Abstract
Objective:
To test the hypothesis that PPI use is associated with an increased risk of being diagnosed with toxoplasmic retinochoroiditis
Design:
Retrospective, matched case-control study using data from 2000–2020
Participants:
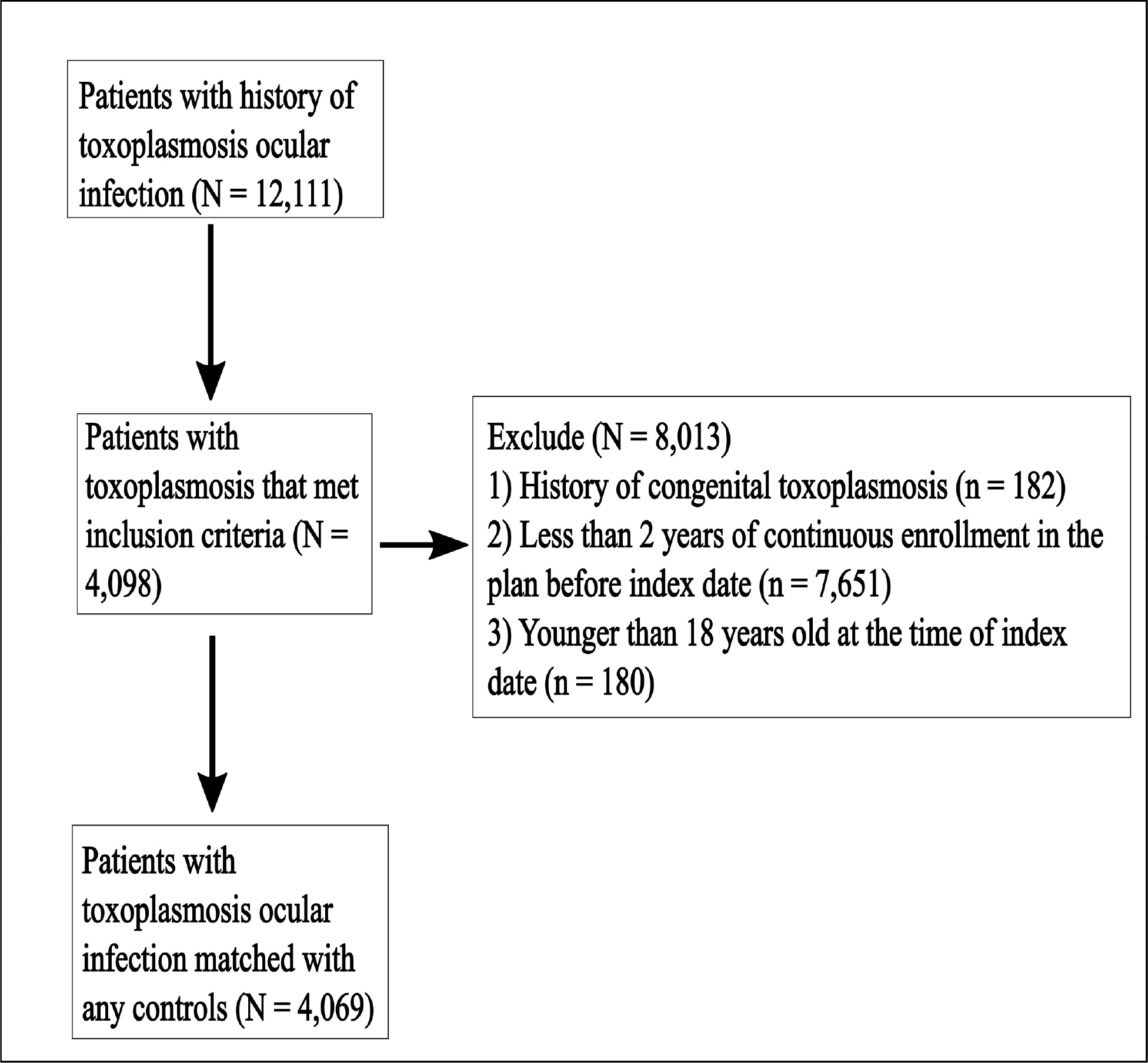
Cases of ocular toxoplasmosis and controls were matched 5:1 for age, gender, race, with eligibility date ±3 months from index date of exposed match. Exclusion occurred for being <18 years old, having congenital toxoplasmosis, having <2 years in the insurance plan prior to the index date and not having at least one visit to an eyecare provider prior to the index date.
