Abstract
Chronic cadmium (Cd) exposure severely affects the structural integrity of the heart, leading to cardiovascular disease. This study investigates the protective role of ascorbic acid (AA) and resveratrol (Res) in cellular defense against Cd-induced cardiomyocyte damage and myocardial hypertrophy in H9c2 cardiomyocytes. Experimental results showed that AA and Res treatment significantly increased cell viability, reduced ROS production, attenuated lipid peroxidation, and increased antioxidant enzyme activity in Cd-induced H9c2 cells. AA and Res decreased the mitochondrial membrane permeability and protected the cells from Cd induced cardiomyocyte damage. This also suppressed the pathological hypertrophic response triggered by Cd, which increased the cell size of cardiomyocytes. Gene expression studies revealed that cells treated with AA and Res decreased the expression of hypertrophic genes ANP (two-fold), BNP (one-fold) and β- MHC (two-fold) compared to Cd exposed cells. AA and Res promoted the nuclear translocation of Nrf2 and increased the expression of antioxidant genes (HO-1, NQO1, SOD and CAT) during Cd mediated myocardial hypertrophy. This study proves that AA and Res play a significant role in improving Nrf2 signaling, thereby reversing stress-induced injury, and facilitating the regression of myocardial hypertrophy.
Keywords: Ascorbic acid, Resveratrol, Cadmium, ROS, Myocardial hypertrophy, Nrf2
Introduction
Cadmium (Cd) is a non-essential heavy metal with no biological role and poses greatest threat to human health. Exposure to Cd is inevitable, as this extremely toxic element enters the food chain through diet, occupation, and smoking (Atsdr 2012). Cd has high affinity to metallothionein, a low molecular weight Cd-binding protein and the half-life of Cd inside human body ranges from 20 to 30 years. Chronic exposure to Cd results in multiorgan toxicities such as hepatotoxicity, nephrotoxicity, and cardiotoxicity. Most of the studies have focused on hepatotoxicity and nephrotoxicity and data on Cd-induced cardiotoxicity are very scarce. In order to overcome cardiovascular pathologies, more studies on Cd-induced cardiotoxicity are essential. Studies have reported that acute and chronic Cd toxicities directly affect myocardial structures and cause tissue injury due to oxidative stress and impaired mitochondrial function (Cuypers et al. 2010; Ferramola et al. 2012; Borné et al. 2015; Sundaresan et al. 2021). Epidemiological evidence supports the association between Cd and incidence of cardiovascular diseases (CVD) such as sudden cardiac arrest, stroke, peripheral arterial diseases, and myocardial infarction (Cheng et al. 2015; Barregard et al. 2021).
Cardiac hypertrophy of the heart is a substantial response to various factors characterized by specific molecular and phenotypic changes. Heart failure due to chronic myocardial hypertrophy has been a prominent cause of mortality worldwide (Cox and Marsh 2014; Bajaj et al. 2020). Pathological cardiac hypertrophy results in the activation of panel of fetal genes such as atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), α-skeletal muscle actin, and β-myosin heavy chain (β-MHC), which can be used as consistent biomarkers of cardiac hypertrophy (Dolinsky and Dyck 2011). Unlike oxidative burst from leukocytes that acts as an indication for systemic inflammatory response, excessive production of reactive oxygen species (ROS) and mitochondrial dysfunction that occur exclusively and specifically in cardiomyocytes act both as an indication and as a contributor for the incidence of pathological cardiac hypertrophy due to Cd exposure (Donpunha et al. 2011).
Ascorbic acid (AA) and resveratrol (Res) are the two major antioxidants present in plant-based diet. AA is a water-soluble vitamin widely distributed in fresh fruits (especially in citrus fruits) and in leafy vegetables, and Res is a natural polyphenol mostly present in red wine and peanuts (Eybl et al. 2006; Moser and Chun 2016). Several reports have deciphered the potential of dietary intake of AA and Res in the prevention of cardiovascular diseases including oxidative stress–induced cardiac hypertrophy (Goszcz et al. 2015; Collins et al. 2021). Studies have correlated the cardioprotective effect of AA and Res with their antioxidant activity due to the activation of redox-sensitive transcription factor nuclear erythroid-2 like factor-2 (Nrf2) and subsequent transcriptional activation of antioxidant response element (ARE)-bearing antioxidants, including NAD(P)H dehydrogenase (quinone 1) (NQO1), heme oxygenase-1 (HO-1), catalase (CAT), superoxide dismutases (SODs), and glutathione peroxidases (GPx), which in turn antagonize oxidative stress-induced tissue inflammation and injury (Kim et al. 2015; Mostafavi-Pour et al. 2017).
Nrf-2-activation-mediated phase 2 enzymes were shown to be protective against lethal concentrations of Cd in various cell lines and in mice. It has been reported that higher AA concentration in patients with Cd toxicity protects them from developing cardiovascular disease, whereas lower AA concentrations make them more susceptible to coronary artery disease (Kleszczewski et al. 2016). Moreover, data have shown that dietary supplementation of AA reverses oxidative stress, hypertension, and vascular dysfunction in Cd-induced mice. Although more studies are available on the effect of AA and Res on Cd-induced toxicities, studies exclusively on the protective effect of AA and Res in ameliorating Cd-induced cardiac hypertrophy are scarce. Therefore, based on the previously established association between dietary antioxidants and oxidative stress-induced cardiovascular pathologies, in this study, we aimed to prove that AA and Res-induced activation of Nrf2 signaling and successive expression of phase 2 enzymes to be an effective approach to restore Cd-induced cardiomyocyte damage and myocardial hypertrophy in H9c2 cells.
Materials and methods
Chemicals
AA, Res, cadmium chloride (CdCl2), dichlorofluorescein diacetate (DCFH-DA), bovine serum albumin (BSA), rhodamine-123(R-123), Triton X 100, dimethyl sulphoxide (DMSO), radioimmunoprecipitation assay buffer (RIPA buffer), and Tris base were purchased from Sigma-Aldrich, India. Dulbecco’s modified Eagle’s medium (DMEM), antibiotic–antimycotic solution (100X), fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT), and trypsin–EDTA solution were purchased from Hi-Media Laboratories, India. Agarose was procured from Invitrogen, USA, and Sybr Green master mix was purchased from Bioline, India. Nitrocellulose membrane was purchased from Pall Life Sciences, USA, and GAPDH primary antibody and secondary antibody were purchased from Santa Cruz, USA.
Cell culture and treatment
Rat cardio myoblast cell line, H9c2 was procured from the National Centre for Cell Science (NCCS), Pune, India. Cells were cultured in high-glucose DMEM and supplemented with 10% FBS and 1% antibiotic antimycotic solution in a humidified atmosphere of 95% air with 5% CO2 at 37 °C. The cells were sub-cultured at 70–80% confluence, and the medium was changed once in two days. Then H9c2 cells were treated with different concentrations of Cd, and the cell viability was determined by MTT assay (Ferrari et al. 1990). The optimal cytotoxic concentration of Cd was determined by using different concentration of Cd (1, 3, 5, 7, and 9 µM). Based on the IC50 values obtained, 3 µM of Cd was used throughout the experiment. Following the determination of optimal concentration, the experimental group was divided into (a) Control, (b) H9c2 cells exposed to 3 µM CdCl2 for 24 h (Cd-exposed group), (c) H9c2 cells co-exposed to 3 µM CdCl2 and 150 µM AA for 24 h (AA-treated group), and (d) H9c2 cells co-exposed to 3 µM CdCl2 and 20 µM Res for 24 h (Res-treated group).
