Abstract
Background
GLPG1205 is a selective functional antagonist of G-protein-coupled receptor 84, which plays an important role in fibrotic processes. This study assessed the efficacy, safety and tolerability of GLPG1205 for treatment of idiopathic pulmonary fibrosis (IPF).
Methods
PINTA (ClinicalTrials.gov: NCT03725852) was a phase 2, randomised, double-blind, placebo-controlled, proof-of-concept trial. Patients with IPF were randomised 2:1 to once-daily oral GLPG1205 100 mg or placebo for 26 weeks and stratified to receive GLPG1205 alone or with local standard of care (nintedanib or pirfenidone). The primary end-point was change from baseline in forced vital capacity (FVC); other end-points were safety and tolerability, and lung volumes measured by imaging (high-resolution computed tomography). The study was not powered for statistical significance.
Results
In total, 68 patients received study medication. Least squares mean change from baseline in FVC at week 26 was −33.68 (95% CI −112.0–44.68) mL with GLPG1205 and −76.00 (95% CI −170.7–18.71) mL with placebo (least squares mean difference 42.33 (95% CI −81.84–166.5) mL; p=0.50). Lung volumes by imaging declined −58.30 versus −262.72 mL (whole lung) and −33.68 versus −135.48 mL (lower lobes) with GLPG1205 versus placebo, respectively. Treatment with GLPG1205 versus placebo resulted in higher proportions of serious and severe treatment-emergent adverse events and treatment-emergent discontinuations, most apparent with nintedanib.
Conclusions
Treatment with GLPG1205 did not result in a significant difference in FVC decline versus placebo. GLPG1205 demonstrated a poorer safety and tolerability profile than placebo.
Short abstract
The PINTA trial (NCT03725852) did not find a significant difference between GLPG1205 and placebo on change in FVC in patients with idiopathic pulmonary fibrosis. GLPG1205 demonstrated a poorer safety and tolerability profile versus placebo. https://bit.ly/3EQGst7
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrotic interstitial lung disease of unknown aetiology that primarily affects males aged ≥60 years [1]. Median survival following IPF diagnosis is ∼3–4 years [2, 3]. Two oral treatments, pirfenidone and nintedanib, are approved worldwide as therapies for IPF and are the current standard of care (SoC) [4–9]. While both drugs slow decline of forced vital capacity (FVC), they do not halt disease progression [10, 11] and are frequently associated with adverse drug reactions, potentially limiting use in clinical practice. Thus, an unmet need exists for additional treatments among patients with IPF [12]. Evaluation of potential treatments on top of local SoC is aligned with real-world clinical practice and allows detection of potential additive or synergistic effects.
G-protein-coupled receptor 84 (GPR84) plays an important role in the innate immune response and fibrotic processes [13, 14]. GPR84 knockout mice have shown reduced kidney fibrosis in a model of nephropathy, suggesting GPR84 could represent a promising molecular target in other fibrotic diseases such as IPF [13]. GLPG1205 is a selective functional antagonist of GPR84 that has demonstrated antifibrotic effects in murine models of bleomycin- or radiation-induced lung fibrosis [13, 15]. In healthy volunteers, GLPG1205 resulted in extensive and sustained reduction of ligand binding to GPR84; multiple doses ≤100 mg once daily for 14 days were well tolerated [16].
Here we report findings from a phase 2 trial that evaluated the efficacy, safety and tolerability of GLPG1205 in patients with IPF.
Methods
Study design
The PINTA study (ClinicalTrials.gov: NCT03725852) was a phase 2, randomised, double-blind, placebo-controlled, proof-of-concept trial conducted at 28 centres in nine countries (supplementary material). Patients with IPF received oral GLPG1205 100 mg (substance manufacturer: PCAS, Limay, France; product capsules used in study: Glatt Pharmaceuticals, Binzen, Germany) or placebo once daily alone or on top of local SoC (nintedanib or pirfenidone) for 26 weeks.
This study was conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonisation. The protocol was approved prior to study initiation by relevant local independent institutional review boards/ethics committees. All patients provided written informed consent.
Participants
To be included, patients were required to be males or females of non-child-bearing potential aged ≥40 years with: a diagnosis of IPF ≤5 years prior to screening per relevant clinical guidelines [1]; a diagnosis of IPF confirmed by central review of both high-resolution computed tomography (HRCT) images taken ≤12 months prior to or during screening and lung biopsies, if available; FVC ≥50% of predicted normal; forced expiratory volume in 1 s (FEV1)/FVC ≥0.7; and disease progression, defined as a decline in FVC during the 9 months prior to screening per the investigator's discretion.
Patients were excluded if they had a current immunosuppressive condition, history of lung transplant or recent/current use of non-evidence-based medicines for IPF (see supplementary material for further details of eligibility criteria and study population size calculations).
Randomisation and masking
Patients were randomised 2:1 to receive oral GLPG1205 100 mg or placebo once daily for 26 weeks. In case of an adverse event (AE) possibly related to, or intolerance of, study treatment, per the investigator's judgement, patients could have their dose reduced to 50 mg once daily (or corresponding placebo) for the remainder of the study. Patients were stratified based on prior SoC. Participants, personnel, investigators and outcome assessors were blinded to treatment allocation.
Assessments
Study visits took place at screening (≤28 days before day 1) and then on day 1 (baseline) and at weeks 2, 4, 8, 12, 16, 20 and 26; a follow-up assessment was conducted at week 30, as described in supplementary table S1. As PINTA was partly conducted during the coronavirus disease 2019 (COVID-19) pandemic, appropriate steps were taken to ensure patients’ safety while maintaining study integrity, including reasonable accommodations to assessment timings (supplementary material).
Pharmacokinetic assessments conducted to evaluate plasma concentrations of GLPG1205, nintedanib and pirfenidone are described in supplementary figure S1.
