatory molecule CD80/86 on APCs. This process impedes the binding of CD28 on T cells to CD80/86, thereby inhibiting T-cell activation [80].
UTMD treatment could reduce Tregs in the tumour [48], and the proliferation rate of Tregs gradually decreases with increasing ultrasound irradiation time due to apoptosis [81]. Interestingly, the combination of UTMD and doxorubicin (Dox) was able to induce the translocation of calreticulin (CRT) and ER-associated protein disulfide isomerase ERp57 and upregulate the expression of chromatin-binding protein high-mobility group B1 (HMGB-1) in tumour cells, suggesting the activation of ICD. This response reversed the suppressive immune microenvironment so that a lower percentage of Tregs was observed [53]. Pro-inflammatory factors have been reported to differentiate Tregs into an unstable subpopulation. This subpopulation decreased the expression of Forkhead family transcription factor P3 (Foxp3) and Treg-related markers, raised the expression of TNF-α and IL17A, and elevated both glycolysis and proliferation [82]. UTMD boosts the expression of pro-inflammatory factors and may deregulate the immunosuppressive function of partially Tregs in this manner. Additionally, concomitant UTMD and microRNA was also used to knock down Foxp3 to inhibit the negative effect of Tregs by decreasing the level of TGF-β and IL-10 [83]. Mature Tregs maintain sustained Foxp3 transcription through the autoregulatory transcriptional circuit [84]. Once Foxp3 is knocked down, this circuit is disrupted and Tregs lose immunosuppressive function. Thus, UTMD combined with drugs or microRNAs dysfunction the proliferation and maturation of Foxp3+ Tregs, thereby alleviating the unfavourable tumour microenvironment.
Effects of UTMD on intratumoral natural killer cells
Natural killer cells (NK cells) as critical components of the innate immune response are a class of lymphocytes that lyse tumour cells and infected cells non-specifically without pre-sensitisation [85]. NK cells recognise tumour cells through two modes: missing-self and antibody-dependent cell-mediated cytotoxicity (ADCC), depending on the balance between activatory and inhibitory receptors [86–88].
Considering the non-antigen-specific killing mechanism, NK cells may not only resist tumour escape caused by antigenic drift [89], but also have the potential to exert broad antitumor effects on a wide range of tumour species [90]. However, suppressed activity immensely limits the advancement of NK cells’ clinical applications. Alkins et al. injected HER2-specific NK-92 cells into tumour-bearing mice via tail vein. Then they found a 5-fold increase in the ratio of NK-92 cells to tumour cells compared to the control group with ultrasonication performed 30 seconds after injection. However, the detailed mechanism leading to increased NK cell infiltration is not precise. One possibility is that ultrasound transiently enlarges the endothelial cell gap to facilitate the passage of NK cells [91]. What’s more, pro-inflammatory chemokines recruit circulating NK cells to tumour sites [87]. UTMD could trigger the expression of chemokine (C-X3-C motif) ligand 1 (CX3CL1), thus attracting NK-92MI cells into the tumour regions [92]. The mechanical stimulation generated by cavitation promoted Ca2+ influx through the mechanosensitive channel Piezo1 [93]. In the presence of Ca2+, the perforin released from NK cells is embedded in the tumour cell membrane [94], thus killing tumour cells. Therefore, UTMD expands the endothelial cell gap, elicits pro-inflammatory chemokine expression, and promotes Ca2+ influx to potentiate intratumoral NK cell recruitment and lytic effects.
Effects of UTMD on tumour vasculature
Tumour vasculature is tortuous, tangled, branched irregularly and unevenly distributed, with low pericyte coverage and loose connections between endothelial cells, which augments interstitial fluid pressure (IFP) [95]. Blood does not flow in a steady, unidirectional course through tumours, and high interstitial pressure collapses blood vessels and reduces tumour perfusion [96]. Furthermore, as the main cytokine, VEGF effectively stimulates the pathological process, forming abnormal tumour blood vessels [97, 98]. VEGF has also been utilised as a target to normalise tumour vasculature [99]. Normalisation of tumour vasculature improves the supply of partial nutrients and oxygen, leading to a return of tumour sensitivity to drugs [100]. In addition, this modifies the acidic and hypoxic hostile environment and diminishes the number of immunosuppressive cells [101]. On the other hand, normalisation alleviates high IFP in the tumour, facilitating the delivery of ICBs and infiltration of effector immune cells, thus improving the efficacy of CIT [99].
UTMD significantly increased perfusion according to ultrasound imaging and decreased tumour microvascular density (MVD) [48], demonstrating vascular normalisation. Previous studies have revealed that ultrasound using microbubble cavitation increased shear-dependent ATP. ATP release mediated calcium wave propagation to activate calcium-dependent purinergic signalling pathways. Endothelial nitric oxide synthase and vasoactive prostanoids were produced to augment skeletal muscle blood flow [102]. The expression of CD34 (positioned on tumour vascular endothelium), VEGF and tumour endothelial marker (TEM8) decreased, suggesting the inhibition of tumour angiogenesis [103, 104]. Consistently, histological staining revealed vasodilation rather than angiogenesis mediated by UTMD [48, 105]. Besides, Liu reported that UTMD allowed FITC-labelled dextran to leak from the vasculature into the extravascular tumour area, and no increase in apoptotic cells was distinctly observed [49]. Tumour vascular permeability was better enhanced without significant cellular damage, facilitating homogeneous drug distribution in the tumour’s central and peripheric regions [49, 106, 107]. What’s more, UTMD promotes the expression of the adhesion molecule ICAM-1, which is crucial for T-cell accumulation [48]. Tumour vascular normalisation has been reported to upregulate the expression of adhesion molecules and thus facilitate the localisation of antitumor T cells [108]. UTMD potentially increases the accumulation of T cells by contributing to the normalisation of tumour vasculature. Taken together, UTMD fosters vasodilation, strengthens vascular permeability, restrains tumour angiogenesis, and normalises tumour vasculature, thereby accumulating and seeding antitumor effector immune cells.
Effects of UTMD on barrier structures
The blood-brain barrier (BBB) strictly limits the entry and exit of substances to protect the brain from toxic molecules [109]. When brain tumours occur (both primary and secondary brain tumours), components of the BBB will be modified to develop the blood-tumour barrier (BTB) [110], such as increased VEGF expression, loss of tight junctions and pericytes [111], and loss of basement membrane protein components [112]. Although some drugs can enter the tumour through BTB, the therapeutic effect is still unsatisfactory [110].
