Abstract
Purpose:
To assess rates of diagnostic conversion from anatomical narrow angle (ANA) to primary angle closure glaucoma (PACG) in the United States and identify factors associated with diagnostic conversion.
Design:
Retrospective case-control study.
Subjects, Participants, and/or Controls:
Patients diagnosed with ANA between the years 2007 to 2019 were identified based on International Classification of Diseases (ICD) codes in the Optum Clinformatics® Data Mart Database. Inclusion was limited to newly diagnosed ANA, defined as 1) continuous enrollment during a 2-year lookback period and 6-year study period from index (first) date of ANA diagnosis; 2) diagnosis by an ophthalmologist or optometrist and record of gonioscopy; 3) no history of intraocular ocular pressure (IOP) lowering drops, laser peripheral iridotomy (LPI), or intraocular surgery.
Methods, Intervention, or Testing:
Cox proportional hazards (CPH) models were developed to assess factors associated with diagnostic conversion, defined as a change in ICD code from ANA to PACG.
Main Outcome Measures:
New diagnosis of PACG within the 6-year study period recorded after an index diagnosis of ANA.
Results:
Among 3,985 patients meeting inclusion criteria, 459 (11.52%) had detected diagnostic conversion to PACG within the study period. The conversion rate was stable at 3.54%/year after the first 6 months of ANA diagnosis. In the CPH model, age over 70 years and early (within 6 months of ANA diagnosis) need for LPI or IOP-lowering drops were positively associated with diagnostic conversion (HR>1.59; p<0.02). Cataract surgery at any time and late (after 6 months of ANA diagnosis) need for IOP-lowering drops appeared protective against diagnostic conversion (HR<0.46; p<0.004).
Conclusions:
Annual risk of diagnostic conversion from ANA to PACG is relatively low overall; elderly patients are at higher risk while patients receiving cataract surgery are at lower risk. The utility of long-term monitoring appears low for most ANA patients, highlighting the need for improved clinical methods to identify patients at higher risk for PACG.
Précis
Healthcare claims data reveals the diagnostic conversion of anatomical narrow angle (ANA) to primary angle closure glaucoma is low over a 6-year period. This highlights the need for improved methods to risk-stratify patients with ANA.
Introduction
Primary angle closure glaucoma (PACG) is a leading cause of irreversible vision loss worldwide, affecting an estimated 20 million people. This number is expected to nearly double by the year 2040 due to the aging of the world’s population.1 Any rise in PACG prevalence is problematic as PACG accounts for almost half of glaucoma-related blindness globally and over 90% of bilateral blindness in Asia.2 Progression from anatomical narrow angle (ANA), a broad term for angle closure without glaucoma used by clinicians, to PACG results from crowding of the iridocorneal angle leading to impeded aqueous outflow and elevated intraocular pressure (IOP).3 Established treatments for ANA, including laser peripheral iridotomy (LPI) and cataract surgery, alleviate angle crowding and improve aqueous outflow, thereby reducing IOP and risk of glaucomatous optic neuropathy. Recent research has focused on improving the efficiency and cost-effectiveness of ANA care and identifying high-risk patients who would benefit from treatment.4–7 However, there is sparse knowledge about clinical outcomes based on broad disease definitions and practice patterns adopted by clinicians at large for detecting, monitoring, and treating patients with ANA.
Research studies on angle closure typically stratify ANA into two specific categories of severity: primary angle closure suspect (PACS) and primary angle closure (PAC).8 While ANA broadly encompasses PACS and PAC, it is likely not exclusive to these specific definitions of angle closure without glaucoma. PACS is more common than PAC, and its progression to PACG is relatively slow and rare; therefore, longitudinal studies on progression are time- and resource-intensive. A set of studies in India using strict epidemiological definitions of PACD estimated a progression rate of 1.25% per year from PACS to PACG and 5.70% per year from PAC to PACG over 5 years.9,10 However, the sociodemographic scope of the study population was limited, and the generalizability of study findings in more diverse patient populations or based on broad definitions of ANA is unclear. There is also limited knowledge about the effect of treatments for ANA administered outside of experimental settings.4,5 The paucity of real-world data on the clinical course of patients with ANA is especially glaring in the United States, which has a diverse populace and the highest total health expenditure per capita of any country.11
In this study, we attempt to address these deficiencies in knowledge about patients diagnosed with ANA and PACG using longitudinal national healthcare claims data from the United States. While it is difficult to stratify broad real-world definitions of ANA and PACG based on strict epidemiological definitions of PACD , there remains clinical and scientific value in better understanding long-term rates of diagnostic conversion from ANA to PACG in diverse multi-racial/ethnic patient populations. We also assess sociodemographic and clinical factors associated with diagnostic conversion, including treatment with IOP-lowering drops, LPI, and cataract surgery.
Methods, Intervention, or Testing
Optum’s Clinformatics® Data Mart (CDM) is derived from a database of de-identified administrative health claims data warehouse of commercial and Medicare Advantage health claims. The database includes approximately 17 to 19 million annual covered lives, for a total of over 65 million unique lives over a 13-year period (1/2007 through 12/2019). Available sociodemographic data included sex, race/ethnicity, education level, income, net worth, insurance product, and census division regions. Available clinical data includes first dates of ANA and PACG diagnoses, healthcare provider type, IOP-lowering medications, in-office procedures including gonioscopy and LPI, surgeries including cataract and glaucoma surgery, and first/last date of enrollment in Optum. The University of Southern California Institutional Review Board exempted this study from IRB approval. The study adhered to the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act.
