Abstract
Animal models of podocytopathy and chronic kidney diseases (CKD) help elucidate these pathologies. Adriamycin (ADR)-induced nephropathy is a common rodent model of podocytopathy. BALB/c mice are sensitive to ADR, whereas C57BL/6 (B6) mice, the most commonly used strain, are resistant to ADR. Therefore, mouse strains with the B6 genetic background cannot be used as an ADR nephropathy model. We previously generated DNA-dependent protein kinase catalytic subunit (Prkdc) mutant B6 mice (B6-PrkdcR2140C) carrying the R2140C mutation that causes ADR nephropathy. However, whether ADR nephropathy in the novel strain progresses to CKD after ADR administration has not been evaluated. Therefore, we examined whether the B6-PrkdcR2140C mice develop CKD after ADR administration. We also evaluated whether differences existed in the genetic background in ADR nephropathy by comparing the B6-PrkdcR2140C mice with BALB/c mice. Our findings demonstrated that B6-PrkdcR2140C progresses to CKD and is resistant to nephropathy compared with the BALB/c mice. The B6-PrkdcR2140C and BALB/c mice differed in the expression of genes related to inflammatory mediators, and further analysis is required to identify factors that contribute to resistance to nephropathy.
Keywords: adriamycin, chronic kidney diseases, genetic background, mouse, nephropathy
Introduction
An increase in the number of patients with chronic kidney disease (CKD) and the number of patients requiring dialysis worldwide burdens the healthcare budgets of many countries. More than 10% adults in developed countries have CKD, and many of these CKD patients have podocytopathy, which is attributable to a primary disease such as diabetes or hypertension [1]. Podocytopathy may be the main driver of focal segmental glomerulosclerosis, a major glomerular disease that progresses to end-stage renal failure [2]. Podocyte damage and dysfunction lead to glomerulosclerosis, proteinuria, and renal dysfunction [3]. Therefore, podocyte-associated molecules related to kidney disease have been extensively studied. Adriamycin (ADR)-induced nephropathy is a highly reproducible rodent model of podocytopathy; a single ADR injection induces podocyte reduction, persistent proteinuria, focal glomerulosclerosis, and renal fibrosis [4, 5]. Among mouse inbred strains used as disease models, only BALB/c and 129/SvJ mice are susceptible to ADR nephropathy, while the C57BL/6J (B6J) strain, the gold standard in many studies, is resistant [6, 7]. Therefore, in our previous study [8], we generated a B6-PrkdcR2140C mouse strain containing the R2140C mutation in DNA-dependent protein kinase catalytic subunit (Prkdc). This mutation is believed to be responsible for ADR nephropathy susceptibility in BALB/c. Prkdc encodes the catalytic subunit of the trimeric DNA-dependent protein kinase (DNA-PKcs) and plays vital roles in maintenance of the mitochondrial genome, DNA repair, signal transduction, and transcriptional activation [9, 10]. ADR administration causes mitochondrial DNA damage in podocytes, and Prkdc mutation results in nephropathy susceptibility due to the inability to repair damaged mitochondrial DNA [10].
The mutant strain developed similar levels of ADR susceptibility and acute kidney injury (AKI) due to glomerular disorders as the BALB/c mice [11]. However, we had not tested whether the novel strain B6-PrkdcR2140C would progress to CKD after ADR administration. In addition, BALB/c and B6J mice have been reported to differ in various phenotypes, but no known genetic background-dependent diversity exists in renal disease, particularly ADR nephropathy. Therefore, in the present study, we tested whether B6-PrkdcR2140C mice develop CKD after ADR administration and whether differences exist in diverse genetic backgrounds in ADR nephropathy by comparing the results with those of the BALB/c mice.
Materials and Methods
Ethics statement
All animal experiments complied with the ARRIVE guidelines and were conducted according to the Act on Welfare and Management of Animals in Japan. The research was conducted according to the Regulations for the Care and Use of Laboratory Animals of Kitasato University. The President of Kitasato University approved the animal experimentation protocol on the basis of the judgment made by the Institutional Animal Care and Use Committee of Kitasato University (Approval ID: 19-163). A humane endpoint was applied when the mice with severe anemia became moribund.
