Abstract
Introduction
Cystic fibrosis (CF), especially CF lung disease, is characterized by chronic infection, immune dysfunction including impairment of regulatory T cells (Tregs) and an exaggerated inflammatory response. CF transmembrane conductance regulator (CFTR) modulators have shown to improve clinical outcomes in people with CF (PwCF) with a wide range of CFTR mutations. However, it remains unclear whether CFTR modulator therapy also affects CF-associated inflammation. We aimed to examine the effect of elexacaftor/tezacaftor/ivacaftor therapy on lymphocyte subsets and systemic cytokines in PwCF.
Methods
Peripheral blood mononuclear cells and plasma were collected before and at three and six months after the initiation of elexacaftor/tezacaftor/ivacaftor therapy; lymphocyte subsets and systemic cytokines were determined using flow cytometry.
Results
Elexacaftor/tezacaftor/ivacaftor treatment was initiated in 77 PwCF and improved percent predicted FEV1 by 12.5 points (p<0.001) at 3 months. During elexacaftor/tezacaftor/ivacaftor therapy, percentages of Tregs were enhanced (+18.7%, p<0.001), with an increased proportion of Tregs expressing CD39 as a marker of stability (+14.4%, p<0.001). Treg enhancement was more pronounced in PwCF clearing Pseudomonas aeruginosa infection. Only minor, non-significant shifts were observed among Th1-, Th2- and Th17-expressing effector T helper cells. These results were stable at 3- and 6-month follow-up. Cytokine measurements showed a significant decrease in interleukin-6 levels during treatment with elexacaftor/tezacaftor/ivacaftor (–50.2%, p<0.001).
Conclusion
Treatment with elexacaftor/tezacaftor/ivacaftor was associated with an increased percentage of Tregs, especially in PwCF clearing Pseudomonas aeruginosa infection. Targeting Treg homeostasis is a therapeutic option for PwCF with persistent Treg impairment.
Keywords: pulmonary infection, cytokines, immunophenotyping, CFTR modulator therapy, cystic fibrosis - immunology, Pseudomonas aeruginosa
1. Introduction
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene resulting in dysfunctional or absent CFTR protein. CFTR modulators directly target this underlying protein defect and improve clinical outcomes in individuals with a wide range of CFTR mutations. Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) is the first approved triple combination CFTR modulator therapy that is indicated for people with CF (PwCF) who have a F508del mutation in at least one allele (1, 2).
While recent studies analyzing the efficacy of CFTR modulators have focused on clinical outcome parameters, there is a relative lack of data about whether CFTR modulators also dampen CF-associated inflammation. Exaggerated inflammation is a major driver of disease progression in CF that primarily, but not exclusively, affects the lungs (3). Increased levels of acute-phase proteins and pro-inflammatory cytokines are also present in peripheral blood from PwCF (3–5). Among these, interleukin [IL]-6, IL-8 and IL-1β have been reported to be elevated in the systemic circulation of PwCF compared with healthy controls (4, 6, 7). In addition, T cells are involved in inducing and maintaining chronic inflammation in CF (8, 9). Regulatory T cells (Tregs), which are known to counterbalance inflammatory processes and help to maintain homeostasis between pro- and anti-inflammatory mediators, are reduced in peripheral blood from PWCF (10, 11). CFTR dysfunction and chronic Pseudomonas aeruginosa infection are major mediators of the reported Treg impairment (10).
Given that CFTR is widely expressed on epithelial cells and on immune cells, highly effective CFTR modulators might resolve immune dysregulation and inflammation that result from a lack of CFTR activity. Current evidence on the potential anti-inflammatory effects of CFTR modulators is limited and is predominantly based on studies investigating the effects of mono- (ivacaftor) or dual combination (ivacaftor/lumacaftor, ivacaftor/tezacaftor) CFTR modulators (12, 13). Decreased sputum levels of IL-8 and IL-1β, and decreased serum levels of tumor necrosis factor (TNF) and IL-1β have been reported in PwCF receiving ivacaftor or ivacaftor/tezacaftor, respectively (14–16). However, these studies involve only a small number of participants and conflicting data exist (17). We previously compared PwCF receiving versus not receiving mono or dual combination CFTR modulators in a cross-sectional study and did not find any difference in either lymphocyte subsets including Tregs or cytokines in peripheral blood (18).
Available lines of evidence indicate a significantly higher efficacy of the triple combination compared with dual combination therapies with ivacaftor/teazacaftor or ivacaftor/lumacaftor in PwCF homozygous for the F508del mutation (2, 19). We therefore hypothesized that there would be effects on the composition of immune cell subsets and systemic cytokines in PwCF receiving highly effective triple combination CFTR modulators. Therefore, this longitudinal, observational study analyzed the effects of treatment with ELX/TEZ/IVA on immune cell subsets and systemic cytokines in PwCF
2. Methods
2.1. Study design and participants
Eligible participants were all PwCF starting on ELX/TEZ/IVA (T0) between March 2020 and January 2021. Those with clinical signs of a pulmonary exacerbation were excluded. Study participants were invited to follow-up consultations including pulmonary function tests, six-minute walk test and sweat chloride measurement at three (T1) and six (T2) months after treatment initiation. Results from sputum and throat swab cultures provided during 12 months treatment with ELX/TEZ/IVA therapy were studied retrospectively. P. aeruginosa infection status was classified according to Leeds criteria: chronic P. aeruginosa infection was defined as >50% of months with P. aeruginosa-positive sputum cultures in the preceding 12 months. Written informed consent was obtained from all study participants prior to enrolment. This study was approved by the local ethics committee (no. 17-7365-BO).
2.2. PBMC isolation
Whole blood samples were collected at T0, T1 and T2 and prepared as previously described (18). In brief, 12 mL of whole blood was collected from each participant and processed within four hours after collection. Following centrifugation, plasma samples (for cytokine measurement) and peripheral blood mononuclear cells (PBMC, for lymphocyte phenotyping) were obtained. PBMC were then adjusted to 1x107 cells/mL phosphate-buffered saline (PBS) for further use.