Study Definitions
Inclusion in the study population required an index diagnosis of ANA based on International Classification of Diseases Ninth Revision (ICD-9) or Tenth Revision (ICD-10) codes (Supplementary Table 1). The index date of diagnosis was defined as the date of the first claim associated with an ANA diagnosis. Inclusion also required an 8-year (96-month) period of continuous enrollment in Optum, comprised of a 2-year (24-month) lookback period prior to the index date and a 6-year (72-month) study period after the index date.
Newly diagnosed ANA cases met the following criteria: (1) continuous enrollment during the lookback and study periods, (2) ANA diagnosis provided by an ophthalmologist or optometrist with at least one recorded gonioscopy based on Current Procedural Terminology (CPT) code (Supplementary Table 1), (3) no history of pseudophakia, IOP-lowering medications, laser treatments, cataract or glaucoma surgery before the index date based on CPT codes (Supplementary Table 1), and (4) no PACG diagnosis prior to or concurrent with the index date. Criterion 2 was implemented to increase the fidelity of diagnoses. Criterion 3 was implemented to ensure patients who received conventional ANA or PACG treatments prior to the index date would not be designated as newly diagnosed ANA. Diagnostic conversion from ANA to PACG, the primary study endpoint and outcome variable, was defined as a new and distinct diagnosis of PACG by an ophthalmologist based on ICD-9 or ICD-10 codes within the 6-year study period recorded after an index diagnosis of ANA.
Primary (first) ANA treatments administered after the index date were included in the analyses. These treatments included IOP-lowering drops, LPI, and cataract surgery. IOP-lowering drops include alpha agonists, beta blockers, carbonic anhydrase inhibitors, miotics, prostaglandin analogs, and miscellaneous antiglaucoma agents (Supplementary Table 1). Timing of treatments were categorized as never, early, or late. Early intervention was defined as treatment initiated within 6 months of the index date while late intervention was defined as treatment initiated more than 6 months after the index date. Treatments initiated after diagnosis of PACG were not considered. By nature of healthcare claims data, clinical factors, including gonioscopy findings and IOP measurements, necessary to conform patients to strict epidemiological definitions of PACD were not available for analysis.
Statistical Analysis
The proportion of ANA with diagnostic conversion to PACG among all ANA was calculated and stratified by sex, age, and race/ethnicity. Analyses were conducted on the patient level rather than eye level due to lack of laterality data in ICD-9. Continuous data were expressed as means and standard deviations while categorical data were expressed as proportions and percentages. Survival times were calculated as the duration between index date of ANA diagnosis and last recorded appointment date or first date of PACG diagnosis, whichever came first. Univariable Cox proportional hazards (CPH) models were developed with sociodemographic and treatment variables to determine hazard ratios (HR) for diagnostic conversion. A multivariable CPH model was developed using variables significant at p < 0.15 in univariable analysis. Age, sex, and race/ethnicity were included in the model regardless of significance. From this multivariable model, clinically and statistically significant variables were defined as having a hazard ratio greater than 1.25 or less than 0.80 and a p-value < 0.05. Statistical analysis was completed with R version 4.0.3.