Animals
B6-PrkdcR2140C mice were generated as described previously [8]. Male 8-week-old mice were used for all experiments. BALB/c mice were purchased from CLEA Japan (Tokyo, Japan), acclimatized for 2 weeks, and then used in the experiments. The animal facility was air-conditioned at 22 ± 2°C and maintained at 40–60% relative humidity. The mice were maintained under a 12-h light/dark cycle. A standard laboratory diet, CE-2 (CLEA Japan), and tap water were available ad libitum.
Blood sampling and blood test
ADR (doxorubicin hydrochloride, Fujifilm Wako Pure Chemical Corp., Osaka, Japan) was injected via the tail vein at a 13 mg/kg concentration according to the standard protocol [8]. The control group received the same volume of saline. At 28 days after ADR administration, the mice were anesthetized intraperitoneally by administering a triad of mixed anesthesia (0.5 mg/kg medetomidine, 30 mg/kg alphaxalone, and 5.0 mg/kg butorphanol) [11]. Then, the abdomen was opened, and 500–1,000 µl of blood was collected from the caudal vena cava using a syringe. The blood was centrifuged at 1,700 × g for 15 min to obtain the supernatant. The supernatant was centrifuged at 1,700 × g for 15 min. Serum blood urea nitrogen (BUN) and creatinine (Cre) levels were measured using a Hitachi 7180 automatic analyzer (Hitachi, Yokohama, Japan).
Measurement of urinary albumin
Urine samples were collected from the mice 28 days after administering 13 mg/kg ADR. Albumin was measured in the urine samples diluted with sample buffer containing 2% sodium dodecyl sulfate (SDS), 5% 2-mercaptoethanol, 10% glycerol, 60 mM Tris-HCl (pH 6.8), and bromophenol blue and then heated at 95°C for 5 min. Samples containing 1 µl of urine were applied to 10% SDS-polyacrylamide gel electrophoresis. As a positive control, 10 µg of bovine serum albumin was loaded simultaneously. The gel was fixed and stained with Coomassie brilliant blue (CBB, Nacalai Tesque, Kyoto, Japan) according to the manufacturer’s instructions and scanned using a standard commercial scanner. The CBB-stained band corresponding to urinary albumin was quantified using ImageJ software (https://imagej.nih.gov/ij/, National Institutes of Health, Bethesda, MD, USA). Urinary Cre was measured using a Cre colorimetric assay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions. Urinary albumin excretion was normalized against urinary Cre excretion.
Histology
Kidneys were collected from the mice on day 28 after ADR administration and were fixed with 4% paraformaldehyde (PFA) at 4°C overnight. The 2-µm PFA-fixed paraffin sections were subjected to normal histological processes and stained with periodic acid-Schiff (PAS) solution. Glomerular injury (GI) was scored by average score of randomly selected 50 glomeruli per individual according to the following criteria: 0, no disability; 1, mild disability; 2, segmental glomerulosclerosis; and 3, global glomerulosclerosis. Tubular injury (TI) was scored by grading the percentage of affected proximal tubules per 10 randomly chosen, non-overlapping fields (magnification, 100×) according to the following criteria: tubular dilation, loss of brush border, tubular necrosis, and cast formation. The tubular injury score was estimated on a scale from 0 to 5: 0, none; 1, 0–10%; 2, 11–25%; 3, 26–45%; 4, 46–75% and 5, 76–100% [12]. For Picrosirius red staining, slides were deparaffinized, stained 1 h with Picrosirius red in saturated aqueous picric acid, and washed with 0.5% acetic acid. To score fibrosis areas through Picrosirius red staining, the target areas were photographed randomly in eight views of renal cortex (100×), and the positive areas were quantified using ImageJ to calculate their occupancy rate with respect to the total area. Only areas in the tubulointerstitium that stained red with Picrosirius red were determined to be picrosirius-positive fibrosis areas.
Immunohistochemistry for α-SMA
Kidney sections (5 µm) were deparaffinized and subjected to antigen retrieval in citrate buffer at 121°C for 15 min. After washing with Tris-buffered saline (TBS), the sections were immersed in 0.3% hydrogen peroxide/methanol for 20 min and then incubated overnight at 4°C with anti-α-SMA antibody (ab5694, Abcam, Cambridge, UK). The sections were washed with TBS and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Histofine SimpleStain MAX PO rabbit, Nichirei Biosciences, Tokyo, Japan) for 30 min at room temperature. After washing the sections with TBS, they were incubated with 3,3′-diaminobenzidine (Fujifilm Wako Pure Chemical Corp., Osaka, Japan). Counter staining was then performed through hematoxylin staining.
