Abstract
Slow growth has been hypothesized to be an essential aspect of bacterial physiology within biofilms. In order to test this hypothesis, we employed two strains of Escherichia coli, ZK126 (ΔlacZ rpoS+) and its isogenic ΔrpoS derivative, ZK1000. These strains were grown at two rates (0.033 and 0.0083 h−1) in a glucose-limited chemostat which was coupled either to a modified Robbins device containing plugs of silicone rubber urinary catheter material or to a glass flow cell. The presence or absence of rpoS did not significantly affect planktonic growth of E. coli. In contrast, biofilm cell density in the rpoS mutant strain (ZK1000), as measured by determining the number of CFU per square centimeter, was reduced by 50% (P < 0.05). Deletion of rpoS caused differences in biofilm cell arrangement, as seen by scanning confocal laser microscopy. In reporter gene experiments, similar levels of rpoS expression were seen in chemostat-grown planktonic and biofilm populations at a growth rate of 0.033 h−1. Overall, these studies suggest that rpoS is important for biofilm physiology.
In their natural environments, bacteria often adhere to surfaces on which they form biofilm communities that may be several millimeters thick. Within biofilms, individual bacteria are encased in a polysaccharide matrix, which functions to bind cells together and facilitates adhesion to the underlying surface. Bacteria are not distributed uniformly throughout a biofilm but rather aggregate into microcolonies, which are typically a few micrometers in diameter (6). Studies employing scanning confocal laser microscopy (SCLM) have shown a wide range of bacterial growth rates throughout a biofilm. The fastest growth was observed at the biofilm-liquid interface. Bacteria in the biofilm interior, particularly those inside microcolonies, grew much more slowly, presumably due to limited access to nutrients (10, 13). These and other studies have led to the hypothesis that slow growth is a major aspect of bacterial biofilm physiology (4). In order to test this hypothesis, we investigated whether the absence of a slow-growth-activated gene, rpoS (7), could affect the biofilm formation of Escherichia coli under defined growth conditions (15). Here we report that deletion of rpoS greatly reduces the ability of E. coli to grow in biofilms yet has little effect on the growth of planktonic (i.e., unattached) bacteria.
(This research was conducted by J. L. Adams in partial fulfillment of the requirements for an M.S. from Southwest Texas State University.)
Strains and culturing conditions.
The strains of E. coli used in this study are ZK126 (ΔlacZ), ZK1000 (ZK126 ΔrpoS) (1), and DS526 (ZK126 λRZ5 rpoS742::lacZ) (13a). Cultures were stored frozen at −80°C in Luria-Bertani (LB) broth containing 15% (vol/vol) glycerol as described elsewhere (14). Prior to each experiment, the appropriate E. coli strain was streaked from a frozen stock culture onto LB agar, checked for purity, and grown overnight in 5 ml of glucose-limited, defined medium (GDM) (15) containing 0.25 g of glucose per liter.
Biofilm chemostat experiments.
Chemostats were coupled to a modified Robbins device (MRD; Tyler Research, Edmonton, Alberta, Canada) as described by Whiteley et al. (15). Briefly, this consisted of filling a chemostat with sterile GDM and inoculating it with 1 ml of an overnight E. coli culture in GDM. This culture was allowed to grow overnight under batch conditions, after which continuous culture was commenced at a dilution rate (DR) of either 0.033 or 0.0083 h−1. The chemostat cultures were allowed to equilibrate for 1 generation time (121 h at a DR of 0.0083 h−1 and 30 h at a DR of 0.033 h−1), after which time the chemostat was connected to an MRD containing 7-mm-diameter silicone rubber plugs. A peristaltic pump was used to circulate the chemostat culture through the MRD at a flow rate of 100 ml min−1. After 48 h, the experiment was stopped and nine plugs were removed from the MRD, sonicated, serially diluted in phosphate-buffered saline, and plated onto LB agar (Difco Laboratories, Detroit, Mich.) as previously described (8, 15). Each chemostat-MRD culture experiment was replicated a minimum of three times. Within each chemostat-MRD replicate, a minimum of five measurements were taken.
When biofilm cultures were to be examined by SCLM, the chemostat was established as described previously and attached by capillary tubing to a flow cell (2) (Water Technologies, Bozeman, Mont.) to which was attached a glass microscope slide. A Pharmacia peristaltic pump (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.) was used to circulate the chemostat culture through the flow cell at a rate of 8.3 ml min−1. For SCLM examination, the glass slide was removed and stained with BacLite Live/Dead viability stain (Molecular Probes, Eugene, Oreg.) in order to estimate the viability of individual cells. Biofilm formation in flow cells was examined by SCLM with an Olympus IX-70 inverted microscope (Olympus America Inc., Melville, N.Y.) coupled with a Bio-Rad 1024 SCLM System (Bio-Rad Laboratories, Hercules, Calif.). The slides were placed with the biofilm side facing the 60× Uplan Apo (Olympus) oil immersion objective lens.
rpoS expression assay.