Methods:
Cases of ocular toxoplasmosis were identified using ICD 9/10 codes and PPI use or diseases highly associated with PPI were identified utilizing national drug codes from an administrative medical claims database.
Main Outcome Measures:
The primary outcome was defined as having a prescription for PPI or H2 blocker. Multivariable logistic regression analyses were performed controlling for demographic and systemic health variables.
Results:
4069 cases and 19177 controls met eligibility criteria. 24.3% (989/4069) of ocular toxoplasmosis cases were on PPI/H2 blockers compared to 19.2% (3763/19177) of controls. An adjusted logistic regression model demonstrated 1.28 greater odds of PPI/H2 blocker use in ocular toxoplasmosis cases compared to matched controls (95% CI: 1.17–1.40, p < 0.001).
Conclusions:
PPI/H2 blocker exposure was associated with an increased risk of being diagnosed with ocular toxoplasmosis, corroborating findings from a prior case series.
Keywords: toxoplasmosis, retinochoroiditis, uveitis, proton pump inhibitors, histamine2 blockers
Introduction
Toxoplasma gondii is a ubiquitous, obligate intracellular protozoan that sexually reproduces in felines but can infect most warm-blooded animals. The parasite is most commonly acquired following contact with infected fecal matter of cats, undercooked meats, or contaminated water.1,2 In humans, congenital infection may result in subclinical presentations, induce stillbirths, or lead to the development of the classic triad of bilateral retinochoroiditis, hydrocephalus, and intracranial calcifications.2 In contrast to congenital disease, most acquired infections in adults are asymptomatic; however, approximately 2% of immunocompetent adults will develop ocular complications, typically a unilateral, unifocal area of necrotizing retinochoroiditis with overlying dense vitritis, after exposure to the pathogen.3 This is in contrast to immunocompromised patients in which acquired ocular disease may be bilateral and multifocal with systemic manifestations ranging from a flu-like illness to encephalitis, seizures, and death.4 With high seroprevalence rates of toxoplasmosis in humans and wild animals worldwide, it remains the most common cause of infectious posterior uveitis and a significant healthcare burden.2
We previously reported in a small, retrospective case series that 3 of 7 otherwise healthy patients that developed toxoplasmic retinochoroiditis following exposure to wild game were using antacids and/or protein pump inhibitors (PPIs) for acid reflux.5 This rate of usage of PPIs appeared to be higher than prior general population estimates in the United States of approximately 20%.6 PPIs are the most effective gastric acid-suppressing agents on the market and are first line therapy for treatment of many gastric acid-related disorders. While they are relatively well tolerated in the short term, prolonged use has been associated with Clostridium difficile infections, community acquired pneumonia, micronutrient deficiencies, and kidney dysfunction among others.7 The underlying mechanism is unclear in these associations; however, there has been speculation that the increased risk of infection while using these medications, whether it be C. difficile or community acquired pneumonia, is related to gut flora changes and/or impaired immune cell function.7 Thus, we hypothesized that the use of PPIs increased susceptibility to the acquisition of acquired toxoplasmic retinochoroiditis in lieu of our original observation and the associated risks of acquisition of other infectious organisms.
In order to test this hypothesis, we reviewed a large healthcare claims database using ICD-9 and −10 codes and identified all adult patients who were diagnosed with new toxoplasmic retinochoroiditis infections and compared them with matched controls for previous history of PPI use or having an ailment likely to be associated with PPI use.
