Abstract
Introduction
Complete resection is essential for the treatment of teratoma with malignant transformation, and if metastasis occurs, it will be difficult to cure. We report a case of primary mediastinal teratoma with differentiation into angiosarcoma that caused bone metastases but was cured by multidisciplinary treatment.
Case presentation
A 31‐year‐old man with a primary mediastinal germ cell tumor underwent primary chemotherapy followed by post‐chemotherapy resection, with angiosarcoma due to malignant transformation found in the surgical specimen. Femoral diaphyseal metastasis was manifested, and he underwent femur curettage followed by radiation therapy of 60 Gy in parallel with 4 cycles of chemotherapy combining gemcitabine and docetaxel. Although thoracic vertebral bone metastasis emerged 5 months after treatment, intensity‐modulated radiation therapy was successful, and metastatic lesions have remained shrunken for 39 months after treatment.
Conclusion
Even if complete resection is difficult, teratoma with malignant transformation may be cured by multidisciplinary treatment based on histopathology.
Keywords: angiosarcoma, chemotherapy, extragonadal germ cell tumor, malignant transformation, radiation therapy
Abbreviations & Acronyms
- AFP
α‐fetoprotein
- BEP
bleomycin, etoposide, and cisplatin
- CR
complete response
- CT
computed tomography
- FDG
18F‐fluorodeoxyglucose
- GCT
germ cell tumor
- HCG
human chorionic gonadotropin
- IMRT
intensity‐modulated radiation therapy
- LDH
lactate dehydrogenase
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- PR
partial response
- STS
soft tissue sarcoma
- TIN
paclitaxel, ifosfamide and nedaplatin
- TMT
teratoma with malignant transformation
Keynote message.
TMT is rare, and complete surgical resection is essential for its cure. 1 , 2 However, even if such resection is difficult, TMT may be effectively treated by multidisciplinary treatment based on the pathological diagnosis.
Introduction
We report a case of primary mediastinal GCT with TMT in the surgical specimen resected during post‐chemotherapy surgery and the occurrence of metachronous bone metastases, which were effectively treated by chemotherapy and radiation therapy.
Case presentation
A 31‐year‐old man visited a hospital complaining of anterior chest discomfort. CT revealed an anterior mediastinal tumor (diameter, 70 mm), and multiple lung metastases (Fig. 1a,b). A thoracoscopic biopsy was performed and a pathological examination showed teratoma. Serum concentrations of AFP, total HCG and LDH were elevated (849 ng/mL, 26548 mIU/mL, and 314 IU/L, respectively). Four cycles of BEP followed by two cycles of TIN were administered, confirming that the tumor marker level was normalized. CT showed a 6% reduction in the mediastinal tumors and a 35% reduction in the lung metastases (Fig. 1). One month after chemotherapy, residual mediastinal tumor resection and partial lung resection were performed. The histopathological findings showed teratoma with a slight angiosarcoma component in the mediastinal tumor and no viable cancer in the lung (Fig. 2a,b).
Fig. 1.
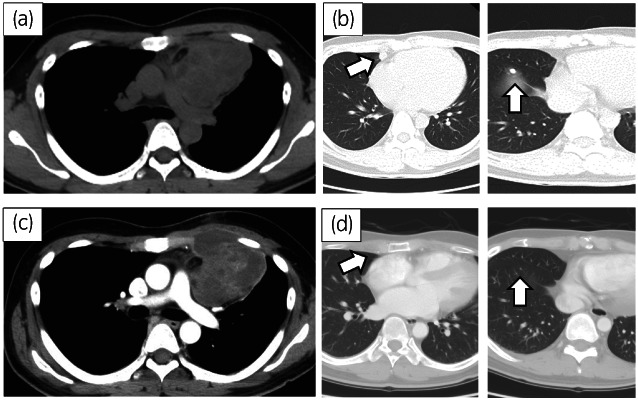
CT showed mediastinal tumor (a) and multiple lung metastases (b, arrow). After 4 cycles of BEP and following 2 cycles of TIN, there was a slight reduction in the mediastinal tumor (c) and a marked reduction in lung metastases (d, arrow).
Fig. 2.