Recent studies demonstrated that UTMD was a technology worth exploring for opening BBB/BTB. For instance, researchers found a temporary increase in BBB permeability by UTMD comparing MRI signal intensity change (SIC) at different time points, including a significant increase in CD4+ and CD8+ lymphocytes in the rat glioma model [113]. In addition, in intracranial melanomas, UTMD successfully disturbed BTB, enhanced the expression of the pro-inflammatory cytokines and enriched the inflammatory gene sets. More importantly, UTMD elicited immune response due to the raised expression of H2-K1 and H2-D1, the class I MHC molecules [62]. In brain metastases, some investigators showed that UTMD increased the extravascular concentration and penetration distance of chemotherapeutic agents, suggesting the possibility of improved efficacy [114]. Another modified critical component is the transporter, Permeability-glycoprotein (Pgp), which overexpresses on the BBB/BTB. Aryal reported that using UTMD could transiently downregulate Pgp expression, supported by being suppressed for 48 hours and restored after 72 hours at the ultrasound intensity of 0.55MPa [115]. Similarly, UTMD significantly increased paclitaxel-induced apoptosis by strengthening the permeability of the blood-prostate barrier [116].
Enhanced antitumor efficacy in different cancer species with UTMD
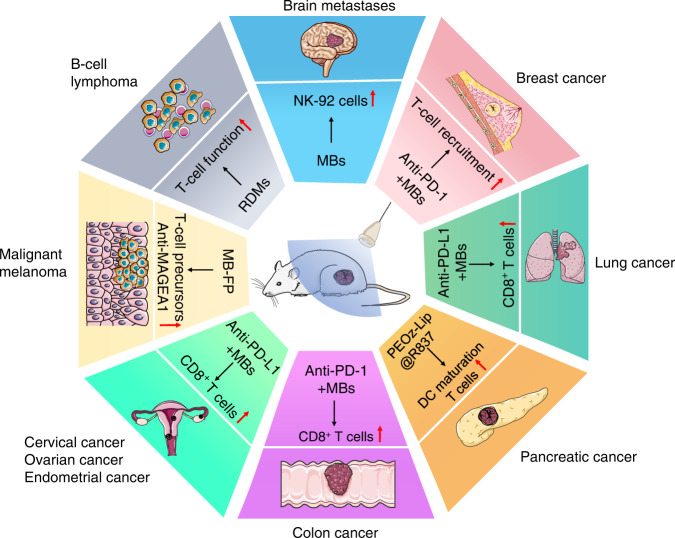
As previously mentioned, UTMD can increase vascular permeability, normalise tumour vasculature, increase infiltration of antitumor effector cells and reduce immunosuppressive cells in the TME. This technology has led to investigating its antitumor efficacy and promising results have been achieved (Fig. 3).
Fig. 3. UTMD strengthens antitumor efficacy in different cancer species.
UTMD has been tested in animals and achieved enhanced antitumor effects in a variety of cancers by augmenting immune responses.
UTMD reduced mean brain metastasis volumes by 3-fold (measured at 28 days) and prolonged the mean survival time by 3-fold of rats with the front-loaded treatment arm (5 treatments in the first week, 2 treatments in the second week, and 1 treatment in the third week) by injecting Definity microbubbles, which was attributed to the localisation of NK-92 cells within the tumour [10]. UTMD with pIFN-β and anti-PD-1 also slowed tumour growth by 4-fold and even showed complete tumour regression by enhancing T-cell recruitment in a mouse model of breast cancer [51]. For the Lewis lung cancer (LLC) model, UTMD and anti-PD-L1 combination therapy induced an increase in IFN-γ production, causing an increase in CD8+ T-cell frequency to inhibit LLC progression [74]. Moreover, researchers constructed TLR agonist (R837)-loaded pH-responsive liposomes (PEOz-Lip@R837) to treat pancreatic cancer. They observed a 1.5-fold lower mean fluorescence intensity of primary tumour, a 5-fold smaller mean distant tumour volume compared to the control group and reduced secondary tumour weight value due to DC maturation, and activation and proliferation of T cells after UTMD [117].
Colon cancer was also treated using UTMD technology with anti-PD-1, and the number and effector function of CD8+ T cells were systemically increased, thereby augmenting anti-PD-1 efficacy [118]. For gynaecologic tumours, UTMD combined with bevacizumab or plasmid pCMV-IL-12 or anti-PD-L1 antibody enhanced efficacy in cervical, endometrial, and ovarian cancers by increasing the migration and infiltration of CD8+ T cells [52, 119, 120]. In addition, UTMD has also been studied for the therapy of malignant melanoma. Gao et al. reported that UTMD with MB-FP (gas-filled ultrasound microbubbles with HSP70-melanoma associated antigen A1 fusion protein) stimulated the generation of T-cell precursors and boosted the titre of anti-MAGEA1, indicating both boosted cellular and humoural immune responses [121]. Another study on the treatment of B-cell lymphoma found that rituximab-conjugated and Dox-loaded microbubbles (RDMs) significantly reduced tumour proliferation, which two out of five mice achieved tumour-free, and promoted apoptosis of malignant cells through restoring T-cell function, suggesting effective antitumor immune responses [122].
UTMD in delivery
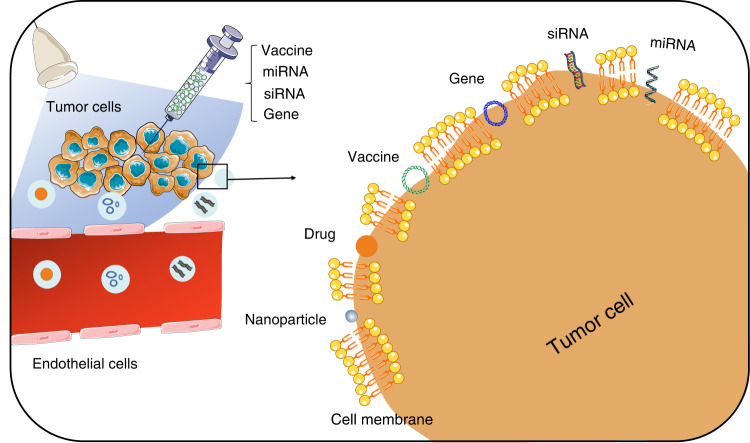
UTMD reversibly opens tight junctions between cells and temporarily expands the endothelial cell gap, providing accessible pathways for drugs, genes, plasmids, and nanoparticles [25] (Fig. 4). It is a non-invasive and efficient delivery process. Therefore, it may be a novel and promising delivery technique that combines microbubbles with relevant effector substances and then delivers them to the target location.
Fig. 4. UTMD for delivery applications.
UTMD improves the efficiency of drugs, nanoparticles, vaccine delivery and gene, siRNA and miRNA transfection.
Drugs
Drugs are widely utilised in the preoperative and postoperative treatment of malignant tumours and have achieved certain efficacy. However, the urgent problem is that drugs penetrate weakly into the tumour, resulting in a relatively high concentration when achieving the expected antitumor effect, which may cause severe toxic side effects. UTMD has been conducted to study to improve this situation. The sonoporation not only normalises tumour vasculature in TME, but also opens the endothelial cell gap and augments the permeability of the tumour cell membrane, which facilitate the delivery of drugs into the tumour. For example, paclitaxel combined with folate-targeted microbubbles was delivered to ovarian cancer sites. Then the higher drug concentrations were detected in tumour and lymphoid tissues, while lower in blood, implying better efficacy and less severe side effects [123]. Consistently, tumour cells accumulated seven times higher Dox with an exceeding five times penetration distance [53, 114]. In addition, the higher delivery efficiency of anti-PD-L1 was discovered with UTMD treatment by detecting fluorescent signals in a mouse intracranial glioma model [124]. UTMD was also able to improve the consistency of Dox distribution, reducing the difference in concentration between the central and peripheral regions of the tumour [107].