Methods
Dataset
Data were abstracted from Optum’s de-identified Clinformatics® Data Mart Database. The database contains all outpatient medical claims (office visits, procedures, and medications given) as well as demographic data and some laboratory values for all patients enrolled in commercial and Medicare Advantage insurance plans. The subset of data available for this study included all patients in the database from January 1, 2000, to June 30, 2020. The institutional review board of the University of Pennsylvania deemed this study exempt from review due to the de-identified nature of the data.
Cases and Controls
All patients over 18 years old with a diagnosis of toxoplasmosis ocular infection were identified using ICD9 and ICD10 codes (please see Supplemental Table 1 for all codes used in this study). The index date was set to the earliest date of toxoplasmosis diagnosis. Patients were excluded for having ≤2 years in the insurance plan or not having an eye exam prior to the index date. Also, exclusion occurred for any diagnosis of congenital toxoplasmosis at any point during their time in the plan. As many as 5 controls were selected for every case and matched on age (±3 years), gender, race and insurance start date (±3 months). Each control was assigned the index date of the matched case and required to meet the same exclusion criteria as controls.
Analysis and Covariates
The primary outcome was the odds of having filled a prescription for a PPI or H2 blocker prior to the index date. Multivariable logistic regression was used to determine the odds of having a gastric hyperacidic condition. Covariates used in the model were several demographic and systemic health variables, including alcohol abuse, smoking, prescription nonsteroidal anti-inflammatories, oral steroids, or pregnancy in the 2 years prior to the index date8 (see Table 1 for the full list of covariates). Two sensitivity analyses were run, including one that broadened the definition of the primary outcome to also include either filling a prescription for a PPI/H2 blocker or having a diagnosis that would likely to lead to PPI/H2 blocker use (esophagitis, gastritis, etc.; please see Supplemental Table 1 for the full list of diagnoses used). The other analysis included all toxoplasmosis diagnoses that did not specify ocular disease.
Table 1:
Descriptive of the study cohort for the primary analysis
| Control (N=19177) | Case (N=4069) | |
|---|---|---|
|
| ||
| Age (years) | ||
| N | 19177 | 4069 |
| Mean (SD) | 54.3 (18.2) | 54.1 (18.1) |
| Median | 54.0 | 54.0 |
| Q1, Q3 | 39.0, 70.0 | 39.0, 70.0 |
| Range | (18.0–90.0) | (18.0–90.0) |
| Gender | ||
| Male | 7495 (39.1%) | 1596 (39.2%) |
| Female | 11677 (60.9%) | 2472 (60.8%) |
| Unknown | 5 (0.0%) | 1 (0.0%) |
| Race/Ethnicity | ||
| White | 11979 (62.5%) | 2536 (62.3%) |
| Black | 2096 (10.9%) | 441 (10.8%) |
| Hispanic | 2773 (14.5%) | 591 (14.5%) |
| Asian | 653 (3.4%) | 138 (3.4%) |
| Unknown | 1676 (8.7%) | 363 (8.9%) |
| Education level | ||