2.3. Lymphocyte phenotyping
PBMC were adjusted to 2x105 cells and washed with flow cytometry wash buffer (4% PFA [Carl Roth, Karlsruhe, Germany], 1% fetal calf serum [FCS; PAN-Biotech, Aidenbach, Germany] in PBS). PBMC were then incubated with a mix of antibodies purchased from BioLegend® (Koblenz, Germany) for multicolor cytofluorometric analyses. Antibodies and the gating strategy used to identify T cell subsets have been described previously and were adapted from Rühle et al. ( Figure S1 ; 18, 20). Tregs were defined as CD4+CD127lowCD25+ cells and further characterized as CD39+ “stable” Tregs ( Figure S1 ). In addition, Tregs were subdivided into FoxP3+ (BioLegend® PE α-human FoxP3 clone:206D) Tregs in stored samples from 10 PwCF (before and 3 months after receiving ELX/TEZ/IVA, Figure S2 ). Intracellular staining against the transcription factors FoxP3 was performed on surface-stained PBMC using the FoxP3/Transcription Factor Staining Buffer Set (Invitrogen®, Darmstadt, Germany). Flow cytometric measurements were performed with a CytoFLEX LX (Beckman Coulter, Krefeld, Germany) and corresponding software (CytExpert V2.3). Finally, data were analyzed using FlowJo Software V10 (Tree Star, Ashland, USA).
2.4. Quantification of cytokines
Concentrations of T helper cell-associated cytokines were quantified in plasma samples from PwCF before and after 3 months’ treatment with ELX/TEZ/IVA using the bead-based immunoassay LEGENDplex® (LEGENDplex® Human Th Panel Standard V02, BioLegend, Koblenz, Germany) according to the manufacturers’ instructions (21). Samples were processed as described previously (18).
2.5. Statistical analyses
Two-tailed (un)paired Student-t-test was used for parametric data. Nonparametric data were analysed using the Mann–Whitney U-test for unpaired data or the Wilcoxon signed-rank test for paired data. Pearson Chi-squared test was used to assess frequency distributions of categorical data. Correlations were analysed using the pairwise Spearman correlation test. Data are displayed as mean and standard deviation or median with first and third quartile, as indicated. Statistical significance was defined as p<0.05. GraphPadPrism version 9, IBM SPSS version 28 and/or R studio (version 1.4.1106) were used for statistical operations. Plots and graphs were generated with various R packages (“ggplot2”, “ggiraph”, “ggpubr”, “factoextra”, “ggradar”, “fmsb”) in its latest versions as of August 2022.
3. Results
3.1. Study population
The study population consisted of 77 PwCF (mean age 34 years). The majority of PwCF (74/77, 96.10%) were either homozygous or heterozygous for the F508del mutation. Three PwCF with other CFTR mutations received off-label treatment with ELX/TEZ/IVA. Although ELX/TEZ/IVA therapy was not initiated in PwCF with recent pulmonary exacerbation, several PwCF showed signs of systemic inflammation at T0, as shown by elevated C-reactive protein (mean 1.25 mg/dl) and leukocytes (mean 10.60/nL) ( Table 1 ). Among analyzed PwCF 59/77 (76.62%) received either inhaled antibiotics, intravenous antibiotic treatment in the last 12 months or both.
Table 1.
Study population and results from immunophenotyping measurements.
| 0 (n=77) | T1 (n=77) | T2 (n=77) | p-value (T0-T1) | p-value (T1-T2) | p-value (T0-T2) | |
|---|---|---|---|---|---|---|
| Age, years | 33.77 ± 11.52 | |||||
| Female sex, n (%) | 34 (44.2) | |||||
| ppFEV1 | 44.86 ± 20.27 | 57.37 ± 22.33 | 56.92 ± 22.11 | <0.001 | 0.261 | <0.001 |
| BMI, kg/m2 | 20.09 ± 2.58 | 21.13 ± 2.69 | 21.64 ± 2.63 | <0.001 | <0.001 | <0.001 |
| Six-minute walk test, m | 493.4 ± 104.7 | 578.1 ± 118.6 | 589.2 ± 114.7 | <0.001 | 0.079 | <0.001 |
| Sweat chloride, mmol/L | 105.52 ± 12.16 | 56.37 ± 19.06 | 52.24 ± 17.97 | <0.001 | 0.083 | <0.001 |
| Leukocytes,/nL | 10.60 ± 3.50 | 7.34 ± 2.49 | 7.75 ± 2.63 | <0.001 | 0.046 | <0.001 |
| CRP, mg/dL | 1.25 ± 1.81 | 0.02 ± 0.13 | 0.05 ± 0.25 | <0.001 | 0.864 | <0.001 |
| P. aeruginosa infection, n (%) | ||||||
| Chronic | 37 (48.1) | |||||
| Non chronic | 40 (52.0) | |||||
| CFTR genotype, n (%) | ||||||
| Homozygous dF508 | 40 (52.0) | |||||
| Heterozygous dF508 | 34 (44.2) | |||||
| Other* | 3 (3.9) | |||||
| Prior CFTR modulator therapy, n (%) | ||||||
| Tezacaftor/Ivacaftor | 34 (44.2) | |||||
| Lumacaftor/Ivacaftor | 3 (3.9) | |||||
| Ivacaftor | 0 (0.0) | |||||
| None | 40 (52.0) | |||||
| Immunophenotyping | ||||||
| CD3+ T cells, % PBMC | 42.20 ± 16.65 | 42.58 ± 15.35 | 45.23 ± 13.81 | 0.831 | 0.126 | 0.128 |
| CD8+ T cells, % T cells | 24.50 ± 8.52 | 26.10 ± 7.67 | 25.48 ± 6.90 | 0.037 | 0.240 | 0.168 |
| CD4+ T helper, % T cells | 63.65 ± 10.40 | 62.29 ± 10.35 | 63.16 ± 8.99 | 0.121 | 0.279 | 0.602 |
| CD25+CD127- Treg, % Th | 8.30 ± 2.37 | 9.85 ± 3.18 | 9.70 ± 2.75 | <0.001 | 0.645 | <0.001 |
| CD39+ Treg, % Treg | 45.16 ± 14.06 | 51.68 ± 12.72 | 51.84 ± 11.41 | <0.001 | 0.895 | <0.001 |
| FoxP3+ Treg, % Treg** | 82.39 ± 6.99 | 79.86 ± 10.38 | 0.221 | |||
| Effector Th, % Th | 87.90 ± 3.11 | 86.69 ± 4.20 | 86.56 ± 3.70 | 0.011 | 0.781 | 0.001 |
| Th1, % effector Th | 10.02 ± 4.90 | 10.23 ± 4.39 | 11.15 ± 4.97 | 0.735 | 0.133 | 0.063 |
| Th2, % effector Th | 68.07 ± 13.14 | 67.60 ± 13.61 | 65.54 ± 14.21 | 0.648 | 0.106 | 0.221 |
| Th17, % effector Th | 10.14 ± 4.22 | 10.12 ± 4.39 | 9.47 ± 4.15 | 0.975 | 0.147 | 0.150 |
| Th1-17, % effector Th | 7.72 ± 4.57 | 7.80 ± 4.41 | 8.91 ± 5.47 | 0.792 | 0.071 | 0.087 |
Values are mean ± standard deviation. p-values were obtained using Wilcoxon test or t test for paired samples. BMI, body mass index; CFTR, cystic fibrosis transmembrane conductance regulator; CRP, C-reactive protein; PBMC, peripheral blood mononuclear cell; ppFEV1, percent predicted forced expiratory volume in 1 second; Th, helper T cells; Treg, regulatory T cells. *Other: R553X/I336K, G542X/3849+10KbC->T, R1162X/A455E. **FoxP3 expression determined in stored samples from a subcohort of 10 PwCF.