Cancer vaccines
Cancer vaccines are designed to trigger the specific immune response and establish immune memory to prevent tumour metastasis and recurrence [125]. Cancer vaccines work primarily by processing tumour-associated antigens (TAAs) or tumour-specific antigens (TSAs) through APCs to activate effector cells [126]. DNA and RNA vaccines are also common types. Although APCs can internalise DNA and RNA, the low uptake efficiency limits the clinical effectiveness of these vaccines [127]. Moreover, insufficient intratumoral CD8+ T-cell infiltration and local accumulation of immunosuppressive cells such as Tregs, MDSCs, and TAMs are essential factors compromising vaccine efficacy [125]. Given its remodelling of TME, UTMD is a promising novel platform for vaccine delivery to improve cancer vaccine efficacy. Some progress has been made in applying UTMD technology to deliver DNA or RNA vaccines. For instance, antigen mRNA was co-cultured with DCs and then treated with sonication for 30 seconds to obtain mRNA sonoporated DCs. After being injected with tumour cells, the tumour-bearing mice received two inoculations of therapeutic mRNA sonoporated DCs. Eventually, the mice had 58% slower tumour growth, 41% longer median survival and elicited immune memory by reinjection 42 days following the initial tumour injection [128]. What’s more, pGM-CSF-loaded microbubbles with UTMD mediated more cytokine secretion and recruited more T cells on both the treated and untreated sides of the bilateral tumour models [54]. Moreover, pUb-M-loaded bubble lipoplexes (containing DNA encoding melanoma-specific antigens) were constructed to immunise mice. Furthermore, they found that 7/10 of melanoma mice developed complete tumour rejection. Inhibition was observed not in subcutaneous tumours but pulmonary metastases when tumour cells were reinjected 100 days after the initial implantation, suggesting the successful establishment of immune memory [129].
Molecules
MicroRNA, short interfering RNA, genes and nanoparticles have also made some progress in tumour therapy [130, 131]. miRNAs have been shown to participate in reprogramming the TME, including tumour-infiltrating lymphocytes, MDSCs, cancer-associated endothelial cells, to accelerate or dampen tumour progression [132]. UTMD has the potential to augment the antitumor effect and alleviate the immunosuppressive environment through the delivery of miRNAs. What’s more, traditional viral vectors often raise concerns about their immunogenicity in humans, while physical methods such as electroporation are less specific and with low transfection efficiency [133]. UTMD may be a novel and adequate technology due to its reversibility, non-invasiveness and repeatability. The delivery of miR-34a into cervical cancer cells using UTMD was found to have a stronger fluorescence emission, suggesting a higher gene transfection efficiency. Simultaneously, the binding of PD-L1 Ab to miR-34a lorded microbubbles was observed to cause an increase in apoptosis by detecting the mRNA expression of Bax and Bcl2 [134, 135]. Besides, UTMD was reported to enhance anti-miR21 delivery efficiency by 5-fold through modified nanoparticles. The distribution of the Cy5 fluorescence signal showed that hepatocellular carcinoma cells successfully absorbed miRNAs released by nanoparticle rupture [136]. In addition to miRNA, UTMD is also used to deliver siRNA, indicating a 10-fold increase in siRNA delivery in brain TME [137]. Moreover, researchers constructed a suspension of DNA plasmids and microbubbles to deliver endostatin (ED) and CRT DNA to muscle tissue. They observed a significant increase in ED and CRT expression and a decrease in tumour size combined with Dox in orthotopic hepatocellular carcinoma and lung cancer [138].
Clinical application potential of UTMD
In the background of the popularity of precision medicine and targeted therapy, UTMD is attracting attention for its unique advantages. It also demonstrates substantial prospects for clinical application. First, UTMD is safe and harmless to the heart, spleen, kidneys and other organs [74, 92]. Studies have shown that UTMD did not cause significant weight loss or a decrease in cellular activity in experimental animals [116, 139]. Second, the technique is repeatable in opening the BBB, and the microbubbles remain well stabilised during the movement in the circulatory system in vivo [91, 140, 141]. Third, UTMD is highly specific and targeted by modifying microbubbles to confer them localisation ability [25, 142]. Furthermore, boosting CIT is one of the most clinically valuable aspects. For instance, compared to using them alone, UTMD strengthened the antitumor effects of ICIs and cytokines, such as anti-PD-1/PD-L1 and IL-12 [143–145]. What’s more, UTMD lessened the toxic effects of chemotherapy drugs by encapsulating them in microbubbles, creating a barrier between them and organs or tissue [107]. More interestingly, this technology could regulate the release rate of the drugs or molecules and precisely kill cells in the tumour area without disturbing the level of immune cells throughout the body, which may alleviate the immune deficiency caused by chemotherapy [68, 146].
Discussion
UTMD exerts a notable effect on TME by increasing infiltration of CD8+ T cells and NK cells, facilitating maturation of DCs and conversion of macrophage phenotype, diminishing the number of Tregs and MDSCs and normalising tumour vasculature, thereby remodelling TME. In addition, UTMD can also break down barrier structures, promote drug delivery, gene transfection, and sensitise cancer vaccines.
While focusing on the substantial promise for clinical application, some limitations and difficulties need to be further addressed. (a) Currently, the majority of UTMD trials are still being conducted in animals. Although a few clinical trials are available, they evaluate whether to improve the sensitivity of chemotherapy and radiotherapy [147–149]. Considering the complexity and heterogeneity of the human environment, the application of UTMD combined with CIT necessitates further clinical trials to discover. (b) Neoplasms at varying regions or organs have either mild or pronounced differences in the TME, which affects the selection of immunotherapy regimens. Accordingly, taking into account the immune heterogeneity of the organs and individual genetic background and then choosing the well-matched microbubbles is a part of the UTMD treatment strategy that cannot be overlooked and merits further investigation. (c) The current commercially available microbubbles are only used for enhanced imaging, and the “next generation” microbubbles integrated with drugs, genes and vaccines need to be optimised and adapted for clinical application in the future. In other words, the new generation microbubbles are not only a complex containing multifunctional substances, but also convenient to produce, preserve and transport so that they can be successfully used in clinical patients. (d) The optimal parameters of UTMD are continuously being investigated. The therapeutic effect is not only correlated with ultrasound parameters such as frequency, mechanical index, duty cycle and irradiation time, but also with non-ultrasound elements such as microbubble dose, ambient temperature, air humidity and tissue type. The appropriate microbubble type and acoustic parameters are pivotal segments of the treatment protocol. (e) Most animal treatments utilise ultrasound to irradiate solid subcutaneous tumours in mice. For the more prominent human body, the suitable ultrasound probe and irradiation modality for treating haematologic and cavernous organ tumours such as colorectal cancer is a topic worth investigating. (f) The precise mechanisms that UTMD remodels the components of TME have not been elucidated. Previous studies have only focused on surface phenomena, so an in-depth analysis of the regulatory agency causing enhanced immune response may provide fresh insights on CIT sensitisation.