| Less than or equal to HS Diploma | 4387 (22.9%) | 1073 (26.4%) |
| Less than Bachelor Degree | 9558 (49.8%) | 1873 (46.0%) |
| Bachelor Degree Plus | 4009 (20.9%) | 874 (21.5%) |
| Unknown | 1223 (6.4%) | 249 (6.1%) |
| Household income | ||
| <$40K | 3016 (15.7%) | 679 (16.7%) |
| $40K – $49K | 1005 (5.2%) | 217 (5.3%) |
| $50K – $59K | 1140 (5.9%) | 212 (5.2%) |
| $60K – $74K | 1632 (8.5%) | 334 (8.2%) |
| $75K – $99K | 2309 (12.0%) | 480 (11.8%) |
| $100K+ | 5469 (28.5%) | 1072 (26.3%) |
| Unknown | 4606 (24.0%) | 1075 (26.4%) |
| Geographic location | ||
| Upper Midwest | 5189 (27.1%) | 752 (18.5%) |
| Southern Midwest | 3254 (17.0%) | 639 (15.7%) |
| Northeast | 2373 (12.4%) | 668 (16.4%) |
| Mountain | 1512 (7.9%) | 262 (6.4%) |
| Pacific | 1983 (10.3%) | 407 (10.0%) |
| South Atlantic | 4835 (25.2%) | 1328 (32.6%) |
| Unknown | 31 (0.2%) | 13 (0.3%) |
| History of hypertension | ||
| No | 9756 (50.9%) | 2027 (49.8%) |
| Yes | 9421 (49.1%) | 2042 (50.2%) |
| History of hypercholesterolemia | ||
| No | 8538 (44.5%) | 1772 (43.5%) |
| Yes | 10639 (55.5%) | 2297 (56.5%) |
| Ischemic heart disease | ||
| No | 16117 (84.0%) | 3292 (80.9%) |
| Yes | 3060 (16.0%) | 777 (19.1%) |
| Heart failure | ||
| No | 17902 (93.4%) | 3753 (92.2%) |
| Yes | 1275 (6.6%) | 316 (7.8%) |
| Diabetes Mellitus | ||
| No | 14669 (76.5%) | 3089 (75.9%) |
| Yes | 4508 (23.5%) | 980 (24.1%) |
| Alcohol Abuse | ||
| No | 18687 (97.4%) | 3944 (96.9%) |
| Yes | 490 (2.6%) | 125 (3.1%) |
| Smoking | ||
| No | 15596 (81.3%) | 3236 (79.5%) |
| Yes | 3581 (18.7%) | 833 (20.5%) |
| Pregnancy in the 2 years prior to index date | ||
| No | 18366 (95.8%) | 3678 (90.4%) |
| Yes | 811 (4.2%) | 391 (9.6%) |
| NSAID use in the 2 years prior to the index date | ||
| No | 15762 (82.2%) | 3238 (79.6%) |
| Yes | 3415 (17.8%) | 831 (20.4%) |
| Oral steroid use in the 2 years prior to the index date | ||
| No | 15715 (81.9%) | 3114 (76.5%) |
| Yes | 3462 (18.1%) | 955 (23.5%) |
| A prescription for PPI or H2 blocker | ||
| No | 15504 (80.8%) | 3080 (75.7%) |
| Yes | 3673 (19.2%) | 989 (24.3%) |
| A prescription for PPI or H2 blocker OR hx of a disease that would be likely to lead to use of a PPI/H2 blocker | ||
| No | 12493 (65.1%) | 2433 (59.8%) |
| Yes | 6684 (34.9%) | 1636 (40.2%) |
H2, histamine; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump in inhibitor
Results
Four thousand and sixty-nine cases of toxoplasmic retinochoroiditis and 19177 unaffected, matched controls met all inclusion/exclusion criteria (Table 1). Cases and controls were similar in age (54.1 years old (standard deviation±18.1) vs. 54.3 (SD±18.2), respectively), gender composition (39.2% male vs. 39.1%) and racial mixture (62.3% White vs. 62.5%). Of ocular toxoplasmosis cases, 24.3% (989/4069) were on PPI/H2 blockers compared to 19.2% (3763/19177) of controls. After adjusting for all covariates of interest, primary analysis showed a an increased odds ratio of 1.28 (95% CI: 1.17–1.40, p<0.001) for known PPI use among toxoplasmosis cases (Table 2).