Flow cytometry analysis
Cell viability was determined by flow cytometry as described by Johnson et al. (2013). H9c2 cardiomyocytes were cultured in 6 well plates with 3 µM of CdCl2, 150 µM of AA, and 20 µM of Res, respectively, for 24 h. The cells in plates were washed with PBS, stained with 1 µL of 2 mg/mL propidium iodide, and incubated for 10 min in dark at room temperature. Unstained cells were used as control, and cell viability was determined using a fluorescence-activated cell sorter (FACS Aria III, BD Biosciences, USA) with excitation at 488 nm and emission at 550 nm.
Detection of intracellular ROS
The production of ROS was observed by using a fluorescent probe 2’,7’-dichlorofluorescein diacetate (DCFH-DA). In brief, H9c2 cells were seeded in 24-well plates containing DMEM medium and incubated at 37 °C with 5% CO2 for 24 h. The cells in the plates were then treated with 3 µM of CdCl2, 150 µM of AA, and 20 µM of Res, respectively, for 24 h. Afterward, 10 µM of DCFH-DA was added to the plates and kept in dark at 37 °C for 30 min. Post-staining, the plates were rinsed with PBS, and the images were examined by using a fluorescent microscope (Nikon Eclipse Ti200). The mean fluorescence intensity (MFI) of DCFH-DA from five randomly selected fields was analyzed using Image J software, which represented the level of ROS.
Measurement of lipid peroxidation and enzymatic antioxidants
Cell lysates were prepared according to the manufacturer’s protocol. The level of malondialdehyde (MDA) in the homogenate was identified with thiobarbituric acid (TBA) reagent using a spectrophotometer with the commercially available kit (HiMedia Laboratories, India). The results were expressed as nanomoles per milligram of protein. The total protein of the samples was quantified using Bradford assay kit (Sigma Aldrich, USA). Standard protocols were used to measure enzymatic antioxidants such as catalase and SOD (Sinha 1972; Kono 1978).
Analysis of mitochondrial transmembrane potential (ΔΨm)
The outcome of the antioxidant effect on the inner mitochondrial membrane potential (MMP) of cardiomyocytes was resolved using rhodamine-123 (R-123) (Wang et al. 2011). H9c2 cardiomyocytes were cultured in 96 well plates with 3 µM of CdCl2, 150 µM of AA, and 20 µM of Res, respectively, for 24 h. Then, the cells were washed with PBS and 10 µg/mL of R-123 solution was added and incubated at 37 °C for 30 min in dark. Cell images after PBS wash were captured using fluorescence microscope (Nikon Eclipse Ti200) at 20× magnification. The MFI of R-123 from five randomly selected fields was determined with ImageJ software, which represents the level of MMP. The experiments were performed in triplicate.
Scanning Electron Microscopy
H9c2 cells (5 × 104 cells/chamber) were cultured in a cell culture chamber slide. After they were exposed to Cd, AA, and Res, a primary fixative and secondary fixative were added according to Samuel et al. (2011). Different graded ethanol (20%, 50%, 75%, 90%, 95%, and 100%) was used to desiccate the cells for 10 min each. The samples were dried in a vacuum desiccator and visualized using a VEGA3 TESCAN scanning electron microscope (SEM).
Cell size measurement
After treating the H9c2 cells with Cd, AA, and Res for 24 h, the size of the cardiomyocytes was determined by staining the cells with acridine orange dye for 3 min. Afterward, they were washed with PBS and observed under a fluorescent microscope with 20X magnification as described previously by Ramasamy et al. (2018). The area and size of the H9c2 cells were measured using Image J software.
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was isolated from cardiomyocytes using TRIzol Reagent (Sigma, USA), and cDNA was synthesized with Moloney Murine Leukemia Virus reverse transcriptase. RT-PCR was performed by Mastercycler ep realplex2 system (Eppendorf AG, Germany) using specific primers as listed in Table 1. Real-time cycling parameters include initial activation step (95 °C, 5 min), cycling step (denaturation 95 °C, 20 s; annealing at 59 °C, 20 s; and final extension at 68 °C, 20 s × 40 cycles), followed by melting curve analysis. PCR products were detected using SensiFast SYBR Green PCR Master Mix (Bioline). Relative mRNA levels were calculated after normalization to GAPDH mRNA levels using the comparative cycle threshold 2-ΔΔCt method (Livak and Schmittgen 2001).
Table 1.
Primers used in real-time polymerase chain reaction analysis
| Gene | Orientation | Sequence (5′–3') |
|---|---|---|
| Nrf2 |
Forward Reverse |
GCAACTCCAGAAGGAACAGG AGGCATCTTGTTTGGGAATG |
| NQO-1 |
Forward Reverse |
AGCAGCGGCTCCATGTACTC CCAGTTGAGGTTCTAAGACCTGTAA |
| HO-1 |
Forward Reverse |
GCATGAACACTCTGGAGATGACC GGCGAAGAAACTCTGTCTGTG |
| SOD |
Forward Reverse |
GGTGGTCCACGAGAAACAAG CAATCACACCACAAGCCAAG |
| CAT |
Forward Reverse |
GAATGGCTATGGCTCACACA CAAGTTTTTGATGCCCTGGT |
| ANP |
Forward Reverse |
GGGAAGTCAACCCGTCTCA GGGCTCCAATCCTGTCAAT |
| BNP |
Forward Reverse |
CTGGGAAGTCCTAGCCAGTCTCCA GCGACTGACTGCGCCGATCCGGTC |
| βMHC |
Forward Reverse |
GCAGACAGAGAATGGGGAGCTGTCC TCGCAATCATGCCGGGCTGAC |
| GAPDH |
Forward Reverse |
CTGAGAATGGGAAGCAGGTC GAAGGGGCAGAGATGATGAC |
Nrf2 Nuclear factor erythroid 2-related factor 2, NQO1 NAD(P)H dehydrogenase (quinone 1), HO-1 heme oxygenase-1, SOD superoxide dismutase, CAT Catalase, ANP arterial natriuretic peptide, BNP brain natriuretic peptide, β-MHC beta- myosin heavy chain, GAPDH Glyceraldehyde 3 Phosphate dehydrogenase
Western blot analysis
Protein samples from H9c2 cells were extracted by using RIPA buffer (Sigma-Aldrich) at 2–8 °C and the total protein of the samples was quantified using the Bradford method. An equal amount of protein was separated in 12% sodium dodecylsulfate-polyacrylamide gel and transferred onto nitrocellulose membranes. After blocking in 5% fat-free milk, the membranes were incubated at 4 °C overnight with the following primary antibodies: anti- Nrf2 (Sigma, USA) and anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA). All the antibodies were diluted at 1:1000 before use. The membranes were then incubated for 1 h at room temperature with horseradish peroxidase conjugated-secondary antibodies (Santa Cruz Biotechnology). The blots were observed using an Enhanced Chemiluminescence (ECL) Western blotting reagent (GE Healthcare Life Sciences, USA) and the protein band intensities were quantified using Image Lab Software version 5 (Bio-Rad, USA).