Western blot analysis
Western blot analysis was performed as previously described [13]. The primary antibodies used were anti-α-SMA and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (HRP-60004, Proteintech, Rosemont, IL, USA). The secondary antibody used was HRP-conjugated anti-rabbit IgG (Cell signaling Technology, Danvers, MA, USA). After incubation with ECL Prime detection reagent (GE Healthcare Chicago, IL, USA), the blots were imaged using an Omega Lum C imaging system (Gel Co., San Francisco, CA, USA). The band intensity of western blot was measured using ImageJ software.
Quantitative reverse transcription-PCR (qRT-PCR)
Total RNA was extracted from whole kidney tissue by using TRI Reagent (Molecular Research Center, OH, USA) according to the manufacturer’s instructions. cDNA was synthesized from the RNA using ReverTra Ace (Toyobo, Osaka, Japan). qRT-PCR was performed using Thunderbird SYBR qPCR Mix (Toyobo) according to the manufacturer’s instructions. The reaction was analyzed using CFX Maestro (Bio-Rad Laboratories Inc., Hercules, CA, USA). Primer sequences used were as follows: Nuclear factor κB (Nfkb1) forward, 5′-CCAGAAGAGGGTGTCAGAGC-3′and Nfkb1 reverse, 5′-ACATTTGCCCAGTTCCGTAG-3′, Transforming growth factor-β1 (Tgfb1) forward, 5′-TGCTTCAGCTCCACAGAGAA-3′ and Tgfb1 reverse, 5′-TGGTTGTAGAGGGCAAGGAC-3′ and Gapdh forward, 5′-CGACTTCAACAGCAACTC-3′ and Gapdh reverse, 5′-GCCGTATTCATTGTCATACCAG-3′. To measure gene expression, the Gapdh gene was chosen as a reference, and the delta-delta Ct (ΔΔCT) method was applied for relative quantification.
Statistics
All statistical analyses were performed using software EZR [14]. Student’s t-test was used to determine statistically significant differences between the two groups, and the Mann-Whitney U-test, a nonparametric test, was used for comparing histological scoring data. One-way ANOVA was used for multiple group comparisons, followed by the Tukey-Kramer post hoc test. Survival curves were generated using the Kaplan-Meier method, and the log-rank test was used for testing. Results with a P value of <0.05 were considered significantly different. Error bars indicate SD.
Results
Survival rate and clinical symptoms
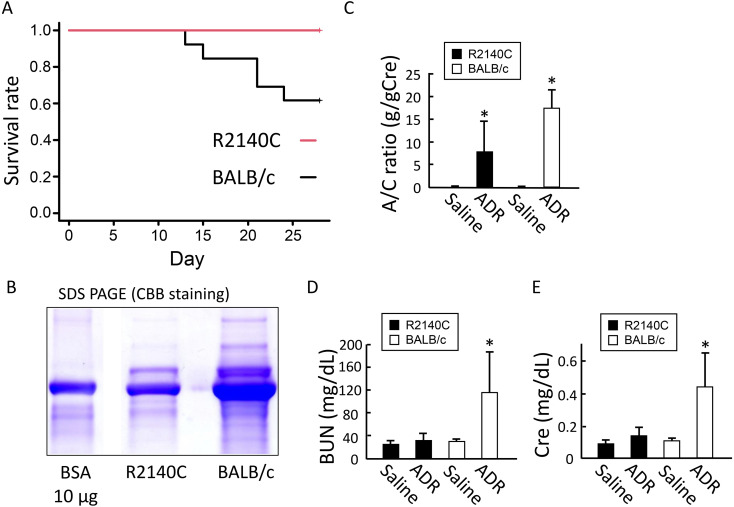
To compare the levels of ADR susceptibility, the number of survivors on post-ADR administration day 28 was compared between the B6-PrkdcR2140C and BALB/c mice. All 14 B6-PrkdcR2140C mice survived, whereas 6 of the 11 BALB/c mice died (Fig. 1A). Renal function was evaluated by measuring urinary albumin levels on post-ADR administration day 28. Severe albuminuria was observed in the two strains. The urinary albumin level was significantly lower in the B6-PrkdcR2140C mice than in the BALB/c mice (Figs. 1B and C). Blood tests performed on post-ADR administration day 28 indicated that the renal impairment markers, BUN and Cre levels in the blood, were significantly increased in the BALB/c mice, whereas remarkable increases were not observed in the B6-PrkdcR2140C mice (Figs. 1D–F).