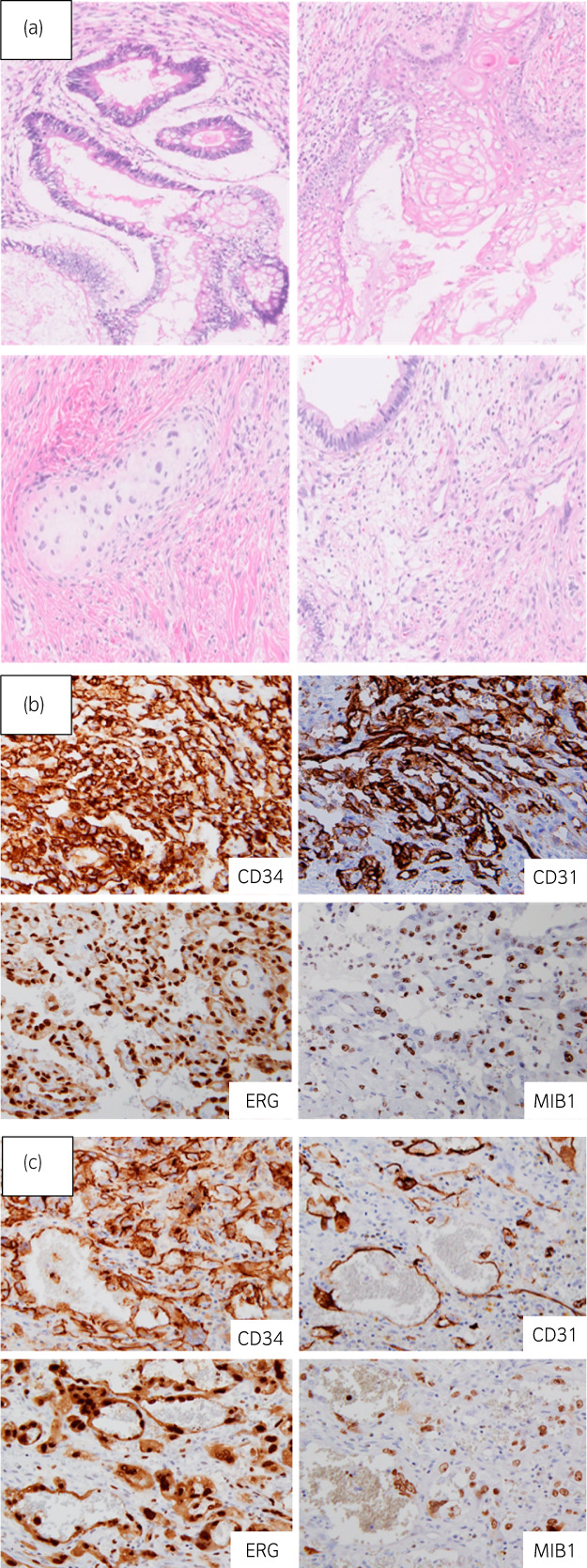
In the mediastinal tumor, tissues containing epithelial and mesenchymal components were found (a), and the diagnosis of teratoma was made. There were heterozygous cells with a cleft‐like structure in a very small area, and immunostaining was strongly positive for CD31, CD34, and ERG (b), and angiosarcoma was diagnosed. The histopathological findings of the femur also showed angiosarcoma (c).
One month after surgery, the patient developed left lower limb pain due to a left intrafemoral tumor (Fig. 3a). Curettage of the left femoral tumor was performed, and a pathological examination showed angiosarcoma. We proposed total hip arthroplasty in addition to femoral tumor resection, but the treatment was refused due to poor patient tolerance. He, therefore, received radiation therapy of 60 Gy for the intrafemoral tumor in parallel with 4 cycles of chemotherapy consisting of gemcitabine (450 mg/m2 on days 1 and 8) and docetaxel (70 mg/m2 on day 8). After chemotherapy, abnormal intrafemoral FDG uptake disappeared (Fig. 3b). Five months later, metastasis to the 4th thoracic vertebra appeared without elevated tumor markers (Fig. 3c). He refused a tumor biopsy and underwent IMRT of 60.2 Gy for the vertebral metastasis, which shrank it (Fig. 3d). The metastatic lesions have remained shrunken for 39 months after treatment.
Fig. 3.