Statistical analysis
Data analysis was performed with Graph Pad Prism (GraphPad Software, Inc., San Diego, CA, United States). The results were presented as mean ± SD. Comparisons between multiple groups were performed using one-way ANOVA followed by Bonferroni test. A statistical difference of p < 0.05 was considered significant for all experiments.
Results
AA and Res improve cell viability of H9c2 cardiomyocytes
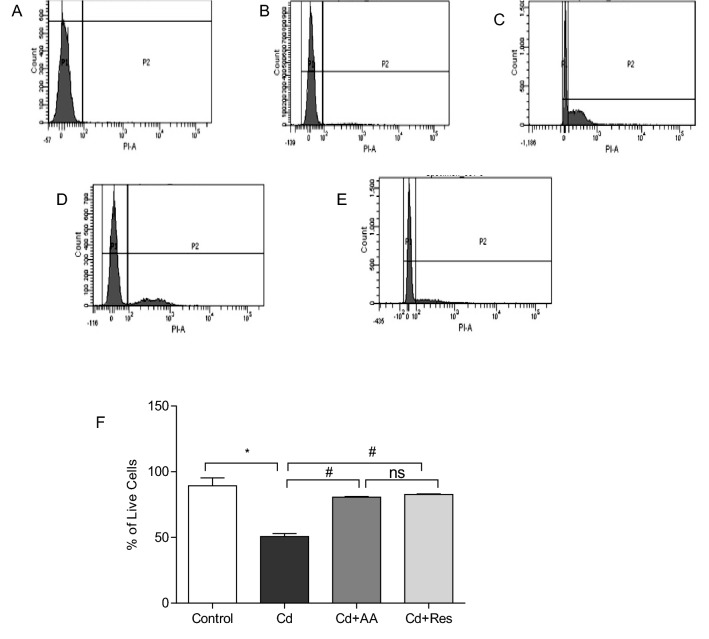
The optimal concentration of Cd to induce toxicity in H9c2 cardiomyocytes was determined by treating the cells with different concentrations (1, 3, 5, 7, and 9 µM) of CdCl2 for 24 h. The results showed that Cd-induced cytotoxicity was dose dependent (Fig. 1). Based on the IC50 values obtained, 3 µM of Cd used as a working concentration throughout the study. In this study, we examined the ameliorative effect of AA and Res in H9c2 cardiomyocytes exposed to CdCl2. Flow cytometry graphs of cardiomyocytes showed that AA and Res significantly increased the live cell population of Cd exposed cells (p < 0.001) (Fig. 2A–E). The percentage of cell viability obtained for dietary antioxidants AA and Res is shown in Fig. 2F. The results of this study showed that dietary antioxidants AA and Res can ameliorate cell viability and antagonize Cd-induced cardiotoxicity, which emphasizes that they exert a protective effect on cardiotoxicity. However, no significant difference was observed between control and AA and Res treated groups.
Fig. 1.

Dose-dependent cytotoxicity of Cd in H9c2 cardiomyocytes. Cell viability of cardiomyocytes after 24 h of induction of CdCl2 as measured by MTT. Results are expressed as mean ± SD percentage compared to control from three independent experiments
Fig. 2.
Live/dead cell assay in Cd-induced cardiomyocytes after 24 h of incubation. The X-axis corresponds to the propidium iodide (PI) positive H9c2 cells and Y-axis to the total cell count. A H9c2 PI negative control, 99.8% viability, B control, C H9c2 treated with 3 µM CdCl2, D H9c2 cells treated with 3 µM CdCl2 and 150 µM of AA, and E cells treated with 3 µM of CdCl2 and 20 µM of Res. F Percentage of live cells represented as mean ± SD (n = 3). Statistically significant differences are indicated as follows: *p < 0.0001, #p < 0.001, ns no significance
Dietary antioxidants AA and Res decrease ROS and myocardial oxidative stress markers in H9c2 cells exposed to Cd
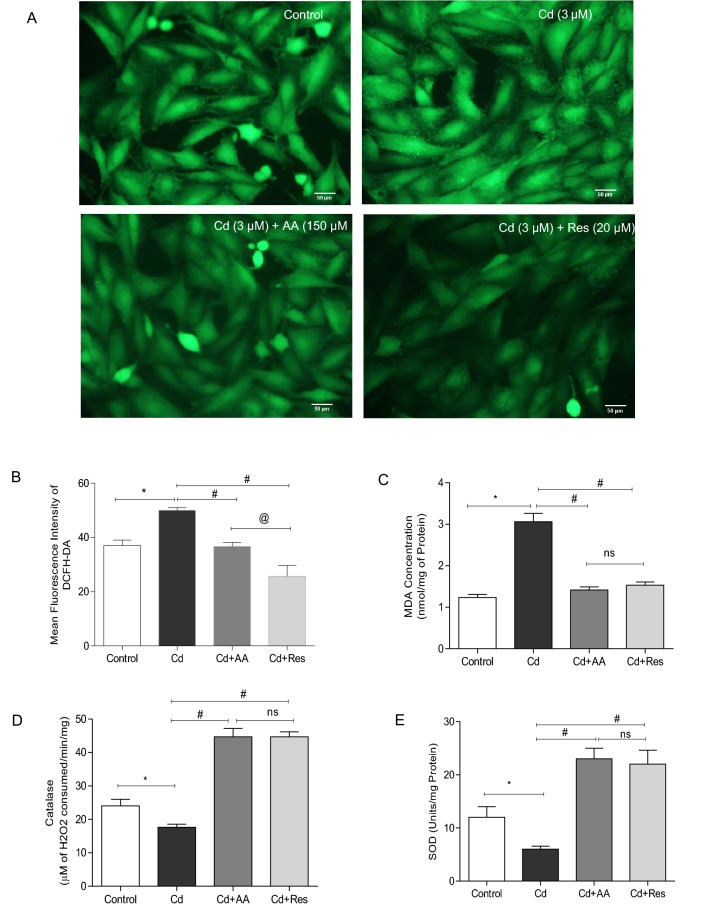
The generation of ROS was assessed in all the experimental groups by using DCFH-DA. The results showed a significant increase (p < 0.0001) in the intensity of green fluorescence in cardiomyocytes exposed to 3 µM CdCl2 compared to control. AA and Res-treated groups exhibited a significant decrease in fluorescence compared to Cd-exposed group (Fig. 3A, B). Cd-exposed group exhibited significantly increased levels of MDA and decreased levels of antioxidant enzymes SOD and catalase compared to control. AA and Res-treated groups exhibited significantly lower levels of MDA and increased levels of SOD and catalase compared to Cd-exposed cells (Fig. 3C, D). These results show that AA and Res improve antioxidant status and mitigate Cd-induced oxidative damage.
Fig. 3.