Treatment-emergent AEs (TEAEs), serious TEAEs, discontinuations due to TEAEs and deaths were monitored throughout the study. AEs were coded according to MedDRA (Medical Dictionary for Regulatory Activities).
End-points
The primary end-point was the change in FVC from baseline to week 26 versus placebo. Secondary end-points were: safety and tolerability from baseline to 26 weeks; the number and proportion of patients with a major event (all-cause and respiratory related), including death and first hospitalisation (whichever occurred first); change in functional exercise capacity from baseline to 26 weeks, assessed by the 6-min walk test (6MWT); change in St George's Respiratory Questionnaire (SGRQ) total score from baseline to 26 weeks; the proportion of SGRQ responders at 26 weeks (e.g. those with an absolute change from baseline in SGRQ total score ≤ –4 units, at least once); and plasma concentrations of GLPG1205, nintedanib and pirfenidone. Other end-points included change from baseline in diffusing capacity of the lung for carbon monoxide (DLCO) % pred corrected and change in lung volume (lobar volume at full inspiration in the total lung region and in the lower lobes specifically given their correlation with the study's primary end-point, FVC [17]), assessed by HRCT (supplementary material).
Statistical analysis
This was a proof-of-concept study to identify a trend in treatment difference for the primary end-point; no formal sample size calculation was performed (supplementary material). The study was not powered for statistical significance.
The full analysis set included all randomised patients who received ≥1 doses of GLPG1205 or placebo. The primary efficacy analysis was performed using an ANCOVA model including treatment, sex and SoC stratum as categorical covariates and age, height and baseline FVC value as continuous covariates. A similar prespecified ANCOVA model with an additional term for treatment-by-stratum interaction was used to estimate the least squares (LS) mean treatment difference by SoC stratum. No imputation was performed for missing data. For FVC, the mean of the analysis values before the first treatment dose (including screening, baseline and unscheduled values, as applicable) was used to define the baseline value to reduce variability. A sensitivity analysis was performed to assess the annualised rate of decline in FVC with GLPG1205 and placebo, using a random coefficient linear regression model including treatment, sex and SoC stratum as categorical fixed effects; time, age and height as continuous fixed effects; and a random intercept and slope. A mixed effect repeated measures model (MMRM) was performed to compare change from baseline in FVC across treatment groups, including sex, SoC stratum and a treatment-by-time-point interaction as categorical fixed effects; age, height and baseline FVC as continuous fixed effects; and correlated within-subject residuals. Lastly, sensitivity analyses were conducted to evaluate the impact of delaying primary end-point assessments from week 26 to week 30 because of the COVID-19 pandemic. A post hoc analysis was performed to assess the impact of excluding outliers (defined as measurements with an absolute change from baseline in FVC >600 mL). Secondary efficacy analyses are described in the supplementary material.
Results
Patients
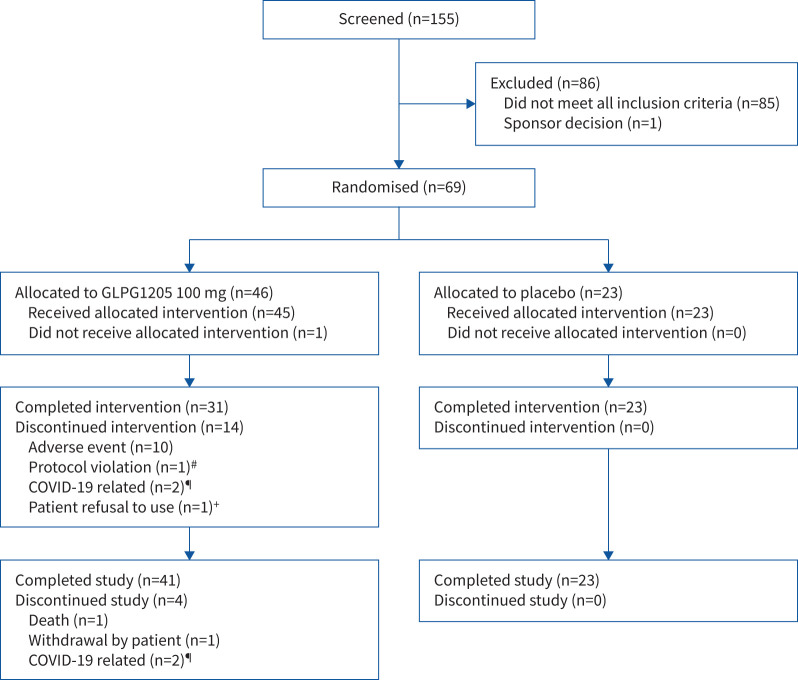
Of the 69 patients randomised, 68 received ≥1 doses of study medication (GLPG1205 100 mg, n=45; placebo, n=23) (figure 1). One patient randomised to GLPG1205 met an exclusion criterion before dosing began and did not receive study treatment. Of the 68 patients treated, 19 were receiving pirfenidone, 24 were receiving nintedanib and 25 were receiving neither medication as background treatment for IPF.
FIGURE 1.
Patient disposition (full analysis set). #: patient did not meet the 12-lead ECG eligibility criteria and was randomised in error, then terminated early from the study; ¶: travel restrictions related to COVID-19; +: patient had a serious adverse event of interstitial lung disease and refused to take the investigational product (patient later died).
In total, 54/68 patients (79.4%) completed 26 weeks of treatment. The remaining 14 patients (all randomised to GLPG1205) discontinued treatment before week 26: 10 because of TEAEs (nintedanib, n=6; pirfenidone, n=2; neither SoC, n=2) (supplementary tables S2 and S3), one because of a protocol violation, two because of COVID-19-related travel restrictions, and one who refused treatment after hospitalisation for a serious AE of interstitial lung disease and later died. Overall treatment compliance was 96.1%, with no major differences between treatment groups. Median total duration of treatment was 183 days for both the GLPG1205 and placebo groups. Overall, the study data were considered minimally impacted by the COVID-19 pandemic (supplementary material).