Characteristics at baseline (T0), at 3 months (T1) and at 6 months (T2).
Bold values denote statistical significance.
3.2. Clinical efficacy
After 3 months’ treatment with ELX/TEZ/IVA (T1), PwCF showed increased percent predicted FEV1 (ppFEV1; +12.5 points, p<0.001 vs. baseline), then ppFEV1 remained stable between T1 and T2 (p=0.261). Sweat chloride levels decreased by 46.6% between T0 and T1 (p<0.001), with sweat chloride levels below 30 mmol/L in seven PwCF (7/77, 9.1%) at T2 ( Table 1 ). Furthermore, the three PwCF with off-label ELX/TEZ/IVA therapy showed improved ppFEV1 (+4.7 points) and reduced sweat chloride levels (-52.6%). Thirty-seven PwCF (37/77, 48.1%) had chronic pulmonary P. aeruginosa infection. At follow-up, sputum cultures were negative for P. aeruginosa in six of these participants (6/37, 16.2%) ( Table 2 ).
Table 2.
Sputum culture results.
| Follow up results | Sputum culture (n=26) | Throat culture (n=10) | All (n=36) |
|---|---|---|---|
| Patients with >50% of cultures positive for P. aeruginosa (mean Treg increase T0-T1)= chronic | 18 (+16.7%) | 3 (+11.9%) | 21 (+16.0%) |
| Patients with <50% of cultures positive for P. aeruginosa (mean Treg increase T0-T1)= intermittent | 6 (+33.7%) | 3 (+28.9%) | 9 (+32.1%) |
| All negative, P. aeruginosa potentially cleared (mean Treg increase T0-T1) | 2 (+43.8%) | 4 (+37.8%) | 6 (+39.8%) |
| p-value | 0.039 |
Follow-up results from sputum and throat swab cultures and regulatory T cell (Treg) dynamics in PwCF with pre-treatment chronic P. aeruginosa infection status (n=37). At least two follow-up sputum samples or throat swabs were available from 36/37 (97.30%) study participants. PwCF who cleared P. aeruginosa infection were those with strongest Treg enhancement (+39.8%). Statistics: ANOVA.
Bold values denote statistical significance.
3.3. Effects of ELX/TEZ/IVA on immune cell subsets
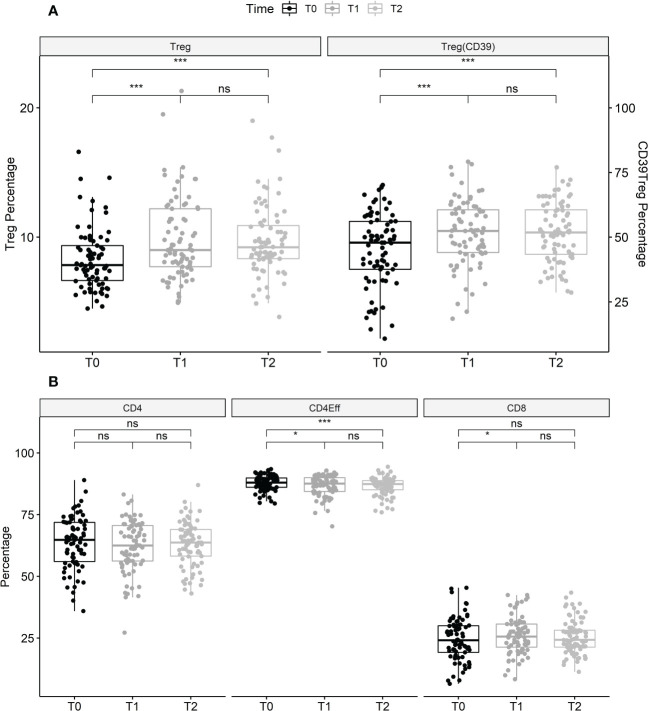
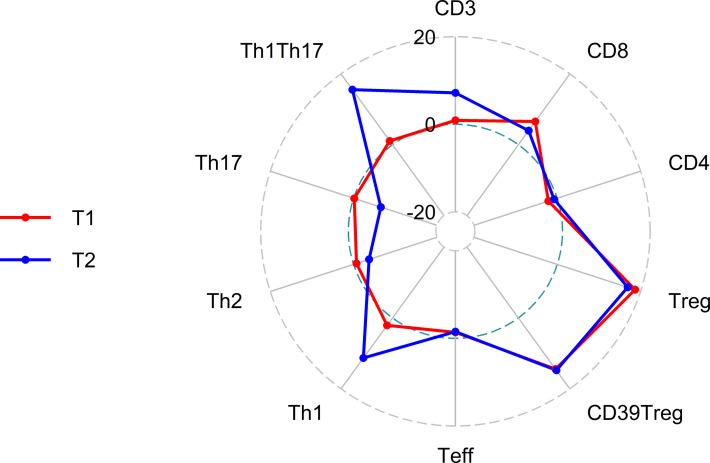
Treatment with ELX/TEZ/IVA had no significant effect on the proportion of total CD3+ T cells (mean 42.2% of PBMC at T0 vs. 42.6% at T1 and 45.2% at T2) or the proportion of CD4+ T helper cells (mean 63.7% of T cells at T0 vs. 62.3% at T1 and 63.2% at T2). Modest changes were observed in the percentage of CD8+ T cells between T0 and T1 (mean 24.5% vs. 26.1% of T cells; +6.5%, p=0.037), but not between T0 and T2 (p=0.168). However, treatment with ELX/TEZ/IVA was associated with a significant increase of Tregs (CD4+CD127lowCD25+cells) from T0 to T1 (mean 8.3% vs. 9.9% of T helper cells, +18.7%; p<0.001), while percentages of Tregs remained stable between T1 and T2 (p=0.645; Figure 1 ). Treg enhancement was found in PwCF with elevated baseline CRP and leukocyte levels and in those with normal baseline CRP and leukocytes ( Figure S3 ). CD39 further characterizes a stable subset of Tregs under inflammatory conditions (22). The proportion of CD39+Tregs disproportionately increased during ELX/TEZ/IVA therapy (mean 45.2% of Tregs at T0 vs. 51.7% at T1, +14.4%; p<0.001), with no significant change between T1 and T2 (p=0.895). FoxP3 expression of Tregs was determined in a subcohort of 10 PwCF. Overall, FoxP3 expression was found in 82.39% (mean) of Tregs at baseline and remained stable within the increased share of Tregs at T1(p=0.221). FoxP3 expression was higher in CD39+Tregs compared with CD39-Tregs