Effect of AA and Res on cellular ROS generation and oxidative stress markers in Cd-exposed cells. A Representative image of DCFH-DA-stained H9c2 cells exposed to Cd and treated with AA or Cd with Res for quantification of ROS generation. B Quantitative analysis of mean fluorescence intensity of DCFH-DA in control cells and Cd-exposed cells with AA or Res using Image J software. Scale bar corresponds to 50 µm C MDA levels against Cd induction in cardiomyocytes after 24 h. D Catalase activity. E SOD-1 activity after Cd exposure. All values are presented as mean ± SD (n = 3). Statistically significant differences are indicated as *p < 0.0001, #p < 0.0001, @p < 0.05, ns no significance
AA and Res improve MMP in Cd-exposed cells
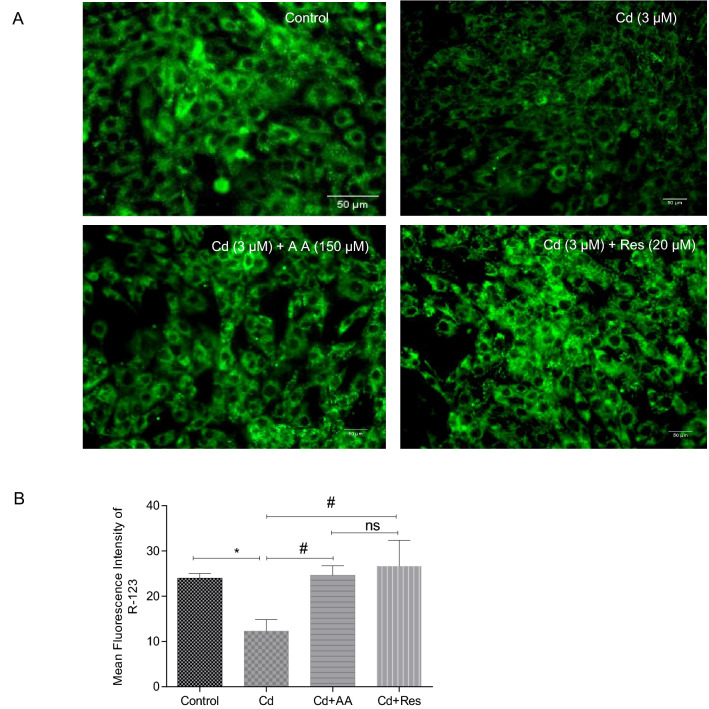
Differences in MMP were observed between Cd-exposed groups and antioxidant-treated groups. Cd-exposed cells (3 µM) showed a significant reduction (p < 0.001) in MMP (Fig. 4A), whereas AA and Res-treated groups showed a significant restoration of MMP as observed from the higher fluorescence intensity in contrast to Cd exposed cells (Fig. 4B).
Fig. 4.
Effect of AA and Res on mitochondrial membrane potential using rhodamine-123 in Cd-exposed H9c2 cells. A Representative images of mitochondrial membrane potential in AA- and Res-treated groups, respectively. B Quantitative analysis of mean fluorescence intensity of rhodamine-123 in Cd-exposed and control cells analyzed using Image J software. Scale bar corresponds to 50 µm. Values are expressed as mean ± SD (n = 3). Statistically significant differences are shown as follows: *p < 0.001, #p < 0.0001, ns no significance
AA and Res reduce Cd-induced structural abnormalities in H9c2 cells
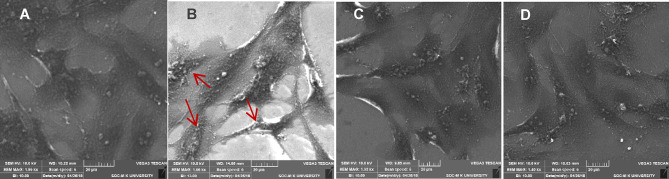
Morphological analysis of H9c2 cells was carried out by scanning electron microscopy. The results showed that the control H9c2 cells were elongated and spindle in shape with dense surface. Cd-exposed H9c2 cells showed morphological alterations such as loss of structural integrity, cell shrinkage, rounding up of cells, and membrane blebbing, similar to the characteristics of apoptosis. However, AA- and Res-treated groups showed significant reduction in Cd-induced morphological modifications in H9c2 cells (Fig. 5).
Fig. 5.
Morphological examinations of H9c2 cells by scanning electron microscopy. A Control cells with regular morphological architectures—elongated spindle shape with dense surface. B Cd-exposed cells with morphological changes like loss of spindle shape, cell shrinkage, and membrane blebbing in H9c2 cells. The results showed reduced structural abnormalities C Cd-exposed cells treated with ascorbic acid and D resveratrol. Scale bar corresponds to 20 µm
AA and Res inhibit pathological modifications in Cd-induced cardiomyocytes
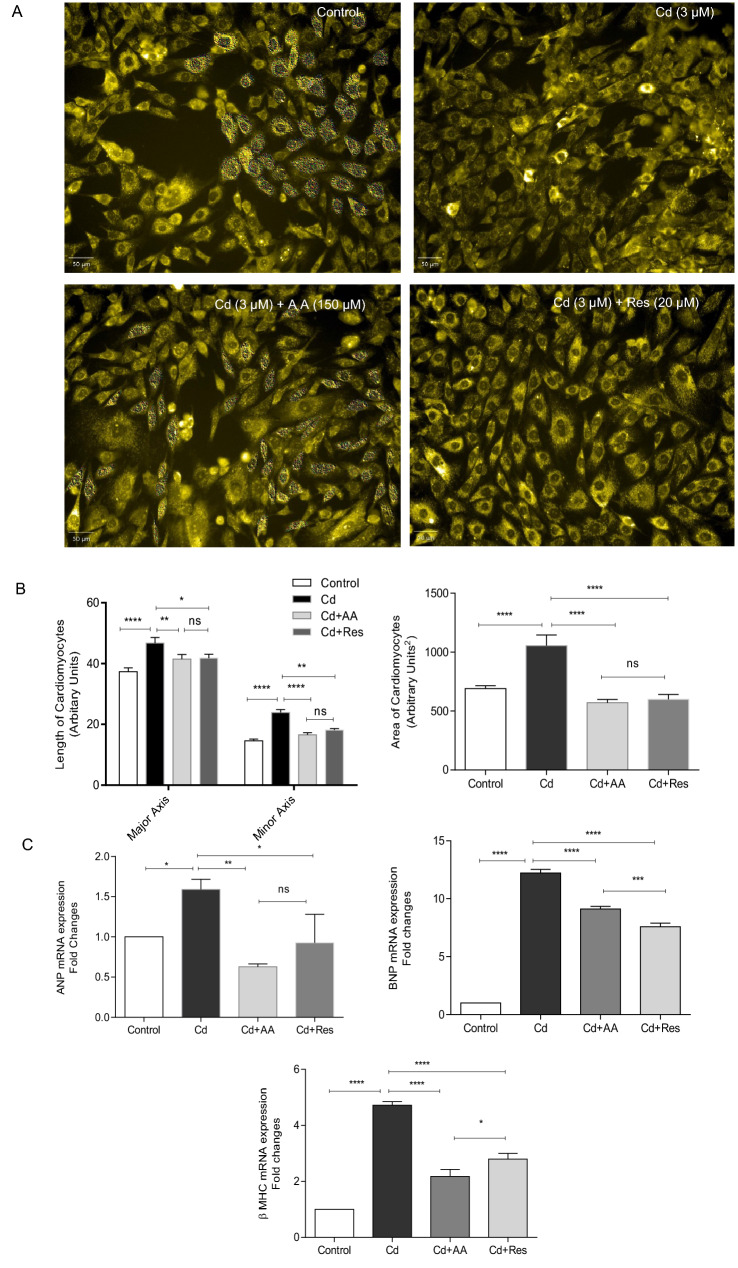
In order to quantify the effects of AA and Res on Cd-induced pathological modifications, cell size or area was observed after 24 h of treatment. H9c2 cells stained with acridine orange showed a significant increase in cell surface area of Cd-exposed cells compared to control. In contrast, a two-fold decrease was observed in the cell surface area of AA and Res-treated cells (Fig. 6A). Subsequently, myocardial hypertrophy markers were also quantified using RT-PCR. The results showed that mRNA levels of ANP, BNP, and βMHC were upregulated in Cd-exposed H9c2 cells compared to control (Fig. 6B), which indicates myocardial hypertrophy in cardiomyocytes. However, AA and Res-treated groups reversed the expression of myocardial hypertrophic genes (ANP-two-fold, BNP- one-fold and βMHC-two-fold) when compared to Cd-exposed cells (p < 0.05). This shows that AA and Res play a significant role in the downregulation of hypertrophic genes, thereby aiding in the mitigation of myocardial hypertrophy in cardiomyocytes.