Baseline characteristics are shown in table 1. The study population was representative of a contemporary IPF population; the majority were male (73.5%) with a mean age of 69.8 years. The proportions of patients receiving nintedanib, pirfenidone or neither as background therapy were largely balanced within the GLPG1205 and placebo arms. The mean duration of IPF was 2.51, 2.16 and 1.19 years for patients on a background of pirfenidone, nintedanib and no background treatment, respectively.
TABLE 1.
Baseline characteristics (full analysis set)
| GLPG1205 100 mg (n=45) | Placebo (n=23) | |
| Age, years | 70.5±6.8 | 68.3±5.5 |
| Sex | ||
| Male | 33 (73.3) | 17 (73.9) |
| Female | 12 (26.7) | 6 (26.1) |
| Race | ||
| Asian | 2 (5.9) | 0 |
| White | 32 (94.1) | 17 (100) |
| Smoking status | ||
| Current | 0 | 0 |
| Ex-smoker | 21 (46.7) | 13 (56.5) |
| Never-smoker | 24 (53.3) | 10 (43.5) |
| Background standard of care | ||
| Currently receiving nintedanib | 17 (37.8) | 7 (30.4) |
| Currently receiving pirfenidone | 11 (24.4) | 8 (34.8) |
| Never received nintedanib/pirfenidone | 16 (35.6) | 8 (34.8) |
| Stopped pirfenidone/nintedanib | 1 (2.2) | 0 |
| Duration of IPF, years | 1.9±1.3 | 2.0±1.2 |
| Duration of IPF# | ||
| <0.5 years | 10 (22.2) | 2 (8.7) |
| ≥0.5– <1 years | 6 (13.3) | 3 (13.0) |
| ≥1– <2 years | 11 (24.4) | 9 (39.1) |
| ≥2– <3 years | 10 (22.2) | 4 (17.4) |
| ≥3 years | 8 (17.8) | 5 (21.7) |
| FVC, mL | 2865.4±694.6 | 2817.1±804.6 |
| FVC, % pred | 80.3±17.1 | 75.8±15.4 |
| 6-min walk distance , m | 412.6±124.3 | 391.8±123.4 |
| SGRQ total score | 45.4±19.1 | 48.6±18.9 |
| Corrected DLCO, mmol·min−1·kPa−1 | 3.7±1.4 | 3.4±1.3 |
| Corrected DLCO, % pred | 48.0±14.8 | 46.2±15.2 |
| CPI | 46.4±11.7 | 48.6±10.8 |
Data are presented as n (%) or mean±sd. IPF: idiopathic pulmonary fibrosis; FVC: forced vital capacity; SGRQ: St George's Respiratory Questionnaire; DLCO: diffusing capacity of the lung for carbon monoxide; CPI: composite physiologic index. #: (date of first administration of investigational product minus date of the initial diagnosis)/365.25.
Efficacy
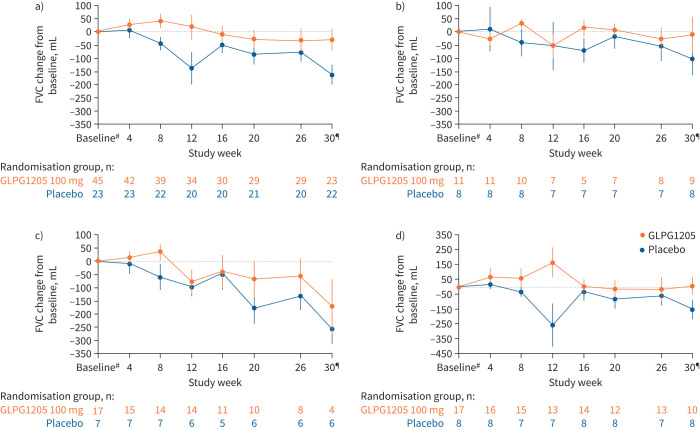
Primary end-point results, i.e. change in FVC from baseline to week 26 versus placebo, are presented over time and by background therapy (figure 2). The primary end-point was not met. The LS mean decline in FVC from baseline to 26 weeks from the ANCOVA model was −33.68 mL for GLPG1205 and −76.00 mL for placebo (LS mean treatment difference 42.33 (95% CI −81.84–166.5) mL; p=0.50) (table 2). The LS mean difference between GLPG1205 and placebo for FVC % pred at week 26 was 1.20% (p=0.48). FVC change from baseline showed comparable trends for GLPG1205 versus placebo in the three SoC strata (table 2). From the sensitivity analysis using a random coefficient linear regression model, the LS mean difference in annualised rate of FVC decline between GLPG1205 and placebo was 115.6 mL (p=0.29).
FIGURE 2.
Forced vital capacity (FVC) by time in a) the overall population and b–d) stratified by standard of care: b) pirfenidone, c) nintedanib or d) neither nintedanib nor pirfenidone. Error bars represent standard error. #: baseline values were defined as the mean of the analysis values before first treatment dose; ¶: safety follow-up visits were conducted at week 30.
TABLE 2.