Apart from CIT, UTMD has shown potential in augmenting chemotherapy and radiotherapy. Clinical trials revealed that UTMD combined with nab-paclitaxel, gemcitabine or technetium 99 m macroaggregated albumin had a greater prevalence of tumour response, prolonged quality of life, and extended survival [147–149]. In addition, UTMD has been disclosed to treat cardiovascular diseases, such as avoiding thrombosis after mechanical heart valve replacement, preventing cardiomyopathy, and relieving acute cellular rejection after heart transplantation [150]. More interestingly, UTMD augmented proliferation and chondrogenic and osteogenic differentiation, demonstrating activation of mesenchymal stem cells [151]. What’s more, using UTMD better to diagnose is under ongoing study. Conventional ultrasound with contrast imaging primarily focuses on the perfusion status due to the size of microbubbles and the permeability of the blood vessels. UTMD combined with targeted microbubbles breaks through vascular restrictions to bind to markers of extravascular tissues or tumours, thereby facilitating early diagnosis, clinical staging, and disease monitoring [152, 153].
In short, we conclude that UTMD plays an essential part in remodelling the TME and has an augmenting action on CIT. We believe that as the research on UTMD technology continues, the mystery of its mechanism will gradually unravel and bring a new revolution in tumour immunotherapy.
Supplementary information
Author contributions
ZS, LL and CW provided direction and guidance throughout the preparation of this manuscript. SL, YZ and YL drafted the manuscript and drew the figures. WW and SG collected the related references and participated in discussion. WY revised the manuscript. All authors contributed to this manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by The National Natural Science Foundation of China (81972663, 82173055, U2004112), The Excellent Youth Science Project of Henan Natural Science Foundation (212300410074), The Youth Talent Innovation Team Support Program of Zhengzhou University (32320290), The Provincial and Ministry co-constructed key projects of Henan medical science and technology (SBGJ202102134), Key scientific and technological research projects of Henan Provincial Department of Science and Technology (212102310117), Henan Provincial Health Commission and Ministry of Health Co-construction Project, and Henan Provincial Health and Health Commission Joint Construction Project (LHGJ20200158), the Natural Science Foundation of Henan Province (202300410446).
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have approved to publish this manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Senbo Liu, Yan Zhang, Yang Liu.
Contributor Information
Zhenqiang Sun, Email: fccsunzq@zzu.edu.cn.
Lin Liu, Email: liulin@zzu.edu.cn.
Chengzeng Wang, Email: czw202112@zzu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-02076-y.
References
- 1.Sun JY, Lu XJ. Cancer immunotherapy: current applications and challenges. Cancer Lett. 2020;480:1–3. doi: 10.1016/j.canlet.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell. 2019;36:471–82. doi: 10.1016/j.ccell.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang H, Qiao J, Fu YX. Immunotherapy and tumor microenvironment. Cancer Lett. 2016;370:85–90. doi: 10.1016/j.canlet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bejarano L, Jordao MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021;11:933–59. doi: 10.1158/2159-8290.CD-20-1808. [DOI] [PubMed] [Google Scholar]
- 6.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 7.Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52:17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Unga J, Hashida M. Ultrasound induced cancer immunotherapy. Adv Drug Deliv Rev. 2014;72:144–53. doi: 10.1016/j.addr.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Snipstad S, Vikedal K, Maardalen M, Kurbatskaya A, Sulheim E, Davies CL. Ultrasound and microbubbles to beat barriers in tumors: improving delivery of nanomedicine. Adv Drug Deliv Rev. 2021;177:113847. doi: 10.1016/j.addr.2021.113847. [DOI] [PubMed] [Google Scholar]
- 10.Alkins R, Burgess A, Kerbel R, Wels WS, Hynynen K. Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro Oncol. 2016;18:974–81. doi: 10.1093/neuonc/nov318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baetke SC, Rix A, Tranquart F, Schneider R, Lammers T, Kiessling F, et al. Squamous cell carcinoma xenografts: use of VEGFR2-targeted microbubbles for combined functional and molecular US to monitor antiangiogenic therapy effects. Radiology. 2016;278:430–40. doi: 10.1148/radiol.2015142899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song HW, Lee HS, Kim SJ, Kim HY, Choi YH, Kang B, et al. Sonazoid-conjugated natural killer cells for tumor therapy and real-time visualization by ultrasound imaging. Pharmaceutics. 2021;13:1689. [DOI] [PMC free article] [PubMed]
- 13.Tu J, Zhang H, Yu J, Liufu C, Chen Z. Ultrasound-mediated microbubble destruction: a new method in cancer immunotherapy. Onco Targets Ther. 2018;11:5763–75. doi: 10.2147/OTT.S171019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu W, Zhang X, Hu X, Zhiyi C, Huang P. Translational prospects of ultrasound-mediated tumor immunotherapy: preclinical advances and safety considerations. Cancer Lett. 2019;460:86–95. doi: 10.1016/j.canlet.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Zhang Y, Shu H, Lv W, Su C, Nie F. Highlights in ultrasound-targeted microbubble destruction-mediated gene/drug delivery strategy for treatment of malignancies. Int J Pharm. 2022;613:121412. doi: 10.1016/j.ijpharm.2021.121412. [DOI] [PubMed] [Google Scholar]
- 16.Paliwal S, Mitragotri S. Ultrasound-induced cavitation: applications in drug and gene delivery. Expert Opin Drug Deliv. 2006;3:713–26. doi: 10.1517/17425247.3.6.713. [DOI] [PubMed] [Google Scholar]
- 17.Suo D, Govind B, Zhang S, Jing Y. Numerical investigation of the inertial cavitation threshold under multi-frequency ultrasound. Ultrason Sonochem. 2018;41:419–26. doi: 10.1016/j.ultsonch.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury SM, Abou-Elkacem L, Lee T, Dahl J, Lutz AM. Ultrasound and microbubble mediated therapeutic delivery: underlying mechanisms and future outlook. J Control Release. 2020;326:75–90. doi: 10.1016/j.jconrel.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Ho YJ, Li JP, Fan CH, Liu HL, Yeh CK. Ultrasound in tumor immunotherapy: Current status and future developments. J Control Release. 2020;323:12–23. doi: 10.1016/j.jconrel.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Al-Jawadi S, Thakur SS. Ultrasound-responsive lipid microbubbles for drug delivery: a review of preparation techniques to optimise formulation size, stability and drug loading. Int J Pharm. 2020;585:119559. doi: 10.1016/j.ijpharm.2020.119559. [DOI] [PubMed] [Google Scholar]