Fig. 1.
Survivors and clinical symptoms in adriamycin (ADR)-treated mice. (A) Log-rank survival analysis of B6-PrkdcR2140C and BALB/c mice. ADR-treated BALB/c (n=11) and B6-PrkdcR2140C (n=14) mice were analyzed. (B and C) Urinary albumin quantified by Coomassie brilliant blue (CBB) staining was corrected for creatinine (Cre). ADR-treated BALB/c (n=6) and B6-PrkdcR2140C (n=10) mice, and non-treated BALB/c (n=4) and B6-PrkdcR2140C (n=4) mice were analyzed. (D and E) Serum from the collected blood was separated and subjected to biochemical tests; blood urea nitrogen (BUN) and Cre levels in the blood were evaluated. ADR-treated BALB/c (n=6) and B6-PrkdcR2140C (n=10) mice, and non-treated BALB/c (n=4) and B6-PrkdcR2140C (n=4) mice were analyzed. Data are expressed as mean ± SD. *P<0.05.
Histological examination of podocytopathy and tubular injury
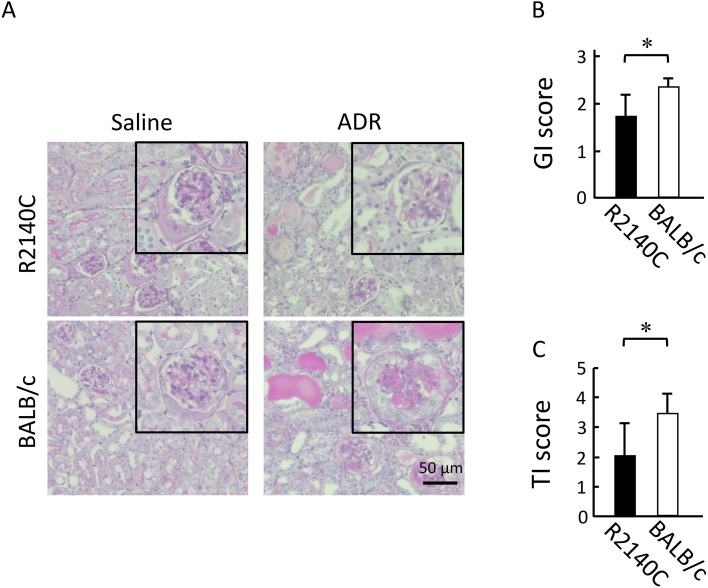
As noted in many human nephropathy cases, tubular injury is often observed following podocytopathy [15]. Here, the severity of podocytopathy and tubular injury was compared between the two strains. Renal histopathological analysis performed on post-ADR administration day 28 showed expansion of the mesangial region, reduced podocytes, rupture of glomerular capillaries, and segmental to global glomerulosclerosis (Fig. 2A). The results of podocytopathy severity scoring indicated that the proportion of impaired glomeruli was significantly increased in the BALB/c mice (Fig. 2B). The severity of tubular injury was also scored; a significantly high severity was noted in the BALB/c mice (Fig. 2C).
Fig. 2.
Histological analyses of kidney tissue samples from adriamycin (ADR)-treated mice. (A) Representative periodic acid-Schiff-stained renal sections from ADR-treated mice. Inserts are magnified images of a typical glomerulus. Scale bar, 50 µm. (B) GI scores. ADR-treated BALB/c (n=5) and B6-PrkdcR2140C (n=5) mice were analyzed. (C) Tubular injury (TI) scores. ADR-treated BALB/c (n=5) and B6-PrkdcR2140C (n=5) mice were analyzed. Data are expressed as mean ± SD. *P<0.05.