Efficacy end-points (full analysis set)
| GLPG1205 100 mg | Placebo | Treatment difference# | p-value | |
| Primary end-point: change from baseline in FVC at week 26, mL | ||||
| Overall population | n=29 | n=20 | ||
| LS mean (95% CI) | −33.68 (−112.0–44.68) | −76.00 (−170.7–18.71) | 42.33 (−81.84–166.5) | 0.50 |
| Pirfenidone SoC stratum | n=8 | n=7 | ||
| LS mean (95% CI) | 12.96 (−150.64–176.57) | −47.44 (−211.58–116.70) | 60.40 (−169.12–289.92) | 0.60 |
| Nintedanib SoC stratum | n=8 | n=6 | ||
| LS mean (95% CI) | −74.46 (−233.14–84.21) | −127.67 (−303.32–47.98) | 53.21 (−184.25–290.66) | 0.65 |
| Neither pirfenidone nor nintedanib stratum | n=13 | n=7 | ||
| LS mean (95% CI) | −39.46 (−167.05–88.13) | −56.24 (−226.20–113.71) | 16.78 (−199.52–233.09) | 0.88 |
| Secondary end-points | ||||
| Major events through week 26 | ||||
| Respiratory-related mortality, %¶ | 3.1 | 0 | 0.40 | |
| All-cause hospitalisation, %¶ | 16.6 | 4.3 | 0.13 | |
| Respiratory-related hospitalisation, %¶ | 2.8 | 4.3 | 0.76 | |
| Change from baseline in 6MWT distance over 26 weeks, m | n=26 | n=19 | ||
| LS mean (95% CI) | −17.11 (−37.70–3.47) | −8.00 (−32.09–16.09) | −9.11 (−40.87–22.64) | 0.57 |
| Change from baseline in SGRQ total score over 26 weeks | n=29 | n=19 | ||
| LS mean (95% CI) | −3.5 (−8.2–1.2) | −1.9 (−7.7–3.9) | −1.6 (−9.1–5.9) | 0.67 |
| Lung lobar volumes measured by HRCT | ||||
| Change from baseline in image-based lung volume at full inspiration, mL | n=25 | n=16 | ||
| Total lung region, mean±se | −58.30±130.927 | −262.72±110.155 | –+ | |
| Lower lobes, mean±se | −33.68±56.632 | −135.48±55.498 | –+ |
FVC: forced vital capacity; LS: least squares; SoC: standard of care; 6MWT: 6-min walk test; SGRQ: St George's Respiratory Questionnaire; HRCT: high-resolution computed tomography. #: GLPG1205 minus placebo; ¶: cumulative percentage based on Kaplan–Meier estimates; +: correlation between image-based lung volume and change in FVC from baseline to week 26 (Spearman correlation coefficient) was 0.64 for the total lung region and 0.67 for the lower lobes; see supplementary table S4 for further details.
The MMRM and ANCOVA models gave similar results for FVC change from baseline; at week 26, the LS mean change from baseline was −38.79 (95% CI −118.92–41.33) mL for GLPG1205 and −101.78 (95% CI −203.14– −0.41) mL for placebo (LS mean treatment difference 62.98 (95% CI −66.86–192.83) mL; p=0.33). Sensitivity analyses showed that primary end-point results were unaffected by outliers (supplementary figure S2) or the COVID-19 pandemic (supplementary material).
One respiratory-related death occurred in the GLPG1205 arm, in a patient receiving nintedanib; no deaths occurred in the placebo arm. The cumulative percentage of patients with an all-cause hospitalisation at week 26 was 16.6% for GLPG1205 and 4.3% for placebo; the difference in the time to first all-cause hospitalisation between the groups was not statistically significant (p=0.13) (table 2). One respiratory-related hospitalisation occurred in each of the two arms.
Both treatment groups demonstrated a decrease from baseline in mean total distance walked in the 6MWT at 26 weeks (table 2). A decrease in SGRQ total score from baseline was observed in both arms (table 2) but was not considered clinically relevant and between-patient variability in SGRQ scores was high. The LS mean±se decline from baseline in DLCO % pred corrected at week 26 was 1.2±2.22% for GLPG1205 and 1.8±2.49% for placebo (supplementary figure S3).
The mean decline from baseline to 26 weeks in HRCT-based lobar volume at full inspiration in patients receiving GLPG1205 versus placebo was −58.30 versus −262.72 mL (total lung region) and −33.68 versus −135.48 mL (lower lobes) (table 2 and supplementary table S4). At week 26, a significant correlation was observed between changes in total lung volume and lower lobe volume (Spearman correlation 0.64 and 0.67, respectively) with change in FVC.
Safety and tolerability
A similar proportion of patients reported TEAEs in the GLPG1205 and placebo groups (80.0% (36/45 patients) and 78.3% (18/23 patients), respectively) (tables 3 and 4). Gastrointestinal disorders, including nausea and diarrhoea, were the most commonly reported TEAEs and occurred in 53.3% (24/45) of GLPG1205-treated patients versus 21.7% (5/23) of placebo-treated patients. TEAEs of weight decrease occurred in 13.3% (6/45) of GLPG1205-treated patients and 4.3% (1/23) of placebo-treated patients. At week 26, mean change from baseline in weight was −2.5 kg (–2.9%) for GLPG1205 and +0.2 kg (+0.4%) for placebo.
TABLE 3.
Summary of treatment-emergent adverse events (TEAEs) (full analysis set)
| GLPG1205 100 mg (n=45)# | Placebo (n=23)# | |
| TEAEs | 36 (80.0) | 18 (78.3) |
| Serious TEAEs | 9 (20.0) | 1 (4.3) |
| TEAEs leading to death | 1 (2.2) | 0 |
| TEAEs with worst severity | ||
| Mild | 10 (22.2) | 7 (30.4) |
| Moderate | 14 (31.1) | 10 (43.5) |
| Severe | 8 (17.8) | 1 (4.3) |
| Life-threatening | 3 (6.7) | 0 |
| Death | 1 (2.2) | 0 |
| TEAEs related to GLPG1205/placebo | 20 (44.4) | 3 (13.0) |
| Pirfenidone SoC stratum | 5/11 (45.5) | 2/8 (25.0) |
| Nintedanib SoC stratum | 12/17 (70.6) | 1/7 (14.3) |
| Neither pirfenidone nor nintedanib stratum | 3/17 (17.6) | 0/8 (0.0) |
| Study treatment GLPG1205/placebo | ||
| Reduced¶ | 2 (4.4) | 1 (4.3) |
| Temporarily stopped | 4 (8.9) | 2 (8.7) |
| Permanently stopped | 10 (22.2) | 0 |
Data are presented as n (%). SoC: standard of care. #: except where noted otherwise; ¶: dose reduction was not preceded by treatment interruption for the same reported event. In addition, three patients receiving GLPG1205 100 mg (two on a background of nintedanib and one on a background of pirfenidone) and none on placebo interrupted treatment because of TEAEs and restarted on a reduced dose.