Fig. 6.
Effect of AA and Res on pathological hypertrophic modifications induced by Cd. A H9c2 cells stained with acridine orange for the quantification of length and area of cardiomyocytes. The experimental group represents control, cells exposed to Cd, and cells treated with AA and Res. B 30 cells per group were counted in each experiment and the bar diagram represents the length and area of cardiomyocytes. Scale bar corresponds to 50 µm. C Expression of pathological hypertrophic genes (ANP, BNP, and βMHC) by qPCR, and GAPDH served as housekeeping gene. All values represent mean of biological triplicates with technical duplicates. Data are represented as mean ± SD; ****p < 0.0001, **p < 0.001, *p < 0.05, ns no significance
AA and Res upregulate the expression of Nrf2 transcription factors and their downstream genes and attenuate Cd-induced myocardial hypertrophy in H9c2 cells
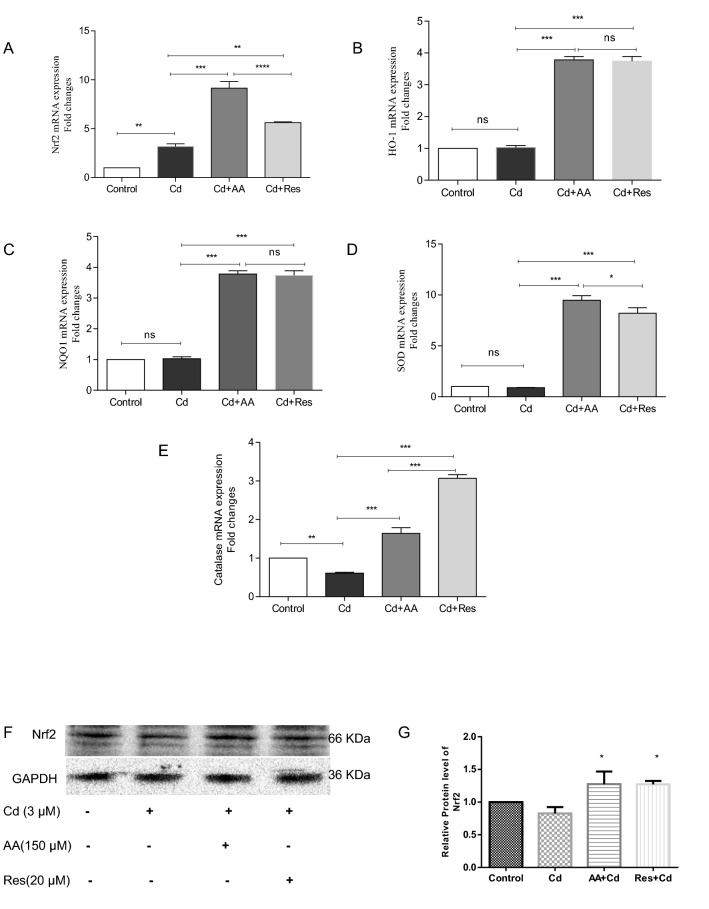
In order to find out the impact of AA and Res against Cd-induced myocardial hypertrophy, the mRNA levels of Nrf2-regulated genes such as HO-1, NQO1, SOD, and CAT was evaluated by RT-PCR. The results showed a basal level expression of Nrf2 in control, but a moderate expression of Nrf2 in Cd-exposed cardiomyocytes. However, AA-treated group showed a nine-fold increase in the expression of Nrf2 and Res-treated group a 5.6-fold increase in the expression of Nrf2 (Fig. 7A). Moreover, a significant increase (p < 0.001) in the expression of HO-1, NQO1, SOD, and CAT was observed in AA and Res-treated groups compared to other groups. Furthermore, Cd-exposed cells showed no significant difference in the expression of HO-1, NQO1, and SOD but downregulated the expression of CAT compared to control (Fig. 7B–E).
Fig. 7.
AA and Res activate Nrf2 expression in Cd-induced H9c2 cells. Relative mRNA expression of A Nrf2, B HO-1, C NQO1, D SOD, and E CAT normalized with GAPDH expression. AA and Res activate Nrf2 expression in Cd-induced H9c2 cells F Expression of Nrf2 protein was analyzed by Western blotting. Data are presented as mean ± SD. Assays are performed in triplicates. Statistically significant differences are indicated as follows: ***p < 0.001, **p < 0.01, *p < 0.05, ns no significance
Discussion
Rapid industrialization and urbanization have led to uncontrolled release of Cd into the environment and have contaminated drinking water and food materials derived from both animals and plants (Luo et al. 2021; Mohammadi et al. 2022; Portugal and Flores-Quispe 2022). Chronic exposure to Cd causes serious health issues, the primary being cancer (Genchi et al. 2020; Tarhonska et al. 2022). Of the various hazardous outcomes of chronic Cd exposure, cardiotoxicity is a serious life-threatening outcome and recently being recognized as an alarming threat to the society (Peters et al. 2010; Borné et al. 2015; Barregard et al. 2016; Chen et al. 2018). Cd molecules tend to accumulate in various parts of the body which includes organs of cardiovascular system (Tai et al. 2022). Cardiomyocytes are very sensitive to Cd and even a very low concentration of Cd can elicit a toxic effect on cardiovascular system (McMahon et al. 2003; Diaz et al. 2021). Unlike other cells, complete differentiation of myofibroblast into cardiomyocyte is a slow process, which reduces the healing ability of cardiovascular system when exposed to Cd. Cd toxicity inhibits mitochondrial function in cardiomyocytes by inactivating respiratory chain enzymes which results in the induction of ferroptosis and apoptosis (Hao et al. 2022) Additionally, Cd interact with endogenous and exogenous antioxidants and compromise the redox potential of the cells, which causes a series of complications like lipid peroxidation of membranous structures, lysosomal leakage, proteotoxicity associated ER-stress, DNA damage–associated genotoxicity, and other oxidation-associated cellular anomalies (Belyaeva et al. 2006; Zhang et al. 2019).