Sensitivity analysis A was conducted to assess the effect of the censoring methodology. This analysis assumed all cases without diagnostic conversion within 6 years did not develop PACG regardless of whether they followed up or not (all censored at 6 years). This differed from the primary analysis, in which cases without diagnostic conversion were censored on the date of their last recorded appointment.
Results
A total of 263,422 patients diagnosed with ANA were identified in the Optum database (Figure 1), and 3,985 patients (1.51%) met the eligibility criteria for newly diagnosed ANA. The mean (standard deviation) age of study patients at index was 65.4 ± 11.9 years (range 17 to 84 years). There were 2,620 (65.75%) females and 1,364 (34.23%) males. Despite being continuously enrolled in Optum for the entire 6-year study period, 1,281 (32.15%) patients did not follow up after the index date of ANA diagnosis. All other patients (67.85%) returned at least once, and 646 (16.21%) patients had at least one visit recorded beyond the 6-year study period (Table 1).
Figure 1.
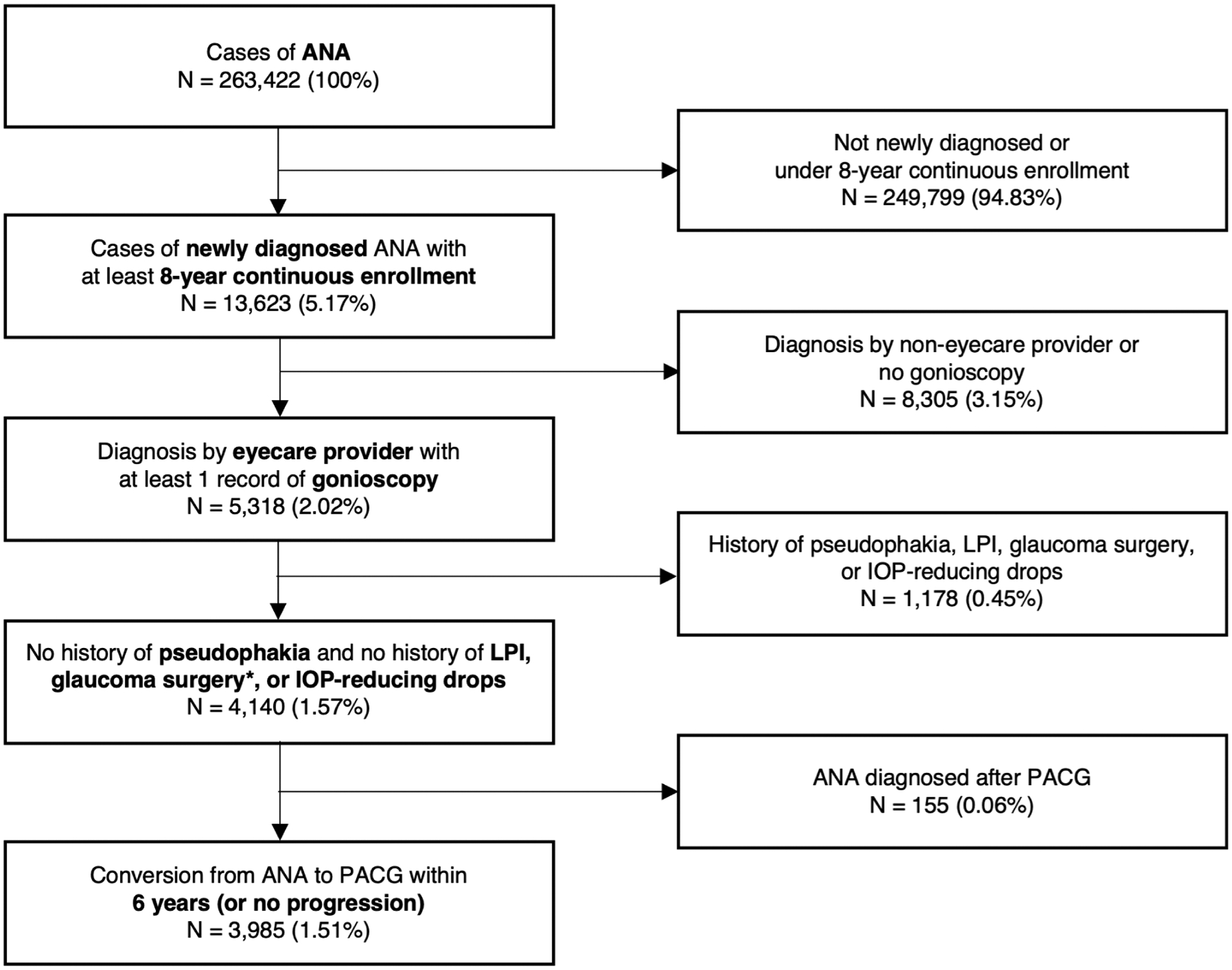
Attrition diagram of eligible patients with newly diagnosed anatomical narrow angle (ANA).
*Including iridoplasty, iridotomy, iridectomy, lens extraction cataract surgery, gonisynechiolysis, trabeculectomy, aqueous shunt, and cyclophotocoagulation
Table 1.
Proportion with diagnostic conversion from anatomical narrow angle (ANA) to primary angle closure glaucoma (PACG).
| N | n | % | ||
|---|---|---|---|---|
| Sex | Female | 2620 | 303 | 11.56% |
| Male | 1364 | 156 | 11.44% | |
| Age | <40 | 96 | 14 | 14.58% |
| 40–49 | 380 | 29 | 7.63% | |
| 50–59 | 769 | 85 | 11.05% | |
| 60–69 | 953 | 120 | 12.59% | |
| 70–79 | 1372 | 155 | 11.30% | |
| 80+ | 415 | 36 | 8.67% | |
| Race/Ethnicity | Asian | 203 | 30 | 14.78% |
| Black | 353 | 33 | 9.35% | |
| Hispanic | 472 | 60 | 12.71% | |
| White | 2705 | 298 | 11.02% | |
| Unknown | 252 | 27 | 10.71% | |
| Education | H.S. degree or less | 834 | 95 | 11.39% |
| Some college | 2160 | 249 | 11.53% | |
| College graduate | 846 | 96 | 11.35% | |
| Unknown | 145 | 19 | 13.10% | |
| Income | <$40K | 774 | 80 | 10.34% |
| $40K-49K | 228 | 29 | 12.72% | |
| $50K-59K | 281 | 42 | 14.95% | |
| $60K-74K | 394 | 46 | 11.68% | |
| $75K-99K | 593 | 68 | 11.47% | |
| $100K+ | 1189 | 125 | 10.51% | |
| Unknown | 526 | 69 | 13.12% | |
| Location | Northeast | 802 | 93 | 11.60% |
| South | 1607 | 189 | 11.76% | |
| Midwest | 882 | 98 | 11.11% | |
| Mountain | 178 | 26 | 14.61% | |
| Pacific | 515 | 53 | 10.29% | |
| LPI | None | 2390 | 141 | 5.90% |
| Early | 1248 | 244 | 19.55% | |
| Late | 347 | 74 | 21.33% | |
| Cataract Surgery | None | 2698 | 426 | 15.79% |
| Early | 470 | 18 | 3.83% | |
| Late | 817 | 15 | 1.84% | |
| IOP-lowering Drops | None | 3154 | 334 | 10.59% |
| Early | 536 | 104 | 19.40% | |
| Late | 295 | 21 | 7.12% | |
| Duration of follow up a | 0 yearsb | 1281 | 0 | 0 |
| 0–1 year | 858 | 112 | 13.05% | |
| 1–2 years | 312 | 43 | 13.78% | |
| 2–3 years | 243 | 42 | 17.28% | |
| 3–4 years | 196 | 51 | 26.02% | |
| 4–5 years | 210 | 49 | 23.33% | |
| 5–6 years | 239 | 39 | 16.32% | |
| > 6 years | 646 | 123 | 19.04% |
N = newly diagnosed ANA; n = detected conversion to PACG
duration of time between index date and last appointment
patient was not seen again after index date
Within the study period, 459 (11.52%) reached the primary endpoint of diagnostic conversion to PACG (Table 1). The overall conversion rate was 4.13%/year. The conversion rate was 10.59%/year during the first 6 months and stable at around 3.54%/year thereafter (Figure 2). In sensitivity analysis A, the overall progression rate was 2.01%/year, which dropped to 1.23%/year after the first 6 months.
Figure 2.