TABLE 4.
Most common treatment-emergent adverse events (full analysis set)
| GLPG1205 100 mg (n=45) | Placebo (n=23) | |
| Gastrointestinal disorders | 24 (53.3) | 5 (21.7) |
| Diarrhoea | 12 (26.7) | 3 (13.0) |
| Nausea | 13 (28.9) | 2 (8.7) |
| Abdominal pain | 5 (11.1) | 0 |
| Vomiting | 4 (8.9) | 0 |
| Abdominal pain (upper) | 3 (6.7) | 0 |
| Infections and infestations | 11 (24.4) | 10 (43.5) |
| Nasopharyngitis | 6 (13.3) | 4 (17.4) |
| Bronchitis | 3 (6.7) | 3 (13.0) |
| Nervous system disorders | 13 (28.9) | 5 (21.7) |
| Headache | 11 (24.4) | 4 (17.4) |
| Dizziness | 6 (13.3) | 2 (8.7) |
| Respiratory, thoracic and mediastinal disorders | 12 (26.7) | 5 (21.7) |
| Cough | 6 (13.3) | 2 (8.7) |
| Dyspnoea | 5 (11.1) | 2 (8.7) |
| Epistaxis | 0 | 2 (8.7) |
| Interstitial lung disease | 2 (4.4) | 0 |
| General disorders and administration site conditions | 14 (31.1) | 0 |
| Asthenia | 9 (20.0) | 0 |
| Fatigue | 6 (13.3) | 0 |
| Investigations | 12 (26.7) | 1 (4.3) |
| Weight decreased | 6 (13.3) | 1 (4.3) |
| Alanine aminotransferase increased | 3 (6.7) | 0 |
| Aspartate aminotransferase increased | 3 (6.7) | 0 |
| γ-glutamyltransferase increased | 3 (6.7) | 0 |
| Blood bilirubin increased | 2 (4.4) | 0 |
| Blood lactate dehydrogenase increased | 2 (4.4) | 0 |
| Blood pressure increased | 2 (4.4) | 0 |
| Musculoskeletal and connective tissue disorders | 9 (20.0) | 3 (13.0) |
| Back pain | 3 (6.7) | 1 (4.3) |
| Myalgia | 2 (4.4) | 1 (4.3) |
| Metabolism and nutrition disorders | 8 (17.8) | 0 |
| Decreased appetite | 5 (11.1) | 0 |
| Skin and subcutaneous tissue disorders | 4 (8.9) | 4 (17.4) |
| Pruritus | 3 (6.7) | 0 |
| Rash | 2 (4.4) | 1 (4.3) |
| Psychiatric disorders | 6 (13.3) | 0 |
| Anxiety | 2 (4.4) | 0 |
| Insomnia | 2 (4.4) | 0 |
| Blood and lymphatic system disorders | 4 (8.9) | 1 (4.3) |
| Leukocytosis | 2 (4.4) | 0 |
| Neutrophilia | 2 (4.4) | 0 |
| Vascular disorders | 3 (6.7) | 1 (4.3) |
| Hypertension | 3 (6.7) | 1 (4.3) |
| Hepatobiliary disorders | 2 (4.4) | 1 (4.3) |
| Injury, poisoning and procedural complications | 3 (6.7) | 0 |
| Renal and urinary disorders | 3 (6.7) | 0 |
| Polyuria | 2 (4.4) | 0 |
Data are presented as n (%). Only MedDRA system organ classes and preferred terms reported in ≥2 patients in any treatment group are included.
A greater proportion of patients experienced serious TEAEs and permanent treatment discontinuations due to TEAEs in the GLPG1205 group versus placebo (serious TEAEs: 20.0% (9/45 patients) versus 4.3% (1/23 patients); permanent treatment discontinuations: 22.2% (10/45 patients) versus 0%). Additionally, a higher proportion of patients in the GLPG1205 group than the placebo group experienced severe or life-threatening TEAEs (severe: 17.8% (8/45 patients) versus 4.3% (1/23 patients); life-threatening: 6.7% (3/45 patients) versus 0%). One death occurred in the GLPG1205 arm. This patient experienced a severe serious AE of an acute exacerbation of IPF and severe non-serious TEAEs of gastric ulcer, pyrexia and bronchitis. The patient received GLPG1205 on a background of nintedanib and died of worsening IPF, which was considered by the investigator not to be related to GLPG1205 or nintedanib treatment.
Coadministration of GLPG1205 with nintedanib seemed to result in a less favourable safety and tolerability profile than its coadministration with pirfenidone or neither SoC. GLPG1205 treatment was permanently discontinued in 6/17 patients (35.3%) in the GLPG1205 with nintedanib stratum (compared with two patients each in the pirfenidone and neither SoC strata (18.2% and 11.8%, respectively)) (supplementary tables S2 and S3). Serious TEAEs occurred in 7/17 patients (41.2%) given GLPG1205 with nintedanib, 0/11 patients given GLPG1205 with pirfenidone and 2/17 patients (11.8%) given GLPG1205 with neither SoC. Life-threatening TEAEs only occurred in the GLPG1205 with nintedanib stratum (17.6% (3/17 patients)).