Table 2:
Association of H2/PPI use with diagnosis of ocular toxoplasmosis for the primary analysis
| Control | Case | aOR (95% CI)* | P value | |
|---|---|---|---|---|
| A prescription for PPI/H2 blocker | 3673 (19.2%) | 989 (24.3%) | 1.28 (1.17, 1.40) | <0.001 |
| A prescription for PPI/H2 blocker OR hx of disease that would be likely to lead to use of PPI/H2 blocker | 6684 (34.9%) | 1636 (40.2%) | 1.20 (1.11, 1.30) | <0.001 |
Conditional logistic regression adjusting for age, education, income, geographic location, hypertension, hypercholesterolemia, Ischemic heart disease, Heart failure, Diabetes Mellitus, Alcohol Abuse, Smoking, Pregnancy in the 2 years prior to index date, NSAID use in the 2 years prior to the index date and oral steroid use in the 2 years prior to the index date
aOR, adjusted odds ratio; CI, confidence interval; H2, histamine; hx, history; PPI, proton pump inhibitor
For the sensitivity analysis with a broader definition to also include those with diseases likely lead to PPI/H2 blocker use, 40.2% (1636/4069) of the toxoplasmosis cases and 34.9% (6684/19177) of controls had exposure. After multivariable analysis, the odds of having exposure more broadly defined was 1.20 (95% CI: 1.11–1.30, p<0.001) for the toxoplasmosis cases versus controls. The other sensitivity analysis which broadened the definition of cases to all patients diagnosed with ocular or systemic toxoplasmosis included 4931 cases and 23293 matched controls. After multivariable analysis the odds of a toxoplasmosis case having a filled a prescription for a PPI/H2 blocker was again higher at 1.23 (95% CI: 1.13–1.33, p<0.001) (Table 3).
Table 3:
Association of H2/PPI use with diagnosis of ocular toxoplasmosis for the sensitivity analysis
| Control | Case | aOR (95% CI)* | P value | |
|---|---|---|---|---|
| A prescription for PPI/H2 blocker | 4557 (19.6%) | 1199 (24.3%) | 1.23 (1.13, 1.33) | <0.001 |
| A prescription for PPI/H2 blocker OR hx of disease that would be likely to lead to use of PPI/H2 blocker | 8152 (35.0%) | 1997 (40.5%) | 1.19 (1.11, 1.28) | <0.001 |
Conditional logistic regression adjusting for age, education, income, geographic location, hypertension, hypercholesterolemia, Ischemic heart disease, Heart failure, Diabetes Mellitus, Alcohol Abuse, Smoking, Pregnancy in the 2 years prior to index date, NSAID use in the 2 years prior to the index date and oral steroid use in the 2 years prior to the index date
aOR, adjusted odds ratio; CI, confidence interval; H2, histamine; hx, history; PPI, proton pump inhibitor
Discussion
We evaluated 4069 cases of ocular toxoplasmosis and compared them to matched controls to find that cases were at significantly elevated odds of having filled a prescription for a PPI or H2 blocker prior to the date of the toxoplasmosis diagnosis. When sensitivity analyses were run to test assumptions made in the case and outcome definitions, our findings were essentially unchanged, suggesting a robust association. Together, these findings are consistent with our hypothesis that PPI/H2 blocker use increases the risk of toxoplasmosis infection.
It is likely that PPIs may be only one of many risk factors for the development of ocular toxoplasmosis in adults. There are previously reported environmental and behavioral risk factors that have been associated with developing systemic toxoplasmosis. Such factors include living at lower elevations, having contact with raw meat, consuming unfiltered water or unpasteurized milk, and living in a community with an increased density of cats.1,9 Similarly, severity and recurrences of ocular toxoplasmosis have been positively correlated with increasing age and the use of steroids without concurrent antibiotics.10–12 These host risk factors do not include pathogen-associated attributes such as tropism of certain T. gondii strains to the eye and the pathogen’s expression of specific neuroinvasive genes that have been associated with ocular complications.13 However, we are unaware of prior reports identifying PPI use as a potential risk factor for developing toxoplasmic retinochoroiditis. It is unclear at this time whether this finding is simply due to an increased risk of developing acquired infections or if this finding is specific to ocular disease alone.