- 21.Wang R, Bian Q, Xu Y, Xu D, Gao J. Recent advances in mechanical force-assisted transdermal delivery of macromolecular drugs. Int J Pharm. 2021;602:120598. doi: 10.1016/j.ijpharm.2021.120598. [DOI] [PubMed] [Google Scholar]
- 22.Bouakaz A, Zeghimi A, Doinikov AA. Sonoporation: concept and mechanisms. Adv Exp Med Biol. 2016;880:175–89. doi: 10.1007/978-3-319-22536-4_10. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Li Q, Guo X, Tu J, Zhang D. Mechanisms underlying sonoporation: Interaction between microbubbles and cells. Ultrason Sonochem. 2020;67:105096. doi: 10.1016/j.ultsonch.2020.105096. [DOI] [PubMed] [Google Scholar]
- 24.Schoen S, Jr., Kilinc MS, Lee H, Guo Y, Degertekin FL, Woodworth GF, et al. Towards controlled drug delivery in brain tumors with microbubble-enhanced focused ultrasound. Adv Drug Deliv Rev. 2022;180:114043. doi: 10.1016/j.addr.2021.114043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omata D, Unga J, Suzuki R, Maruyama K. Lipid-based microbubbles and ultrasound for therapeutic application. Adv Drug Deliv Rev. 2020;154-155:236–44. doi: 10.1016/j.addr.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Snipstad S, Sulheim E, de Lange Davies C, Moonen C, Storm G, Kiessling F, et al. Sonopermeation to improve drug delivery to tumors: from fundamental understanding to clinical translation. Expert Opin Drug Deliv. 2018;15:1249–61. doi: 10.1080/17425247.2018.1547279. [DOI] [PubMed] [Google Scholar]
- 27.Fan CH, Ting CY, Liu HL, Huang CY, Hsieh HY, Yen TC, et al. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials. 2013;34:2142–55. doi: 10.1016/j.biomaterials.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 28.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–54. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 29.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arneth B. Tumor microenvironment. Medicina (Kaunas). 2019;56:15. [DOI] [PMC free article] [PubMed]
- 31.Frankel T, Lanfranca MP, Zou W. The role of tumor microenvironment in cancer immunotherapy. Adv Exp Med Biol. 2017;1036:51–64. doi: 10.1007/978-3-319-67577-0_4. [DOI] [PubMed] [Google Scholar]
- 32.Zhou S, Gravekamp C, Bermudes D, Liu K. Tumour-targeting bacteria engineered to fight cancer. Nat Rev Cancer. 2018;18:727–43. doi: 10.1038/s41568-018-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14:642–62. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015;15:361–70. doi: 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh S, Hassan D, Aldawsari HM, Molugulu N, Shukla R, Kesharwani P. Immune checkpoint inhibitors: a promising anticancer therapy. Drug Discov Today. 2020;25:223–9. doi: 10.1016/j.drudis.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol. 2019;94:S3–S9. doi: 10.1002/ajh.25418. [DOI] [PubMed] [Google Scholar]
- 37.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2:e192535. doi: 10.1001/jamanetworkopen.2019.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18:635–47. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 39.Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234:8509–21. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 40.Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21:298–312. doi: 10.1038/s41568-021-00339-z. [DOI] [PubMed] [Google Scholar]
- 41.Tan YN, Li YP, Huang JD, Luo M, Li SS, Lee AW, et al. Thermal-sensitive lipid nanoparticles potentiate anti-PD therapy through enhancing drug penetration and T lymphocytes infiltration in metastatic tumor. Cancer Lett. 2021;522:238–54. doi: 10.1016/j.canlet.2021.09.031. [DOI] [PubMed] [Google Scholar]
- 42.Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. 2021;591:645–51. doi: 10.1038/s41586-020-03045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golstein P, Griffiths GM. An early history of T cell-mediated cytotoxicity. Nat Rev Immunol. 2018;18:527–35. doi: 10.1038/s41577-018-0009-3. [DOI] [PubMed] [Google Scholar]
- 44.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaaf MB, Garg AD, Agostinis P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 2018;9:115. doi: 10.1038/s41419-017-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vodnala SK, Eil R, Kishton RJ, Sukumar M, Yamamoto TN, Ha NH, et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science. 2019; 363:eaau0135. [DOI] [PMC free article] [PubMed]
- 47.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–76. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li N, Tang J, Yang J, Zhu B, Wang X, Luo Y, et al. Tumor perfusion enhancement by ultrasound stimulated microbubbles potentiates PD-L1 blockade of MC38 colon cancer in mice. Cancer Lett. 2021;498:121–9. doi: 10.1016/j.canlet.2020.10.046. [DOI] [PubMed] [Google Scholar]
- 49.Liu HL, Hsieh HY, Lu LA, Kang CW, Wu MF, Lin CY. Low-pressure pulsed focused ultrasound with microbubbles promotes an anticancer immunological response. J Transl Med. 2012;10:221. doi: 10.1186/1479-5876-10-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Das JK, Xiong X, Ren X, Yang JM, Song J. Heat shock proteins in cancer immunotherapy. J Oncol. 2019;2019:3267207. doi: 10.1155/2019/3267207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ilovitsh T, Feng Y, Foiret J, Kheirolomoom A, Zhang H, Ingham ES, et al. Low-frequency ultrasound-mediated cytokine transfection enhances T cell recruitment at local and distant tumor sites. Proc Natl Acad Sci USA. 2020;117:12674–85. doi: 10.1073/pnas.1914906117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki R, Namai E, Oda Y, Nishiie N, Otake S, Koshima R, et al. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J Control Release. 2010;142:245–50. doi: 10.1016/j.jconrel.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 53.Huang FY, Lei J, Sun Y, Yan F, Chen B, Zhang L, et al. Induction of enhanced immunogenic cell death through ultrasound-controlled release of doxorubicin by liposome-microbubble complexes. Oncoimmunology. 2018;7:e1446720. doi: 10.1080/2162402X.2018.1446720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang N, Foiret J, Kheirolomoom A, Liu P, Feng Y, Tumbale S, et al. Optimization of microbubble-based DNA vaccination with low-frequency ultrasound for enhanced cancer immunotherapy. Adv Ther (Weinh). 2021;4:2100033. [DOI] [PMC free article] [PubMed]
- 55.Kovacs ZI, Kim S, Jikaria N, Qureshi F, Milo B, Lewis BK, et al. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci USA. 2017;114:E75–E84. doi: 10.1073/pnas.1614777114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chin EN, Sulpizio A, Lairson LL. Targeting STING to promote antitumor immunity. Trends Cell Biol. 10.1016/j.tcb.2022.06.010 2022. [DOI] [PubMed]