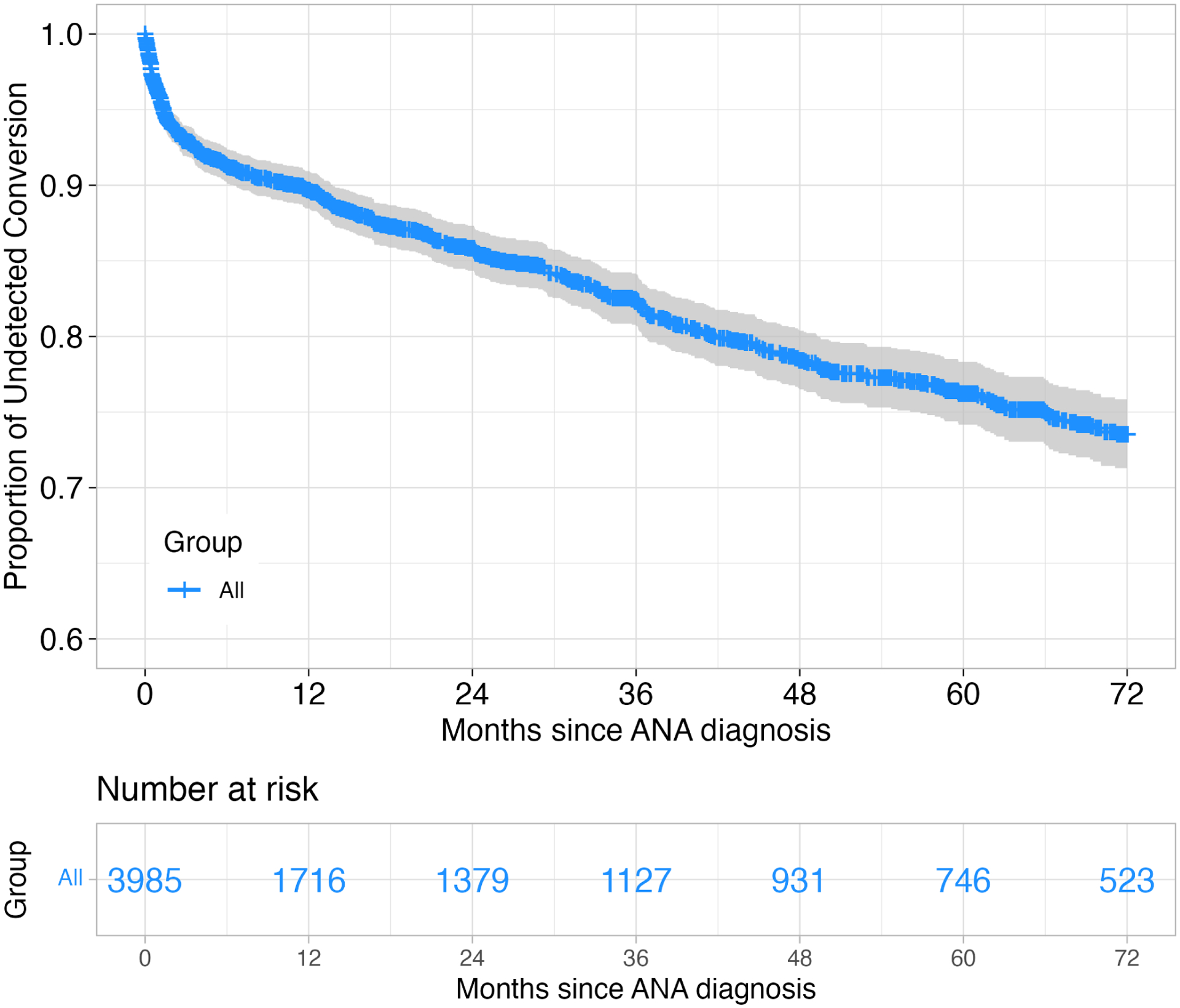
Kaplan-Meier survival curves of diagnostic conversion to primary angle closure glaucoma (PACG).
There was weak correlation between treatment variables among patients receiving multiple treatments (r = −0.01 to 0.12, Supplementary Table 2). Among all 3,985 cases of ANA, 1,595 (40.03%) received primary LPI, 1,287 (32.30%) received primary cataract surgery, and 831 (20.85%) received primary IOP-lowering drops. 1,001 (25.20%) patients had more than one treatment, and 217 (5.45%) had all three treatments at some point in the study period. Among the treatments, bivariable logistic regression models identified cataract surgery as a confounding factor in the association between age and risk of diagnostic conversion (Supplementary Table 3).
On univariable analysis (Table 2), location in the Mountain region (HR = 1.70), early or late LPI (HR > 1.36), and early IOP-lowering drops (HR = 1.79) were associated with significantly higher hazard (p < 0.03) of diagnostic conversion. Late cataract surgery (HR = 0.12) and late IOP-lowering drops (HR = 0.49) appeared significantly protective (p < 0.001) against diagnostic conversion. Age, household income, location, LPI, cataract surgery, and IOP-lowering drops were included in the multivariable analysis due to p < 0.15. Sex and race/ethnicity were included in the multivariable analysis despite p > 0.15.
Table 2.
Univariable and multivariable analysis of factors associated with diagnostic conversion to primary angle closure glaucoma (PACG).