While the pathogenic mechanism underlying this association is unclear, PPIs are known to significantly reduce gastric acid levels, thereby increasing gut pH. Omeprazole, an over-the-counter PPI, has been shown to inhibit acidification of phagolysosomes in vitro.14,15 T. gondii tachyzoites are extremely susceptible to acidic conditions (low pH) and acidification of the phagosome is one of the primary defense mechanisms of the host innate immune response to toxoplasmosis invasion.16 Taken together, we hypothesize that the reduction in gut acidity and neutralization of the phagolysosome resulting from PPI use may be responsible for the increased risk of being diagnosed with toxoplasmic retinochoroiditis. In further support of this hypothesis and the importance of pH changes in the host-pathogen interplay is the observation that T. gondii has evolved specific mechanisms to inhibit acidification and fusion of the phagosome to other endocytic cellular compartments.17,18 Thus, control of pH changes are central to both host defense and T. gondii invasiveness.
Our retrospective, population-based study has several strengths and weaknesses. The size of our patient population is substantial, including over 4,000 cases of ocular toxoplasmosis and 19,000 matched controls in the United States. This large patient population allowed us to identify a new, potential risk factor for the diagnosis of ocular toxoplasmosis that could not have been recognized in smaller cohorts. Nevertheless, we acknowledge that retrospective analyses cannot prove causality and that ICD-based studies are limited by proper use of diagnostic codes. Further, it is unclear what impact specific strains or clades of T. gondii may have on our reported association. This consideration would be especially important in places such as Brazil, where rates of toxoplasmic retinochoroiditis are higher and predominant toxoplasmosis strains differ from those found in the United States.2,13,19 Lastly, it should be noted that PPIs became readily available in over-the-counter formulations in 2003, and PPI usage was likely underestimated in both cases and controls in our study due to the inability of claims data to capture over-the-counter medication uses. Despite this, there is no reason to believe that an impactful differential in exposure measurement would have occurred across the study groups, since both groups had exposure defined by prescription fill and/or associated diagnoses.
In summary, we found that receiving a diagnosis of ocular toxoplasmosis was associated with increased odds of having filled a PPI and/or H2 blocker prescription prior to the diagnosis. Future work should be directed at elucidating the mechanism by which PPI or H2 blocker use can increase the risk of toxoplasmosis infection.
Supplementary Material
Figure 1:
Study design
Precis.
Toxoplasmic retinochoroiditis is the most common cause of infectious posterior uveitis. In the following manuscript, we show that proton pump inhibitor/histamine2 blocker use is associated with the diagnosis of the condition.
Acknowledgements:
FUNDING:
University of Pennsylvania Core Grant for Vision Research (2P30EYEY001583). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional funding was provided by Research to Prevent Blindness and the Paul and Evanina Mackall Foundation. Funding from each of the above sources was received in the form of block research grants to the Scheie Eye Institute. CDC was supported in part by a Knights Templar Eye Foundation early career award. None of the funding organizations had any role in the design or conduction of the study.