at baseline (p=0.002) and T1 (p=0.007, Figure S2 ). ELX/TEZ/IVA therapy neither changed the FoxP3 expression among CD39+Tregs (p=0.846) nor among CD39-Tregs (p=0.770, Figure S2 ). Moreover, percentages of effector T helper cells decreased from T0 to T1 (mean 87.9% of T helper cells vs. 86.7%, –1.38%; p=0.011) and no changes were observed between T1 and T2 ( Figure 1 ). Minor shifts occurred among effector T helper cell subpopulations. Percentages of Th1 effector cells tended to increase while effector T helper cells with Th17 phenotype tended to decrease. Th2 effector cells seem to be almost unaffected by treatment with ELX/TEZ/IVA ( Table 1 and Figure 2 ).
Figure 1.
Changes of lymphocyte subsets during treatment with elexacaftor/tezacaftor/ivacaftor. (A) Data shown are the proportion of Tregs (CD4+, CD25+, CD127- as a proportion of T helper cells) at T0, T1 and T2, and Tregs with CD39+ expression (as a proportion of total Tregs) as a marker of stability in pro-inflammatory environments. Tregs (+18.7%) and CD39+ Tregs (+14.4%) significantly increased between T0 and T1, and between T0 and T2, then remained stable between T1 and T2. (B): CD4+ T helper (as a percentage of T cells), effector helper T cells (Th; as a percentage of total Th) and CD8+ T cells (as a percentage of T cells) before, and after 3 months (T1) and 6 months (T2) of treatment with elexacaftor/tezacaftor/ivacaftor in PwCF. Percentages of effector Th cells significantly decreased after initiation of elexacaftor/tezacaftor/ivacaftor therapy and remained lower at T2. Percentages of CD4+ and CD8+ T cells were stable between T0 and T2. Statistics: Values are median, Wilcoxon signed rank test. ns, not statistically significant. *p < 0.05; ***p < 0.001.
Figure 2.
Radar plot of percentage change in lymphocyte subsets at T1 (3 months after initiation of elexacaftor/tezacaftor/ivacaftor, red) and T2 (6 months after initiation of elexacaftor/tezacaftor/ivacaftor, blue) compared with baseline (T0).
3.4. Effects of ELX/TEZ/IVA on P. aeruginosa infection status
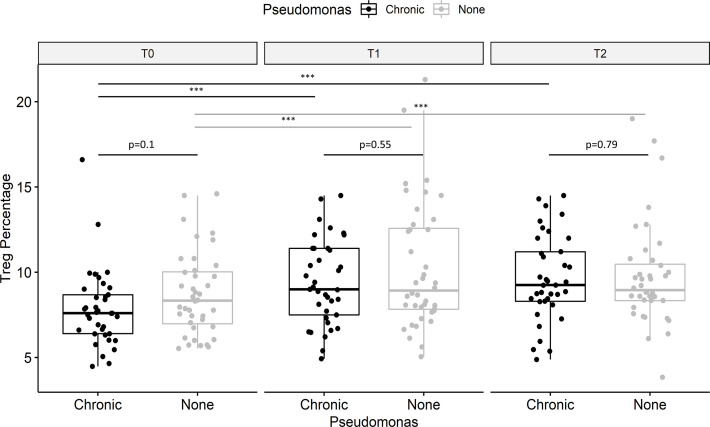
Tregs were impaired in PwCF with versus without chronic pulmonary P. aeruginosa infection (7.9% vs. 8.7% of T helper cells), but the between-group difference did not reach statistical significance (p=0.10; Figure 3 ). The increase in Tregs during treatment with ELX/TEZ/IVA was 23.3% in PwCF with chronic P. aeruginosa infection and 18.8% in those with no or intermittent P. aeruginosa infection. Results from sputum cultures were studied in 37 PwCF with chronic pulmonary P. aeruginosa infection over 12 months’ follow-up to determine post-treatment infection status. Data from 36/37 PwCF who provided at least two sequential sputum or throat swab samples were included in this subanalysis ( Table 2 ). Sputum samples were available from 26/36 study participants (average 5.3 samples/participant). Throat swabs in case of reduced sputum production after treatment initiation were available from another 10/36 participants (average 3.7 samples/participant). Tregs increased by 39.8% in six PwCF who potentially cleared the infection compared with 16.0% in twenty-one PwCF who had persisting evidence of chronic P. aeruginosa infection ( Table 2 ). The Treg percentage increased by 32.1% in the nine PwCF who had intermittent post-treatment evidence of P. aeruginosa infection.