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| HR (CI) | P-value | HR (CI) | P-value | |
| Age | ||||
| <40 | 1.65 (0.91–2.99) | 0.098 | 1.38 (0.64–3.01) | 0.412 |
| 40–49 | REF | REF | ||
| 50–59 | 0.87 (0.62–1.24) | 0.456 | 0.94 (0.63–1.40) | 0.754 |
| 60–69 | 1.01 (0.73–1.41) | 0.938 | 1.20 (0.82–1.76) | 0.342 |
| 70–79 | 1.04 (0.75–1.43) | 0.811 | 1.59 (1.09–2.32) | 0.016 |
| 80+ | 1.11 (0.72–1.70) | 0.643 | 1.82 (1.10–3.01) | 0.020 |
| Sex | ||||
| Female | REF | REF | ||
| Male | 1.14 (0.94–1.38) | 0.198 | 1.04 (0.84–1.29) | 0.694 |
| Race/Ethnicity | ||||
| White | REF | REF | ||
| Asian | 1.30 (0.89–1.89) | 0.176 | 1.27 (0.82–1.97) | 0.278 |
| Black | 1.22 (0.89–1.67) | 0.223 | 1.00 (0.70–1.43) | 0.994 |
| Hispanic | 1.18 (0.89–1.55) | 0.251 | 1.00 (0.73–1.37) | 0.982 |
| Education | ||||
| H.S. degree or less | REF | - | ||
| Some college | 0.91 (0.72–1.15) | 0.422 | - | |
| College graduate | 0.82 (0.62–1.09) | 0.168 | ||
| Income | ||||
| <$40K | REF | REF | ||
| $40K-49K | 1.03 (0.67–1.57) | 0.901 | 1.15 (0.75–1.77) | 0.523 |
| $50K-59K | 1.06 (0.73–1.54) | 0.754 | 1.10 (0.75–1.61) | 0.614 |
| $60K-74K | 0.94 (0.66–1.36) | 0.754 | 1.02 (0.70–1.48) | 0.908 |
| $75K-99K | 0.87 (0.63–1.20) | 0.381 | 0.90 (0.64–1.26) | 0.535 |
| $100K+ | 0.77 (0.58–1.02) | 0.07 | 0.81 (0.59–1.10) | 0.170 |
| Insurance | ||||
| HMO | REF | - | ||
| EPO | 1.36 (0.89–2.07) | 0.158 | - | |
| Other | 1.15 (0.93–1.43) | 0.188 | - | |
| PPO | 0.83 (0.57–1.21) | 0.329 | - | |
| Location | ||||
| Northeast | REF | REF | ||
| South | 1.14 (0.89–1.46) | 0.303 | 0.94 (0.71–1.23) | 0.641 |
| Midwest | 1.03 (0.78–1.37) | 0.839 | 0.85 (0.62–1.17) | 0.312 |
| Mountain | 1.70 (1.10–2.63) | 0.017 | 1.31 (0.81–2.12) | 0.268 |
| Pacific | 1.06 (0.76–1.49) | 0.723 | 0.67 (0.45–1.00) | 0.048 |
| LPI | ||||
| None | REF | REF | ||
| Early | 2.31 (1.88–2.85) | <0.001 | 2.49 (1.97–3.16) | 0.001 |
| Late | 1.36 (1.03–1.80) | 0.032 | 1.28 (0.93–1.75) | 0.126 |
| Cataract Surgery | ||||
| None | REF | REF | ||
| Early | 0.69 (0.43–1.10) | 0.118 | 0.46 (0.27–0.78) | 0.004 |
| Late | 0.12 (0.07–0.20) | <0.001 | 0.10 (0.06–0.17) | 0.001 |
| IOP-lowering Drops | ||||
| None | REF | REF | ||
| Early | 1.79 (1.44–2.24) | <0.001 | 1.64 (1.28–2.09) | 0.001 |
| Late | 0.49 (0.31–0.76) | 0.001 | 0.43 (0.26–0.71) | 0.001 |
Statistically significant p-values and odds ratios are bolded; OR = odds ratio; CI = confidence interval; HMO = health maintenance organization; EPO = exclusive provider organization; PPO = preferred provider organization.
On multivariable analysis (Table 2), age greater than 70 years (HR > 1.59), early LPI (HR = 2.49), and early IOP-lowering drops (HR = 1.64) were associated with significantly higher hazard (p < 0.02) of diagnostic conversion. Cataract surgery at any time (HR < 0.46), late IOP-lowering drops (HR = 0.43), and location in the Pacific region (HR = 0.67) appeared significantly protective (p < 0.048) against detected conversion.
The multivariable model developed in sensitivity analysis A (patients lost to follow up censored at 6 years) included the same variables as the primary analysis with the addition of insurance product. The model produced similar results to the primary analysis with the exception of late LPI (HR = 3.21, p = 0.001) and “Other” insurance product (HR = 1.61; p = 0.001) being associated with increased hazard of detected conversion (Supplementary Table 4).
Discussion
In this paper, we assessed 6-year rates and patterns of diagnostic conversion from ANA to PACG based on ICD code analysis of healthcare claims data from the United States. The overall conversion rate was relatively low at 4.13%/year. Our analyses also demonstrated an association between the need for early treatment with IOP-lowering drops or LPI and diagnostic conversion. Cataract surgery performed any time after ANA diagnosis appeared strongly protective against diagnostic conversion. These findings provide insights into the real-world clinical course of patients in the United States with newly diagnosed ANA and help contextualize broad disease definitions and practice patterns related to patients at risk for PACG.