- 57.Appleton E, Hassan J, Chan Wah Hak C, Sivamanoharan N, Wilkins A, Samson A, et al. Kickstarting immunity in cold tumours: localised tumour therapy combinations with immune checkpoint blockade. Front Immunol. 2021;12:754436. doi: 10.3389/fimmu.2021.754436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engblom C, Pfirschke C, Pittet MJ. The role of myeloid cells in cancer therapies. Nat Rev Cancer. 2016;16:447–62. doi: 10.1038/nrc.2016.54. [DOI] [PubMed] [Google Scholar]
- 59.Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. 2021;184:792–809.e723. doi: 10.1016/j.cell.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 60.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balan S, Saxena M, Bhardwaj N. Dendritic cell subsets and locations. Int Rev Cell Mol Biol. 2019;348:1–68. doi: 10.1016/bs.ircmb.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Curley CT, Stevens AD, Mathew AS, Stasiak K, Garrison WJ, Miller GW, et al. Immunomodulation of intracranial melanoma in response to blood-tumor barrier opening with focused ultrasound. Theranostics. 2020;10:8821–33. doi: 10.7150/thno.47983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park SJ, Ye W, Xiao R, Silvin C, Padget M, Hodge JW, et al. Cisplatin and oxaliplatin induce similar immunogenic changes in preclinical models of head and neck cancer. Oral Oncol. 2019;95:127–35. doi: 10.1016/j.oraloncology.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sheybani ND, Witter AR, Garrison WJ, Miller GW, Price RJ, Bullock TNJ. Profiling of the immune landscape in murine glioblastoma following blood brain/tumor barrier disruption with MR image-guided focused ultrasound. J Neurooncol. 2022;156:109–22. doi: 10.1007/s11060-021-03887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66:97–110. doi: 10.1146/annurev-med-051013-052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laviron M, Boissonnas A. Ontogeny of tumor-associated macrophages. Front Immunol. 2019;10:1799. doi: 10.3389/fimmu.2019.01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11:583084. doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xue VW, Chung JY, Tang PC, Chan AS, To TH, Chung JS, et al. USMB-shMincle: a virus-free gene therapy for blocking M1/M2 polarization of tumor-associated macrophages. Mol Ther Oncolytics. 2021;23:26–37. doi: 10.1016/j.omto.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–9. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 70.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–8. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 71.Lopez-Pelaez M, Lamont DJ, Peggie M, Shpiro N, Gray NS, Cohen P. Protein kinase IKKβ-catalyzed phosphorylation of IRF5 at Ser462 induces its dimerization and nuclear translocation in myeloid cells. Proc Natl Acad Sci USA. 2014;111:17432–7. doi: 10.1073/pnas.1418399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller CL, Sagiv-Barfi I, Neuhöfer P, Czerwinski DK, Artandi SE, Bertozzi CR, et al. Systemic delivery of a targeted synthetic immunostimulant transforms the immune landscape for effective tumor regression. Cell Chem Biol. 2022;29:451–.e458. doi: 10.1016/j.chembiol.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kheirolomoom A, Ingham ES, Mahakian LM, Tam SM, Silvestrini MT, Tumbale SK, et al. CpG expedites regression of local and systemic tumors when combined with activatable nanodelivery. J Control Release. 2015;220:253–64. doi: 10.1016/j.jconrel.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan X, Yi C, Zhang Y, Tang N, Xu Y, Liu Z. Ultrasound-targeted microbubble destruction alleviates immunosuppression induced by CD71(+) erythroid progenitor cells and promotes PDL-1 blockade immunotherapy in the lewis lung cancer model. Front Oncol. 2021;11:768222. doi: 10.3389/fonc.2021.768222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends Immunol. 2013;34:33–40. doi: 10.1016/j.it.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–9. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 77.Tanaka A, Sakaguchi S. Targeting Treg cells in cancer immunotherapy. Eur J Immunol. 2019;49:1140–6. doi: 10.1002/eji.201847659. [DOI] [PubMed] [Google Scholar]
- 78.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–46. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 79.Han S, Toker A, Liu ZQ, Ohashi PS. Turning the tide against regulatory T cells. Front Oncol. 2019;9:279. doi: 10.3389/fonc.2019.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 81.Shi C, Zhang Y, Yang H, Dong T, Chen Y, Xu Y, et al. Combined effect of ultrasound/SonoVue microbubble on CD4(+)CD25(+) regulatory T cells viability and optimized parameters for its transfection. Ultrasonics. 2015;62:97–102. doi: 10.1016/j.ultras.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 82.Yi G, Zhao Y, Xie F, Zhu F, Li B. Single-cell RNA-seq unveils critical regulators of human FOXP3+ regulatory T cell stability. Sci Bull. 2020;65:1114–24. [DOI] [PubMed]
- 83.Shi C, Zhang Y, Yang H, Dong T, Chen Y, Xu Y, et al. Ultrasound-targeted microbubble destruction-mediated Foxp3 knockdown may suppress the tumor growth of HCC mice by relieving immunosuppressive Tregs function. Exp Ther Med. 2018;15:31–8. doi: 10.3892/etm.2017.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ono M. Control of regulatory T-cell differentiation and function by T-cell receptor signalling and Foxp3 transcription factor complexes. Immunology. 2020;160:24–37. doi: 10.1111/imm.13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 87.Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18:85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lieberman NAP, DeGolier K, Haberthur K, Chinn H, Moyes KW, Bouchlaka MN, et al. An uncoupling of canonical phenotypic markers and functional potency of ex vivo-expanded natural killer cells. Front Immunol. 2018;9:150. doi: 10.3389/fimmu.2018.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cerwenka A, Lanier LL. Natural killers join the fight against cancer. Science. 2018;359:1460–1. doi: 10.1126/science.aat2184. [DOI] [PubMed] [Google Scholar]
- 91.Alkins R, Burgess A, Ganguly M, Francia G, Kerbel R, Wels WS, et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013;73:1892–9. doi: 10.1158/0008-5472.CAN-12-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang C, Du M, Yan F, Chen Z. Focused ultrasound improves NK-92MI cells infiltration into tumors. Front Pharm. 2019;10:326. doi: 10.3389/fphar.2019.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song Y, Chen J, Zhang C, Xin L, Li Q, Liu Y, et al. Mechanosensitive channel Piezo1 induces cell apoptosis in pancreatic cancer by ultrasound with microbubbles. iScience. 2022;25:103733. doi: 10.1016/j.isci.2022.103733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940–52. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 95.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 96.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–8. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–75. doi: 10.1146/annurev.pathol.2.010506.134925. [DOI] [PubMed] [Google Scholar]