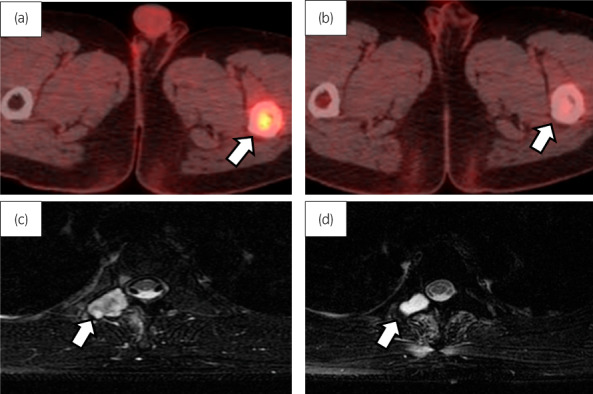
Imaging findings of the metastatic tumor. PET/CT fusion images showed abnormal FDG uptake in the left thigh bone that declined after chemotherapy and radiotherapy (b). T2WI MRI showed the 4th thoracic vertebral metastasis (c), which shrank after IMRT (d).
Discussion
GCTs frequently arise in the testes, but a small subset of 2–5% arises extragonadally. 3 Even rarer is a GCT with somatic malignant transformation, occurring in around 2.2–4.1% of all GCTs in males. Malignant transformation is regarded as the dedifferentiation of a component of the teratoma, with overgrowth or invasion. 4 Primary mediastinal GCT is more prone to malignant transformation than primary testicular and retroperitoneal GCT. 5 Both epithelial and sarcomatous malignancies can arise in a GCT. Malignant transformation into the sarcoma component results most commonly in rhabdomyosarcoma, followed by angiosarcoma. 6 TMT has a worse prognosis than that of GCT. Although complete resection has a pivotal role in a better prognosis, an optimal chemotherapy regimen remains to be established. 1
Angiosarcoma is a rare malignant soft tissue neoplasm of the vascular endothelium, accounting for only 1% of all STS. 7 It can arise anywhere in the body and has a poor prognosis. 8 Surgery, if possible, is the primary treatment for angiosarcoma, and the role of adjuvant chemotherapy is unclear. 9 In the present case, the primary site of angiosarcoma was a small region in the mediastinal tumor, which was assumed to have metastasized to the femoral bone.
Generally, the residual tumor should be resected as surgically as possible in the GCT therapy setting, but our patient refused surgical resection due to poor tolerance. Although metastatic angiosarcoma is incurable in most cases as no chemotherapy regimen supported by randomized trials exists, we successfully treated this patient with multidisciplinary treatment including a cytotoxic agent. 9 Generally, anthracycline‐based chemotherapy is often administered as a first‐line treatment for STS, but its therapeutic effect on angiosarcoma is poor. 8 However, taxanes have antiangiogenic activity and are therefore of particular interest in the management of angiosarcoma. Recently, the efficacy of taxane‐based chemotherapy has been shown for the treatment of angiosarcoma. 8 The efficacy of gemcitabine for metastatic angiosarcoma has also been noted. In a retrospective study of 25 cases, Stacchiotti et al. reported an overall response rate of 68%, median overall survival of 17 months, and median progression‐free survival of 7 months. 10 Combination therapy of gemcitabine and docetaxel is a typical second‐line regimen in the management of advanced STS. There have been no case series studies of this combination therapy for metastatic angiosarcoma. However, a few reports have shown efficacy. 11 , 12 , 13 In a retrospective study involving 4 cases of angiosarcoma among 35 cases of STS, Leu et al. showed CR in 2 of the 4 cases and PR in 1 of them with combination therapy of gemcitabine and docetaxel. 11 The present patient was found to have poorly tolerated previous chemotherapy, so we administered a reduced dose of gemcitabine and docetaxel. The combination therapy of gemcitabine and docetaxel may be considered one of the multidisciplinary treatments for TMT pathologically diagnosed as angiosarcoma.
Recently, the efficacy of concurrent chemoradiation using taxanes as a treatment for localized angiosarcoma with or without radical resection was reported. 14 , 15 In the present patient, it was difficult to perform complete resection of the metastatic lesion. However, as it was a solitary metastasis, we considered that the administration of radiation in addition to chemotherapy contributed to disease control. IMRT was also effective for the subsequent vertebral metastasis because it was a solitary metastasis.
In conclusion, we described a case of mediastinal TMT transforming into angiosarcoma occurring as a bone metastasis effectively treated by multidisciplinary treatment. Conventionally, TMT has been considered to have no effective treatment other than surgical resection, but treatment based on pathological diagnosis might be effective.