Our findings indicated the conversion rate from ANA to PACG over a 6-year period was stable and relatively low at around 3.54%/year after the first 6 months of ANA diagnosis. This rate is higher than the estimated 0.34%/year and 1.25%/year rates of progression from PACS to PACG based on 5-year studies from Singapore and India, respectively, but lower than the estimated 5.70%/year rate of progression from PAC to PACG based on a 5-year study from India.9,10,12 While it is tempting to speculate on the proportion of PACS and PAC in our study cohort, it is important to acknowledge that broad definitions of ANA utilized in clinical settings by clinicians lack granularity compared to strict definitions of PACD utilized in study settings by scientific researchers; therefore, direct comparisons must be made cautiously. While PACS is more common than PAC in population-based epidemiological studies, a bias toward PAC in our study cohort is reasonable as healthcare claims data is inherently skewed towards individuals with more severe disease who tend to utilize healthcare services and follow up after initial diagnosis. There was also a high rate of loss to follow up in our study population, which may have further biased our sample toward conversion. This possibility led us to conduct sensitivity analysis A, which produced a more conservative conversion rate of 1.23%/year, and served to mitigate this effect. Based on the primary analysis, only one out of every 24 patients diagnosed with ANA is expected to be diagnosed with PACG, which suggests that most patients with ANA are at low risk of PACG. Therefore, long-term monitoring of most ANA patients appears to be of low clinical yield, highlighting the need for clearer practice guidelines on the management of low-severity ANA without PAS or elevated IOP.13
While our results indicate that early LPI and early IOP-lowering eyedrops are positively associated with diagnostic conversion, we emphasize that this association does not indicate a causative relationship. Rather, we speculate that patients receiving early IOP-lowering drops LPI have higher disease severity at diagnosis, including cases with peripheral anterior synechiae (PAS) or elevated IOP, that would fit the definition of PAC if gonioscopy and IOP data were available. This theory is also supported by our finding that need for early treatment with IOP-lowering drops or LPI was positively associated with detected progression whereas late treatment was not. This differential effect of treatment timing suggests that early treated ANA cases reflect higher disease severity compared to cases where treatment was deemed safe to delay or defer.14,15 One limitation of healthcare claims data is that clinical factors motivating treatment cannot be directly assessed through broad categorical ICD diagnoses; therefore, these hypotheses cannot be tested using data analyzed in this study.
One factor that was consistently associated with reduced risk of diagnostic conversion was cataract surgery performed at any time after ANA diagnosis. One interpretation of the data is that cataract surgery produces more dramatic angle widening than LPI, leading to better IOP and disease control. However, it is important to recognize that LPI is performed almost exclusively to alleviate pupillary block and ANA, whereas cataract surgery is more commonly performed to treat vision loss secondary to cataract formation. Therefore, there may be confounding factors that place patients receiving cataract surgery at inherently lower risk of diagnostic conversion compared to those who require early treatment with LPI. The protective effect of cataract surgery is especially strong among patients receiving late cataract surgery, where the delayed timing of surgery is consistent with a non-urgent process (i.e. visually significant cataract). Regardless of motivating factors by treating physicians, our findings suggest that cataract surgery is strongly protective against PACG and support the trend toward earlier consideration of lens extraction in patients with ANA.4,16
We found the risk of conversion from ANA to PACG increases with older age; however, this association emerges only after controlling for cataract surgery in the multivariable analysis. While prior epidemiological studies reported higher prevalence of PACG in older patients, our findings suggest that this increased risk of PACG is partially offset by higher rates of cataract surgery among the elderly.17–22 One population-based study in Myanmar estimated a 38% relative reduction in PACG burden if cataract surgery were performed for all visually significant cataracts.23 Therefore, regional and global rates of cataract surgery could be important factors to consider when forecasting future changes in PACG prevalence.
Our study provides the first evidence that race/ethnicity has no significant effect on risk of conversion from ANA to PACG. Although it is well-documented that angle closure disease is endemic among certain ethnicities/races, most notably Asians, and there are racial disparities in disease detection, there is a paucity of longitudinal studies on disease progression among non-Asians.1,24–26 Our results suggest that the higher prevalence of PACG among Asians may be related to a higher burden of ANA rather than a racial tendency toward disease progression. However, our sample size of Asians was smaller than other races and ethnicities, and a larger sample size may have revealed a significant difference in conversion rate. Therefore, larger and more rigorous longitudinal studies on incidence and progression of PACD in multi-racial/ethnic cohorts using strict definitions of ANA (i.e. PACS and PAC) and PACG are necessary to demonstrate this point conclusively.27,28 Other sociodemographic variables, including sex, were not significantly associated with conversion to PACG, which is consistent with previous longitudinal studies on PACD.2,21,29
Although diagnostic conversion rates were stable after the first 6 months of ANA diagnosis, these rates were higher (10.59%/year) within 6 months of ANA diagnosis. One possible explanation for this difference in conversion rates is that some patients with PACG had mild disease or received incomplete glaucoma testing on the index data of ANA diagnosis, leading to a brief delay in the diagnosis of PACG.17,30–34 While a similar explanation was proposed by previous studies on delayed glaucoma diagnoses among patients in the United States and Japan, it is difficult to confirm without access to additional clinical data.17,30–34 Therefore, further study is necessary to determine if patients with newly diagnosed ANA may benefit from closer monitoring within the first 6 months of ANA diagnosis.
Our study has several limitations. First, we relied on diagnoses of ANA and PACG provided by a wide range of clinicians using broad real-world rather than strict epidemiological disease definitions. While ICD-9 and ICD-10 diagnoses of ANA likely encompass both PACS and PAC, there is insufficient clinical data available in the Optum database to stratify ANA diagnoses by severity based on strict definitions of PACD. However, there are currently no alternative data sources to study the clinical course of a diverse, multi-racial/ethnic cohort of patients with ANA. In addition, diagnostic conversion was defined based on a change in ICD code rather a static ICD code; this approach mitigates the effects of inaccurate coding and increases the fidelity of our study findings. Second, our study cohort represented a small proportion of all cases of ANA in the Optum database, primarily due to the long lookback and study periods and requisite record of gonioscopy. Therefore, higher-risk cases of ANA may be over-represented among patients who followed up for the entire 6-year study period, which could limit the generalizability of our findings. However, the rate of diagnostic conversion remained stable after the first 6 months of ANA diagnosis despite a relatively high attrition rate, which gives us confidence regarding this estimate. Third, our analyses of treatment effects are purely observational and not meant to simulate a randomized controlled trial; therefore, we cannot comment on the relative benefits of studied treatments. Fourth, the lack of eye laterality and clinical data made eye-level assessment of disease severity difficult. Consequently, detection of conversion could be confounded by diagnosis or treatment in the contralateral eye. Finally, only primary treatments were included in the analyses as correlation between treatments was weak.