- 98.Nagy JA, Chang SH, Dvorak AM, Dvorak HF. Why are tumour blood vessels abnormal and why is it important to know? Br J Cancer. 2009;100:865–9. doi: 10.1038/sj.bjc.6604929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–40. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–54. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 101.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–22. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Belcik JT, Davidson BP, Xie A, Wu MD, Yadava M, Qi Y, et al. Augmentation of muscle blood flow by ultrasound cavitation is mediated by ATP and purinergic signaling. Circulation. 2017;135:1240–52. doi: 10.1161/CIRCULATIONAHA.116.024826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y, Hu B, Diao X, Zhang J. Antitumor effect of microbubbles enhanced by low frequency ultrasound cavitation on prostate carcinoma xenografts in nude mice. Exp Ther Med. 2012;3:187–91. doi: 10.3892/etm.2011.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hua X, Ding J, Li R, Zhang Y, Huang Z, Guo Y, et al. Anti-tumor effect of ultrasound-induced Nordy-loaded microbubbles destruction. J Drug Target. 2016;24:703–8. doi: 10.3109/1061186X.2016.1144058. [DOI] [PubMed] [Google Scholar]
- 105.Xiao N, Liu J, Liao L, Sun J, Jin W, Shu X. Improved delivery of doxorubicin by altering the tumor microenvironment using ultrasound combined with microbubbles and chemotherapy. J BUON. 2019;24:844–52. [PubMed] [Google Scholar]
- 106.Wang G, Zhuo Z, Xia H, Zhang Y, He Y, Tan W, et al. Investigation into the impact of diagnostic ultrasound with microbubbles on the capillary permeability of rat hepatomas. Ultrasound Med Biol. 2013;39:628–37. doi: 10.1016/j.ultrasmedbio.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 107.Ho YJ, Yeh CK. Concurrent anti-vascular therapy and chemotherapy in solid tumors using drug-loaded acoustic nanodroplet vaporization. Acta Biomater. 2017;49:472–85. doi: 10.1016/j.actbio.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 108.Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Wyser Rmili C, Kiialainen A, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. 2017;9:eaak9670. [DOI] [PubMed]
- 109.Deprez J, Lajoinie G, Engelen Y, De Smedt SC, Lentacker I. Opening doors with ultrasound and microbubbles: Beating biological barriers to promote drug delivery. Adv Drug Deliv Rev. 2021;172:9–36. doi: 10.1016/j.addr.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 110.Steeg PS. The blood-tumour barrier in cancer biology and therapy. Nat Rev Clin Oncol. 2021;18:696–714. doi: 10.1038/s41571-021-00529-6. [DOI] [PubMed] [Google Scholar]
- 111.Zhou W, Chen C, Shi Y, Wu Q, Gimple RC, Fang X, et al. Targeting glioma stem cell-derived pericytes disrupts the blood-tumor barrier and improves chemotherapeutic efficacy. Cell Stem Cell. 2017;21:591–603.e594. doi: 10.1016/j.stem.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lyle LT, Lockman PR, Adkins CE, Mohammad AS, Sechrest E, Hua E, et al. Alterations in pericyte subpopulations are associated with elevated blood-tumor barrier permeability in experimental brain metastasis of breast cancer. Clin Cancer Res. 2016;22:5287–99. doi: 10.1158/1078-0432.CCR-15-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen KT, Chai WY, Lin YJ, Lin CJ, Chen PY, Tsai HC, et al. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci Adv. 2021;7:eabd0772. [DOI] [PMC free article] [PubMed]
- 114.Arvanitis CD, Askoxylakis V, Guo Y, Datta M, Kloepper J, Ferraro GB, et al. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood-tumor barrier disruption. Proc Natl Acad Sci USA. 2018;115:E8717–E8726. doi: 10.1073/pnas.1807105115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aryal M, Fischer K, Gentile C, Gitto S, Zhang YZ, McDannold N. Effects on P-glycoprotein expression after blood-brain barrier disruption using focused ultrasound and microbubbles. PLoS ONE. 2017;12:e0166061. doi: 10.1371/journal.pone.0166061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xia H, Yang D, He W, Zhu X, Yan Y, Liu Z, et al. Ultrasound-mediated microbubbles cavitation enhanced chemotherapy of advanced prostate cancer by increasing the permeability of blood-prostate barrier. Transl Oncol. 2021;14:101177. doi: 10.1016/j.tranon.2021.101177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang X, Shi Z, Luo J, Zeng Y, He L, Chen L, et al. Ultrasound improved immune adjuvant delivery to induce DC maturation and T cell activation. J Control Release. 2022;349:18–31. doi: 10.1016/j.jconrel.2022.06.054. [DOI] [PubMed] [Google Scholar]
- 118.Hu J, He J, Wang Y, Zhao Y, Fang K, Dong Y, et al. Ultrasound combined with nanobubbles promotes systemic anticancer immunity and augments anti-PD1 efficacy. J Immunother Cancer. 2022;10:e003408. [DOI] [PMC free article] [PubMed]
- 119.Ma Y, Han J, Jiang J, Zheng Z, Tan Y, Liu C, et al. Ultrasound targeting of microbubble-bound anti PD-L1 mAb to enhance anti-tumor effect of cisplatin in cervical cancer xenografts treatment. Life Sci. 2020;262:118565. doi: 10.1016/j.lfs.2020.118565. [DOI] [PubMed] [Google Scholar]
- 120.Yamaguchi K, Matsumoto Y, Suzuki R, Nishida H, Omata D, Inaba H, et al. Enhanced antitumor activity of combined lipid bubble ultrasound and anticancer drugs in gynecological cervical cancers. Cancer Sci. 2021;112:2493–503. doi: 10.1111/cas.14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gao X, Nan Y, Yuan Y, Gong X, Sun Y, Zhou H, et al. Gas‑filled ultrasound microbubbles enhance the immunoactivity of the HSP70‑MAGEA1 fusion protein against MAGEA1‑expressing tumours. Mol Med Rep. 2018;18:315–21. doi: 10.3892/mmr.2018.9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zheng S, Song D, Jin X, Zhang H, Aldarouish M, Chen Y, et al. Targeted microbubbles with ultrasound irradiation and PD-1 inhibitor to increase antitumor activity in B-cell lymphoma. Nanomed (Lond) 2018;13:297–311. doi: 10.2217/nnm-2017-0296. [DOI] [PubMed] [Google Scholar]
- 123.Luo T, Sun J, Zhu S, He J, Hao L, Xiao L, et al. Ultrasound-mediated destruction of oxygen and paclitaxel loaded dual-targeting microbubbles for intraperitoneal treatment of ovarian cancer xenografts. Cancer Lett. 2017;391:1–11. doi: 10.1016/j.canlet.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 124.Ye D, Yuan J, Yue Y, Rubin JB, Chen H. Focused ultrasound-enhanced delivery of intranasally administered anti-programmed cell death-ligand 1 antibody to an intracranial murine glioma model. Pharmaceutics. 2021;13:190. [DOI] [PMC free article] [PubMed]