Gastrointestinal TEAEs seemed to be more common when GLPG1205 was coadministered with nintedanib (64.7% (11/17 patients)) or neither SoC (58.8% (10/17 patients)) than with pirfenidone (27.3% (3/11 patients)). A higher proportion of TEAEs of weight decrease was observed when GLPG1205 was coadministered with nintedanib (23.5% (4/17 patients)) than with pirfenidone (18.2% (2/11 patients)) or neither SoC (0 patients). Mean body weight change from baseline was more pronounced in patients who received GLPG1205 and nintedanib (–4.5 kg (–4.9%) versus +1.2 kg (+1.7%) for placebo and nintedanib) than GLPG1205 and pirfenidone (–3.3 kg (–4.2%) versus −0.6 kg (–0.8%) for placebo and pirfenidone) or GLPG1205 and neither SoC (–0.6 kg (–0.9%) versus +0.1 kg (+0.4%) for placebo and neither SoC). Treatment discontinuations in all SoC strata were primarily related to gastrointestinal side-effects (vomiting, nausea and diarrhoea). Two patients receiving GLPG1205 with nintedanib reported clinically meaningful increases in transaminases (supplementary table S3). In both cases, the treatment with the study medication was withdrawn (i.e. patients permanently discontinued from the study) and both cases resolved.
Three patients, all on a background of nintedanib, had their study treatment dose reduced to 50 mg once daily (or corresponding placebo) because of TEAEs during the study (without preceding interruption for the same reported event; two were receiving GLPG1205 and one was receiving placebo) (supplementary table S2). Three patients receiving GLPG1205 100 mg (two on a background of nintedanib and one on a background of pirfenidone) interrupted treatment because of TEAEs and restarted on a reduced dose of GLPG1205 50 mg. By study end, all non-fatal serious TEAEs and TEAEs leading to treatment discontinuation had resolved/were resolving (supplementary table S3). Plasma concentrations of GLPG1205 were not affected by coadministration of nintedanib or pirfenidone, although coadministration of GLPG1205 numerically increased median nintedanib plasma levels at 2 h post-dose of SoC (results of pharmacokinetic assessments are presented in supplementary figure S1).
Discussion
The PINTA study did not meet its primary or secondary end-points. An imbalance in the incidence of higher-grade TEAEs, serious TEAEs and early discontinuations due to TEAEs was observed in patients receiving GLPG1205, and was more pronounced in patients receiving GLPG1205 with nintedanib. While increased median nintedanib plasma levels were seen 2 h post-dose of SoC when coadministered with GLPG1205, there is no current mechanistic explanation for this observation and this initial finding was based on a small sample size with high between-patient variability. Additional investigations would be needed to fully evaluate this finding; however, other studies have found similar trends of increased AEs with nintedanib coadministration [18].
Although no difference between groups was found in FVC, HRCT-measured lung lobar volumes were numerically greater in patients receiving GLPG1205 versus placebo, with a stronger signal in the lower lobes. This is in line with IPF disease distribution and consistent with numerical trends seen in FVC, supporting treatment potential of GPR84 antagonism. While lung volume by HRCT is not validated in clinical trials, lung volume has been shown to be strongly correlated with HRCT and FVC [17, 19]. Results from the PINTA trial support the use of lung volume assessment by HRCT as a measure of IPF disease progression alongside FVC in IPF studies, pending validation as an end-point, which could help further contextualise low or moderate numerical change in FVC in clinical trials with small patient populations.
In the PINTA study, after 26 weeks, a 42 mL treatment difference in FVC was seen with GLPG1205 versus placebo. This is consistent with published data from the 24-week INSTAGE study, in which sildenafil coadministered with nintedanib demonstrated a treatment effect of 46 mL [20]. The LS mean treatment difference in FVC % pred change from baseline to 26 weeks was 1.20%. Extrapolating the results to a 1-year period, assuming linearity, would produce an estimated annual effect size of 2.4%. In comparison, a 28-week study comparing pentraxin with placebo found a 2.3% difference in LS mean change from baseline in FVC % pred [21]. Studies of nintedanib in treatment-naïve patients with IPF found mean observed declines from baseline in FVC of ∼50 mL after 24 weeks of treatment [11], whereas in this 26-week study, the LS mean value for patients receiving GLPG1205 was 34 mL.
This was a well-designed, randomised, controlled trial and is the first 6-month study in IPF evaluating a new small molecule in addition to SoC treatment. This method allows for therapeutic response beyond that of SoC to be predicted in a population of patients already receiving treatment. Pentraxin, a biologic, has also been evaluated on top of SoC (nintedanib or pirfenidone) for 24 weeks and showed positive FVC efficacy signals, although a higher proportion of patients were on background therapy (78.4% versus 63.3% in this study) [21]. A smaller phase 2 trial evaluating PBI-4050 in IPF employed a similar approach, but was limited by an open-label design and shorter duration (12 weeks) [22]. While study methodologies evaluating a therapy “on top” of SoC have some benefits (e.g. broadening of the potential recruitment pool to include patients already receiving treatment), the risk of increased patient heterogeneity and potential recruitment bias is notable. Patients showing IPF progression despite SoC treatment may differ from treatment-naïve patients and patients who are more stable on SoC, with trial recruitment of the latter group being unlikely. One lesson learned is that allowing background SoC therapy makes the evaluation of both efficacy and safety/tolerability more complex. In the present trial, it was challenging to evaluate the safety/tolerability of GLPG1205 in the absence of SoC.