Nrf2-antioxidant response element pathway is a well-known cellular defense pathway against damage that is mediated by oxidative stress. Nrf2 also helps in establishing cellular homeostasis by upregulating cellular autophagy and anti-inflammatory type-2 immune response. The therapeutic potential of Nrf2 activation was well documented in toxified animal models and cell lines against pathological conditions associated with oxidative stress (Ma 2013; Uruno et al. 2020). Thus, potentiating the antioxidant response through Nrf2 activation would be a novel ideal therapeutic strategy for alleviating cardiotoxicity and multiorgan damage caused due to Cd exposure. In addition, many natural compounds are reported to induce Nrf2 pathway and alleviate oxidative stress. The cumulative benefit of Nrf2-activating natural compounds accompanied by the lack of adverse side effects made them an ideal candidate for therapeutic purposes. Therefore, based on the previous studies, this study aimed to unravel the impact of AA compared to Res (an established Nrf2-activating natural polyphenol) in upregulating Nrf2-dependent phase 2 antioxidant enzymes and their implications in H9c2 cardiomyocytes subjected to oxidative stress due to Cd exposure.
Under normal conditions, intracellular Nrf2 protein is maintained under lower concentrations by ubiquitination mediated by Keap1-Cul2 ubiquitin complex. Nrf2 has two motifs, DLGex and ETGE motifs, which exhibit different affinity toward Keap1 protein by binding to them in a lock and key fashion (Padmanabhan et al. 2006; Horie et al. 2021). Res, a natural phenol, acts as an antioxidant and protects the body against damage that results in heart diseases and cancer (Dyck et al. 2019). Res exerts its activity by destabilizing the disulfur bridges (mainly cys151) in cysteine-rich regions of Nrf-2-bound Keap-1 dimers. This in turn reduces the grip over Nrf2 DLGex and ETGE domains, and releases Nrf-2 free from Keap-1-mediated ubiquitination. A study by Yu et al. (2009) has shown that 20 µM of Res is capable of protecting cardiomyocytes from H2O2-induced apoptosis. Furthermore, AA is a water-soluble vitamin capable of actively scavenging free radicals directly from the cellular environment. AA was also shown to mediate nuclear translocation of Nrf-2 and to modulate phase 2 enzyme transactivation (Labib et al. 2022). Yet, it is not known whether AA-mediated Nrf-2 activation takes place via Keap-1 destabilization during Cd exposure.
In this study, we analyzed the protective effects of AA and Res on Cd-exposed H9c2 cells. The results showed that H9c2 cardiomyocytes exposed to Cd showed significant reduction in cell viability and increase in intracellular ROS level. The cells also exhibited elevated levels of MDA, which demonstrates high levels of cardiomyocyte damage caused by Cd through lipid peroxidation. The results of this study also showed that Cd exposure modulated inner mitochondrial membrane permeability. Similarly studies reported that Cd exerts obliteration of respiration sites by damaging Fe-S complex and leads to apoptosis (Shih et al. 2005; Cao et al. 2021). Increased cell surface area, induction of apoptosis, and re-expression of fetal genes such as ANP, BNP, and β-MHC are the important phenotypic and molecular markers for cardiac hypertrophy (Barry et al. 2008; Varshney et al. 2021). Phenotypic modification of cardiomyocytes like cell shrinkage coupled with increased surface area, rounding up, and membrane blebbing and expression of cardiac hypertrophy markers (ANP, BNP, and β-MHC) highlight the development of pathological conditions induced by Cd toxicity.
Notably, Cd is capable of directly inducing structural alteration in cysteine-rich domains of KEAP1 to free the Nrf2 from KEAP1 bound state and facilitate its nuclear translocation to mediate the expression of downstream phase 2 genes containing ARE (promotor having Nrf2-binding site), including the one that is present on nrf2-gene itself. Similarly, in this study, upregulation of Nrf2 mRNA was observed even in Cd-exposed group. This interesting observation was also documented in a previous report by He et al. (2008). But the level of Nrf2 protein expression may not correlate with its mRNA level due to excessive endoplasmic reticulum stress coupled with Cd-induced oxidative stress (Wu et al. 2021). This was exactly reflected in our experiment where the quantified Nrf2 protein level in Cd-exposed cells was found to be slightly lower than the control group. Donpunha et al. (2011) and Cheng et al. (2015) previously reported the efficacy of AA in inhibiting oxidative stress, lipid peroxidation, cell death, and also in mediating cellular antioxidant signaling (catalase activity and SOD activity) through Nrf-2 activation compared to Res. Supplementation of AA and Res was able to prevent the reduction of mitochondrial membrane potential in Cd-exposed cells. AA and Res significantly reduced the levels of cellular ROS and MDA (a by-product of membrane lipid peroxidation) in Cd-exposed cells compared to untreated cells which can be directly correlated to their beneficial effect during cardiac hypertrophy. Moreover, AA and Res greatly reduced the structural abnormalities and attenuated the hypertrophic phenotype of H9c2 cells exposed to Cd. This was evident from the regulated expression of ANP, BNP, and β-MHC and preservation of the cellular morphology back to near-normal condition. While quantification of serine phosphorylated Nrf2 (S40) from nuclear fraction could accurately describe Nrf2 activation by any polyphenol, upregulation of phase 2 enzyme expression could also reflect the status of Nrf2 signaling. Also, the potential of Res in activating Nrf2 signaling and the dependency of Nrf2 for Res-mediated antioxidant response in cardiomyocytes was already demonstrated in earlier reports involving Nrf2 knockout studies by measuring the nuclear translocated Nrf2 (S-40) and the mRNA level of phase 2 enzyme, thus easing us from the need for Nrf2-KO group and the need for quantifying Nrf2 from nuclear fraction (Wang et al. 2018; Song et al. 2020). In our study, Res was shown to upregulate the expression of Nrf2 downstream genes (NQO-1, HO-1, SOD-1, and CAT) and increase the cellular level of Nrf2 protein. AA was also shown to be highly potential compared to Res in activating Nrf2 signaling and antioxidant response to protect cells from Cd-induced oxidative stress. Similarly, Shabani et al. (2019) reported that AA improves the regeneration of embryonic stem cell-derived cardiac lineage cells injected into the ischemic myocardium and prevents early death of engrafted cells during cell therapy in the presence of severe oxidative stress.
Taken together, our results clearly indicated that AA at 150 µM (physiological concentration in plasma) induced Nrf2-dependent phase 2 enzymes and effectively exerted antioxidant response to protect Cd-exposed cells. Moreover, our experiments confirmed that significant increase in the protein level of Nrf2 and the transcriptional level of NQO-1, HO-1, SOD, and CAT by AA treatment is on par with Res treatment (15 µM). Notably, AA has the advantage of being highly bioavailable when compared to other polyphenols. Since AA can directly scavenge ROS irrespective of Nrf2 signaling, adopting AA as a better therapeutic choice over Res for ameliorating Cd toxicity is plausible.
Conclusions
Overall, our findings highlight the importance of dietary antioxidants AA and Res in preventing oxidative damage and myocardial hypertrophy caused by Cd exposure. AA and Res protect mitochondrial function and reduce apoptosis which may improve the cardiac function. AA and Res exhibited the protective effects on cardiomyocyte loss in Cd mediated myocardial hypertrophy by stabilizing Nrf2 signaling and restoring the depleted antioxidant enzymes. This study provides evidence that AA activates Nrf2 besides scavenging free radicles directly and its supplementation could reestablish the failing heart’s function. Further this study suggests that targeting Nrf2 could provide novel therapeutic strategy to treat Cd-induced adverse vascular and cardiac complications.