Evaluation of interstitial fibrosis
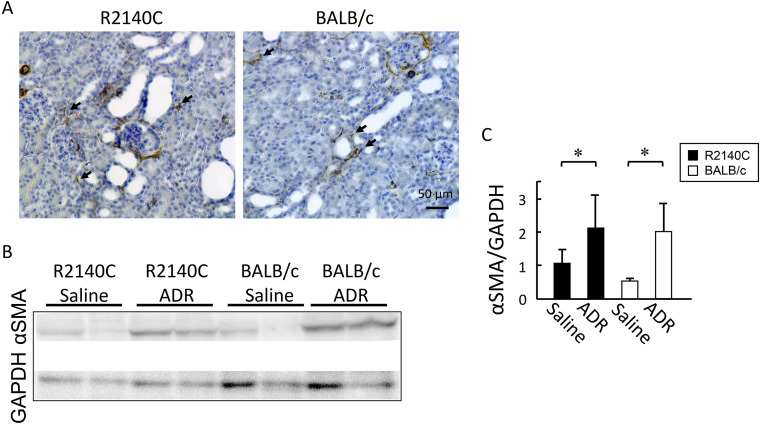
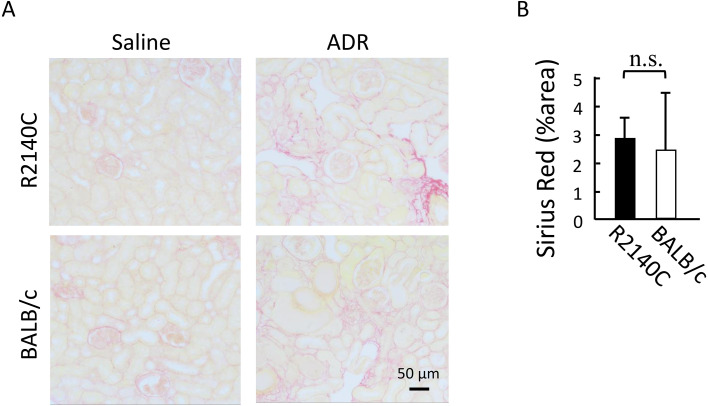
Immunohistochemical staining and western blot analysis using anti-α-SMA antibody revealed no significant differences in the number of α-SMA-positive interstitial cells on post-ADR administration day 28 between the two strains (Figs. 3A–C). Although Picrosirius red staining indicated an increase in the collagen fiber-positive region in the renal interstitium after ADR administration in both strains, no significant difference was observed (Figs. 4A and B).
Fig. 3.
Expression of α-smooth muscle actin (SMA) protein. (A) Representative immunohistochemical staining of α-SMA in kidney tissue samples from adriamycin (ADR)-treated mice. α-SMA-positive interstitial cells (arrows). Scale bar, 50 µm. (B, C) Western blot analysis of α-SMA from ADR-treated BALB/c (n=5) and B6-PrkdcR2140C (n=5) mice, and non-treated BALB/c (n=4) and B6-PrkdcR2140C (n=4) mice were analyzed. Data are expressed as mean ± SD. *P<0.05.
Fig. 4.
Evaluation of interstitial fibrosis with Sirius red staining. (A) Representative Picrosirius red-stained images of kidney sections from adriamycin (ADR)-treated mice. Scale bar, 50 µm. (B) Graph comparing the fibrosis area vs. total area. ADR-treated BALB/c (n=6) and B6-PrkdcR2140C (n=6) mice were analyzed. Data are expressed as mean ± SD. n.s: not significant.
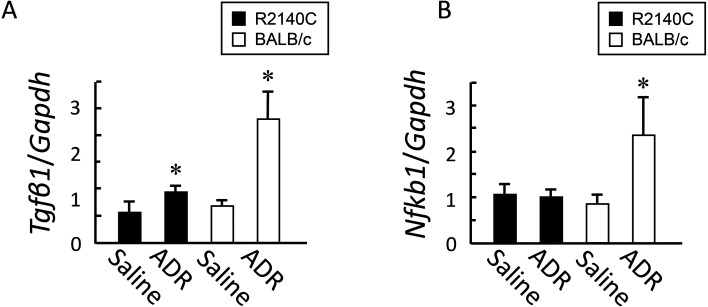
Evaluation of inflammation through qRT-PCR
On post-ADR administration day 28, expression levels of the two inflammatory mediators, Tgfb1 and Nfkb1, significantly increased in the BALB/c strain, compared with the B6-PrkdcR2140C strain (Figs. 5A and B).