Figure 3.
Effect of Pseudomonas aeruginosa infection on regulatory T cells (Tregs). Data showed are for CD25+ CD127- Treg (as a proportion of T helper cells), stratified for P. aeruginosa infection. PwCF with chronic P. aeruginosa infection tended to have lower percentages of Tregs (black) compared to PwCF without chronic P. aeruginosa infection (grey) before therapy (T0). Treg impairment in PwCF with P. aeruginosa infection appeared to be rebalanced at T1 and T2. Values are median, and p-values were determined using the Wilcoxon signed rank test. ***p < 0.001.
3.5. Effects of ELX/TEZ/IVA on cytokine levels
Twelve T cell-associated cytokines were measured in plasma from 52 participating PwCF (67.5%) before and three months after the initiation of ELX/TEZ/IVA. IL-6 was elevated in 43/52 (82.7%) PwCF at baseline. Plasma IL-6 levels were significantly decreased from baseline at 3-month follow-up (T1) in 38/43 (88.4%) PwCF (median 13.9 pg/mL vs. 3.48 pg/mL; -50.2%; p<0.001), independent of P. aeruginosa infection status (p=0.970). ELX/TEZ/IVA had no significant effects on plasma levels of Th1- (IL-2, interferon-γ, TNF-α), Th2- (IL-4, IL-5, IL-13) or Th17- (IL-17A, IL-17F, IL-22) associated cytokines ( Table S1 , Figure S4 ). Although the proportion of Tregs significantly increased during ELX/TEZ/IVA therapy, no statistically significant effects were observed on plasma IL-10 levels (median 2.97 pg/mL at T0 vs. 3.52 pg/mL at T1, p=0.579).
3.6. Correlation analysis
During treatment with ELX/TEZ/IVA there were significant improvements in ppFEV1, six-minute walk test and sweat chloride, an increase of Tregs, and a reduction in IL-6 levels. Testing the relation between the percentage change of these parameters revealed only weak correlations. After 3 months’ treatment with ELX/TEZ/IVA, increased ppFEV1 was not significantly correlated with Treg enhancement (R=0.007, p=0.096), IL-6 decrease (R-0.116, p=0.465) or reduced sweat chloride (R=–0.082, p=0.508). Likewise, changes in sweat chloride levels were not correlated with Treg enhancement (R=0.022, p=0.856) or with reduction of IL-6 levels (R=0.072, r=0.667). There was a weak, but statistically significant, positive correlation between percentage changes in six-minute walk test and Tregs (R=0.304, r=0.024; Table S2 ). Treg enhancement was linked to a downregulation of pro-inflammatory cytokines, predominantly to a reduction of the Th2-related cytokines IL-4 (r=-0.487, p=0.001) and IL-5 (r=-0.415, p=0.005, Figure S5 ).
4. Discussion
The results of this longitudinal study show the systemic anti-inflammatory effects of ELX/TEZ/IVA therapy in PwCF, based on an increased proportion of Tregs in peripheral blood and a decreased in plasma IL-6 levels. Furthermore, P. aeruginosa infection-mediated Treg impairment appeared to resolve in those with reduced or cleared P. aeruginosa infection after treatment with ELX/TEZ/IVA.
The clinical effects of ELX/TEZ/IVA in the current study (a 13 points increase in ppFEV1 and a 47% reduction in sweat chloride levels) were consistent with results from the phase 3 trial (1). Moreover, treatment with ELX/TEZ/IVA was associated with a marked increase in Treg percentages (+19%) and, to a lesser extent, a decrease in effector T helper cells (–6%). These changes remained stable at 6-month follow-up. In this study, Tregs were defined as CD4+CD127lowCD25+cells and showed a high expression of FoxP3 in a subcohort of 10 PwCF. Treg enhancement was observed in PwCF independently of CRP/leukocyte levels suggesting that Treg enhancement is not only based on a simple shift from effector T helper cells to Tregs. Additional mechanisms might favor an induction of Tregs. Previous studies reported a general Treg impairment in blood and bronchoalveolar lavage fluid from children with CF compared with healthy controls (10, 11). Reduced Tregs have also been found in spleens and lungs from CFTR-/- mice compared with CFTR+/+ littermates (10, 23). In addition, pharmacologic inhibition of CFTR dampened Tregs in cultures of human PBMCs indicating a direct link between Treg quantities and CFTR function (10). Therefore, restoration of CFTR function might trigger the Treg enhancement seen during treatment with ELX/TEZ/IVA. We previously did not find any differences in lymphocyte subsets, including Tregs, between PwCF receiving versus not receiving mono or dual combination CFTR modulator therapy (18). However, that cross-sectional study was potentially insufficient to detect differences in immune cell subsets due to missing samples for a longitudinal analysis. Moreover, treatment with mono or dual combination CFTR modulators are less effective to improve CFTR function compared with the triple-combination ELX/TEZ/IVA (18, 19). Experimental data by Gu et al. demonstrated that the subset of CD39+ Tregs maintained its suppressive Treg function and FoxP3 expression under inflammatory conditions while CD39-Tregs lost their FoxP3 expression (22). We observed a higher proportion of CD39+Tregs (in % of Tregs) in response to ELX/TEZ/IVA therapy which probably represents a favorable outcome. CD39+Tregs exhibited an enhanced FoxP3 expression compared with CD39-Tregs at baseline. At 3-month follow-up, FoxP3 expression levels of CD39+/CD39-Tregs remained stable. Thus, we detected no disproportional increase of FoxP3 expression under the reduced inflammatory environment that we observed in pwCF receiving ELX/TEZ/IVA therapy. Perhaps, we were unable to find significant differences due to a low number of available samples for FoxP3 staining (n=10) or other, unknown factors regulate FoxP3 expression in our real-world cohort.