Acknowledgements
S. Sasikumar expresses his sincere thanks to University Grants Commission (UGC), New Delhi, India for providing Rajiv Gandhi National Fellowship (RGNF). The authors acknowledge UGC-CEGS, UGC-CAS, UGC-NRCBS, DST-FIST, and DST-PURSE program for the central instrumentation facility at SBS, MKU. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
- Cd
Cadmium
- AA
Ascorbic Acid
- Res
Resveratrol
- CVD
Cardiovascular disease
- ROS
Reactive oxygen species
- MDA
Malondialdehyde
- ΔΨm
Mitochondrial transmembrane potential
- Nrf2
Nuclear erythroid-2 like factor-2
- ARE
Antioxidant Response Element
- NQO1
NAD(P)H dehydrogenase quinone 1
- HO-1
Heme oxygenase-1
- CAT
Catalase
- SOD
Superoxide dismutase
- GPx
Glutathione peroxidase
- ANP
Atrial natriuretic peptide
- BNP
Brain natriuretic peptide
- β-MHC
β-Myosin heavy chain
- GAPDH
Glyceraldehyde 3 Phosphate dehydrogenase
Author contributions
SSK and GSS conceived and designed the experiments; SSK, SY performed the experiments; SS, AKP, SSK, JSGC and GSS analyzed the data; SSK wrote the manuscript: SSK, VP, NT and GSS revised the manuscript. All authors read and approved the final manuscript.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Sundaresan Sasikumar, Email: sasigene@gmail.com.
Subramani Yuvraj, Email: yuvaraj.biochem@gmail.com.
Pattapulavar Veilumuthu, Email: veilumuthu.p@vit.ac.in.
John Samuel Godwin Christopher, Email: godwinj@vit.ac.in.
Purushothaman Anandkumar, Email: anandkumarbiogene@gmail.com.
Tamilmaran Nagarajan, Email: nagarajantamilmaran@gmail.com.
Selvaraj Sureshkumar, Email: Sureshss85@gmail.com.
Govindan Sadasivam Selvam, Email: drselvamgsbiochem@rediffmail.com.
References
- Atsdr (2012) Toxicological Profile For Cadmium. https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf [PubMed]
- Bajaj NS, Singh A, Zhou W, et al. Coronary microvascular dysfunction, left ventricular remodeling, and clinical outcomes in patients with chronic kidney impairment. Circulation. 2020;141:21–33. doi: 10.1161/CIRCULATIONAHA.119.043916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barregard L, Sallsten G, Fagerberg B, et al. Blood cadmium levels and incident cardiovascular events during follow-up in a population-based cohort of Swedish Adults: the Malmö Diet and cancer study. Environ Health Perspect. 2016;124:594–600. doi: 10.1289/EHP.1509735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barregard L, Sallsten G, Harari F, et al. Cadmium exposure and coronary artery atherosclerosis: a cross-sectional population-based study of swedish middle-aged adults. Environ Health Perspect. 2021;129:067007. doi: 10.1289/EHP8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry SP, Davidson SM, Townsend PA. Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol. 2008;40:2023–2039. doi: 10.1016/j.biocel.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Belyaeva EA, Dymkowska D, Wieckowski MR, Wojtczak L. Reactive oxygen species produced by the mitochondrial respiratory chain are involved in Cd2+-induced injury of rat ascites hepatoma AS-30D cells. Biochim Biophys Acta. 2006;1757:1568–1574. doi: 10.1016/J.BBABIO.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Borné Y, Barregard L, Persson M, et al. Cadmium exposure and incidence of heart failure and atrial fibrillation: a population-based prospective cohort study. BMJ Open. 2015;5:7366. doi: 10.1136/bmjopen-2014-007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Fu M, Bi R, et al. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere. 2021 doi: 10.1016/J.CHEMOSPHERE.2020.128346. [DOI] [PubMed] [Google Scholar]
- Chen C, Xun P, Tsinovoi C, et al. Urinary cadmium concentration and the risk of ischemic stroke. Neurology. 2018;91:e382. doi: 10.1212/WNL.0000000000005856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Jin Z, Zhao R, et al. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: Role of Nrf2/ARE pathway. Int J Clin Exp Med. 2015;8:10420–10428. [PMC free article] [PubMed] [Google Scholar]
- Collins BJ, Mukherjee MS, Miller MD, Delaney CL. Effect of dietary or supplemental vitamin C intake on vitamin C levels in patients with and without cardiovascular disease: a systematic review. Nutrients. 2021 doi: 10.3390/NU13072330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox EJ, Marsh SA. A systematic review of fetal genes as biomarkers of cardiac hypertrophy in rodent models of diabetes. PLoS ONE. 2014;9:e92903. doi: 10.1371/journal.pone.0092903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers A, Plusquin M, Remans T, et al. Cadmium stress: an oxidative challenge. Biometals. 2010;23:927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- Diaz D, Ujueta F, Mansur G, et al. Low-level cadmium exposure and atherosclerosis. Curr Environ Heal Reports. 2021;8:42–53. doi: 10.1007/S40572-021-00304-W. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Dyck JRB. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim Biophys Acta - Mol Basis Dis. 2011;1812:1477–1489. doi: 10.1016/j.bbadis.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Donpunha W, Kukongviriyapan U, Sompamit K, et al. Protective effect of ascorbic acid on cadmium-induced hypertension and vascular dysfunction in mice. Biometals. 2011;24:105–115. doi: 10.1007/s10534-010-9379-0. [DOI] [PubMed] [Google Scholar]
- Dyck GJB, Raj P, Zieroth S, et al. The effects of resveratrol in patients with cardiovascular disease and heart failure: a narrative review. Int J Mol Sci. 2019 doi: 10.3390/IJMS20040904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eybl V, Kotyzova D, Koutensky J. Comparative study of natural antioxidants - curcumin, resveratrol and melatonin - in cadmium-induced oxidative damage in mice. Toxicology. 2006;225:150–156. doi: 10.1016/j.tox.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Ferramola ML, Pérez Díaz MFF, Honoré SM, et al. Cadmium-induced oxidative stress and histological damage in the myocardium. Effects of a soy-based diet. Toxicol Appl Pharmacol. 2012;265:380–389. doi: 10.1016/j.taap.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Fornasiero MC, Isetta AM. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods. 1990;131:165–172. doi: 10.1016/0022-1759(90)90187-Z. [DOI] [PubMed] [Google Scholar]
- Genchi G, Sinicropi MS, Lauria G, et al. The effects of cadmium toxicity. Int J Environ Res Public Health. 2020 doi: 10.3390/IJERPH17113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goszcz K, Deakin SJ, Duthie GG, et al. Antioxidants in cardiovascular therapy: panacea or false hope? Front Cardiovasc Med. 2015;2:6. doi: 10.3389/FCVM.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao R, Ge J, Song X, et al. Cadmium induces ferroptosis and apoptosis by modulating miR-34a-5p/Sirt1axis in PC12 cells. Environ Toxicol. 2022;37:41–51. doi: 10.1002/TOX.23376. [DOI] [PubMed] [Google Scholar]
- He X, Chen MG, Ma Q. Activation of Nrf2 in defense against cadmium-induced oxidative stress. Chem Res Toxicol. 2008;21:1375–1383. doi: 10.1021/tx800019a. [DOI] [PubMed] [Google Scholar]