Fig. 5.
Expression of inflammatory mediators after adriamycin (ADR) administration. (A) Tgfb1 and Nfkb1 expression in kidney tissue samples from ADR-treated and non-treated mice was measured through quantitative reverse transcription-PCR (qRT-PCR). ADR-treated BALB/c (n=4) and B6-PrkdcR2140C (n=4) mice, and non-treated BALB/c (n=3) and B6-PrkdcR2140C (n=3) mice were analyzed. Data are expressed as mean ± SD. *P<0.05.
Discussion
The severity of ADR administration-induced nephropathy was compared between two mouse strains: one was the B6-PrkdcR2140C strain with R2140C mutation in Prkdc that is responsible for ADR susceptibility, and the other was the BALB/c strain, a traditional ADR susceptible model. Histopathological features similar to CKD in humans were observed (i.e., the onset of glomerulosclerosis and tubulointerstitial fibrosis) in the B6-PrkdcR2140C mice on post-ADR administration day 28, thus confirming the validity of the B6-PrkdcR2140C mouse model as a human CKD model. The severity of nephropathy was compared between the B6-PrkdcR2140C and BALB/c mice. All B6-PrkdcR2140C mice survived, whereas 6 of the 11 BALB/c mice died. ADR directly induces glomerular and renal tubular toxicity, causing inflammation and fibrosis in the tubulointerstitium [16]. Thus, ADR administration increases BUN and Cre levels and decreases serum albumin, urea, and Cre clearance in the kidney, ultimately resulting in severe proteinuria [17, 18]. Podocytopathy severity, tubular disorder frequency, and serum BUN and Cre levels were significantly higher in the BALB/c mice than in the B6-PrkdcR2140C mice, which led to a high mortality rate of the BALB/c mice. In other words, nephropathy was more severe in the BALB/c mice than in the B6-PrkdcR2140C mice, indicating that the genetic background of B6 was resistant to ADR nephropathy.
Continuous albuminuria due to podocytopathy generally causes tubular disorders and interstitial fibrosis, thereby decreasing renal function. Renal fibrosis is caused when fibroblasts are transformed into α-SMA-positive myofibroblasts, and extracellular matrices such as collagen are excessively produced and accumulated proportional to the increase in the number of α-SMA-positive cells [19]. Contrary to expectations, the expression of α-SMA in the renal cortex and the severity of tubulointerstitial fibrosis were not significantly different between B6-PrkdcR2140C and BALB/c mice. A possible explanation for this result is that the severity of fibrosis may have reached a plateau or the severity in the tested BALB/c mice was relatively low because they were post-ADR administration survivors. However, the reason for such a similar result between the two strains is not completely clear; a further study is thus warranted.
ADR administration causes podocytopathy and tubular disorders. Studies evaluating ADR-induced nephrotoxicity are fewer than those examining the adverse effects of anthracyclines. ADR induces nephropathy through a complicated mechanism. Key mediators of nephropathy include free radicals, lipid peroxidation, and decreased activity of antioxidant enzymes [20, 21]. A drug with anti-inflammatory and antioxidative effects can attenuate the effects of ADR. Such findings suggest that ADR nephropathy is caused by inflammation (i.e., local cytokines and effects of other cytotoxic factors) [4]. TGF-β1, synthesized by several cells such as macrophages, T and B cells, fibroblasts, lymphocytes, and renal resident cells, is the main inflammatory mediator in kidney diseases. Its renal expression causes structural damage and fibrosis in the glomeruli, leading to the onset of several types of podocytopathy; therefore, TGF-β1 is considered a common target [22]. NF-kB is a transcription factor regulating several genes responsible for kidney disease onset and is involved in inflammatory responses by mediating TNF-α, IL-1β, and IL-6 expression [23, 24]. Expression levels of Tgfb1 and Nfkb1 were increased in the BALB/c mice, suggesting that the BALB/c strain is highly susceptible to developing nephropathy. Since no pathological differences were observed in the tubulointerstitium and Tgfb1 and Nfkb1 are also expressed by mesangial cells [25, 26], these differences in inflammatory cytokines may reflect differences in glomerular pathology.