In order to compare the levels of rpoS expression in biofilm cells and planktonic cells, reporter strain DS526, containing an rpoS::lacZ fusion on a λ phage, was constructed by D.A. Siegele, Texas A&M University, as previously described (5). This strain was cultured in the chemostat-MRD apparatus at a DR of 0.033 h−1 as described above. After 48 h of biofilm growth, biofilm and planktonic samples were removed and frozen at −80°C for 2 weeks until analyzed. We permeabilized E. coli cells with chloroform and sodium dodecyl sulfate and quantified the β-galactosidase activity with o-nitrophenyl-β-d-galactopyranoside (ONPG) as described by Miller (9). The cell number was determined on the basis of direct cell counts of 4′,6-diamidino-2-phenylindole (DAPI)-stained biofilm and planktonic cell suspensions. β-Galactosidase activity was expressed as nanomoles of ONPG cleaved per cell per minute.
Data analysis.
Biofilm cell densities, expressed as log10 CFU per square centimeter, and planktonic cell densities, expressed as log10 CFU per milliliter, were analyzed by one-way analysis of variance.
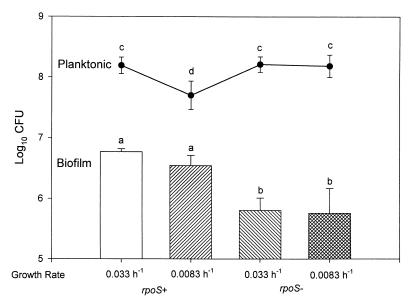
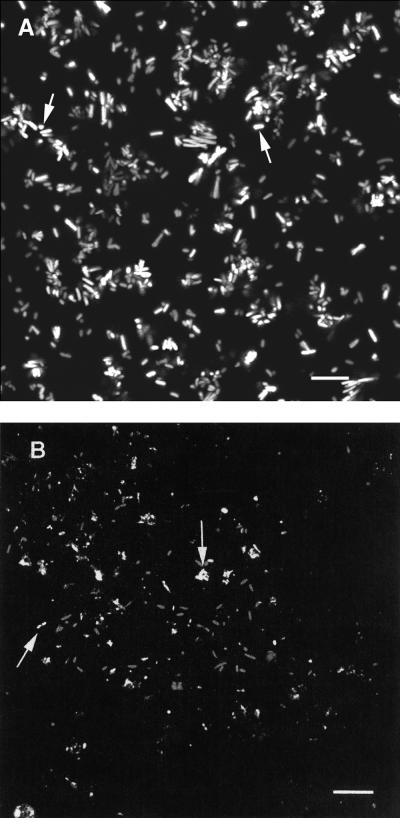
The influences of rpoS deletion and growth rate on E. coli biofilms and planktonic cultures are shown in Fig. 1. As can be seen, deletion of rpoS had a major impact on biofilm populations and less of an impact on planktonic populations. SCLM examinations (Fig. 2) showed differences in E. coli biofilm structures in the presence (Fig. 2A) and absence (Fig. 2B) of rpoS. When bacteria were grown at a DR of 0.033 h−1, similar levels of β-galactosidase activity were seen in biofilm (3.04 × 10−6 nmol of ONPG min−1 cell−1) and planktonic (3.08 × 10−6 nmol of ONPG min−1 cell−1) populations of E. coli DS526 containing an rpoS::lacZ fusion.
FIG. 1.
Graph showing effects of rpoS deletion and growth rate on planktonic (expressed as log10 CFU per milliliter) and biofilm (expressed as log10 CFU per square centimeter) cultures. Error bars represent standard deviations. Values with the same letter are not significantly different (P = 0.05).
FIG. 2.
SCLM micrographs of E. coli biofilms stained with Live/Dead viability stain in the presence (A) and absence (B) of rpoS. The viable (brightly stained) cells are indicated by an arrow. Bars, 2 μm in panel A and 3 μm in panel B.
Several lines of evidence support the role of slow growth in biofilm physiology. Due to their enhanced access to nutrients, bacteria on the periphery of biofilm microcolonies grow much more quickly than do the nutrient-limited organisms in the interior (10). One striking feature of biofilm growth is that bacteria are significantly more resistant to antimicrobial agents than they are during planktonic growth (11). To investigate this finding, Evans et al. (3) compared the antibiotic resistance of planktonic chemostat cultures at various growth rates. They found antibiotic susceptibility to be correlated with growth rate and thus attributed biofilm antimicrobial resistance to a reduced growth rate. The study of Evans et al. (3) provides additional impetus for studying biofilms at reduced growth rates.