In summary, the real-world rate of diagnostic conversion from ANA to PACG is relatively low and does not appear to vary by sex or race/ethnicity. Based on our findings, we suggest that elderly patients with ANA be monitored more closely, especially if requiring treatment with IOP-lowering drops or LPI. In addition, earlier cataract surgery should be strongly considered in qualifying patients with ANA. We emphasize that these suggestions are meant to complement rather than replace clinical measures of angle closure severity that normally guide monitoring or treatment, including presence of PAS or elevated IOP. Current clinical practice patterns for lower-severity ANA appear to lack precision and cost-effectiveness, although additional study is needed on this topic. Given these current deficiencies, future work should be directed at developing convenient and quantitative methods to better risk-stratify patients with ANA for PACG.
Supplementary Material
Supplementary Table 1. Diagnosis, procedure, and treatment codes used in the study.
Supplementary Table 2. Pearson correlation matrix between treatments and age at anatomical narrow angle (ANA) diagnosis.
Supplementary Table 3. Bivariable analyses between age and treatments for risk of detected conversion to primary angle closure glaucoma (PACG).
Supplementary Table 4. Sensitivity analysis A: Factors associated with diagnostic conversion to primary angle closure glaucoma (PACG) with non-converters censored at 6 years.
Financial Support:
This work was supported by grant K23 EY029763 from the National Eye Institute, National Institute of Health, Bethesda, Maryland and an unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflicting relationship exists for any author.
Prior Presentations: American Glaucoma Society Annual Meeting, 2022.
References
- 1.Quigley H, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster PJ. The epidemiology of primary angle closure and associated glaucomatous optic neuropathy. Semin Ophthalmol. 2002;17(2):50–58. doi: 10.1076/soph.17.2.50.14718 [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA, Friedman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003;12(2):167–180. doi: 10.1097/00061198-200304000-00013 [DOI] [PubMed] [Google Scholar]
- 4.Azuara-Blanco A, Burr J, Ramsay C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet. 2016;388(10052):1389–1397. doi: 10.1016/S0140-6736(16)30956-4 [DOI] [PubMed] [Google Scholar]
- 5.He M, Jiang Y, Huang S, et al. Laser peripheral iridotomy for the prevention of angle closure: a single-centre, randomised controlled trial. Lancet. 2019;393(10181):1609–1618. doi: 10.1016/S0140-6736(18)32607-2 [DOI] [PubMed] [Google Scholar]
- 6.Xu BY, Friedman DS, Foster PJ, et al. Anatomical Changes and Predictors of Angle Widening After Laser Peripheral Iridotomy: The Zhongshan Angle Closure Prevention Trial. Ophthalmology. Published online 2021:1–8. doi: 10.1016/j.ophtha.2021.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu BY, Friedman DS, Foster PJ, et al. Ocular Biometric Risk Factors for Progression of Primary Angle Closure Disease: The Zhongshan Angle Closure Prevention Trial. Ophthalmology. 2021;0(0). doi: 10.1016/J.OPHTHA.2021.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238–242. doi: 10.1136/bjo.86.2.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas R, George R, Parikh R, Muliyil J, Jacob A. Five year risk of progression of primary angle closure suspects to primary angle closure: A population based study. Br J Ophthalmol. 2003;87(4):450–454. doi: 10.1136/bjo.87.4.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas R, Parikh R, Muliyil J, Kumar RS. Five-year risk of progression of primary angle closure to primary angle closure glaucoma: A population-based study. Acta Ophthalmol Scand. 2003;81(5):480–485. doi: 10.1034/j.1600-0420.2003.00135.x [DOI] [PubMed] [Google Scholar]
- 11.Health resources - Health spending - OECD Data.