Changes downstream of CFTR dysfunction might also play a role in Treg homeostasis. CFTR dysfunction has been associated with an imbalance of sphingolipids (24, 25). Sphingolipids such as ceramides are not only structural components of cell membranes, but also bioactive molecules that are, for example, involved in T cell differentiation (26–28). Interestingly, Treg frequencies were increased in acid sphingomyelinase-deficient mice compared with wildtype mice, and in human PBMC after in vitro treatment with acid sphingomyelinase inhibitors such as sertraline (26, 27). Quantification of ceramide subtypes showed a reduced ratio of ceramide species C16/C24 in these cells after treatment (supplementary material of 26). In the context of CF, an elevated ratio of ceramide subtypes C16/C24 correlates with inflammation and disease severity (24, 29). Recently, we reported a 35.5% decrease in the C16/C24 ratio in blood plasma from PwCF treated with ELX/TEZ/IVA (30). These sphingolipid-modulating effects of ELX/TEZ/IVA therapy could potentially be involved in the induction of Tregs in PwCF in the present study.
Tregs have been found to be impaired in blood and bronchoalveolar lavage from PwCF who have chronic P. aeruginosa infection and in a mouse model of chronic P. aeruginosa lung infection (10, 18, 31). P. aeruginosa seems to regulate Treg differentiation independent of direct cell-cell interactions. In particular, cell-free supernatants from virulent and flagellin-deficient P. aeruginosa strains impaired Tregs in vitro (10). In the present study, Tregs were impaired in PwCF who had chronic P. aeruginosa infection at baseline, but differences compared to PwCF who did not have chronic P. aeruginosa infection were not statistically significant. So far, conflicting data exist concerning the effects of CFTR modulators on CF-related lung infections. An immediate reduction in P. aeruginosa density followed by a rebound at pre-treatment levels has been observed in PwCF treated with ivacaftor (14, 32). Recent studies reported a significant decrease of culture positivity for P. aeruginosa in PwCF treated with ELX/TEZ/IVA for 6-12 months (33, 34). We observed a culture clearance of P. aeruginosa in 17% of participants in the current study, and intermittent evidence of P. aeruginosa infection in another 25% of PwCF with previous chronic P. aeruginosa infection. In the present study, PwCF who experienced reduced or cleared chronic P. aeruginosa infection were those who showed the strongest increases in Treg percentages, emphasizing the important role of P. aeruginosa infection in regulating Treg homeostasis.
Our analysis of T cell-associated systemic cytokines showed a marked reduction in plasma IL-6 levels in PwCF with and without chronic P. aeruginosa infection, but stable levels of several other pro-inflammatory cytokines. Thus, we could not attribute anti-inflammatory effects to a reduction of a specific Th2-, Th1- or Th17-type of inflammation in this study. Elevated levels of IL-6 have been detected in both plasma and in bronchoalveolar lavage fluid, and represent a characteristic component of the pro-inflammatory environment in CF that seems to be significantly dampened by ELX/TEZ/IVA (6, 7, 35). Induction of IL-10 has been reported from human PBMCs treated with dual combination CFTR modulators in vitro (15). However, in our study, increases of anti-inflammatory plasma IL-10 did not reach statistical significance. Another group reported reduced levels of pro-inflammatory IL-6, IL-8 and IL-17A and stable levels of six other cytokines in blood from PwCF after ELX/TEZ/IVA therapy (33). Lepissier et al. found reduced IL-8 and IL-1β sputum levels in adolescents with mild CF lung disease under ELX/TEZ/IVA therapy (36). These data suggest that CFTR modulators dampen pro-inflammatory cytokines that are predominately associated with neutrophilic inflammation.
In almost all participating PwCF, treatment with ELX/TEZ/IVA improved ppFEV1, sweat chloride levels and six-minute walk test. However, correlations between these outcome parameters were limited and not statistically significant, indicating a high variability in treatment responses to ELX/TEZ/IVA. Lung function and (CD39+) Tregs were positively correlated at baseline and in previous studies (10, 18), but there was no relationship between the percentage change in ppFEV1 and Tregs in response to ELX/TEZ/IVA therapy in the present study. There was a positive correlation between the percentage change in six-minute walk test and Tregs at 3 months, suggesting that Treg enhancement may contribute to the clinical improvement in response to ELX/TEZ/IVA. Weak correlations between outcome parameters have been reported by other groups analyzing various clinical parameters in PwCF receiving ELX/TEZ/IVA (37, 38). Possible explanations are a heterogenous study population in terms of pre-existing structural lung damage, infection status and established therapy with mono or dual combination CFTR modulators, different time intervals until full therapeutic effects are achieved and/or complementary, non-correlating effects being captured by a parameter.
The current study is the largest to date investigating the composition of immune cell subsets and systemic cytokines in PwCF receiving highly effective triple combination CFTR modulators. However, the study also has some limitations. Although our cohort included PwCF with mild to severe lung disease, the average ppFEV1 was lower than in the approval study for ELX/TEZ/IVA, and therefore our results might not be directly applicable to PwCF who have milder disease. Our cohort was heterogeneous regarding several baseline characteristics (ppFEV1, genotype, infection status, previous CFTR modulator therapy). Nonetheless, our main finding of Treg enhancement was found in all subgroups. Leukocytosis and elevated CRP were present in several PwCF at baseline due to chronic infection and/or CF-related inflammation. Therefore, studying the natural course and immunological reaction to ELX/TEZ/IVA therapy is complicated by the individual, complex inflammatory state of each study participant. A more detailed characterization of Tregs was outside the initial scope of the present study. The number of stored PBMC samples limited additional retrospective FACS analyses. Furthermore, we did not conduct in vitro Treg suppression assays to analyze a possible, altered Treg function in response to ELX/TEZ/IVA therapy. Future studies may provide a more comprehensive analysis of Treg phenotypes and function in PwCF under ELX/TEZ/IVA therapy.