- 125.Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21:360–78. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 126.Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun B, Zhao X, Wu Y, Cao P, Movahedi F, Liu J, et al. Mannose-functionalized biodegradable nanoparticles efficiently deliver DNA vaccine and promote anti-tumor immunity. ACS Appl Mater Interfaces. 2021;13:14015–27. doi: 10.1021/acsami.1c01401. [DOI] [PubMed] [Google Scholar]
- 128.Dewitte H, Van Lint S, Heirman C, Thielemans K, De Smedt SC, Breckpot K, et al. The potential of antigen and TriMix sonoporation using mRNA-loaded microbubbles for ultrasound-triggered cancer immunotherapy. J Control Release. 2014;194:28–36. doi: 10.1016/j.jconrel.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 129.Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M. Suppression of melanoma growth and metastasis by DNA vaccination using an ultrasound-responsive and mannose-modified gene carrier. Mol Pharm. 2011;8:543–54. doi: 10.1021/mp100369n. [DOI] [PubMed] [Google Scholar]
- 130.Cabral H, Kinoh H, Kataoka K. Tumor-targeted nanomedicine for immunotherapy. Acc Chem Res. 2020;53:2765–76. doi: 10.1021/acs.accounts.0c00518. [DOI] [PubMed] [Google Scholar]
- 131.Xu J, Shao T, Song M, Xie Y, Zhou J, Yin J, et al. MIR22HG acts as a tumor suppressor via TGFβ/SMAD signaling and facilitates immunotherapy in colorectal cancer. Mol Cancer. 2020;19:51. doi: 10.1186/s12943-020-01174-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kohlhapp FJ, Mitra AK, Lengyel E, Peter ME. MicroRNAs as mediators and communicators between cancer cells and the tumor microenvironment. Oncogene. 2015;34:5857–68. doi: 10.1038/onc.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cross D, Burmester JK. Gene therapy for cancer treatment: past, present and future. Clin Med Res. 2006;4:218–27. doi: 10.3121/cmr.4.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Y, Jiang J, Liu C, Zhao W, Ma Y, Zheng Z, et al. Synergistic anti-tumor effect of anti-PD-L1 antibody cationic microbubbles for delivery of the miR-34a gene combined with ultrasound on cervical carcinoma. Am J Transl Res. 2021;13:988–1005. [PMC free article] [PubMed] [Google Scholar]
- 135.Qin YE, Tang WF, Xu Y, Wan FR, Chen AH. Ultrasound-mediated co-delivery of miR-34a and sPD-1 complexed with microbubbles for synergistic cancer therapy. Cancer Manag Res. 2020;12:2459–69. doi: 10.2147/CMAR.S238643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kumar SU, Telichko AV, Wang H, Hyun D, Johnson EG, Kent MS, et al. Acoustically driven microbubbles enable targeted delivery of microrna-loaded nanoparticles to spontaneous hepatocellular neoplasia in canines. Adv Ther (Weinh). 2020;3:2000120 (2020). [DOI] [PMC free article] [PubMed]
- 137.Guo Y, Lee H, Fang Z, Velalopoulou A, Kim J, Thomas MB, et al. Single-cell analysis reveals effective siRNA delivery in brain tumors with microbubble-enhanced ultrasound and cationic nanoparticles. Sci Adv. 2021;7:eabf7390. [DOI] [PMC free article] [PubMed]
- 138.Liao ZK, Tsai KC, Wang HT, Tseng SH, Deng WP, Chen WS, et al. Sonoporation-mediated anti-angiogenic gene transfer into muscle effectively regresses distant orthotopic tumors. Cancer Gene Ther. 2012;19:171–80. doi: 10.1038/cgt.2011.73. [DOI] [PubMed] [Google Scholar]
- 139.Wu Y, Sun T, Tang J, Liu Y, Li F. Ultrasound-targeted microbubble destruction enhances the antitumor efficacy of doxorubicin in a mouse hepatocellular carcinoma model. Ultrasound Med Biol. 2020;46:679–89. doi: 10.1016/j.ultrasmedbio.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 140.McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72:3652–63. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gong Q, Gao X, Liu W, Hong T, Chen C. Drug-loaded microbubbles combined with ultrasound for thrombolysis and malignant tumor therapy. Biomed Res Int. 2019;2019:6792465. doi: 10.1155/2019/6792465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beguin E, Bau L, Shrivastava S, Stride E. Comparing strategies for magnetic functionalization of microbubbles. ACS Appl Mater Interfaces. 2019;11:1829–40. doi: 10.1021/acsami.8b18418. [DOI] [PubMed] [Google Scholar]
- 143.Curley CT, Sheybani ND, Bullock TN, Price RJ. Focused ultrasound immunotherapy for central nervous system pathologies: challenges and opportunities. Theranostics. 2017;7:3608–23. doi: 10.7150/thno.21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bulner S, Prodeus A, Gariepy J, Hynynen K, Goertz DE. Enhancing checkpoint inhibitor therapy with ultrasound stimulated microbubbles. Ultrasound Med Biol. 2019;45:500–12. doi: 10.1016/j.ultrasmedbio.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 145.Chen PY, Hsieh HY, Huang CY, Lin CY, Wei KC, Liu HL. Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J Transl Med. 2015;13:93. doi: 10.1186/s12967-015-0451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shi F, Li M, Wu S, Yang F, Di W, Pan M, et al. Enhancing the anti-multiple myeloma efficiency in a cancer stem cell xenograft model by conjugating the ABCG2 antibody with microbubbles for a targeted delivery of ultrasound mediated epirubicin. Biochem Pharm. 2017;132:18–28. doi: 10.1016/j.bcp.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 147.Dimcevski G, Kotopoulis S, Bjånes T, Hoem D, Schjøtt J, Gjertsen BT, et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Control Release. 2016;243:172–81. doi: 10.1016/j.jconrel.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 148.Castle J, Kotopoulis S, Forsberg F. Sonoporation for augmenting chemotherapy of pancreatic ductal adenocarcinoma. Methods Mol Biol. 2020;2059:191–205. doi: 10.1007/978-1-4939-9798-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Eisenbrey JR, Forsberg F, Wessner CE, Delaney LJ, Bradigan K, Gummadi S, et al. US-triggered microbubble destruction for augmenting hepatocellular carcinoma response to transarterial radioembolization: a randomized pilot clinical trial. Radiology. 2021;298:450–7. doi: 10.1148/radiol.2020202321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alphandéry E. Nanomaterials as ultrasound theragnostic tools for heart disease treatment/diagnosis. Int J Mol Sci. 2022;23:1683. [DOI] [PMC free article] [PubMed]
- 151.Xia P, Shi Y, Wang X, Li X. Advances in the application of low-intensity pulsed ultrasound to mesenchymal stem cells. Stem Cell Res Ther. 2022;13:214. doi: 10.1186/s13287-022-02887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Köse G, Darguzyte M, Kiessling F. Molecular ultrasound imaging. Nanomaterials (Basel). 2020;10:1935. [DOI] [PMC free article] [PubMed]
- 153.Wang Y, Cong H, Wang S, Yu B, Shen Y. Development and application of ultrasound contrast agents in biomedicine. J Mater Chem B. 2021;9:7633–61. doi: 10.1039/D1TB00850A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.