A study that conducted linkage analysis between the BALB/c and B6 strains suggested that an ADR nephropathy-resistant gene exists in B6, and the causative locus is mapped to D9Mit182-D9Mit229 [7]. Furthermore, this region overlaps with a resistance region (Renf1) of the B6 strain with Alport syndrome [27]. The present study showed that an inflammation-associated gene may be a candidate because a difference was observed in the levels of inflammatory mediators. Moreover, this study indicated that the B6-PrkdcR2140C mice had an ADR-resistant gene; however, whether the inflammatory-related gene is this ADR-resistant gene is unknown and requires further evaluation. The relevant gene is expected to be used as a nephropathy biomarker and in facilitating drug development for CKD treatment.
Conflict of Interest
The authors declare no conflict interest.
Acknowledgments
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Glant-in-Aid for Scientific Research, KAKENHI (16K16606) to HS and KAKENHI (20302614) to N.S. This study was partially supported by the National Center for Global Health and Medicine (20A1019).
References
- 1.López-Novoa JM, Martínez-Salgado C, Rodríguez-Peña AB, López-Hernández FJ. Common pathophysiological mechanisms of chronic kidney disease: therapeutic perspectives. Pharmacol Ther. 2010; 128: 61–81. doi: 10.1016/j.pharmthera.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 2.Jefferson JA, Shankland SJ. The pathogenesis of focal segmental glomerulosclerosis. Adv Chronic Kidney Dis. 2014; 21: 408–416. doi: 10.1053/j.ackd.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol. 2012; 74: 299–323. doi: 10.1146/annurev-physiol-020911-153238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee VW, Harris DC. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology (Carlton). 2011; 16: 30–38. doi: 10.1111/j.1440-1797.2010.01383.x [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Wang YP, Tay YC, Harris DCH. Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int. 2000; 58: 1797–1804. doi: 10.1046/j.1523-1755.2000.00342.x [DOI] [PubMed] [Google Scholar]
- 6.Lombardi D, Lasagni L. Transgenic strategies to study podocyte loss and regeneration. Stem Cells Int. 2015; 2015: 678347. doi: 10.1155/2015/678347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Z, Schmidt-Ott KM, Chua S, Foster KA, Frankel RZ, Pavlidis P, et al. A Mendelian locus on chromosome 16 determines susceptibility to doxorubicin nephropathy in the mouse. Proc Natl Acad Sci USA. 2005; 102: 2502–2507. doi: 10.1073/pnas.0409786102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe M, Takahashi Y, Hiura K, Nakano K, Okamura T, Sasaki H, et al. A single amino acid substitution in PRKDC is a determinant of sensitivity to Adriamycin-induced renal injury in mouse. Biochem Biophys Res Commun. 2021; 556: 121–126. doi: 10.1016/j.bbrc.2021.03.150 [DOI] [PubMed] [Google Scholar]
- 9.Dip R, Naegeli H. More than just strand breaks: the recognition of structural DNA discontinuities by DNA-dependent protein kinase catalytic subunit. FASEB J. 2005; 19: 704–715. doi: 10.1096/fj.04-3041rev [DOI] [PubMed] [Google Scholar]
- 10.Papeta N, Zheng Z, Schon EA, Brosel S, Altintas MM, Nasr SH, et al. Prkdc participates in mitochondrial genome maintenance and prevents Adriamycin-induced nephropathy in mice. J Clin Invest. 2010; 120: 4055–4064. doi: 10.1172/JCI43721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsukamoto Y, Yamada N, Miyoshi K, Yamashita K, Ohsugi T. Anesthetic effect of a mixture of alfaxalone, medetomidine, and butorphanol for inducing surgical anesthesia in ICR, BALB/c, and C57BL/6 mouse strains. J Vet Med Sci. 2019; 81: 937–945. doi: 10.1292/jvms.18-0712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du J, Sun Q, Wang Z, Wang F, Chen F, Wang H, et al. Tubular epithelial cells derived-exosomes containing CD26 protects mice against renal ischemia/reperfusion injury by maintaining proliferation and dissipating inflammation. Biochem Biophys Res Commun. 2021; 553: 134–140. doi: 10.1016/j.bbrc.2021.03.057 [DOI] [PubMed] [Google Scholar]