5. Conclusions
We report stable Treg enhancement in peripheral blood from PwCF receiving ELX/TEZ/IVA therapy. This effect is probably mediated by a restoration of CFTR function and a reduction in P. aeruginosa airway colonization. Our results underline the anti-inflammatory effects of ELX/TEZ/IVA therapy, with implications for future clinical trials evaluating anti-inflammatory therapies for PwCF, especially for those with persistent pulmonary infections.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethik-Kommission, Universität Duisburg-Essen (no. 17-7365-BO). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Study conception: SR, CT, DW. Data acquisition and analysis: DW, HU, JR, MS, JP, SS, SSu, MW. Data interpretation: DW, SR, JR, CT. Writing the original manuscript: DW, SR. Revising the work for important intellectual content: SR, CT, DW, SSu, MW, HU, JR, MS. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank all participating individuals with CF for their willingness to contribute to this study. Sample collection and processing were supported by the West German Biobank. English language editing assistance was provided by Nicola Ryan, independent medical writer, funded by the University of Essen. We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Funding Statement
Supported by a German Research Foundation (DFG)-initiated clinician scientist program FU 356/12–1 (DW).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1107437/full#supplementary-material
References
- 1. Middleton PG, Mall MA, Dřevínek P, Lands LD, McKone EF, Polineni D, et al. Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med (2019) 381:1809–19. doi: 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet (2019) 394:1940–8. doi: 10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cantin AM, Hartl D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros (2015) 14:419–30. doi: 10.1016/j.jcf.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 4. Ngan DA, Wilcox PG, Aldaabil M, Li Y, Leipsic JA, Sin DD, et al. The relationship of systemic inflammation to prior hospitalization in adult patients with cystic fibrosis. BMC Pulm Med (2012) 12:3. doi: 10.1186/1471-2466-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matouk E, Nguyen D, Benedetti A, Bernier J, Gruber J, Landry J, et al. C-reactive protein in stable cystic fibrosis: an additional indicator of clinical disease activity and risk of future pulmonary exacerbations. J Pulm Respir Med (2016) 6:1000375. doi: 10.4172/2161-105X.1000375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nixon LS, Yung B, Bell SC, Elborn JS, Shale DJ. Circulating immunoreactive interleukin-6 in cystic fibrosis. Am J Respir Crit Care Med (1998) 157:1764–9. doi: 10.1164/ajrccm.157.6.9704086 [DOI] [PubMed] [Google Scholar]
- 7. Randhawa I, Nussbaum E, Ahdoot R, Rees H, Yu A, Cihn T. Clinical outcomes associated with interleukin 6 and interleukin 8 cytokine production in cystic fibrosis. Br J Med Med Res (2015) 6:164–72. doi: 10.9734/BJMMR/2015/12588 [DOI] [Google Scholar]
- 8. Mulcahy EM, Hudson JB, Beggs SA, Reid DW, Roddam LF, Cooley MA. High peripheral blood Th17 percent associated with poor lung function in cystic fibrosis. PloS One (2015) 10:e0120912. doi: 10.1371/journal.pone.0120912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tiringer K, Treis A, Fucik P, Gona M, Gruber S, Renner S, et al. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for pseudomonas aeruginosa infection. Am J Respir Crit Care Med (2013) 187:621–9. doi: 10.1164/rccm.201206-1150OC [DOI] [PubMed] [Google Scholar]
- 10. Hector A, Schäfer H, Pöschel S, Fischer A, Fritzsching B, Ralhan A, et al. Regulatory T-cell impairment in cystic fibrosis patients with chronic pseudomonas infection. Am J Respir Crit Care Med (2015) 191:914–23. doi: 10.1164/rccm.201407-1381OC [DOI] [PubMed] [Google Scholar]
- 11. Anil N, Singh M. CD4+CD25 high FOXP3+ regulatory T cells correlate with FEV1 in north Indian children with cystic fibrosis. Immunol Invest (2014) 43:535–43. doi: 10.3109/08820139.2014.888447 [DOI] [PubMed] [Google Scholar]
- 12. Harwood KH, McQuade RM, Jarnicki A, Schneider-Futschik EK. Anti-inflammatory influences of cystic fibrosis transmembrane conductance regulator drugs on lung inflammation in cystic fibrosis. Int J Mol Sci (2021) 22:7606. doi: 10.3390/ijms22147606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keown K, Brown R, Doherty DF, Houston C, McKelvey MC, Creane S, et al. Airway inflammation and host responses in the era of CFTR modulators. Int J Mol Sci (2020) 21:6379. doi: 10.3390/ijms21176379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hisert KB, Heltshe SL, Pope C, Jorth P, Wu X, Edwards RM, et al. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med (2017) 195:1617–28. doi: 10.1164/rccm.201609-1954OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jarosz-Griffiths HH, Scambler T, Wong CH, Lara-Reyna S, Holbrook J, Martinon F, et al. Different CFTR modulator combinations downregulate inflammation differently in cystic fibrosis. Elife (2020) 9:e54556. doi: 10.7554/eLife.54556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mainz JG, Arnold C, Wittstock K, Hipler U-C, Lehmann T, Zagoya C, et al. Ivacaftor reduces inflammatory mediators in upper airway lining fluid from cystic fibrosis patients with a G551D mutation: Serial non-invasive home-based collection of upper airway lining fluid. Front Immunol (2021) 12:642180. doi: 10.3389/fimmu.2021.642180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kopp BT, Fitch J, Jaramillo L, Shrestha CL, Robledo-Avila F, Zhang S, et al. Whole-blood transcriptomic responses to lumacaftor/ivacaftor therapy in cystic fibrosis. J Cyst Fibros (2020) 19:245–54. doi: 10.1016/j.jcf.2019.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Westhölter D, Beckert H, Straßburg S, Welsner M, Sutharsan S, Taube C, et al. Pseudomonas aeruginosa infection, but not mono or dual-combination CFTR modulator therapy affects circulating regulatory T cells in an adult population with cystic fibrosis. J Cyst Fibros (2021) 20:1072–9. doi: 10.1016/j.jcf.2021.05.001 [DOI] [PubMed] [Google Scholar]