The PINTA study was limited by its small sample size, high between-patient variability of the primary end-point (FVC) and high rate of permanent treatment discontinuations. This proof-of-concept study was designed to examine the feasibility of larger studies to evaluate GLPG1205 in IPF treatment. Missing data were not imputed; this limitation was addressed by MMRM and linear mixed model sensitivity analyses, which both accounted for missing-at-random data. While most missing data were due to treatment discontinuation (implying study discontinuation and no further data collection), the linear mixed model assumed a linear decline in FVC over time and implicitly imputed missing data based on individual patient's estimated rate of decline before treatment discontinuation, resulting in a reasonably conservative estimate. Therefore, further sensitivity analyses of patients who discontinued treatment before week 26 were not performed due to the limited size of the study. The optimal design of future studies combining SoC with novel agents is yet to be fully elucidated, specifically in the context of recent phase 2 studies of pentraxin [21] and BI1015550 [23] showing a larger than expected decline in FVC in IPF in patients on SoC. In this context, the forthcoming results of the two prematurely terminated Galapagos ISABELA phase 3 studies (ClinicalTrials.gov: NCT03711162 and NCT03733444) [24] of the autotaxin inhibitor ziritaxestat, which has a different therapeutic target than GLPG1205, will be relevant.
Conclusions
The primary end-point for the study was not met. Compared with placebo, treatment with GLPG1205 resulted in higher proportions of serious and severe TEAEs and treatment-emergent discontinuations, most apparent in the nintedanib stratum. Additional studies are required to further characterise the effect of GPR84 modulation in IPF.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01794-2022.Supplement (774.2KB, pdf)
Shareable PDF
Acknowledgements
We thank the patients and investigators (N. Al Busaidi, L. Bjermer, V. Blazhko, V. Cottin, M. Diakopoulou, E. Docheva, E. Ekbom, V. Gavrysyuk, S. Hrebenar, C. Hugo Marquette, D. Israel Biet, Y. Ivanov, K. Lebed, M. Man, S. Marchand-Adam, T. Mihaescu, M. Myllarniemi, M. Ostrovskyy, J. Plutinsky, G. Prevot, S. Quetant, M. Samarzija, O. Smolianyi, I. Strambu, I. Strander and L. Wemeau Stervinou) who participated in this study. We give special thanks to Silke Hüttner and Ellen Jansen (Galapagos NV, Mechelen, Belgium) for designing the study, writing the protocol, setting up the study and initiating the study until first patient enrolment. We thank Ralph Preiss (Galapagos GmbH, Basel, Switzerland) and Bernt van den Blink (Galapagos NV, Leiden, The Netherlands) for reviewing the manuscript and providing critical feedback. Medical writing support was provided by Kristian Clausen (Aspire Scientific Ltd, Bollington, UK), funded by Galapagos NV.
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.02355-2022
This study was prospectively registered at ClinicalTrials.gov with identifier number NCT03725852. Individual participant data are not currently publicly available.
Author contributions: I.R. Strambu was involved in study investigation and recruitment. C.A. Seemayer contributed to study conceptualisation, design and methodology, and data interpretation. L.M-C.A. Fagard contributed to study design and methodology, and data interpretation. P.A. Ford contributed to study conceptualisation, design and methodology, and data interpretation. T.A.K. Van der Aa contributed to study design and methodology, and study recruitment. A.A. de Haas-Amatsaleh contributed to study design and methodology, and data interpretation. V. Modgill contributed to study design and methodology, and data interpretation. E. Santermans contributed to study conceptualisation, design and methodology, formal analysis and visualisation, and data interpretation. E.N. Sondag contributed to study conceptualisation and data interpretation. E.G. Helmer contributed to study conceptualisation, design and methodology, formal analysis and visualisation, and data interpretation. T.M. Maher contributed to data interpretation and served as a study advisory board member. U. Costabel contributed to data interpretation and served as a study advisory board member. V. Cottin contributed to study investigation and recruitment, data interpretation, and served as a study advisory board member. E. Santermans and A.A. de Haas-Amatsaleh verified the data in this manuscript. All authors had full access to the study data, reviewed the manuscript and accepted responsibility to submit the manuscript for publication.
Conflict of interest: I.R. Strambu is an investigator in the PINTA study and received an investigator's fee and support for travel costs to a PINTA investigator meeting from Galapagos; she has also received investigator's fees from Novartis and GlaxoSmithKline; has been paid as a speaker by AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis, Roche and Teva; and has been an advisory board member for Boehringer Ingelheim. C.A. Seemayer, T.A.K. Van der Aa, A.A. de Haas-Amatsaleh and E. Santermans are employees of Galapagos and have received warrants from Galapagos. L.M-C.A. Fagard, E.N. Sondag, E.G. Helmer and P.A. Ford were employees of Galapagos at the time of the study and have received warrants from Galapagos. V. Modgill is an employee of Galapagos. T.M. Maher has, via his institution, received industry-academic funding from AstraZeneca and GlaxoSmithKline R&D; and has received consultancy and/or speakers’ fees from AstraZeneca, Bayer, Blade Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Galapagos, Galecto, GlaxoSmithKline R&D, IQVIA, Pliant, Respivant, Roche and Theravance. U. Costabel has received personal fees from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Fibrogen, Galapagos, Novartis, Pliant Therapeutics and Roche, and has served on data safety monitoring boards for Boehringer Ingelheim, Galapagos (including for the PINTA study), Roche and Sanofi. V. Cottin reports personal fees and non-financial support from Actelion and Roche/Promedior; grants, personal fees and non-financial support from Boehringer Ingelheim; and personal fees from AstraZeneca, Bayer/MSD, Celgene/Bristol Myers Squibb, Fibrogen, Galapagos, Galecto, Novartis, PureTech, RedX, Sanofi and Shionogi, outside the submitted work.