Several notable effects of rpoS deletion were observed in the present study. These include significant differences in biofilm cell density (Fig. 1) and differences in biofilm structure (Fig. 2). The influence of rpoS deletion on planktonic cells was minimal at either DR (Fig. 1). One possible explanation for this phenomenon was that rpoS was expressed only during biofilm growth. We measured patterns of rpoS expression in E. coli DS526, which contains an rpoS-lacZ fusion, at a DR of 0.033 h−1. In this experiment, rpoS expression, as indicated by the amount of β-galactosidase activity per cell, was equivalent in both planktonic (3.08 × 10−6 nmol of ONPG min−1 cell−1) and biofilm (3.04 × 10−6 nmol of ONPG min−1 cell−1) populations. Our observations are consistent with those of Notley and Ferenci (12), who observed rpoS expression in chemostat cultures of E. coli at a DR of ≤0.2 h−1. Deletion of rpoS had an impact on biofilm cell density (Fig. 1) and cell arrangement (Fig. 2), so it is likely that rpoS expression is more important to biofilm populations than to planktonic populations.
Acknowledgments
This project was supported in part by a grant from the Advanced Research Program of the Texas Higher Education Coordinating Board and by the Biology Department at Southwest Texas State University. The scanning confocal microscope used in this study was purchased with funds from an NSF-ILI grant.
We thank Debby Siegele, Texas A&M University, and Grant Balzer, Joe Koke, and Jim Ott, Southwest Texas State University, for their help and suggestions.
REFERENCES
- 1.Bohannon D E, Connell N, Keener J, Tormo A, Espinosa-Urgel M, Zambrano M M, Kolter R. Stationary-phase-inducible “gearbox” promoters: differential effects of katF mutations and role of ς70. J Bacteriol. 1991;173:4482–4492. doi: 10.1128/jb.173.14.4482-4492.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 3.Evans D J, Brown M R W, Allison D G, Gilbert P. Susceptibility of bacterial biofilms to tobramycin: role of specific growth rate and phase in the division cycle. J Antimicrob Chemother. 1990;25:585–591. doi: 10.1093/jac/25.4.585. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert P, Das J, Foley I. Biofilm susceptibility to antimicrobials. Adv Dent Res. 1997;11:160–167. doi: 10.1177/08959374970110010701. [DOI] [PubMed] [Google Scholar]
- 5.Lange R, Hengge-Aronis R. The cellular concentration of the sigma-S subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation and protein stability. Genes Dev. 1994;8:1600–1612. doi: 10.1101/gad.8.13.1600. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence J R, Korber D R, Hoyle B D, Costerton J W, Caldwell D E. Optical sectioning of microbial biofilms. J Bacteriol. 1991;173:6558–6567. doi: 10.1128/jb.173.20.6558-6567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loewen P C, Hu B, Strutinsky J, Sparling R. Regulation in the rpoS regulon of Escherichia coli. Can J Microbiol. 1998;44:707–717. doi: 10.1139/cjm-44-8-707. [DOI] [PubMed] [Google Scholar]
- 8.McLean, R. J. C., M. Whiteley, B. C. Hoskins, P. D. Majors, and M. M. Sharma. Laboratory techniques for studying biofilm growth, physiology, and gene expression in flowing systems and porous media. Methods Enzymol., in press. [DOI] [PubMed]
- 9.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. pp. 352–355. [Google Scholar]
- 10.Møller S, Pedersen A R, Poulsen L K, Arvin E, Molin S. Activity and three-dimensional distribution of toluene-degrading Pseudomonas putida in a multispecies biofilm assessed by quantitative in situ hybridization and scanning confocal laser microscopy. Appl Environ Microbiol. 1996;62:4632–4640. doi: 10.1128/aem.62.12.4632-4640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nickel J C, Ruseska I, Wright J B, Costerton J W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985;27:619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Notley L, Ferenci T. Induction of RpoS-dependent functions in glucose-limited continuous culture: what level of nutrient limitation induces the stationary phase of Escherichia coli? J Bacteriol. 1996;178:1465–1468. doi: 10.1128/jb.178.5.1465-1468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulsen L K, Ballard G, Stahl D A. Use of rRNA fluorescence in situ hybridization for measuring the activity of single cells in young and established biofilms. Appl Environ Microbiol. 1993;59:1354–1360. doi: 10.1128/aem.59.5.1354-1360.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Siegele, D. A. Unpublished data.
- 14.Siegele D A, Hu J C. Gene expression from ParaBAD plasmids at subsaturating inducer concentrations represents mixed populations. Proc Natl Acad Sci USA. 1997;94:8168–8172. doi: 10.1073/pnas.94.15.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiteley M, Brown E, McLean R J C. An inexpensive chemostat apparatus for the study of microbial biofilms. J Microbiol Methods. 1997;30:125–132. [Google Scholar]