- 19. Graeber SY, Vitzthum C, Pallenberg ST, Naehrlich L, Stahl M, Rohrbach A, et al. Effects of elexacaftor/tezacaftor/ivacaftor therapy on CFTR function in patients with cystic fibrosis and one or two F508del alleles. Am J Respir Crit Care Med (2022) 205:540–9. doi: 10.1164/rccm.202110-2249OC [DOI] [PubMed] [Google Scholar]
- 20. Rühle PF, Fietkau R, Gaipl US, Frey B. Development of a modular assay for detailed immunophenotyping of peripheral human whole blood samples by multicolor flow cytometry. Int J Mol Sci (2016) 17:1316. doi: 10.3390/ijms17081316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lehmann JS, Zhao A, Sun B, Jiang W, Ji S. Multiplex cytokine profiling of stimulated mouse splenocytes using a cytometric bead-based immunoassay platform. J Vis Exp (2017) 129):56440. doi: 10.3791/56440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu J, Ni X, Pan X, Lu H, Lu Y, Zhao J, et al. Human CD39hi regulatory T cells present stronger stability and function under inflammatory conditions. Cell Mol Immunol (2017) 14:521–8. doi: 10.1038/cmi.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iannitti RG, Carvalho A, Cunha C, De Luca A, Giovannini G, Casagrande A, et al. Th17/Treg imbalance in murine cystic fibrosis is linked to indoleamine 2,3-dioxygenase deficiency but corrected by kynurenines. Am J Respir Crit Care Med (2013) 187:609–20. doi: 10.1164/rccm.201207-1346OC [DOI] [PubMed] [Google Scholar]
- 24. Garić D, De Sanctis JB, Wojewodka G, Houle D, Cupri S, Abu-Arish A, et al. Fenretinide differentially modulates the levels of long- and very long-chain ceramides by downregulating Cers5 enzyme: Evidence from bench to bedside. J Mol Med (2017) 95:1053–64. doi: 10.1007/s00109-017-1564-y [DOI] [PubMed] [Google Scholar]
- 25. Becker KA, Riethmüller J, Lüth A, Döring G, Kleuser B, Gulbins E. Acid sphingomyelinase inhibitors normalize pulmonary ceramide and inflammation in cystic fibrosis. Am J Respir Cell Mol Biol (2010) 42:716–24. doi: 10.1165/rcmb.2009-0174OC [DOI] [PubMed] [Google Scholar]
- 26. Wiese T, Dennstädt F, Hollmann C, Stonawski S, Wurst C, Fink J, et al. Inhibition of acid sphingomyelinase increases regulatory T cells in humans. Brain Commun (2021) 3:fcab020. doi: 10.1093/braincomms/fcab020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hollmann C, Werner S, Avota E, Reuter D, Japtok L, Kleuser B, et al. Inhibition of acid sphingomyelinase allows for selective targeting of CD4+ conventional versus Foxp3+ regulatory T cells. J Immunol (2016) 197:3130–41. doi: 10.4049/jimmunol.1600691 [DOI] [PubMed] [Google Scholar]
- 28. Zhou Y, Salker MS, Walker B, Münzer P, Borst O, Gawaz M, et al. Acid sphingomyelinase (ASM) is a negative regulator of regulatory T cell (Treg) development. Cell Physiol Biochem (2016) 39:985–95. doi: 10.1159/000447806 [DOI] [PubMed] [Google Scholar]
- 29. Scholte BJ, Horati H, Veltman M, Vreeken RJ, Garratt LW, Tiddens HAWM, et al. Oxidative stress and abnormal bioactive lipids in early cystic fibrosis lung disease. J Cyst Fibros (2019) 18:781–9. doi: 10.1016/j.jcf.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 30. Westhölter D, Schumacher F, Wülfinghoff N, Sutharsan S, Strassburg S, Kleuser B, et al. CFTR modulator therapy alters plasma sphingolipid profiles in people with cystic fibrosis. J Cyst Fibros (2022) 21:713–20. doi: 10.1016/j.jcf.2022.02.005 [DOI] [PubMed] [Google Scholar]
- 31. Ding F-M, Zhu S-L, Shen C, Ji X-L, Zhou X. Regulatory T cell activity is partly inhibited in a mouse model of chronic pseudomonas aeruginosa lung infection. Exp Lung Res (2015) 41:44–55. doi: 10.3109/01902148.2014.964351 [DOI] [PubMed] [Google Scholar]
- 32. Einarsson GG, Ronan NJ, Mooney D, McGettigan C, Mullane D, NiChroinin M, et al. Extended-culture and culture-independent molecular analysis of the airway microbiota in cystic fibrosis following CFTR modulation with ivacaftor. J Cyst Fibros (2021) 20:747–53. doi: 10.1016/j.jcf.2020.12.023 [DOI] [PubMed] [Google Scholar]
- 33. Sheikh S, Britt R, Khan A, Johnson T, McCoy K, Kopp B. Impact of cystic fibrosis transmembrane conductance regulator modulator elexacaftor-tezacaftor-ivacaftor on lung function, BMI, bacterial colonization, and adaptive immune responses in patients with cystic fibrosis. Chest (2021) 160:A1446–7. doi: 10.1016/j.chest.2021.07.1325 [DOI] [Google Scholar]
- 34. Lenhan B, Fitzgerald L, Gifford A, Jia S. Clearance of colonized bacterial species in CF patients before and after elexacaftor/tezacaftor/ivacaftor. J Cyst Fibros (2021) 20:S219. doi: 10.1016/S1569-1993(21)01887-7 [DOI] [Google Scholar]
- 35. Shanthikumar S, Ranganathan SC, Saffery R, Neeland MR. Mapping pulmonary and systemic inflammation in preschool aged children with cystic fibrosis. Front Immunol (2021) 12:733217. doi: 10.3389/fimmu.2021.733217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lepissier A, Bonnel AS, Wizla N, Weiss L, Mittaine M, Bessaci K, et al. Moving the dial on airway inflammation in response to trikafta® in adolescents with cystic fibrosis. Am J Respir Crit Care Med (2023). doi: 10.1164/rccm.202210-1938LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Graeber SY, Renz DM, Stahl M, Pallenberg ST, Sommerburg O, Naehrlich L, et al. Effects of elexacaftor/tezacaftor/ivacaftor therapy on lung clearance index and magnetic resonance imaging in patients with cystic fibrosis and one or two F508del alleles. Am J Respir Crit Care Med (2022) 206:311–20. doi: 10.1164/rccm.202201-0219OC [DOI] [PubMed] [Google Scholar]
- 38. Nichols DP, Paynter AC, Heltshe SL, Donaldson SH, Frederick CA, Freedman SD, et al. Clinical effectiveness of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis: A clinical trial. Am J Respir Crit Care Med (2022) 205:529–39. doi: 10.1164/rccm.202108-1986OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.