- Horie Y, Suzuki T, Inoue J, et al. Molecular basis for the disruption of Keap1-Nrf2 interaction via Hinge & Latch mechanism. Commun Biol. 2021 doi: 10.1038/S42003-021-02100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Nguyen V, Coder D. Assessment of cell viability. Curr Protoc Cytom. 2013 doi: 10.1002/0471142956.cy0902s64. [DOI] [PubMed] [Google Scholar]
- Kim SR, Ha YM, Kim YM, et al. Ascorbic acid reduces HMGB1 secretion in lipopolysaccharide-activated RAW 264.7 cells and improves survival rate in septic mice by activation of Nrf2/HO-1 signals. Biochem Pharmacol. 2015;95:279–289. doi: 10.1016/j.bcp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Kleszczewski T, Kleszczewska E, Buzun L, Modzelewska B. Levels of l-ascorbic acid and cadmium in the saphenous vein of patients with coronary artery disease are negatively correlated. J Trace Elem Med Biol. 2016;36:22–26. doi: 10.1016/j.jtemb.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–195. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- Labib H, Badr AM, Abdelgwad M, Abd El-Galil TI. Keap1/Nrf2 pathway in sodium fluoride-induced cardiac toxicity and the prophylactic role of vitamin C versus platelet-rich plasma. Folia Morphol (warsz) 2022;81:663–678. doi: 10.5603/FM.A2021.0053. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/METH.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo L, Wang B, Jiang J, et al. Heavy metal contaminations in herbal medicines: determination, comprehensive risk assessments, and solutions. Front Pharmacol. 2021;11:2016. doi: 10.3389/FPHAR.2020.595335/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/ANNUREV-PHARMTOX-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- Mohammadi S, Shafiee M, Faraji SN, et al. (2022) Contamination of breast milk with lead, mercury, arsenic, and cadmium in Iran: a systematic review and meta-analysis. Biometals. 2022;354(35):711–728. doi: 10.1007/S10534-022-00395-4. [DOI] [PubMed] [Google Scholar]
- Moser MA, Chun OK. Vitamin C and heart health: A review based on findings from epidemiologic studies. Int J Mol Sci. 2016 doi: 10.3390/ijms17081328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi-Pour Z, Ramezani F, Keshavarzi F, Samadi N. The role of quercetin and vitamin c in NRF2-dependent oxidative stress production in breast cancer cells. Oncol Lett. 2017;13:1965–1973. doi: 10.3892/OL.2017.5619/HTML. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nazimabashir MV, Miltonprabu S. Cadmium induced cardiac oxidative stress in rats and its attenuation by GSP through the activation of Nrf2 signaling pathway. Chem Biol Interact. 2015;242:179–193. doi: 10.1016/j.cbi.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Padmanabhan B, Tong KI, Ohta T, et al. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/J.MOLCEL.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Peters JL, Perlstein TS, Perry MJ, et al. Cadmium exposure in association with history of stroke and heart failure. Environ Res. 2010;110:199–206. doi: 10.1016/j.envres.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal ÓB, Flores-Quispe M. Concentrations of arsenic, cadmium, and lead in herbal infusion tea bags marketed in Tacna, Peru. Environ Monit Assess. 2022;194:534. doi: 10.1007/S10661-022-10232-3. [DOI] [PubMed] [Google Scholar]
- Ramasamy S, Velmurugan G, Rekha B, et al. Egr-1 mediated cardiac miR-99 family expression diverges physiological hypertrophy from pathological hypertrophy. Exp Cell Res. 2018;365:46–56. doi: 10.1016/j.yexcr.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Samuel JB, Stanley JA, Princess RA, et al. Gestational cadmium exposure-induced ovotoxicity delays puberty through oxidative stress and impaired steroid hormone levels. J Med Toxicol. 2011;7:195. doi: 10.1007/S13181-011-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani P, Ghazizadeh Z, Gorgani-Firuzjaee S, et al. Cardioprotective effects of omega-3 fatty acids and ascorbic acid improve regenerative capacity of embryonic stem cell-derived cardiac lineage cells. BioFactors. 2019;45:427–438. doi: 10.1002/biof.1501. [DOI] [PubMed] [Google Scholar]
- Shih YL, Lin CJ, Hsu SW, et al. Cadmium toxicity toward caspase-independent apoptosis through the mitochondria-calcium pathway in mtDNA-depleted cells. Ann NY Acad Sci. 2005 doi: 10.1196/annals.1338.043. [DOI] [PubMed] [Google Scholar]
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Song YJ, Bin ZC, Wu W. Resveratrol and diabetic cardiomyopathy: focusing on the protective signaling mechanisms. Oxid Med Cell Longev. 2020 doi: 10.1155/2020/7051845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan S, John S, Paneerselvam G, Andiapppan R, Christopher G, Selvam GS. Gallic acid attenuates cadmium mediated cardiac hypertrophic remodelling through upregulation of Nrf2 and PECAM-1signaling in rats. Environ Toxicol Pharmacol. 2021;87:103701. doi: 10.1016/j.etap.2021.103701. [DOI] [PubMed] [Google Scholar]
- Tai YT, Chou SH, Cheng CY, et al. The preferential accumulation of cadmium ions among various tissues in mice. Toxicol Rep. 2022;9:111–119. doi: 10.1016/J.TOXREP.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarhonska K, Lesicka M, Janasik B, et al. Cadmium and breast cancer – current state and research gaps in the underlying mechanisms. Toxicol Lett. 2022;361:29–42. doi: 10.1016/J.TOXLET.2022.03.003. [DOI] [PubMed] [Google Scholar]
- Uruno A, Matsumaru D, Ryoke R, et al. Nrf2 suppresses oxidative stress and inflammation in App Knock-In Alzheimer’s disease model mice. Mol Cell Biol. 2020 doi: 10.1128/MCB.00467-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney R, Ranjit R, Chiao YA, et al. Myocardial hypertrophy and compensatory increase in systolic function in a mouse model of oxidative stress. Int J Mol Sci. 2021;22:1–15. doi: 10.3390/IJMS22042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Song X, Zhao L, et al. Resveratrol prevents diabetic cardiomyopathy by increasing Nrf2 expression and transcriptional activity. Biomed Res Int. 2018 doi: 10.1155/2018/2150218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao X, Gao X, et al. Development of fluorescence imaging-based assay for screening cardioprotective compounds from medicinal plants. Anal Chim Acta. 2011;702:87–94. doi: 10.1016/j.aca.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Wu H, Zheng S, Zhang J, et al. Cadmium induces endoplasmic reticulum stress-mediated apoptosis in pig pancreas via the increase of Th1 cells. Toxicology. 2021;457:152790. doi: 10.1016/J.TOX.2021.152790. [DOI] [PubMed] [Google Scholar]
- Yu W, Fu YC, Zhou XH, et al. Effects of resveratrol on H2O2-induced apoptosis and expression of SIRTs in H9c2 cells. J Cell Biochem. 2009;107:741–747. doi: 10.1002/JCB.22169. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hao S, Qiu Z, et al. Cadmium disrupts the DNA damage response by destabilizing RNF168. Food Chem Toxicol. 2019 doi: 10.1016/J.FCT.2019.110745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.