Support statement: This work was funded by Galapagos NV. The funders were involved in study design, data collection, data analysis and interpretation, and reviewed the manuscript for accuracy. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. doi: 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 2.Strongman H, Kausar I, Maher TM. Incidence, prevalence, and survival of patients with idiopathic pulmonary fibrosis in the UK. Adv Ther 2018; 35: 724–736. doi: 10.1007/s12325-018-0693-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G, Chen SY, Yeh WS, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med 2014; 2: 566–572. doi: 10.1016/S2213-2600(14)70101-8 [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Agency . Summary of product characteristics – Esbriet. 2011. www.ema.europa.eu/en/documents/product-information/esbriet-epar-product-information_en.pdf Date last accessed: 7 February 2022.
- 5.European Medicines Agency . Summary of product characteristics – Ofev. 2015. www.ema.europa.eu/en/documents/product-information/ofev-epar-product-information_en.pdf Date Last accessed: 7 February 2022.
- 6.US Food and Drug Administration . Summary of prescribing information – Esbriet. 2014. www.accessdata.fda.gov/drugsatfda_docs/label/2014/022535s000lbl.pdf Date Last accessed: 7 February 2022.
- 7.US Food and Drug Administration . Summary of prescribing information – Ofev. 2014. www.accessdata.fda.gov/drugsatfda_docs/label/2014/205832s000lbl.pdf Date Last accessed: 7 February 2022.
- 8.Ministry of Health, Labour and Welfare . Report on the Deliberation Results: Ministry of Health, Labour and Welfare; 16 September 2008. 2008. www.pmda.go.jp/files/000153687.pdf Date last accessed: 7 February 2022.
- 9.Ministry of Health, Labour and Welfare . Report on the Deliberation Results: Ministry of Health, Labour and Welfare; 3 June 2015. 2015. www.pmda.go.jp/files/000214531.pdf Date last accessed: 7 February 2022.
- 10.King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014: 370: 2083–2092. doi: 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 11.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. doi: 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 12.Wuyts WA, Antoniou KM, Borensztajn K, et al. Combination therapy: the future of management for idiopathic pulmonary fibrosis? Lancet Respir Med 2014; 2: 933–942. doi: 10.1016/S2213-2600(14)70232-2 [DOI] [PubMed] [Google Scholar]
- 13.Gagnon L, Leduc M, Thibodeau JF, et al. A newly discovered antifibrotic pathway regulated by two fatty acid receptors: GPR40 and GPR84. Am J Pathol 2018; 188: 1132–1148. doi: 10.1016/j.ajpath.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 14.Labeguere F, Dupont S, Alvey L, et al. Discovery of 9-cyclopropylethynyl-2-((S)-1-[1,4]dioxan-2-ylmethoxy)-6,7-dihydropyrimido[6,1-a]isoquinolin-4-one (GLPG1205), a unique GPR84 negative allosteric modulator undergoing evaluation in a phase II clinical trial. J Med Chem 2020; 63: 13526–13545. doi: 10.1021/acs.jmedchem.0c00272 [DOI] [PubMed] [Google Scholar]
- 15.Saniere L, Marsais F, Jagerschmidt C, et al. Characterization of GLPG1205 in mouse fibrosis models: a potent and selective antagonist of GPR84 for treatment of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2019; 199: A1046. doi:0.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A1046 [Google Scholar]
- 16.Vanhoutte F, Dupont S, Van Kaem T, et al. P612. Human safety, pharmacokinetics and pharmacodynamics of the GPR84 antagonist GLPG1205, a potential new approach to treat IBD. J Crohns Colitis 2015; 9: S387. doi: 10.1093/ecco-jcc/jju027.730 [DOI] [Google Scholar]
- 17.Clukers J, Lanclus M, Mignot B, et al. Quantitative CT analysis using functional imaging is superior in describing disease progression in idiopathic pulmonary fibrosis compared to forced vital capacity. Respir Res 2018; 19: 213. doi: 10.1186/s12931-018-0918-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vancheri C, Kreuter M, Richeldi L, et al. Nintedanib with add-on pirfenidone in idiopathic pulmonary fibrosis. results of the INJOURNEY trial. Am J Respir Crit Care Med 2018; 197: 356–363. doi: 10.1164/rccm.201706-1301OC [DOI] [PubMed] [Google Scholar]
- 19.Si-Mohamed SA, Nasser M, Colevray M, et al. Automatic quantitative computed tomography measurement of longitudinal lung volume loss in interstitial lung diseases. Eur Radiol 2022; 32: 4292–4303. doi: 10.1007/s00330-021-08482-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolb M, Raghu G, Wells AU, et al. Nintedanib plus sildenafil in patients with idiopathic pulmonary fibrosis. N Engl J Med 2018; 379: 1722–1731. doi: 10.1056/NEJMoa1811737 [DOI] [PubMed] [Google Scholar]
- 21.Raghu G, van den Blink B, Hamblin MJ, et al. Effect of recombinant human pentraxin 2 vs placebo on change in forced vital capacity in patients with idiopathic pulmonary fibrosis: a randomized clinical trial. JAMA 2018; 319: 2299–2307. doi: 10.1001/jama.2018.6129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalil N, Manganas H, Ryerson CJ, et al. Phase 2 clinical trial of PBI-4050 in patients with idiopathic pulmonary fibrosis. Eur Respir J 2019; 53: 1800663. doi: 10.1183/13993003.00663-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richeldi L, Azuma A, Cottin V, et al. Trial of a preferential phosphodiesterase 4B inhibitor for idiopathic pulmonary fibrosis. N Engl J Med 2022; 386: 2178–2187. doi: 10.1056/NEJMoa2201737 [DOI] [PubMed] [Google Scholar]
- 24.Galapagos NV/Gilead Sciences . Galapagos and Gilead discontinue ISABELA phase 3 trials in IPF. 2021. www.glpg.com/press-release/2118/galapagos-and-gilead-discontinue-isabela-phase-3-trials-in-ipf Date last accessed: 22 October 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01794-2022.Supplement (774.2KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01794-2022.Shareable (336KB, pdf)