Brian VanderBeek had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflicts of Interest:
Brian VanderBeek has a consulting contract with EyePoint Pharmaceuticals Mark Johnson serves on Independent Data Monitoring Committees for Aura Biosciences and Amgen (not related to current work) No other conflicts exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Jones JL, Dargelas V, Roberts J, et al. Risk Factors for Toxoplasma gondii Infection in the United States. Clinical Infectious Diseases. 2009;49:878–884. [DOI] [PubMed] [Google Scholar]
- 2.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39:1385–1394. [DOI] [PubMed] [Google Scholar]
- 3.Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol. 2003;136:973–988. [DOI] [PubMed] [Google Scholar]
- 4.Holland GN, Engstrom RE Jr, Glasgow BJ, et al. Ocular Toxoplasmosis in Patients with the Acquired Immunodeficiency Syndrome. American Journal of Ophthalmology. 1988;106:653–667. [DOI] [PubMed] [Google Scholar]
- 5.Conrady CD, Besirli CG, Baumal CR, et al. Ocular Toxoplasmosis after Exposure to Wild Game. Ocul Immunol Inflamm. 2021:1–6. [DOI] [PubMed] [Google Scholar]
- 6.Qato DM, Wilder J, Schumm LP, et al. Changes in Prescription and Over-the-Counter Medication and Dietary Supplement Use Among Older Adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaynes M, Kumar AB. The risks of long-term use of proton pump inhibitors: a critical review. Ther Adv Drug Saf. 2018;10:2042098618809927–2042098618809927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai RJ, Solomon DH, Shadick N, et al. Identification of smoking using Medicare data--a validation study of claims-based algorithms. Pharmacoepidemiol Drug Saf. 2016;25:472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Retmanasari A, Widartono BS, Wijayanti MA, Artama WT. Prevalence and Risk Factors for Toxoplasmosis in Middle Java, Indonesia. Ecohealth. 2017;14:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aleixo ALQ do C, Vasconcelos C. de Oliveira R, Cavalcanti Albuquerque M, et al. Toxoplasmic retinochoroiditis: The influence of age, number of retinochoroidal lesions and genetic polymorphism for IFN-γ +874 T/A as risk factors for recurrence in a survival analysis. PLOS ONE. 2019;14:e0211627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de-la-Torre A, Rios-Cadavid AC, Cardozo-García CM, Gomez-Marín JE. Frequency and factors associated with recurrences of ocular toxoplasmosis in a referral centre in Colombia. Br J Ophthalmol. 2009;93:1001. [DOI] [PubMed] [Google Scholar]
- 12.Johnson MW, Greven CM, Jaffe GJ, et al. Atypical, Severe Toxoplasmic Retinochoroiditis in Elderly Patients. Ophthalmology. 1997;104:48–57. [DOI] [PubMed] [Google Scholar]
- 13.Grigg ME, Ganatra J, Boothroyd JC, Margolis TP. Unusual Abundance of Atypical Strains Associated with Human Ocular Toxoplasmosis. The Journal of Infectious Diseases. 2001;184:633–639. [DOI] [PubMed] [Google Scholar]
- 14.Laine L, Shah A, Bemanian S. Intragastric pH with oral vs intravenous bolus plus infusion proton-pump inhibitor therapy in patients with bleeding ulcers. Gastroenterology. 2008;134:1836–1841. [DOI] [PubMed] [Google Scholar]
- 15.Agastya G, West BC, Callahan JM. Omeprazole inhibits phagocytosis and acidification of phagolysosomes of normal human neutrophils in vitro. Immunopharmacol Immunotoxicol. 2000;22:357–372. [DOI] [PubMed] [Google Scholar]
- 16.Koethe M, Schade C, Fehlhaber K, Ludewig M. Survival of Toxoplasma gondii tachyzoites in simulated gastric fluid and cow’s milk. Veterinary Parasitology. 2017;233:111–114. [DOI] [PubMed] [Google Scholar]
- 17.Sibley LD, Weidner E, Krahenbuhl JL. Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature. 1985;315:416–419. [DOI] [PubMed] [Google Scholar]
- 18.Joiner KA, Fuhrman SA, Miettinen HM, et al. Toxoplasma gondii: Fusion Competence of Parasitophorous Vacuoles in Fc Receptor-Transfected Fibroblasts. Science. 1990;249:641–646. [DOI] [PubMed] [Google Scholar]
- 19.Khan A, Jordan C, Muccioli C, et al. Genetic divergence of Toxoplasma gondii strains associated with ocular toxoplasmosis, Brazil. Emerg Infect Dis. 2006;12:942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