- 13.Sasaki H, Takahashi Y, Ogawa T, Hiura K, Nakano K, Sugiyama M, et al. Deletion of the Tensin2 SH2-PTB domain, but not the loss of its PTPase activity, induces podocyte injury in FVB/N mouse strain. Exp Anim. 2020; 69: 135–143. doi: 10.1538/expanim.19-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48: 452–458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopp JB, Anders HJ, Susztak K, Podestà MA, Remuzzi G, Hildebrandt F, et al. Podocytopathies. Nat Rev Dis Primers. 2020; 6: 68. doi: 10.1038/s41572-020-0196-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeansson M, Björck K, Tenstad O, Haraldsson B. Adriamycin alters glomerular endothelium to induce proteinuria. J Am Soc Nephrol. 2009; 20: 114–122. doi: 10.1681/ASN.2007111205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohebbati R, Khajavi RA, Naser SM, Soukhtanloo M, Hosseinian S, Beheshti F, et al. The Effects of Vitamin C on Adriamycin-Induced Hypercholesterolemia in Rat. Curr Nutr Food Sci. 2015; 11: 309–314. doi: 10.2174/1573401311666150729230335 [DOI] [Google Scholar]
- 18.Wu Q, Li W, Zhao J, Sun W, Yang Q, Chen C, et al. Apigenin ameliorates doxorubicin-induced renal injury via inhibition of oxidative stress and inflammation. Biomed Pharmacother. 2021; 137: 111308. doi: 10.1016/j.biopha.2021.111308 [DOI] [PubMed] [Google Scholar]
- 19.Asada N, Takase M, Nakamura J, Oguchi A, Asada M, Suzuki N, et al. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest. 2011; 121: 3981–3990. doi: 10.1172/JCI57301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rafiee Z, Moaiedi MZ, Gorji AV, Mansouri E. P-coumaric acid mitigates doxorubicin-induced nephrotoxicity through suppression of oxidative stress, inflammation and apoptosis. Arch Med Res. 2020; 51: 32–40. doi: 10.1016/j.arcmed.2019.12.004 [DOI] [PubMed] [Google Scholar]
- 21.Ayla S, Seckin I, Tanriverdi G, Cengiz M, Eser M, Soner BC, et al. Doxorubicin induced nephrotoxicity: protective effect of nicotinamide. Int J Cell Biol. 2011; 2011: 390238. doi: 10.1155/2011/390238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016; 12: 325–338. doi: 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- 23.Choi M, Schreiber A, Eulenberg-Gustavus C, Scheidereit C, Kamps J, Kettritz R. Endothelial NF-κB blockade abrogates ANCA-induced GN. J Am Soc Nephrol. 2017; 28: 3191–3204. doi: 10.1681/ASN.2016060690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Socha MW, Malinowski B, Puk O, Wartęga M, Stankiewicz M, Kazdepka-Ziemińska A, et al. The role of NF-κB in uterine spiral arteries remodeling, insight into the cornerstone of preeclampsia. Int J Mol Sci. 2021; 22: 704. doi: 10.3390/ijms22020704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziyadeh FN. Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am Soc Nephrol. 2004; 15:(Suppl 1): S55–S57. doi: 10.1097/01.ASN.0000093460.24823.5B [DOI] [PubMed] [Google Scholar]
- 26.Satriano J, Schlondorff D. Activation and attenuation of transcription factor NF-kB in mouse glomerular mesangial cells in response to tumor necrosis factor-alpha, immunoglobulin G, and adenosine 3′:5′-cyclic monophosphate. Evidence for involvement of reactive oxygen species. J Clin Invest. 1994; 94: 1629–1636. doi: 10.1172/JCI117505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews KL, Mudd JL, Li C, Miner JH. Quantitative trait loci influence renal disease progression in a mouse model of Alport syndrome. Am J Pathol. 2002; 160: 721–730. doi: 10.1016/S0002-9440(10)64892-4 [DOI] [PMC free article] [PubMed] [Google Scholar]