- 12.Baskaran M, Kumar RS, Friedman DS, et al. The Singapore Asymptomatic Narrow Angles Laser Iridotomy Study (ANA-LIS): 5 year results of a Randomized Controlled Trial. Ophthalmology. 2021;0(0). doi: 10.1016/j.ophtha.2021.08.017 [DOI] [PubMed] [Google Scholar]
- 13.Gedde SJ, Chen PP, Muir KW, et al. Primary Angle-Closure Disease Preferred Practice Pattern®. Ophthalmology. 2021;128(1):P30–P70. doi: 10.1016/j.ophtha.2020.10.021 [DOI] [PubMed] [Google Scholar]
- 14.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment. Evidence-Based Eye Care. 2003;4(4):196–197. doi: 10.1097/00132578-200310000-00007 [DOI] [Google Scholar]
- 15.Kass MA, Gordon MO, Gao F, et al. Delaying treatment of ocular hypertension: The ocular hypertension treatment study. Arch Ophthalmol. 2010;128(3):276–287. doi: 10.1001/archophthalmol.2010.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tham C, Kwong Y, Leung D, et al. Phacoemulsification vs phacotrabeculectomy in chronic angle-closure glaucoma with cataract complications (Archives of Ophthalmology (2010) 128, 3, (303–311)). Arch Ophthalmol. 2010;128(9):1128. doi: 10.1001/archophthalmol.2010.197 [DOI] [PubMed] [Google Scholar]
- 17.Sawaguchi S, Sakai H, Iwase A, et al. Prevalence of primary angle closure and primary angle-closure glaucoma in a southwestern rural population of Japan: The Kumejima study. Ophthalmology. 2012;119(6):1134–1142. doi: 10.1016/j.ophtha.2011.12.038 [DOI] [PubMed] [Google Scholar]
- 18.Salmon JF, Mermoud A, Ivey A, Swanevelder SA, Hoffman M. The Prevalence of Primary Angle Closure Glaucoma and Open Angle Glaucoma in Mamre, Western Cape, South Africa. Arch Ophthalmol. 1993;111(9):1263–1269. doi: 10.1001/archopht.1993.01090090115029 [DOI] [PubMed] [Google Scholar]
- 19.Liang Y, Friedman DS, Zhou Q, et al. Prevalence and characteristics of primary angle-closure diseases in a rural adult Chinese population: The Handan eye study. Investig Ophthalmol Vis Sci. 2011;52(12):8672–8679. doi: 10.1167/iovs.11-7480 [DOI] [PubMed] [Google Scholar]
- 20.Day AC, Baio G, Gazzard G, et al. The prevalence of primary angle closure glaucoma in European derived populations: A systematic review. Br J Ophthalmol. 2012;96(9):1162–1167. doi: 10.1136/bjophthalmol-2011-301189 [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Huang W, Huang S, et al. Ten-year incidence of primary angle closure in elderly Chinese: The Liwan Eye Study. Br J Ophthalmol. 2019;103(3):355–360. doi: 10.1136/bjophthalmol-2017-311808 [DOI] [PubMed] [Google Scholar]
- 22.Keenan TDL, Salmon JF, Yeates D, Goldacre M. Trends in rates of primary angle closure glaucoma and cataract surgery in England from 1968 to 2004. J Glaucoma. 2009;18(3):201–205. doi: 10.1097/IJG.0b013e318181540a [DOI] [PubMed] [Google Scholar]
- 23.Chan W, García JA, Newland HS, et al. Killing two birds with one stone: The potential effect of cataract surgery on the incidence of primary angle-closure glaucoma in a high-risk population. Clin Exp Ophthalmol. 2012;40(4):128–134. doi: 10.1111/j.1442-9071.2011.02607.x [DOI] [PubMed] [Google Scholar]
- 24.He M, Foster PJ, Johnson GJ, Khaw PT. Angle-closure glaucoma in East Asian and European people. Different diseases? Eye. 2006;20(1):3–12. doi: 10.1038/sj.eye.6701797 [DOI] [PubMed] [Google Scholar]
- 25.Apolo G, Bohner A, Pardeshi A, et al. Racial and Sociodemographic Disparities in the Detection of Narrow Angles before Detection of Primary Angle-Closure Glaucoma in the United States. Ophthalmol Glaucoma. Published online January 2022. doi: 10.1016/j.ogla.2022.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson AC, Vu DM, Cowan LA, Asrani S. Risk Factors Associated with Missed Diagnoses of Narrow Angles by the Van Herick Technique. Ophthalmol Glaucoma. 2018;1(2):108–114. doi: 10.1016/J.OGLA.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 27.Vijaya L, Asokan R, Panday M, et al. Six-Year Incidence of Angle-Closure Disease in a South Indian Population: The Chennai Eye Disease Incidence Study. Am J Ophthalmol. 2013;156(6):1308–1315.e2. doi: 10.1016/J.AJO.2013.07.027 [DOI] [PubMed] [Google Scholar]
- 28.Teo ZL, Soh Z Da, Tham YC, et al. Six-Year Incidence and Risk Factors for Primary Angle-Closure Disease: The Singapore Epidemiology of Eye Diseases Study. Ophthalmology. 2022;129(7):792–802. doi: 10.1016/J.OPHTHA.2022.03.009 [DOI] [PubMed] [Google Scholar]
- 29.Vijaya L, George R, Arvind H, et al. Prevalence of angle-closure disease in a rural southern Indian population. Arch Ophthalmol. 2006;124(3):403–409. doi: 10.1001/archopht.124.3.403 [DOI] [PubMed] [Google Scholar]
- 30.Shaikh Y, Yu F, Coleman AL. Burden of undetected and untreated glaucoma in the United States. Am J Ophthalmol. 2014;158(6):1121–1129.e1. doi: 10.1016/j.ajo.2014.08.023 [DOI] [PubMed] [Google Scholar]
- 31.Gordon-Bennett P, Ung T, Stephenson C, Hingorani M. Misdiagnosis of angle closure glaucoma. BMJ. 2006;333:1157–1158. doi: 10.1136/bmj.39024.570313.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siriwardena D, Arora AK, Fraser SG, Mcclelland HK, Claoué C. Misdiagnosis of acute angle closure glaucoma. Age Ageing. 1996;25(6):421–423. doi: 10.1093/ageing/25.6.421 [DOI] [PubMed] [Google Scholar]
- 33.Nolan WP, Foster PJ, Devereux JG, Uranchimeg D, Johnson GJ, Baasanhu J. YAG laser iridotomy treatment for primary angle closure in east Asian eyes. Br J Ophthalmol. 2000;84(11):1255–1259. doi: 10.1136/bjo.84.11.1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng PH, Nguyen H, Lin HS, Nguyen N, Lin S. Long-term outcomes of laser iridotomy in Vietnamese patients with primary angle closure. Br J Ophthalmol. 2011;95(9):1207–1211. doi: 10.1136/bjo.2010.181016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Diagnosis, procedure, and treatment codes used in the study.
Supplementary Table 2. Pearson correlation matrix between treatments and age at anatomical narrow angle (ANA) diagnosis.
Supplementary Table 3. Bivariable analyses between age and treatments for risk of detected conversion to primary angle closure glaucoma (PACG).
Supplementary Table 4. Sensitivity analysis A: Factors associated with diagnostic conversion to primary angle closure glaucoma (PACG) with non-converters censored at 6 years.