Author contributions
Masaru Tani: Visualization; writing – original draft. Akira Nagahara: Visualization; writing – review and editing. Shingo Takada: Investigation. Kazutoshi Fujita: Investigation; supervision. Shinichiro Fukuhara: Supervision. Motohide Uemura: Supervision. Hiroshi Kiuchi: Supervision. Ryoichi Imamura: Supervision. Norio Nonomura: Supervision.
Conflict of interest
The authors declare no conflict of interest.
Approval of the research protocol by an Institutional Reviewer Board
Not applicable
Informed consent
Written informed consent was obtained.
Registry and the Registration No. of the study/trial
Not applicable
References
- 1. Giannatempo P, Pond GR, Sonpavde G et al. Treatment and clinical outcomes of patients with teratoma with somatic‐type malignant transformation: an international collaboration. J. Urol. 2016; 196: 95–100. [DOI] [PubMed] [Google Scholar]
- 2. Daneshmand S. Role of surgical resection for refractory germ cell tumors. Urol. Oncol. Semin. Orig. Investig. 2015; 33: 370–8. [DOI] [PubMed] [Google Scholar]
- 3. Schmoll HJ. Extragonadal germ cell tumors. Ann. Oncol. 2002; 13: 265–72. [DOI] [PubMed] [Google Scholar]
- 4. Idrees MT, Kuhar M, Ulbright TM et al. Clonal evidence for the progression of a testicular germ cell tumor to angiosarcoma. Hum. Pathol. 2010; 41: 139–44. [DOI] [PubMed] [Google Scholar]
- 5. Mustafa OM, Mohammed SF, Aljubran A, Saleh WN. Immature mediastinal teratoma with unusual histopathology: a case report of multi‐lineage, somatic‐type malignant transformation and a review of the literature. Medicine 2016; 95: e3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malagón HD, Valdez AMC, Moran CA, Suster S. Germ cell tumors with sarcomatous components: a clinicopathologic and immunohistochemical study of 46 cases. Am. J. Surg. Pathol. 2007; 31: 1356–62. [DOI] [PubMed] [Google Scholar]
- 7. Guadagnolo BA, Zagars GK, Araujo D, Ravi V, Shellenberger TD, Sturgis EM. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck 2011; 33: 661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010; 11: 983–91. [DOI] [PubMed] [Google Scholar]
- 9. Cioffi A, Reichert S, Antonescu CR, Maki RG. Angiosarcomas and other sarcomas of endothelial origin. Hematol. Oncol. Clin. North Am. 2013; 27: 975–88. [DOI] [PubMed] [Google Scholar]
- 10. Stacchiotti S, Palassini E, Sanfilippo R et al. Gemcitabine in advanced angiosarcoma: a retrospective case series analysis from the Italian rare cancer network. Ann. Oncol. 2012; 23: 501–8. [DOI] [PubMed] [Google Scholar]
- 11. Leu KM, Ostruszka LJ, Shewach D et al. Laboratory and clinical evidence of synergistic cytotoxicity of sequential treament with gemcitabine followed by docetaxel in the treatment of sarcoma. J. Clin. Oncol. 2004; 22: 1706–12. [DOI] [PubMed] [Google Scholar]
- 12. Wilson R, Glaros S, Brown RK, Michael C, Reisman D. Complete radiographic response of primary pulmonary angiosarcomas following gemcitabine and taxotere. Lung Cancer 2008; 61: 131–6. [DOI] [PubMed] [Google Scholar]
- 13. Shkoukani MA, Carron MA, Tulunay O, Kucuk O, Lin HS. Angiosarcoma of the scalp with complete response to a biweekly gemcitabine and docetaxel (GEMDOC) chemotherapy regimen. Ear Nose Throat J. 2011; 90: E26–9. [DOI] [PubMed] [Google Scholar]
- 14. Ihara H, Kaji T, Katsui K et al. Single institutional experience of radiation therapy for angiosarcoma of the scalp without cervical lymph node metastases: impact of concurrent chemoradiation with maintenance chemotherapy using taxanes on patient prognosis. Mol. Clin. Oncol. 2019; 11: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roy A, Gabani P, Davis EJ et al. Concurrent paclitaxel and radiation therapy for the treatment of cutaneous angiosarcoma. Clin. Transl. Radiat. Oncol. 2021; 27: 114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


